INTRODUCTION
Autologous cells obtained from adult patients might deliver a less difficult route to regenerative-cell therapies. In the past, many tissues were assumed incapable of self-regeneration upon its damage, mainly because they do not possess any endogenous stem cells. However, recent discoveries show that more adult tissues harbor cells with capacity for regenerative repair[1]. Tissue engineering comprises of suitable cells, scaffolds and appropriate biochemical/physical factors, such that it function as a strategy towards the engineering of tissues and cellular delivery. Encapsulating stem cells within biocompatible scaffolds provide a matrix which result in cell adhesion, migration and differentiation suitable for transplantation and tissue regeneration[2-4].
Stem cells have uncovered a new perspective as therapeutic tools in regenerative medicine. These cells with the ability to proliferate indefinitely (in vitro) and the capacity to differentiate into any somatic cell type are a potential cell source. Stem cells are originated in the early embryo, the fetus, placenta, umbilical cord, and it occurs in many different tissues of the body. Also they have been engineered from somatic cells, termed as induced pluripotent stem cells (iPSCs). iPSCs give rise to different cell types including the neural cell types (neurons, oligodendrocytes, and glia), cardiomyocytes, osteoblasts, hepatocytes, and haemopoietic progenitors cells[5-8]. The two main stem cell types are the embryonic stem cells (ESCs) and the adult stem cells. ESCs are derived from fetal tissue or from the inner cell mass of the blastocytes with ability for unlimited growth in culture that could be associated to a high risk of teratoma formation. Several researches have been done on ESCs pluripotency to obtain specific cell lineages for tissue engineering and regenerative medicine, as well as for therapeutic treatments[8-13]. Adult stem cells are specialized cells including the mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and neural stem cells (NSCs)[9]. MSCs are self-replicating cells that are capable of differentiating in multidirectional pathways, resulting in cell lineages such as the osteoblasts, chondrocytes, myocytes, marrow stromal cells, tendon-ligament fibroblasts, adipocytes, neural cells, oligodendrocytes and haemopoietic cells[9,14,15]. These cells have been used for the engineering of different tissues such as the cardiac[16] , tendon[17] , cartilage[18-20] , vascular[11,21] Meniscus[22], bone[23], Ligament[24], myocardia[25], fat[26], and neural tissues[5,16,17,27].
While a routine source of human MSCs is the bone marrow, they have been also derived from multiple adult tissues comprising of adipose tissue (ADSCs), umbilical cord blood, placenta, thymus, and dental pulp[28]. In comparison to MSCs derived from the bone marrow[29], ADSCs have several advantages such that they are easy to obtain after minor donor site morbidity, possess high proliferating capacity and can be preserved for longer period of time in cell banks[30]. Under appropriate culture conditions, ADSCs can differentiate to classical mesenchymal lineages, which include adipogenesis, chondrogenesis, osteogenesis, and myogenesis[31,32]. Moreover, the ability of ADSCs differentiation to numerous lineages when seeded into polymeric scaffolds has been well documented in several literatures[33,34].
The sources of HSCs are bone marrow, peripheral blood, umbilical cord blood, fetal hematopoietic system[35], ESCs and embryonic germ cells[11]. These cells are also a suitable cell source for blood vessel engineering[12]. Avoidance of anaesthesia without the need for hospitalization or blood transfusion, and low risk of serious adverse events are the major advantages of the peripheral stem cells, which make them the favourable source of stem cells worldwide.On the other hand, NSCs are defined as self-renewing multipotent progenitors existing in the developing and adult CNS. Generally, they are considered by their capacity to symmetrically self-renew and their ability to discriminate into neurons, oligodendrocytes, and astrocytes through asymmetrical fate-committed division[27]. Neural stem or progenitor cells can be obtained in different approaches such as direct isolation of these cells from embryonic or adult brain tissue[36,37]. NSCs have been used for tissue engineering of different tissues such as multiple cell types of the adult nervous system[5,38]. It was shown in several studies that stem cells behavior such as their adherence, proliferation and differentiation on scaffolds, depends on the material itself. Additionally, the surface chemistry, surface geometry/micro architecture of scaffolds, and the mechanical properties of scaffolds[39-42] play a significant role in determining the cellular responses after culture of stem cells on them[43].
Therefore, recent studies have focused on the selection of an appropriate biomaterial, whit focusing on the surface modification of the scaffolds, creating appropriate microstructures and controlling the mechanical properties of scaffolds to recapitulate the in vivo microenvironment suitable for regenerating tissues or for the differentiation stem cells to specific cell lineages[44].
This review will discuss on the influence of different aspects of scaffolds including scaffold composition, surface modification, micro-nano architecture of scaffolds and mechanical properties of scaffolds pertaining to stem cells differentiation. An emphasis is also given to the effect of mechanical properties of scaffolds towards stem cells differentiation.
EFFECT OF SCAFFOLD COMPOSITION ON STEM CELLS DIFFERENTIATION
The interaction of stem cells with their surrounding microenvironment is fundamental to multiple processes such as cell migration, proliferation, lineage specificity, and tissue morphogenesis[45]. Biomaterials play an important role in directing tissue growth and chemical properties of the scaffolds have been shown to influence the behavior of stem cells whereas the scaffold composition has an significant role in stem cells differentiation towards preferred lineages[42,43,45]. The ability to selectively guide stem cells differentiation by merely changing the properties of an underlying biomaterial scaffold is a smart approach in tissue engineering, which can help compliment or potentially eliminate the use of exogenous differentiation inducers like the viral gene vectors and small molecule drugs[46].
Natural polymers such as the collagen, fibrinogen, hyaluronic acid, glycosaminoglycans, cellulose, chitosan, silk fibroin, etc., consist of components found in the native extracellular matrix, such that they can assist cells to attach to cell surface receptors and provide a physical environment to control cell function compared to synthetic materials[40,47]. However these materials suffer from fast degradation rates, difficulty in sterilization and purification, high variability, high contamination potential, and induce immune response upon implantation, while synthetic materials offer the potential for improved control, repeatability and safety[40,47]. Scaffolds fabricated by synthetic materials can be chemically modified by natural materials to carefully regulate the differentiation of stem cells[47]. Immune response is an important concern in synthetic biomaterials used for scaffold fabrication and it can be reduced by selection of materials which are inert inherently or by modification of scaffolds to avoid detection by the immune system. Hydrophobic materials usually tend to increase monocyte adhesion compared to hydrophilic materials leading to a local immune reaction at the scaffold site[48].
Awad et al[34] compared the chondrogenic differentiation of human adipose-derived adult stem cells seeded in alginate, agarose and gelatin scaffolds and their findings revealed that the growth and differentiation potential of adult stem cells seeded on the scaffolds varied and the cells on gelatin scaffolds showed better proliferation and differentiation. Collagen has attracted much interest as a biomaterial for fabrication of scaffolds in tissue engineering as a key structural protein found in the extracellular matrix of many connective tissues[49,50]. In spite of the several advantages of collagen as a biodegradable, biocompatible, safe and multifunctional material, its potential to evoke immune response has raised concerns as an animal-derived biomaterial[51].
Meinel et al[52] investigated the osteogenic differentiation of human MSCs on different protein substrates including unmodified collagen with fast degradation rate, cross-linked collagen with slow degradation rate and silk; in both static and dynamic culture conditions. Under dynamic condition, MSCs on cross-linked collagen and silk scaffolds deposited more calcium and had a higher alkaline phosphatase activity than MSCs on unmodified collagen scaffolds indicating the importance of scaffold properties in causing osteogenesis of the cultured MSCs[52].
Jäger et al[39] cultivated MSCs on the D,D,L,L-polylactide (PLLA), collagen I/III, and polygalactin-910/polydioxanone (PGPD) scaffolds and their results showed significant differences in the proliferation, differentiation, and Ca2+ accumulation of MSCs on different scaffolds. Cell adhesion on PGPD was lower compared to the cell adhesion on PLLA and collagen I/III scaffolds, which was related to the significant biodegradation rate of PGPD scaffolds compared to other scaffolds. They found significantly higher cell numbers on collagen I/III membrane compared to that for PLLA scaffolds after 4 h cell culture while the differences were less significant after 28 d of cell seeding. Lower cell number on PLLA scaffolds compared to collagen I/III after 4 h could be attributed to the hydrophobic nature of PLLA while an increasing deposition of serum proteins from culture media on the PLLA scaffold may reduce the effect of its hydrophobic nature and decreased the quantitative differences in cell attachment between collagen I/III and PLLA scaffolds after 28 d cell seeding[39].
PEG is a relatively inert ,biocompatible and hydrophilic polymer[53]. Hwang et al[41] encapsulated MSCs in PEG based hydrogels containing type I collagen, type II collagen and hyaluronic acids (HA) which are the main components of musculoskeletal tissue matrix. Chondrogenic differentiation of MSCs was slightly enhanced in collagen-matrix-based hydrogels, whereas osteogenic differentiation was induced in HA-containing hydrogels suggesting the potential of ECM components to modulate the fate of MSCs differentiation[41].
Ravichandran et al[54] fabricated a composite scaffold containing PLLA and Poly-benzyl-L-glutamate (PBLG), a polymer of glutamic acid in which the carboxyl groups have been benzoylated, by electrospinning. Nanohydroxyapatite (n-HA) was also deposited on the surface of the nanofibers by calcium-phosphate dipping method. Their results proved that the incorporation of PBLG along with n-HA deposition on the surface of scaffolds promoted greater osteogenic differentiation of ADSCs in the absence of an induction medium highlighting the chemical composition of the scaffold as a determinant factor towards ADSCs differentiation[54].
Shah et al[46] reported selective differentiation of NSCs into oligodendrocytes by seeding NSCs in PCL nanofibrous scaffolds coated by graphene oxide (GO). Higher concentration of GO was found to promote NSCs differentiation into mature oligodendrocytes without introducing differentiation inducers in the culture media. Their results showed that the presence of GO in the structure of nanofibrous scaffolds was a crucial factor that determined the stem cell-scaffold interactions in directing NSCs towards oligodendrocyte differentiation[46].
One of the major challenges in cartilage and disc-tissue engineering using MSCs is the rapid expression of type X collagen as a marker of chondrocyte hypertrophy associated with endochondral ossification. Nelea et al[55] used polypropylene and nylon-6 polymers for the fabrication of scaffolds and modified the surface of scaffolds by glow discharge plasma treatment in ammonia gas to investigate the potential of each polymer in its abilityto inhibit the expression of type X collagen. They cultured MSCs on modified and un-modified polypropylene and nylon-6 and evaluated the chondrogenic differentiation pathway using aggrecan and types I, II, and X collagens. Their results showed that MSCs did not adhere to unmodified PP while they observed cell adhesion on modified PP, nylon 6 and unmodified nylon 6 and concluded that the nature of the surface should be vitalfor the interaction of MSCs. Both modified and un-modified nylon-6 suppressed the expression of type X collagen, while the modified polypropylene scaffold almost completely inhibited its expression indicating the impact of polymer type and surface characteristics on the fate of stem cells[55].
Hosseinkhani et al[56] encapsulated DNA nanoparticles into collagen sponge reinforced with poly (glycolic acid) fiber for bone tissue engineering. More osteogenic differentiation of MSCs and formation of homogeneous bone upon implantation of scaffolds containing DNA nanoparticles was observed by these researchers[56].
In yet in another study, semi-interpenetrating polymer networks made of collagen type I fibers combined with fibronectin or laminin were fabricated to investigate the effect of matrix composition towards ESCs differentiation. Results showed that scaffold composition has an important role in ESCs development and differentiation whereas the presence of fibronectin in collagen scaffolds strongly induces endothelial cell differentiation and vascularization. On the contrary, laminin stimulated ESCs to differentiate into beating cardiomyocytes[57]. Costa-Pinto et al[58] prepared chitosan-poly (butylene succinate) scaffolds in different weight ratios of chitosan (50%, 25%, and 0%) and compared the potential of osteogenic differentiation of human bone marrow stromal cells on these scaffolds. Their results revealed higher cell viability, adhesion, proliferation, and osteogenic differentiation on scaffolds with more ratios of chitosan[58]. Overall, it was clear that the scaffold material and its properties played a critical role in directing the stem cells towards differentiation, with proteins and peptides had a positive influence towards this effect.
EFFECT OF SURFACE MODIFICATION OF SCAFFOLDS ON STEM CELLS DIFFERENTIATION
Cell-biomaterial interactions has a significant role on the growth and differentiation of stem cells within scaffolds[34]. It is well known that the surface properties of scaffolds have an important role in stem cells- biomaterials interactions, ultimately controlling the stem cell adhesion, proliferation, and differentiation after its attachment on the surface[59,60]. Stem cells differentiation can be regulated by presenting suitable biological or chemical signals within the structure of the scaffolds[47]. Therefore the chemical and biological modification of the scaffolds can affect stem cells behavior which is also defined by the substrate properties and their degradation rate, eventually manipulating the signal transduction pathways in stem cells[47]. It can be concluded that the surface chemistry of materials can affect protein adsorption and the binding of different integrins and influence the cell behavior[59]. Surface modification of scaffolds is therefore an important issue in tissue engineering in order to control the cellular behavior[59]. More recently different methods have been established for the modification of scaffolds thorough incorporation of biomolecules within the scaffold structure by method like simple physical adsorption or by covalent conjugation[47,61].
Ayala et al[45] demonstrated the influence of interfacial matrix hydrophobicity on stem cells behavior. They fabricated tunable synthetic scaffolds with control over the hydrophobicity of the scaffolds, by copolymerizing acrylamide with acryloyl amino acids and their results showed that the small changes in matrix hydrophobicity can dramatically alter the cell-matrix interaction and can influence various cellular behaviors[45].
Chua et al[62] investigated the effect of surface-functionalized polyethersulfone (PES) scaffolds including the hydroxylated, carboxylated and aminated PES nanofibers and films and their results showed that the surface-bound amino groups highly influenced the proliferation and differentiation of hematopoietic stem/progenitor cells. The effect of coating of polycaprolactone-co-lactide scaffolds with collagen I (coll I) and coll I/chondroitin sulfate (CS) on osteogenic differentiation potential of ovine bone marrow MSCs has been investigated. The results showed surface modification of scaffolds using coll I/CS induce osteogenic differentiation of cells, without using any differentiation supplements such as dexamethasone, revealing the osteoinductive characteristics of the modified scaffolds[63].
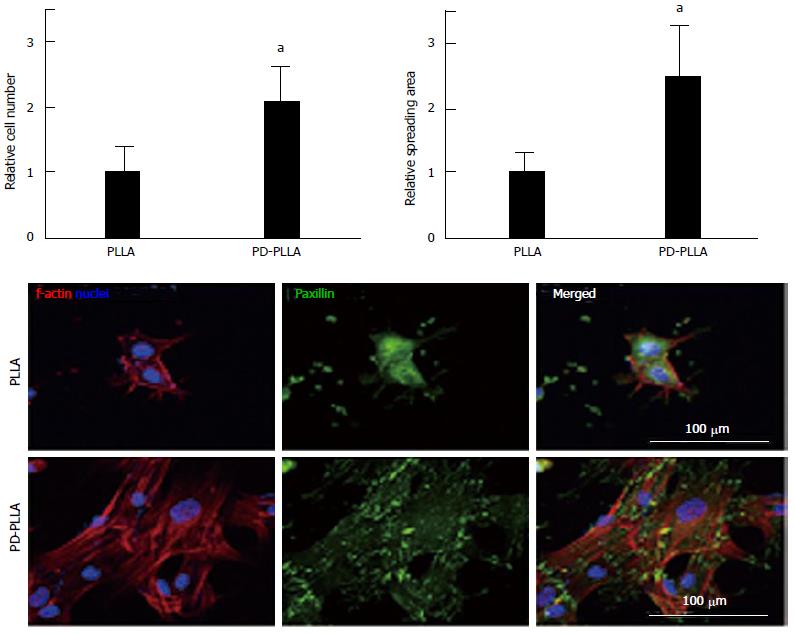
Rim et al[64] fabricated electrospun PLLA nanofibrous scaffolds and coated scaffolds with polydopamine through immersion in an aqueous solution of dopamine with mild shaking for 1 h. Their results showed more adhesion and proliferation of MSCs cultured on surface modified nanofibrous scaffolds with dopamine relative to cells seeded on unmodified scaffolds (Figure 1). More osteogenic differentiation as well as angiogenesis was also observed for MSCs seeded on modified scaffolds compared to unmodified scaffolds[64].
Figure 1 Quantitative analysis of initial cell adhesion from human mesenchymal stem cells cultured on the fibers.
Relative adherent cell numbers and spreading area of human mesenchymal stem cells (hMSCs) cultured on PLLA and PD-PLLA fibers were analyzed after 12 h of culture. aP < 0.05, PD-PLLA vs PLLA group. Adherent morphology of hMSCs on PLLA and PD-PLLA fibers was observed by confocal microscopy. Scale bars represent 100 μm. Reproduced with permission from Rim et al[64]. PLLA: Poly(l-lactide); PD-PLLA: Poly(l-lactide) (PLLA) fibers coated with polydopamine.
In yet another study, electrospun PCL nanofibers were coated using polydopamine by simple immersion of substrates in an alkaline dopamine solution. RE-1 silencing transcription factor (REST) was then absorbed onto PCL nanofibers coated with polydopamine to induce scaffold-mediated gene knockdown for enhanced neuronal differentiation of neural stem/progenitor cells. The results showed significant enhanced neuronal commitment and decreased glial cells differentiation due to presence of the silencing of REST[44]. Controlled release of insulin-like growth factor I (IGF-I) from silk fibroin scaffolds for chondrogenic differentiation of human MSCs has been studied by Uebersax et al[65] and the results showed that IGF-I loaded silk fibroin scaffolds have the potential to provide chondrogenic stimuli to human MSCs, thus beneficial for cartilage repair.
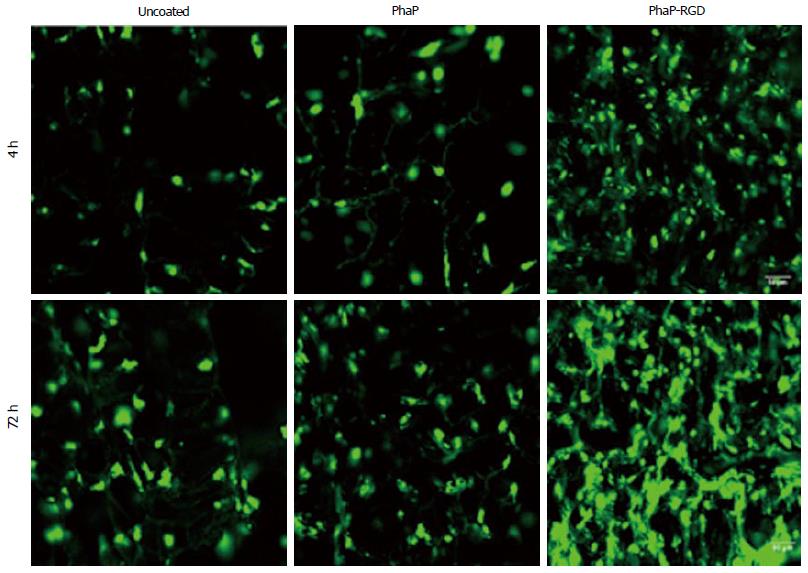
In yet another study, copolyester of 3-hydroxybutyrate-cohydroxyhexanoate (PHBHHx) scaffolds were fabricated and coated with PHA granule-associated proteins (PhaP) and PHA granule-associated proteins PhaP fused with RGD peptide (PhaP-RGD) for investigating the effect of surface modification towards chondrogenic differentiation of human bone marrow mesenchymal stem cells (hBMSCs). Their results showed that the surface modification of scaffolds with PhaP-RGD promote chondrogenic differentiation of hBMSCs compared to PhaP coated or uncoated scaffolds even without the presence ofchondrogenic induction medium (Figure 2). Hyaline cartilage regeneration, and inhibited fibrocartilage formation in hBMSCs derived chondrocytes was also observed on PhaP-RGD coated PHBHHx scaffolds indicating suitability of this substrate for cartilage tissue engineering compared to the uncoated ones[61].
Figure 2 Confocal microscopic imaging of human bone marrow mesenchymal stem cells grown in uncoated, PhaP and PhaP-RGD coated 3-hydroxybutyrate-cohydroxyhexanoate scaffolds after 4 or 72 h of incubation, respectively.
Phalloidin-fluorescein isothiocyanate was used to F-actin of cells grown in the scaffolds (Green). Reproduced with permission from You et al[61]. PhaP-RGD: PhaP binding protein fused with arginyl-glycyl-aspartic acid.
In yet another study, poly (ethylene glycol) (PEG) hydrogels were functionalized with heparin and osteogenic differentiation of human MSCs was evaluated. The results through increased ALP production and gene expression of osteopontin and collagen type I showed that functionalization of hydrogel with heparin induce osteogenic differentiation which is likely due to improvement of cell-scaffold interactions due to the presence of heparin[66]. Surface modification of poly-(lactic-co-glycolic acid) (PLGA) (75:25) scaffolds with RGD peptides also enhanced the human osteoprogenitor differentiation and osteogenesis[67]. While RGD peptide immobilized macroporous alginate scaffolds showed enhanced chondrogenesis properties of human MSCs compared to the un-modified ones[68].
In vitro osteogenic differentiation of human MSCs was also observed on RGD peptide functionalized PLLA nanofibersby Paletta et al[69]. Their results showed more osteogenic differentiation of human MSCs on modified scaffolds revealing the osteoinductive effect of the scaffolds functionalized with RGD[69].
Kuo et al[70] fabricated PLGA /chitosan scaffolds and functionalized it with type I collagen, whereby these researchers were able to improve the cell adhesion and viability on PLGA/chitosan/collagen scaffolds. Moreover, MSCs differentiated towards osteoblasts in the modified scaffolds without induction procedures, while neural differentiation was observed on the scaffolds by the induction MSCs with neuron growth factor (NGF)[70].
In another study Yang et al[71] fabricated porous poly l-lactide-co-ε-caprolactone (PLCL) and did surface modification via crosslinking of chitosan on the surface of scaffold. Their finding showed elongated morphology of MSCs on modified scaffolds while cells on unmodified scaffolds showed more spherical morphology with lower spreading. Moreover, the surface modified scaffolds provide surfaces for early differentiation of MSCs with more in vitro cartilage tissue formation revealing more condrogenic differentiation of MSCs on modified scaffolds compared to the unmodified PLCL scaffold[71].
In yet another study, Budiraharjo et al[72] obtained carboxymethyl chitosan scaffolds (CMCS), coated with hydroxyapatite and compared the behavior of osteoblasts and human MSCs on both modified and un-modified scaffolds. Coating the scaffold with HA substantially enhanced the osteogenic differentiation of the human MSCs.
Surface modification using plasma has been frequently used to improve surface properties of scaffolds fabricated with synthetic polymeric materials through formation of functional groups on the surface[73]. Lin et al[74] investigated the effect of modification of PLLA scaffolds by gas plasma towards the differentiation of ADSCs and their results showed that the cells seeded on modified scaffolds displayed significantly increased differentiation into endothelial cells[74].
Wang et al[60] fabricated porous nanocrystalline HA/chitosan scaffolds using a lyophilization technique, further treated them with cold atmospheric plasma as a simple, quick, and inexpensive method to modify the surface of scaffolds. Their results showed enhanced stem cell growth and osteogenic differentiation on modified scaffolds, which might be due to higher amounts of protein adsorption on the scaffold surfaces due to improves surface roughness and wettability after its modification[60].
An ideal bone implant should recruit osteoblasts or progenitor cells from the adjacent native tissue and induce cell proliferation and osteogenic differentiation for new bone formation. Hu et al[75] modified Ti6Al4V (TC4) implants using biofunctional multilayer coating of gelatin, chitosan, gelatin and human recombinant BMP2. In vitro results showed that such modifications of the implant surface stimulated the adhesion and osteogenic differentiation of MSCs and in vivo results showed improved induction of new bone formation at the interface of bone and implant and the integration of the implant within the surrounding living tissues[75] .
EFFECT OF SCAFFOLD TOPOGRAPHY ON STEM CELLS DIFFERENTIATION
The topography of the extracellular matrix is known to influence cell attachment, morphology, proliferation and differentiation[76]. Topography can also enhance differentiation of progenitor cells into their programmed pathways[77].
Topographic surfaces with micro and nano scale structures have been shown to stimulate the cell alignment, polarization, elongation, migration, proliferation, gene expression, etc., while the mammalian cells have been shown to react to nanoscale structures on a synthetic surface[77,78]. Nano scale topographies are obtained by different fabrication methods such as electrospinning, polymer phase separation, photolithography, chemical vapor deposition, electron beam lithography, etc.[2]. The similarity of electrospun nanofibers to the morphology of native extracellular matrix has attracted interest in the application of these scaffolds as a substrate for stem cell attachment and differentiation[2].
Smith et al[79] compared the in vitro osteogenic differentiation of ESCs on nanofibrous scaffolds and traditional scaffolds with two and three dimensional structure without the nanofibrous features. The results showed that the nanofibrous features enhanced the osteogenic differentiation and mineralization of ESCs compared to traditional substrates. Moreover, the osteogenic differentiation was observed on nanofibrous scaffolds without osteogenic supplements, while cells on other scaffolds required osteogenic supplements and growth factors for osteogenic differentiation[79].
Ragetly et al[80] evaluated the chondrogenic differentiation of MSCs on chitosan microfibrous scaffolds and chitosan sponges. More chondrogenesis differentiation was observed on chitosan microfibers compared to that on sponges indicating the effect of scaffold microstructure towards the cell behavior[80].
Christopherson et al[81] evaluated the effect of topographical features of nanofibrous scaffolds in view point of fiber diameter on rat adult neural stem/progenitor cell (rNSC) differentiation and proliferation. They fabricated different nanofibrous scaffolds with average fiber diameters of 283 ± 45 nm, 749 ± 153 nm and 1452 ± 312 nm and their findings showed that the fiber diameter of PES nanofibrous scaffolds significantly influenced the differentiation of rNSCs, whereas by decreasing of fiber diameter, more cell spreading and proliferation was observed. Additionally, the differentiation of stem cells towards oligodendrocytes was observed on nanofibrous scaffolds with fiber diameter of 283 ± 45 nm while morphology similar to neuronal progenitors was observed on nanofibers with fiber diameter of 749 ± 153 nm. Although, the number of cells on nanofibers with fiber diameter of 1452 ± 312 nm was found to be low which may be due to the difficulty of adhesion and migration of rNSCs on fibers with large diameter. However, the cells present within these nanofibrous scaffolds showed the morphology of neurons or neuronal progenitors[81].
In yet another study, aligned PLLA nanofibrous scaffolds with a mean diameter ranging from 307 to 917 nm and random PLLA nanofibrous scaffolds with a mean diameter ranging from 327 to 1150 nm were fabricated and the behavior of NSCs were evaluated on these scaffolds. The results showed that the depending on the fiber diameter and pattern, NSCs behaved differently revealing that the fiber alignment and diameter had significant effects on cellular behavior[82].
Yin et al[76] examined the effect of alignment of PLLA nanofibrous scaffolds on differentiation of stem cells from human tendon andthe differentiation of the cells seeded on aligned nanofibrous scaffolds was found to be higher with either normal or osteogenic media than on the randomly-oriented nanofibers[76].
Subramony et al[83] also evaluated the effect of PLGA nanofiber alignment and mechanical stimulation towards MSCs differentiation. Their results showed fibroblastic differentiation of MSCs in the absence of chemical induction factors on aligned nanofibers together with mechanical stimulation under tensile loading. No fibroblastic differentiation was observed on the non-aligned scaffolds even after the application of mechanical stimulation, indicating the effect of topography of scaffold towards stem cells differentiation[83].
Bakeine et al[84] investigated the effect of nanoscale surface topography on adhesion, proliferation and neural differentiation of mouse ESCs by fabrication of thin films of gold with different root mean square (RMS) surface roughness of 10, 21, 30 nm and 50 nm. Their results showed that surface topography influencedthe neural differentiation whereas highest differentiation was observed on gold films with RMS surface roughness of 21 nm after five days of cell seeding without addition of the traditional soluble neurotrophic factors[84].
The effect of topography with different sizes (groove width: 350 nm/2 μm/5 μm, depth = 300 nm) on the differentiation of human induced pluripotent stem cells (hiPSCs) towards neuronal lineage was studied by Pan et al[78]. Their results showed the obvious effect of topography on directing differentiation of hiPSCs towards neuronal lineage with noticeable up-regulation of neuronal marker expression on surfaces with nanostructured topography[78].
Yim et al[77] studied the proliferation and differentiation of human MSCs on nano-gratings of 1 μm, 10 μm, 350 nm width and their results showed the alignment of cytoskeleton and nuclei of human MSCs along the nano-gratings. Significant up-regulation of neuronal markers was also observed on patterned surface compared to un-patterned and micro-patterned controls[77].
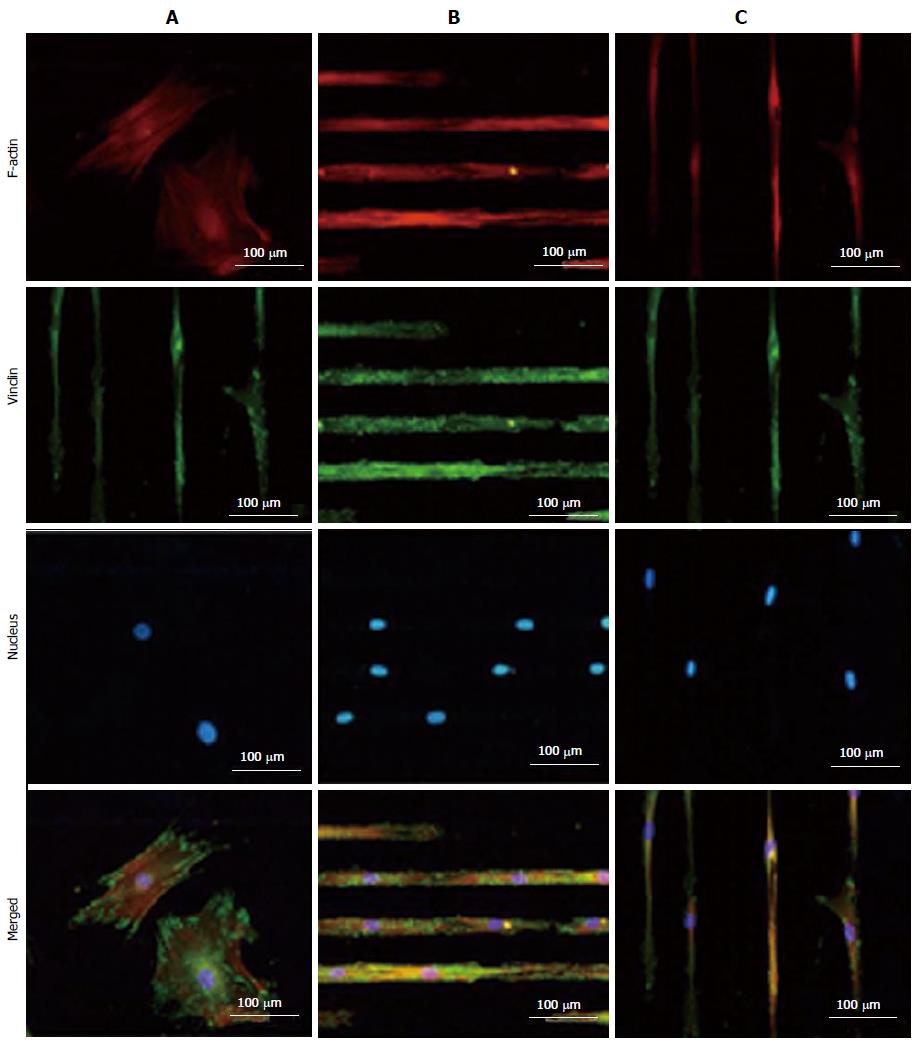
Chaubey et al[85] evaluated the effect of the surface topography on the differentiation of multipotent mouse bone marrow stromal precursors (D1 cells) to adipocytes. They compared the differentiation potential of D1 cells on patterned PLLA films, plain PLLA films and tissue culture-plate and their results showed the impact of patterned surfaces on cell differentiation[85]. Tay et al[86] printed fibronectin on PLGA thin films and investigated the differentiation of human MSCs on patterned and un-patterned scaffolds, where they revealed the differentiation of human mesenchymal stem cells (hMSCs) into myocyte like cells on the micropatterned films (Figure 3).
Figure 3 TRITC-Phalloidin labeled F-actin (red), AlexaFluor 488 labeled vinculin (green), diamidino-2-phenylindole nuclear staining (blue) and overlaid fluorescent image of immuno-stained cellular components (merged) for the unpatterned (A) and patterned human mesenchymal stem cells (B and C).
Samples were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic solution for 4 d before they were fixed and stained. All images were taken with a 20 × objective lens. (Scale bar = 100 μm) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.). Reproduced with permission from Tay et al[86].
Shi et al[87] fabricated two nano-grating substrates with a period of 250 and 500 nm and with a depth of 120 nm on quartz, and investigated the effect of substrate nanotopography on differentiation of ADSCs into endothelial cells which play an important role in vascularization. Decreased cell proliferation along with enhanced endothelial gene expression of the ADSCs and in vitro angiogenesis was observed on nanograting ssuggested the role of nanotopography on ADSCs differentiation to endothelial cells[87]. Oh et al[88] fabricated PCL cylindrical scaffolds with different pore size (90-400 μm range) along the longitudinal direction; and investigated the effect of pore size on the chondrogenic differentiation of ADSCs. Their results showed that the pore size of 370-400 μm provided a more suitable environment for chondrogenic differentiation than other pore size groups[88].
Teo et al[89] applied nanotopography technique along with NGF controlled release from polydimethylsiloxane substrate for neuronal tissue engineering and their results showed enhanced expression of neuronal genes on nanopatterned substrate combined with NGF delivery[89].
The influence on nanotopography, whether in the form of fibers, porous microstructures or pits and grates, was studied extensively by various researchers. However, comparison of the results between such studies was difficult due to the differences in various factors involved in each study, where some researchers used mechanical stimulation, while others used a specific biomolecule for the differentiation to take place. A systematic evaluation is therefore necessary, to clearly define the fiber length or the pore size that might specifically cause the differentiation of stem cells to nerve, osteolineages or endothelial cells.
EFFECT OF CULTURE ENVIRONMENT ON STEM CELLS DIFFERENTIATION
Bioreactors have been used in tissue engineering to overcome the drawbacks of static culturing conditions and to obtain uniform distribution of cells within the scaffolds and provide sufficient levels of oxygen, nutrients, cytokines, and growth factors, and to expose the cultured cells to mechanical stimuli[90]. Dynamic condition of cell culture has also been shown to influence stem cells differentiation.
Gomes et al[90] evaluated the effect of static and dynamic culturing conditions on the proliferation and osteogenic differentiation of rat bone marrow stromal cells seeded on two starch-based scaffolds. Calcium content analysis, VonKossa-stained sections, tetracycline fluorescence and histological analysis revealed the enhancement of osteogenic differentiation under dynamic condition created by flow perfusion bioreactor[90]. Mauney et al[91] also showed increased levels of alkaline phosphatase activity, bone-specific protein transcript levels, and mineralized matrix production by human bone marrow stromal cells under dynamic culture condition indicating increase inosteogenic differentiation of human BMSCs using mechanical stimulation applied by bioreactor. However, the influence of mechanical stimulation towards stem cells differentiation is not studied highly and more investigations are required.
EFFECT MATRIX MECHANICAL PROPERTIES TOWARDS STEM CELL DIFFERENTIATION
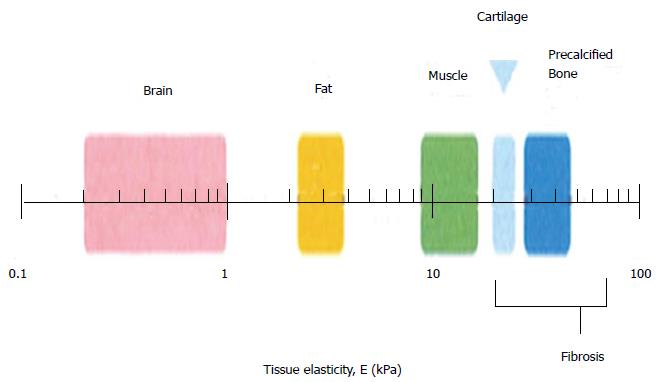
Matrix stiffness varies with respect to different organs present in the human body, and Figure 4 shows the difference in matrix stiffness of a few organs, as depicted by Vincent et al[92]. It explains how the matrix stiffness varies throughout the body: the brain is the softest tissue with an elasticity ranging between 0.2 to 1 kPa, while the precalcified bone is the hardest tissue with an elasticity value of more than 30 kPa[87], explaining the differences in the tissue matrix stiffness. Cells of different lineages survive in distinct natural environment, and they possess different stiffness. This is also the main reason why we believe that the stiffness have a higher influence towards the differentiation of stem cells. For tissue engineering, the most considered factors are the structure and the components used for scaffold fabrication, which determine the physical, chemical and biological properties of the scaffolds, and these properties will furthermore affect the differentiationof stem cells.
Figure 4 Schematic depicting the normal variation in elasticity of the indicated tissue[92].
Bone
Various strategies has been acknowledged and selectively chosen to alter the biomaterial surfaces or to create micro-environment to establish both mechanical and biochemical cues for the differentiation of stem cells, especially for application in regenerative medicine. Chemical factors have been individually studied, suggesting the ability of BMP-2 to promote pro-osteogenesis or IGF to promote tenogenic phenotype[93,94]. However, aligning the mechanical properties of the scaffolds with the specific tissue of interest was an interesting strategy applied to hydrogels, nanofibers and ceramics, in order to advance the field of stem cell differentiation on bioengineered substrates. Among these, injectable biodegradable hydrogels eliminate the need for surgical interventions or they allow for minimally invasive procedures causing minimal harm to the patients. However, physically crosslinked hydrogels lack strength and stability, while the chemically crosslinked hydrogels are more appreciated due to their high mechanical strength and stiffness. Hydrogels are useful substrates that assist to determine the interplay between physical cues/stiffness and their relationship with the fate of stem cell differentiation. A thiolene chemistry was utilized by Gandavarapu et al[95] to functionalize poly(ethylene glycol) hydrogels with a pendant peptide moiety, c(RRETAWA) and gels with Young’s Modulus (YM) of 2 kPa (soft) and 25 kPa (Stiff) were prepared. The stiffer substrate was found to give 3.5 fold higher hMSCs attachment. The ALP activity of hMSCs cultured on stiff gels containing 0.1 mmol/L and 1 mmol/L c(RRETAWA) increased by 2.5 and 3.5 fold, respectively after 14 d of cell culture. At the same time, the expression of osteopontin and collagen-1a for cells cultured on stiffer gels were higher, suggesting the application of high substrate stiffness gels for osteogenic differentiation of MSCs. In order to adjust the matrix stiffness, crosslinking of the thiol functionalized hyaluronic acid (HA-SH) and thiol functionalized recombinant human gelatin (Gtn-SH), were performed by using varied amounts of poly (ethylene glycol) tetra-acrylate (PEGTA)[96]. Further hMSCs were seeded on the hydrogels to assess their potential towards osteo-lineage differentiation. MSCs grown on gels with 1.5 kPa stiffness, was found to express the early bone protein, namely osteopontin, while the cells on stiffer (4 kPa) gel expressed late osteogenic gene or the bone sialoprotein (BSP). This highlights the potential of higher osteogenic differentiation on stiffer hydrogels, mainly because the focal adhesion structures might have increased and F-actin might have got organized on such substrates, supporting the differentiation of MSCs to osteo lineages.
In order to study the role of substrate stiffness towards cell differentiation, Sun et al[97] prepared crosslinked gelatin scaffolds, where by the influence of scaffold strength was demonstrated in vitro and in vivo during this study. Crosslinking of gelatin scaffolds was performed by these researchers, using 1-Ethyl-3-[3- dimethylaminopropyl] carbodiimide hydrochloride (EDC) to prepare three dimension gelatin scaffolds with high stiffness properties. The elastic modulus of the EDC crosslinked scaffolds (2.5 kP) was significantly higher than those of the non-crosslinked scaffolds (0.6 kP). High mechanical strength of cross-linked scaffolds was suggested to promote stem cell mediated bone regeneration via endochondral ossification process, than the non-crosslinked scaffolds. In vivo studies using Inbred C57BJL/6 mice, showed significantly high trabecular bone formation for the crosslinked gelatin implanted animals, by micro-computed tomography and histology analysis[92]. Wang et al[98] prepared gelatin-hydroxyphenylpropionic acid-tyramine (Gtn-HPA-Tyr) conjugates to simulate osteogenic differentiation of hMSCs, by conjugation of Tyr to Gtn-HPA conjugate by means of carbodiimide/active ester mediated coupling reaction. Oxidative coupling of phenol moieties were further carried out by hydrogen peroxide (H2O2) and horseradish peroxidase (HRP), whereby the concentration of H2O2 was varied producing Gtn-HPA-Tyr hydrogels of varying storage modulus (G0). Hydrogels with G0 > 20000 was found suitable for the osteo-specific differentiation of MSCs, with osteocalcinupregulation and high calcium accumulation was observed on these gels compared to hydrogels with G0 of 13500. Stiffer hydrogels were suggested beneficial for the repair of bone defects. In yet another attempt, the ability of cells to differentiate to osteogenic cells were studied on transglutaminase cross-linked gelatin (TG-Gel) of varying rigidity and these researchers found that thesofter matrix promote cell proliferation, while stiffer matrix promote osteogenesis[99]. Osteogenic differentiation was found to occur in 6% and 9% TG-Gels with rigidity of 13.51-2.13 kPa and 32.32-1.9 kPa, respectively than using 3% TG-Gels. However, little or no effect of BMP-2 was found in the case of stiffer (6% and 9%) TG-Gelstowards MSC differentiation. The 9% TG-Gels with and without BMP-2 demonstrated high calcium deposition with expression of osteogenic markers during in vitro studies. On the other hand, the ALP activity and early markers of osteogenic differentiation were observed in 6% TG-Gel and these cells differentiated to osteoblast-like cells at a later time point. Stiffer substrates are also demonstrated to direct the osteogenic differentiation of ADSCs, even in the absence of any growth factors[100]. PDGF and BMP-2 were covalently immobilized to collagen-Glycosoaminoglycan (CG) membrane in defined patterns, by combined photolithography-carbodiimide crosslinking strategy and the elastic modulus (stiffness) of the matrix was orthogonally manipulated. These researchers explained that by increasing the ratios of EDC:NHS:COOH, the stiffness of the CG got increased. Photoimmobilized BMP-2 was demonstrated to have a little effect on lineage-specific gene expression of ADSCs during this study, while the matrix stiffness had a higher impact. In yet another study, using BMP-2 modified poly(acrylamide-co-acrylic acid) hydrogels, BMP-2 was found to have little or no effect towards stem cell differentiation if grafted on softer gels[101]. Hence the mechanical properties of the substrate dictated the potential for stem cell differentiation than the biochemical reagents, explaining the diversity of stem cell behaviors. Efforts to leverage approaches to explore a wider range of combinatorial environments and biochemical pathways, in a systematic manner is necessary, such that the results obtained by different kinds of material systems can be compared. In yet another study, CG scaffolds were prepared by crosslinking treatment via dehydrothermal and 1-ethyl-3-3-dimethyl aminopropylcarbodiimide methods, with varying stiffness of 0.5 to 1.5 kPa[102]. MSCs differentiation studies on these scaffolds, showed higher level of RUNX2 expression on the stiffer scaffolds (1.5 kPa) indicating MSCs differentiation to osteogenic lineage. Chondroitin sulphate and hyaluronic acid was found to have a positive influence towards the MSCs differentiation towards osteogenic and chondrogenic lineages, respectively.
Actin filaments of cells are structures for force transmission in response to the substrate with which it comes in contact[103,104]. Increased cell spreading in response to geometric features of the substrate, could result in enhanced actomyosin contractility associated with osteogenic differentiation[103]. The expression of osteogenic markers was demonstrated as stiffness dependent, with maximum osteogenesis observed for fibronectin coated micropatterned polyacrylamide gels with stiffness of 30 kPa.
Studies on the rate of proliferation of mouse ESCs suggest higher proliferation on stiffer substrates of polyacrylamide hydrogels compared to the proliferation on soft substrates, favoring mESC colonization with high levels of Oct3/4 and the Nanog biomarker production[105]. In maintaining the stemness of mESCs on soft hydrogels with varying topography, no significant advantages were observed, suggesting the insensitiveness of topography effect towards mESC stemness. However, surface topography might have a role on substrates with hexagonal or pillar shapes topology or even on stiffer surfaces. It is also believed that either stiffness or topography might play an independent decision factor towards colony formation on 3D substrates. Geometric control of osteogenesis was also studied on polyacrylamide gels prepared by microcontact printing of adhesion proteins where cell spreading and cell geometry are suggested to have some influence towards MSCs differentiation[106]. Stiffer matrices can assist to increase cell spreading, thus promote osteogenesis through enhanced actomyosin contractility and this was observed up to a stiffness of 30 kPa. However, the density of seeded cells were described to over-ride the effect of gel stiffness to cause osteogenic differentiation, according to Xue et al[107]. However, this effect was not pronounced on soft gel, where chondrogenic differentiation was induced. Overall, their study highlights the importance of cell-matrix and cell-cell interactions in causing MSCs differentiation. Substrate stiffness was also found to play a predominant role in regulating the growth of rat BM-MSCs, while substrate topography manipulate the cell morphology and spreading, with spherically shaped cells were observed on pillar substrates but not on grooved substrate[108].
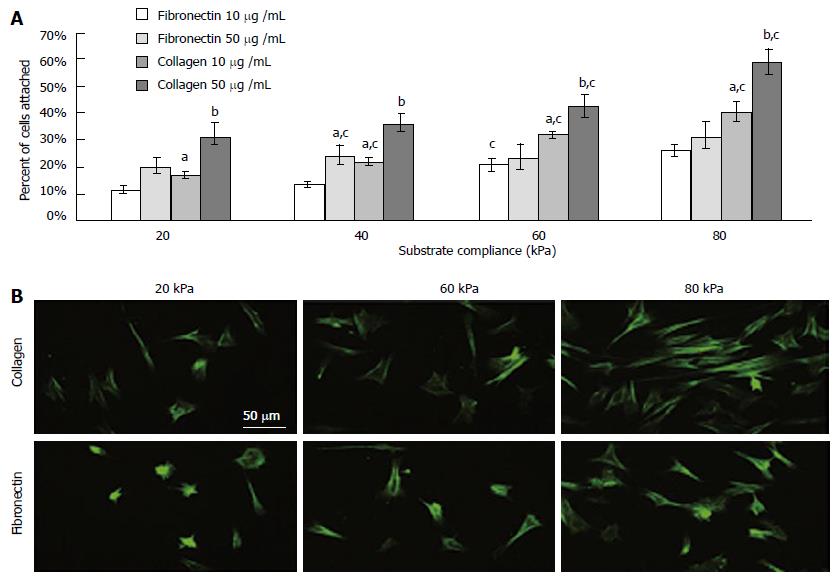
In general, studies using mechano-variant polyacrylamide substrates showed that the osteogenic differentiation reduced on substrates with low stiffness and ligand density. However, compared to fibronectin, BMSCs were demonstrated to have a higher binding affinity towards an equivalent bulk of collagen (Figure 5). Such comparisons were further delineated by Sharma et al[109], and they found the occurrence of osteogenic differentiation on fibronectin substrates, while tenoblast gene expression was only found on collagen substrates. Two entirely different microsystems namely, sponges and hydrogels were compared by Zhang et al[110] where they studied the behavior of MSCs and they found fast cell proliferation in sponges than in hydrogels. Sponges might have encouraged cell-cell and cell-matrix interaction and upon inducing them with media containing TGF-β3, the chondrogenic differentiation of MSCs were better compared to those in hydrogels.
Figure 5 (A) As the rigidity increased, so did cell attachment.
Collagen elicited a greater degree of cell attachment, and increased bulk density of the ligand further enhanced this (B) Morphology on functionalized substrates at 8000 cells/cm2 for 24 h. astatistical difference compared to fibronectin at 10 mg/mL, bdifference compared to collagen at 10 mg/mL, cdifference compared to preceding substrate compliance. Cells were stained with FITC-phalloidin. (Scale bar represents 50 μm). Reproduced with permission from Sharma et al[109].
Nerve
From Figure 4 and the abovementioned researches, it can be indicated that for neural differentiation, scaffolds with relatively small stiffness is required. The fate of MSCs towards neural or glial lineages could be manipulated by adjusting the substrate stiffness. Three dimensional collagen-HA scaffolds (fabricated by lyophilization) with higher elasticity (E approximately 1 kPa) induced MSCs into neuronal differentiation, while those with less elasticity (E approximately 10 kPa) induced MSCs into glial differentiation. The elasticity was adjusted by using different concentration of the crosslinking agent, namely 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide (EDC)[111]. Tuning the modulus of hydrogels affects neurite outgrowth and ESCs differentiation; with higher modulus gels offered better substrate for neural differentiation and neurite outgrowth, while less modulus enhanced astrocyte formation and cell migration. Thus, ESCs differentiation into neural and glial lineages is influenced by the type, composition, architecture and stiffness of the matrix and the type and concentration of signaling molecules comprehensively[112]. Neuronal differentiation was favored on softer surfaces such as the photopolymerizablemethacrylamide chitosan hydrogel with Young’s modulus less than 1000 Pa as confirmed by both immunohistochemistry and qRT-PCR. Oligodendrocyte differentiation was favored on stiffer scaffolds with Young’s modulus more than 7000 Pa. However, myelin oligodendrocyte glycoprotein gene expression suggested that oligodendrocyte maturation and myelination was best on scaffolds with modulus less than 1000 Pa where more mature neurons were present. Astrocyte differentiation was only observed on scaffolds with modulus less than 1000 Pa and 3500 Pa and represented less than 2% of the total cell population[113]. Elastic moduli, even in the range of neural tissue, would have a great impact on adult neural stem cell differentiation. The adult NSCs expressed a high level of the neuronal marker, β-tubulin III, on variable moduli interpenetrating polymer networks of 500 Pa, which is similar to the physiological stiffness of brain tissue. Furthermore, under mixed differentiation conditions with serum, softer gels (approximately 100-500 Pa) greatly favored neurons, whereas harder gels (approximately 1000-10000 Pa) promoted glial cultures[114].
To achieve the scaffold with favorable stiffness for nerve tissue regeneration, the fabrication method and the materials should be carefully considered. Hydrogels possess low modulus, and they are suitable as substrates for neuronal differentiation of stem cells. Alternatively, polymers with low modulus might be appropriate for this purpose. Within the category of the neuronal tissue structures, glial differentiation of stem cells might be gained on stiffer scaffolds, and neural differentiation can be achieved on softer scaffolds. By fine tuning the stiffness of the matrix, there is a great possibility to control the differentiation of stem cells into appropriate nerve cells.
Cardio-vascular
Lineage commitment of MSCs towards specific vascular cells (MSCs to ECs or MSCs to SMCs) on electrospun scaffolds could be manipulated by controlling the substrate modulus, from 2 to 15 kPa. Around 95% of MSCs seeded on soft scaffolds (3 kPa) showed Flk-1 endothelial markers within 24 h, while only 20% of MSCs seeded on the rigid scaffolds (> 8 kPa) showed Flk-1 marker. In contrast, about 80% of MSCs seeded on rigid scaffolds (> 8 kPa) showed smooth muscle α-actin marker within 24 h, while fewer than 10% of MSCs seeded on soft scaffold (< 5 kPa) showed α-actin markers[115]. Besides, the synergistic effect of scaffold elasticity and growth factors has been studied for the differentiation of MSCs. In one such study, MSCs were seeded on soft nanofibrousmatrices with or without VEGF, and in Petri dishes with or without VEGF, where these researchers found that MSCs in soft matrices with VEGF showed significant increase in the expression of endothelia markers (vWF, eNOS, Flt-1, and Flk-1), with faster up-regulation of the endothelial markers. The results indicate that it is critical to control both mechanical factors and biochemical factors to regulate vascular endothelial regeneration of MSCs[116]. MSCs gained very low rate of cardiogenic differentiation after transplantation to infarcted heart, partly because stiffer scar tissue lack the capacity to support cardiogenic differentiation. Thermosensitive and injectable hydrogels with different moduli (16, 45 and 65 kPa) were achieved by controlling the concentration of the hydrogel solution. After 14 d, more than 76% MSCs differentiated into cardiac cells in gels with 45 and 65 kPa, while MSCs in the 65 kPa gel had the highest differentiation efficiency[117]. But the effect of stiffness towards the differentiation of stem cells might be time-dependent. Human embryonic stem cells are sensitive to substrate stiffness during early mesodermal specification, but not late fate choices during cardiac differentiation, when they are seeded on polyacrylamide hydrogels. An intermediate stiffness was most beneficial for cardiomyogenic differentiation than soft or stiff substrates[118].
CONCLUSION
Selection of appropriate biomaterials for scaffold fabrication and their modification by incorporation of biological constitutes, or by patterning, thus altering the topography and mechanical properties of the scaffolds, might have a significant effect on stem cells behavior. Major literature review shows that MSCs are in favor of soft substrate to differentiate to neuronal cells, with moderately stiffer materials promote myogenic differentiation and more stiffer substrates support osteogenic differentiation, suggesting the effect of substrate mechanical properties in influencing the differentiation of stem cells toward specific lineages. The stiffness of mature tendon range between 500-1000 MPa and bone falls in the range of 20 GPa and this might be a reason for such specific behaviors of stem cells on substrates with proportionate mechanical properties. Prioritizing the stiffness of the substrate alone might not serve as a smart approach, but the presence of specific ligands along with the matrix mechanical compliance, might assist for more specific stem cell differentiations. There exists great difficulty in comparing the results obtained by different research groups, mainly because the parameters such as the ligand type, their concentrations, surface topology or matrix type and their mechanics, and even the cell numbers differ between studies. Hence more uniform and systematically organized studies, are required for a thorough understanding of the stem cell differentiation effect. The extent of differentiation or the % of differentiated cells is another parameter that deserves great attention, such that the cell-substrate can be used for its maximum potential.
The cells on a 2-D platform might be less sensitive to mechanical properties than when they are in 3-D environment. The 3-D structures might provide an additional dimension to mechanical properties, such that the cell related factors such as the integrin ligation, cell contraction, and associated intracellular signaling might be influenced.