Published online Feb 26, 2017. doi: 10.4252/wjsc.v9.i2.26
Peer-review started: June 17, 2016
First decision: July 27, 2016
Revised: September 9, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 26, 2017
Processing time: 255 Days and 8.6 Hours
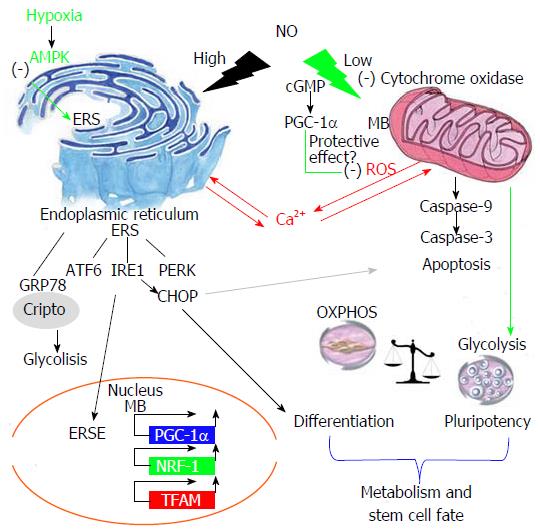
Mitochondrial dysfunction and endoplasmic reticulum stress (ERS) are global processes that are interrelated and regulated by several stress factors. Nitric oxide (NO) is a multifunctional biomolecule with many varieties of physiological and pathological functions, such as the regulation of cytochrome c inhibition and activation of the immune response, ERS and DNA damage; these actions are dose-dependent. It has been reported that in embryonic stem cells, NO has a dual role, controlling differentiation, survival and pluripotency, but the molecular mechanisms by which it modulates these functions are not yet known. Low levels of NO maintain pluripotency and induce mitochondrial biogenesis. It is well established that NO disrupts the mitochondrial respiratory chain and causes changes in mitochondrial Ca2+ flux that induce ERS. Thus, at high concentrations, NO becomes a potential differentiation agent due to the relationship between ERS and the unfolded protein response in many differentiated cell lines. Nevertheless, many studies have demonstrated the need for physiological levels of NO for a proper ERS response. In this review, we stress the importance of the relationships between NO levels, ERS and mitochondrial dysfunction that control stem cell fate as a new approach to possible cell therapy strategies.
Core tip: Several studies have focused on the role of nitric oxide (NO) in regulating many physiological functions, such as metabolism and pluripotency. NO has been established to act as a potent agent for the control of stemness by promoting the expansion of pluripotent cells. NO regulates mitochondrial function and endoplasmic reticulum stress. In pluripotent stem cells, both of these factors are related to the control of cell fate and may contribute to the mechanism by which NO regulates the maintenance of pluripotency. This provides additional evidence supporting the use of NO as an alternative small molecule for the conservation and expansion of cultured pluripotent cell lines necessary for implementing a cell therapy programme.
- Citation: Caballano-Infantes E, Terron-Bautista J, Beltrán-Povea A, Cahuana GM, Soria B, Nabil H, Bedoya FJ, Tejedo JR. Regulation of mitochondrial function and endoplasmic reticulum stress by nitric oxide in pluripotent stem cells. World J Stem Cells 2017; 9(2): 26-36
- URL: https://www.wjgnet.com/1948-0210/full/v9/i2/26.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i2.26
Nitric oxide synthase (NOS) modulates the L-arginine-to-L-citrulline pathway, by which nitric oxide (NO) is synthesized[1,2]. NO, a short-lived free radical, reacts with oxygen, superoxide, cytochrome c oxidase (CcO) and other molecules when it acts independently of cyclic guanosine monophosphate (cGMP), and this interaction is dose-dependent. Therefore, at high levels of NO, reactive oxygen species and NO interact to contribute to protein posttranslational modifications through S-nitrosylation and S-nitration[3-5]. When it acts in concert with cGMP, NO activates soluble guanylate cyclase (sGC), catalysing the conversion of GTP into cGMP, which controls a variety of physiological effects in multiple tissues[6,7]. The role of NO/cGMP in embryonic development and cell differentiation is a subject of intensive investigation; however, it is not clear whether the action of NO in stem cell biology is mediated via the cGMP pathway. It has been reported that bone marrow stem cell potency and differentiation are independent of the sGC/cGMP pathway[8-12]. Moreover, many effects of NO on stem cell pluripotency and differentiation are independent of this pathway, and thus the mechanism by which NO modulates the differentiation of embryonic stem cells (ESCs) remains unclear[13,14].
NO has been described to have important roles as a regulator of multiple physiological functions: It is a principal mediator of the immune system in the inflammatory response and a neurotransmitter in the central nervous system; acting as a second messenger, it has multiple biological effects that have been implicated in numerous physiological functions in mammals, such as regulation of blood pressure via smooth muscle relaxation and inhibition of platelet aggregation[13,15-17]. NO has been reported to affect gene expression at the levels of transcription and translation and has been associated with the regulation of cell survival and proliferation in diverse cell types[14,18]. Moreover, important processes such as growth, survival, proliferation, differentiation, and the pathologies of various diseases such as cancer, diabetes, and neurodegenerative diseases are mediated by functions of NO[19,20]. NO has also been shown to be involved in the control of heart functions and cardiac differentiation/development[21,22].
NO is considered one of many molecules that act on specific cell signalling pathways involved in embryonic development, specifically playing a dual role in the control of ESC differentiation and morphogenesis[23].
This dual role is determined by the NO concentration: It has been demonstrated that a low NO concentration maintains pluripotency, whereas a high concentration induces differentiation[14,18,23]. A high NO concentration has been reported to cause oxidative and nitrosative stress and apoptosis, processes partly responsible for cell death during chronic and degenerative disease. Moreover, embryonic stem cell differentiation is promoted by pharmacological treatment with high NO concentrations[18,24,25]. Our group has reported[18] that exposure to high concentrations of a NO donor (DETA-NO) promotes the differentiation of mouse ESCs induced by down-regulation of the pluripotency genes Nanog and Oct4. However, low NO concentration has been shown to promote cell proliferation and survival. Specifically, our group has demonstrated that the exposure of ESCs to a low concentration of DETA-NO promotes the expression of self-renewal genes and prevents the differentiation of mouse and human ESCs[14]. Therefore, NO is also considered a regulator of cellular respiration (oxygen-sensitive pathways) and metabolism, with a major role in regulating the hypoxia response by modulating the activity of CcO, a component of complex IV, which is involved in the final processes of the mitochondrial electron transport chain[4,16,26-28].
NO is a small molecule that has dual roles in the control of ESC differentiation and tissue morphogenesis. High concentrations of NO promote differentiation. It has been reported that in ESCs, the production of NO is necessary for cardiomyogenesis because the maturation of terminally differentiated cardiomyocytes is prevented by NOS inhibitors[22]. Mouse ESC (mESC) differentiation is promoted by high DETA-NO concentrations, which induce the down-regulation of Nanog and Oct4 expression. NO represses Nanog via the activation of p53, which is associated with covalent modifications such as Ser315 phosphorylation and Lys379 acetylation. Moreover, the expression of the definitive endoderm markers FoxA2, Gata4, Hfn1-β and Sox17 is increased by exposure to high concentrations of DETA-NO[8,18]. It has been reported that the NO concentration regulates signalling pathways implicated in the survival and homeostasis of RINm5F cells. Thus, high NO can cause oxidative and nitrosative stress and apoptosis[18,24,25]. Several studies report that NO induces apoptosis in various cell types, such as pancreatic beta cells[29,30], thymocytes[31] and hepatocytes[32]. In ESCs, high levels of DETA-NO promote nitrosative stress, inducing apoptotic events in part of the ESC population. The remaining ESC population will be resistant to nitrosative stress and express the cytoprotective genes haeme oxygenase-1 and HSP70, representing the start of a differentiation programme[18].
Mitochondrial modulation is emerging as a mediator of stem cell proliferation and differentiation. Mitochondrial function is known to be fundamental to cellular health. The two actions that maintain mitochondrial function are fission and fusion processes, collectively termed mitochondrial dynamics (MD). Altering the balance of MD results in changes to mitochondrial morphology and increases the incidence of age-related disorders, such as neuromuscular degeneration, and of metabolic disorders, such as obesity, impaired glucose tolerance, and diabetes[33,34]. Many of these disorders have been shown to originate due to alterations in the function, morphology and number of mitochondria. The volume and efficacy of the mitochondrial mass is considered a determining factor in the production of reactive oxygen species (ROS) and the response to the oxidative stress level[35]. It has been reported that ROS levels are lower in undifferentiated cells. ESCs have been reported to resist oxidative stress better than differentiated cells and to contain a large complement of active mitochondria[36]. In addition, it has been shown that the expression levels of pluripotency markers are downregulated in mESCs when the mitochondrial DNA copy number is increased[37]. In general, pluripotent stem cells (PSCs) have a low mitochondrial population with low energy potential; most of the energy comes from glycolysis, which is limited only by a low ATP reservoir that precludes glucose phosphorylation to glucose 6-phosphate, which is required for uptake into the cells. Several types of differentiated cells, such as those that have differentiated into the trophectoderm of mice and rats, have been described as having more elongated mitochondria, with higher membrane potential and more O2 consumption[38]. When cells are differentiated, the number of mitochondria is observed to increase, and the mitochondrial morphology shows characteristics observed in mature cells[39-41]. This behaviour is similar to that described in tumour cells, which have a decreased respiratory rate associated with an enhancement of anaerobic glycolysis due to a uniform transcriptional reduction of mitochondrial components. Human ESCs (hESCs) have been reported to have only a few mitochondria with immature morphology, and it has been found that the mitochondrial mass, the intracellular ROS level and the expression of antioxidant enzymes increase with differentiation[41]. Thus, the ROS produced in the differentiated cells might play an important role in cell signalling and differentiation[41-43]. Dynamic changes in mitochondrial energy metabolism, ROS and antioxidant enzymes have been shown to affect differentiation propensity. In fact, mitochondrial biogenesis is controlled by the expression of oxidative metabolism genes, among which are mitochondrial transcription factor A (Tfam), nuclear respiratory factor (NRF-1) and peroxisome proliferator activated receptor γ co-activator 1α (PGC-1α)[44,45], which has been described to regulate mtDNA transcription and replication.
NO has been described as a physiological regulator of the mitochondrial respiratory chain and has been reported to interact with oxygen bound to CcO located in the inner mitochondrial membrane. CcO has a higher affinity for NO than for oxygen, which suggests that this interaction and its biological consequences are dependent on the redox state and turnover of CcO[4,46]. It has been shown that NO maintains normal cellular ATP levels by inhibiting mitochondrial respiration and increasing glycolysis[28]. This activity of NO is an important mechanism by which NO can modulate cellular responses to hypoxia in mammalian cells.
Mitochondrial biogenesis (MB) can be defined as the growth and division of pre-existing mitochondria as a mechanism to adjust the cellular energy balance in response to an environmental change or a change in the general status[47,48]. This process is regulated by a wide range of substances, including benzodiazepine, Ca2+ fluxes, and thyroid hormones such as T3, which controls metabolic rates in vertebrates[49,50]. Growing evidence suggests that the delicate equilibrium between mitochondrial fission and fusion is vital for many mitochondrial functions, including metabolism, energy production, Ca2+ signalling, ROS production and apoptosis[49,51-53]. For example, in some neurodegenerative diseases, there is a reduction in the expression levels of fusion proteins such as optic atrophy type 1, mitofusin-1 and mitofusin-2 and an increase in the expression of fission proteins such as dynamin-related protein-1 and fission related protein-1[54]. It has been reported that mitochondrial fusion permits inter-mitochondrial interaction and the exchange of membrane and matrix components; fusion can help to maintain the mitochondrial membrane potential and mitochondrial function[55]. Moreover, it has been demonstrated that mitochondria and mtDNA can be transferred between cells through cytoplasmic projections[56], which is a compensatory mechanism that allows mammalian cells with non-functional mitochondria to restore aerobic respiration. In addition, mitochondria also require numerous proteins from the cytosol that are transported through the matrix[57].
It is well known that treatment with low concentrations of NO induces MB in various mammalian cells types as well as in animal tissues. The MB induced by low concentrations of NO is mediated by activation of guanylate cyclase-dependent cGMP and is related to the increased expression of PGC-1α, NRF-1 and Tfam (Figure 1), and it also affects the mitochondrial function[58] (Figure 1). It has also been reported that NO/cGMP-dependent MB activates oxygen consumption and ATP production and subsequently increases mitochondrial function in U937, L6 and PC12 cells, among other cell types[59]. However, a previous study showed that during spontaneous differentiation of hESCs, the expression of MB markers was increased. In addition, that study found a discrepancy between regulatory factors of MB and mitochondrial activity: The mitochondrial activity in differentiated cells was higher than in undifferentiated cells, and differentiated cells showed no relationship between the expression of PGC-1α, NRF-1 and Tfam expression and mitochondrial function. Moreover, transmission electron microscopy showed that cellular organelles were immature in undifferentiated hESCs compared to cells undergoing differentiation. They concluded that hESCs possess immature machinery for the assembly of functional mitochondria[41]. Therefore, we consider in this review that low NO induces MB, but the expression of mitochondrial biogenesis markers alone may not be indicative of mitochondrial function.
Signalling for MB is activated by peroxisome PGC-1α and involves the expression of several transcription factors, resulting in the upregulation of proteins encoded by both nuclear and mitochondrial genomes[60].
PGC-1α is a transcription factor that regulates oxidative metabolism and adaptive thermogenesis and is the main inducer of MB in cells[61]. PGC-1α regulates the activities of cAMP response element binding protein and nuclear respiratory factors (NRFs). It provides a direct link between external physiological stimuli and the regulation of MB. PGC-1α has been described to have an important role in aging-related diseases, including neurodegenerative diseases such as Alzheimer’s. PGC-1α activates a protective response through the induction of many antioxidant enzymes to decrease the ROS level, including superoxide dismutase and glutathione peroxidase 1. In addition, Ca2+ and ROS regulate MB by activating PGC-1α, leading to an increase in the mitochondrial mass[62]. It is very important to mention that PGC-1α was found to regulate the adaptive response at sub-lethal levels of different toxins that improve mitochondrial function in a process known as mitohormesis[60,63].
Interestingly, this hormetic response is controlled by the cell-tolerated increasing expression of PGC-1α, which in turn induces a balanced expression of fusion/fission genes. However, deregulation of PGC-1α expression through either stable down-regulation or overexpression renders cells more susceptible to toxic insult, leading to mitochondrial fragmentation and cell death. This result has also been supported by the activation of Mtn2 expression[64]. In contrast, while PGC-1α permits the tolerance of a certain level of toxins in the cell, the prolonged expression to non-physiological levels of PGC-1α has a negative effect on mitochondrial function and the viability of the cells[65]. This suggests that the maintenance of a homeostatic PGC-1α expression level may offer a promising strategy for neuroprotective therapies against some toxicants[60].
NO plays a very important role in regulating mitochondrial function and cell metabolic activity. It has been described that endothelial nitric oxide synthase (eNOS) is associated with the outer mitochondrial membrane in neurons and endothelial cells, which suggests that NOS regulates mitochondrial function[66] (Figure 1). The chemical structure of NO allows the interaction with haemoglobin and the release of O2 for mitochondrial consumption[67]. As introduced earlier in this review, a mechanism for the regulation of mitochondrial function is the binding of NO to CcO, the terminal enzyme in the electron transport chain[68]. It competes with O2 as the last electron receiver, inhibiting the activity of the enzyme and preventing water formation and ROS generation[69,70]. Furthermore, at a low concentration of O2, both physiological and low concentrations of NO inhibit CcO and induce a switch to glycolysis that permits adaptation to hypoxic conditions[28,71]. However, a high concentration of NO also inhibits other mitochondrial complexes of the respiratory chain (complexes I, complexes II and complexes III), increasing superoxide anion (O2-) production and inducing cell death[70,72] (Figure 1).
The hypoxia response is mediated by hypoxia inducible factor (HIF). HIF1α is the isoform that regulates oxygen homeostasis and cell metabolism in the short-term hypoxia response. Both HIF1α and NO help to restore energy metabolism at a low oxygen concentration[73]. High NO has been described to induce HIF1α expression under normoxic conditions in a mitochondria-dependent manner, but the effect of low NO under normoxia is unknown[27]. It is very important for us to evaluate this effect because we have evidence that low NO under normoxia induces HIF1α and can activate a similar hypoxia response.
The increase in anaerobic respiration and the decrease in oxidative phosphorylation in the presence of available oxygen is known as the Warburg effect[74]. This effect was considered a particular feature of cancer cells due to the typical hypoxic environment of tumours, but currently, it is considered a metabolic shift that permits cells to divide and proliferate[75]. Because of this, reduction of the ROS levels by the reduction of oxidative phosphorylation permits the activity of proliferative kinases, such as ERK1/2 and Akt, which inhibits the activation of the apoptotic machinery via activation of anti-apoptotic control mechanisms and the non-activation of pro-apoptotic kinases, such as c-Jun-NH2-terminal kinase and p38 mitogen-activated protein kinase[76].
It has been reported that somatic cells require a shift from oxidative to glycolytic metabolism for the reprogramming process. Both HIF1α and HIF2α are necessary in the early state of the reprogramming for this metabolic change and for the recovery of the pluripotent state[77]. The bioenergetics of pluripotent cells can vary depending on their developmental stage. hECSs present highly glycolytic metabolism and share this feature with cancer cells (Warburg effect)[78]. Because the stem cell niche presents an hypoxic environment, the glycolytic metabolism of undifferentiated cells could be an adaptation to low oxygen concentrations in vivo[79]. In addition, the efficiency of the reprogramming process is reduced by glycolysis inhibition and an increase in glycolytic potency during the generation of inducible PSCs (iPSCs)[80]. Furthermore, it has been described that hypoxia enhances the generation of iPSCs. HIF1α and HIF2α are essential for the metabolic changes required for early iPSC generation in humans. However, HIF2α is detrimental at later stages of reprogramming because of the upregulation of TNF-related apoptosis-inducing ligand[77].
Cell metabolism is remarkably important for determining the stem cell fate and the role of NO in the regulation of metabolism. Almeida et al[28] described that NO activates glycolysis in astrocytes via the phosphorylation of AMP-activated protein kinase (AMPK), which activates Phosphofructokinase 2 and protects cells from apoptosis.
The relationship between NO and cell metabolism could be vital for the expansion of pluripotent cells when NO is used as a supplement in the design of culture medium, and it seems reasonable that NO may be used as a pluripotency inducer (Figure 1).
The endoplasmic reticulum (ER) is the organelle designated for the synthesis and folding of proteins that are directed for secretion or to the Golgi apparatus. Because proper protein synthesis and protein folding are the key functions of the ER, the interruption of this physiological process ends in a complex ERS response, with a goal of recovering physiological function. The ER also functions as a store for Ca2+ and regulates its homeostasis through Ca2+-pumping and Ca2+-releasing proteins located in its membrane. Ca2+ is also an essential ion for ER function. Many chaperones, such as calreticulin or protein disulphide isomerase, are dependent on Ca2+ concentration, and therefore, any variation in ER intra-organelle Ca2+ concentration leads to changes in ER function[81,82].
There are many states that can lead to an unfolded protein response (UPR), the primary role of which is to recover internal homeostasis and adapt to the new conditions in the ER. Any alterations in Ca2+ homeostasis can involve the UPR due to the malfunctioning of proteins responsible for protein folding in the ER lumen; nevertheless, the possible causes of the UPR are not restricted to this process, as, for example, glucose deprivation can also lead to ERS[83].
The first stage in the UPR implies an attempt at adaptation triggered by the release of chaperones anchored in membrane proteins, mainly glucose regulate protein 78 (Grp78, also known as BiP), to prevent the accumulation of misfolded proteins in the ER lumen. This release of Grp78 entails the aggregation of membrane proteins to which Grp78 was attached as well as Grp78 auto phosphorylation. Among these proteins, we primarily found protein kinase-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (Ire-1) and transcription factor 6 (ATF6)[84] (Figure 1).
PERK is a protein kinase that is able to auto phosphorylate when it oligomerizes, activating its kinase domain and inactivating eukaryotic initiation factor 2 α (eIF2α) after its phosphorylation. This leads to a decrease of mRNA translation with the aim of reducing the protein mass in the ER. In turn, genes related with the UPR are preferentially translated, as is the case of the transcription factor ATF4[85].
ATF4 is a CHOP activator and an apoptotic enhancer[86]. CHOP has been involved in many processes that lead to cellular apoptosis through the regulation of genes related with the cell cycle and ROS generation[87]. CHOP dimerizes with C/EBP, preventing its binding to gene promoters and blocking genes such as Bcl2, apoptotic suppressor, or peroxisome proliferator-activated receptor gamma, which promotes cell proliferation. This uniformly activates the expression of genes such as IL-6 and GADD34, which promotes cell differentiation and ROS synthesis[82,86].
ATF6 is a transcription factor that acts in the ERS response. ATF6 is cleaved when unfolded proteins are accumulated in the ER, releasing its cytoplasmic domain, which translocates to the nucleus. The inactive form of ATF6 (p90ATF6) is transported to the Golgi and is activated via two-step cleavage by Site-1 protease and Site-2 protease. Then, the active form of ATF6 is transported to nucleus[88] and functions as a transcriptional activator for ERS-related genes such as CHOP[89] (Figure 1).
Ire1 is a protein that shares similar pathways with PERK. Upon its oligomerization after Grp78 is released, its kinase activity is activated, and Ire1 is able to process an intron from Xbox protein-1 (XBP-1) mRNA to trigger its translation. XBP-1 activates genes related to protein transport from the ER to the cytosol, resulting in its degradation[82]. XBP-1 has also been correlated with apoptotic processes[84] and lymphocyte differentiation[83].
The release of Grp78 from the end of ATF6 also leads to its release towards the Golgi apparatus, where proteases are in charge of its cleavage, after which ATF6 is able to migrate to the nucleus and regulate genes, including the activation of XBP-1, which, as mentioned before, is an upregulator of CHOP[85].
Despite previous research on the ERS response and the effects of NO in cell regulation, the connection between these two processes was not clarified until 2001, when the first relationships between NO and CHOP were established through Ca2+ release and the subsequent release of ER Ca2+ to the cytosol induced by NO. Increases in the cell NO concentration lead to Ca2+ release from the mitochondria due to Cyt c inhibition, and this unleashes Ca2+ release in the ER. This response to NO is triggered as a result of protein misfolding in the ER because many chaperones are Ca2+-dependent, which causes a UPR that over time ends in an apoptotic response[90-92] (Figure 1).
High concentrations of NO have been frequently associated with differentiation and apoptotic processes; nevertheless, the genetic mechanisms that initiate these processes are barely defined[93]. Recently, many reports have suggested the relevance of the ERS response in the differentiation of several cell lines, which suggests the possibility of studying this response as a possible differentiation mechanism triggered by high concentrations of NO (Figure 1).
Previous studies have demonstrated the importance of ERS for the differentiation of chondrocytes[94-96], plasma cells[95,97], adipocytes[98] and myoblasts[18]. Nonetheless, recently, the differentiating potential of ERS has been identified in many other cell lines. Saito et al[99] demonstrated the effect of ERS in the terminal differentiation of osteoblasts through the activation of the PERK-eIF2α-ATF4 pathway. The results concluded that PERK activation triggered by the ERS response is required for ATF4 activation in osteoblast differentiation and for the proper expression of genes that are essential for osteogenesis, such as Ocn and Bsp[99]. Heijmans et al[100] studied the loss of pluripotency in epithelial intestinal cells upon activation of the UPR. They proposed that the ERS response causes the loss of stemness in a manner dependent on Perk-eIF2α-ATF4, a pathway activated in the UPR. Their findings demonstrate that inhibition of this signalling pathway results in stem cell accumulation in the epithelium, suggesting that the ER stress response is a key factor in the differentiation of this cell type[101,102]. More recently, Matsuzaki et al[101] evaluated the effect of physiological ER stress in fibroblast differentiation, suggesting that fibroblasts subjected to RER are more susceptible to differentiation signals and implying that UPR signalling could be essential for this differentiation. This is significant, considering that these cells are subject to a high protein load in the ER lumen in their final differentiation state due to the high amount of protein directed to secretion[96,100].
These studies suggest the possibility of studying the effect of NO in differentiation through this response because the precise control of this molecule in this cellular process has been at least partially characterized.
Although NO is a molecule that in high concentrations can lead to apoptosis and differentiation processes, some studies have suggested its protective effect and have demonstrated that endogenous levels of NO produced by different types of NOS are necessary for proper cell function and pluripotency maintenance. Previous studies have shown the need for physiological levels of NO to avoid the generation of free radicals or the activity of many proteases or to increase the antioxidant potential of GSH[103].
In 2007, Kitiphongspattana et al[97] demonstrated that the constitutive production of NO by cNOS was essential for a proper ER response in β pancreatic cells. Its inhibition repressed the expression of genes involved in protein folding and antioxidant defence, such as Gclc, Grp78, Prx-1 or Gpx-1[88,97]. Moreover, a protective effect of physiological NO in β-cells through the activation of Nrf2, a transcription factor for antioxidant proteins, in the RER has been reported previously, as has the importance of the UPR in resistance to oxidative stress[104-106].
ERS activates apoptosis through several stimuli, including hypoxia, oxidative stress and Ca2+ depletion. AMPK activation has been shown to protect cardiomyocytes against hypoxic injury through the attenuation of ERS[107].
ERS promotes apoptotic signalling or cell survival in different cell types. Therefore, the decision between survival and apoptosis may depend on the balance between survival signalling and apoptotic signalling.
Three apoptotic pathways are known to be related to ERS. Among others, the induction of the gene for CHOP promotes apoptosis, and CHOP deficiency can protect cells from ER stress-induced apoptosis, suggesting that CHOP is involved in the process of cell death caused by ERS. Other authors proposed that the mechanism of ERS induction during hypoxia is the alteration of the Ca2+ balance via the inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase pump due to intracellular ATP depletion[108]. Because of this, Terai et al[107] hypothesized that conservation of the intracellular ATP content during hypoxia would protect cardiomyocytes from ERS-induced cell death. They concluded that AMPK would be a cell protector against ERS-induced cell death during hypoxia because AMPK acts as an intracellular energy sensor, maintaining the energy balance within cells during ischaemia. The mechanism by which AMPK protects cells during hypoxia is attributed to the suppression of protein synthesis due to the phosphorylation of eEF2. This protective effect mediated by AMPK has also been described in another recent study. Hwang et al[109] reported that AMPK mediated cell survival induced by resveratrol in H9c2 cells, and this may exert a novel therapeutic effect on the oxidative stress induced in cardiac disorders.
It is interesting to consider the effects of AMPK with regard to the role of NO in the activation of this kinase. NO has a protective role against ROS and ERS, and this action could be mediated by AMPK. As we have demonstrated, NO regulates cell metabolism, and this effect can help to maintain pluripotency. NO has become known as a potent molecule that regulates cell protection.
In this literature review, we would like to highlight the role of NO as a regulator of stem cell properties. Low NO concentrations have been shown to help to maintain pluripotency, but the molecular mechanisms of this effect are not yet known.
We have analysed the role of NO in mitochondrial function and ERS because this pathway is interrelated with stem cell fate and could be an explanation for the mechanism by which NO regulates embryonic development.
We have described that NO has an important role in mitochondrial biogenesis and the induction of PGC1α expression. Notably, PGC1α has a neuro-protective effect against certain levels of different toxins. Due to the relationship between NO and PGC1α, the information in this review suggests the importance of studying the potential protective role of NO in cells.
NO is highly involved in the ERS response through its effect on mitochondria initiation of an ERS response, initially triggered by the release of Ca2+ in the mitochondria. This ERS response originates differentiation processes in many cell lines. This suggests the importance of studying the effect of NO in the ERS response to clarify its different effects through this pathway.
Likewise, NO in the ER is also relevant at physiological concentrations because many studies have shown that a proper ERS response is not feasible without sufficient physiological intracellular NO synthesis.
NO is involved in the regulation of cell respiration at a physiological level. NO inhibits cytochrome c and induces glycolysis, which can help to regulate stem cell fate. Pluripotent cells have been reported to have a highly glycolytic metabolism. In the reprogramming process, it is necessary that restoration of glycolytic metabolism is mediated by hypoxia-inducible factors. The induction of glycolysis by NO is vital for using this molecule as an inducer of pluripotency. NO is a strong tool for culturing pluripotent cell lines in an undifferentiated state. Moreover, NO can be used for biotechnology applications in the design of a culture medium for pluripotent cell expansion and for the creation of a cell therapy programme.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gharaee-Kermani M, Kan L, Kiselev SL, Louboutin JP S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Mannick JB, Miao XQ, Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125-24128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 222] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Lee SK, Kim HS, Lee HJ, Lee J, Jeon BH, Jun CD, Lee SK, Kim EC. Dual effect of nitric oxide in immortalized and malignant human oral keratinocytes: induction of apoptosis and differentiation. J Oral Pathol Med. 2006;35:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1395] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 4. | Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 763] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 5. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 6. | Krumenacker JS, Murad F. NO-cGMP signaling in development and stem cells. Mol Genet Metab. 2006;87:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Krumenacker JS, Katsuki S, Kots A, Murad F. Differential expression of genes involved in cGMP-dependent nitric oxide signaling in murine embryonic stem (ES) cells and ES cell-derived cardiomyocytes. Nitric Oxide. 2006;14:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Mujoo K, Krumenacker JS, Murad F. Nitric oxide-cyclic GMP signaling in stem cell differentiation. Free Radic Biol Med. 2011;51:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Mujoo K, Krumenacker JS, Wada Y, Murad F. Differential expression of nitric oxide signaling components in undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev. 2006;15:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Napoli C, Paolisso G, Casamassimi A, Al-Omran M, Barbieri M, Sommese L, Infante T, Ignarro LJ. Effects of nitric oxide on cell proliferation: novel insights. J Am Coll Cardiol. 2013;62:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Wong JC, Fiscus RR. Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Iα (PKG-Iα) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Iα autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells. J Cell Biochem. 2011;112:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Chu L, Jiang Y, Hao H, Xia Y, Xu J, Liu Z, Verfaillie CM, Zweier JL, Liu Z. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur J Pharmacol. 2008;591:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Tejedo JR, Tapia-Limonchi R, Mora-Castilla S, Cahuana GM, Hmadcha A, Martin F, Bedoya FJ, Soria B. Low concentrations of nitric oxide delay the differentiation of embryonic stem cells and promote their survival. Cell Death Dis. 2010;1:e80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Kots AY, Bian K, Murad F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr Med Chem. 2011;18:3299-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Foster MW, Stamler JS. New insights into protein S-nitrosylation. Mitochondria as a model system. J Biol Chem. 2004;279:25891-25897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Enikolopov G, Banerji J, Kuzin B. Nitric oxide and Drosophila development. Cell Death Differ. 1999;6:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Mora-Castilla S, Tejedo JR, Hmadcha A, Cahuana GM, Martín F, Soria B, Bedoya FJ. Nitric oxide repression of Nanog promotes mouse embryonic stem cell differentiation. Cell Death Differ. 2010;17:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Kojda G, Laursen JB, Ramasamy S, Kent JD, Kurz S, Burchfield J, Shesely EG, Harrison DG. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Bloch W, Fleischmann BK, Lorke DE, Andressen C, Hops B, Hescheler J, Addicks K. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc Res. 1999;43:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Li J, Bombeck CA, Yang S, Kim YM, Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem. 1999;274:17325-17333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593-615. [PubMed] |
| 25. | Cahuana GM, Tejedo JR, Hmadcha A, Ramírez R, Cuesta AL, Soria B, Martin F, Bedoya FJ. Nitric oxide mediates the survival action of IGF-1 and insulin in pancreatic beta cells. Cell Signal. 2008;20:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Mateo J, García-Lecea M, Cadenas S, Hernández C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 29. | Ankarcrona M, Dypbukt JM, Brüne B, Nicotera P. Interleukin-1 beta-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp Cell Res. 1994;213:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Kaneto H, Fujii J, Seo HG, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes. 1995;44:733-738. [PubMed] |
| 31. | Fehsel K, Kröncke KD, Meyer KL, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858-2865. [PubMed] |
| 32. | Kim YM, Bergonia H, Lancaster JR. Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS Lett. 1995;374:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312-318. [PubMed] |
| 34. | De Flora S, Izzotti A. Modulation of genomic and postgenomic alterations in noncancer diseases and critical periods of life. Mutat Res. 2009;667:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Lyu BN, Ismailov SB, Ismailov B, Lyu MB. Mitochondrial concept of leukemogenesis: key role of oxygen-peroxide effects. Theor Biol Med Model. 2008;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, Yoon CJ, Kim DW, Kim SH, Moon SY. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. 2005;23:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park DJ, Park KS, Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 42. | Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 816] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 44. | Furman JM, Becker JT. Vestibular responses in Wernicke’s encephalopathy. Ann Neurol. 1989;26:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 636] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 45. | Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 968] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 46. | Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993-C2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Klaus S, Casteilla L, Bouillaud F, Ricquier D. The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int J Biochem. 1991;23:791-801. [PubMed] |
| 48. | Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 978] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 49. | Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 617] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 50. | Goglia F, Moreno M, Lanni A. Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Lett. 1999;452:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 215] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 567] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 53. | Mannella CA, Marko M, Buttle K. Reconsidering mitochondrial structure: new views of an old organelle. Trends Biochem Sci. 1997;22:37-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Su B, Wang X, Zheng L, Perry G, Smith MA, Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 55. | Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185-26192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1053] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 56. | Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 787] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 57. | Wickner W, Schekman R. Protein translocation across biological membranes. Science. 2005;310:1452-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 58. | Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA. 2004;101:16507-16512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 59. | Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 850] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 60. | Dabrowska A, Venero JL, Iwasawa R, Hankir MK, Rahman S, Boobis A, Hajji N. PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging (Albany NY). 2015;7:629-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 61. | Austin S, St-Pierre J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963-4971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 62. | Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O, Zorzano A. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 318] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 65. | Ciron C, Lengacher S, Dusonchet J, Aebischer P, Schneider BL. Sustained expression of PGC-1α in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet. 2012;21:1861-1876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 66. | Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, Dhadwal N, Cohen-Gould L, Gross SS, Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279:15968-15974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Wolzt M, MacAllister RJ, Davis D, Feelisch M, Moncada S, Vallance P, Hobbs AJ. Biochemical characterization of S-nitrosohemoglobin. Mechanisms underlying synthesis, no release, and biological activity. J Biol Chem. 1999;274:28983-28990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 69. | Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 394] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 70. | Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95:7631-7636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 682] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 71. | Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 576] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 72. | Iglesias DE, Bombicino SS, Valdez LB, Boveris A. Nitric oxide interacts with mitochondrial complex III producing antimycin-like effects. Free Radic Biol Med. 2015;89:602-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1487] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 74. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11811] [Article Influence: 738.2] [Reference Citation Analysis (0)] |
| 75. | Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal. 2012;16:1150-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 77. | Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 78. | Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3712] [Article Influence: 265.1] [Reference Citation Analysis (0)] |
| 79. | Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 80. | Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 81. | Correction for Dillin, Profile of Kazutoshi Mori and Peter Walter, 2014 Lasker Basic Medical Research Awardees: The unfolded protein response. Proc Natl Acad Sci USA. 2015;112:E2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Sidrauski C, Chapman R, Walter P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell Biol. 1998;8:245-249. [PubMed] |
| 83. | Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2260] [Cited by in RCA: 2366] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 84. | Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 338] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 85. | Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1342] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 86. | Shen X, Zhang K, Kaufman RJ. The unfolded protein response--a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004;28:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 87. | Tapia-Limonchi R, Díaz I, Cahuana GM, Bautista M, Martín F, Soria B, Tejedo JR, Bedoya FJ. Impact of exposure to low concentrations of nitric oxide on protein profile in murine and human pancreatic islet cells. Islets. 2014;6:e995997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355-1364. [PubMed] |
| 89. | Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol. 2001;21:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 90. | Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897-904. [PubMed] |
| 91. | Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881-891. [PubMed] |
| 92. | Zeng L, Zampetaki A, Margariti A, Pepe AE, Alam S, Martin D, Xiao Q, Wang W, Jin ZG, Cockerill G. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci USA. 2009;106:8326-8331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 93. | McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1536] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 94. | Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 95. | Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it’s a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491-R511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 96. | Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 97. | Kitiphongspattana K, Khan TA, Ishii-Schrade K, Roe MW, Philipson LH, Gaskins HR. Protective role for nitric oxide during the endoplasmic reticulum stress response in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2007;292:E1543-E1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108-20117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 99. | Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286:4809-4818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 100. | Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, Ferrante M, Lee AS, Onderwater JJ, Paton JC. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 101. | Matsuzaki S, Hiratsuka T, Taniguchi M, Shingaki K, Kubo T, Kiya K, Fujiwara T, Kanazawa S, Kanematsu R, Maeda T. Physiological ER Stress Mediates the Differentiation of Fibroblasts. PLoS One. 2015;10:e0123578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 102. | Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 103. | Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1004] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 104. | Horndasch M, Lienkamp S, Springer E, Schmitt A, Pavenstädt H, Walz G, Gloy J. The C/EBP homologous protein CHOP (GADD153) is an inhibitor of Wnt/TCF signals. Oncogene. 2006;25:3397-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 106. | Xu W, Charles IG, Moncada S. Nitric oxide: orchestrating hypoxia regulation through mitochondrial respiration and the endoplasmic reticulum stress response. Cell Res. 2005;15:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554-9575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 108. | Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca(2+) uptake is impaired in coronary smooth muscle distal to coronary occlusion. Am J Physiol Heart Circ Physiol. 2001;281:H223-H231. [PubMed] |
| 109. | Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |