Published online Oct 26, 2016. doi: 10.4252/wjsc.v8.i10.355
Peer-review started: January 15, 2016
First decision: March 1, 2016
Revised: July 21, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 26, 2016
Processing time: 279 Days and 23.6 Hours
To identify differences between primed mouse embryonic stem cells (ESCs) and fully functional naive ESCs; to manipulate primed cells into a naive state.
We have cultured 3 lines of cells from different mouse strains that have been shown to be naive or primed as determined by generating germline-transmitting chimeras. Cells were put through a battery of tests to measure the different features. RNA from cells was analyzed using microarrays, to determine a priority list of the differentially expressed genes. These were later validated by quantificational real-time polymerase chain reaction. Viral cassettes were created to induce expression of differentially expressed genes in the primed cells through lentiviral transduction. Primed reprogrammed cells were subjected to in-vivo incorporation studies.
Most results show that both primed and naive cells have similar features (morphology, proliferation rates, stem cell genes expressed). However, there were some genes that were differentially expressed in the naïve cells relative to the primed cells. Key upregulated genes in naïve cells include ESRRB, ERAS, ATRX, RNF17, KLF-5, and MYC. After over-expressing some of these genes the primed cells were able to incorporate into embryos in-vivo, re-acquiring a feature previously absent in these cells.
Although there are no notable phenotypic differences, there are key differences in gene expression between these naïve and primed stem cells. These differences can be overcome through overexpression.
Core tip: Derivation and culturing of mouse embryonic stem cells (ESCs) from gene targeting to injection into blastocysts for chimera generation is a lengthy process that is difficult to control. Some stem cells might be in a primed state, having lost some of their characteristics, most importantly their pluripotency. These differences between ESC clones are usually only detected after many months by the failure of chimeric males to transmit the ESC genome through the germline. Here we have determined key expression differences between cells in a primed state and those in a presumed ground state. Detection of these differences will give researchers a powerful tool to quickly distinguish these cells, saving time, money and effort by choosing the best clones to go forward with. Furthermore, we were able to rescue the ground state through overexpression, indicating that the fate of these cells may potentially be controlled.
- Citation: Rossello RA, Pfenning A, Howard JT, Hochgeschwender U. Characterization and genetic manipulation of primed stem cells into a functional naïve state with ESRRB. World J Stem Cells 2016; 8(10): 355-366
- URL: https://www.wjgnet.com/1948-0210/full/v8/i10/355.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i10.355
Stem cells are in an early undifferentiated state and have the potential to differentiate into a variety of cell types and tissues, both in-vitro and in-vivo[1]. There are different types of stem cells. Adult stem cells are multipotent cells that exist within the adult tissue[2]. Embryonic stem cells (ESCs) have the potential to be differentiated to any cell type (pluripotent), whereas more differentiated stem cells, such as those in the skin, have a more restricted differentiation potential (unipotent)[3,4]. Induced pluripotent stem cells (iPSCs) can also be differentiated into various cell types[5-8] but a major advantage of iPSCs is that they can be generated from already terminally differentiated cells, such as skin or fibroblasts, of an individual and do not require isolating cells from embryos[9]. Findings that the simple over-expression of four transcription factors (Oct4, Sox2, Klf4 and c-myc) was sufficient to induce iPSCs from adult mice[5] and human[6] cells made the process of generating stem cells much more tractable in certain species, where it was once difficult to generate stem cells (such as in rats[10], pigs[11], and birds[12,13]). Since then, several strategies have been used to manipulate cells into a pluripotent state[14,15].
Derivation of mouse ESCs is a lengthy process[16,17] that often produces cell lines that have all of the features inherent in ESCs, but fail to incorporate into the germline. Similarly, culturing, selection, and expansion of ESC clones for gene targeting experiments results in clones whose potential for germline transmission will only be revealed after months of mouse breeding. This presents a significant limitation, as time invested may not yield the desired results. Identifying the potential of these cells early in the process, in order to make a stop/go decision, could enhance the efficiency in which research is conducted. Furthermore, overcoming identified differences in cells which lost their pluripotency may lead to rescue of valuable cell lines.
Lastly, while the reprogramming of healthy human somatic cells into a stem cell state has been defined[6,14]; there are still important differences being assessed between pluripotent states in derived ESCs, such as the differences between primed and naïve ground states[18].
Our work aims to identify differences, molecular and otherwise, between mouse embryonic stem cells which we are defining as naïve (ESCs that result in germline transmitting chimeras, and thus are fully pluripotent) and primed (ESCs that have all of the features of naïve cells, except that they fail to produce germline transmitting chimeras). These included morphological markers, telomerase activity, MTT assays, and microarray analysis, and incorporation into an embryo. Differences in gene expression can be used as a diagnostic tool to determine if the stem cells are in a fully naïve pluripotent state. In addition, we aim to manipulate primed cells, using lentiviral vectors, in order to induce a naïve state. We determined the differential expression patterns in 3 pairs (naïve/primed) of mouse ESC lines derived from different strains and test the hypothesis that ESC functionality can be restored. Each pair of naïve and primed cell line was generated during a separate gene targeting experiment, each starting from a pluripotent ESC line. Using microarray and bioinformatic analysis, we determined a priority list of differentially expressed genes. The list included genes such as Esrrb, Eras, Klf-5, c-myc, Rnf-17, Atrx, which were significantly downregulated in the primed ESCs; the expression level of these genes was further validated using qRT-PCR. cDNAs for these genes were isolated and used to construct gene cassettes and lentiviral vectors. Primed cells were induced to overexpress some of these genes. Reprogrammed cells were injected into the blastocyst to assess the hypothesis that function, here measured by incorporation into the embryo, could be restored.
Mouse embryonic fibroblasts were collected at embryonic day 12.5 for 129 Sv and C57BL/6 mice strains (Jackson). Briefly, embryos (n = 4) were extracted from the womb, their liver and head were removed, and the remaining contents were minced manually using forceps. The contents were placed in a 15 mL tube and treated with 0.25% trypsin (0.25% Trypsin/EDTA, Gibco; 1-2 mL per embryo) for 30 min at 37 °C, pipetting briefly every 5 min to enhance dissociation. Trypsin was neutralized with complete DMEM media, cells were spun down, counted (hemocytometer), re-suspended in media and plated at a concentration of one embryo per 150 mm dish. When grown to confluent layers, all fibroblasts were passaged in complete media twice before cells were frozen in aliquots. Mouse embryonic stem cells[16] were cultured using KO-DMEM and standard conditions. Cells from two different genetic backgrounds and from three different gene targeting experiments were paired up after they were revealed as naïve (germline transmitting) or primed (no germline transmission), respectively (Table 1).
| Mouse strain of ESC line | Targeted locus | Germline transmission Pluripotent | No germline transmission Not pluripotent |
| 129Sv | POMC | I (QKQR-1E) | II (QKQR-11B) |
| C57BL/6 | IGFR1 | III (IGFR1-152) | IV (IGFR1-R13) |
| C57BL/6 | FGF13 | V (FGF13-1) | VI (FGF13-15) |
Cells or RNA were spun down and RNA isolated using a standard kit (Promega SV total RNA isolation system, Z3105) as before[12]. RNA was quantified using a NanoDrop 2000c (Thermo Scientific) and then stored in -80 °C. RNA was used for microarray (methods) and qRT-PCR experiments.
Microarray analysis was performed in the Microarray Center (Duke University Center for Genomic and Computational Biology), as per their standard protocols (Affymetrix Exon WT Package). Briefly, total RNA (volume 50 μL) was extracted and submitted to the core for analysis on a Mouse Gene 1.0 ST Array (Affymetrix). Results were analyzed using variance stabilization[19].
Complementary DNA (cDNA) was produced by reverse transcription (RT) in a 20 μL reaction using the supplier’s protocol (10 μL of 2 × RT buffer and 1 μL of 20 × Superscript II enzyme; Applied Biosystems). The cDNA was then used as a template to perform PCR gene expression assays in 20 μL reactions containing 1 μL template (approximately 2 μg/μL), 10 μL 2 × Gene Expression Master Mix (BioRad) and forward and reverse TaqMan primer probes (Generated by Applied Biosystems) or in 20 μL reactions containing the same reagents, but in place of TaqMan primers, custom PCR primers and 1 μL SYBR green (BioRad). The reactions were performed in a Cx96 real-time machine (Bio-rad). Cycling conditions were 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. No-template controls were run for each primer set and probe. 18S rRNA endogenous control was run for each sample using TaqMan primers that recognized the RNA in all samples tested (Cat# Eukaryotic 18S RNA HS99999901_S1; Applied Biosystems). The results were normalized to the endogenous 18S expression and to the gene expression level of the control mouse fibroblasts using the 2-DDCT method common for qRT-PCR analyses[20]. All primers showed efficiency levels above 90%, using the protocol in the MIQE guidelines (minimal information for publication of real-time PCR experiments). For statistical analysis, 2-way ANOVAs were performed on two factors [genes and strain type (C57BL/6 and 129 Sv)] on n = 3 independently generated lines (replicates) for each of the groups. Table 2 contains the primer sets utilized in this project.
| Mouse | Gene identification | Fwd primer | Rev primer |
| Oct-4 | NM_013633.2 | CCCCATGTCCGCCCGCATAC | AGGCCCAGTCCAACCTGAGGTC |
| Sox-2 | NM_011443.3 | GAAGAACAGCCCGGACCGCGT | ATGAACGGCCGCTTCTCGGT |
| c-myc | NM_010849.4 | ACCCGCTCAACGACAGCAGC | ACTAGGGGCTCAGGGCTGGC |
| KLF-4 | NM_207209.2 | TAGTGGCGCCCTACAGCGGT | TCGTGTGTGTTGGGCCGGTG |
| KLF-5 | NM_009769.4 | CACCGGATCTAGACATGCCC | ACGTCTGTGGAACAGCAGAG |
| Pax-6 | NM_001244198.1 | CACCAGACTCACCTGACACC | TCACTCCGCTGTGACTGTTC |
| BMP-7 | NM_007557.3 | CTGAGTAAAGGACAGGGGCG | CTGAGTAAAGGACAGGGGCG |
| ESRRB | NM_001159500.1 | CTACGCCACTCAAGAAGCCA | TTGATGAAGGAGCCGCAACT |
| Nanog | NM_028016.1 | GGCTGCCTCTCCTCGCCCTT | GTGCACACAGCTGGGCCTGA |
| ERAS | NM_181548.2 | TGCCCCTCATCAGACTGCTA | CCAAGCCTCGTGACTTTCCT |
| ATRX | NM_009530.2 | CTTGCTTTGTTTCCGTGGCTCT | CTTGTTTCCACTCATGGGCTC |
| RNF17 | XM_006519107.1 | CACCTAGTGGAGAGTGACCA | TCTAAATGCCTGTCAGGGGC |
In order to generate vectors, we used the backbone for the STEMCCA Cassette[20], excising the stem cell genes using restriction enzymes. After evaluating a priority list of differentially expressed genes, we decided to generate cDNAs for two genes, Eras (Embryonic Stem Cell Expressed RAS, ENSMUSG00000031160) and Ring Finger Protein 17 (Rnf-17, ENSMUSG00000000365) and Essrb (Estrogen Related Receptor Beta, ENSMUSG00000021255) were generated in order to incorporate them into the cassette. RNF17 incorporation was not successful, therefore, only the ESRRB and Eras genes were used. Cassettes with c-myc and KlF-4 derived from Sommer et al[20], were also generated. We also generated a cassette with Nanog (NM_028016.1), as a positive control to ESRRB.
Lentiviral vectors were generated in human embryonic kidney 293T cells (Cell Biolabs, Cat # LTV-100), using a third-generation lentiviral system, following a previously described protocol[12]. Prior to transfection, the cells were plated on 10 cm collagen coated plates at a density that resulted in 60%-70% confluency at the time of transfection. A transfection mix was prepared with either 5, 10 or 15 μg of DNA of the genes generated in vector or control GFP lentiviral vectors (EF1alfa-GFP; generated in lab), packaging cassette (REV and Gag/Pol, 10 μg) and the VSV-G (5 μg) envelope expression cassette, respectively. The cells were then transduced with the mix, using 40 μL of Lipofectamine (Invitrogen) per plate. Eight hours after the addition of DNA, the transduced cells were washed with PBS and fresh complete media as used for mouse cells. Media with viral particles were collected every 24 h for the next 48 h and stored at 4 °C until complete. Viral particles were separated from cellular debris by centrifugation at 4000 g for 5 min followed by filtration through a 0.45-micron filter. The titer was measured using Quick-Titer (Cell Biolabs Inc, Cat # VPK-112) and promptly stored at -80 °C. If necessary, titer concentrations were increased by ultracentrifugation (SW-29 rotor) at 50000 g for 2 h, followed by re-suspension in PBS (pH = 7.2).
Transduction was performed in the Comprehensive Cancer Center of Puerto Rico, using the ViraDuctin system, as per supplier’s protocol (Cell Biolabs, Cat # LTV-201) in KO medium. Before transduction, cells were thawed and cultured in complete media until 80% confluent. After transduction, cells were grown for 10 d, then passaged (1st passage), and let to grow for approximately 10 d in KO medium. Viral transduction efficiency values were assessed at different vector concentrations in 48 well plates and cell colony-forming units quantified as before (Rosselló et al[12], 2013).
To assess proliferation, we used the MTT [3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] Quantitative Cell Proliferation Assay (ATCC; Cat# 30-1010K). Briefly, tetrazolium salts are reduced metabolically by the cells, resulting in a colorimetric change. The resulting intracellular purple formazan is solubilized and quantified spectrophotometrically (at 570 nm). Cells were plated at 10000 cells/well (in quintuplets) and incubated for 24 h. Ten microliters of the MTT reaction solution was added to each plate and incubated for 3 h. One hundred microliters of detergent was added to each plate, stored for 2 h in the dark (room temperature), and the absorbance was measured at 570 nm using a Molecular Devices Emax Microplate Reader. ANOVA was performed to test for differences between cells and strain (n = 5 lines, per strain). Statistical significance was considered at P < 0.05.
Telomerase enzymatic activity was determined using the Quantitative Telomerase Detection Kit (BioMax, United States, MT3012), following the manufacturer’s protocol. Cell extracts containing proteins and RNA were generated from the ESC, iPSC, and control fibroblast, and then telomerase activity was measured. If telomerase is present, it adds nucleotide repeats to the end of an oligonucleotide substrate of the kit, which is subsequently amplified by real time qPCR. Quantitation was carried out by the PCR software of the BioRad Cx96 system. Positive control (template provided with kit) and negative control (heat inactivated samples) reactions were performed. Cycling conditions for the BioRad Cx96 real-time machine were as follows: 48 °C for 10 min and 95 °C for ten min, followed by 40 cycles of 95 °C for 15 s (denaturation) and 60 °C for 1 min (annealing/extension). All reactions were performed in quintuplets. Paired t-tests were performed to test for differences of telomerase in the induced and control fibroblasts of each cell line. Statistical significance was considered at P < 0.05.
Blastocysts (from strain C57BL/6) were injected with control fibroblasts, primed ESCs, reprogrammed primed ESCs and positive control naive ESCs and implanted into recipient females of the same strain as has been previously done[21]. Briefly, we injected blastocysts, isolated from pregnant C57BLK/6 females, with fibroblasts, primed ESCs, reprogrammed primed ESCs, and positive control naïve ESCs (n = 4). All cells were labeled with GFP through viral transduction. Five days after injection, embryos were extracted and analyzed for incorporation. Embryos were placed in 70% EtOH solution, before being paraffined and sectioned for histological analysis.
GFP labeling (performed by the Duke University Pathology Lab, as before[12]) was performed on mouse embryos, or positive control tissue slides (GFP positive), that were cut at 5 μm on a paraffin block and mounted onto glass slides. These were dried for 40 min at 60 °C in an oven. The slides were deparaffinized in 3 changes of xylene (5 min each), 2 changes of 100% EtOH (3 min each), and 2 changes of 95% EtOH (3 min each). Rehydration was performed in dH2O for 1 min. To block endogenous peroxidase activity, 3% hydrogen peroxide was used for 10 min, followed by a rinse in dH2O to remove antigens. For the primary antibody [anti-Rabbit GFP Abcam ab290, diluted at 1:100 in PBS (pH = 7.1)], 200 mL of the citrate, pH 6.1, antigen-retrieval buffer from Dako (10 × concentrate) were used. The buffer was preheated to 80 °C in a Black and Decker vegetable steamer for 20 min. The slides were then cooled to room temperature. Slides were thoroughly rinsed in water and placed in TBST. After antigen retrieval, 10% normal rabbit serum was applied to the slides and incubated for 60 min at room temperature. Afterwards, they were washed with PBS and the excess was drained. After incubation, Vectastain Elite ABC was used, followed by DAB chromagen (Dako), and incubated for 5 min, followed by washing. All slides were counterstained in hematoxylin for 30 s. Slides were rinsed in tap water until clear and coverslipped.
All appropriate measures were taken to minimize animal discomfort, monitor post operative recovery and establishing humane endpoints per our IACUC protocol A262-12-10.
Biostatistics were reviewed by an expert in biomedical statistics, in order to evaluate methods used, as per suggestions. For the gene comparative, the positive log fold change values mean that the gene expression is lower in the pluripotent cells. The same is true for the t value (which the P value is based on that shows the strength of significance). Although P and t values are linked, we use t values to determine differences between populations, in order to measure the size difference relative to the variation.
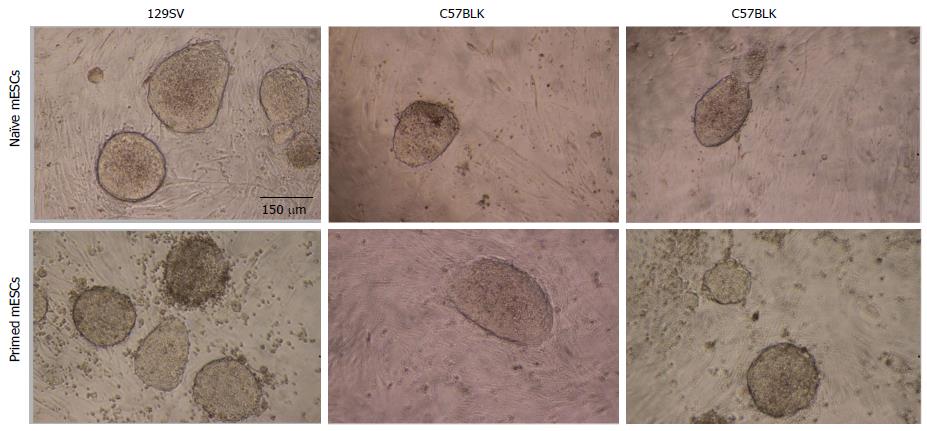
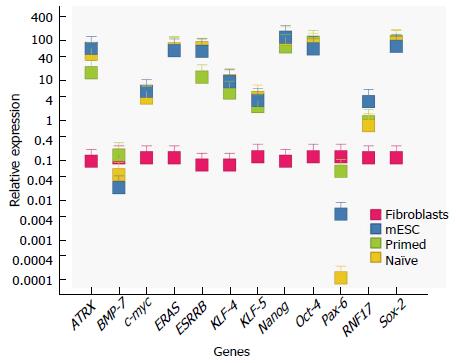
At first glance, all ESC cells exhibit similar morphology. Those that had been determined to be primed, i.e., no germline transmission, showed round, cluster like formation, similar to naïve ESCs (Figure 1), as well as alkaline phosphatase activity (not shown). In addition, qRT-PCR was performed on all samples to determine expression of typical stem cell genes (Oct-4, Sox-2, Klf-4, Nanog). Here, they exhibited similar profiles (relative to control fibroblasts) (Figure 2). Normalization was performed with 18 s expression levels for each sample. In order to compare the expression levels of the different stem cells relative to fibroblasts, fibroblasts expression values were set at 1 (Figure 2). This normalization allows us to visualize and determine the difference between fibroblasts and the stem cell groups, relative to each other.
| ID | Gene symbol (HUGO) | t | P value |
| 1420106_at | Siah1a | 3.397282751 | 0.019877842 |
| 1451158_at | Trip12 | 3.397517988 | 0.019872832 |
| 1425223_at | Birc3 | 3.399188537 | 0.019837294 |
| 1455579_at | Csng | 3.407711727 | 0.019657099 |
| 1416670_at | Setdb1 | 3.40836782 | 0.019643305 |
| 1448406_at | Cri1 | 3.417799448 | 0.019446231 |
| 1420981_a_at | Lmo4 | 3.424023676 | 0.01931741 |
| 1417831_at | Smc1l1 | 3.429111876 | 0.019212822 |
| 1423271_at | Gjb2 | 3.440985605 | 0.01897126 |
| 1425329_a_at | Dia1 | 3.444249087 | 0.018905474 |
| 1438223_at | Grid2 | 3.447575597 | 0.018838686 |
| 1422666_at | Cblc | 3.458197089 | 0.018627226 |
| 1434755_at | Coro2b | 3.458912253 | 0.018613086 |
| 1422812_at | Cxcr6 | 3.459767614 | 0.01859619 |
| 1416515_at | Fscn1 | 3.460527429 | 0.018581195 |
| 1449371_at | Harsl | 3.460876887 | 0.018574304 |
| 1415772_at | Ncl | 3.461800178 | 0.018556109 |
| 1448389_at | Wdr5 | 3.473565946 | 0.018326028 |
| 1426389_at | Camk1d | 3.474454082 | 0.018308793 |
| 1424840_at | Rbks | 3.476474953 | 0.018269645 |
| 1425234_at | 1700051I12Rik | 3.477915552 | 0.018241796 |
| 1452638_s_at | Dnm1l | 3.478750478 | 0.018225678 |
| 1430335_a_at | Pax3 | 3.480374948 | 0.018194365 |
| 1427854_x_at | 3.482574812 | 0.018152058 | |
| 1452402_at | 3.483987577 | 0.018124947 | |
| 1438070_at | Phf3 | 3.487165588 | 0.018064131 |
| 1454061_at | Thumpd3 | 3.488257585 | 0.018043287 |
| 1420053_at | Psmb1 | 3.488906078 | 0.018030922 |
| 1422546_at | Ilf3 | 3.508209824 | 0.017667244 |
| 1418909_at | Ermap | 3.509477595 | 0.017643654 |
| 1424498_at | 5730596K20Rik | 3.515771328 | 0.017527076 |
| 1418065_at | Rag2 | 3.520156261 | 0.017446374 |
| 1425961_at | BC016548 | 3.520765643 | 0.017435193 |
| 1444953_at | 8430423A01Rik | 3.524330508 | 0.017369944 |
| 1418227_at | Orc2l | 3.526672877 | 0.017327223 |
| 1419179_at | Txnl4 | 3.529589461 | 0.017274198 |
| 1423249_at | Nktr | 3.532283103 | 0.01722539 |
| 1427554_at | Hel308 | 3.53288254 | 0.01721455 |
| 1427643_at | 1200009O22Rik | 3.543340398 | 0.01702668 |
| 1421869_at | Trim44 | 3.554867777 | 0.016822308 |
| 1427482_a_at | Car8 | 3.558911802 | 0.016751276 |
| 1432459_a_at | Rog | 3.561412525 | 0.016707523 |
| 1438245_at | Nfib | 3.565204959 | 0.01664142 |
| 1422956_at | D1Pas1 | 3.576076558 | 0.016453576 |
| 1422036_at | Strn | 3.57762187 | 0.016427073 |
| 1453683_a_at | 1200008O12Rik | 3.585757456 | 0.016288346 |
| 1419253_at | Mthfd2 | 3.586846457 | 0.016269879 |
| 1420974_at | Setdb1 | 3.587477754 | 0.016259184 |
| 1431686_a_at | Gmfb | 3.588593964 | 0.016240294 |
| 1430586_at | 2700007P21Rik | 3.588969301 | 0.016233948 |
| 1423553_at | Dnajb3 | 3.599839679 | 0.016051375 |
| AFFX-18SRNAMur/X00686_5_at | 3.612923955 | 0.015834729 | |
| 1438748_at | 2700078E11Rik | 3.613005797 | 0.015833384 |
| 1425605_a_at | Lmbr1 | 3.614705726 | 0.015805487 |
| 1443589_at | Gpm6b | 3.615856465 | 0.015786634 |
| 1425338_at | Plcb4 | 3.61646714 | 0.015776639 |
| 1428132_at | Cdc42se1 | 3.625113293 | 0.015635909 |
| 1450541_at | Pvt1 | 3.628326703 | 0.015583973 |
| 1456565_s_at | Map3k12 | 3.631282846 | 0.015536369 |
| 1419052_at | Ovol1 | 3.635287213 | 0.015472151 |
| 1451456_at | 3.640820903 | 0.015383907 | |
| 1452642_at | Tmem16f | 3.646863541 | 0.015288205 |
| 1451021_a_at | Klf5 | 3.651556285 | 0.015214353 |
| 1421933_at | Cbx5 | 3.652925906 | 0.015192876 |
| 1419241_a_at | Aire | 3.655287704 | 0.015155921 |
| 1456190_a_at | BC031140 | 3.659632397 | 0.015088211 |
| 1436162_at | C730048C13Rik | 3.668343947 | 0.014953486 |
| 1420517_at | 2310010I16Rik | 3.669025757 | 0.01494300 |
| 1452408_at | 3.669339752 | 0.014938174 | |
| 1438215_at | Sfrs3 | 3.674062823 | 0.014865793 |
| 1417757_at | Unc13b | 3.696140227 | 0.014532751 |
| 1417270_at | Wdr12 | 3.706962408 | 0.014372629 |
| 1424942_a_at | Myc | 3.714859557 | 0.014257063 |
| 1422135_at | Zfp146 | 3.722089586 | 0.014152193 |
| 1425270_at | Kif1b | 3.723748371 | 0.014128258 |
| 1428045_a_at | Elf2 | 3.731380905 | 0.014018724 |
| 1427101_at | Metrn | 3.749492087 | 0.013762692 |
| 1419940_at | 4930488L10Rik | 3.761372517 | 0.01359766 |
| 1435626_a_at | Herpud1 | 3.76528356 | 0.01354383 |
| 1423437_at | Gsta3 | 3.765317741 | 0.013543361 |
| 1449416_at | Fzd4 | 3.775661238 | 0.01340218 |
| 1428160_at | Ndufab1 | 3.778355433 | 0.013365684 |
| 1417029_a_at | Trim2 | 3.781146538 | 0.013327996 |
| 1455808_at | 4922502D21Rik | 3.782747632 | 0.013306432 |
| 1430483_a_at | 2310042N02Rik | 3.791162199 | 0.013193759 |
| 1424083_at | Rod1 | 3.791959817 | 0.013183136 |
| 1460725_at | Xpa | 3.792592186 | 0.01317472 |
| 1452318_a_at | Hspa1b | 3.796896622 | 0.013117602 |
| 1421230_a_at | Msi2h | 3.812474518 | 0.012913258 |
| 1418349_at | Hbegf | 3.817881032 | 0.012843195 |
| 1440192_at | 1810054D07Rik | 3.854206413 | 0.012383645 |
| 1452243_at | Kcnj14 | 3.85496781 | 0.012374217 |
| 1451887_at | Lrba | 3.857465589 | 0.012343347 |
| 1419900_at | Sin3a | 3.88287621 | 0.012034288 |
| 1450193_at | Hcn1 | 3.891121372 | 0.011935929 |
| 1417905_at | Prlpf | 3.896151709 | 0.011876375 |
| 1434987_at | Aldh2 | 3.902236841 | 0.011804791 |
| 1449118_at | Dbt | 3.905570325 | 0.011765788 |
| 1420982_at | Rnpc2 | 3.917975152 | 0.011621945 |
| 1424755_at | Hip1 | 3.936108008 | 0.011415316 |
| 1446914_at | Eif2s2 | 3.944832819 | 0.011317406 |
| 1455540_at | 3.954421033 | 0.011210923 | |
| 1456319_at | 3.960519659 | 0.011143796 | |
| 1438741_at | Rbm13 | 3.971304363 | 0.011026221 |
| 1449597_at | 3.9720719 | 0.011017908 | |
| 1416934_at | Mtm1 | 3.975422707 | 0.010981701 |
| 1426196_at | 3.981642563 | 0.010914857 | |
| 1427765_a_at | Tcrb-V13 | 3.988067373 | 0.010846303 |
| 1419277_at | Usp48 | 3.995642623 | 0.010766111 |
| 1438700_at | Fnbp4 | 4.007934467 | 0.010637441 |
| 1451739_at | Klf5 | 4.009661813 | 0.010619502 |
| 1452415_at | Actn1 | 4.013468651 | 0.010580091 |
| 1421595_at | 9630031F12Rik | 4.026451959 | 0.010446942 |
| 1423093_at | Incenp | 4.056991536 | 0.010141312 |
| 1449729_at | 4.059318965 | 0.010118447 | |
| 1448371_at | Mylpf | 4.060423682 | 0.010107615 |
| 1432007_s_at | Ap2a2 | 4.061118175 | 0.010100812 |
| 1426375_s_at | BC019806 | 4.061705841 | 0.01009506 |
| 1421267_a_at | Cited2 | 4.067131851 | 0.010042127 |
| AFFX-18SRNAMur/X00686_3_at | 4.068244684 | 0.01003131 | |
| 1424872_at | 2310001H12Rik | 4.072170362 | 0.009993261 |
| 1448606_at | Edg2 | 4.076478623 | 0.009951696 |
| 1420930_s_at | Catnal1 | 4.093968451 | 0.009784998 |
| 1438082_at | 2310028N02Rik | 4.096841999 | 0.00975792 |
| 1418569_at | 2410043F08Rik | 4.109867254 | 0.009636263 |
| 1417178_at | Semcap2 | 4.111817999 | 0.009618195 |
| 1452620_at | Pck2 | 4.113798961 | 0.009599886 |
| 1419116_at | 5430428G01Rik | 4.127933982 | 0.009470413 |
| 1451902_at | BC021442 | 4.164797416 | 0.009142157 |
| AFFX-18SRNAMur/X00686_M_at | 4.172558479 | 0.009074738 | |
| 1449838_at | Crisp3 | 4.181403551 | 0.008998604 |
| 1450430_at | Mrc1 | 4.194900667 | 0.008883849 |
| 1431893_a_at | Tprt | 4.199779855 | 0.008842784 |
| 1453360_a_at | Tex9 | 4.212874201 | 0.008733659 |
| 1418417_at | Msc | 4.222435464 | 0.008654964 |
| 1456225_x_at | Trib3 | 4.225132709 | 0.008632913 |
| 1427649_at | Wdr22 | 4.23098011 | 0.008585331 |
| 1452502_at | Serf1 | 4.234174345 | 0.008559468 |
| 1452070_at | Dedd2 | 4.27019116 | 0.008274009 |
| 1448457_at | Krt2-6g | 4.278133662 | 0.008212555 |
| 1416268_at | Ets2 | 4.278591968 | 0.008209025 |
| 1425831_at | Zfp101 | 4.280752877 | 0.008192405 |
| 1449693_at | Map3k7 | 4.284119202 | 0.008166592 |
| 1449229_a_at | Cdkl2 | 4.286647093 | 0.008147271 |
| 1452142_at | Slc6a1 | 4.305051867 | 0.008008185 |
| 1430634_a_at | Pfkp | 4.316779598 | 0.007920996 |
| 1428060_at | Cd3z | 4.323698776 | 0.007870073 |
| 1434326_x_at | Coro2b | 4.329579399 | 0.007827092 |
| 1424843_a_at | Gas5 | 4.357574401 | 0.007626183 |
| 1421279_at | Lamc2 | 4.404512781 | 0.007302618 |
| 1421496_at | 2410116I05Rik | 4.40646557 | 0.007289506 |
| 1419921_s_at | Usp7 | 4.409499533 | 0.007269189 |
| 1448348_at | Gpiap1 | 4.411257474 | 0.007257448 |
| 1423809_at | Tcf19 | 4.516818863 | 0.006591172 |
| 1429427_s_at | Tcf7l2 | 4.555365814 | 0.006365827 |
| 1431117_x_at | 1810029B16Rik | 4.593837426 | 0.006149825 |
| 1450791_at | Nppb | 4.610263947 | 0.006060208 |
| 1451524_at | Fbxw2 | 4.619741472 | 0.006009196 |
| 1426458_at | 4.621344819 | 0.006000616 | |
| 1431716_at | Herc4 | 4.621895613 | 0.005997671 |
| 1421257_at | Pigb | 4.628246541 | 0.005963844 |
| 1425285_a_at | Rab27a | 4.63066177 | 0.005951038 |
| 1425709_at | Rnf17 | 4.684098899 | 0.005675746 |
| 1418460_at | Sh3d19 | 4.750024695 | 0.005356347 |
| 1434674_at | Lyst | 4.776236981 | 0.005235221 |
| 1422567_at | Niban | 4.818450492 | 0.005046817 |
| 1423154_at | BC005537 | 4.830514081 | 0.00499444 |
| 1425837_a_at | Ccrn4l | 4.886163806 | 0.004760904 |
| 1419929_at | 4.934007639 | 0.004570291 | |
| 1427285_s_at | 2210401K01Rik | 4.971430915 | 0.004427403 |
| 1424841_s_at | Rbks | 4.999229746 | 0.004324644 |
| 1456511_x_at | Eras | 5.002953677 | 0.004311091 |
| 1460464_at | 2700089E24Rik | 5.011990225 | 0.004278412 |
| 1435106_at | 3732412D22Rik | 5.067838157 | 0.004082764 |
| 1420605_at | Mtag2 | 5.106047884 | 0.003954942 |
| 1438403_s_at | Ramp2 | 5.1302574 | 0.003876383 |
| 1438824_at | Slc20a1 | 5.153244671 | 0.003803479 |
| 1422986_at | Esrrb | 5.181240688 | 0.003716852 |
| 1437867_at | 5.202674306 | 0.003652093 | |
| 1451416_a_at | Tgm1 | 5.218518597 | 0.003605072 |
| 1455930_at | 5.274806147 | 0.003443681 | |
| 1422903_at | Ly86 | 5.294879889 | 0.003388188 |
| 1420947_at | Atrx | 5.29861609 | 0.003377976 |
| 1426267_at | Zbtb8os | 5.321555804 | 0.003316062 |
| 1420946_at | Atrx | 5.339806851 | 0.003267753 |
| 1443949_at | Ppp2r5e | 5.375119299 | 0.003176613 |
| 1418189_s_at | 5.379928117 | 0.003164434 | |
| 1427408_a_at | Thrap3 | 5.479919443 | 0.002923217 |
| 1418188_a_at | 5.510099766 | 0.002854707 | |
| 1416325_at | Crisp1 | 5.528967926 | 0.002812835 |
| 1423411_at | BC013481 | 5.560493925 | 0.002744476 |
| 1449167_at | Epb4.1l4a | 5.632515885 | 0.002595515 |
| 1417755_at | Topors | 5.656705105 | 0.002547636 |
| 1424786_s_at | Wdr45 | 5.706240554 | 0.002452797 |
| 1417548_at | Sart3 | 5.718574747 | 0.002429833 |
| 1420781_at | Etos1 | 5.757749264 | 0.002358561 |
| 1425019_at | Ubxd4 | 5.830010327 | 0.002233456 |
| 1420909_at | Vegfa | 5.932031625 | 0.002069901 |
| 1450051_at | Atrx | 5.978799312 | 0.001999675 |
| 1420169_at | 6.10858383 | 0.001819049 | |
| 1447984_at | Gpatc2 | 6.132886435 | 0.001787407 |
| 1422259_a_at | Ccr5 | 6.259509495 | 0.001632696 |
| 1442566_at | Jarid2 | 6.267516862 | 0.001623457 |
| 1437534_at | 6.415989061 | 0.001462848 | |
| 1428786_at | 4930568P13Rik | 6.897342617 | 0.001057372 |
| 1418350_at | Hbegf | 8.389351415 | 0.00043149 |
| 1449898_at | 1-Sep | 9.133148915 | 0.00029058 |
| Categories | Diseases or functions annotation | P value | No. of molecules |
| Cellular growth and proliferation | Proliferation of cells | 1.08E-08 | 114 |
| Cellular development, cellular growth and proliferation | Proliferation of stem cells | 5.45E-05 | 11 |
| Cellular development, cellular growth and proliferation, embryonic development, development | Proliferation of embryonic cells | 4.41E-04 | 13 |
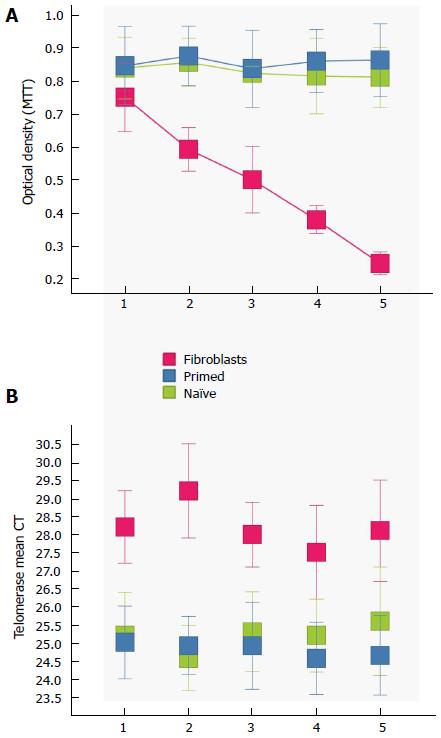
Doubling times were observed to be similar in all six cell types (3 naive, 3 primed), but were also put to the quantitative test with an MTT Assay. After 5 passages, there was no significant difference between cells, and they all maintained steady rates (Figure 3A).
Finally, we assessed telomerase activity in all cell types. Telomerase expression is low or absent in most somatic tissues, such as our control fibroblasts, but not in germ cells, stem cells, and tumors. The telomerase binds to a particular repeat sequence TTAGGG present at the ends of chromosomes of most eukaryotic species and extends them during cell replication. While telomerase activity was significantly lower in the control fibroblast cells, there was no significant difference between the naïve and primed ESC groups (Figure 3B).
The gene array that was utilized (Affymetrix Mouse 1.0 ST Array), evaluated a total of 22690 genes. Our analysis included all of the genes, and a priority list was established for those that were differentially expressed (Table 3). We used a positive log fold change to evaluate the differences. A positive log fold change indicates that gene expression is lower in the naïve cells. The same is true for the t value (which the P-value is based on that shows the strength of significance).
A gene ontology analysis was performed on the top set of genes using the Ingenuity Pathway Analysis. We have provided a detailed list of all significant gene ontology categories and the genes within (Table 4). Cell proliferation is the most significant gene ontology category. The P-value estimated from the current version of the IPA database (October 2015) is 1.08E-8. The gene ontology categories we searched are comprised of thousands of complex overlapping hierarchies. Further analysis was performed examining significant sub-categories listed under proliferation. The two sub-categories that passed our significance threshold were “proliferation of stem cells” at P = 5.45E-5 and “proliferation of embryonic cells” at P = 1.41E-4 (Table 4). These two, more specific, categories further connect the results of our gene ontology analysis to the function of embryonic stem cells. These two candidate genes (ESRRB and ERAS), as well as the others we highlight (KLF5 and MYC) are found in the significant “proliferation” sub-categories. Only 18 distinct genes are found in these two sets. Estrogen-Related Receptor Beta (Esrrb), Eras and myc are found in “proliferation of embryonic cells” (Table 4). The other significant sub-category of proliferation, “proliferation of stem cells”, contained the genes Eras, Kruppel-Like Factor (Klf-5), and myc (Table 4).
Thus, we turned our attention to a particular set of genes that were differentially higher in naïve stem cells. In particular, Klf-5 (5 1451021_a_at, 1451739_at), c-myc (1424942_a_at), Rnf-17 (1425709_at), Esrrb (1422986_at), Eras (ES Cell Expressed Ras 1456511_x_at) have been implicated in stem cell growth and pluripotency. It is important to note that there were several genes that were upregulated in the primed, that are implicated in differentiation, such as bone morphogenetic protein 7 (Bmp-7) and paired box 6 (Pax-6). Microarray results were validated using qRTPCR (Figure 2).
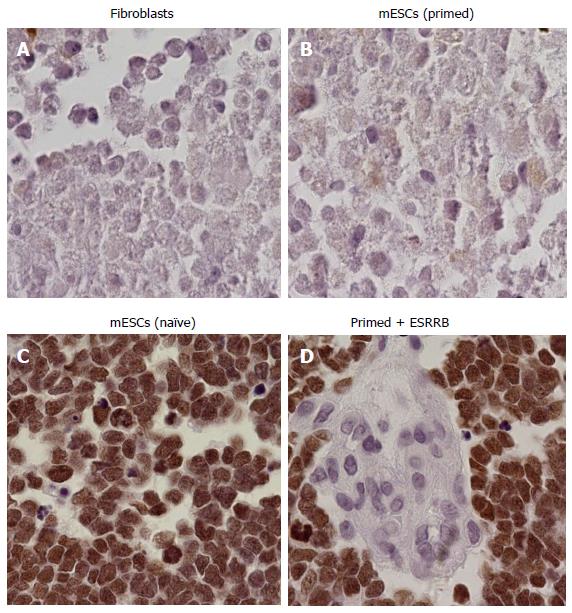
Primed cells were transduced with GFP containing vectors expressing either Essrb or Eras. In addition to these two genes, cells were transduced with c-myc and Klf-4. Gene expression was assessed with RTPCR (Figure 2, only ESRRB + c-myc + Klf-4 transduced cells shown). Embryos injected with primed transduced cells over-expressing ESRRB were able to incorporate into mouse embryos, whereas those same cell controls were not. When ESRRB, c-myc and Klf-4 where expressed in the same primed ESC, cells incorporated into 5 out of 6 of the embryos (Figure 4). However, cells overexpressing Eras alone, or Eras with Klf-4 and c-myc, were not able to incorporate, with the exception of one sample containing all three (1 out of 6). Cells transduced with c-myc and Klf-4 only did not incorporate. Cells overexpressed with Nanog only did not incorporate, demonstrating that the effect is Esrrb dependent. Positive control groups (naïve ESCs, Figure 4C) and negative control groups (fibroblasts, Figure 4A) showed the expected results.
Given the results, we performed expression profiles on Esrrb levels of primed ESCs. The data shows that all of them expressed significantly less Esrrb than their naive counterparts.
Establishing mouse ESC cell lines from blastocysts or after gene targeting experiments can be a laborious endeavor, which may produce naïve or primed ESCs. Here we report that, although there are no significant differences in morphology, proliferation, telomerase activity, there are however some significant differences in the expression level of key genes. Upregulation of key genes is observed in primed cells that indicate differentiation, such as Bmp-7 and Pax-6. Bmp-7 is a bone morphogenetic protein has been shown to be important in development, particularly, bone formation[2,22] and embryogenesis[23]. Pax-6 is a transcription factor that is implicated in embryonic development, particularly the brain and eye[24], ensuring proper tissue formation. Although further studies are necessary, overexpression of these factors, relative to a base ESC range, could provide an early marker to determine if the cell clones are naïve or primed.
Our attention focused on genes that were downregulated in primed ESCs. Ingenuity Pathway Analysis showed that the top gene ontology category was proliferation. Interestingly, there was no significant difference in proliferation rates, when measured by MTT (Figure 3A). However, some of these genes have also been implicated in pluripotency and stem cell self-renewal. This may indicate that either pluripotency genes are the driving force, or that diminishing proliferation rates may be small and biologically significant or may be observed in further cell passages. In any case, downregulation of these genes may serve a similar diagnostic purpose as the upregulated ones.
Specifically we examined several genes that were downregulated in primed cells; namely Eras Esrrb, c-myc, Klf-5, Atrx, and Rnf-17. All of these genes were shown to be significantly downregulated relative to funtional ESCs (Figure 2). Eras produces a constitutively active product that stimulates ESC proliferation[25], while Esrrb has been shown to have an essential role in placental development and has recently been used as a marker for iPSC reprogramming and substitute for Sox-2[26,27]. Eras has been identified to provoke tumorigenic growth, expressed only in stem cells and silenced in somatic cells due to epigenetic changes. Adding Eras exogenously in a constitutively expressed promoter would overcome this limitation. Asides from Sox-2, Esrrb has also been identified as prominent transcription factor that targets Nanog[28]. However, interestingly, when overexpressing Nanog only in primed cells, they did not acquire a naïve phenotype, showing that ESRRB’s role spans beyond only NANOG. In fact, ESRRB’s role interacting with key stem cell master factors, made it a prime candidate to study not only as a diagnostic indicator, but also as a potential reprogramming factor[29]. In addition ESRRB has been implicated as key downstream regulator of self-renewal, downstream of GSK-3[27]. Inhibition of GSK-3 has been implicated in supporting mESC state.
Low induction of endogenous Klf-5 may be due to the redundancy of the Klf family[30], or a lineage specific difference of mammals. It has been shown that the Klf family preferentially regulates genes involved in cell adhesion, either activating or inhibiting adhesion, and that cell adhesion can inhibit proliferation[31]. Myc, in particular c-myc, is known to induce proliferation, by repressing growth arresting genes[32]. This makes it a key contributor in inducing the self-renewal state of the cell. Recently, other factors that are less oncogenic have been shown to be suitable substitutes for c-myc, such as Glis1[14]. However, Glis1 is not differentially regulated between the naïve and primed cell types. Although we were not able to produce Atrx and Rnf-17 vectors, they do serve as key indicators. Atrx [alpha thalassemia/mental retardation syndrome X-linked homolog (human)] is known for its role in mental retardation, but it has recently been shown that it is a key element in maintaining telomere integrity in pluripotent stem cells[33]. Three different times this gene (1420947_at 1420946_at and 1450051_at) is in the top 20 genes downregulated, and differences in expression level were significant (Figure 2 and Table 4). Future studies will look at this particular gene and its novel function. Rnf-17 is involved in early stages of germ cells, such as PGCs[34,35]. It is also known that Rnf-17 enhances c-myc function, through interaction with all four known Mad proteins[36]. Although germline transmission is beyond the scope of this paper, primed cells do not possess this quality. We encourage others to examine the differentially expressed genes to further elucidate important mechanisms in the maintenance and plasticity of ESCs (Table 3).
It is interesting to note that key stem cell “master factor” genes, such as Oct-4, Sox-2 and Nanog[37], are not differentially expressed in cells whose in-vivo function is limited. These results may therefore yield insights into proper reprogramming of iPSCs, as all of these genes may be upregulated, but other key co-regulators may be lagging.
Another important question in our project was to determine if we could restore the fully functional naïve phenotype, by overexpressing some of these key genes. Here we show at least one combination of transfections (Esrrb + Klf-4 + c-myc) in primed cells was able to alter the expression profile and establish functionality as determined by the degree of incorporation of ESCs into embryos (Figure 4). Also, in two cases, Esrrb was sufficient to establish pluripotency in primed stem cells. We do not claim that these vectors will work for every case, but do demonstrate the principle that these cells can be reprogrammed into a naïve state, without the need for the OSCK cassette[20]. This suggests that, through genetic manipulation, it is possible to restore the functional naïve state of a primed mESC. The results may be a translational gateway into reprogramming human ESCs, into a naïve state with full ESC features and function.
Although there were strain differences observed in terms of gene expressions, all of the genes utilized in our experiments were differentially expressed in both C57BL/6 and 129SV derived ESCs. Further studies are needed to assess if there are significant strain differences, and what their implications are.
Our study shows that there is a significant set of genes that are differentially expressed between naïve and primed mESCs. These genes tend to be implicated in proliferation and pluripotency. Overexpression of at least one set of genes restores the functional naive phenotype in the primed cells. Taken together, primed cells can be identified at early stages, allowing the researcher to disregard this cell type or attempt to change it into a naïve state. Future studies into other genes, such as ATRX, should yield further insight into the nature of ESC functionality and phenotypes, providing a platform to study the ESC ground state and iPSC reprogramming fate.
We’d like to thank Erich Jarvis for his support, mentoring and comments throughout this project, and Gustavo Mostoslavsky (Boston University) for providing the STEMCCA cassette vector. We would also like to thank the University of Puerto Rico Comprehensive Cancer Center, for the lab space provided to perform some of the experiments. ES cell targeting experiments, blastocyst injections for generation of chimeras, and mating of chimeric males to test for germline transmission were performed by the Duke Neurotransgenic Laboratory. Microarray analysis was performed in the Duke University DNA analysis facility.
Derivation of mouse embryonic stem cells (ESCs) or gene targeting of ESCs is a lengthy process that sometimes produces cell lines that have all of the features inherent in ESCs, but fail to incorporate into the germline. Identifying this limitation takes many months, from blastocyst injection of ESCs to testing chimeric males for germline transmission of the ESC genome.
Cell plasticity, reprogramming, and maintenance of stem cells are all inherent topics in this research.
No study, that the authors are aware of, had looked at the differences between two phenotypically identical stem cells, and determined the features that make them behave differently. In addition, here the authors demonstrated that the incorporating/pluripotent feature can be induced in these stem cells as well as potentially controlled.
Researchers will be able to detect within days if the stem cells they are working with have the capacity to be functional, i.e., generate germline transmitting chimeras, or not. This is a key feature that will save time, money, and effort.
Several proteins and gene products are discussed in this paper. Importantly, ESRRB is an estrogen related receptor beta that has been implicated as a downstream regulator of self-renewal and embryonic stem cell expressed RAS, has been implied with tumorigenic growth in stem cells.
The paper is well written and addresses an important issue of the ESC functionality. Authors performed transcriptom analysis of functional and non-functional ESC lines and found some differences in gene expression signature. Overexpression of the downregulated ESRRB gene along with Klf-5 and c-myc provided better chimera formation.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Puerto Rico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kiselev SL, Lee Y, Sarkadi B S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Langer RS, Vacanti JP. Tissue engineering: the challenges ahead. Sci Am. 1999;280:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 188] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Rosselló RA, Wang Z, Kizana E, Krebsbach PH, Kohn DH. Connexin 43 as a signaling platform for increasing the volume and spatial distribution of regenerated tissue. Proc Natl Acad Sci USA. 2009;106:13219-13224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10432] [Article Influence: 386.4] [Reference Citation Analysis (0)] |
| 4. | Lavial F, Acloque H, Bertocchini F, Macleod DJ, Boast S, Bachelard E, Montillet G, Thenot S, Sang HM, Stern CD. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development. 2007;134:3549-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18204] [Article Influence: 958.1] [Reference Citation Analysis (0)] |
| 6. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14323] [Article Influence: 842.5] [Reference Citation Analysis (0)] |
| 7. | Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1123] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 8. | Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Li W, Ding S. Generation of novel rat and human pluripotent stem cells by reprogramming and chemical approaches. Methods Mol Biol. 2010;636:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009;1:46-54. [PubMed] |
| 12. | Rosselló RA, Chen CC, Dai R, Howard JT, Hochgeschwender U, Jarvis ED. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. Elife. 2013;2:e00036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Dai R, Rossello R, Chen CC, Kessler J, Davison I, Hochgeschwender U, Jarvis ED. Maintenance and neuronal differentiation of chicken induced pluripotent stem-like cells. Stem Cells Int. 2014;2014:182737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, Kawamura Y, Mochizuki H, Goshima N, Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Lin Z, Perez P, Lei D, Xu J, Gao X, Bao J. Two-phase analysis of molecular pathways underlying induced pluripotent stem cell induction. Stem Cells. 2011;29:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424-8428. [PubMed] |
| 17. | Handyside AH, Hunter S. A rapid procedure for visualising the inner cell mass and trophectoderm nuclei of mouse blastocysts in situ using polynucleotide-specific fluorochromes. J Exp Zool. 1984;231:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1380] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 19. | Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18 Suppl 1:S96-104. [PubMed] |
| 20. | Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543-549. [PubMed] |
| 21. | Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 719] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 22. | Rossello RA, H D. Cell communication and tissue engineering. Commun Integr Biol. 2010;3:53-56. [PubMed] |
| 23. | Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, Granjeiro JM. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 2014;93:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 24. | Nacher J, Varea E, Blasco-Ibañez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res. 2005;81:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 27. | Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell. 2012;11:491-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, Wong F, Yates A, Tomlinson SR, Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell. 2012;11:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 29. | Papp B, Plath K. Pluripotency re-centered around Esrrb. EMBO J. 2012;31:4255-4257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 31. | Swamynathan SK, Davis J, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008;49:3360-3370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Gartel AL, Shchors K. Mechanisms of c-myc-mediated transcriptional repression of growth arrest genes. Exp Cell Res. 2003;283:17-21. [PubMed] |
| 33. | Wong LH, McGhie JD, Sim M, Anderson MA, Ahn S, Hannan RD, George AJ, Morgan KA, Mann JR, Choo KH. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 34. | Antonucci I, Di Pietro R, Alfonsi M, Centurione MA, Centurione L, Sancilio S, Pelagatti F, D’Amico MA, Di Baldassarre A, Piattelli A. Human second trimester amniotic fluid cells are able to create embryoid body-like structures in vitro and to show typical expression profiles of embryonic and primordial germ cells. Cell Transplant. 2014;23:1501-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Zhou J, Leu NA, Eckardt S, McLaughlin KJ, Wang PJ. STK31/TDRD8, a germ cell-specific factor, is dispensable for reproduction in mice. PLoS One. 2014;9:e89471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Yin XY, Grove LE, Prochownik EV. Mmip-2/Rnf-17 enhances c-Myc function and regulates some target genes in common with glucocorticoid hormones. Oncogene. 2001;20:2908-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Li YQ. Master stem cell transcription factors and signaling regulation. Cell Reprogram. 2010;12:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |