Published online Jun 26, 2015. doi: 10.4252/wjsc.v7.i5.873
Peer-review started: October 1, 2014
First decision: October 28, 2014
Revised: January 27, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: June 26, 2015
Processing time: 272 Days and 23.1 Hours
AIM: To determine the effects of transplanting osteogenic matrix cell sheets and beta-tricalcium phosphate (TCP) constructs on bone formation in bone defects.
METHODS: Osteogenic matrix cell sheets were prepared from bone marrow stromal cells (BMSCs), and a porous TCP ceramic was used as a scaffold. Three experimental groups were prepared, comprised of TCP scaffolds (1) seeded with BMSCs; (2) wrapped with osteogenic matrix cell sheets; or (3) both. Constructs were implanted into a femoral defect model in rats and bone growth was evaluated by radiography, histology, biochemistry, and mechanical testing after 8 wk.
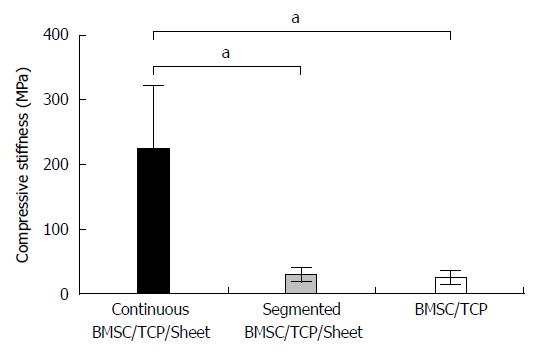
RESULTS: In bone defects, constructs implanted with cell sheets showed callus formation with segmental or continuous bone formation at 8 wk, in contrast to TCP seeded with BMSCs, which resulted in bone non-union. Wrapping TCP constructs with osteogenic matrix cell sheets increased their osteogenic potential and resulting bone formation, compared with conventional bone tissue engineering TCP scaffolds seeded with BMSCs. The compressive stiffness (mean ± SD) values were 225.0 ± 95.7, 30.0 ± 11.5, and 26.3 ± 10.6 MPa for BMSC/TCP/Sheet constructs with continuous bone formation, BMSC/TCP/Sheet constructs with segmental bone formation, and BMSC/TCP constructs, respectively. The compressive stiffness of BMSC/TCP/Sheet constructs with continuous bone formation was significantly higher than those with segmental bone formation and BMSC/TCP constructs.
CONCLUSION: This technique is an improvement over current methods, such as TCP substitution, and is useful for hard tissue reconstruction and inducing earlier bone union in defects.
Core tip: The treatment of bone defects is a common clinical problem for orthopedic surgeons. Artificial bone combined with cultured bone marrow stromal cells (BMSCs) has been examined and applied for the treatment of clinical cases such as small bone defects. In the present study, we developed constructs of BMSCs and beta-tricalcium phosphate (TCP) combined with osteogenic matrix cell sheets (BMSC/TCP/Sheet constructs), which showed a vigorous osteogenic potential compared with conventionally engineered bone tissue structures consisting of BMSC/TCP constructs. The BMSC/TCP/Sheet constructs will be useful for reconstruction of hard tissues, such as bone defects, as new tissue-engineered bone.
- Citation: Ueha T, Akahane M, Shimizu T, Uchihara Y, Morita Y, Nitta N, Kido A, Inagaki Y, Kawate K, Tanaka Y. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J Stem Cells 2015; 7(5): 873-882
- URL: https://www.wjgnet.com/1948-0210/full/v7/i5/873.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i5.873
The treatment of bone defects is a common clinical problem for orthopedic surgeons. Autologous bone grafts including vascularized bone grafts are performed for treatment of osteonecrosis and bone defects in clinical practice[1,2]. However, it is necessary to sacrifice intact bone such as the pelvis or fibula. Moreover, there is limited bone available for autologous transplantation. Artificial bone such as hydroxyapatite (HA) and beta-tricalcium phosphate (TCP) can be applied as a scaffold for the treatment of small bone defects. Although these biomaterials provide a physical matrix for deposition of new bone and may guide the extension of bone, they do not induce ectopic bone formation[3]. To achieve osteogenesis in artificial bone, constructs of artificial bone combined with cultured bone marrow stromal cells (BMSCs), which are designated as engineered bone tissue structures[4-6], have been examined and applied for the treatment of clinical cases such as avascular necrosis of the femoral head and benign tumors[7-9]. However, some cases require a long period of time to replace autologous bone, which subsequently delays bone union.
Previously, we developed a mechanical retrieval method to fabricate cell sheets of cultured BMSCs with osteogenic potential, designated osteogenic matrix cell sheets, that could be transplanted without a scaffold, and resulted in bone formation at subcutaneous sites[9-12]. The utility of transplantation of engineered bone tissue structures for hard tissue reconstruction will increase if such treatment can achieve sufficient bone formation for early bone union after transplantation. In our previous study, we reported that a construct of osteogenic matrix cell sheets and artificial bone results in extensive bone formation compared with a construct containing a BMSC suspension. A construct with osteogenic matrix cell sheets allows bone formation inside of the pores as well as outside of the artificial bone. Outside bone formation can unite two artificial bones by bridging bone formation[11]. These results suggested that transplanting a combination of osteogenic matrix cell sheets and engineered bone tissue structures may advance bone formation for reconstruction of bone defects. Marcacci et al[9] reported the clinical outcomes of long bone repair using macroporous bioceramics with BMSCs. However, they used a BMSC suspension to prepare BMSC/bioceramic composites. Therefore, in the present study, we prepared TCP constructs with or without osteogenic matrix cell sheets and assessed their osteogenic potential after transplantation subcutaneously and at bone defect sites. We compared the bone formation patterns between TCP constructs with or without osteogenic matrix cell sheets.
The experimental procedures were approved by the Animal Experimental Review Board of Nara Medical University. Male Fisher 344 (F344) rats were purchased from Japan SLC (Shizuoka, Japan) for use as donors and recipients. The method for bone marrow cell preparation has been reported previously[12-14]. Briefly, bone marrow cells were obtained from the femoral shafts of 7-wk-old F344 rats by flushing with 10 mL of culture medium. The cells were collected in two T-75 flasks (Costar, Cambridge, MA, United States) containing 15 mL of minimal essential medium (MEM; Nacalai Tesque, Kyoto, Japan) supplemented with 15% fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA, United States) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Nacalai Tesque), and incubated at 37 °C in a humidified atmosphere with 5% CO2. At confluency (approximately day 14), the primary cultured cells were harvested using a 0.25% trypsin/EDTA solution (Nacalai Tesque) to obtain in a cell suspension.
To prepare an osteogenic matrix cell sheet, a cell suspension was seeded at a density of 1 × 104 cells/cm2 in a 100-mm cell culture dish (100 × 20 mm; BD Falcon, NJ, United States) and cultured with medium containing 10 nmol/L dexamethasone (Dex; Sigma, MO, United States) and 82 μg/mL ascorbic acid phosphate (AscP; L-ascorbic acid phosphate magnesium salt n-hydrate; Wako Pure Chemical Industries, Kyoto, Japan) until confluency (approximately 2 wk)[10,13]. The cells were then rinsed twice with phosphate-buffered saline (PBS; Gibco), and the sheet was lifted by a mechanical retrieval method using a scraper. Because the osteogenic matrix cell sheet was sufficiently robust to handle with a scraper and forceps, handling procedures for the cell sheet were performed easily, such as combination of the cell sheet with TCP. Although β-glycerophosphate (β-GP) was added to create osteogenic matrix cell sheets in our initial study[10], recent investigations have revealed that osteogenic matrix cell sheets with high osteogenic potential can be prepared without β-GP[15,16]. Therefore, the osteogenic matrix cell sheets in the present study were created without β-GP.
Sterilized porous TCP ceramics (Osferion; disks: 5 mm diameter and 2 mm thick; cylinders: 5 mm outer diameter, 1 mm inner diameter, 5 mm in length, and 60% porosity) were provided by Olympus Terumo Biomaterials (Tokyo, Japan). After immersion in MEM, the air bubbles in the TCP were aspirated and each ceramic was soaked in a cell suspension (1 × 106 cells/mL) for 2 h in a CO2 incubator at 37 °C[13]. The constructs were then cultured with osteoinductive supplements including Dex, AscP, and 10 mmol/L β-GP (Sigma) for 2 wk in a CO2 incubator at 37 °C to obtain BMSC/TCP constructs.
We prepared three experimental groups according to the type of implant received. The C group received BMSC/TCP constructs, the S group received TCP combined with an osteogenic matrix cell sheet (Sheet/TCP constructs), and the SC group received BMSC/TCP constructs combined with an osteogenic matrix cell sheet (BMSC/TCP/Sheet constructs). Constructs with an osteogenic matrix cell sheet were prepared by simply wrapping the sheet around the constructs just prior to transplantation. The TCP ceramics used for subcutaneous transplantation and bone defect model experiments were the disk and cylinder types, respectively.
Constructs with a TCP disk were implanted subcutaneously onto the back of syngeneic 7-wk-old male rats using a previously described procedure[10,17]. Each group included six disks for one recipient rat as the subcutaneous implantation model. After 4 wk, the implanted constructs were harvested and evaluated radiographically, histologically, and biochemically. For radiographic and histological evaluations, the harvested disks were fixed in buffered formalin (Wako Pure Chemical Industries). After obtaining X-ray images, each disk was decalcified with a KCl solution (K-CX solution; Falma, Tokyo, Japan), embedded in paraffin, cut at the middle of the disk parallel to the base, and then stained with hematoxylin and eosin (HE) for histological evaluation.
The alkaline phosphatase (ALP) activities and osteocalcin contents of harvested constructs were measured as described previously[17,18]. Briefly, each disk was crushed and homogenized using a microhomogenizer in 1 mL of 0.2% Nonidet P-40 (NP-40), and then centrifuged at 12000 rpm for 10 min at 4 °C. A 10-μL aliquot of the supernatant was combined with 56 mmol/L 2-amino-2-methylpropanediol buffer containing 10 mmol/L p-nitrophenylphosphate and 1 mmol/L MgCl2. After incubation for 30 min at 37 °C, 2 mL of 0.2 N NaOH was added to stop the reaction and the ALP activity was calculated by measuring the absorbance of p-nitrophenol released at 410 nm using a spectrophotometer.
After extraction with 0.2% NP-40 in 4 mL of 20% formic acid for 2 wk at 4 °C, osteocalcin was extracted from the sediment. A 0.5-mL aliquot of the formic acid extract was applied to a prepacked Sephadex G-25 column (NAP-5 column; Amersham Pharmacia Biotech AB, Uppsala, Sweden) and eluted with 10% formic acid. The protein fractions were pooled and dried. After dissolution in 0.5 mL of ELISA sample buffer, the osteocalcin content was assayed using a Rat Osteocalcin ELISA Kit (DS Pharma Biomedical, Osaka, Japan)[18].
Bone defects of the femur were created in F344 rats (300-350 g body weight) under anesthesia. Briefly, a lateral incision was made on the hind limb and the vastus muscle was divided longitudinally to expose the femur. The femoral shaft was removed from the distal lesser trochanter to a length of 10 mm to create a bone defect that was filled with a TCP-cylinder construct. The cylinder was fixed with an 18 G needle inserted into the intramedullary femoral shaft to achieve ridged fixation. A BMSC/TCP cylinder (BMSC/TCP construct) was transplanted into the bone defect of the left femur, while a BMSC/TCP cylinder with an osteogenic matrix cell sheet (BMSC/TCP/Sheet construct) was transplanted into the defect of the right femur. To evaluate bridging bone formation, X-ray images were taken under anesthesia at 2, 4, and 8 wk postoperatively.
At 8 wk post-transplantation, the rats were euthanized and both hind limbs were harvested. After removal of the intramedullary pins, micro-CT images were taken using a Microfocus X-ray CT system (SMX-160CTS; Shimadzu, Kyoto, Japan). Five of the harvested femurs were then dissected from the surrounding muscle, fixed in 10% formalin, decalcified with K-CX solution, and embedded in paraffin. For histology, the femurs were cut longitudinally and stained with HE. Eight of the harvested femurs were applied to compression testing using a universal testing machine (5566; Instron, Canton, MA, United States) equipped with a computer for data acquisition. The harvested femur was compressed longitudinally and the primary yield point for fracture of the TCP was calculated. The crosshead speed was 10 mm/min. Compressive stiffness was calculated by dividing the compressive stress by the compressive strain in the linear portion to the primary yield point of the stress-strain curve.
The values for ALP activity, osteocalcin content, and compressive stiffness were calculated as the mean and SD. Statistical significance was determined by one-way ANOVA with post-hoc multiple comparisons using Tukey’s test. A value of P < 0.05 was considered statistically significant.
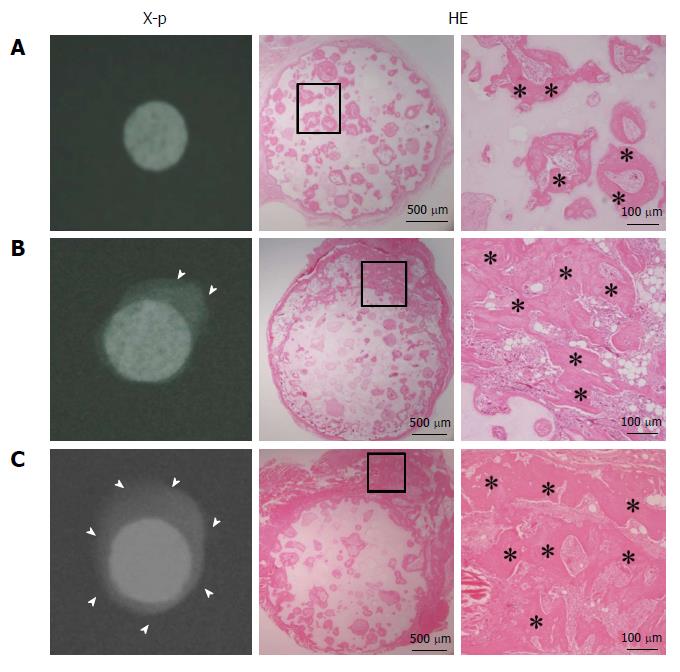
Figure 1 shows the radiographic images and histology (HE staining) at 4 wk after subcutaneous implantation. Abundant calcification around the disks was observed in the radiographic images of the S and SC groups, whereas no calcification was observed in the C group. Histology showed bone formation in all harvested disks. Although the C group showed bone formation only in the pores of the ceramics, the S and SC groups showed bone formation in the pores and around the disks. Less bone formation appeared to be in the central area of the disks in the S group compared with the SC group.
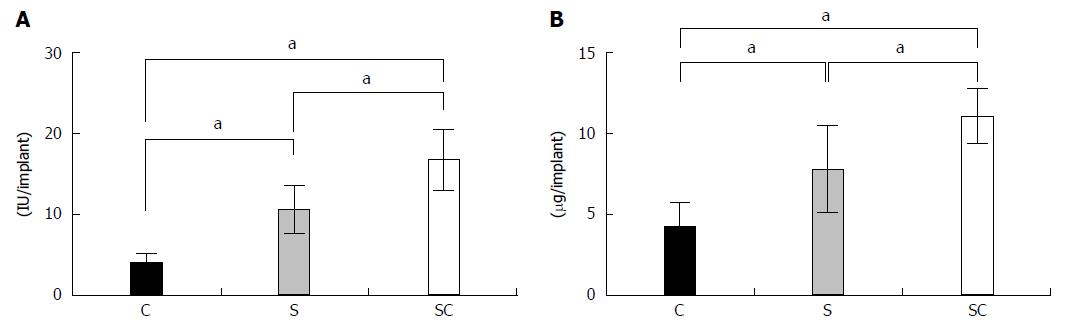
The ALP activities (Figure 2A) and osteocalcin contents (Figure 2B) in the SC group were significantly higher than those in the other groups at 4 wk post-implantation. In the S group, these values were significantly higher than those in the C group.
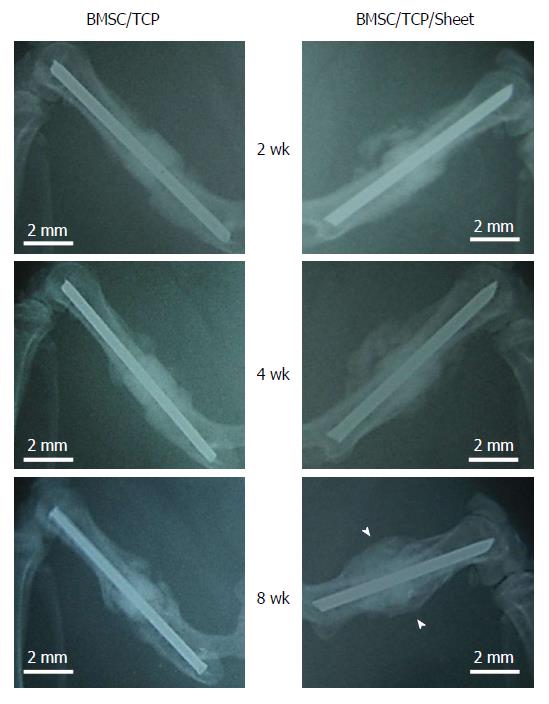
Radiographic images were taken at 2, 4, and 8 wk post-implantation (Figure 3). At 2 wk, obvious callus formation was observed around the implanted constructs with osteogenic matrix cell sheets. At 8 wk post-implantation, callus formation had developed and bridging callus formation between host bones resulted in bone union. In contrast, no bridging callus formation was observed around constructs without osteogenic matrix cell sheets at 8 wk post-implantation.
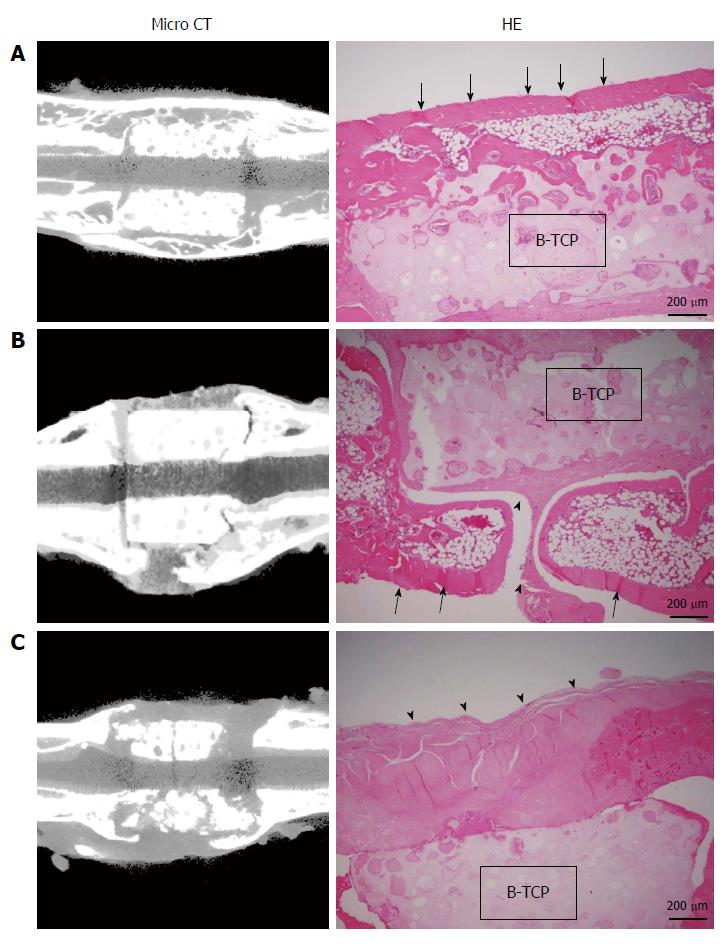
Figure 4 shows the micro-CT images and histology of each construct implanted into femurs at 8 wk. Implanted BMSC/TCP/Sheet constructs showed bone formation around the TCP cylinder (n = 8). However, micro-CT revealed two patterns of bone formation, continuous bone formation to host bones (Figure 4A, n = 4) and segmental bone formation over the cylinder (Figure 4B, n = 4). The ratio of segmental and continuous bone formation among the samples was 1:1. We defined BMSC/TCP/Sheet constructs showing bridging bone formation between host bones across the TCP by micro-CT as continuous bone formation. Bone formation observed around the constructs, but not continuing to the host bones, was defined as segmental bone formation. Bone formation was not observed around implanted BMSC/TCP constructs (n = 8) in which soft tissue interposition between the cylinder and the host bone resulted in non-union (Figure 4C). Micro-CT also revealed that BMSC/TCP/Sheet constructs with either continuous or segmental bone formation were intact, whereas broken TCP cylinders were found in BMSC/TCP constructs (Figure 4C).
Figure 5 shows the results of biomechanical compression testing. The compressive stiffness (mean ± SD) values were 225.0 ± 95.7, 30.0 ± 11.5, and 26.3 ± 10.6 MPa for BMSC/TCP/Sheet constructs with continuous bone formation, BMSC/TCP/Sheet constructs with segmental bone formation, and BMSC/TCP constructs, respectively. The compressive stiffness of BMSC/TCP/Sheet constructs with continuous bone formation was significantly higher than those with segmental bone formation. The stiffness of constructs with segmental bone formation was similar to that of constructs without osteogenic matrix cell sheets (BMSC/TCP constructs).
The present study clearly demonstrated that the osteogenic potential of the BMSC/TCP/Sheet construct was significantly higher than that of the BMSC/TCP construct, namely a conventional engineered bone tissue structure. When the conventional engineered bone tissue structures were combined with an osteogenic matrix cell sheet, the newly formed bone area spread from the center to the surface of the TCP. We observed bridging bone formation over the TCP cylinder implanted between both ends of autologous bones. Moreover, biomechanical examination showed significantly higher strength against compressive stress in the BMSC/TCP/sheet construct with continuous bone formation to host bones, indicating that the BMSC/TCP/sheet construct may be useful for hard tissue reconstruction.
Some scaffolds such as TCP can be replaced with autologous bone by bone remodeling after implantation into bone defects[19]. Although osteogenesis does not occur in artificial bone itself, HA and TCP are common scaffolds for artificial bone in hard tissue reconstruction[20]. Ohgushi et al[21] and Akahane et al[22] reported a transplantation technique using artificial bone combined with cells. The cells combined with artificial bone can be freshly prepared bone marrow cells or cultured mesenchymal stem cells (MSCs). In clinical cases, differentiated osteoblasts have been used in combination with scaffolds for the treatment of patients with osteoarthritis and osteonecrosis[8,9,23]. When a BMSC suspension is combined with such scaffolds, bone formation is observed but only in the pores of the artificial bone. Therefore, when artificial bone is combined with BMSCs for transplantation into a bone defect, soft tissue interposition between the construct and the host bone might occur and result in non-union.
Several studies have reported tissue-engineered bone combined with BMSCs for reconstruction of bone defects[24-26]. Kadiyala et al[26] showed that a critical-size segmental defect of the femur in a canine model can be healed by transplantation of culture-expanded cell-loaded artificial bone. However, defects that received no implant, cell-free artificial bone, or artificial bone loaded with fresh marrow failed to heal at 8 wk. Dallari et al[25] reported critical-size defect reconstruction using BMSCs, platelet-rich plasma (PRP), and freeze-dried bone allografts alone and in combination. They concluded that the combination of freeze-dried bone allografts, BMSCs, and PRP accelerates bone healing and remodeling processes. However, the bone healing was evaluated by radiology, histology, and histomorphometry in these previous studies. In contrast, we evaluated bone reconstruction by mechanical testing (compression testing) as well as micro-CT, radiology, and histology because the mechanical strength of the reconstructed site is an important clinical outcome.
The starting cell numbers were different for the three groups in this study. It would very difficult to control the cell numbers because of the different modalities used for construct preparation. Considering clinical cases of bone defect reconstruction, tissue-engineered bone with a vigorous osteogenic potential should be used for better clinical outcomes. Therefore, we first evaluated the osteogenesis of the three types of constructs, BMSC/TCP, Sheet/TCP, and BMSC/TCP/Sheet, using the subcutaneous implantation model to determine the highest osteogenic construct, and found that the BMSC/Sheet/TCP construct had the most vigorous osteogenic potential. The number of cells that can be combined with artificial bone materials like TCP is limited because suspended cells remain in the pores of the TCP. In contrast, cell sheets can be wrapped around TCP, resulting in a large amount of loaded cells on the TCP. Moreover, the Sheet/TCP constructs showed bone formation on the surface of the construct, which is greatly advantageous for bridging bone formation.
The bone union of conventionally engineered bone tissue structures was improved by combining the construct with an osteogenic matrix cell sheet. The BMSC/TCP construct with an osteogenic matrix cell sheet (BMSC/TCP/Sheet construct) had an excellent ability to form bone, as indicated by bone formation on the TCP surface as well as in the pores of the central area. Bone formation around the TCP was continuous with the host bone when BMSC/TCP constructs with osteogenic matrix cell sheets were implanted into bone defects of the femur. This finding may have occurred because the BMSC suspension entered the pores of the central area and the osteogenic matrix cell sheet was located on the surface of the TCP ceramic. The subsequent combination of osteogenesis by the BMSC suspension and osteogenic matrix cell sheet resulted in vigorous bone formation in the subcutaneous and bone defect sites. Prevention of soft tissue invasion and achieving early bone formation at the bone defect site is clinically important for reconstruction of bone tissue. Accordingly, our transplantation technique using constructs with osteogenic matrix cell sheets is useful because the defect is filled with the osteogenic matrix cell sheet to prevent the invasion of soft tissue, resulting in early bone formation.
MSCs have the potential to replace or regenerate damaged and diseased tissue by multipotent differentiation into osteogenic, chondrogenic, and adipogenic cells among others[4,7,27]. Recently, although the detailed molecular mechanisms remain unclear, it was reported that MSCs, including BMSCs, can interact with cells of the immune system and modulate immune responses in vitro and in vivo[28,29]. MSCs can drive immune responses toward immunosuppression and anti-inflammation. In the present study, we used TCP constructs to repair bone defects in the femur. Scaffolds such as TCP might cause inflammatory responses when they are transplanted in vivo[30]. However, the transplantation model in the present study used a combination of TCP ceramics and cell sheets created from BMSCs, and therefore inflammatory responses could be reduced by the anti-inflammatory function of BMSCs. Further studies are necessary to clarify this point.
We used an osteogenic matrix cell sheet created in a 100-mm cell culture dish to prepare BMSC/TCP/Sheet constructs. When BMSC/TCP/Sheet constructs were transplanted into bone defects of the femur, half of the constructs (four of eight constructs) formed a segmental callus. Similar to the TCP cylinders, the mechanical properties of constructs with segmental bone were still fragile. This result indicates that coverage of the entire TCP cylinder with an osteogenic matrix cell sheet is important for continuous bone formation around the TCP cylinder. Two or more sheets or a larger sheet can be used to cover the TCP surface and increase the compressive stiffness. Further studies will establish the numbers of sheets needed to cover certain sizes of TCP cylinders.
A thermosensitive cell culture plate is commonly used to fabricate cell sheets[31]. Memon et al[32] used culture dishes coated with thermosensitive polymers to fabricate cell sheets for implantation into a heart disease model and for clinical treatment of corneal surface dysfunction[33]. However, other methods have been reported, such as those using magnetite nanoparticles and magnetic force[34]. Anil Kumar et al[35] reported a method for preparation of cell sheet/HA constructs. In their study, they showed rapid and complete cellularization of HA by wrapping it with a cell sheet. Gao et al[36] reported the results of osteogenic cell sheet transplantation using rabbit MSCs. They used osteogenic cell sheets to generate bone grafts, but did not find any bone formation on the outer HA surface in their model. We[10,13] and others[24,37] reported a method to prepare osteogenic cell sheets cultured in osteogenic medium. Our mechanical retrieval method only requires the use of regular cell culture equipment, including a cell scraper, to fabricate the osteogenic matrix cell sheet, in which cells form the sheet during culture with Dex and AscP. On the other hand, the methods of Anil Kumar et al[35] and Gao et al[36] require specialized culture dishes coated with thermosensitive polymers. The osteogenic matrix cell sheets used in this study were sufficiently robust to handle with forceps. The cell sheet could be easily handled for processes such as combination with artificial bone, and has osteogenic potential for osteoblastic differentiation induced by Dex and AscP. Therefore, we propose that this technique can be applied to bone defects that occur because of tumor resection or wounding.
In our study, there are a few limitations to be acknowledged. First, we only used TCP cylinders of 5 mm in length. We need to conduct further studies with longer TCP cylinders to assess their bone formation ability for cell sheet transplantation. Second, the experimental period in the present study was relatively short. Therefore, a further follow-up study is needed to determine whether the implanted TCP is replaced by autologous bone. Third, the TCP was transplanted into a bone defect site that was created by bone resection. We need to assess whether the cell sheet can form bone tissue when it is applied to a bone defect in a region with a poor blood supply, such as a large bone defect with soft tissue injury after severe trauma. Finally, we need to show the osteogenic ability using human BMSCs for future clinical application.
In conclusion, the present study clearly demonstrates that constructs of BMSCs and TCP combined with osteogenic matrix cell sheets show a vigorous osteogenic potential compared with conventionally engineered bone tissue structures consisting of a BMSC/TCP construct. Because bridging bone formation over the implanted TCP can unite the ends of the cylindrical TCP to autologous bone, the construct will be useful for hard tissue reconstruction as new tissue-engineered bone.
We thank F Kunda and M Matsumura (Nara Medical University) for their technical assistance. The TCP disks were kindly provided by Olympus Terumo Biomaterials Corp., Tokyo, Japan. We did not receive any monetary compensation or financial support from the company.
Bone defects are difficult to treat. Effective treatment options for bone defects have been reported that include tissue engineering of bone tissue structures using bone marrow stromal cells (BMSCs). Recently, many researchers have reported methods for creating cell sheets, such as thermo-responsive polymer-grafted culture dishes and mechanical retrieval.
Tissue engineering for regenerative medicine using cell sheets are becoming much more common. The transplantation of cell sheets created from BMSCs will most likely be incorporated into clinical cases in the near future. Osteogenic matrix cell sheets have the potential to greatly advance the treatment of critical size bone defects.
Constructs comprised of BMSCs, tricalcium phosphate (TCP) and osteogenic matrix cell sheets show an increased osteogenic potential compared with conventionally engineered bone tissue structures consisting of a BMSC/TCP construct. Bridging bone formation by placing osteogenic matrix cell sheets over the implanted TCP can unite the ends of the cylindrical TCP to autologous bone.
The construct will be useful for hard tissue reconstruction, such as critical bone defects after tumor resection or traumatic injuries and avascular necrosis of femoral head, by acting as new tissue-engineered bone.
Osteogenic matrix cell sheets are cell sheet created from BMSCs. The cell sheet is lifted by a mechanical retrieval method using a scraper. Beta-TCP is one of the most commonly used artificial bone in clinical treatments.
The article describes about the application of tricalcium phosphate and osteogenic matrix cell sheet for bone defect reconstruction. The efficacy of bone formation with matrix cell sheets, BMSC, and beta-tricalcium phosphate has been examined. Although the result section should be described more in detail, this study provides helpful insights for understating overall regenerative medicine technique.
P- Reviewer: Yao CL, Tanabe S S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Kawate K, Yajima H, Sugimoto K, Ono H, Ohmura T, Kobata Y, Murata K, Shigematsu K, Kawamura K, Kawahara I. Indications for free vascularized fibular grafting for the treatment of osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2007;8:78. [PubMed] |
| 2. | Chen CC, Lin CL, Chen WC, Shih HN, Ueng SW, Lee MS. Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Joint Surg Am. 2009;91:2390-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Letić-Gavrilović A, Scandurra R, Abe K. Genetic potential of interfacial guided osteogenesis in implant devices. Dent Mater J. 2000;19:99-132. [PubMed] |
| 4. | Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res. 1996;32:333-340. [PubMed] |
| 5. | Ito Y, Tanaka N, Fujimoto Y, Yasunaga Y, Ishida O, Agung M, Ochi M. Bone formation using novel interconnected porous calcium hydroxyapatite ceramic hybridized with cultured marrow stromal stem cells derived from Green rat. J Biomed Mater Res A. 2004;69:454-461. [PubMed] |
| 6. | Iida J, Yoshikawa T, Akahane M, Ohgushi H, Dohi Y, Takakura Y, Nonomura A. Osteogenic potential of cultured bone/ceramic construct: comparison with marrow mesenchymal cell/ceramic composite. Cell Transplant. 2004;13:357-365. [PubMed] |
| 7. | Matsushima A, Kotobuki N, Tadokoro M, Kawate K, Yajima H, Takakura Y, Ohgushi H. In vivo osteogenic capability of human mesenchymal cells cultured on hydroxyapatite and on beta-tricalcium phosphate. Artif Organs. 2009;33:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, Kobata Y, Shigematsu K, Kawamura K, Tamai K. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960-962. [PubMed] |
| 9. | Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947-955. [PubMed] |
| 10. | Akahane M, Nakamura A, Ohgushi H, Shigematsu H, Dohi Y, Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Akahane M, Ueha T, Shimizu T, Inagaki Y, Kido A, Imamura T, Kawate K, Tanaka Y. Increased osteogenesis with hydroxyapatite constructs combined with serially-passaged bone marrow-derived mesenchymal stem cells. Stem Cell Discovery. 2012;2:133-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Akahane M, Shigematsu H, Tadokoro M, Ueha T, Matsumoto T, Tohma Y, Kido A, Imamura T, Tanaka Y. Scaffold-free cell sheet injection results in bone formation. J Tissue Eng Regen Med. 2010;4:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Akahane M, Ueha T, Shimizu T, Shigematsu H, Kido A, Omokawa S, Kawate K, Imamura T, Tanaka Y. Cell sheet injection as a technique of osteogenic supply. Int J Stem Cells. 2010;3:138-143. [PubMed] |
| 15. | Inagaki Y, Uematsu K, Akahane M, Morita Y, Ogawa M, Ueha T, Shimizu T, Kura T, Kawate K, Tanaka Y. Osteogenic matrix cell sheet transplantation enhances early tendon graft to bone tunnel healing in rabbits. Biomed Res Int. 2013;2013:842192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Shimizu T, Akahane M, Ueha T, Kido A, Omokawa S, Kobata Y, Murata K, Kawate K, Tanaka Y. Osteogenesis of cryopreserved osteogenic matrix cell sheets. Cryobiology. 2013;66:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Tohma Y, Ohgushi H, Morishita T, Dohi Y, Tadokoro M, Tanaka Y, Takakura Y. Bone marrow-derived mesenchymal cells can rescue osteogenic capacity of devitalized autologous bone. J Tissue Eng Regen Med. 2008;2:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Chazono M, Tanaka T, Komaki H, Fujii K. Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. J Biomed Mater Res A. 2004;70:542-549. [PubMed] |
| 20. | Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990;72:298-302. [PubMed] |
| 21. | Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48:913-927. [PubMed] |
| 22. | Akahane M, Ohgushi H, Yoshikawa T, Sempuku T, Tamai S, Tabata S, Dohi Y. Osteogenic phenotype expression of allogeneic rat marrow cells in porous hydroxyapatite ceramics. J Bone Miner Res. 1999;14:561-568. [PubMed] |
| 23. | Ohgushi H, Kotobuki N, Funaoka H, Machida H, Hirose M, Tanaka Y, Takakura Y. Tissue engineered ceramic artificial joint--ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials. 2005;26:4654-4661. [PubMed] |
| 24. | Geng W, Ma D, Yan X, Liu L, Cui J, Xie X, Li H, Chen F. Engineering tubular bone using mesenchymal stem cell sheets and coral particles. Biochem Biophys Res Commun. 2013;433:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, Cenni E, Baldini N, Cenacchi A, Bassi A. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877-888. [PubMed] |
| 26. | Kadiyala S, Jaiswal N, Bruder S. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3:173-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2010;109:277-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375-392. [RCA] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Fellah BH, Josselin N, Chappard D, Weiss P, Layrolle P. Inflammatory reaction in rats muscle after implantation of biphasic calcium phosphate micro particles. J Mater Sci Mater Med. 2007;18:287-294. [PubMed] |
| 31. | Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, Kobayashi J, Chen G, Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939-4946. [PubMed] |
| 32. | Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333-1341. [PubMed] |
| 33. | Yang J, Yamato M, Nishida K, Hayashida Y, Shimizu T, Kikuchi A, Tano Y, Okano T. Corneal epithelial stem cell delivery using cell sheet engineering: not lost in transplantation. J Drug Target. 2006;14:471-482. [PubMed] |
| 34. | Shimizu K, Ito A, Yoshida T, Yamada Y, Ueda M, Honda H. Bone tissue engineering with human mesenchymal stem cell sheets constructed using magnetite nanoparticles and magnetic force. J Biomed Mater Res B Appl Biomater. 2007;82:471-480. [PubMed] |
| 35. | Anil Kumar PR, Varma HK, Kumary TV. Rapid and complete cellularization of hydroxyapatite for bone tissue engineering. Acta Biomater. 2005;1:545-552. [PubMed] |
| 36. | Gao Z, Chen F, Zhang J, He L, Cheng X, Ma Q, Mao T. Vitalisation of tubular coral scaffolds with cell sheets for regeneration of long bones: a preliminary study in nude mice. Br J Oral Maxillofac Surg. 2009;47:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Ma D, Ren L, Liu Y, Chen F, Zhang J, Xue Z, Mao T. Engineering scaffold-free bone tissue using bone marrow stromal cell sheets. J Orthop Res. 2010;28:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |