INTRODUCTION
Epidemiological studies of amyotrophic lateral sclerosis (ALS) are in progress, but so far we know the incidence is related to aging, primarily those over 60, two in 100000 new cases per year, and men are afflicted 1.5 times more than women. Despite suspected clusters being studied in the United States, including around bodies of water where contaminated seafood was ingested[1] the only cluster of ALS patients was confirmed in the Western Pacific (Guam) in the 1940’s where locals were ingesting neurotoxins[2]. Some physicians report that they are “seeing increasing numbers of younger ALS patients”. Agreeing with what the epidemiology suggests, the etiology of ALS is multifactorial, including exposure to various chemicals[3]. Most ALS experts think these brain afflictions have multiple causes, including genetics, epigenetics[4,5], poor diet, lack of exercise, stress, and a variety of environmental agents, including pathogens[6], pesticides, for example, pentachlorobenzene, and other chemicals, such as PCBs and formaldehyde[7]. A recent New England study found pesticides, solvents, or heavy metals, increased the risk of ALS[8], and Seneff et al[9] propose that glyphosate (Roundup) causes a number of mechanistic abnormalities associated with ALS, including protein misfolding, and elevated levels of glutamate[10]. Surprisingly, even silver nanoparticles 100 nm in diameter, commonly used in personal, commercial, and industrial products can, when in aerosol form, be absorbed by the nasal mucosa and travel to the brain where they elicit a microglial, potentially inflammatory, response[11].
Neurological diseases are very complex, involving many underlying causative pathways, and, as such, using poly pharmacological approaches may increase efficacy when compared with single molecule therapies[12]. Therefore, neurodegenerative disorders such as ALS might require system therapeutic approaches[13] such as S2RM technology to obtain long-lasting, efficacious results[12]. In addition to the motor system dysfunction, deficits in mentation are reported for 50% of the ALS patients[14] demonstrating the complexity of this disease as measured by clinical endpoints.
The mechanisms underlying the destruction of motor neurons and other cell types in ALS are beginning to be understood, with many recent studies demonstrating key irregularities at the molecular and tissue levels in animal models and human ALS patients. Laboratory evidence suggests that stem cell therapy can positively affect ALS[15]. I will explain these distinct irregularities, show how the distinct components are interrelated, and then, based on the evidence, provide a rationale for using the stem cell-based S2RM technology to treat ALS.
In the clinic, the practitioner usually has no way to determine whether the stem cells used for therapy are viable, whether they perfuse to the site of injury following administration, and whether the cells engraft into the tissue and remain functional over time. Limited clinical research suggests that the stem cells often don’t engraft and therefore don’t remain functional in the patient. Laboratory models with detailed analysis show that stem cell injections into host tissue often fail for various reasons, including programmed cell death (apoptosis), a process that may be activated when stem cell numbers (shown for mesenchymal stem cells) become too high in the tissue[16]. As a therapeutic strategy, given that 80% or more of the therapeutic properties of stem cells is derived from the release of SRM, the better choice is to use the molecules derived from stem cells grown in optimal conditions in the laboratory. Those molecules can then be more easily, less expensively, and with better efficacy, directly administered to the patient[17].
Further, recent work in humans shows that stem cell therapy may induce advanced aging because forced cellular regeneration that accompanies re-engraftment induces intrinsic stem cell aging as measured using a p16 biomarker[18]. In mouse models, serial transplant readily “exhausts” stem cells evidenced by a diminished capacity to produce new somatic cells[19]. Using only the molecules released from stem cells means that the S2RM technology will not induced intrinsic aging caused by engraftment of the stem cells. Considering the use of iPSCs for therapeutic use, these cells suffer from genetic and epigenetic reprogramming errors, and the older the patient, the more mutations found in mitochondrial DNA along with increased metabolic problems[20].
I will now describe the mechanisms underlying ALS, and then propose a treatment regimen based on the use of S2RM technology.
MECHANISMS UNDERLYING ALS
The multifactorial disease ALS is a result of some of the mechanisms listed below. Evidence suggest that these many factors, such as exposure to harmful chemicals during conception, development, and throughout life, coupled with aging, infection, and poor lifestyle can lead to the breakdown of the all-important extracellular matrix (ECM) and peri-neuronal nets (PNN) that support the motor tracts. The breakdown of matrix is likely a generalized phenomenon in ALS patients given that in animal models a breakdown of the matrix in the gut leads to “leaky gut,” followed by the induction of inflammation[21]. At the root of the breakdown of the ECM and PNN is a loss of homeostasis, and particularly a loss of proteostasis, meaning a loss of the building blocks for the ECM and PNN. As a major consequence of the ECM breakdown, heat shock proteins (HSP) and chaperone proteins from neighboring cells cannot supply the motor neurons with their necessary proteins that enable autophagy, and proper protein folding. These results are deadly for motor neurons.
ALS IS NOT GENETIC, AND MUTATIONS ARE NOT SPECIFIC TO ONE GENE
First, because of overlapping genes, a mutation at one nucleotide can have effects in multiple genes[22]. The very concept of a gene is in dispute[23], and the nebulous concept of a gene as a cause for disease is in question[24], especially given the “buffering” capacity of a redundant system to preclude simple mutations as disease causative agents[25] so well espoused in a new book by Professor Nobel of Oxford University[26].
“Genetic Factors Are Not the Major Causes of Chronic Diseases” is the title of highly regarded PLoSOne article by Professor Stephen Rappaport at UC Berkeley[27]. Indeed because of the failure of genetic studies to predict disease, where over 2000 genome-wide association studies have infrequently found risks greater than 1.2[24], geneticists are now using whole-genome sequencing (WGS) to search for “missing heritability”. The precision with which we can analyze the genome is very seductive, but as is the case with many seductresses, the outcome may not be positive. Rather than genetics, the empirical evidence suggests that exposures, extrinsic factors, are necessary determinants of disease that may or may not be operating in a background of genetic diversity, intrinsic factors. Thus, the extrinsic factors may be operating “above” the genome, for example, at the level of translation and post-translation. That the symptoms of ALS are largely a result of protein dysfunction is indisputable. Over sixty years ago the Millers[28] first discovered covalent binding of tissue proteins and carcinogen. Today we understand that exogenous (i.e., xenobiotics) and endogenous chemicals (i.e., oxidative stress factors), such as electrophiles, are a primary cause of protein dysfunction through, for example, covalent modifications[29]. Clearly genetics alone will not predict disease, whereas the exposome in general, and protein function specifically will be important in understanding many diseases and indications.
Although reports continue to claim genetic causes for ALs, such as mutations in C9orf72[30], studies of the loss of function of C9orf72 is insufficient to cause motor neuron disease by itself[31]. Likewise in SOD1 mutant mice, a predominant model for ALS, transcription and splicing are normal, suggesting that the abnormality occurs at the level of translation or post-translation[32]. Further, proteins, such as SOD1 can become pathogenic through non-heritable modifications such as oxidation, with characteristics similar to the SOD1 mutant in familial ALS, suggesting that conformational abnormalities due to WT-SOD1 protein translation or post-translational events can underlie sporadic ALS pathogenesis[33]. As MIT’s Evelyn Fox Keller[23] and Oxford’s Denis Noble[26] explain so well, mutations in the DNA often don’t express at the level of protein function, and simple sequence analysis of DNA alone will tell us little or nothing about disease. Let us now look at some of the mechanisms known to underlie ALS.
EXTRACELLULAR MATRIX
First, the extracellular matrix (ECM), particularly the perineuronal nets (PNN), surrounding motor neurons are dysregulated in ALS animal models. Reactive astrocytes and the abnormal accumulation of chondroitin sulfate proteoglycans (CSPGs) in the ECM[34] may create a microenvironment that is non-permissive for neural regeneration in ALS. For example, proteoglycans, specifically Decorin and Perlecan, control cell autophagy, meaning that these ECM components are important in clearing the motor neuron of misfolded proteins[35]. Further, breakdown of the ECM will lead to fragments that upregulate kainate receptors and exacerbate excitatory neurotoxicity[36]. PNNs are specialized for neurons and are condensed versions of ECM, the material that surrounds our individual cells. If these PNNs are restored through mesenchymal stem cell therapy by intrathecal injection (IT), then the motor function of the treated animals can be significantly improved. The mode of action in restoring the PNN is thought to be the release of a multitude of trophic factors, including neurotrophic factors, from the implanted stem cells[15]. In contrast to the success of this study, and like studies where cells or multiple factors are used, administration of only one neurotrophic factor failed to provide any benefit[37,38]. Recent safety studies show that administration (IT or IM) of stem cells to ALS patients is safe, and early indications are that some benefit was provided[39]. The aforementioned data provide excellent evidence that the S2RM methodology will work well in treating ALS.
Evidence suggests that the breakdown of ECM may be generalized phenomenon throughout the body in ALS patients given that the basement membrane breakdowns[40], as does the matrix in the gut, which leads to “Leaky gut” and an ensuing inflammatory response[21].
MUSCLE AND NEUROMUSCULAR JUNCTION
Evidence that pathological mechanisms begin at the neuromuscular junction (NMJ) rather than within the motor neurons[41-43] suggests that ALS may be a distal axonopathy. Cultures of satellite cells from biopsies of ALS patient biopsies have been shown to proliferate similarly to cells obtained from healthy muscle. However, the morphology of the ALS derived cells resembles that of senescent cells[44]. Myoblasts obtained from ALS patients are unable to normally differentiate into myotubes[44,45].
Human ALS samples are rare, but autopsy of an ALS patient demonstrated skeletal muscle changes with clear signs of denervation and reinnervation. However, the patient had normal appearing motor neurons; thus pathological changes in skeletal muscle appear to be present before the motor neurons are affected[41], thus providing human evidence for the “dying-back” phenomenon. In vitro cultures of SCs from ALS patients demonstrate a senescent-like morphology, disturbed differentiation, and an apparent inability to proceed through the myogenic program resulting in a decreased ability to regenerate and mature to functional myofiber[46]. Proteostasis is critical for maintaining aged satellite cells (muscle stem cell) function, preventing the stem cells from becoming senescent[47]. Proteostasis is dependent on a normal ECM and cytoskeleton. Exercise has been shown to increase the number of MSCs in muscle, where the MSCs act to release growth factors, inducing muscle regeneration[48]. At the NMJ, agrin, an ECM protein, is critical to maintenance of the synapse, and can rescue the NMJ in animal models of neuromuscular degeneration[49]. Agrin is expressed by MSCs, including bone marrow stem cells[50]. Again, agrin constitutes but one part of maintaining homeostasis at the NMJ, and if used alone to treat ALS, would represent a minimally effective, reductionist treatment regimen.
SUPEROXIDE DISMUTASE
The superoxide dismutase (SOD) protein is misfolded in the motor neurons of ALS patients, which means that SOD will not clear out oxygen radicals developed in the highly active motor neurons - thus, the motor neuron will die. Although some correlations of SOD misfolding with DNA mutations has been reported, Horwich’s lab at Yale has shown that the transcripts in SOD1 mice are largely normal[32], meaning that the RNA for SOD is normal and that the protein dysfunction is a result of translation, or post-translational mechanisms. Misfolded SOD1 will have many negative consequences, including binding the Voltage Dependent Anion Channel isoform 1 (VDAC1) in mitochondria, creating organelle dysfunction[51].
TDP43
TDP-43 is a protein that helps to regulates gene expression, including RNA transcription, splicing, transport, and translation. CNS cells, in particular motor neurons, are highly vulnerable to TDP-43 dysfunction. In TDP-43 knockdown mice, the effects are predominantly observed in astrocytes within the spinal cord, suggesting that TDP-43 dysfunction in astrocytes may play an important role in motor neuron degeneration. TDP-43 is misfolded in ALS, and spreads in a prion-like fashion[52].
TDP-43 can also impair mitochondrial dynamics and function in motor neurons. Previous studies described how ALS-associated mutations transform the cytosolic SOD1 into a membrane-interacting protein, and therefore are then capable of associating with organelles such as mitochondria. Recently the peptides derived from the TDP-43 prion-like domain were shown to have membrane-damaging capacity[53].
DJ-1 PROTEIN IS MISFOLDED
DJ-1 responds to oxidative stress to protect cells, i.e., motor neurons, from radical oxygen species[54,55]. Proper function of this protein is also controlled by Heat shock proteins[56], that must be donated to the motor neurons from adjacent cells. DJ-1 is also responsible for DNA repair, acting as guanine glycation repair protein[57]. Thus, if DJ-1 is misfolded, guanine glycation occurs and with resultant mutation frequency, DNA strand breaks, and cytotoxicity.
VGF
Fragment depletion of VGF is likely involved in the onset and progression of ALS. In fibroblasts and plasma samples from ALS patients in an advanced stage, VGF C-terminus peptides were reduced in both fibroblast and plasma. In the G93A-SOD1 mice, the same VGF peptides were decreased in plasma in the late-symptomatic stage, while in the spinal cord down-regulation occurred earlier. Immunohistochemistry studies suggests that a large amount of gray matter is VGF C-terminus positive in control mice (including nerve terminals, axons and some somata of motoneurons), while a significant reduction of VGF peptides has already occurred in the pre-symptomatic stage[58].
FUS
In a small number of patients, mutations in fused in sarcoma/translocated in liposarcoma (FUS/TLS or FUS) are associated with ALS. Animal models provide evidence that mutant FUS exerts a gain-of-toxic function in the cytoplasm of motor neurons resulting in cellular degeneration. Recombinant FUS proteins perfused into squid axoplasm inhibits anterograde and retrograde transport. Regardless of whether the mutation affects the nuclear localization signal (e.g., R521G or R495X) or the glycine-rich domain (e.g., G230C) of FUS, each of the disease variants tested inhibited FAT. When mutant FUS is mixed with the chaperone Hsp110, the impairment is reduced suggesting that a misfolded FUS is responsible for the gain-of-toxic function[59].
DIPEPTIDE REPEAT PROTEINS
One of the most common genetic changes associated with ALS and frontotemporal dementia (FTD) are aberrant hexanucleotide repeat expansions in C9orf72. Transcripts containing these expansions undergo repeat-associated non-ATG translation (RAN-T) to form five dipeptide repeat proteins (DPRs). In C9orf72-ALS/FTD patients, DPR aggregates are found throughout the CNS. DPRs can also cause degeneration when expressed in vitro in neuronal cultures and in vivo in animal models. The prion-like spread of the misfolded proteins is thought to lead to the spreading progression of pathology in the patients of many neurodegenerative diseases. Whether DPRs spread has yet to be determined. Using a number of experimental cell culture techniques, including spinal motor neurons derived from iPScs of C9orf72-ALS patients, data suggests cell-to-cell spreading of DPRs by exosome-dependent and exosome-independent pathways[60].
MITOCHONDRIAL DYSFUNCTION
Mitochondria are responsible for efficiently creating energy in our cells, including motor neurons, through a process called oxidative phosphorylation. In ALS, mitochondria function is compromised, and cells go into an inefficient energy producing state called glycolysis[61] that is 16 times less efficient than oxidative phosphorylation.
Inflammation is an essential component of immunity, but excessive response has been shown to damage tissue and lead to autoimmune disease. The excessive response is avoided with parallel, integrated regulatory responses that begin the healing. One of the keys to this regulatory response is interleukin-10 (IL-10). IL-10 is particularly important because it limits macrophage proinflammatory functions. IL-10 modifies macrophage function by enhancing the clearance of damaged mitochondria and modulating cellular metabolism to limit inflammation[62]. IL-10 is part of the molecular mix in the S2RM.
HEAT SHOCK PROTEINS
Neurons, once differentiated from their stem cell precursors, stop producing HSP. Without endogenous HSP, the motor neurons are dependent on surrounding cells to shuttle the HSP from the surrounding cell to the motor neuron (Figure 1). Mesenchymal stem cells release HSP, chaperone proteins[63], and HSP is transferred by exosomes from one cell to another to maintain protein homeostasis[64]. A compromised ECM impedes HSP transfer from one cell to another (Figure 2).
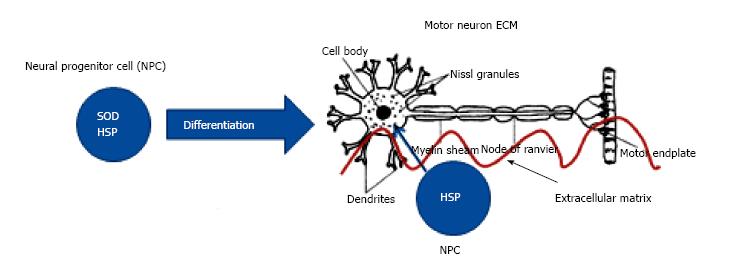
Figure 1 Differentiation of neural progenitor cells into motor neuron.
Once differentiated, only SOD produced by motor neuron. HSP must be supplied to motor neuron from surrounding stem cells. ECM facilitates HSP transfer. SOD: Superoxide dismutase; NPC: Neural progenitor cells; ECM: Extracellular matrix; HSP: Heat shock proteins.
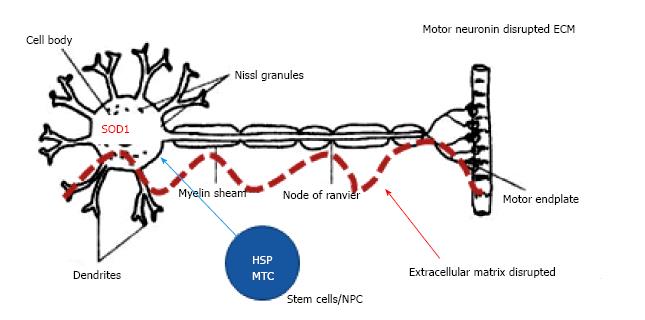
Figure 2 Motor neuron in amyotrophic lateral sclerosis.
ALS Motor neurons have misfolded SOD, reduced HSP and mitochondrial function. ALS: Amyotrophic lateral sclerosis; SOD: Superoxide dismutase; SOD1: Superoxide dismutase - misfolded; HSP: Heat shock proteins - disrupted ECM not permissive to HSP transfer; MTC: Mitochondria; ECM: Extracellular matrix.
EXOSOMES
Secretion of exosomes and their intercellular transmission of chaperone protein are key mechanisms for non-cell-autonomous proteostasis. Hsp40, Hsp70, and Hsp90, are secreted from cells via exosomes under physiological conditions[64]. Thus exosomes from healthy cells, including healthy stem cells, are necessary for the transfer of HSP to motor neurons. Exosome transfer from one cell to another is facilitated by the ECM. Exosomes from MSCs have been shown to alleviate some of the abnormal SOD1 aggregation in neural cells in an in vitro mouse model of ALS[65]. Exosomes that are loaded with the normal set of molecules, and/or the ability of the exosome to travel from healthy stem cells to cells in need of rescue may be compromised in ALS.
GLUTAMATE IS ELEVATED
Astrocytes associated with an ALS mouse model, called AbA cells, do not express detectable levels of the specific glial glutamate transporter GLT-1. Lack of a glutamate transporter in the glial cells of the ALS model may explain, at least partially, excitotoxic damage to motor neurons. Furthermore, the induction of neurotoxicity using the conditioned medium from AbA cells is specific to motor neurons[66]. As part of the support system, the astrocytes and the ECM, including the PNN, are critical to maintaining glutamate levels surrounding the motor neurons and preventing the glutamate induced excitatory neurotoxicity. Breakdown of the ECM also upregulates kainate receptors that are responsive to glutamate and mediate excitotoxicity[36].
ASTROCYTES
Astrocytes derived from the spinal cord of persons with sporadic and familial ALS also can kill motoneurons when co-cultured[67]. AbA cells in culture demonstrate increased proliferation and lack of replicative senescence, thus suggesting aberrant contact inhibition, and therefore a dysregulated ECM that fails to regulate the cells to a normal phenotype[66].
OLIGODENDROCYTES
Oligodendrocytes are severely affected in ALS and their degeneration has been shown to precede motor neuron death in the mutant SOD1 (mSOD1) mouse model[68]. Also, oligodendrocyte progenitors rapidly proliferate in the spinal cord of mSOD1G93A mice, but fail to replace degenerating oligodendrocytes, thus leaving motor neuron axons demyelinated. Recent studies suggest that SOD1 in ALS models is directly or indirectly contributing to oligodendrocyte pathology, and suggest that in this cell type some of the damage is irreversible[69].
PRION-LIKE PROTEINS
Misfolded proteins in the ALS model have similarities to prions. Key to the SOD1 and TDP43 proteins in ALS is that they self-template themselves, meaning that the proteins replicate themselves without the involvement of DNA and RNA, and then spread to other parts of the nervous system. These prion-like proteins will spread not only throughout the cell, but traveling in exosomes spread from cell to cell[70]. However, unlike true prions, the ALS prion-like proteins are able to be refolded or cleared from the body, correcting the destructive properties of the protein[71].
Protein-RNA assemblies serve as the foci of protein misfolding and their maturation into insoluble structures in the ALS state. The assemblies then recruit native proteins, turning them into misfolded forms. This self-perpetuation of misfolding proteins is a “twisted version of classical prion replication” that leads to amplification of pathological protein complexes that spread throughout the neuraxis, offering a pathogenic principle that underlies the rapid disease progression that characterizes ALS, and other neurodegenerative diseases[72].
CYTOSKELETON
The cytoskeleton in general[73] and actin[74], microtubules and neurofilaments[75] in particular, are believed to be dysregulated in ALS. Understanding that the rescue of neurons through the delivery of HSP and mitochondria is dependent on the ECM and actin-based tunnelling nanotubes (TNT) emanating from the cytoskeleton, and conveyed though the ECM of surrounding healthy stem cells, we can well believe that motor neurons in ALS are put at extreme risk by the interruption of the ECM/cytoskeleton.
BASEMENT MEMBRANE - BLOOD BRAIN BARRIER DYSFUNCTION
Basement membrane dysfunction leading to irregularities in blood/nutrient flow to neurons is a hallmark of macular degeneration, and has recently become evident in ALS[76]. Here again, breakdown of ECM can lead to vascular irregularities in the motor tracts and induce an inflammatory state. In the healthy adult with a stable ECM and basement membrane on which to attach, microglia are ramified and relatively quiescent. With breakdown of the basement membrane, microglia become mobile and reactive, and can further degrade the ECM[77]. A chronic para-inflammatory state in the motor tracts ensues. Blood-derived neurotoxic hemoglobin and iron accumulate in the spinal cord, leading to early motor-neuron degeneration in SOD1G93A mice. Chelation of blood-derived iron and antioxidant treatment early in the ALS sequalae mitigated early motor-neuronal injury[78]. Chronic inflammation with MMP-9 presence, as an example, can lead to laminin-III breakdown and a resulting breakdown of the blood brain barrier. Transplant of bone marrow stem cells into a mouse model of ALS repaired some of the vascular pathology[76].
ACHE - LOSS OF FUNCTION AT THE SYNAPSE BECAUSE ACHE BECOMES UNBOUND FROM THE ECM AT NMJ
Interestingly, muscle biopsies revealed a reduction in the AChE level of ALS patients[79], and analysis of the patient’s plasma revealed a large increase in circulating AChE[80]. The increase in circulating AChE may reflect a disruption of extracellularly bound AChE at the NMJ[81], leading to functional deficits in the muscle. Although in a study where nerve-muscle integrity was altered, and a similar reduction of AChE at the animals NMJ was measured, with an associated increase in plasma levels[82,83], the exact source of AChE increase in ALS plasma has not been demonstrated. Again, the data are suggestive of an early disruption of ECM, including that of the NMJ.
STRESS
Traditionally we considered inflammation as a defense in response to infection or injury. Inflammation, however, is often induced by tissue stress and malfunction in the absence of infection or overt tissue damage. Stress as a chronic phenomenon, will elicit a chronic, self-perpetuating para-inflammatory state with an active immune response.
Interestingly, activation of autoreactive T-cell receptors by non-commensal microbes might be a common trigger of autoimmune diseases in the immunoprivileged CNS[84]. This means that stress, and other factors that degrade the microbiota of the gut can have devastating long term effects through chronic inflammation and or elicit a full-blown autoimmune response in the nervous system.
Although the data are poorly collected in third world nations, the incidence of ALS in underdeveloped countries is reportedly lower. This could be due to many factors, including less stress and fewer dangerous chemicals in the environment[85].
EXPOSOME
Chemical exposure is thought to contribute about 70% of our health state[86]. Exposure to various chemicals, including PCBs and pesticides has been correlated to human ALS, but not to their matched controls[3]. The specificity with which pesticides and other environmental chemicals can act to destroy components of the nervous system is exemplified by the chronic exposure of the brain to the lipophilic pesticide rotenone causing a specific loss of dopaminergic neurons[87].
PGC-1α
Mesenchymal stem cells better engraft into host tissue when they overexpress PGC-1α[88]. Exosomes from adipose-derived stem cells ameliorate Huntington’s disease phenotypes in an in vitro model through the activation of PGC-1α[89]. This has yet to be explored in ALS.
SPATACSIN
In one study of Spatacsin, variants of the spatascin gene were associated with amyotrophic lateral sclerosis neuropathology in one member of one of the 10 families studied[90]. Given the limited tissue samples available for genetic analysis, and the lack of RNA or protein analysis, while the data warrant further study, no conclusions from this one study can be made.
LEAKY GUT AND DYSBIOSIS
Intestinal homeostasis and the microbiome have recently been shown to play essential roles in neurological diseases, such as Parkinson’s disease and ALS. Animal model studies suggests a potential role of the intestinal epithelium and microbiome in the very early progression of ALS[21] The ALS mouse model, G93A, which expresses mutant superoxide dismutase (SOD1G93A), showed a number of gut abnormalities. Damaged tight junction structure was present leading to increased permeability. Also, significant reductions in the protein expression levels of tight junction ZO-1 and the adherens junction E-cadherin were seen. Increased numbers of abnormal Paneth cells were reported. Because Paneth cells are specialized epithelial cells that can sense microbes and secrete antimicrobial peptides, they are key to host innate immune responses and helping to shape the gut microbiome. A decreased level of defensin 5 alpha, an antimicrobial peptide, was also found in the ALS intestine. A shift in the profile of the intestinal microbiome was also observed, including reduced levels of Butyrivibrio Fibrisolvens, Escherichia coli, and Fermicus, in the G93A mice. Increased expression of the inflammatory cytokine IL-17, and reduced levels of autophagic lysozyme 1, which leads to a reduced ability to clear misfolded proteins) were additional attributes of a disrupted microbiome. Importantly, the dysbiotic properties of the gut were present when the mice were 2 months old, before any other symptoms of ALS had yet developed. A comparison of healthy subjects vs ALS patients also has shown dysbiosis in the ALS patients with a decrease in healthy microbes and decrease in unhealthy microbes of the intestine[91].
Dysbiosis of the gut may also be involved in the misfolded alpha-synuclein (AS) and other proteins involved in neurodegenerative disorders that display prion-like transmission of protein aggregation. The role of amyloid proteins made by gut microbiota in the development of ALS was studied using aged rats and transgenic C. elegans. Both animals were exposed to E. coli producing the extracellular bacterial amyloid protein curli. Rats exposed to curli-producing bacteria displayed increased neuronal AS deposition in both gut and brain. Also observed was enhanced microgliosis and astrogliosis compared to rats exposed to either mutant bacteria unable to synthesize curli, or to vehicle alone[92]. The study showed that bacterial amyloid functions as a trigger to initiate AS aggregation through cross-seeding, while also priming responses of the innate immune system.
Relevant to ALS, and neurodegeneration in general, a probiotic mixture of 8 different strains of bacteria, namely Streptococcus thermophilus DSM24731, Bifidobacterium breve DSM24732, Bifidobacterium longum DSM24736, Bifidobacterium infantis DSM24737, Lactobacillus acidophilus DSM24735, Lactobacillus plantarum DSM24730, Lactobacillus paracasei DSM 24733, Lactobacillus delbrueckii subspecies Bulgaricus DSM24734, has shown reparation of leaky gut and a concomitant improvement in a number of neural functions in aged animal models of neurodegenerative disease[93].
AGE/RAGE
Fibroblasts are abundant in most tissues and in particular the nervous system. They are crucial for homeostatic maintenance and for building the ECM, as well as pathological ECM alterations observed in tissue[94]. Proteins in the ECM with a long half-life, such as collagen, can nonenzymatically react with high levels of glucose to form advanced glycation end products (AGEs). AGE-modified collagen increases stiffness of the matrix making the matrix resistant to hydrolytic turnover, resulting in ECM proteins accumulating in the microenvironment of cells. In addition, AGE will activate the receptor called RAGE (The receptor for advanced glycation endproducts). RAGE activation in turn can negatively impact normal ECM formation[95].
Many lines of evidence suggest that inflammatory responses play a critical role in the pathogenesis of motor neuron degeneration in ALS. RAGE is a key component in regulation of both innate and adaptive immunity in different pathologies associated with inflammation. RAGE mediates inflammatory responses and microglia stimulation in the brain[96], leading to neuronal damage, neurodegeneration, and resulting in symptomatic brain disorders. Immunohistochemical analysis of RAGE in control and ALS patients showed increased expression in reactive glial cells in both gray (ventral horn) and white matter in the spinal cord[97]. Expression of RAGE is also higher in the SOD1 transgenic mouse model of ALS vs wild-type mouse spinal cord. Further, treatment of SOD1 transgenic mice with soluble RAGE (sRAGE), a natural competitor of RAGE that sequesters RAGE ligands and blocks their interaction with cell surface RAGE, significantly delays the progression of ALS and prolongs life span as compared to vehicle treatment[98].
Part of the therapeutic strategy of S2RM is to return proteostasis to a normal state using the S2RM technology, thus rebuilding the ECM to a normal state, and reducing the deleterious build-up of AGE and the subsequent activation of RAGE.
WHAT LEADS TO BREAKDOWN OF THE ECM
Environmental toxins, xenobiotic electrophiles for example, can disrupt DNA and protein function and degrade ECM[99]. Damage to ECM triggers inflammation and further degradation of the extracellular matrix (ECM). The ECM is an intricately arranged biochemical and mechanical scaffold composed of secreted proteins and complex sugars that collectively support cell function and survival. Some ECM molecules become aberrantly expressed following damage, whereas other proteins are cleaved into bioactive fragments known as damage-associated molecular patterns (DAMPs) or “alarmins”. The DAMPs are able to bind different types of pattern recognition receptors (PRRs), and can influence the phenotype and magnitude of inflammation. Further, the enzymes and inflammatory mediators released by immune cells resulting from the damage further degrade or alter the composition of the ECM[100].
Knowing that the breakdown of the ECM/cytoskeleton is a key determinant in ALS, we can then ask, what causes the breakdown of the ECM/cytoskeleton? The exposome, i.e., chemical exposure, is thought to contribute about 70% of health state[86]. This coupled with lifestyle, stress, and the aging process itself are all risk factors. To summarize, the following factors are known to cause ECM breakdown: (1) Chemical exposure - numerous mechanisms, including breakdown of matrix; (2) stress - signals originating in the sympathetic nervous system can suppress osteoblast function and control the attraction of stem cells to their niche[101]. Stress, acting through cortisol directly breaks down ECM; (3) aging - degradation of ECM, loss of stem cell function; (4) glycation - binding of sugars with ECM causing ECM dysfunction[98]; and (5) inflammation, MMPs - chronic para-inflammation or inflammation as a result of ECM breakdown leads to further inflammation and an activation of macrophages (microglia) and a release of many molecules, including MMPs that will exacerbate the inflammatory state thus damaging neurons and muscle.
Collectively these studies suggest that a key determinant of ALS is a dysfunctional ECM and cytoskeleton. I now present the rationale for our therapeutic approach based on the evidence.
S2RM METHODOLOGY FOR TREATING ALS
Simply stated, I believe the predominant cause of ALS is a breakdown of the ECM/microenvironment in the motor neuron tracts of the nervous system; all other mechanism underlying ALS derive from this breakdown. As a major consequence of the ECM breakdown, HSP and chaperone proteins from neighboring cells cannot supply the motor neurons with their necessary proteins that enable autophagy, and proper protein folding. These results are deadly for motor neurons.
My strategy uses a combination of stem cell types to treat ALS. And instead of injecting cells into the patient, we inject the molecules from the multiple cell types into the patient. Here’s why. The molecules released from the stem cells are doing all of the work in treating ALS, and the cells are difficult and expensive to work with outside of the laboratory setting, making clinical procedures with the stem cells onerous. Further, once the cells are injected into the patient, the functionality of the stem cells is not measurable, and we know that most stem cells injected into patients are not functional shortly after implantation, failing to make a live graft[102]. Therefore, our strategy is to grow and stimulate the stem cells in the laboratory to optimize the set of SRM, collect the therapeutic molecules that the stem cells release, and then use those molecules to directly treat the patient. In this manner, we know the “healing molecules” are being delivered to the patient in a defined manner, both in space and in time. The molecules we use are derived from several types of stem cells known to be active in the brain, to stimulate neuron and glial cell growth[103], build the PNNs, set the ECM to a permissive state for neuro-regeneration, and to correct some of the mechanistic failures and alleviate the symptoms of ALS (Figure 3).
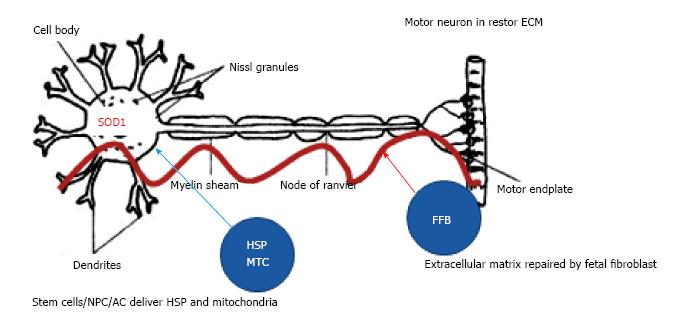
Figure 3 Repairing motor neurons in amyotrophic lateral sclerosis.
SOD, HSP and mitochondrial function are restores to motor neurons. SOD: Superoxide dismutase; SOD1: Superoxide dismutase – misfolded; HSP: Heat shock proteins – disrupted ECM not permissive to HSP transfer; MTC: Mitochondria; NPC: Neural progenitor cells; ECM: Extracellular matrix; FFB: Fetal fibroblast; AC: Fetal astrocyte.
S2RM DELIVERED IN EXOSOMES
As stated above, exosomes are key to this technology given their protective, penetrating, and delivery qualities. They are also immunoprivileged, and have been shown to be used successfully for patient to patient injection without negative immune reaction. Recently, exosomes from human cancer patients have been demonstrated to protect their protein contents from dephosphorylation from endogenous phosphatases. The exosomes were successfully used for protein-based diagnostics of cancer patients for up to 5 years following sampling from the patient[104]. This is strong evidence for the ability of our exosome-based therapeutic to be protected in the body as it courses to the target site in the nervous system of the ALS patient.
CELLS USED TO CREATE S2RM-EXOSOME (1-4 ADMINSTERED INTRANASAL, 1-5 ADMINSTERED INTRATHECAL)
First, the use of allogeneic MSCs or their exosomes have been used to treat a number of patients to treat graft-vs-host disease with success, meaning that stem cells themselves, and especially their exosomes, will not elicit a negative immune reaction[105-107]. Because of their small size, unlike the stem cells themselves, exosomes can easily be sterilized by passing them through a 0.22-µm sterile filter. The cell types used to develop the therapeutic are as follows: (1) bone marrow mesenchymal stem cells (BMSC) - conditioned media induces angiogenesis and rescue mitochondrial function - these cells are stimulated and cultured in a special way, explained in methods section below (Figures 4 and 5); (2) neural progenitor cells - neural growth factors; (3) fetal fibroblasts - less than 24 wk post-gestation (secreting growth factors and building blocks of ECM and PNN, allowing a permissive environment for cellular reprogramming of the afflicted motor neurons and astrocytes); (4) fetal astrocytes - homeostasis of motor neuron support cells; (5) satellite cells (muscle stem cells) - known to express HSP. Administered only at spine through Intrathecal Injection. The SRM from the cells listed as 1-4 are collected and can be dosed intra-nasally to the patient. The SRM from the cells listed in 1-5 are collected and can dosed by intrathecal injection to the patient.
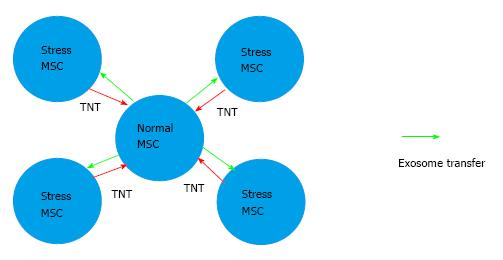
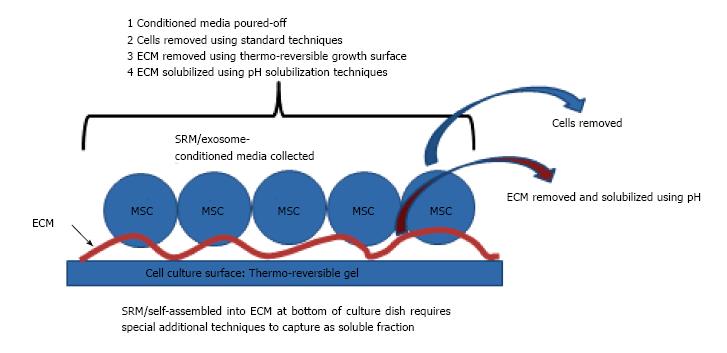
Figure 4 Stimulating and culturing mesenchymal stem cells.
TNT formation between stressed and normal MSCs. TNTs facilitate mitochondrial, exosome and molecule transfer between cells. As part of the ECM, the TNTs are extracted with out thermoreversible gel sxtraction procedure, and included in the S2RM. TNT: Tunnelling nanotubes; ECM: Extracellular matrix.
Figure 5 Techniques to capture soluble SRM, exosomes, and self-assembled molecules in extracellular matrix at bottom of culture dish.
ECM: Extracellular matrix; MSC: Mesenchymal stem cell.
CAN THE DISEASE BE CURED? CONSIDERATIONS OF ALS AS A CHRONIC PARA-INFLAMMATORY STATE AND HOMEOSTATIC RENORMALIZATION
Tissue damage, independent of infection, such as breakdown of the ECM, Basal Lamina, and PeriNeuronal Nets, can induce a tissue-repair response that involves the activation of both innate and adaptive immunity, such as activation of regulatory T cells, group 2 innate lymphoid cells (ILC2 cells), eosinophils, and M2 macrophages[108]. This can lead to a state of chronic parainflammation that is self-perpetuating. Let me explain. Between basal homeostatic conditions and true inflammation a “para-inflammation” state exists[109]. Para-inflammation is an adaptive response of the immune system to low levels of tissue damage, such as in aging whereby oxidative stress or cortisol release for example, accumulates for many decades. The physiological role of para-inflammation is to maintain homeostasis (or re-set the homeostatic threshold of the tissue) and restore tissue functionality. Unfortunately, because of aging, and living in the industrial world with all of the newly created stressors and an exposome that lacks a corresponding evolved homeostatic renormalization function in our bodies, homeostasis is constantly disrupted. This para-inflammation theory helps to explain many phenomena observed in various chronic disease conditions, an example of which is “inflammaging”[110]. Why the ECM and Peri-Neuronal Nets will be differentially affected and cause ALS will be hard to figure, but is likely to involve the exposome, what you’ve been exposed to during your lifetime, at conception, during development, and throughout your adult life, along with other lifestyle factors such as stress, exercise, and diet. Further, epigenetic regulation of your parents, and their parents, by what they did and what they encountered in their environments will also have major consequences to your health[111-114]. This is termed transgenerational epigenetic inheritance. For example, even dietary effects at the epigenetic level can be passed for three generations through an RNA-based mechanism[115,116], and the drugs consumed by the father[117]. The types, quantities, and timing of these factors are likely to be critical in determining whether one will develop ALS, or any other disease of inflammation and aging.
HOMEOSTATIC RENORMALIZATION USING S2RM
The chronic parainflammatory state underlying ALS can best be treated with S2RM technology to renormalize homeostasis. The renormalization process, which includes proteostatic renormalization to rebuild the ECM, TNT, and Peri Neuronal Nets will allow for key HSP and chaperone proteins, along with protein conglomerates called proteasomes, to fold, refold, repair, or expunge the misfolded and damaged proteins, SOD1, DG-1, TDP-43, that lead to ALS. An imbalance in proteostasis leads to many dysfunctions including, for example, inhibition of FGFBP1 expression in muscle cells by increased accumulation of the transforming growth factor-β1 (TGF-β1) in skeletal muscles and at their NMJs. In the absence of FGFBP1, NMJs exhibit structural abnormalities[118].
Further, mitochondrial function will be repaired in the neurons and muscles, and TNTs to transfer healthy mitochondria from healthy cells to compromised cells reestablished - this too is critical in ALS therapy because mitochondria are damaged[119] by phylogenetically bacterial symbionts of early eukaryotic cells, that when damaged, release mitochondrial damage-associated molecular patterns (formyl peptides and mitochondrial DNA) with evolutionarily conserved similarities to bacterial pathogen-associated molecular patterns. These are released into the circulation and are powerful activators of innate immunity and Nlrp3 inflammasome[120,121]. Fitting with our hypothesis and treatment strategy, formyl peptides (chemotactic peptides), for example, rearrange the cytoskeleton and change migration patterns of cells, likely interrupting the cell to cell connections that convey HSP, chaperones, exosomes, and mitochondria from healthy stem cells to damaged motor neurons and muscles. These protein factors, along with key microRNA[122] to renormalize dysregulated microRNA in the ALS patient[123], contained in the exosomes of S2RM, are the major factors in S2RM therapeutics for ALS.
SPECIAL CULTURE TECHNOLOGY
Our strategy is to use only the molecules that are fully formed and secreted by the stem cells. Crushing the cells and extracting the molecules by harsh techniques is inadvisable as incomplete and misfolded proteins and other molecules may be extracted in this manner. Incomplete and misfolded proteins as we have seen in the aforementioned studies are exactly what we want to avoid in ALS. Stem cells secrete many molecules into the media, and also a rich self-assembly of some of those molecules forms an ECM on the bottom of the culture dish.
Figure 4 diagrams how HSP is induced in MSCs, and how TNTs are formed between the stressed cells and the normal cells. The culture procedure has two steps: (1) MSCs are cultured under normal conditions, but pulsed with higher temperatures at 39 °C for 24 h in order to slightly stress the cells so the HSPs are formed. On completing of the 24 h stress period at 39 °C, the cells are then removed from the culture vessel using standard techniques so that they can be co-cultured with normal, non-stressed MSCs; and (2) the stressed MSCs are then co-cultured with normal MSCs at a ratio of 1 normal cell to 4 stressed cells. Under these conditions TNTs will form between the normal and stressed cells using actin and microtubules emanating from the stressed cells and guided through the developing ECM at the bottom of the dish that serves as a matrix for the growing cells. In this manner, HSPs, mitochondria, and exosomes can transfer from one cell to another.
Our strategy is to: (1) Capture the conditioned media with all of its soluble molecules, as well as the molecules in the exosomes; and (2) capture all of the molecules that have formed the ECM at the bottom of the dish by the process shown below in Figure 5. Although our therapeutic is protected by issued and pending patents and trade secrets, we can disclose here that our therapeutic is developed using the SRM from five different types of adult stem cells, including lineages from mesenchyme and nervous system.
CONCLUSION
ALS is a disease of protein dysfunction without an RNA or DNA component. Extrinsic factors, including the exposome, cause disruption of proteins that build and maintain the ECM. Once the ECM is disrupted, the support function of stem cells, including neural stem cells, which maintain the proper folding of proteins in the nervous system is degraded. Once the proteins in the nervous system misfold, the misfolded proteins self-template themselves and act in a prion-like manner to spread and destroy neurons. The best way to treat this disease of degraded ECM and misfolded proteins is to restore homeostasis, and in particular, proteostasis, so that the ECM and proper folding of proteins is restored. Proteostasis will allow, for example, tunneling nanotube formation and the transfer of mitochondria from healthy cells to those in need of recue[124]. Homeostatic renormalization is accomplished by administering S2RM stem cell molecule technology to the nervous system.