Published online Jul 26, 2015. doi: 10.4252/wjsc.v7.i6.899
Peer-review started: December 21, 2014
First decision: February 7, 2015
Revised: February 28, 2015
Accepted: May 8, 2015
Article in press: May 11, 2015
Published online: July 26, 2015
Processing time: 225 Days and 4.3 Hours
Functional synaptogenesis and network emergence are signature endpoints of neurogenesis. These behaviors provide higher-order confirmation that biochemical and cellular processes necessary for neurotransmitter release, post-synaptic detection and network propagation of neuronal activity have been properly expressed and coordinated among cells. The development of synaptic neurotransmission can therefore be considered a defining property of neurons. Although dissociated primary neuron cultures readily form functioning synapses and network behaviors in vitro, continuously cultured neurogenic cell lines have historically failed to meet these criteria. Therefore, in vitro-derived neuron models that develop synaptic transmission are critically needed for a wide array of studies, including molecular neuroscience, developmental neurogenesis, disease research and neurotoxicology. Over the last decade, neurons derived from various stem cell lines have shown varying ability to develop into functionally mature neurons. In this review, we will discuss the neurogenic potential of various stem cells populations, addressing strengths and weaknesses of each, with particular attention to the emergence of functional behaviors. We will propose methods to functionally characterize new stem cell-derived neuron (SCN) platforms to improve their reliability as physiological relevant models. Finally, we will review how synaptically active SCNs can be applied to accelerate research in a variety of areas. Ultimately, emphasizing the critical importance of synaptic activity and network responses as a marker of neuronal maturation is anticipated to result in in vitro findings that better translate to efficacious clinical treatments.
Core tip: During stem cell neuronal differentiation, functional synaptogenesis and the emergence of coordinated, networked activity are critical behaviors in confirming that cells have developed into a relevant neuronal population. As the number of stem cell-derived neuron (SCN) models continues to proliferate, the use of specific functional readouts to evaluate SCN maturity will become increasingly important compared to morphological or proteomic characterization of neuronal maturation. The review provides diverse options for reliably assaying the development of synaptic neurotransmission in derived neurons and describes the strengths, weaknesses and potential applications of several stem cell-based neuron models.
- Citation: Bradford AB, McNutt PM. Importance of being Nernst: Synaptic activity and functional relevance in stem cell-derived neurons. World J Stem Cells 2015; 7(6): 899-921
- URL: https://www.wjgnet.com/1948-0210/full/v7/i6/899.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i6.899
Over the last few decades a large variety of in vitro models have been developed for use in basic and applied neuroscience. These neurogenic models originate from diverse sources, including dissociated primary neurons, immortalized cell lines derived from neuronal and non-neuronal tissues and, most recently, stem cells. The predictive value of these models is critically dependent on their ability to recapitulate fundamental neuronal behaviors exhibited by primary neurons. This is particularly important given the profound effects that subtle changes in neuron development or maturation can have on emergent network properties.
In vivo, the differentiation of neural precursors into synaptically active, post-mitotic neurons involves a complex developmental cascade of gene expression and morphological changes[1-3]. These changes ultimately orchestrate synaptic, neuronal and network behaviors to produce the emergent properties responsible for sustained central nervous system (CNS) function. Given the complex cellular behaviors involved in producing synaptically active neurons, it is not surprising that synaptogenesis and maintenance of synaptic activity are highly sensitive to genetic and environmental perturbation[4,5]. While many dissociated primary neuron cultures reliably form functioning networks that exhibit physiological behaviors, their use is limited by several factors, including the difficulty of dissection, variability among cultures, poor viability for longer-term studies and the regulatory, administrative and ethical burdens imposed by animal studies. In contrast, while immortalized cells have been extensively used as a replacement for primary neurons, they uniformly fail to recapitulate many neurotypic properties[6].
The advent of neurons derived from stem cells offers the potential for a unique experimental platform that combines the relevance of primary neurons with the flexibility and scalability of immortalized cells[6]. Stem cell-derived neuron (SCN) models that produce functionally mature neurons have multiple characteristics that render them exceedingly well-suited to the study of neural development, neuron function and human disease. For example, SCNs can recapitulate functional behaviors that are characteristic of primary neurons, such as synaptic neurotransmission and network emergence. Many stem cell lines can be maintained in culture for prolonged periods prior to differentiation, enabling scalable expansion to accommodate the demands of high-throughput approaches and endowing differentiated neurons with reduced inter-experimental phenotypic and genetic variability. The ability to convert primary cells to patient-specific induced pluripotent stem cells (iPSCs) has kindled the extraordinary potential of personalized medicine, in which iPSC-derived neurons (i-neurons) expressing cellular correlates of particular neurological pathologies can be studied in vitro in the context of the patient’s genome[7]. Finally, SCNs have also been proposed to have a direct application in cell-based therapies, whereby partially differentiated neural progenitor cells or post-mitotic immature neurons can be directly injected into the CNS to integrate into existing architecture, supplement endogenous neurogenic processes and promote the repair of damaged neural tissues[8,9]. However, SCN models must be shown to be competent to form context-appropriate, functioning neurons before these approaches can be used as intended.
The signature characteristic of CNS neurons is action potential (AP)-induced synaptic neurotransmission that synchronizes neuron firing to give rise to emergent circuit behaviors. Since synaptic activity is a principal endpoint of neurogenesis, detection of synaptic events and/or synaptically driven network behaviors serves as a higher-order readout that confirms the proper elaboration of the full range of biochemical, proteomic and morphological properties that are required for neuron function. However, in many cases the rigor and specificity of techniques used to characterize the physiological relevance of SCNs have been highly variable[10,11]. Frequently, characterizations have been limited to expression of small sets of neurotypic genes or electrophysiological assessment of intrinsic electrical excitability, without evaluation of functional synaptogenesis or network formation[12,13]. SCNs are frequently described as physiologically relevant based on insufficient or incomplete characterizations, therefore producing data of uncertain value. These inconsistencies illuminate a critical need for the identification of appropriate assays to evaluate the functional maturity and physiological relevance of derived neuron models.
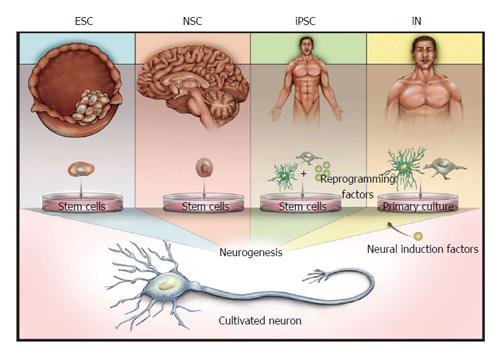
In this review we will discuss methods to characterize the progression of in vitro neurogenesis and propose specific functional assays to confirm the physiological relevance of SCNs. We will focus on SCNs derived from four sources (summarized in Figure 1): embryonic stem cells (ESCs); restricted-potency neural stem cells (NSCs); iPSCs; and direct conversion of post-mitotic cells into induced neurons (iNs). Note that although iNs do not explicitly incorporate a pluripotent phase, the derivation of iNs uses principles and techniques involved in production of other SCN models and therefore will be addressed in this review. We will also describe the current status of existing SCN models, and elaborate on reasons why synapse and network formations are critically important to SCN applications, even in cases where applications may not directly rely on neuronal function.
Developmentally regulated changes in proteomic, transcriptomic, biochemical and functional properties during embryonic neurogenesis can be repurposed to evaluate developmental progression in vitro[14]. The specific markers used may vary depending on the ultimate application, but at a minimum should include morphological characterization of neurotypic compartments; the development of electrical excitability; the detection of post-synaptic responses to pre-synaptic release of neurotransmitter; and, when appropriate, network-level behaviors (Figure 2). While functional confirmation of synaptic neurotransmission represents indisputable evidence of synaptogenesis, supplemental studies such as transcriptomic analysis, planar multielectrode array (MEA) analysis or protein expression studies can also provide valuable contributions to the overall understanding of the maturity and nature of derived neuronal populations, as described below.
Although methods to validate the progression of neuronal maturation have been well-described in dissociated cultures of primary neurons, it is important to recognize that the source tissues for these neurons are often comprised of functionally mature neurons that are dissociated and plated[15]. In these cases, re-establishment of synaptic activity is primarily contingent on the temporal re-elaboration of appropriate morphological structures. In contrast, the differentiation of pluripotent stem cells into mature SCNs requires recapitulation of the full range of transcriptional, morphological and proteomic changes involved in functional neurogenesis. Neurogenic progression is an intricate process that is susceptible to misdirection in vitro, and unlike in dissociated primary neurons, there is a lower correlation between expression of intermediate developmental markers and the probability of functional maturation. Inappropriate differentiation conditions can cause neurogenic models to become developmentally arrested, expressing immature neurotypic properties but lacking the functional correlates of active synapses and network emergence[16]. Dissociated primary neuron cultures can also undergo developmental arrest if not properly maintained, despite having been functionally mature prior to dissociation[1]. Once neurons become functionally mature, their survival continues to depend on synaptic activity-induced neurotrophic signaling[17-20], as demonstrated by neurotoxicity following impaired synaptic function in neurodegenerative diseases[21-23]. Therefore, researchers characterizing SCN differentiation should be careful to avoid phenomenologically conflating the expression of intermediate developmental markers with the eventual likelihood of producing functionally mature neurons. For example, the ability to fire repeated APs with hyperpolarizing after shoots in response to current injection is often associated with neuronal maturity. This may be valid in many well-characterized primary neuron cultures. However, there is no causal requirement that SCNs with this intrinsic behavior will exhibit functional synapses with synaptically driven network responses.
As the number of SCN models continues to proliferate, the deliberate use of specific functional readouts to evaluate SCN maturity will become increasingly important. By precisely and accurately associating neurotypic behaviors with stage-specific markers, researchers will be able to better ascertain the degree of relevance and applicability of SCN populations. Efforts to develop techniques to reliably determine the neurogenic potential of SCN differentiation models are currently underway[11,24]. However, in the absence of such functional validation, claims of neuronal maturation and therefore relevance should be reviewed with care.
Much of the current understanding of neuronal maturation comes from longitudinal studies of the formation of specialized neuronal structures in dissociated primary neurons[25]. These studies established that the progressive establishment of functionally specialized compartments (e.g., axons, hillocks, dendrites, and pre- and post-synaptic compartments) provided the cellular substrates for polarized neurotransmission in vitro. Many of these morphological features can be distinguished by the localization expression of specific proteins and therefore immunocytochemistry (ICC) is often used to characterize developmental progression[26,27]. Morphological characterization may start with general neuronal markers, such as NeuN (post-mitotic neuronal nuclei) or βIII-tubulin (immature neuronal marker), and progress to more specialized markers of cellular compartments, such as synapsin (pre-synaptic), tau (axon), MAP2 (somatodendritic) and gephyrin or PSD-95 (inhibitory or excitatory post-synaptic markers, respectively). It can then extend to the use of well-described antibodies to distinguish among neuronal subtypes, such as markers of glutamatergic (vesicular glutamate transporter-1 or -2), cholinergic (choline acetyltransferase), GABAergic (glutamate decarboxylase 1), serotonergic (serotonin transporter), aminergic (vesicular monoamine transporters 1 or 2), or dopaminergic and/or adrenergic (tyrosine hydroxylase) neurons. Longitudinal studies using such markers are a regular feature of SCN differentiation studies and are an important aspect of verifying neuronal maturation, even though expression of specific markers are not indicative of functional behaviors[28,29].
In addition to providing information on the establishment of synaptic compartments, the morphological apposition of pre- and post-synaptic proteins can provide clear evidence of synaptic assembly[30]. Synaptic assembly begins with the contact of complementary cell adhesion molecules between potential pre- and post-synaptic compartments, which in turn requires the previous elaboration of axons and dendrites (with the exception of axosomatic synapses[31]). From this point, proteins necessary for synaptic architectures and function are recruited to the site of contact in both compartments[32,33]. In mature neuron cultures and in vivo, the establishment of new synapses can occur very quickly, with synaptic activity apparent almost immediately[34,35]. However, as demonstrated in Figure 2 and discussed above, SCNs can form synaptic structures but fail to develop synaptic function. Based on this positional apposition, ICC for synapsin and PSD-95 (for example) have been used to provide a low-resolution confirmation that pre- and post-synaptic compartments are in close proximity. More recently, the mammalian GFP reconstitution across synaptic partners (mGRASP) method has been used as a fluorescent indicator of the co-localization of synaptic adhesion proteins. mGRASP is based on functional complementation of split GFP separately expressed from pre- and post-synaptic membranes, and therefore is a highly specific marker of synaptic proximity[36]. Ultrastructural imaging using transmission electron microscopy (TEM) can also be used for advanced morphological analysis of synaptogenesis. TEM enables a definitive visualization of pre- and post-synaptic compartments, allowing researchers to search for characteristic intra-synaptic structures, such as pre- and post-synaptic densities, the presence of synaptic vesicles in the pre-synaptic compartment and possibly even vesicles docked or fused with the pre-synaptic membrane, suggesting neurotransmitter release. It is important to emphasize that positive confirmation of synaptic architectures by any of these methods is not prima facie evidence of synaptic function. Synapse assembly precedes onset of synaptic activity and is not indicative of the current or future development of synaptic function[37-39]. However, pairing these types of morphological studies with electrophysiology may allow the identification of benchmarks that can be used to correlate synaptic function with gene expression (e.g., the onset of synaptic activity cannot precede the localization of protein X, etc.). While structural characterizations have rarely been used in the study of SCN maturation, they have the potential to offer highly specific and detailed correlations between structural and functional synaptogenesis when combined with synaptic activity assays.
While morphological characterization provides researchers with a visual confirmation of protein expression and compartmentalization, some neuronal markers (e.g., βIII-tubulin[40], synapsin[41] or NeuN[42]) may also be expressed in non-neuronal or neoplastic cells. Morphological characterization of neurogenesis should therefore be used as a secondary method to confirm neurogenesis and be corroborated by more direct methods to measure the production of neurons. For example, we have found transcriptomic analysis to be invaluable in reconciling neuronal fate with expression of neurotypic markers and evaluating neurotypic responses, including: (1) longitudinal characterization of neurogenic progression; (2) identification of neuronal subtypes; (3) determination of which neurotransmitter receptors subunits are expressed; and (4) measurement of activity-dependent responses to treatment with pharmacological regulators of network activity[43].
Functional evaluation of neuron maturation begins with electrophysiological characterization of intrinsic excitability, which is the ability to undergo the rapid, defined changes in ion flux that are necessary to repeatedly fire APs. These include measurements such as quantitation of Na+ and K+ voltage-gated responses, the ability to fire evoked APs, membrane resistance, membrane capacitance, sag (or Ih) currents, hyperpolarization-activated currents and establishment of a stable resting membrane potential. Another intrinsic neuronal characteristic that can be used as a developmental marker of maturation is the γ-aminobutyric acid (GABA) reversal potential (EGABA). Immature neurons have high intracellular Cl- levels, such that GABAA receptor activation triggers Cl- efflux[44]. This excitatory GABA current has been proposed to contribute to the developmental activation of nascent excitatory synapses[45]. Neuron maturation is associated with increased expression of the potassium chloride co-transporter KCC2, which is an electroneutral Cl- exporter that decreases intracellular Cl- concentration, thereby shifting EGABA to a value that is close to the mature resting membrane potential[46]. The developmental shift in EGABA potentiates a transition from excitatory post-synaptic GABA currents to inhibitory GABA currents[47]. While the development of EGABA is rarely used for neuronal characterization because of the relative difficulty in measuring Cl- reversal potentials, the shift in EGABA is an elegant marker of the developmental changes that are required for the establishment of network-level activities in many neuron subtypes.
Once neurons have been determined to exhibit mature characteristics of intrinsic excitability, analysis then expands to measuring cell behaviors under non-evoked conditions, such as spontaneous production of APs and miniature post-synaptic currents. It is important to note that the mere presence of spontaneous APs does not necessitate or signify synaptic function[48]. For example, dissociated primary neurons exhibit mature-appearing intrinsic characteristics and spontaneously fire APs prior to formation of active synapses[27]. In fact, spontaneous, non-evoked AP firing appears to be a developmental phenomenon involved in the maturation of ionic gradients[49,50]. Furthermore, the detection of APs can reflect a neuron with a membrane potential that is near to the threshold potential and therefore fires APs in response to subtle changes in the conductance of membrane ion channels, independent of synaptic activity[51,52]. Since APs may occur in the absence of synaptic function, pharmacological antagonists of neurotransmitter receptors must be used to experimentally confirm that APs originate from synaptically driven events as described below.
In some cases, SCNs produce morphological characteristics of synapse formation, exhibit evidence of intrinsic excitability and fire spontaneous APs, yet fail to develop synaptic activity[29,53]. As discussed below, this is currently a particularly vexing problem with human iPSC-derived neurons, which exhibit a wide array of morphological markers of neurogenesis, but often appear to be developmentally delayed or even arrested prior to the onset of synaptic activity. Consequently, measurements of synaptic activity are critical to providing unimpeachable confirmation of functional synaptogenesis.
The production of excitatory and/or inhibitory post-synaptic currents is a higher-order representation of the spatiotemporally precise elaboration of a large number of developmental processes required for synaptic activity, such as juxtaposition of pre-synaptic and post-synaptic compartments; loading and docking of neurotransmitter vesicles; and the appropriate localization and function of voltage-dependent ion channels. The two dominant modes of synaptic neurotransmission are spontaneous release and AP-evoked release. Under physiological conditions stochastic Ca2+ currents trigger synapses to spontaneously release neurotransmitter at approximate rates of 10-3 quanta per second (Hz) per CNS synapse[54,55] and 0.79 Hz per motor neuron synapse[56]. In a typical central neuron, which has 102-104 synapses, monosynaptic release probabilities result in a post-synaptic current detection rate of 0.1-10 Hz[57,58]. Similar rates have been reported in mouse SCNs[59]. In contrast, AP-induced pre-synaptic depolarization increases instantaneous quantal release rates increase to 103 Hz[54,55]. While either spontaneous or AP-evoked neurotransmission can be used to identify the presence of synaptic neurotransmission, the large currents generated by AP propagation can complicate rigorous characterization of post-synaptic behaviors, as described below. Consequently, detection and characterization of spontaneous post-synaptic events often provides a more reliable and quantifiable indicator of synaptic function than detection of synchronized release from many synapses following an AP.
Detection of post-synaptic events using whole-cell patch-clamp electrophysiology can involve characterization of excitatory post-synaptic potentials (EPSPs) in current-clamp mode or spontaneous miniature post-synaptic currents in voltage-clamp mode. For a number of technical reasons related to quantitation and characterization that will not be addressed here, we recommend the latter approach. Spontaneous release is a salient feature of all synapses in vivo and in vitro and direct measurement of spontaneous monosynaptic activity via detection of miniature excitatory or inhibitory post-synaptic currents (mEPSC or mIPSC, respectively) in the presence of tetrodotoxin (TTX) is an unambiguous indicator of synaptic function in neuron subtypes that utilize ionotropic neurotransmitter receptors (Figure 2)[54,60-62]. TTX blocks voltage-gated Na+ channels, eliminating the large whole-cell currents caused by AP firing and enabling the detection of small post-synaptic currents resulting from the spontaneous activation of individual synapses. The addition of pharmacological agonists or antagonists for specific neurotransmitter receptors allows the contributions of different neuron subtypes to post-synaptic responses to be precisely determined.
Characterization of miniature post-synaptic currents (aka, minis) should include analysis of event rates and kinetics; functional responses to the addition of pharmacological modulators of post-synaptic function; and current:voltage relationships for well-characterized receptors[59]. Much is known about the functional mechanisms of synaptic neurotransmission in primary CNS and peripheral neurons and an extensive pharmacopeia is available to manipulate and characterize post-synaptic responses for most synapse subtypes. Thus, there is a wide array of resources to identify and develop electrophysiological approaches to evaluate synaptic function in SCNs.
While the kinetic analysis of minis can be used to unambiguously distinguish between synaptic and non-synaptic neurotransmission, in some instances (e.g., synaptic potentiation studies) it would be valuable to simultaneously evaluate both pre- and post-synaptic behaviors. Paired recordings offer one means to do so. In paired recordings, two synaptically connected neurons are simultaneously patched, such that stimulation of one neuron elicits post-synaptic responses in the other. While this is a highly rigorous and reliable indicator of connectivity, it is a technically difficult method. Paired recordings are relatively feasible under conditions in which neuronal connectivity is well defined and the polarization of synaptic connections are known, such as in hippocampal slices[63]. In contrast, trans-synaptic connectivity and polarization are stochastic in dissociated neuron cultures, making it difficult to identify and record from synaptically coupled neurons, even when plated at very low densities[64]. Thus, while paired recordings are an effective method to characterize evoked synaptic neurotransmission, it is technically challenging to accomplish in neuron cultures and should be anticipated to have a high failure rate. Alternatively, the use of bipolar stimulating electrodes allows for the repeated stimulation of specific local synapses in vitro without requiring multiple patches[65]. However, this approach relies on the stimulation of multiple processes in proximity to a patched neuron and therefore may result in field stimulus-like artifacts and difficult to interpret post-synaptic responses.
The use of post-synaptic miniature currents to measure synaptic neurotransmission is not feasible in neuron subtypes that rely on metabotropic neurotransmitter receptors, such as dopaminergic or serotonergic neurons. While methods such as ultrafast voltammetry or the use of fluorescent false neurotransmitters can directly measure synaptic release of neurotransmitters[65,66], they are not a confirmation of synaptic neurotransmission, but rather are limited to providing information about the pre-synaptic release of neurotransmitter. Alternatively, indirect measurements of the activation of metabotropic synapses in cultures containing multiple neuron subtypes can be conducted based on changes in network behavior, as described in the next section.
Table 1 lists exemplar SCN models that have been evaluated for the existence of spontaneous post-synaptic currents in the absence of co-cultured primary neurons. The latter were excluded due to the difficulty in disambiguating neurotransmission and neuro-exception between SCNs and primary neurons[67]. Table 1 does not include references for putative SCNs that have only been characterized for morphological markers or for intrinsic excitability, since as described above these are poor surrogates for assaying functional synapses.
| Cell type | Source | Technical approach | Post-synaptic measurement(days after differentiation) | Additional notes | Ref. |
| ESC | Human (BG01, BG03) | Stromal cell factors applied | mEPSCs (21) | Also dopaminergic | [254] |
| Continued expression of NPC marker Msx1 | |||||
| Human (WiCell H9, H1) | SHH application on ESCs or NPCs | mEPSCs, mIPSCs (30) | Also dopaminergic | [110] | |
| Human (H9 and SA121) | Dual SMAD inhibition, variable SHH, several additional neurotrophic factors | sEPSCs, sIPSCs (35) | SHH concentration-dependent GABAergic and glutamatergic, or dopaminergic fates | [240] | |
| Mouse (R1) | RA treatment, suspension culture | mEPSCs, mIPSCs (21) | [6,59,226] | ||
| Mouse (J1) | FACS sorting for dopaminergic NPCs, SHH treatment | sEPSCs (22) | Also dopaminergic | [255] | |
| NSC | Human (NSI-566RSC from fetal spinal cord) | 9 component differentiation media | mEPSCs (12-48) | [256] | |
| Mouse (Adult C57/Bl6 SEZ) | BDNF treatment | mEPSCs, mIPSCs (11-28) | Perforated patch recording | [171] | |
| iPSC | Human (normal and ALS fibroblasts) | Dual SMAD inhibition, RA and purmorphamine treatment | sEPSCs (21-42) | sEPSCs only detected with picrotoxin and 4-aminopyridine | [86] |
| Human (normal and AD fibroblasts) | FACS sorting of NPCs, BDNF, GDNF and cAMP treatment | mIPSCs, mEPSCs (26) | Currents detected in approximately 40% of cells | [148] | |
| iN | Human (postnatal and adult fibroblasts) | Two-stage Dox, bFGF, Noggin, GDNF, BDNF, forskolin treatments | mEPSCs (30) | Currents detected in 25%-43% of converted cells | [158] |
| Mouse (perinatal tail tip fibroblasts) | Glial co-culture, FACS sorting | sEPSCs (15-27) | [156,157] | ||
| ESC + iPSC | Human (HES3, BioTime ESI, and WiCell H9 ESCs + melanocyte hiPSCs) | 5 morphogenic factors, FACS sorting, glial co-culture | sIPSCs (42-84) | % of cells with currents increases over time | [28] |
| Implanted neurons also received EPSCs | |||||
| Human (HUES9 ESCs + fibroblast hiPSCs) | Noggin and dorsomorphin treatment, recombinant human Dkk1 treatment of hiPSC EBs, glial co-culture | mEPSCs (35) | Currents detected in 33% of differentiated ESCs and 46% of differentiated iPSCs tested | [111] | |
| Human (WiCell H9 and SHEF4 ESCs + fibroblast hiPSCs) | 3% O2 | mEPSCs (35) | Mature AMPA and GABA profiles | [47,116] |
Similar to synaptic activity, emergence of network activity confirms successful elaboration of the developmental processes required for functional synaptogenesis. We define “network potential” as the ability of derived neurons to integrate post-synaptic events into APs (excitability), to transmit outgoing synaptic signals to other neurons (neurotransmission), and to elicit activity-dependent post-synaptic behaviors (adaptability). While the emergence of network activity is a function of the neuronal subtypes present, and therefore is not a required feature of mature neuron cultures, it does provide unambiguous confirmation of synaptic activity. Ideally, neuron cultures will incorporate both excitatory and inhibitory neuron subtypes whose activity is modulated by feedback mechanisms to produce an excitatory/inhibitory (E/I) balance. This condition is maintained by adaptation of pre- and post-synaptic properties and can be considered functional evidence of synaptic plasticity[68]. Alternatively, monotypic cultures comprised of excitatory or inhibitory neurons will result in unbalanced signaling and network inactivity[69] or seizurogenic bursting[70], conditions in which long-term neuronal viability may be compromised[71,72].
Mature network activity is typically observed in SCNs as synchronous AP propagation among multiple neurons, a phenomenon often referred to as network bursting. Since AP production is a stochastic process caused by the near-simultaneous occurrence of a sufficient number of excitatory EPSPs to exceed the firing threshold, in vitro quantitation of APs is influenced by a number of factors which determine neuron connectivity, such as the number, weight and type of functioning synapses. Several methods are available to quantify network activity. The most specific is the use of electrophysiological methods to directly measure AP production[59,73,74]. This can occur via simultaneous, multiple patch-clamp experiments, where the synchronicity of spontaneous AP production is compared among patched neurons. Alternatively, the effects of network modulators (e.g., bicuculline, aminopyridine or CNQX, as described below) on the frequency or nature of synaptically driven APs can be evaluated in a single neuron in current-clamp mode, providing indirect evidence of the contribution of synaptic activity to AP production. A relatively new approach is the use of MEAs to quantify synchronized bursting behavior in two- and three-dimensional cultures, which provides a unique method for characterizing the effects of neuromodulatory compounds on longitudinal network responses[75,76]. A more limited alternative to MEAs is the use of live-imaging assays to quantify bursting behavior using fluorescence-based detection of neuronal activity reporters, such as fluorescent genetically encoded calcium indicators (GECIs) or voltage-gated dyes[77-79]. Calcium imaging is particularly useful for studying network activity, as it is capable of simultaneously monitoring up to thousands of neurons and their associated processes[80]. While the temporal resolution and sensitivity of calcium imaging can be limited by fluorophore kinetics, level of gene expression or equipment, it is an excellent complement to electrophysiology[81]. Recently described GECIs have been found to be able to detect individual APs[82]; however, improved detection of the small potential differences driven by sub-threshold events is still needed before calcium imaging can approach the resolution offered by whole-cell patch-clamp electrophysiology. The development of optical electrophysiology techniques that offer the throughput of GECIs with the sensitive of whole-cell recordings would represent a transformative approach in large population analyses that would be immediately applicable to synaptically active SCN models.
In all cases the presence of network activity must be confirmed by treatment with pharmacological modulators that alter synaptic activity without directly activating post-synaptic receptors. This is particularly important for MEAs and calcium imaging, in which it is currently impractical to directly measure synaptic function. Such treatments could include bicuculline, which disinhibits the network by blocking GABAergic inhibitory activity; aminopyridines, which prolong APs by blocking K+ efflux; or CNQX, which blocks AMPAR activation and therefore eliminates excitatory signaling in glutamatergic networks[83-85]. Depending on neuron maturity and the relative proportions of neuron subtypes in SCN cultures, the use of network modulators may be the only possible means to reveal excitatory synaptic activity exceeding the threshold of AP detection[86]. Thus, the inability to detect APs by methods such as fluorescent calcium imaging or MEAs should not be considered evidence of the absence of synaptic activity.
A signature in vivo characteristic of network function is the elaboration of activity-induced changes in post-synaptic neurons. In healthy networks a variety of mechanisms are used to balance excitatory and inhibitory inputs, producing circuits that can respond to increases or decreases in input[68,87]. The best-described of these is Hebbian plasticity, which allows for the constant formation and strengthening of synchronously active synapses and weakening of asynchronous synapses and is mediated by altered phosphoproteomic signaling, changes in receptor function and expression and differential gene expression[88,89]. Functional Hebbian plasticity can be indirectly evaluated in cultured neurons by measuring changes in the biochemical correlates of plasticity under conditions that up- or down-regulate network activity. Molecular correlates of plasticity include the surface localization of glutamate-responsive AMPA receptors, which can be measured by surface biotinylation or antibody labeling of extracellular residues[47,90], or the phosphorylation state of post-synaptic kinases such as CaMKII, Akt or CREB, which can be quantified using routine immunoblot methods[91-93]. Activity-induced changes in the expression of plasticity-related immediate early genes (IEGs) can be monitored by transcriptomic and/or proteomic methods, including Arc, the EGR family, c-Fos, Jun and Homer-1a[69,70,94-96]. However, it is important to note that many of these genes have multiple functions and are regulated during development and other neuromodulatory treatments[97,98]. Thus, researchers should be careful to ensure they are evaluating IEG expression under conditions that precisely modulate network activity without inducing acute cytotoxicity or other forms of neuronal stress. Synaptic plasticity can be directly measured by whole-cell patch-clamp electrophysiology in neuron cultures using trans-synaptic patch clamping or bipolar stimulating electrode techniques[65]. Plasticity- and activity-related assays have rarely been described in SCN models or in primary neuron cultures, but do represent relevant physiological correlates of network function and they should be considered to be a valuable confirmatory tool when characterizing and validating activity in SCNs.
The yield, neuronal subtype(s) and purity of SCNs are heavily influenced by an array of biochemical factors that affect the differentiation process by modulating progression through neurodevelopmental stages. In some cases these factors can be controlled very precisely, such as by the addition of small molecules to promote exit from the pluripotency state, whereas others are less amenable to rational control, such as longitudinal changes in neurogenic potential caused by the spontaneous emergence of mutated subpopulations during routine culturing of stem cell lines. Thus identifying confirmatory markers of neurogenic progression is critically important to validate that differentiation of specific stem cell lines successfully results in the desired neuronal products. Derivation of neurons from human and mouse stem cells lines has been shown to recapitulate many ontological markers described during in vivo neurogenesis[43]. These include markers of the different stages of neurodevelopment, starting from a stem cell state and expressing characteristics of a NSCs stage, neural progenitor cell (NPC) stage and an immature neuron stage before ultimately developing into functional mature neurons.
Neuronal differentiation typically starts with the induction of a neuroepithelial NSC phenotype by withdrawal of pluripotency factors, such as LIF (mouse stem cells) or FGF-2 (human stem cells). The resulting NSCs may be expanded in culture for a limited number of passages to increase neuronal yield[99-101]. Identifying compounds that sustain the neurogenic competency of NSCs in culture will be valuable for manufacturing purposes, since this will both streamline the differentiation process and increase the total yield. Methods of neural patterning vary wildly. Many protocols involve the use of retinoic acid (RA), which is an early developmental signal for rostral-caudal patterning of the embryonic brain[102-104]. Differentiation can be further enhanced or directed by a variety of techniques, including supplementation with growth factors, such as brain-derived neurotrophic factor or glia-derived growth factor[100,105-107]; forced expression of transcription factors that control neuronal fates[108,109]; or addition of small molecules such as Noggin, Sonic Hedgehog or dorsomorphin[74,110,111]. Neural patterning can also be influenced by secreted factors, such as the increased production of motor neurons by co-culture of NSCs with muscle cells[112]. While neuronal maturation can proceed without the deliberate addition of conditioned media or the presence of support cells, some studies suggest that synaptogenesis is significantly enhanced by co-culture with astrocytes[113,114].
ESCs are derived from totipotent cells collected from the inner cell mass of embryos at the blastocyst stage[115]. Mouse ESC are frequently differentiated to neurons via the 4/4 method, which involves neural induction by the withdrawal of LIF and neural patterning by the addition of RA[116,117]. The resulting ESC-derived neurons (e-neurons) have been shown to produce highly active glutamatergic and GABAergic synapses with emergent network responses[59] and activity-dependent gene expression[74]. ESC differentiation protocols can be modified by the addition of exogenous growth factors to generate other neuronal phenotypes, ranging from a mixture of dopaminergic, glutamatergic, cholinergic and andrenergic neurons[118] to a homogenous GABAergic neuron population with immature, excitatory GABA signaling[119]. Mouse e-neurons have been found to reliably form functioning synapses and robust emergent networks within weeks, whereas methods to produce comparable levels of network activity in human e-neurons are still under investigation[28]. It has recently been suggested that many extant human ESC lines exhibit epigenetic markers of partially differentiated epiblasts rather than naïve ESCs, which could provide a plausible mechanism for their relatively poor and inconsistent neurogenic potential[120]. In contrast, neurons derived from monkey, canine, pig, chicken, worm or fly ESCs[121-127] have been found to develop neurotypic morphologies, although characterization of the resulting neurons has not included functional measure of synaptic activity and network emergence to date.
NSCs are proliferating stem cells that are restricted to neural lineages. NSCs are isolated from fetal and adult CNS, and therefore their availability and use is limited and subject to ethical concerns[128-131]. One advantage of NSCs is that they appear to replicate in vivo mechanisms of adult neurogenesis and therefore may represent a more physiologically appealing model than neurons derived from pluripotent cell lines[132]. In the brain, slow cycling NSCs in the sub-ventricular zones and in the subgranular zone of the dentate gyrus produce neurogenic progeny that pass through sequential developmental stages with structurally and functionally distinct cellular properties[133]. For example, in the well-studied rodent hippocampal subgranular zone, GFAP+ NSCs progressively differentiate into Trb2+ transiently amplifying NPCs, DCX+ migratory neuroblasts and finally into post-mitotic NeuN+-neurons which functionally integrate into existing hippocampal circuits within 3 wk and adopt mature dentate gyrus characteristics within 6-8 wk[134]. A similar developmental progression has been shown to occur in cultured NSCs based on morphological analysis[132]. Some NSCs have been observed to be restricted to specific neural lineages in vitro, such as lines that only generate astrocytes and oligodendrocytes[135,136]. The mechanisms underlying this limited potency are unknown, and whether this is a consequence of culture conditions or representative of in situ behaviors is unclear. As with iPSC- or ESC-derived NSCs, primary NSCs have a limited ability to proliferate in culture before losing their neurogenic competence[137,138]. Despite the potential value of NSC models, few functional assays have been performed on neurons differentiated from these sources, making it unclear whether they reliably produce active synapses. Furthermore, the collection of human NSCs is only possible under limited conditions, such as from aborted fetuses, during brain surgery or immediately post-mortem, and consequently their use is likely to have limited clinical utility[139].
iPSCs are reprogrammed from differentiated tissues through the exogenous expression of pluripotency genes, such as the original Yamanaka reprogramming factors Oct4, Sox2, Klf4 and cMyc or subsequently described variants[140,141]. While neurogenic iPSCs have been generated from a wide variety of animals and tissues, this review will specifically focus on human iPSCs, predominantly derived from fibroblasts. For reasons that appear to be attributable to stochastic variability in epigenetic changes that occurs during reprogramming, even iPSC clones derived from the same tissue typically exhibit a range of neurogenic potentials, requiring neurogenesis to be characterized from each individual line[142,143]. As with human ESCs, there has been a recent focus on converting human iPSCs to a naïve epigenetic state (aka, ground-state), with the objectives of establishing a truly pluripotent phenotype that exhibits greater reliability and reduced variability during differentiation[144-146]. Despite their current technical limitations, two characteristics of iPSCs render iPSC-derived neurons (i-neurons) highly valuable for clinical research. First is the ability to generate patient-specific i-neurons that express the specific genotypic and phenotypic characteristics for a given disease in the context of the patient’s own genome. Second, the decreased risk of immunoreactivity following engraftment of autologous cells back to the patient renders iPSCs a viable model for cell replacement therapies. The clinical implications of these characteristics are described further in next section.
The production of i-neurons is relatively new and protocols to reliably generate synaptically active i-neurons are not yet currently available[147]. For example, neurons differentiated from both normal and Alzheimer’s disease patient iPSCs display neurotypic intrinsic electrophysiological properties within a week after differentiation from NPC cultures, but derived neurons were unable to fire repetitive APs following current injection, so they were considered immature[148]. Similarly, iPSCs differentiated for 10 wk with standard stem cell derivation and differentiation directed towards cortical neurons were found to express abundant synaptic markers, but electron micrographs indicated sparse vesicles in pre-synaptic compartments, and no activity was reported[16]. In another example, the ability to fire repeated APs was not observed in differentiated iPSC neurons until about 12 wk after plating[28]. These findings suggest that i-neurons produced using current methods tend to be developmentally arrested or undergo functional synaptogenesis much less quickly than rodent SCNs. In contrast, there are reports suggesting the robust production of synaptically active i-neurons; however, reproducible protocols have not been published nor have other labs validated these findings, making it unclear exactly what element(s) of the differentiation protocols promoted functional synaptogenesis[111,116].
Rationally designed improvements to the speed and efficiency of i-neuron differentiation would be exceedingly valuable for a wide variety of uses, including pharmaceutical screening and cell replacement strategies. The ideal approach would enable the reproducible yield of large quantities of synaptically active neurons of defined lineages in a relatively short period of time. Recent experiments have focused on the ability of transgenic transcription factors and/or small molecules to increase the speed and efficiency of differentiation. For example, overexpression of neurogenin (Ngn1 or 2) transcription factors accelerates maturation of intrinsic neuronal characteristics in i-neurons, but has mixed results on the appearance of post-synaptic currents, with one study finding such currents at 2-3 wk[149] while another did not find any until 7 wk[150]. The addition of small molecules to iPSCs during neuronal differentiation increases the efficiency of differentiation into intermediate neuronal precursors[151]. Small molecules may also be used to influence neuronal pattering, but to date this approach has only been successful in directing cells towards dopaminergic populations, and the resulting neurons have not been assessed for network activity[152,153]. While these approaches are still immature, the identification of methods that improve the speed and efficiency/specificity of neuronal differentiation has the potential to render i-neurons a transformative tool in basic and clinical sciences.
Recent work suggests that a neuronal phenotype can be differentiated directly from non-neuronal tissue by the expression of specific genes. Direct conversion, or trans-differentiation, works on many of the same principles as iPSCs, involving the forced expression of transcription factors associated with the target neurons or neuronal intermediates. As with iPSCs, a variety of transcriptional and biochemical factors have been used as neural induction factors for iNs. Initially, the transcription factors Ngn2, Ascl1 (Mash1), and Dlx2 were found to directly convert non-proliferative mouse astrocytes into populations of glutamatergic and GABAergic neurons[154,155]. Ascl1, along with the factors Brn2 and Mytl1 (collectively BAM), can convert mouse fibroblasts into active neuronal populations[156,157]. Human postnatal and adult fibroblasts appear to require expression of Brn2, Mytl1l and microRNA-124 to produce active glutamatergic synapses[158]. Alternatively, Nurr1, Lmx1a, Ngn2, Sox2 and Pitx3 have been found to induce fibroblasts to express dopaminergic markers[159-161], while BAM plus Lhx3, Hb9, Isl1, and Ngn2 promotes expression of motor neuron markers[162]. Like iPSC models, iNs can be used to replicate functional pathologies. For example, neuroligin-3 mutations associated with autism cause post-synaptic dysfunction in iNs when co-cultured with primary neurons[163].
The iN field is still new, and many of the challenges facing iPSC-based neuronal differentiation are likely to affect iN approaches as well. Only a small percentage of cells are successfully converted to neurons and most iNs retain epigenetic features of their non-neuronal origins[161]. Unlike differentiation of iPSCs which can be expanded during the stem cell stage iN conversion has frequently proven to have low efficiency, with only 1%-20% of starting cells successfully reprogrammed to become neurons, although some more recent work indicates conversion rates that exceed 200% (indicating a period of proliferation during the conversion process)[162,164]. Low success rates may complicate functional neurogenesis, leading to a sparser neuron population, variable intrinsic properties and little to no network activity, as demonstrated in electrophysiological profiling of iN populations with as few as five neuron-like conversions per coverslip[165]. Conversely, the limited scalability may also render iNs less likely to become tumorigenic following implantation or conversion in vivo. One potential way around limited scalability might be to induce differentiated cells into neuronal precursor cells rather than directly into neurons. While this approach has been used to produce presumptive precursors (based on gene expression studies and morphology), it remains to be seen if induced progenitors cells can differentiate into functionally mature neurons[166,167].
In addition to the clinical potential, SCNs have extensive potential for basic and applied neuroscience studies. In many cases, stem cells are genetically tractable, typically clonal and amenable to culturing in large numbers, facilitating the production of scalable quantities of synchronized SCNs that are genetically homogenous and amenable to forward and reverse genetics approaches as well as functional engineering for biotechnological studies[101,168,169]. While the stem cell stage of SCN differentiation is the most obvious target for genetic manipulation, allowing for isolation and expansion of genetically identical clones, post-mitotic neurons can also be modified using a variety of techniques that are well-described in primary neuron cultures[170]. Genetic manipulations have been used for a variety of studies. For example, exogenous expression of developmentally regulated transcription factors has been used to explicate the genetic regulators that influence neuronal patterning and neuron subtype specification[171,172]. In addition to influencing cell type or development, functionality can be added to SCNs by the expression of transgenic proteins. These functional modifications enable a particular readout, such as fluorescent reporters of gene expression, synaptic assembly, synaptic activity, or metabolic function[77,173-175]. Recent advances that provide control over neuron activity via biologically orthogonal inducers, such as biophotonic or chemogenetic approaches, make it possible to measure and control synaptic activity in networked neuron cultures[176,177]. For example, expression of channelrhodopsin under the control of a dopaminergic promoter allows for the light-activated stimulation of grafted dopaminergic neurons[67]. The effects of these controlled stimuli on in vitro network behavior could be measured by whole-cell patch clamp or in MEAs, providing an indirect electrophysiological measure of the function of metabotropic synapses. Genetic manipulation may also be used to improve the therapeutic potential of SCNs. Targets include repairing the mutated huntingtin protein in models of Huntington’s disease[178,179] or expressing factors enhancing neuron growth and repair in implanted SCNs[180,181]. Despite the utility of genetic modulation of stem cells, care must be taken in characterizing the resultant lines. For example, genetic modifications may alter differentiation outcomes, potentially producing cells that are morphologically and structurally neuronal, but lack intrinsic or synaptic electrophysiological characteristics[182]. Although below we will focus on applications that are not dependent on genetic modification, it is important to remember that in most applications the incorporation of genetically modified SCNs would complement basic and clinical studies.
The in vitro analysis of i-neurons produced from patient-derived iPSCs is expected to be one of the early successes of personalized medicine. Autologous SCN models are already in use for applications such as population-wide phenotypic comparisons and drug screening studies[183]. Shortly after protocols became widespread to generate iPSCs, laboratories rushed to establish cell banks of iPSCs derived from patients with a wide variety of neurodevelopmental and neurodegenerative disorders, with the goal of developing diagnostics and assessing treatment efficacies in vitro[184-186]. This approach has the distinct advantage of studying the phenotypic disease in the native genetic environment and avoiding confounds that may influence disorder penetrance and prognosis. One example of a disease-specific approach is i-neurons derived from a patient with Dravet syndrome epilepsy, which is caused by a single protein mutation in the Nav1.1 sodium channel. Dravet SCNs exhibited altered excitability, with reductions in evoked AP amplitude and probability of firing[187]. Autologous iPSCs also facilitate the study of neuropsychiatric diseases with complex polygenic or unknown genetic origins, something difficult or impossible by the rational introduction of mutations[188]. For example, i-neurons derived from schizophrenia (SZ) patients have fewer post-synaptic markers and less neurite growth than controls, despite similar intrinsic electrophysiological profiles[189]. When maintained in an immature NPC-like state, SZ cells were also found to have abnormal cytoskeletal development, increased oxidative stress, and aberrant migration, indicative of developmental abnormalities[190]. These discoveries would have been considerably more difficult without access to a developmental SCN model.
Many neurodegenerative disorders originate from multiple genetic loci, including Alzheimer’s disease, Parkinson’s disease and other dementias[191]. They often exhibit variable penetrance, which is further compounded by a lifetime of unknown environmental interactions. Patient-derived iPSCs offer the potential to study the effects of complex and subtle genetic contributions to these variable disease phenotypes by replicating the genomic context from which the disease is expressed. In some cases, disorders have displayed relatively consistent pathophysiologies in SCN models, enabling high resolution characterization of the disease pathophysiology. For example, i-neurons derived from Parkinson’s disease patients from diverse genetic backgrounds exhibit initial morphological characteristics of synaptogenesis, but subsequently degenerate in vitro, accumulating α-synuclein and other toxic intermediaries[192,193]. Other disorders have shown inconsistent pathophysiology in vitro, including iPSCs derived from Huntington’s disease patients[194,195] and multiple sclerosis patients[196]. However, while sometimes confounding, this inconsistent pathology may also contribute to an improved understanding of disease mechanisms. For example, iPSC-derived motor neurons from a subset of amyotrophic lateral sclerosis (ALS) patients exhibit pathophysiological characteristics, while others ALS patient-derived motor neurons appear normal[86,197-199]. Derived ALS motor neurons cocultured with astrocytes have suggested that some ALS neuropathology is due to aberrant glial function[200-202]. Together, these studies suggest that ALS may originate from the interactions of multiple cell types and genetic loci, with potentially tissue-specific diagnoses and treatment requirements.
Two potential caveats to the general appeal of iPSCs for disease modeling are the possibility of selecting a non-representative line during reprogramming and increased risk of genomic changes during maintenance of reprogrammed iPSCs. For example, cultured iPSCs have been found to exhibit increased copy number variation, which is associated with a wide range of neuropsychiatric diseases[203]. Unfortunately, while a variety of assays have been used to characterize the phenotype of neuropsychiatric i-neuron models, few have emphasized differences in synaptic and network activity, in large part because reproducible protocols to generate synaptically active i-neurons are not widely available. In fact, recent reviews have noted that functional assays, such as calcium imaging or electrophysiology are infrequently performed compared to gene expression studies[192]. This lack of rigor in characterizing SCN models may further contribute to discrepancies in translational interpretation. While i-neurons are not likely to be viable models for all neuropsychiatric diseases, particularly those that rely on multi-tissue interactions, the pathophysiological relevance of patient-specific SCN models has extraordinary potential to explicate complex disease mechanisms.
Unlike the comparatively robust reparative mechanisms of the peripheral nervous system, intrinsic regenerative processes in the CNS do not appear to be capable of repairing damaged nervous tissue and restoring normal brain function in cases of severe or large-scale neural disease or injury[204]. Consequently, clinical management of traumatic brain injury or degenerative neurological diseases is predominantly limited to supportive or palliative care. One of the most promising treatment strategies for regeneration of neural function is targeted cell replacement (TCR), in which exogenously derived cells are administered to the CNS to ameliorate the disease or injury process, protect from further decline of function and possibly facilitate repair of lost capacity. In one aspect of TCR, in vitro-derived neural cells of defined maturity and fate are delivered to specific CNS locations, where in theory they will appropriately integrate into existing circuits. This approach is complicated by several factors, including the intricate and poorly understood physiology of the nervous system; the fact that ontogenic processes such as axonal pathfinding and circuit formation may not be appropriately recapitulated in adult brains; and the difficulty in specifying the character of the grafted cells. Nonetheless, in preliminary studies centrally administered SCNs have exhibited potential as a regenerative CNS therapy. Administration of human NSCs, such as the NSI-566RSC line, have proven safe and effective in animal models of ALS, spinal cord injury, and ischemia[205], and are currently undergoing clinical trials[206]. These NSCs appear to form functioning neurons that integrate into existing neuronal networks in vivo[207]. Studies using more mature dopaminergic neurons derived from fetal NSCs have also found that long-term integration and transplanted neurons conferred resistance to PD in mouse models[208].
TCR has been made more technically feasible by the recent development of autologous iPSCs, which are less likely to evoke undesired immune responses. The development of autologous SCN-based TCR therapies would be immensely valuable for traumatic CNS injury as well as for neurodegenerative diseases such as Parkinson’s disease and ALS. However, despite the promise of SCN therapies, early studies suggest that implantation of stem cells and stem cell-derived tissue suffers from several complications, most notably cell survival and functional integration into target circuits[209,210]. For example, inappropriately integrated cells may disrupt existing circuits while cell death may trigger counterproductive inflammatory responses that further degrade central function[211,212]. More severe complications may arise if mitotically active donor cells proliferate to form non-neuronal, neoplastic tissues[213] or otherwise damage the engraftment site, provoking responses that impair the function of implants over time[214,215]. Despite these complications, SCN therapies have transformative potential now, and as protocols are established for improved graft survival and integration with reduced adverse host responses, such therapeutic modalities will be realized.
One of the most attractive uses of SCNs is as a scalable, relevant cell model of neuronal responses in pharmacological and toxicological testing[216]. For example, the physiological relevance imparted by SCNs for in vitro toxicology is anticipated to significantly reduce dependence on the use of animals to detect, diagnose or study lethal toxins, such as with the mouse lethality assay, or to conduct initial efficacy testing of candidate therapeutics[217,218]. Neuron-like immortalized cell lines, such as neuroblastomas and pheochromocytomas, have historically been used in in vitro toxicity studies and countermeasure screening protocols for the Tier 1 select agents collectively known as botulinum toxins (BoNTs)[219-222]. However, as described above, these cell lines have poor neurogenic competence and thus are not suitable to study neurotoxic substances whose modes of action are mediated by neurotypic behaviors, act in specific neuronal compartments or are evidenced through altered neurotransmission[223,224]. The various BoNT serotypes are good examples of neuron specific agents, as they are internalized at the pre-synaptic compartment and then specifically target and cleave SNARE proteins responsible for neurotransmitter release[225]. In the absence of a functioning pre-synaptic compartment, it is doubtful that non-neuronal models faithfully reflect the biochemistry and host interactions involved in intoxication. In contrast, mouse e-neurons differentiated into a synaptically active population of glutamatergic and GABAergic neurons with emergent network behavior undergo synaptic blockade within hours after intoxication with femtomolar concentrations of botulinum neurotoxins serotype A[6,226,227]. This represents the first in vitro-derived, cell-based model to manifest the functional pathophysiology responsible for the clinical symptoms of botulism, enabling novel in vitro studies on host:toxin interactions. This e-neuron model has also been shown to be highly sensitive to other neurotoxins, such as glutamate and latrotoxin, with mechanisms of injury, toxin sensitivities and cellular responses that are identical to those of primary neurons[59]. Similar glutamate excitotoxicity has been demonstrated in human e-neurons and i-neurons[228]. As these models are shown to be increasingly relevant neurotoxicological models, they are anticipated to rapidly become the primary approach for target discovery, drug screening and detection/diagnosis of neurotoxins and neurotoxic effects.
Another valuable application of SCNs in neurotoxin studies is the modeling of developmental neurotoxicity. While primary neuron cultures may not recapitulate developmental vulnerabilities that occur during early stages of differentiation, SCNs are well-suited for studying specific mechanisms of early developmental toxicity, such as the effects of reactive oxygen production and alterations to specific cell signaling pathways during early stages of neurogenesis[229-232]. SCNs can also be used to evaluate toxicant-induced changes to relative neurogenic and gliogenic outcomes during neural differentiation, as well as to test for persistent changes in synaptic function, such as the chronic functional deficits resulting from developmental exposure to ethanol[233-235]. Taking advantage of the scalability of SCN models and high throughput detection platforms such as MEAs or calcium imaging, multiple neurotoxic compounds can be screened and compared in SCN systems over multiple stages of development to assess vulnerable stages and mechanisms[236-238]. In addition, the use of MEAs facilitates high-throughput monitoring of the development and interruption of network activity[75,76,239]. Finally by combining scalability with transgene expression, moderate-to-high throughput assays can be developed to rapidly assess the efficacy of candidate therapeutics on injury or disease at various stages of neurogenesis.
Relevance is a key goal of neuronal models. In addition to functional relevance of neuronal monolayers, a relevant histotypic model can be generated by simultaneously producing or combining multiple nervous tissue types in two- or three-dimensional cultures. Depending on the differentiation protocol, multiple neural types, including glia and oligodendrocytes, can be produced simultaneously with neurons[137,138]. Multiple neuronal subtypes are generated in many SCN differentiation protocols[43,110,171,240]. The introduction of physicobiological techniques to differentiation methods may further enable the defined production of diverse neural cell types, such as the three-dimensional culture of neocortical-like organoids[241-245] and use of chemical or physical micro-patterning of plates, including microfluidics[246,247].
Histogenic neuronal models are anticipated to produce more complex neuronal circuits by facilitating synaptic interactions among multiple neuron types. For instance, complex retinal tissue has been generated from iPSCs[248-250], including the formation of three-dimensional superstructures that facilitate the development of photoreceptor cells, multiple neuron types and other cells in the retina. The cells themselves respond to incident light with electrochemical and biochemical responses that are similar to those produced in vivo. Further elaboration of this retinal model would require optic nerve tissue as well as visual cortex tissue to more comprehensively model the circuits involved in sight. It may be that in producing SCN models with multiple active neuronal and glial types, researchers have incidentally begun to form specific regional patterning and developmental forms of neuroectodermal tissue that essentially produce many of the outcomes of a histogenic model. For example, the rosettes formed during neurogenesis in vitro may be patterned similar to neural tubes or cortical plates in vivo[251]. Similarly, three-dimensional cultures of some ESCs allow for the formation of stratified structures similar to fetal cortex[252], and even recapitulates the folding of neuroepithelium[253]. The functional generation of complex tissues such as retina and multi-layered cortex and neuroepithelium solely from stem cells suggests that generation of other complex CNS tissues may soon be feasible.
With available stem cell derivation and SCN differentiation techniques growing rapidly, researchers must be able to critically analyze and compare these models to in vivo neurons and circuits. Many SCN models, particularly those derived from human origins, still require optimized protocols to reproducibly generate synaptically active neurons. It may be a challenge for some researchers to functionally validate SCN models, but validation is essential for physiological relevance and to increase the translatability of findings regarding neurological development, function and dysfunction. It is encouraging to see newer research in the literature recognizing that functional endpoints, such as the establishment of a neuronal network, are highly sensitive and powerful tools. This review presents some variations in the approaches used to establish synaptic and network activity, but for now it should be apparent to those pursuing electrophysiological approaches that post-synaptic activity must be measured alongside intrinsic characteristics, followed by assays for networked activity. It is hoped that as techniques improve, the means of proving functional synaptogenesis and networked activity in SCNs will also expand. With expanding collections of validated models available, researchers will have excellent options for both basic neuroscience and therapeutic applications.
We would like to acknowledge Kyle Hubbard and Megan Lyman (USAMRICD) for providing cells and exemplar images; Phillip Beske (USAMRICD) for providing exemplar traces; and Tracey Hamilton (USAMRICD) for electron microscopy images; James Abraham (USAMRICD) for illustration work; and Katie Hoffman and Cindy Kronman (USAMRICD) for editorial assistance.
P- Reviewer: Sarkadi B S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Dodla MC, Mumaw J, Stice SL. Role of astrocytes, soluble factors, cells adhesion molecules and neurotrophins in functional synapse formation: implications for human embryonic stem cell derived neurons. Curr Stem Cell Res Ther. 2010;5:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Dieni CV, Chancey JH, Overstreet-Wadiche LS. Dynamic functions of GABA signaling during granule cell maturation. Front Neural Circuits. 2012;6:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Kaur P, Karolina DS, Sepramaniam S, Armugam A, Jeyaseelan K. Expression profiling of RNA transcripts during neuronal maturation and ischemic injury. PLoS One. 2014;9:e103525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Zuko A, Kleijer KT, Oguro-Ando A, Kas MJ, van Daalen E, van der Zwaag B, Burbach JP. Contactins in the neurobiology of autism. Eur J Pharmacol. 2013;719:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Telias M, Segal M, Ben-Yosef D. Neural differentiation of Fragile X human Embryonic Stem Cells reveals abnormal patterns of development despite successful neurogenesis. Dev Biol. 2013;374:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | McNutt P, Celver J, Hamilton T, Mesngon M. Embryonic stem cell-derived neurons are a novel, highly sensitive tissue culture platform for botulinum research. Biochem Biophys Res Commun. 2011;405:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lin M, Hrabovsky A, Pedrosa E, Wang T, Zheng D, Lachman HM. Allele-biased expression in differentiating human neurons: implications for neuropsychiatric disorders. PLoS One. 2012;7:e44017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC Transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. 2014;32:3075-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Boucherie C, Hermans E. Adult stem cell therapies for neurological disorders: benefits beyond neuronal replacement? J Neurosci Res. 2009;87:1509-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Telias M, Ben-Yosef D. Modeling neurodevelopmental disorders using human pluripotent stem cells. Stem Cell Rev. 2014;10:494-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Panchision DM. Meeting report: using stem cells for biological and therapeutics discovery in mental illness, April 2012. Stem Cells Transl Med. 2013;2:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS One. 2010;5:e11853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Li H, Liu H, Corrales CE, Risner JR, Forrester J, Holt JR, Heller S, Edge AS. Differentiation of neurons from neural precursors generated in floating spheres from embryonic stem cells. BMC Neurosci. 2009;10:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Verstraelen P, Pintelon I, Nuydens R, Cornelissen F, Meert T, Timmermans JP. Pharmacological characterization of cultivated neuronal networks: relevance to synaptogenesis and synaptic connectivity. Cell Mol Neurobiol. 2014;34:757-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 879] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 16. | Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12770-12775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 17. | Lilienbaum A, Israël A. From calcium to NF-kappa B signaling pathways in neurons. Mol Cell Biol. 2003;23:2680-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Hagenston AM, Bading H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb Perspect Biol. 2011;3:a004564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 587] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 20. | Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009;5:e1000604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Nie CL, Wang XS, Liu Y, Perrett S, He RQ. Amyloid-like aggregates of neuronal tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci. 2007;8:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 874] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 23. | Léveillé F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623-2635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Kozhich OA, Hamilton RS, Mallon BS. Standardized generation and differentiation of neural precursor cells from human pluripotent stem cells. Stem Cell Rev. 2013;9:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 595] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Cáceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617-1626. [PubMed] |
| 28. | Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 29. | Belinsky GS, Moore AR, Short SM, Rich MT, Antic SD. Physiological properties of neurons derived from human embryonic stem cells using a dibutyryl cyclic AMP-based protocol. Stem Cells Dev. 2011;20:1733-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68:639-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Burton SD, Johnson JW, Zeringue HC, Meriney SD. Distinct roles of neuroligin-1 and SynCAM1 in synapse formation and function in primary hippocampal neuronal cultures. Neuroscience. 2012;215:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Bury LA, Sabo SL. Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons. Neural Dev. 2014;9:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: a cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci USA. 2008;105:20947-20952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6:e219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Kim J, Zhao T, Petralia RS, Yu Y, Peng H, Myers E, Magee JC. mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat Methods. 2012;9:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18:1217-1229. [PubMed] |
| 38. | Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Valor LM, Charlesworth P, Humphreys L, Anderson CN, Grant SG. Network activity-independent coordinated gene expression program for synapse assembly. Proc Natl Acad Sci USA. 2007;104:4658-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Dráberová E, Lukás Z, Ivanyi D, Viklický V, Dráber P. Expression of class III beta-tubulin in normal and neoplastic human tissues. Histochem Cell Biol. 1998;109:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Bustos R, Kolen ER, Braiterman L, Baines AJ, Gorelick FS, Hubbard AL. Synapsin I is expressed in epithelial cells: localization to a unique trans-Golgi compartment. J Cell Sci. 2001;114:3695-3704. [PubMed] |
| 42. | Darlington PJ, Goldman JS, Cui QL, Antel JP, Kennedy TE. Widespread immunoreactivity for neuronal nuclei in cultured human and rodent astrocytes. J Neurochem. 2008;104:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Hubbard KS, Gut IM, Lyman ME, McNutt PM. Longitudinal RNA sequencing of the deep transcriptome during neurogenesis of cortical glutamatergic neurons from murine ESCs. F1000Res. 2013;2:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414-6423. [PubMed] |
| 45. | Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33:6614-6622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 47. | Livesey MR, Bilican B, Qiu J, Rzechorzek NM, Haghi G, Burr K, Hardingham GE, Chandran S, Wyllie DJ. Maturation of AMPAR composition and the GABAAR reversal potential in hPSC-derived cortical neurons. J Neurosci. 2014;34:4070-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Moore AR, Zhou WL, Jakovcevski I, Zecevic N, Antic SD. Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci. 2011;31:2391-2398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol. 2003;89:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Sipilä ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280-5289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Beatty JA, Sullivan MA, Morikawa H, Wilson CJ. Complex autonomous firing patterns of striatal low-threshold spike interneurons. J Neurophysiol. 2012;108:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Ovsepian SV, Dolly JO, Zaborszky L. Intrinsic voltage dynamics govern the diversity of spontaneous firing profiles in basal forebrain noncholinergic neurons. J Neurophysiol. 2012;108:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Sonntag KC, Simantov R, Kim KS, Isacson O. Temporally induced Nurr1 can induce a non-neuronal dopaminergic cell type in embryonic stem cell differentiation. Eur J Neurosci. 2004;19:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol. 2014;76:333-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 55. | Peled ES, Newman ZL, Isacoff EY. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr Biol. 2014;24:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 56. | Dolly JO, Lande S, Wray DW. The effects of in vitro application of purified botulinum neurotoxin at mouse motor nerve terminals. J Physiol. 1987;386:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 1093] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 58. | Otsu Y, Murphy TH. Miniature transmitter release: accident of nature or careful design? Sci STKE. 2003;2003:pe54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Gut IM, Beske PH, Hubbard KS, Lyman ME, Hamilton TA, McNutt PM. Novel application of stem cell-derived neurons to evaluate the time- and dose-dependent progression of excitotoxic injury. PLoS One. 2013;8:e64423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 61. | Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109-128. [PubMed] |
| 62. | Paré D, Lebel E, Lang EJ. Differential impact of miniature synaptic potentials on the soma and dendrites of pyramidal neurons in vivo. J Neurophysiol. 1997;78:1735-1739. [PubMed] |
| 63. | Debanne D, Boudkkazi S, Campanac E, Cudmore RH, Giraud P, Fronzaroli-Molinieres L, Carlier E, Caillard O. Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat Protoc. 2008;3:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464-10472. [PubMed] |
| 65. | Maximov A, Pang ZP, Tervo DGR, Südhof TC. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Meth. 2007;161:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378-4385. [PubMed] |
| 67. | Tønnesen J, Parish CL, Sørensen AT, Andersson A, Lundberg C, Deisseroth K, Arenas E, Lindvall O, Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6:e17560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Beggs JM. The criticality hypothesis: how local cortical networks might optimize information processing. Philos Trans A Math Phys Eng Sci. 2008;366:329-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 69. | Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell. 2012;149:886-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 70. | DiFebo F, Curti D, Botti F, Biella G, Bigini P, Mennini T, Toselli M. Neural precursors (NPCs) from adult L967Q mice display early commitment to “in vitro” neuronal differentiation and hyperexcitability. Exp Neurol. 2012;236:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Habas A, Hahn J, Wang X, Margeta M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci USA. 2013;110:18291-18296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1631] [Cited by in RCA: 1687] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 73. | Barth L, Sütterlin R, Nenniger M, Vogt KE. Reduced synaptic activity in neuronal networks derived from embryonic stem cells of murine Rett syndrome model. Front Cell Neurosci. 2014;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Copi A, Jüngling K, Gottmann K. Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network. J Neurophysiol. 2005;94:4538-4543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Weiss D. Neurotoxicity assessment by recording electrical activity from neuronal networks on microelectrode array neurochips. In: Aschner M, Suñol C, Bal-Price A. Cell culture techniques. Humana Press 2011; 467-480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | van Vliet E, Stoppini L, Balestrino M, Eskes C, Griesinger C, Sobanski T, Whelan M, Hartung T, Coecke S. Electrophysiological recording of re-aggregating brain cell cultures on multi-electrode arrays to detect acute neurotoxic effects. Neurotoxicology. 2007;28:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Ikegaya Y, Le Bon-Jego M, Yuste R. Large-scale imaging of cortical network activity with calcium indicators. Neurosci Res. 2005;52:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Chen Q, Cichon J, Wang W, Qiu L, Lee SJ, Campbell NR, Destefino N, Goard MJ, Fu Z, Yasuda R. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron. 2012;76:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 79. | Leão RN, Reis A, Emirandetti A, Lewicka M, Hermanson O, Fisahn A. A voltage-sensitive dye-based assay for the identification of differentiated neurons derived from embryonic neural stem cell cultures. PLoS One. 2010;5:e13833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Blum R, Heinrich C, Sánchez R, Lepier A, Gundelfinger ED, Berninger B, Götz M. Neuronal network formation from reprogrammed early postnatal rat cortical glial cells. Cereb Cortex. 2011;21:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Matsumoto K, Ishikawa T, Matsuki N, Ikegaya Y. Multineuronal spike sequences repeat with millisecond precision. Front Neural Circuits. 2013;7:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Badura A, Sun XR, Giovannucci A, Lynch LA, Wang SS. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics. 2014;1:025008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 83. | Odawara A, Saitoh Y, Alhebshi AH, Gotoh M, Suzuki I. Long-term electrophysiological activity and pharmacological response of a human induced pluripotent stem cell-derived neuron and astrocyte co-culture. Biochem Biophys Res Commun. 2014;443:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Piper DR, Mujtaba T, Rao MS, Lucero MT. Immunocytochemical and physiological characterization of a population of cultured human neural precursors. J Neurophysiol. 2000;84:534-548. [PubMed] |
| 85. | Song M, Mohamad O, Chen D, Yu SP. Coordinated development of voltage-gated Na+ and K+ currents regulates functional maturation of forebrain neurons derived from human induced pluripotent stem cells. Stem Cells Dev. 2013;22:1551-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci USA. 2012;109:5803-5808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 87. | Levina A, Herrmann JM, Geisel T. Dynamical synapses causing self-organized criticality in neural networks. Nat Phys. 2007;3:857-860. [DOI] [Full Text] |
| 88. | Cullen DK, Gilroy ME, Irons HR, Laplaca MC. Synapse-to-neuron ratio is inversely related to neuronal density in mature neuronal cultures. Brain Res. 2010;1359:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 90. | Whitney NP, Peng H, Erdmann NB, Tian C, Monaghan DT, Zheng JC. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 2008;22:2888-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Barcomb K, Buard I, Coultrap SJ, Kulbe JR, O’Leary H, Benke TA, Bayer KU. Autonomous CaMKII requires further stimulation by Ca2+/calmodulin for enhancing synaptic strength. FASEB J. 2014;28:3810-3819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 2014;159:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 93. | Akchiche N, Bossenmeyer-Pourié C, Pourié G, Koziel V, Nédélec E, Guéant JL, Daval JL. Differentiation and neural integration of hippocampal neuronal progenitors: signaling pathways sequentially involved. Hippocampus. 2010;20:949-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Wang CC, Held RG, Hall BJ. SynGAP regulates protein synthesis and homeostatic synaptic plasticity in developing cortical networks. PLoS One. 2013;8:e83941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 96. | Inoue Y, Udo H, Inokuchi K, Sugiyama H. Homer1a regulates the activity-induced remodeling of synaptic structures in cultured hippocampal neurons. Neuroscience. 2007;150:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Sanders JD, Happe HK, Bylund DB, Murrin LC. Differential effects of neonatal norepinephrine lesions on immediate early gene expression in developing and adult rat brain. Neuroscience. 2008;157:821-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 98. | Saito TH, Uda S, Tsuchiya T, Ozaki Y, Kuroda S. Temporal decoding of MAP kinase and CREB phosphorylation by selective immediate early gene expression. PLoS One. 2013;8:e57037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Axell MZ, Zlateva S, Curtis M. A method for rapid derivation and propagation of neural progenitors from human embryonic stem cells. J Neurosci Methods. 2009;184:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Cohen MA, Itsykson P, Reubinoff BE. Neural differentiation of human ES cells. Curr Protoc Cell Biol. 2007;Chapter 23:Unit 23.7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 102. | Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 826] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 103. | McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 539] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 104. | Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 655] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 105. | Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 106. | Gaspard N, Bouschet T, Herpoel A, Naeije G, van den Ameele J, Vanderhaeghen P. Generation of cortical neurons from mouse embryonic stem cells. Nat Protoc. 2009;4:1454-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 107. | Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 108. | Braun SM, Machado RA, Jessberger S. Temporal control of retroviral transgene expression in newborn cells in the adult brain. Stem Cell Reports. 2013;1:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Hong S, Chung S, Leung K, Hwang I, Moon J, Kim KS. Functional roles of Nurr1, Pitx3, and Lmx1a in neurogenesis and phenotype specification of dopamine neurons during in vitro differentiation of embryonic stem cells. Stem Cells Dev. 2014;23:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 111. | Kim JE, O’Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, Deerinck TJ, Goldstein LS, Gage FH, Ellisman MH. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci USA. 2011;108:3005-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Anjomshoa M, Karbalaie K, Mardani M, Razavi S, Tanhaei S, Nasr-Esfahani MH, Baharvand H. Generation of motor neurons by coculture of retinoic acid-pretreated embryonic stem cells with chicken notochords. Stem Cells Dev. 2009;18:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069-3077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 114. | Jordan PM, Cain LD, Wu P. Astrocytes enhance long-term survival of cholinergic neurons differentiated from human fetal neural stem cells. J Neurosci Res. 2008;86:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10427] [Article Influence: 386.2] [Reference Citation Analysis (0)] |
| 116. | Bilican B, Livesey MR, Haghi G, Qiu J, Burr K, Siller R, Hardingham GE, Wyllie DJ, Chandran S. Physiological normoxia and absence of EGF is required for the long-term propagation of anterior neural precursors from human pluripotent cells. PLoS One. 2014;9:e85932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Okada Y, Shimazaki T, Sobue G, Okano H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol. 2004;275:124-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 118. | Raye WS, Tochon-Danguy N, Pouton CW, Haynes JM. Heterogeneous population of dopaminergic neurons derived from mouse embryonic stem cells: preliminary phenotyping based on receptor expression and function. Eur J Neurosci. 2007;25:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Chatzi C, Scott RH, Pu J, Lang B, Nakamoto C, McCaig CD, Shen S. Derivation of homogeneous GABAergic neurons from mouse embryonic stem cells. Exp Neurol. 2009;217:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Schnerch A, Cerdan C, Bhatia M. Distinguishing between mouse and human pluripotent stem cell regulation: the best laid plans of mice and men. Stem Cells. 2010;28:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Chen X, Li T, Li X, Xie Y, Guo X, Ji S, Niu Y, Yu Y, Ding C, Yao R. Neural progenitors derived from monkey embryonic stem cells in a simple monoculture system. Reprod Biomed Online. 2009;19:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 122. | Wilcox JT, Lai JK, Semple E, Brisson BA, Gartley C, Armstrong JN, Betts DH. Synaptically-competent neurons derived from canine embryonic stem cells by lineage selection with EGF and Noggin. PLoS One. 2011;6:e19768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Yang JY, Mumaw JL, Liu Y, Stice SL, West FD. SSEA4-positive pig induced pluripotent stem cells are primed for differentiation into neural cells. Cell Transplant. 2013;22:945-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Boast S, Stern CD. Simple methods for generating neural, bone and endodermal cell types from chick embryonic stem cells. Stem Cell Res. 2013;10:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Wu Y, Ge C, Zeng W, Zhang C. Induced multilineage differentiation of chicken embryonic germ cells via embryoid body formation. Stem Cells Dev. 2010;19:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 126. | Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331:304-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 127. | Schmidt H, Lüer K, Hevers W, Technau GM. Ionic currents of Drosophila embryonic neurons derived from selectively cultured CNS midline precursors. J Neurobiol. 2000;44:392-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 128. | Horiguchi S, Takahashi J, Kishi Y, Morizane A, Okamoto Y, Koyanagi M, Tsuji M, Tashiro K, Honjo T, Fujii S. Neural precursor cells derived from human embryonic brain retain regional specificity. J Neurosci Res. 2004;75:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 129. | Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727-8735. [PubMed] |
| 130. | Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599-7609. [PubMed] |
| 131. | Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Fan G. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 132. | Hermann A, Maisel M, Wegner F, Liebau S, Kim DW, Gerlach M, Schwarz J, Kim KS, Storch A. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Frisén J, Johansson CB, Lothian C, Lendahl U. Central nervous system stem cells in the embryo and adult. Cell Mol Life Sci. 1998;54:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 295] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 135. | Rao MS, Noble M, Mayer-Pröschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA. 1998;95:3996-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 136. | Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883-13888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 562] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 137. | Piao JH, Odeberg J, Samuelsson EB, Kjaeldgaard A, Falci S, Seiger A, Sundström E, Akesson E. Cellular composition of long-term human spinal cord- and forebrain-derived neurosphere cultures. J Neurosci Res. 2006;84:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 138. | Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 139. | Schultz SS, Lucas PA. Human stem cells isolated from adult skeletal muscle differentiate into neural phenotypes. J Neurosci Methods. 2006;152:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 141. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7242] [Article Influence: 402.3] [Reference Citation Analysis (0)] |
| 142. | Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335-4340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 756] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 143. | Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 380] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 144. | Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 834] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 145. | Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:4484-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 372] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 146. | Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports. 2014;2:366-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 147. | Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 148. | Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 920] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 149. | Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1123] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 150. | Busskamp V, Lewis NE, Guye P, Ng AH, Shipman SL, Byrne SM, Sanjana NE, Murn J, Li Y, Li S. Rapid neurogenesis through transcriptional activation in human stem cells. Mol Syst Biol. 2014;10:760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 151. | Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci USA. 2011;108:19240-19245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 152. | Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3036] [Cited by in RCA: 2676] [Article Influence: 167.3] [Reference Citation Analysis (0)] |
| 153. | Mak SK, Huang YA, Iranmanesh S, Vangipuram M, Sundararajan R, Nguyen L, Langston JW, Schüle B. Small molecules greatly improve conversion of human-induced pluripotent stem cells to the neuronal lineage. Stem Cells Int. 2012;2012:140427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 154. | Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Götz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654-8664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 303] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 155. | Heinrich C, Gascón S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, Götz M, Berninger B. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 156. | Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2235] [Cited by in RCA: 2274] [Article Influence: 151.6] [Reference Citation Analysis (0)] |
| 157. | Chanda S, Ang CE, Davila J, Pak C, Mall M, Lee QY, Ahlenius H, Jung SW, Südhof TC, Wernig M. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Reports. 2014;3:282-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 289] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 158. | Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 159. | Liu X, Huang Q, Li F, Li CY. Enhancing the efficiency of direct reprogramming of human primary fibroblasts into dopaminergic neuron-like cells through p53 suppression. Sci China Life Sci. 2014;57:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 160. | Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2012;22:321-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 161. | Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 777] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 162. | Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 519] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 163. | Chanda S, Marro S, Wernig M, Südhof TC. Neurons generated by direct conversion of fibroblasts reproduce synaptic phenotype caused by autism-associated neuroligin-3 mutation. Proc Natl Acad Sci USA. 2013;110:16622-16627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 164. | Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 165. | Koppensteiner P, Boehm S, Arancio O. Electrophysiological profiles of induced neurons converted directly from adult human fibroblasts indicate incomplete neuronal conversion. Cell Reprogram. 2014;16:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 166. | Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109:2527-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 167. | Tian C, Liu Q, Ma K, Wang Y, Chen Q, Ambroz R, Klinkebiel DL, Li Y, Huang Y, Ding J. Characterization of induced neural progenitors from skin fibroblasts by a novel combination of defined factors. Sci Rep. 2013;3:1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 168. | Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1014] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 169. | Hong S, Kang UJ, Isacson O, Kim KS. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;104:316-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Dhara SK, Gerwe BA, Majumder A, Dodla MC, Boyd NL, Machacek DW, Hasneen K, Stice SL. Genetic manipulation of neural progenitors derived from human embryonic stem cells. Tissue Eng Part A. 2009;15:3621-3634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Berninger B, Guillemot F, Götz M. Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur J Neurosci. 2007;25:2581-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 172. | Sánchez-Danés A, Consiglio A, Richaud Y, Rodríguez-Pizà I, Dehay B, Edel M, Bové J, Memo M, Vila M, Raya A. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Hum Gene Ther. 2012;23:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 173. | McNeish J, Roach M, Hambor J, Mather RJ, Weibley L, Lazzaro J, Gazard J, Schwarz J, Volkmann R, Machacek D. High-throughput screening in embryonic stem cell-derived neurons identifies potentiators of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate-type glutamate receptors. J Biol Chem. 2010;285:17209-17217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 238] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 175. | Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 176. | Dranias MR, Ju H, Rajaram E, VanDongen AM. Short-term memory in networks of dissociated cortical neurons. J Neurosci. 2013;33:1940-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 177. | Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L. Remote control of induced dopaminergic neurons in parkinsonian rats. J Clin Invest. 2014;124:3215-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 178. | Nguyen GD, Molero AE, Gokhan S, Mehler MF. Functions of huntingtin in germ layer specification and organogenesis. PLoS One. 2013;8:e72698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 179. | Fink KD, Crane AT, Lévêque X, Dues DJ, Huffman LD, Moore AC, Story DT, Dejonge RE, Antcliff A, Starski PA. Intrastriatal transplantation of adenovirus-generated induced pluripotent stem cells for treating neuropathological and functional deficits in a rodent model of Huntington’s disease. Stem Cells Transl Med. 2014;3:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 180. | Cenciarelli C, Budoni M, Mercanti D, Fernandez E, Pallini R, Aloe L, Cimino V, Maira G, Casalbore P. In vitro analysis of mouse neural stem cells genetically modified to stably express human NGF by a novel multigenic viral expression system. Neurol Res. 2006;28:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 181. | Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 182. | Donato R, Miljan EA, Hines SJ, Aouabdi S, Pollock K, Patel S, Edwards FA, Sinden JD. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 183. | Haggarty SJ, Perlis RH. Translation: screening for novel therapeutics with disease-relevant cell types derived from human stem cell models. Biol Psychiatry. 2014;75:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 184. | Wray S, Self M, Lewis PA, Taanman JW, Ryan NS, Mahoney CJ, Liang Y, Devine MJ, Sheerin UM, Houlden H. Creation of an open-access, mutation-defined fibroblast resource for neurological disease research. PLoS One. 2012;7:e43099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 185. | Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters Ca2+ dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicol Sci. 2012;130:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 186. | Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1775] [Cited by in RCA: 1608] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 187. | Higurashi N, Uchida T, Lossin C, Misumi Y, Okada Y, Akamatsu W, Imaizumi Y, Zhang B, Nabeshima K, Mori MX. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol Brain. 2013;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 188. | Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6882] [Cited by in RCA: 5698] [Article Influence: 518.0] [Reference Citation Analysis (0)] |
| 189. | Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109-R115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 190. | Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 191. | Zou F, Chai HS, Younkin CS, Allen M, Crook J, Pankratz VS, Carrasquillo MM, Rowley CN, Nair AA, Middha S. Brain expression genome-wide association study (eGWAS) identifies human disease-associated variants. PLoS Genet. 2012;8:e1002707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 192. | Badger JL, Cordero-Llana O, Hartfield EM, Wade-Martins R. Parkinson’s disease in a dish - Using stem cells as a molecular tool. Neuropharmacology. 2014;76 Pt A:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 193. | Peng J, Liu Q, Rao MS, Zeng X. Using human pluripotent stem cell-derived dopaminergic neurons to evaluate candidate Parkinson’s disease therapeutic agents in MPP+ and rotenone models. J Biomol Screen. 2013;18:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 194. | Camnasio S, Delli Carri A, Lombardo A, Grad I, Mariotti C, Castucci A, Rozell B, Lo Riso P, Castiglioni V, Zuccato C. The first reported generation of several induced pluripotent stem cell lines from homozygous and heterozygous Huntington’s disease patients demonstrates mutation related enhanced lysosomal activity. Neurobiol Dis. 2012;46:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 195. | Niclis JC, Pinar A, Haynes JM, Alsanie W, Jenny R, Dottori M, Cram DS. Characterization of forebrain neurons derived from late-onset Huntington’s disease human embryonic stem cell lines. Front Cell Neurosci. 2013;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 196. | Song B, Sun G, Herszfeld D, Sylvain A, Campanale NV, Hirst CE, Caine S, Parkington HC, Tonta MA, Coleman HA. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012;8:259-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 197. | Burkhardt MF, Martinez FJ, Wright S, Ramos C, Volfson D, Mason M, Garnes J, Dang V, Lievers J, Shoukat-Mumtaz U. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2013;56:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 198. | Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4:145ra104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 199. | Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1404] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 200. | Christou YA, Ohyama K, Placzek M, Monk PN, Shaw PJ. Wild-type but not mutant SOD1 transgenic astrocytes promote the efficient generation of motor neuron progenitors from mouse embryonic stem cells. BMC Neurosci. 2013;14:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 201. | Hedlund E, Isacson O. ALS model glia can mediate toxicity to motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 202. | Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 203. | Närvä E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 204. | Gomez-Nicola D, Suzzi S, Vargas-Caballero M, Fransen NL, Al-Malki H, Cebrian-Silla A, Garcia-Verdugo JM, Riecken K, Fehse B, Perry VH. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain. 2014;137:2312-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 205. | Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, Hayama KL, Hazel TG, Johe K, Wu MC. Behavioral and histopathological assessment of adult ischemic rat brains after intracerebral transplantation of NSI-566RSC cell lines. PLoS One. 2014;9:e91408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 206. | Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75:363-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 207. | Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 208. | Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 2014;7:1755-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 209. | Rong Z, Fu X, Wang M, Xu Y. A scalable approach to prevent teratoma formation of human embryonic stem cells. J Biol Chem. 2012;287:32338-32345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 210. | Parsons XH. Constraining the Pluripotent Fate of Human Embryonic Stem Cells for Tissue Engineering and Cell Therapy - The Turning Point of Cell-Based Regenerative Medicine. Br Biotechnol J. 2013;3:424-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 211. | Weerakkody TN, Patel TP, Yue C, Takano H, Anderson HC, Meaney DF, Coulter DA, Wolfe JH. Engraftment of nonintegrating neural stem cells differentially perturbs cortical activity in a dose-dependent manner. Mol Ther. 2013;21:2258-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 212. | Hunt JA, Fok M, Bryan N. Impact of cell purification technique of autologous human adult stem cells on inflammatory reaction. Biomaterials. 2013;34:7626-7631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 213. | Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 214. | Dlouhy BJ, Awe O, Rao RC, Kirby PA, Hitchon PW. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J Neurosurg Spine. 2014;21:618-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 215. | Molcanyi M, Riess P, Bentz K, Maegele M, Hescheler J, Schäfke B, Trapp T, Neugebauer E, Klug N, Schäfer U. Trauma-associated inflammatory response impairs embryonic stem cell survival and integration after implantation into injured rat brain. J Neurotrauma. 2007;24:625-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 216. | McKim JM. Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb Chem High Throughput Screen. 2010;13:188-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 217. | Bitz S. The botulinum neurotoxin LD50 test - problems and solutions. ALTEX. 2010;27:114-116. [PubMed] |
| 218. | Scott CW, Peters MF, Dragan YP. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett. 2013;219:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 219. | Laurenza I, Pallocca G, Mennecozzi M, Scelfo B, Pamies D, Bal-Price A. A human pluripotent carcinoma stem cell-based model for in vitro developmental neurotoxicity testing: effects of methylmercury, lead and aluminum evaluated by gene expression studies. Int J Dev Neurosci. 2013;31:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 220. | Lundqvist J, El Andaloussi-Lilja J, Svensson C, Gustafsson Dorfh H, Forsby A. Optimisation of culture conditions for differentiation of C17.2 neural stem cells to be used for in vitro toxicity tests. Toxicol In Vitro. 2013;27:1565-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 221. | Ma S, Liu H, Jiao H, Wang L, Chen L, Liang J, Zhao M, Zhang X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca(2+) influx. Neurotoxicology. 2012;33:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 222. | Munir M, Lu L, Mcgonigle P. Excitotoxic cell death and delayed rescue in human neurons derived from NT2 cells. J Neurosci. 1995;15:7847-7860. [PubMed] |
| 223. | Eubanks LM, Hixon MS, Jin W, Hong S, Clancy CM, Tepp WH, Baldwin MR, Malizio CJ, Goodnough MC, Barbieri JT. An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proc Natl Acad Sci USA. 2007;104:2602-2607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 224. | Purkiss JR, Friis LM, Doward S, Quinn CP. Clostridium botulinum neurotoxins act with a wide range of potencies on SH-SY5Y human neuroblastoma cells. Neurotoxicology. 2001;22:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 225. | Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999;354:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 226. | Hubbard KS, Gut IM, Lyman ME, Tuznik KM, Mesngon MT, McNutt PM. High yield derivation of enriched glutamatergic neurons from suspension-cultured mouse ESCs for neurotoxicology research. BMC Neurosci. 2012;13:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 227. | Hubbard K, Beske P, Lyman M, McNutt P. Functional evaluation of biological neurotoxins in networked cultures of stem cell-derived central nervous system neurons. J Vis Exp. 2015;e52361. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 228. | Gupta K, Hardingham GE, Chandran S. NMDA receptor-dependent glutamate excitotoxicity in human embryonic stem cell-derived neurons. Neurosci Lett. 2013;543:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 229. | Bai X, Twaroski D, Bosnjak ZJ. Modeling anesthetic developmental neurotoxicity using human stem cells. Semin Cardiothorac Vasc Anesth. 2013;17:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 230. | Bosnjak ZJ. Developmental neurotoxicity screening using human embryonic stem cells. Exp Neurol. 2012;237:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 231. | Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 232. | Li T, Wang W, Pan YW, Xu L, Xia Z. A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation, and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci. 2013;134:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 233. | Sánchez-Alvarez R, Gayen S, Vadigepalli R, Anni H. Ethanol diverts early neuronal differentiation trajectory of embryonic stem cells by disrupting the balance of lineage specifiers. PLoS One. 2013;8:e63794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 234. | Nash R, Krishnamoorthy M, Jenkins A, Csete M. Human embryonic stem cell model of ethanol-mediated early developmental toxicity. Exp Neurol. 2012;234:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 235. | Taléns-Visconti R, Sanchez-Vera I, Kostic J, Perez-Arago MA, Erceg S, Stojkovic M, Guerri C. Neural differentiation from human embryonic stem cells as a tool to study early brain development and the neuroteratogenic effects of ethanol. Stem Cells Dev. 2011;20:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 236. | Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J, Coecke S. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27:2591-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 237. | Choi YS, Lee MC, Kim HS, Lee KH, Park YG, Kim HK, Jeong HS, Kim MK, Woo YJ, Kim SU. Neurotoxicity screening in a multipotent neural stem cell line established from the mouse brain. J Korean Med Sci. 2010;25:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 238. | Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008;105:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 239. | Ylä-Outinen L, Heikkilä J, Skottman H, Suuronen R, Aänismaa R, Narkilahti S. Human cell-based micro electrode array platform for studying neurotoxicity. Front Neuroeng. 2010;3:pii: 111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 240. | Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 241. | Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 242. | Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296-4303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 243. | Dubois-Dauphin ML, Toni N, Julien SD, Charvet I, Sundstrom LE, Stoppini L. The long-term survival of in vitro engineered nervous tissue derived from the specific neural differentiation of mouse embryonic stem cells. Biomaterials. 2010;31:7032-7042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 244. | Preynat-Seauve O, Suter DM, Tirefort D, Turchi L, Virolle T, Chneiweiss H, Foti M, Lobrinus JA, Stoppini L, Feki A. Development of human nervous tissue upon differentiation of embryonic stem cells in three-dimensional culture. Stem Cells. 2009;27:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 245. | Ylä-Outinen L, Joki T, Varjola M, Skottman H, Narkilahti S. Three-dimensional growth matrix for human embryonic stem cell-derived neuronal cells. J Tissue Eng Regen Med. 2014;8:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 246. | Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC. Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput. 1998;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 247. | Brunello CA, Jokinen V, Sakha P, Terazono H, Nomura F, Kaneko T, Lauri SE, Franssila S, Rivera C, Yasuda K. Microtechnologies to fuel neurobiological research with nanometer precision. J Nanobiotechnology. 2013;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 248. | Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769-12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 249. | Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 250. | Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 251. | Karus M, Blaess S, Brüstle O. Self-organization of neural tissue architectures from pluripotent stem cells. J Comp Neurol. 2014;522:2831-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 252. | Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3083] [Cited by in RCA: 3515] [Article Influence: 292.9] [Reference Citation Analysis (0)] |
| 253. | Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci USA. 2013;110:20284-20289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 254. | Vazin T, Becker KG, Chen J, Spivak CE, Lupica CR, Zhang Y, Worden L, Freed WJ. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS One. 2009;4:e6606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 255. | Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y, Park S, Li Y, Bolshakov VY, Lamonerie T. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci USA. 2011;108:9703-9708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 256. | Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med. 2010;4:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |