Published online Aug 26, 2017. doi: 10.4252/wjsc.v9.i8.127
Peer-review started: February 8, 2017
First decision: April 17, 2017
Revised: June 30, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: August 26, 2017
Processing time: 211 Days and 3.1 Hours
To investigate whether human embryonic stem cells (hESCs) could be made to attach, grow and differentiate on a human Descemet’s membrane (DM).
Spontaneously differentiated hESCs were transferred onto a human corneal button with the endothelial layer removed using ocular sticks. The cells were cultured on a DM for up to 15 d. The genetically engineered hESC line expressed green fluorescent protein, which facilitated identification during the culture experiments, tissue preparation, and analysis. To detect any differentiation into human corneal endothelial-like cells, we analysed the transplanted cells by immunohistochemistry using specific antibodies.
We found transplanted cells form a single layer of cells with a hexagonal shape in the periphery of the DM. The majority of the cells were negative for octamer-binding transcription factor 4 but positive for paired box 6 protein, sodium potassium adenosine triphosphatase (NaKATPase), and Zona Occludens protein 1. In four of the 18 trials, the transplanted cells were found to express CK3, which indicates that the stem cells differentiated into corneal epithelial cells in these cases.
It is possible to get cells originating from hESCs to become established on a human DM, where they grow and differentiate into corneal endothelial-like cells in vitro.
Core tip: This is the first report on the interaction between human embryonic stem cells (hESCs) and the inner parts of the human cornea. When hESCs were transplanted onto Descemet’s membrane (DM) in vitro we found that they were able to attach to and grow on DM in a single cell layer. Furthermore, the stem cells changed their morphology from small round cells to flat hexagonal cells. The transplanted hESCs also started to express the proteins paired box 6, Zona Occludens protein 1 and sodium potassium adenosine triphosphatase (NaKATPase), which also are expressed in human corneal endothelial cells.
- Citation: Hanson C, Arnarsson A, Hardarson T, Lindgård A, Daneshvarnaeini M, Ellerström C, Bruun A, Stenevi U. Transplanting embryonic stem cells onto damaged human corneal endothelium. World J Stem Cells 2017; 9(8): 127-132
- URL: https://www.wjgnet.com/1948-0210/full/v9/i8/127.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i8.127
The cornea is the first point of contact for visual stimuli entering the nervous system. It is responsible for approximately two-thirds of the refractive power of the eye, and its transparency and a regular shape are pivotal for accuracy of perception. The adult cornea is made up of three cellular layers, the epithelium, stroma, and endothelium, separated by two basement membranes, Bowman’s and Descemet’s membrane (DM). From both a physiological and a pathological perspective, the endothelium is the most important corneal layer. The single layer of hexagonal cells that makes up the endothelium is in direct contact with the aqueous humour in the anterior segment of the eye, and preserves the transparency of the cornea by pumping water out of the stroma. Endothelial dysfunction leads to oedematous accumulation of water in the stroma, disruption of collagen fibrils, and opacification of the cornea[1,2].
Serious corneal damage can in many cases only be treated with transplantation. Due to the global shortage of donated human tissue, there is a growing interest in developing stem cell technology as an alternative. Stem cells are undifferentiated cells that can self-renew and generate one or more differentiated daughter cells. Embryonic stem cells are considered precursor cells for all cells in the body, and therefore are ultimately the origin of all tissue. Their presence is not limited to foetal development, but is preserved along with precursor cells into adulthood. In normal or damaged adult tissue, stem cells maintain a balance between cell production and cell loss by continuously replacing older cells with newer ones. Human embryonic stem cells (hESCs) are derived from the inner cell mass of the blastocyst. They are pluripotent, which means that under certain specific circumstances they can potentially form cells of all three germ layers (ectoderm, mesoderm, and endoderm)[3,4]. hESCs can preserve this pluripotency during long-term culture, and hence may be useful in various scientific and clinical applications[5].
We have previously shown that it is possible to transplant pre-cultured cells derived from hESCs onto exposed (partly stripped from epithelial cells) Bowman’s membrane of the human cornea[6]. In our experiments, the transplanted cells attached efficiently to the membrane and were not detached by the sliding forces from the recipient epithelial cells during wound healing. Furthermore, one to four cell layers of epithelial-like cells were formed by the transplanted cells. Given this initial success in facilitating wound healing of the epithelium, we wanted to expand our research to include the corneal endothelium.
In the current study we attempted to get hESCs to differentiate into corneal endothelial-like cells in vitro on human corneas that had been partially or completely cleared of all existing endothelial cells. The overall aim was to further develop techniques for reconstructing the corneal endothelium.
The study was approved by the Ethics Committee at the Universities of Gothenburg and Malmö (approval number 067-04 and M155-14). In total we conducted experiments on 18 corneas. In all cases, undifferentiated hESCs were prepared by Cellectis AB (Gothenburg, Sweden), using a protocol previously described in detail[7]. In short, hESCs of line SA121 (Cellectis, Gothenburg, Sweden)[4] were genetically modified to constitutively express green fluorescent protein (GFP) under the human elongation factor 1-alpha (EF1-α) promoter. The genetically modified cell line had previously been characterized to confirm that the cells had remained pluripotent and diploid through the transfection procedures. This clone was used to facilitate the identification of the transplanted cells in the immunohistochemistry (IHC) analysis.
Initially, the hESCs were cultured in Cellectis DEF-Culture System (DEF-CS™; Cellectis AB, Sweden), which includes neither feeder cells nor any type of membrane. Before transplantation, the homogeneously undifferentiated hESCs were allowed to initiate differentiation. For this, the cells were cultured in KnockOut™ Dulbecco’s Modified Eagle’s Medium (D-MEM) with 20% knock-out serum replacement (SR) and 5% foetal bovine serum (FBS) (Life Technologies Europe BV), 10 μg/mL Hygromycin (Invitrogen, Carlsbad, CA, United States) and 5 μmol/L of Rho-associated protein kinase (ROCK) inhibitor (Y-27632, Sigma-Aldrich, Stockholm, Sweden) for 14-22 d. On the day of the transplantation, the differentiation medium was removed and the cells were rinsed once with 1 × phosphate buffer saline (PBS) (Ca-/Mg-). Subsequently, the cells were disassociated by trypsin (TrypLE™ Express, ThermoFisher Scientific Company, Waltham, MA, United States) added into the well, and incubated in 37 °C for 3 min × 5 min. The well was gently tapped after each 5-min interval to get the cells to detach. The suspension was centrifuged and the cell pellet resuspended in 1000 μL of the same culture medium as used for differentiation. The final cell concentration was 4 million cells/mL. About 120000 cells were put on each cornea. Human corneal tissue was obtained from patients undergoing penetrating keratoplasty for keratoconus or for corneal decompensation at Mölndal University Hospital in Gothenburg, Sweden. The removed corneal button, 7.5-8.0 mm in diameter, was kept in Modified Eagle’s Medium (MEM) (Invitrogen, Paisley, United Kingdom) until the surgery was completed, and then transferred to the laboratory for further processing. Prior to each experiment, the endothelial cells were removed using an ocular stick (Pro-ophta ocular stick, Lohmann and Rauscher, Regensdorf, Germany). First using the short end, all fluid was removed from the surface. Then the cells were removed with short, gentle strokes parallel to the surface. By adjusting the pressure the cells were made to detach without causing damage to the DM. Repeating this motion six to eight times removed close to all endothelial cells. The GFP-expressing cells were cultured on the corneas for 10-15 d. The medium was changed every 2-3 d throughout the period.
After the 18 corneas were cultured they were fixed in 4% formaldehyde for 24 h and then placed in 70% ethanol (EtOH) until paraffin embedding. They were then embedded in paraffin and tissue slides prepared using methods previously described[6].
The tissue sections were deparaffinized by sequential immersion in Tissue-Clear (Sakura Finetek, Tokyo, Japan) for 2 min × 10 min, 99% isopropanol for 2 min × 5 min, 95% isopropanol for 1 min × 5 min, 70% isopropanol for 1 min × 5 min, and distilled water for 1 min × 5 min, after which they were gently dried.
The tissue sections were rinsed twice with PBS Ca-/Mg- for 5 min each time, and then treated with Proteinase K (Sigma-Aldrich, St. Louis, MS, United States) for 10 min, followed by rinsing with PBS. Next, a 5% mixture of normal goat serum (Vector Laboratories, Inc., Burlingame, CA, United States) in PBS was applied for 30 min, after which the primary antibodies were applied overnight in a refrigerator.
The tissue sections were rinsed by applying PBS for 2 min × 5 min. The secondary antibodies used were CK3 [mouse monoclonal antibody, AE5 (ab77869); Abcam, Cambridge, United Kingdom], a marker for corneal epithelial cells (Abcam); paired box 6 (PAX-6) [mouse monoclonal antibody, AD2.38 (ab78545), Abcam], a marker for a transcription factor important in the development of the eye; octamer-binding transcription factor 3/4 (OCT-3/4) [mouse monoclonal antibody, C-10 (sc-5279), Santa Cruz Biotechnology, Inc., Santa Cruz, CA, United States], a marker for embryonic stem cell activity; sodium potassium adenosine triphosphatase (NaKATPase) [rabbit monoclonal antibody (ab58475), Abcam], a marker for NaKATPase pump function; and Zona Occludens protein 1 (ZO-1) [Guinea pig polyclonal antibody (NBP1-49669), Novus Biologicals, Littleton, CO, United States], a marker for tight junctions.
Slides were washed in PBS Ca-/Mg- and incubated with a secondary antibody (Alexa Fluor® goat anti-mouse or anti-rabbit antibody; Invitrogen, Eugene, OR, United States) for 2 h at room temperature and in the dark. The slides were then rinsed once more with PBS Ca-/Mg- before a small drop of mounting medium for fluorescence with 4’,6-diamidino-2-phenylindole (DAPI) (Vectashield, Vector Laboratories) was applied and the tissue sealed with a thin glass slide. The analysis was performed on a Nikon fluorescence microscope equipped with DAPI, tetramethylrhodamine (TRITC) and fluorescein isothiocyanate (FITC) filters (360, 490, and 570 nm).
This study was conducted in accordance with the tenets of the Declaration of Helsinki and with permission from the Ethics Committee of the University of Gothenburg.
The GFP signal was easily detected in the specimens harbouring stem cells, making it easy to identify the transplanted hESCs. In the tissue sections, it was obvious where the GFP-positive donor cells ended and the recipient cells began.
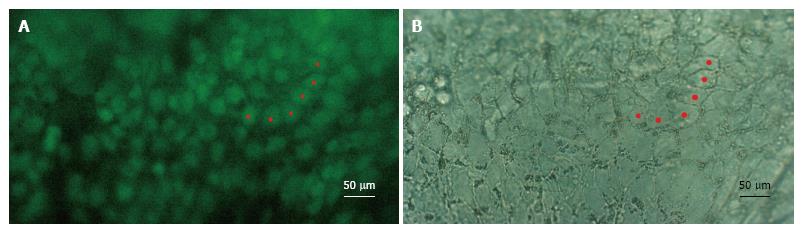
Several experiments were performed in an attempt to optimize the time required for culturing the hESCs on the donor cornea (data not shown). All of the 18 experiments revealed hESCs attached to the donor cornea. Briefly, the course of events can be described as follows: The transplanted hESCs attached to the DM within 6 h, and cells started to grow in different directions. The cells grew as a monolayer (confirmed later with IHC) and eventually covered the entire surface of the DM.
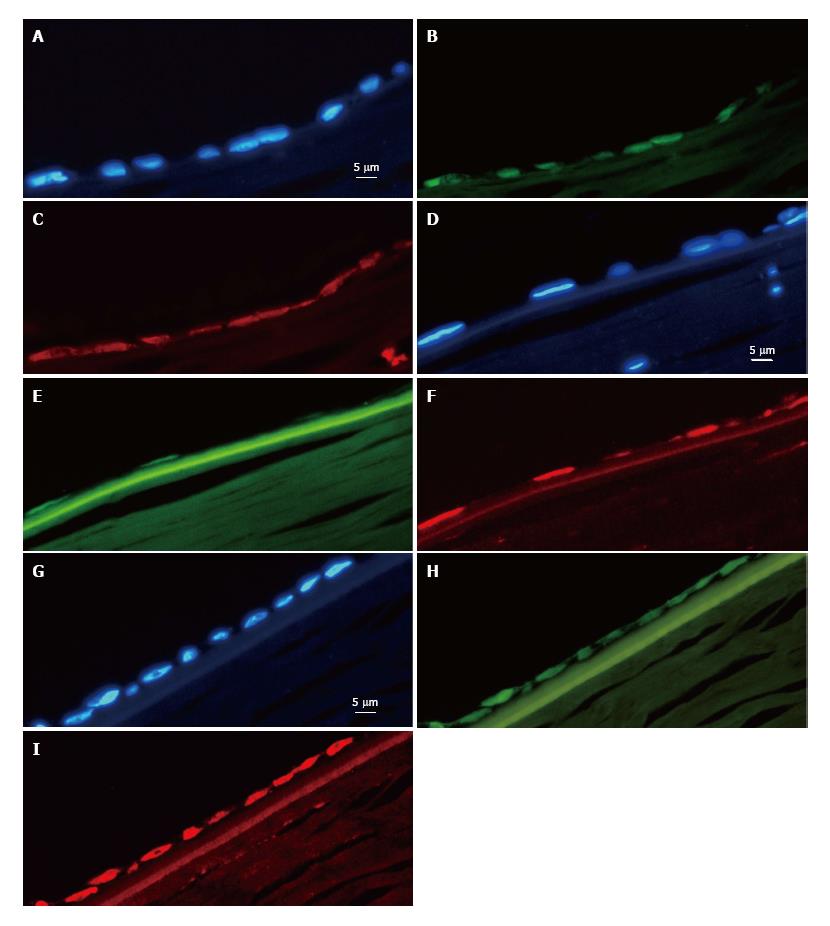
In all of the experiments where hESCs attached to the DM, they formed clusters of between one and eight cells in thickness. However, as they spread out towards the periphery, they did so in a single cell layer and assumed a flat form as found in intact endothelia. Importantly, the hESCs also lost their roundness and assumed a more polygonal form reminiscent of their intact hexagonal shape (Figure 1). Furthermore, sections of the cells in the single cell layer revealed the flattened morphology typical of endothelial cells (Figure 2). Expression of octamer-binding transcription factor 4 (OCT-4), PAX-6, NaKATPase, CK3 and ZO-1 in transplanted hESCs.
We found expression of octamer-binding transcription factor 4 (OCT-4) in ten out of 18 trials, two with clear signals all over the transplanted area but the other eight showing a very weak signal in 5%-20% of the cells in multilayers. In the remaining eight trials, OCT-4 was completely absent. The hESCs were found to express PAX-6 clearly in nine cases (Figure 2), weakly in five and not at all in four. Out of 18 experiments, 14 were positive for NaKATPase (Figure 2); in two experiments we only found NaKATPase faintly expressed and in another two not at all. Fifteen out of 18 corneas were negative for CK-3, two had a weak signal and one was deemed positive. Expression of ZO-1 was found in all of the transplanted corneas (Figure 2).
We have shown that it is possible to culture hESCs on partially decellularized human corneal DM, were they assume the form of endothelial cells and start expressing relevant proteins.
The sparse OCT-4 expression in the hESCs attached to the DM indicates that the hESCs have started to lose their stem cell qualities and are beginning to adopt features associated with corneal endothelial cells, as shown by the expression of PAX-6, and NaKATPase. The former, PAX-6, is a non-specific indicator of development in the eye. The frequent NaKATPase expression seen in the transplanted hESCs points to high activity of the NaKATPase pump, as would be expected in endothelial cells responsible for actively maintaining the fluid balance within the cornea. We found no correlation between the number of days in pre-culture and expression of the studied proteins. Furthermore, there were no correlations between the expression of OCT-4, PAX-6, NaKATPase, ZO-1 and CK3 and the time during which the cells were grown on DM.
To distinguish between corneal endothelial activity, on the one hand, and epithelial cells, on the other, we used CK3 marker for epithelial cells. As there were some cases that were positive for CK3, we conclude that the differentiation of hESCs towards corneal endothelial cells is not as straightforward as differentiation towards corneal epithelial cells. Hence, there is a need for further optimization of the culture system to ensure differentiation into corneal endothelial cells.
Although there are published studies on culturing of adult endothelial cells (for a review, see Mimura et al[8]) and on transplantation of hESC-derived, endothelial-like cells onto rabbit corneas[9], there are no published data on studies of hESCs transplanted onto human corneal endothelial cells.
The knowledge of stem cell culturing (embryonic, foetal, and adult stem cells) for clinical applications is still rudimentary. Future developments within the field of stem cell research will probably lead to different options for different applications. For the treatment of certain diseases, adult stem cells will be the first choice; but for others, embryonic or foetal stem cells will be more suitable. Stem cell research may in the future give us a toolbox of specialized cells which can be matched to different clinical and individual conditions. The current study is a step towards better understanding of how embryonic stem cells can contribute to improved function in the human cornea and to finding new treatment strategies for patients with corneal disorders or damage. We have shown that hESCs attach and grow on DM, and that the cells have the potential to differentiate into corneal endothelial-like cells. However, further optimization of the process is needed. We suspect that the “pre-differentiation” period is key in solving the problem of variation obtained. It seems that just adding ROCK inhibitor is not sufficient for a 100% success rate in differentiation; other, still unknown factors may be used to elevate the efficiency of the differentiation into corneal endothelial cells.
It is possible to get cells originating from hESCs to establish onto a human DM where they grow, and differentiate into corneal endothelial-like cells in vitro.
Serious corneal damage can in many cases only be treated with transplantation. Because of a global shortage of donated human tissue, there is a growing interest in developing stem cell technology as an alternative. Human embryonic stem cells (hESCs) could be an inexhaustible source in corneal damage treatment.
There are several ongoing studies on culturing adult corneal endothelial cells for transplantaion. Some of them seem very promising. However, hESCs would be a complement to these adult cells and have the advantage of being of an earlier origin with the potential for lifelong survival after transplantation.
This is the first report on the interaction between hESCs and the inner parts of the human cornea. When hESCs were transplanted onto the Descemet’s membrane (DM) in vitro the authors found that the stem cells were able to attach to and grow on the DM in a single cell layer. Furthermore, they changed their morphology from small round cells to flat hexagonal cells. The transplanted stem cells also started to express the proteins paired box 6, Zona Occludens protein 1 and sodium potassium adenosine triphosphatase (NaKATPase) which are also expressed in human corneal endothelial cells.
These results point to the possibility of using embryonic stem cell-derived corneal cells for reconstruction of damaged corneas.
This is a well written manuscript evaluating the results of an attempt to get hESCs to differentiate into corneal endothelial like cells in vitro on human corneas that had been partially or completely cleared of all existing endothelial cells.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Altinors DD, O'Connor TR, Shawcross SG, Vladimir H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Levis H, Daniels JT. New technologies in limbal epithelial stem cell transplantation. Curr Opin Biotechnol. 2009;20:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Peh GS, Beuerman RW, Colman A, Tan DT, Mehta JS. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91:811-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Boulton M, Albon J. Stem cells in the eye. Int J Biochem Cell Biol. 2004;36:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Heins N, Englund MC, Sjöblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Mountford JC. Human embryonic stem cells: origins, characteristics and potential for regenerative therapy. Transfus Med. 2008;18:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Hanson C, Hardarson T, Ellerström C, Nordberg M, Caisander G, Rao M, Hyllner J, Stenevi U. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013;91:127-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Thyagarajan B, Liu Y, Shin S, Lakshmipathy U, Scheyhing K, Xue H, Ellerström C, Strehl R, Hyllner J, Rao MS. Creation of engineered human embryonic stem cell lines using phiC31 integrase. Stem Cells. 2008;26:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res. 2013;35:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |