INTRODUCTION
In 2006, Takahashi et al[1] reported that they successfully reprogrammed skin fibroblasts into pluripotent stem cells, which are undistinguishable from embryonic stem (ES) cells, using oct4, sox2, klf4 and c-myc. They called this new pluripotent stem cell type “induced pluripotent stem cells (iPSCs)”. Human induced pluripotent stem cells (hiPSCs) are rejuvenated, proliferate in vitro keeping their pluripotency, and differentiate into multipotent cell lineages. As a result, the induced pluripotent stem (iPS) technology was expected to advance the study of pathogenesis, drug screening, and regenerative medicine. However, in the field of skeletal muscle disease, the use of iPSCs has been relatively limited due to the difficulty of inducing skeletal muscle cells from human iPSCs in large quantities with sufficient purity. In addition, skeletal muscle derived from human iPSCs generally show embryonic phenotypes. In this review, we try to summarize the recent progress and remaining problems to be solved in inducing muscle cells from human iPSCs and their application.
MUSCLE SATELLITE CELLS/MYOBLAST-BASED CELL THERAPY
Muscle satellite cells are skeletal muscle-specific stem cells that reside between the muscle basement membrane and the plasma membrane of myofibers in a G0 state in adult muscle. When muscle is damaged, satellite cells are activated, proliferate (myoblasts), and fuse with injured myofibers to repair muscle tissue. In Duchenne muscular dystrophy (DMD), however, muscle satellite cells are exhausted by repeated cycles of muscle degeneration and regeneration[2,3]. As a result, myofibers are replaced by fibrotic tissue and adipocytes. In 1989, Partridge et al[4] demonstrated that direct injection of normal myoblasts into mdx muscle converted dystrophin-negative myofibers to dystrophin-positive ones. Based on this finding, clinical trials of myoblast transplantation therapy (MTT) were performed. However, MTT for DMD conducted between 1991 and 1997 was not successful[5-7]. Experiments using mouse models suggested the rapid and massive death of a substantial fraction of injected myoblasts after transplantation[8]. It was also demonstrated that satellite cells lose their regenerative ability during expansion in culture[9,10]. Because it is not possible to prepare fresh myoblasts in large quantities from limited donor muscle tissues, MTT is now applied to myopathies that affect specific muscles, such as those in oculo-pharyngeal muscular dystrophy[11].
IPSC-BASED CELL THERAPY
Although it has long been difficult to induce skeletal muscle from human ES/iPSCs, several groups have recently reported successful derivation of skeletal muscle[12]. Many researchers expect that iPS technology will overcome the limitations of MTT because iPSCs are expected to provide a large quantity of muscle progenitor/precursor cells without invasive procedures. It is also expected that more proliferative and regenerative stem/progenitor cells can be induced from hiPSCs than from postnatal myoblasts.
INDUCTION OF MYOGENIC PROGENITORS AND PRECURSOR CELLS FROM HUMAN IPSCS
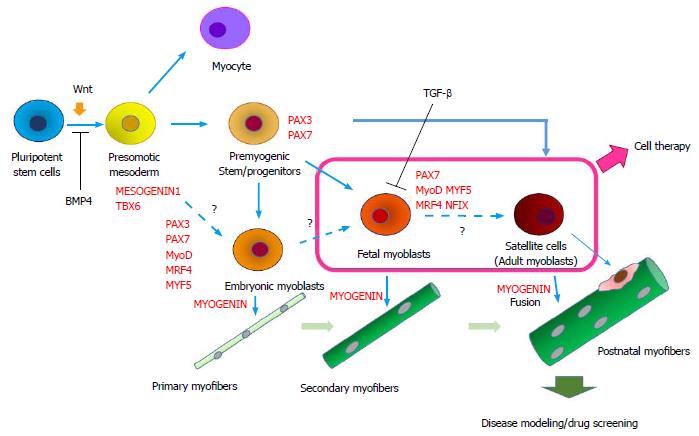
The protocols for the derivation of skeletal muscle from human ES/iPSCs can be roughly divided into two categories: (1) direct reprogramming with muscle-specific transcription factors, such as PAX3, PAX7; and MYOD; and (2) the step-wise induction of skeletal muscle using small molecules and cytokines to inhibit or activate relevant signaling pathways in myogenesis (Figure 1).
Figure 1 Step-wise induction of skeletal muscle from human embryonic stem/induced pluripotent stem cells and their application.
In many protocols, pluripotent stem cells are first induced to differentiate into paraxial mesoderm using a GSK3 inhibitor (activation of Wnt signal) and a BMP4 inhibitor, and they then differentiate into premyogenic progenitors in serum-free DMEM/F12-ITS (or KSR) medium supplemented with growth factors such as FGF-2, IGF-1, or HGF. After differentiation into muscle progenitors, the cells are induced to precursor cells (myoblasts) and then differentiate into multinucleated myotubes (in vitro) and myofibers (in vivo). The transition from embryonic to fetal myoblasts and finally into adult myoblasts is thought to occur sequentially in a dish, but the mechanisms and modes are largely unknown. FGF: Fibroblast growth factor; IGF: Insulin-like growth factor; HGF: Hepatocyte growth factor.
Forced expression of MYOD or PAX7
More than 25 years ago, Weintraub et al[13] found that MyoD can convert non-myogenic cells to skeletal muscle cells[13]. Rao et al[14] lentivirally transduced human ES cells with a doxycycline (DOX)-inducible MyoD. Within 10 d after addition of DOX to the culture, multinucleated myotubes were formed. The induction efficiency was over 90%. Tanaka et al[15] used a Piggy Bac transposon vector to overexpress MYOD and showed robust induction of skeletal muscle from Miyoshi myopathy-iPSCs. Akiyama et al[16] reported that transient ectopic expression of a catalytic domain of histone demethylase JMJD3, which reduces H3K27me, together with synthetic MyoD mRNAs, further accelerates the differentiation of human pluripotent stem cells into myogenic cells. Thus, MyoD-mediated muscle induction is fast and efficient. A limitation of the method would be that a high level or long expression of MyoD protein induces cell cycle arrest. In addition, MyoD cannot induce PAX3+PAX7+ muscle progenitors. For in vitro disease modeling, the properties of myotubes induced by the forced expression of MyoD remain to be compared with myotubes induced by Stepwise methods via the paraxial mesoderm and somite stage.
Pax3 and Pax7 regulate skeletal muscle formation during development, but play distinct roles in the post-natal period (reviewed in Ref.[17]). Forced expression of PAX7 in embryoid bodies successfully induces transplantable myogenic cells from human ES cells[18]. In contrast to MYOD, however, PAX7 alone does not convert adult fibroblasts to skeletal muscle. Therefore, the embryoid body would be the best stage in which to transduce myogenic cells with a PAX7-expression vector. Although random integration into the genome of over-expression vectors is not suitable for cell therapy, MyoD-induced skeletal muscle is now widely used for in vitro muscle disease modeling and drug screening.
Sphere-based culture method
Hosoyama et al[19] reported a sphere-based culture method for the derivation of myogenic progenitor cells from human ES/iPSCs (EZ sphere method). Human ES/iPSCs are cultured as spheres in serum-free medium for neurospheres supplemented with relatively high concentrations of fibroblast growth factor-2 and epidermal growth factor. After a six-week free-floating culture, cells plated onto MatrigelTM-coated dishes start to form multinucleated myotubes and finally start to twitch. EZ-sphere cells contain both myogenic cells and neural cells, requiring the purification of myogenic cells for further application. In addition, whether the EZ-sphere method can induce transplantable myogenic cells or not remains to be shown.
Embryoid body-based induction
Awaya et al[20] reported a method for the selective expansion of mesenchymal cells from cell aggregation called embryonic bodies (EBs). The resulting cells express CD56 (N-CAM) and the mesenchymal markers CD73, CD105, CD166, and CD29. The cells are transplanted into the muscle of immune-deficient mice and regenerate myofibers, as well as replenish the satellite cells. This method and the EZ sphere method require a lengthy culture and are not highly efficient.
Induction of skeletal muscle via activation of Wnt signaling
Many successful methods to induce skeletal muscle progenitors use a GSK-3β inhibitor (which activates Wnt signaling) in the first phase of culture[21-25]. Chal et al[24,25] monitored the induction process using reporter iPSC lines and comprehensive gene expression analysis, and established a stepwise induction of skeletal muscle. Paraxial mesoderm specification was achieved using a GSK3 inhibitor (CHIR-99021) together with BMP4 inhibition (LDN-193189) because BMP4 inhibition prevents the cells from differentiating into lateral mesoderm. The method induces myogenin(+) myogenic cells with 25%-30% efficiency[24,25]. The induced myotubes express titin, a fast perinatal myosin heavy chain, have a sarcomere structure, and spontaneously contract[24,25].
Heterogeneity of differentiation potential of human iPS clones
Human iPSCs are heterogeneous in myogenic differentiation potential. Some iPS clones efficiently differentiate into the skeletal muscle lineage, while others do not. The heterogeneity is found even among iPS clones derived from the same donor using the same method. Although the molecular basis is largely unknown, one possibility is that some clones are incompletely reprogrammed and cannot respond to differentiation signals properly. If the induction protocol is appropriate, completely reprogrammed iPS clones are expected to efficiently differentiate into the skeletal muscle lineage. Recently, using integrative analysis of reprogramming in a human isogenic system, Shutova et al[26] identified criteria to select the best iPS line.
CHARACTERIZATION OF INDUCED MUSCLE CELLS
In humans, the myogenesis process can be divided into 3 developmental stages: primary myogenesis (6-8 wk of development), secondary myogenesis (8-18 wk of development) and adult-type myogenesis (muscle growth in later myogenesis and regeneration). In primary myogenesis, embryonic myoblasts form primary myofibers. In secondary myogenesis, fetal myoblasts form secondary myofibers. Postnatally, satellite cells fuse with growing myofibers or fuse with injured myofibers[27,28]. During regeneration, a fraction of satellite cells return to their niche (self renewal) and maintain quiescence until the next turn. Importantly, the developmental stage of the myogenic progenitors largely determines the types of myofibers they form.
Morphology and gene expression of hiPSC-derived muscle
Human embryonic myoblasts show a limited proliferation capacity and are more prone to differentiation than fetal myoblasts. Isolated embryonic myoblasts form thinner myofibers with fewer nuclei than fetal myoblasts in vitro[27-29]. Because embryonic and fetal myoblasts express quite different gene sets in mice[28,30], gene expression analysis would be informative to determine the properties of the myogenic cells induced from human ES/iPSCs. For example, research in mice has revealed that embryonic myoblasts express PAX3, Paraxis, Meox1, Eya2, and Cadherin11, while fetal myofibers express NFIX, a key transcriptional regulator in fetal myoblasts[31], MCK, PKC theta, HeyL, and integrin α7[27,28,30]. These genes are good markers to determine the developmental stages of hiPSC-derived myogenic cells.
Cell surface markers
Cell surface markers to prospectively enrich myogenic progenitor cells with a highly myogenic and long-term expansion potential are under investigation. Barberi et al[32] reported the sorting of CD73(+) cells enriched in adult mesenchymal stem cell-like cells, and after 4-wk culture in ITS medium, NCAM(+) cells were collected and successfully transplanted into immunodeficient mice. Borchin et al[22] reported that the sorting of cMet(+) CXCR4(+) ACHR(+) cells enriched myogenic progenitors. After the screening of more than 300 antibodies, Uezumi et al[33] found novel surface markers on adult myoblasts (CD82 and CD318) and succeeded in the enrichment of myogenic cells induced from hiPSCs using CD82. The new marker CD82 ensures expansion and preservation of myogenic progenitors by suppressing excessive differentiation of adult myoblasts. Alexander et al[34] also reported that CD82 is a marker for prospectively isolating stem cells from human fetal and adult skeletal muscle and is possibly involved in the pathogenesis of muscular dystrophies. The function of CD318 in myogenesis and whether CD318 is helpful for purification of hiPSC-derived myogenic cells are now under investigation.
Response to TPA, BMP-4, TGF-β and Notch
In mice, embryonic, fetal, and adult myoblasts have been demonstrated to respond differently to extracellular signals such as TPA, BMP-4, and TGF-β[27,28,35]. It was also shown that an activated Notch pathway is necessary for TGF-β- or BMP-4-mediated inhibition of differentiation in fetal myoblasts[27,28]. By contrast, embryonic myoblasts are insensitive to TGF-β and BMP-mediated inhibition of differentiation[27,28]. TPA inhibits the differentiation of fetal myoblasts, but not that of embryonic myoblasts and satellite cells, possibly through the activation of PKC[27,28,36]. The PDGF receptor was reported to be expressed in embryonic myoblasts and adult myoblasts, but not in fetal myoblasts in the chick, suggesting that PDGF is involved in regulation of the transition of myogenesis[27,28,37]. Such different sensitivities to external stimuli not only explain the different timings of the differentiation of embryonic, fetal, and adult myoblasts during development but are also informative to make engrafted myoblasts participate efficiently in muscle repair.
CELL TRANSPLANTATION OF IPSC-DERIVED MUSCLE PROGENITOR CELLS
Allogeneic transplantation of immune-compatible donor cells vs genome-edited autologous cell transplantation
Although the extent to which patient-derived iPSCs and their derivatives evoke immune reactions when transplanted into the same patient is still unclear[38,39], recent tools for genome editing, such as CRISPR/Cas9, help in the preparation of gene-corrected cells from patients for autologous cell transplantation. For DMD, gene correction by homologous recombination is ideal, but restoration of the reading frame by exon skipping at the genomic level or by inserting a small DNA fragment is another option to obtain autologous, functional cells[40]. Recently, Young et al[41] demonstrated a large CRISPR/Cas9-mediated deletion of 725 kb of DMD (deletion of DMD exon 45-55), resulting in reframed and functional DMD iPSCs. Genome editing can also generate PAX7 or MYF5 reporter iPSC lines to monitor muscle differentiation[42,43] or disease-specific iPSCs carrying various gene mutations in the same genetic backgrounds. On the other hand, hiPS stocks are under construction for allogeneic transplantation of immune-compatible donor cells (https://http://www.cira.kyoto-u.ac.jp/e/research/stock.html). The use of iPS stock of a guaranteed quality is less time consuming and more economical.
Xenotransplantation
Thus far, a limited number of reports have described the efficient engraftment of human iPSCs-derived myogenic cells in animal models[18,20,21,24]. Most studies have used immune-deficient, dystrophin-deficient mdx mice as recipients. Recently, NSG-mdx4Cv mice have been developed for xenotransplantation. NSG mice were generated by mating NOD/Scid mice with IL2 receptor gamma chain-null mice. NSG mice were then crossed with mdx4Cv mice[44]. The Central Institute for Experimental Animals in Japan established NOG (NOD/Shi-scid/IL-2Rγnull)-mdx mice, which have a different mutation in the IL-2 receptor gamma gene, and are also expected to be good recipients of human iPSC-derived muscle progenitor cells (https://http://www.ciea.or.jp/about/reports/pdf/report/59_report.pdf). In many studies of xenogeneic transplantation, the hindlimb muscles of host mice are X-irradiated to kill endogenous satellite cells. A highly toxic venom, cardiotoxin, or BaCl2 is also used to damage the TA muscle 24 or 48 h before cell transplantation. Both X-irradiation and cardiotoxin injection effectively increase the contribution of engrafted cells to muscle regeneration; however, the effect is not physiological and cannot be applied to human recipients.
Delivery
Because most muscular dystrophies affect muscles of the whole body, the final goal of cell therapy is to deliver myogenic progenitors via the circulation. However, satellite cells and myoblasts cannot be delivered via the circulation. Mesoangioblasts have been reported to be systemically delivered and ameliorate dystrophic phenotypes in murine and canine models[45,46]. Therefore, the induction of mesoangioblasts from human ES/iPSCs is an attractive strategy to target the whole musculature. Tedesco et al[47] reported induction of mesoangioblast-like myogenic cells from iPSCs. Because the iPSC-derived mesoangioblast-like cells did not spontaneously differentiate into skeletal muscle, the authors overexpressed MyoD-estrogen receptor fusion protein in them and induced myogenic differentiation by tamoxifen administration after intramuscular transplantation.
EVALUATION METHOD FOR PROOF-OF-CONCEPT IN XENOTRANSPLANTATION
Histological and immunohistochemical analysis
Reduced necrotic fibers (H and E staining), fibrosis (Masson’s trichrome), and adipogenesis (oil red O), increased fiber diameter and muscle mass, and reduced inflammation are all indicative of the therapeutic effects of cell therapy. The percentage of central nuclei is not suitable for evaluation because, once myofibers regenerate, nuclei stay in the central position for a long time. Myofibers formed by transplanted cells are immunohistochemically detected using antibodies against human proteins, such as human laminA/C (nuclear membrane) or human spectrin (sarcolemma). The widely used human spectrin antibody (clone RBC2/3D5) reacts with mouse utrophin[48], and dystrophin recognizes revertant fibers. In fact, we experienced high levels of dystrophin expression in NSG-mdx4Cv mice (0.84% in the TA muscle of 6-mo-old males) (data not shown). Rozkalne et al[48] advised against relying on the detection of a single protein, but performing multiple human-specific labels and detecting dystrophin and dystrophin-associated proteins at the sarcolemma instead.
Muscle function
The improvement of muscle function is the most reliable proof-of-concept for cell therapy of muscular dystrophy. The measurement of the tetanic and specific force of an isolated single myofiber or muscle tissues in vitro is one of the widely used evaluation methods, but it is technically difficult. To obtain reproducible data, a system was developed in which the torque of the ankle of mice (planter flexion) is measured after the injection of myogenic stem/progenitor cells into gastrocnemius muscles. The measurement can be performed under anesthesia at different time points (http://www.brck.co.jp/application/files/3614/1523/5703/BRCsogo2011_P145.pdf).
IN VIVO SURVIVAL AND DIFFERENTIATION OF TRANSPLANTED CELLS
The efficiency of the transplantation of muscle stem/progenitor cells depends both on the intrinsic properties of the transplanted cells and on the microenvironment in the diseased muscle. Sakai et al[49] reported that mouse satellite cells showed many more dystrophin-positive fibers than mouse fetal muscle progenitors after intramuscular transplantation into dystrophin-deficient mdx mice. By contrast, Tierney et al[50] demonstrated that fetal muscle stem cells expand and contribute to muscle repair more efficiently than satellite cells after transplantation. Although the reasons for the discrepancy in the results are unclear, the studies suggest that the efficiency of transplantation depends largely on the intrinsic properties of the cells. Therefore, it is important to determine the signals that differently regulate the survival, proliferation, and differentiation of muscle stem/progenitor cells derived from hiPSCs. In addition, the microenvironment of diseased muscle might inhibit the survival and differentiation of engrafted cells. For example, fibrosis, an impaired blood supply, an inflammatory environment, and an activated immune response all inhibit the ability of engrafted cells to survive, proliferate, and differentiate to fuse host myofibers. The reconstitution of a regeneration-friendly microenvironment using a scaffold filled with regeneration-supportive ECM and cytokines, and the suppression of inflammatory responses would be effective.
TUMOR FORMATION BY IPS CELL-DERIVED MYOGENIC CELLS
Tumor-like growth in the host muscle after the transplantation of hiPS-derived muscle progenitor cells is occasionally observed. However, few publications have examined this problem extensively. In our opinion, the causes of tumorigenesis can be divided into at least three categories. The first cause is residual pluripotent stem cells in the transplanted population, which form teratomas. Teratoma is rare, and the elimination of undifferentiated pluripotent cells using FACS and human ES/iPSC markers such as SSEA4 or TRA-1-60 or by a recombinant lectin-toxin fusion protein would be effective[51]. The second cause is genetic abnormalities of human iPSCs ranging from gross karyotypic abnormalities to sub-chromosomal abnormalities (gene duplication, deletions, point mutation, de novo copy number variations (CNVs). Mutations are reported to occur during the derivation and culture of human ES/iPSCs and are supposed to be responsible for tumor formation after the transplantation of hiPS-derived progenitor cells[52,53]. Re-activation of transgenic oncogenes like c-Myc or KLF4 used for reprogramming is often related to the overgrowth of transplanted cells. These genetic abnormalities should be carefully examined before clinical application. The third cause is immature progenitors that fail to differentiate into mature cells for unknown reasons and continue to proliferate in transplanted tissues. In fact, we occasionally observed that hiPSC-derived myogenic cells overgrew in the muscle of immune-compromised mice. A similar phenomenon was observed in the transplantation of neurogenic cells. Interestingly, Ogura et al[54] reported that a Notch inhibitor promoted the differentiation of immature, actively proliferating progenitors, resulting in reduced tumor-like growth and engraftment of mature neurons in animal models of Parkinson’s disease. Similar results have been reported in a mouse model of spinal cord injury[55]. Whether such differentiation-resistant neuronal progenitor cells carry specific genetic abnormalities is not clear. Detailed characterization of cells that proliferate without terminal differentiation in transplanted muscle and investigation of the signaling pathways controlling self-renewal and differentiation of progenitors would be needed.
DISEASE MODELING IN VITRO USING PATIENT-DERIVED IPSCS
Successful examples of disease modeling
iPSCs derived from patients are useful for the elucidation of molecular pathogenesis and drug discovery. Various muscle disease-specific iPSCs have already been generated and deposited in a cell bank (e.g., http://cell.brc.riken.jp/en/; https://catalog.coriell.org/). The CRISPR/Cas9 technique further widened the possibility of examining the molecular pathology of ultra-rare diseases. For Duchenne muscular dystrophy (DMD), several groups have already reported that DMD-iPSCs-derived muscle cells show disease-specific phenotypes in vitro; Choi et al[56] reported the aberrant expression of inflammation or immune-response genes and reduced fusion competence of DMD-iPS-derived myogenic cells. Shoji et al[57] reported a pronounced calcium ion influx only in DMD myotubes, which were rescued by morpholino-mediated exon-skipping to skip a premature stop codon. Chal et al[24] reported that fibers derived from the ES cells of mdx mice exhibited an abnormal branched phenotype resembling that described in vivo. For other muscular dystrophies, Tanaka et al[15] demonstrated defective membrane repair in hiPSC-derived myotubes from a Miyoshi myopathy patient and phenotypic rescue by the expression of full-length DYSFERLIN. Snider et al[58] reported that hiPSCs express full-length DUX4, and the differentiation of control iPSCs to embryoid bodies suppresses the expression of full-length DUX4, whereas the expression of full-length DUX4 persists in differentiated iPSCs derived from patients with FSHD (facio-scapulo-humeral muscular dystrophy). Iovino et al[59] have created a novel cellular model of human muscle insulin resistance by differentiating iPSCs from individuals with mutations in the insulin receptor into functional myotubes and characterizing their response to insulin compared with controls. These successful in vitro disease models using patient-iPSCs are encouraging and useful for screening new drugs.
Neuromuscular junction
Maturation of skeletal muscle derived from human iPSCs in vitro is generally limited, partly because myofibers mature under innervation. However, neuromuscular junction (NMJ) formation in vitro is still challenging[60]. Morimoto et al[61] reported three-dimensional (3D) free-standing skeletal muscle fibers co-cultured with motor neurons. Yoshida et al[62] generated an NMJ-like structure using motor neurons derived from SMA patient-specific iPSCs and myotubes formed by C2C12 cells. Importantly, the clustering of acetylcholine receptors (AChR) is severely impaired. The authors further showed that valproic acid or antisense oligonucleotide-targeting splice-silencing motifs in intron 7 of SMN2 ameliorated the AChR clustering defects, by increasing the level of SMN2 transcripts[62].
Mechanical stress
Mechanical stress is needed for the maturation of hiPSC-derived muscle. A decellularized ECM scaffold filled with hiPSC-derived muscle progenitor cells might help us to obtain functional skeletal muscle tissue under physiological mechanical stress.
Induction of diverse myofibers in the body
Our musculature is composed of many types of muscle in the body: Cranial muscle, trunk muscle, and limb muscle. They have different developmental origins and programs. Each muscle is composed of slow or fast myofibers expressing different types of myosin heavy chain genes[63]. To faithfully mirror the physiology and pathology in vivo, such differences should be considered, although an induction method for diverse types of myofibers is at present challenging.
CONCLUSION
To maximally utilize the benefits of iPS technology for the cell therapy of devastating muscle disorders, a standardized protocol to constantly and efficiently induce skeletal muscle stem/progenitor cells from hiPSCs in a short time at low cost is desirable. Reduction of the risk of tumorigenesis and systemic delivery of therapeutic cells to the wider musculature are also required, and they are still highly challenging. For the modeling of disease, maturation of myotubes into adult-type myofibers in vitro, including the reconstitution of the neuromuscular junction, would be helpful.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kiselev SLL S- Editor: Kong JX L- Editor: A E- Editor: Wu HL