INTRODUCTION
Multipotent mesenchymal stromal cells
Multipotent mesenchymal stromal cells (MSCs), also referred to as mesenchymal stem cells, were originally described by Alexander Friedenstein in 1976 as nonhaematopoietic marrow cells in culture[1]. MSCs were identified as stromal cells that present plastic adherent characteristics and the ability to form in vitro fibroblast-like colonies (CFU-F). In 1991, Caplan defined MSCs as a supportive cell population capable of differentiating into several mesodermal cell lineages including muscle, bone marrow stroma, fibroblasts, osteocytes, adipocytes and chondrocytes[2].
Phenotypically, MSCs are characterized by the expression of surface membrane molecules such as endoglin (CD105), NT5E (CD73), and Thy-1 (CD90) and the lack of expression of haematopoietic (CD45, CD34, CD11b/c and CD19) and endothelial (CD31, KDR) markers and of HLA-DR, an immune-associated molecule linked to major histocompatibility complex class II (MHC II)[3]. In addition, MSCs resemble vascular pericytes, and due to their wide perivascular distribution[4,5], these cells can be identified and expanded ex vivo from a multitude of tissues and organs, for instance: (1) Bone marrow[6]; (2) the umbilical cord[6]; and (3) adipose tissue[7], highlighting MSCs as a very attractive cell subpopulation for several clinical applications.
From a therapeutic perspective, MSCs possess advantages such as low immunogenicity, migration to injured tissues and the production of various trophic/growth factors (e.g., cytokines, chemokines and diverse growth factors), which may be related primarily to the mechanisms of immunoregulation, anti-fibrosis, the induction of endogenous tissue progenitor cells, anti-apoptosis, pro-angiogenesis and chemoattraction. Moreover, MSCs may act as effector agents in the modulation of internal gene expression by releasing extracellular microvesicles enriched with small regulatory RNAs[8-10].
In light of their functional multipotentiality, MSCs are essentially distinguished from other cells by retaining immunomodulatory properties that globally reduce the inflammation process, suppressing cellular alloreactivity. In this regard, studies have shown that the infusion of MSCs reduces local and systemic tissue injury in distinct experimental models, e.g., neural encephalomyelitis[11], pulmonary fibrosis[12], kidney injury[13] and heart inflammation[14], mainly via shifting from a pro-inflammatory to an anti-inflammatory profile. Thus, the immunosuppressive abilities of MSCs may be useful to repair tissue damaged by immune system aggression, for instance: (1) Crohn’s disease[15]; (2) ulcerative colitis[16]; (3) graft-versus-host disease (GVHD) followed by halogen transplantation[17]; and (4) organ rejection in transplants[18]. However, the majority of clinical trials with MSCs remain in phase I/II studies, and most have not clearly described a precise therapeutic response[19]. In this context, the complete elucidation of the mechanisms associated with the in vivo therapeutic effects of MSCs remains a target of intense investigation.
To date, scientists have considered MSCs a heterogenous population with several factors that can interfere in their therapeutic efficacy, such as phenotype, proliferation, secretory profile, tissue origin, donor age, culture and expansion method conditions (i.e., growth factors, cell confluence, passages, oxygen pressure and biomaterials)[20,21]. Considering MSCs a manufactured “product” for cell-based therapy, it is essential to standardize operational processes, which must be in accordance with guidelines assigned by the international programme of good manufacturing practices, also known as “GMP”. Thus, given the high heterogeneity of cultured MSCs, it is not surprising that MSC-based therapies have not yet become a reality in operating centers distributed in several countries.
In an attempt to establish a global organizational process for MSC therapeutic programmes, there are potential strategies for refining the preparation and application of MSC cultures. According to several described approaches, the activation of MSCs via specific receptors is an innovative and accessible methodology for standardizing the use of these cell populations. Studies have found that MSCs express certain key receptors (e.g., TLRs, TNFRs, INFRs) that are activated by the inflammatory microenvironment, modulating its immunosuppressive activity[22,23]. This phenomenon was already demonstrated in vitro and in vivo, where important molecules (i.e., TNF-α, INF-γ, PAMPs, DAMPs, IDO, iNOS, PGE-2) and signalling pathways (i.e., PKR, STAT-1, NF-κB) were shown to be regulated during MSCs activation. In fact, one study found that MSCs exposed to IFN-γ became activated and efficiently suppressed the deleterious effects of an in vivo GVHD experimental model almost five-fold more strongly than unstimulated MSCs[24]. However, the precise role of each receptor, its molecular interactions and its impact on the biology of MSCs yet remain to be investigated.
ARYL-HYDROCARBON RECEPTOR
The aryl-hydrocarbon receptor (AhR) is a member of the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors and is characterized as ligand-dependent transcriptional regulator acting on the modulation of a distinct number of genes associated with several biological processes including: (1) The cell cycle; (2) apoptosis; (3) hypoxia; (4) the circadian cycle; (5) differentiation; (6) haematopoiesis; (7) migration; and (8) the immune response[25]. AhR is considered a multifunctional sensor that responds to toxic/pollutant signals from the environment (e.g., dioxins, pollutants and by-products of metabolism), promoting the regulation of gene expression in responsive cells. AhR can be stimulated by a myriad of specific endogenous or exogenous ligands called hydrophobic aromatic hydrocarbons [e.g., polycyclic aromatic hydrocarbons (PAHs), halogenated aromatic hydrocarbons (HAHs) and planar polychlorinated biphenyls (PCBs)], which can be represented by two main classes: (1) Synthetic and non-biological: e.g., dioxins and dibenzofurans; and (2) natural and biological: e.g., carotenoids, flavonoids and tryptophan-derived metabolites, such as kynurenines[26,27].
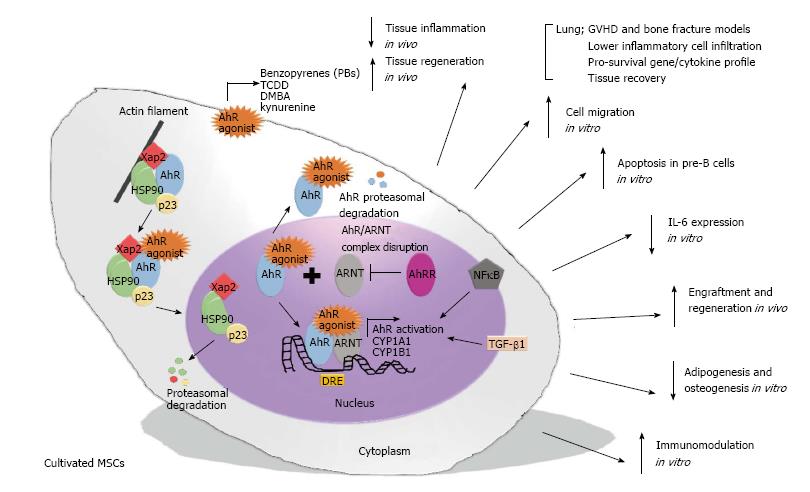
AhR activation starts when a chemical signal enters the target cells and binds with strong affinity to the AhR cytosolic multiprotein complex, which is associated with actin filaments in the cytoplasm. This complex is composed of two Hsp90 chaperone molecules, along with co-chaperones such as hepatitis B virus X-associated protein (XAP2 or AIP) and p23 protein. After stimulation, AhR changes its conformational structure to present the nuclear localization sequence, which promotes its own translocation from the cytoplasm to the nucleus via the importin β protein. In the nucleus, the AhR-ligand complex detaches from the triplex protein (hsp90/XAP2/p23) to form a dimer with a nuclear protein responsible for AhR translocation, ARNT, which converts AhR to an active isoform with elevated affinity for DNA. Then, the AhR-ligand-ARNT complex binds to a specific promoter regulatory region on DNA [5’-T (N) GCGTG-3’] known as the dioxin-responsive element/sequence (DRE), which is located upstream of the specific CYP1A1 locus or other genes responsive to the AhR signal. In contrast, the dimerization of ARNT with AhR repressor protein (AhRR) leads to non-association of the AhR-ligand complex and ARNT protein, and consequently, the AhR-ligand complex exposes its nuclear export sequence to the cytoplasm and is further conducted to the ubiquitination and proteasome degradation process (Figure 1)[28,29].
Figure 1 Illustration demonstrating a hypothetical summary of the potential effect of aryl-hydrocarbon receptor activation on multipotent mesenchymal stromal cell function.
AhR-mediated MSC activation occurs by a cascade of events that substantially modulate the function of the MSCs by mechanisms associated with: (1) The induction of death signalling in pro-inflammatory cells, i.e., pre-B cells; (2) suppression of pro-inflammatory cytokines, i.e., IL-6; (3) the improvement of migration and regenerative potential in acute inflammatory models, i.e., asthma; (4) the inhibition of mesodermal differentiation, i.e., adipogenesis; and (5) the up-regulation of global immunosuppression, i.e., the up-regulation of immunoregulatory genes. AhR: Aryl-hydrocarbon receptor; MSC: Multipotent mesenchymal stromal cell.
AhR is closely linked to the regulation and control of immunity, and there is a substantial amount of evidence supporting the hypothesis that AhR may influence PAH/HAH/PPB-mediated immunoregulation[27,30]. Thus, some reports have shown that AhR activation by particular ligands (i.e., LPS, tetrachlorodibenzo-p-dioxin or TCDD, tryptophan metabolites) can differentially modulate various effects on immunological cells, for example: (1) The function and development of regulatory T cells; (2) the differentiation of Th17 cells; (3) the generation and activity of monocytes and dendritic cells[31-33]; (4) the growth and maturation of mast cells; (5) differentiation/maturation and antibody production by B cells; (6) polarization and cytokine production in macrophages[34,35]; and (7) haematopoietic stem cell expansion, migration, and plasticity[36,37]. Another emerging aspect associated with AhR transcriptional biology involves its cooperative relationship with other signalling pathways, which may interact with AhR or by antagonism, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), or by synergism, such as the signal transducer and activator of transcription 1 (STAT-1) and the nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2). These multiple interactions of different signalling pathways can generate distinct responses according to the nature of the stimulus and the cell type target and thus qualifies as a tissue-specific molecular interchange[26,29,38].
Functionally, AhR can regulate an extensive number of protein-coding genes, specifically those associated with xenobiotic metabolizing enzymes, such as CYP1A1, which is a member of the superfamily of oxidative enzymes called cytochrome P-450 monooxygenases[28]. Among the potential ligands related to AhR activation, the tryptophan degradation products (i.e., tryptamine and kynurenine) are considered natural endogenous stimuli. Under normal conditions, these metabolites are classified as weak inducers, but after a physiological disturbance, their concentration may rise abruptly, leading to strong activation via CYP1A1 signalling[28]. In this sense, we can assume that an environment of intense inflammation and tissue injury may contain sufficient tryptophan-derived products for MSC activation via AhR, improving the MSC-mediated immunotherapeutic responses. According to these findings, we believe that the immunomodulatory potential of MSCs can be strictly regulated by AhR, and their activation may be essential for MSCs to exert their immunosuppressive response. Indeed, some PAH/HAH-derived metabolites themselves can, either directly or indirectly via AhR, down-regulate immune-associated pathways such as the antigen-specific T and B cell responses, compromising lymphocyte development. However, the influence of AhR on the regulation of MSC-induced immunosuppression remains poorly investigated[30].
AhR ACTIVATION IN MSCs
To explore the participation of AhR in MSC activation, it was predicted that MSC priming by AhR is a mechanism intimately associated with its immunotherapeutic response. According to this perspective, it has been shown in vitro that MSCs, under standard conditions, support the growth/differentiation of B lymphocytes, but when the MSCs are pre-stimulated by AhR agonist (i.e., DMBA), these cells exert an inverse immunoregulatory response, inducing apoptosis by cell-cell contact in CD43+ pro/pre-B cells. This cell death signal is regulated mainly via a specific soluble stromal cell-dependent death signal that is presumably regulated by its responsive AhR gene, CYP1A1[10,30,39,40]. Later, the authors of the same study reported that the addition of a precise and competitive inhibitor of AhR, α-naphthoflavone (α-NF), blocked DMBA-induced pre-B cell apoptosis in these bone marrow cell co-cultures[39].
Subsequently, another work showed that the activation of AhR in MSCs can also modulate their secretory profile. In this report, the MSCs were stimulated with AhR-specific ligands (i.e., DMBA and TCDD), and after stimulation, these cells had their production of mRNA/protein of interleukin-6 (IL-6) suppressed through a process partially regulated by the coactivation of NF-κB signalling pathways[41]. IL-6 is required for the growth and terminal differentiation of progenitor blood cells, and its aberrant expression is reportedly associated with autoimmune-related disorders (i.e., systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis)[42-44]. Thus, this evidence illustrates the intrinsic importance of AhR-mediated MSC activation, highlighting the role of the IL-6/AhR axis in the regulation of the immune system.
Additionally, it was observed that the therapeutic abilities of MSCs can be modulated by AhR activation. The MSCs were activated by AhR-specific agonists (i.e., TCDD and cockroach allergen extract) and showed increased CYP1A1 and CYP1B1 expression. This process was accompanied by an elevated migration potential in vitro. Later, the authors also demonstrated in mouse models of experimental asthma that MSCs activated by AhR efficiently engrafted to injury sites and attenuated allergen-induced lung inflammation (i.e., reduced cell infiltrate and change cytokine profile), mainly via TGF-β1 modulation[45].
Moreover, it was determined that AhR stimulation in MSCs can also prevent their multipotent differentiation potential. It was shown that treatment with benzo(a)pyrene (BPs), a specific AhR agonist, markedly inhibited the terminal adipogenic differentiation of MSCs in an AhR-dependent manner, with reduced expression of classical adipogenic markers (FABP4), triglyceride enzymes (G3PDH) and adipogenic transcription factors (PPARγ and CEBPβ)[31]. Despite the decreased expression of AhR in differentiated MSCs, the expression of its target gene CYP1B1 remained elevated, indicating that AhR activation was fully functional during adipogenesis. Later, this same study demonstrated that the use of α-NF, an AhR antagonist, abrogated the AhR-mediated inhibition of MSC adipogenesis[31]. Complementarily, another report demonstrated that BP treatment inhibited adipocyte differentiation in vitro by down-regulating the PPARγ signal and increased the expression of cytochrome P450 (CYP1A1) in canine MSCs[46]. In addition, it was detected in vitro that TCDD-stimulated MSCs suppressed the mRNA levels of osteoblastic markers (i.e., Runx2, Ocn and Alp) in a dose-dependent manner through a process mediated by the inhibition of β-catenin expression. Later, similar observations in MSCs derived from inflamed collagen-induced arthritis mice (a possible environment for AhR activation) showed elevated nuclear expression and translocation of AhR and, in consequence, inhibition of osteogenesis-associated genes as well as reduced β-catenin expression[47]. In fact, an additional study verified that AhR activation by BPs inhibited the MSC mesodermal differentiation, and when these activated MSCs were applied in a mouse model of bone fracture, the tibial ossification was affected mainly via SMAD-dependent (e.g., TGF-β1/SMAD4) and SMAD-independent (e.g., TGF-β1/ERK/AKT) signals[48]. Therefore, these results illustrate that the adipogenesis and osteogenesis signalling pathways are also potential targets for AhR regulation in MSCs.
Finally, another group found that the activation of MSCs through kynurenine, a natural AhR agonist, can enhance its immunosuppressive response. The authors detected that MSCs stimulated by kynurenine were more effective in suppressing in vitro lymphocyte proliferation than MSCs stimulated by IFN-γ and TGF-β separately. Further, the analysis of cytokines in the supernatants of lymphocyte/MSC co-cultures demonstrated that the combination of kynurenine with IFN-γ and TGF-β stimuli significantly reduced IL-6 and IL-17 secretion. In line with these findings, the authors also found that the combination of three effector stimuli (IFN-γ, TGF-β and kynurenine) promoted the overexpression of important immunomodulatory genes in MSCs (e.g., iNOS, IDO, COX2, HO-1, PGE-2, LIF and PD-L1). Later, when these triple-activated MSCs were used in the treatment of an experimental model of GVHD, the stimulated MSCs substantially decreased the inflammation and tissue injury score at a more significant level than normal unstimulated MSCs[49].
Altogether, these recent studies suggest that AhR activation can substantially modulate the function of MSCs by mechanisms associated with: (1) The induction of the death signal in pro-inflammatory cells, i.e., pre-B cells; (2) the suppression of pro-inflammatory cytokines, i.e., IL-6; (3) the improvement of migration and regenerative potential in acute inflammatory models, i.e., asthma and GVHD; (4) the inhibition of mesodermal differentiation, i.e., adipogenesis and osteogenesis; and (5) the up-regulation of global immunosuppression, i.e., the up-regulation of immunoregulatory genes (Figure 1).
CONCLUSION
The immunosuppressive properties of MSCs are of great interest for cellular therapy; however, randomized double-blind clinical studies have not shown clear benefits to date[42,50]. This inconclusive large-scale clinical result may be associated with the variety of cytokines/agonists in the distinct environments that MSCs encounter in vivo. In this context, the molecular mechanisms involved in the reparative status of MSCs through the activation of sensitive immune-associated receptors are so far unclarified, and, therefore, they are indispensable parameters for investigation. Thus, MSC activation is currently considered a sine qua non condition for MSCs and their bioproducts (i.e., trophic factors and microvesicles) to exert their immunoregulatory response.
Considering this perspective, the quality of the immunoregulatory profile of MSCs can be considerably improved when these cells are exposed to sufficient levels of sensitive ligands (i.e., cytokines/growth factors). On the other hand, MSCs not subjected to pre-stimulation tend to decrease or lose their intrinsic immunosuppressive potential, promoting an undesired inflammatory response[49]. In this context, we hypothesized that the optimal immunomodulatory potential of MSCs can be obtained by establishing a steady regulatory phenotype in MSCs using precise MSC-responsive ligands as AhR agonists. Thus, the activation of AhR in MSCs should be extensively explored as a mechanism in relevant pre-clinical and experimental studies, in the attempt to improve the applicability of MSCs in a set of degenerative and immunological diseases.
However, questions regarding the mechanisms of the MSC immunoregulatory response remain inconclusive. In this sense, MSC immunoregulation can vary among species, for instance, IDO up-regulation in MSCs is better described in humans, while inducible nitric oxide synthase (iNOS) is a key regulatory enzyme in mouse MSC immunomodulation[49]. In addition, the elucidation of the cross-talk between AhR agonists and other sensitive molecules (e.g., IFNγ, TGF-β, TNF-α, LPS and others) is a detrimental factor in applying the immunosuppressive response of MSCs. Moreover, the influence of MSCs in another set of experimental models is also important to consider. In line with this purpose, Aleman et al[49] (2015) reported that kynurenine, in combination with other effector stimuli (IFNγ and TGF-β), can induce elevated IDO, COX2, iNOS, and PGE-2 expression in MSCs and, at the same time, reduce the expression of EGFR, MHC II and IL-6. Thus, further investigations should focus on identifying the major components that trigger the activation of the AhR signal and its cross-talk with other signalling pathways, to precisely understand the regulatory mechanism of AhR influence on MSC function. In line with this goal, aspects of this mechanism have begun to be investigated, such as the impact of AhR activation on MSC adipogenesis or osteogenesis; nevertheless, the specific AhR-dependent signalling pathways by which AhR agonists affect MSC-associated mesodermal differentiation also remain to be determined.
In conclusion, we hope that the findings discussed here in this minireview will contribute to better comprehension of the major mechanisms behind MSC immunoregulation and provide a basic background for the development of innovative studies focused on the molecular cascade associated with AhR activation in MSCs. In summary, the study of AhR activation can promote new insights for the better investigation of molecular signalling pathways associated with the regenerative and immunosuppressive potential of MSCs, and consequently, these studies will support the development of potential MSC-derived therapies for a wide variety of immune-associated disorders.
ACKNOWLEDGMENTS
We would like to thank all the professionals who contributed to the discussion and elaboration of this minireview.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu L, Liu SH, Miyagoe-Suzuki Y, Saeki K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ