INTRODUCTION
The initial few days following fertilization of the human egg, all stem cells in the developing egg are able to create any tissue in the human body, i.e., the stem cells are totipotent. However, about four days following the fertilization of a human egg, the stem cells in the blastocyst begin to differentiate and become pluripotent instead of totipotent, thus being able to differentiate into a more limited set of adult cell types[1]. At this point in time, many different stem cell types are beginning to form that will have unique function related to the development, maintenance, and healing of various tissues throughout the body. The degree to which stem cells differentiate into specific adult phenotypes is only recently beginning to be understood. For example, cell types, such as the progenitor cell preadipocyte and adipose-derived mesenchymal stem cells, each of which was previously classified as one cell type, have now been shown to have phenotypic differences depending on the location of the preadipocyte niches or mesenchymal stem cell niches[2,3]. The signaling factors controlling the development and function of the stem cell types, and indeed the signaling factors that each stem cell type releases, are relatively unknown, but progress is being made. For example, we know that adult stem cells release hundreds of types of proteins within the molecular pool[4], called the stem cell released molecules (SRM), and that each cell type will release a unique pool of molecules[5,6]. These molecules in the SRM will prove to be important for developing many types of therapeutics, including, for example, immunoregulators for organ transplantation[7].
The more differentiated the stem cell, the more specialized the SRM will become. Further, that unique pool of molecules from one stem cell type can change in composition, including the types of molecular species, depending on intrinsic and extrinsic regulatory factors. For example, intrinsic factors related to simple passage number of a stem cell will change the composition of the SRM[8,9] and mesenchymal stem cells (MSCs) in different parts of the body will secrete unique pools of SRM[10]. Telomere dysfunction, whether the cause is intrinsic or extrinsic, will change the nature of the SRM[11]. Likewise, when MSCs derived from fat tissue are conditioned with TNF-α, a significant effect on the SRM is observed with an increased release of factors such as Cathepsin L, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1, matrix metalloproteinase (MMPs), and pentraxin-related protein 3[12]. Further, signaling conditions during the immune modulating responses of human MSCs through Toll-like receptors (TLRs) on the MSCs leads to two basic phenotypic changes of the cells (MSC1 and MSC2) and a consequent dramatic difference in their SRM[13]. Phenotypic changes in the MSC are consequent to: (1) low-level exposure of TLR4 agonists that drives hMSCs toward a pro-inflammatory MSC1 phenotype important for early injury responses; and (2) the TLR3 agonist exposure of hMSCs driving the phenotype to an immunosuppressive MSC2 phenotype that is important to later anti-inflammatory responses that help repair the wound. Culture conditions can also have dramatic effects on SRM. A significant increase in SRM [Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), phosphatidylinositol-glycan biosynthesis class F protein, and TGF-β] was observed after subjecting hMSCs to 72 h hypoxia compared with normoxic conditions[14]. Serum deprivation is another model for ischemia, and was shown to increase the secretion of angiogenic factors released by hMSCs, although the results could have been attributed in full, or part, to differences in cell proliferation rates[15]. Glucose levels have been shown to differentially affect the phenotype of endothelial progenitor cells and mesenchymal progenitor cells[16]. Indeed, subtle variations in cell culture conditions can have significant consequences to the phenotype of stem cells[17].
The state of the extracellular matrix in the stem cell niche is also an important regulator of stem cell phenotype, where, for example, the absence of the SPARC protein in the extracellular matrix (ECM) can drive hematopoietic stem cells into a state of quiescence[18]. Antioxidants and FGF-2 were shown to cause rapid proliferation and a retention of stem cell properties in MSCs, and even enhanced their adipogenic and osteogenic potentials[19]. Interestingly, new studies suggest that adult stem cells, and even somatic cells, may exist in a state of dynamic transition between different levels of potency[20,21] that is dependent on many factors, including paracrine and autocrine factors in the SRM from surrounding stem cells in the same niche, and through the physical state of the surrounding stem cell niche[22]. The state of the oxidative stress in the stem cell may be a contributing factor in phenotype, including the state of pluripotency where the antioxidants curcumin and sesamin were shown to decrease oxidative stress and increase pluripotency[23]. Stem cell derived control factors for determining the fate of stem cells and the potency of cells, including the dedifferentiation of somatic cells, their proliferation, and subsequent differentiation, may include growth differentiation factor 11, a protein secreted by bone marrow mesenchymal stem cells[24] that has been shown to be involved in stem cell fate and proliferation[25], and has recently been shown to induce a number of regenerative effects, including neurogenesis[26]. Recent studies also demonstrate that NANOG, a pluripotency transcription factor in embryonic stem cells, is also present in at least some adult tissues further raising the possibility that a dynamic state of pluripotency is a naturally occurring process in adults[27]. Again, these shifts in the state of pluripotency will have concomitant shifts in the composition of the SRM released from the stem cell.
Given the differentiation of stem cells into distinct phenotypes, each of which releases a distinct pool of molecules with each distinct pool of molecules inducing a specific set of functions, a knowledge base of the secreted factors (SRM) from each stem cell type and the resulting actions from each pool of molecules will be instructive in the development of therapeutics. The resulting therapeutics that can be developed using the combination of many types of molecules has been termed “systems therapeutics”[28]. The “systems therapeutic” approach, where multiple molecule types target multiple pathways, is in contradistinction to the more traditional approach of small molecule development for perturbation of one pathway.
Moreover, as the stem cell types are cultured ex vivo in the laboratory and driven to state-dependent specific phenotypes through genetic, epigenetic, and other state-dependent variables, the concentration and composition of the SRM as a result can be experimentally manipulated for the purposes of therapeutic development. In addition, distinct pools of SRM from two or more stem cell types cultured in state-dependent conditions can be combined into a collective pool of molecules called S2RM, mimicking the collective actions of multiple stem cell types in their native state in the human body.
TWO OR MORE TYPES OF STEM CELL INDUCE HEALING
There are two basic forms of cell replacement and regeneration: (1) a maintenance function where renewal of damaged cells during tissue homeostasis (homeostatic growth) are restored; and (2) a response to external injury, such as traumatic wounding, burn, ulceration, or surgery. A given healing response will require many processes acting through a well-orchestrated concert of mechanisms and molecules in the given tissue, and the results of these processes depend on many factors, including the developmental age of the organism. Fundamental factors, such as caspases released from cells undergoing apoptosis, will activate both stem cells and progenitor cells in the wound healing process[29], where apoptosis may be a key factor in cell proliferation during tissue regeneration[30]. Wounds occurring in early to mid-gestational fetal skin have been shown to heal through regeneration without the formation of a scar[31], whereas adult wounds heal by a fibroproliferative response that emphasizes repair over regeneration. The complexity of this process, where fetal wounds differ from adult wounds in inflammatory responses, ECM components, growth factor expression and responses, and profiles of gene expression is exemplified by the observation that adult skin in a fetal environment will still exhibit scar formation[32]. The state dependency of stem cells is so critical as shown in diabetes where the adipose stem cell niche in situ is altered, and the stem cells in the diabetic state are compromised in their ability to establish a vascular network both in vitro and in vivo[33] where glucose itself has profound direct effects on stem cells[34].
Following injury, wound healing begins rapidly and involves resident and migratory stem cell types, ECM, and soluble factors, including SRM. Stem cells and progenitor cells resident in the skin are certainly involved, but recruitment of stem cells from other sources, including bone marrow, is thought to be important[35]. The mechanisms underlying wound healing include: (1) a rapid release of pro-inflammatory mediators; (2) cell to cell, and cell to extracellular matrix interactions that help mediate cell proliferation, migration, and differentiation; (3) a cascade of events including epithelialization, fibroplasia and angiogenesis[36]; (4) contraction of the wound; and (5) remodeling of the tissue. These events begin at the time of physical injury and proceed continuously throughout the process of tissue repair. Although the processes of repair begin immediately after an injury in all tissues, and all wounds proceed with a similar cascade of healing, some tissues, for example, liver, skeletal tissue, and the eye have different forms of regeneration and repair with variations on the underlying mechanisms[37]. Severe injury has been shown to increase the number of circulating stem cells[38,39] and that these stem cells will participate in the wound healing process[6].
At the onset of trauma bone marrow stem cells will sense histamine released from platelets at H1 receptors and change their phenotype to one of releasing more IL-6 and more IL-8. The increased IL-8 will attract polymorphonuclear neutrophil cells, and the increased IL-6 will facilitate their survival through antiapoptotic functions[40]. When the trauma inducing the injury has stopped, and hemostasis is achieved with an immune response activated, the tissue repair phase will then begin[41]. On the third day after wounding the proliferative phase starts and continues for two or more weeks thereafter. Proliferation begins with fibroblast migration and deposition of newly synthesized ECM, elaborating the initial network of tissue built by fibrin and fibronectin. This phase of wound healing can be clinically observed as an abundant formation of granulation tissue. The complex nature of the proliferative phase is briefly described below[42].
First, fibroblasts and myofibroblasts in the tissue surrounding the wound are stimulated to proliferate for 3 d[43]. The fibroblasts and myofibroblasts then migrate from the surrounding tissue into the wound, attracted by soluble factors TGF-β and platelet-derived growth factor (PDGF) that are released by platelets and inflammatory cells[44]. Appearing in the wound on the third day after injury, the accumulation of fibroblasts in the wound requires their phenotypic modulation. Within the wound, the fibroblasts greatly proliferate, producing and locally releasing the matrix proteins hyaluronan, fibronectin, proteoglycans, and type 1 and type 3 procollagen[45]. Abundant new ECM has accumulated at the end of the first week, further supporting cell migration that is essential for the repair process. Fibroblasts then change to a myofibroblast phenotype. The myofibroblast phenotype contains thick actin bundles that extend below the plasma membrane with pseudopodia attaching to fibronectin and collagen in the ECM. Wound contraction, critical to the reparative process by closing the wound margins, then takes place as the actin bundles begin to retract. Any overabundance of unneeded fibroblasts are then eliminated by apoptosis[44].
The three phases of wound healing involve MSCs to varying degrees, whereby, for example, they recruit macrophages to the wound site[46], induce the fibroblast response to injury[47], and remodel the wound site[48], including a preferential release of collagen type III at the site[35]. While the MSCs contribute directly to wound repair by releasing molecules such as collagen to the wound[35], the MSCs also act indirectly by releasing an instruction set to other cells thus initiating, for example, progenitor cell migration to the wound[49]. The MSCs are key to the wound’s ability to proceed beyond the inflammatory phase and not maintain a chronic wound state. A significant early component of the mechanism of action of MSCs is their attenuation of the inflammatory response. The addition of MSCs to an active immune response decreases secretion of the proinflammatory cytokines TNF-α and interferon-γ (IFN-γ) while simultaneously increasing the production of anti-inflammatory cytokines IL-10 and IL-4. These anti-inflammatory properties of MSCs impart a particular benefit to chronic wound treatment through SRM, given the SRM can restart healing in chronic wounds by advancing the wound past a chronic inflammatory state into the next stage of healing.
Many studies have shown that MSCs possess antimicrobial activity, critical for clearance of infection in the wound. The antimicrobial activity of MSCs is mediated by two mechanisms: (1) direct secretion of antimicrobial factors such as LL-37[50]; and (2) indirect, by secreting immune-modulating SRM that will upregulate the bacterial killing and phagocytosis of immune cells[51]. Further, the phenotype of macrophages can be regulated by MSCs into various M1 and M2 classes directed to either antimicrobial, phagocytic activity, or one of controlling inflammation[52].
Endogenous MSCs migrate to sites of injury in response to chemotactic signals where they can then modulate inflammation, repair damaged tissue, and facilitate tissue regeneration. Furthermore, bone marrow stem cells home to the injury where cells in the wounded area secrete a protease that interacts with collagen matrix to produce a homing agent[53]. Differentiation and paracrine signaling are two key mechanisms used by MSCs for tissue maintenance and repair. While differentiation of MSCs contributes by directly regenerating damaged tissue, the paracrine signaling by MSCs regulates the local cellular responses to injury, including the differentiation process itself. However, studies of exogenous MSCs show that the contribution of differentiation of these stem cells is limited due to poor engraftment and survival of MSCs at the site of injury, whereas the activation of endogenous stem cells by SRM may provide better results for the differentiation pathway[54], Paracrine signaling by MSCs appears to be the primary mechanism for the beneficial effects of MSCs in wound healing, including the reduction of inflammation, enhanced angiogenesis, and induction of cell migration and proliferation[55].
An analyses of the conditioned medium indicate that MSCs secrete many known SRM mediators of tissue repair including growth factors, cytokines, and chemokines, including VEGF, PDGF, bFGF, EGF, keratinocyte growth factor (KGF), and TGF-β. Stem cells are also known to release exosomes[56], and exosomes from mesenchymal stem cells have been shown to contain factors, including miRNA, that switch cancer stem cells into a dormant state[57]. Such a mechanism is important to dampen the cells in a wound from moving into a state of cancer[58]. Many cell types, including epithelial cells, endothelial cells, fibroblasts, and keratinocytes are responsive to MSC paracrine signaling, where a number of different cellular responses including cell survival, proliferation, migration, and gene expression are regulated. The SRM from MSCs acts as a chemoattractant for dermal fibroblasts, macrophages, endothelial cells, and epidermal keratinocytes, in vitro. The presence of either MSCs or the SRM from MSCs have been shown to promote wound closure through the activation of dermal fibroblasts. MSCs also secrete mitogens, leading to the proliferation of keratinocytes, dermal fibroblasts, and endothelial cells in vitro. Further, dermal fibroblasts secrete increased amounts of collagen type I and alter gene expression in response to either MSCs in co-culture or the SRM from MSCs. These data suggest that SRM from MSCs stimulate proliferation and migration of the key cell types in the wound. In addition, the SRM of MSCs imparts anti-scarring properties to wound healing through the secretion of VEGF and hepatocyte growth factor (HGF), and through maintaining a normal balance between TGF-β1 and TGF-β3. The pathways underlying MSC processes in wound healing are complex, and further details of these processes can be found in recent reviews. Stem cell niches in other regions of the body, including the hematopoietic stem cell niche, appear to be equally complicated as the skin stem cell niche with a rich interaction amongst many cell types, including a number of stem cell types and their respective SRM[59].
NATURALLY INDUCED PLURIPOTENT STEM CELL WITHIN THE STATE DEPENDENT STEM CELL NICHE
Natually occurring endogenous iPSs, or naturally induced pluripotent stem cells (NiPSs) occur within the state dependent stem cell niche. While induction to a totipotent state has not been realized, dedifferentiation seems to be an important adaptive mechanism in both the animal[60] and plant kingdoms[61] where cells can be induced to become pluripotent. In addition to the therapeutic development of embryonic stem cells and iPSs, the use of adult stem cells and the molecules that they release have been intensively investigated and have current therapeutic applications. Further, SRM from stem cells or other molecules from neighboring cells, such as ciliary neurotrophic factor (CNTF), have been shown to dedifferentiate myoblasts into multipotent progenitor cells. The dedifferentiated myoblasts were then able to differentiate into several new phenotypes[62].
The endogenous mechanisms of adult stem cells, and possibly somatic cells in the stem cell niche, seem to include the ability to reprogram themselves into more primordial states that are pluripotent. That is, the adult stem cell, and even somatic cells, may exist in a state of dynamic transition between different levels of potency that is dependent on many factors, including paracrine and autocrine factors in the SRM from surrounding cells in the stem cell niche, and by the physical, chemical, and electrical state of the stem cell niche[63-65]. Recently, treatment with reversine, a type of purine, transformed 3T3-L1 preadipocytes into MSC-like cells, as evidenced by the expression of MSCs marker genes. The transform allowed differentiation of lineage-committed 3T3-L1 preadipocytes to osteoblasts under the osteogenic condition in vitro[66]. Beyond transcription factors contained in the SRM, physical manipulation through the cytoskeleton is known to transmit signals to the chromatin[67] and reprogram cells[68], and may represent an additional biophysical, in addition to biochemical means, for driving cells to varying levels of potency. Reprogramming of differentiated cells to stem-like cells has been described in several tissues and is well studied in the epithelial-mesenchymal transition where a differentiated epithelial cell transforms to a mesenchymal cell with a stem cell-like phenotype. Thus, by understanding adult stem cell function, we may develop the means to use these cells in many ways to maintain and heal the body, including a means of controlling naturally occurring iPSs (NiPSs).
The physical, chemical, and electrical state of the stem cell niche will have profound influences on stem cell function. Alterations of the stem cell niche in diseases such as diabetes will decrease the ability of endogenous stem cells, or autologous administered stem cells, to increase neovascularization and promote wound healing[33].
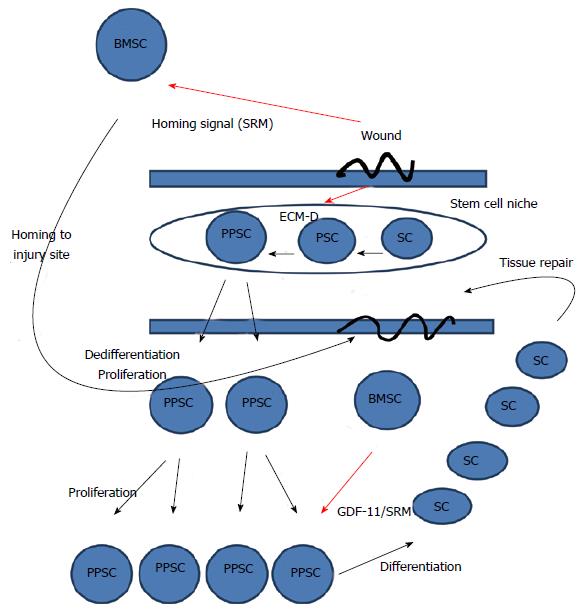
In Figure 1, we see levels of interactions that may control the natural iPSC state. Considering wound healing as described in the aforementioned section, many factors, such as histamine, an important regulator of cell fate, including neurons[69,70] are released at the site of injury. As an example of the actions of these factors, histamine will activate TRPM4 calcium channels in the mesenchymal stem cells and bias the dynamic transition of the stem cells toward differentiation into the needed mature cells types at the injury site[71], including osteoclastogenesis[72]. Similarly, exposure to sunlight will stimulate vitamin D3 levels and induce differentiation of stem cells, doing so through a downstream pathway that includes histamine[73].
Figure 1 General model of wound healing.
The wounded state sends a homing signal to bone marrow stem cells and disrupts the ECM. Disruption of the ECM will shift the dynamic transition of potency towards dedifferentiation and the more pluripotent state. The more pluripotent state will cause the cells to proliferate. After proliferation, the migration of bone marrow stem cells to the wound site will release stem cell released molecules, including GDF-11, that stops proliferation and induces differentiation allowing newly differentiated somatic cells to repair the tissue. Thus, in our model, GDF11 is released from BMSCs and is a master regulator of stem cell transcription that inhibits cell proliferation and migration by down-regulating the expression of numerous genes involved in both these processes[93]. ECM-D: Extracellular matrix disruption; SC: Somatic cell; PPSC: Pluripotent stem cell; BMSC: Bone marrow stem cell; PSC: Potent stem cell.
Reprogramming of cells to push the dynamic transition towards more potency has been specifically shown in mammalian cells whereby muscle cells[74] and pancreas cells[75] will dedifferentiate into a more pluripotent state following injury, and where fibroblasts were incubated in the SRM of adipose-derived stem cells. The fibroblasts displayed gene expression that was indicative of pluripotency in which repressive histone modifications were reduced, and increased global demethylation was present. The Col1a1 and Col1a2 genes, typically found in differentiated cells only, demonstrated reduced expression, and also demonstrated increased methylation in the 5′-flanking regulatory regions[76]. Of the many factors released by mesenchymal stem cells, microRNA is one of the factors that have been shown to induce pluripotency in mouse and human somatic cells[77]. In general, stress is a key factor that can naturally induce pluripotency. For example, simple isolation of mammalian cells from contact with other cells and their normal niche, originally exhibiting a limited differentiation potential, may become multipotent[78]. Pluripotent cells can reside in the naïve state or the primed state where the naïve state is more potent than the primed state[79]. Dedifferentiation under hypoxic conditions can drive committed cells beyond the primed state fully back to the naive state of potency where the pluripotent cells are then capable of forming teratomas[80].
Cancer cells and pluripotent stem cells follow certain common rules. Both cell types, when placed in a dysregulated extracellular matrix, will exhibit an increased state of potency. Cancer cells, when returned to a regulated ECM, will revert to a normal phenotype[58,81]. Likewise, dedifferentiation of cells into a pluripotent state can occur when the cell is isolated and loses connections with other cells and the ECM[78], and stem cells that have differentiated can revert to a more pluripotent state by changes in the concentration of the ECM associated protein, L-proline[82]. Thus, induction of pluripotent stem cells is a naturally occurring phenomenon that can be controlled in vivo for therapeutic effect by manipulating the state of the stem cell niche.
CONCEPTS OF A SYSTEMS THERAPEUTIC
An abnormality in one pathway, or even at one level of the organism, such as at the level of genes, does not explain a disease. Rather, disease reflects the perturbations of the complex system of biological pathways acted on by a complex set of environmental regulators. Most previous work to understand disease and drug response traits have focused on single dimensions, and even single pathways, of the system. Achieving a more comprehensive and predictive understanding of disease and drug response requires examining living systems in multiple dimensions and at multiple scales. Although biological engineering principles are necessary, with the requirement to remove superfluous complexity for the development of a particular therapeutic, the individual components of complex systems are highly coupled such that the individual components cannot be analyzed in isolation. This predicament in biology, such as the desire to place the sequencing of the genome as the singular predictor of disease, is similar to that dictum in physics where electrodynamics was broken down into the misbegotten particles and fields theorem by Bohr and his Copenhagen interpretation[83]. Biological complexity is an extreme example of complexity, arising from a biological system that includes active, plastic components, nested feedback loops, flexible design principles, component multi-functionality, and multiple layers of system dynamics developed through evolutionary processes that are, at least partially, driven through the downward causation of environmental regulators. The power of the dynamic biological system has been recognized in engineering where, for example, neuromorphic engineering[84] has become an important player in the development of new computer chip technologies such as TrueNorth[85].
Despite the use of systems analysis in the fields of biology and therapeutic development, therapeutic development has often remained as one using systems biology techniques for finding the one pathway, or the one target, that is best perturbed to develop the therapeutic. “Finding the magic bullet” is a common phrase that describes this common problem. A shift in mindset to one of finding the minimum set of pathways, or the minimum set of targets, using the “minimum molecule set” to perturb these targets in order to best develop a therapeutic is now needed. Thus, biological function results from a system, and a particular disease state is the result of multiple perturbations in that system, not just one perturbation. Therefore, through an understanding of complex pathways in normal and disease states, and using computationally intensive biological design-build-test-analyze cycles, with therapeutic molecule production batches based on this process, we can hope to develop safe and efficacious therapeutics. This will occur through a multi-targeted, “systems therapeutic” approach. The approach then is to use a system of molecules, the minimum molecule set, that is not overly reductionist so as to be ineffective, but instead use the optimal number of molecule species that are sufficient to realize a safe and efficacious therapeutic. Recognizing that diseases are the result of complex interactions among many networks has significant implications for drug discovery, leading to the design of combinations of molecular species that impact entire network states, rather than designing reductionist drugs that target specific genes that are associated, often weakly, with disease.
DEVELOPMENT OF SYSTEMS ANTIMICROBIALS
The attempt to develop animal-derived antimicrobials is not new. For example, in the 1990s great hope, and many dollars spent, was placed on the development of a small peptide from frog (Xenopus laevis) skin as an antibiotic[86]. The observation that frog skin heals itself, despite the frog living in a very septic environment, led to the formation of Magainin Pharmaceuticals. After years, and millions of dollars, spent on development and Phase II clinical trials, today Magainin’s assets are the auction block (Magainin changed names to Genera and then liquidated: http://www.fiercebiotech.com/press-releases/genaera-corporation-announces-approval-plan-liquidation-and-dissolution-board-direc-0). Why? Because the frog’s skin does not heal itself through a reductionist approach with only one molecule (a peptide), and Magainin didn’t fully learn the frog’s lesson. The lesson not learned was that Magainin developed their antibiotic based on one peptide, a reductionist approach, instead of a mix of antimicrobial factors, a systems antimicrobial approach.
Lipids were first demonstrated by Koch[87] to have antibiotic activity, and exists in human skin, for example, as a wide range of molecule types comprising a significant part of the innate immune system[88]. Like Magainin, a similar reductionist approach was used in the development of squalamine, a lipid compound (aminosterol) derived from the dogfish shark (Squalus acanthias). Squalamine was initially discovered on the basis of its anti-bacterial activity, and has broad spectrum antimicrobial activity against fungi, protozoa, and many viruses[89]. Sadly, isolated squalamine was never approved for antimicrobial use and is now sold as a nutritional product by a number of companies in capsule form. Once again, the “Copenhagen reductionist” approach to therapeutic development has failed us. Here again, instead, an approach to developing antimicrobials using a collection of molecules, including peptides and lipids, is in development.
DEVELOPMENT OF CANCER SYSTEMS THERAPEUTIC
Cancer is strongly associated with a deregulated ECM[58,90]. While cancer and stem cells are regulated by many factors, both cancer cells and pluripotent stem cells follow certain common rules such as regulation by the ECM. Both cell types, when placed in a dysregulated extracellular matrix, will exhibit an increased state of potency. Cancer cells, when returned to a regulated ECM, will revert to a normal phenotype[58,81]. As beautifully explained by Mina Bissell at University of California Berkeley, during development, cells can spatially arrange themselves, differentiate, and change their SRM composition in response to a variety of signals in the microenvironment, including morphogens, biophysical manipulation, juxtacrine signals, and the ECM. All of these components in the microenvironment are sensitive to signals from other tissues and organs of the developing embryo as well as through downward causation from the macroenvironment. However, following organ formation, the microenvironment/ECM integrates and constrains the organ architecture and function, thus ensuring structural and functional homeostasis and therefore, a normal organ phenotype. However, when the organ architecture in adults is insulted by mutations and/or changes in the microenvironment such as ECM dysregulation and/or inflammation, that organ is transformed by the initiation of developmental and embryonic circuits. However, in the adult, the microenvironment is no longer embryonic in nature, and the ECM dysregulation and inflammation leads to a pluripotent state, i.e., the cancerous state. Bissell argues that tumors become new evolutionary organs searching for homeostasis[58]. Recent work fits the paradigm of Bissell, such as that of Liou et al[91] who describes the detailed steps that Kras-mutated acinar cells follow as they change into duct-like cells with a more potent state. They observed that Kras proteins in the acinar cells switched on intercellular adhesion molecule-1. This in turn attracts macrophages. The macrophages then release a variety of proteins, including MMPs that degrade ECM. Following dysregulation of ECM, the acinar cells then transform into the stem cell-like phenotype. Thus, the direct link between Kras mutations, the inflammatory environment, and dysregulated ECM that drive the initiation of pancreatic cancer is demonstrated.
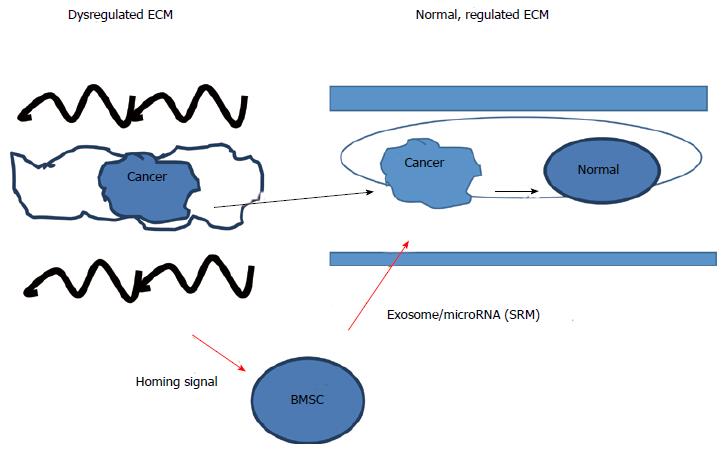
Similar to the cancer state, dedifferentiation of cells into a pluripotent state can occur when the cell is isolated and loses connections with other cells and the ECM[78], and stem cells that have differentiated can revert to a more pluripotent state by changes in the concentration of the ECM associated protein, L-proline[82]. Given that that the ECM can act through mechanical and biochemical mechanisms to regulate the cancer phenotype, one important means to revert the cancer phenotype to the normal somatic cell phenotype is to use S2RM technology to reestablish a normal ECM microenvironment for the cancer cell. That is, using one progenitor cell type to release the building blocks of the ECM, such as collagen, and using another stem cell type to release other building blocks and the instruction sets to build the architecture of the ECM, the normal state of the ECM can be rebuilt and lead to the reversion of the cancer cell phenotype to a more normal somatic cell phenotype as depicted in Figure 2. Thus building on the model developed by Mina Bissell, our model suggests that that the microenvironment/ECM, which is largely comprised of, and developed by S2RM, is key to the regulation of the initiation and degree of pluripotency of cells, controls the “stop” signals for driving potency and then initiates differentiation of the pluripotent stem cells. The S2RM thus controls homeostasis whereby the state of dynamic transition between different levels of potency[92] is set to a proper level in which to produce enough new cells to maintain and heal tissue, but not too much so as to allow uncontrolled, cancerous growth of the tissue.
Figure 2 Regulation of the cancer/pluripotent phenotype by stem cells and extracellular matrix.
The cancer/pluripotent cell phenotype can be regulated by the extracellular matrix (ECM) and stem cells, where cancer cells can be removed from a dysregulated ECM and placed into a normal ECM and the cancer/pluripotent phenotype will revert to a normal, somatic cell phenotype. Likewise, if a dysregulated ECM is reconstructed into a normal state, the cancer/pluripotent phenotype will revert to the normal somatic cell phenotype. Further regulation of the cancer/pluripotent phenotype can be regulated by a number of factors, including microRNA contained within exosomes that were released from mesenchymal stem cells serving to change the state of the cancer cell into one of quiescence. MBSC: BM-stroma cell.
In summary, the S2RM technology provides a natural means for mimicking and stimulating the healing properties of the human body. Instead of using foreign molecules, natural molecules are used that will induce the initiation of natural processes with little or no side-effects. Further, instead of using a small molecule approach where one molecule interacts at one pathway underlying a multi-pathway disease is used, here the S2RM approach uses multiple molecules to perturb multiple pathways underlying the disease, thus yielding a more efficacious result than the one molecule-one pathway reductionist approach.
The S2RM approach will introduce all of the needed molecules to the tissue to induce a full wound healing cascade of events, unlike an approach using the molecules from one stem cell type that will introduce only a portion of the needed molecules and thus provide a fraction of the efficacy that the S2RM provides. And, S2RM uses the particular molecules from the particular stem cells types relevant to the particular tissue to be healed. This is distinct from the “one size fits all” approach where one stem cell type is used to develop therapeutics for the whole body. Therefore, S2RM provides all of the building blocks, such as the different collagen types, to rebuild the tissue, and also provides the instruction set molecules, such as microRNA, that will deliver the needed architectural commands that will lay the building blocks in their proper places for that particular tissue. During this rebuilding process, the immune response will also be modulated by S2RM, so that inflammation is quelled, allowing the rebuilding to proceed within a normalized framework that is not swollen. The S2RM rebuilding process institutes two fundamental stem cell healing processes: (1) Mimicking the actions of multiple stem cell types and the molecules that they release in the relevant tissue, and (2) reconditioning the endogenous stem cell niche itself and driving the niche to a more primordial, potent state, allowing endogenous stem cell processes to better induce a healing response. Thus, a systems therapeutic approach using multiple molecules from multiple stem cell types called S2RM is used to develop a safer, more natural, and more efficacious therapeutic that both mimics and facilitates the natural adult stem cell healing processes of our body.