Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.521
Peer-review started: August 22, 2014
First decision: September 4, 2014
Revised: October 30, 2014
Accepted: November 27, 2014
Article in press: December 1, 2014
Published online: March 26, 2015
Processing time: 210 Days and 11.9 Hours
AIM: To investigate collagen patches seeded with mesenchymal stem cells (MSCs) and/or tenocytes (TCs) with regards to their suitability for anterior cruciate ligament (ACL) repair.
METHODS: Dynamic intraligamentary stabilization utilizes a dynamic screw system to keep ACL remnants in place and promote biological healing, supplemented by collagen patches. How these scaffolds interact with cells and what type of benefit they provide has not yet been investigated in detail. Primary ACL-derived TCs and human bone marrow derived MSCs were seeded onto two different types of 3D collagen scaffolds, Chondro-Gide® (CG) and Novocart® (NC). Cells were seeded onto the scaffolds and cultured for 7 d either as a pure populations or as “premix” containing a 1:1 ratio of TCs to MSCs. Additionally, as controls, cells were seeded in monolayers and in co-cultures on both sides of porous high-density membrane inserts (0.4 μm). We analyzed the patches by real time polymerase chain reaction, glycosaminoglycan (GAG), DNA and hydroxy-proline (HYP) content. To determine cell spreading and adherence in the scaffolds microscopic imaging techniques, i.e., confocal laser scanning microscopy (cLSM) and scanning electron microscopy (SEM), were applied.
RESULTS: CLSM and SEM imaging analysis confirmed cell adherence onto scaffolds. The metabolic cell activity revealed that patches promote adherence and proliferation of cells. The most dramatic increase in absolute metabolic cell activity was measured for CG samples seeded with tenocytes or a 1:1 cell premix. Analysis of DNA content and cLSM imaging also indicated MSCs were not proliferating as nicely as tenocytes on CG. The HYP to GAG ratio significantly changed for the premix group, resulting from a slightly lower GAG content, demonstrating that the cells are modifying the underlying matrix. Real-time quantitative polymerase chain reaction data indicated that MSCs showed a trend of differentiation towards a more tenogenic-like phenotype after 7 d.
CONCLUSION: CG and NC are both cyto-compatible with primary MSCs and TCs; TCs seemed to perform better on these collagen patches than MSCs.
Core tip: Commercially available porcine or bovine-derived collagen type I and type III patches are cytocompatible for anterior cruciate ligament derived tenocytes (ACL-TCs) and for bone-marrow derived mesenchymal stem cells (MSCs). The combination of commercially available collagen patches with MSCs and ACL-TCs seems to be suitable for application with a dynamic intraligamentary stabilization system, which represents a novel technique to fix a ruptured ACL. However, co-culture of MSCs with ACL-TCs did show signs of differentiation of MSCs towards a more TC-like phenotype after a short-term culture of 7 d.
- Citation: Gantenbein B, Gadhari N, Chan SC, Kohl S, Ahmad SS. Mesenchymal stem cells and collagen patches for anterior cruciate ligament repair. World J Stem Cells 2015; 7(2): 521-534
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/521.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.521
Anterior cruciate ligament (ACL) injuries represent one of the most common ligament injuries, estimated to impact 100000 to 200000 people annually in the United States[1-4]. In Germany, the incidence of ACL ruptures is estimated at 32 per 100000 in the general population; in the active sports community, this rate is more than twice as high, at 70 per 100000[1]. The ACL provides an essential mechanism for ensuring stability in the knee joint[5]. The instability resulting from ACL injury not only impairs the functionality of the joint, but can also result in secondary damage to other structures, leading to arthritic changes[1,6,7]. Current surgical treatment methods are thus focused on restoring stability of the knee, reducing pain and preventing osteoarthritis[1,8,9]. The cruciate ligaments do not heal spontaneously; this has been attributed to a lack of a fibrin-platelet clot formation in ACL defects, which may be due to the synovial fluid surrounding the cruciate ligaments into which blood from the ruptured stumps disperses, as well as to high levels of plasmin concentrations known to be circulating in the synovial fluid[10]. Lack of spontaneous healing necessitates intervention to provide for alternative options to restore stability.
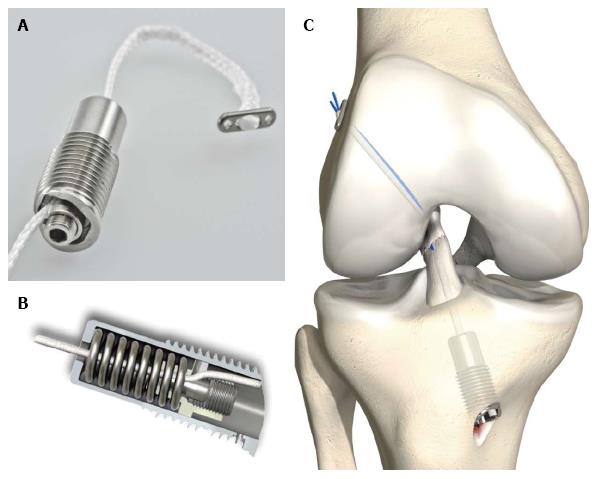
Given the clinical success of the dynamic intraligamentary stabilization approach in ACL repair[11-13], the aim of this study was to provide insight as to whether the collagen patches-which are currently approved and marketed for cartilage and meniscus repair–are also suitable for ACL repair, and elucidate the extent to which they impact and interact with cells. For this purpose, we tested two commercially available collagen scaffolds with regards to cell viability, adherence and proliferation of seeded tenocytes (TCs), the primary cell type in ACL tissue[14]. TCs are considered to be a specific subset of the category of fibroblasts[14]. Ideally, the cells seeded on the scaffold would proliferate and modify the matrix, slowly reconstructing the original tissue.
Considering the difficulty of obtaining autologous ACL-derived TCs for common surgical use, we also used bone-marrow derived mesenchymal stem cells (MSCs), which can be easily harvested through bone marrow aspiration, cultured and stimulated to differentiate into a fibroblast phenotype[2,6,15]. Several studies propose MSCs proliferate faster than TCs, and also exhibit a higher survivability in vivo[15]. Additionally, current literature suggests that MSCs may have an anti-inflammatory effect, providing an additional benefit for their use in tissue engineering[16]. We were particularly interested whether the MSCs would be able to stimulate the TCs in this in vitro experiment set up.
Due to the composition of the scaffold materials, we hypothesized that the patches would provide a suitable environment for TCs and lead to cell adherence as well as proliferation and collagen deposition. Furthermore, we expected MSCs to differentiate into a TCs phenotype in co-culture and even more so if cultured on collagen scaffolds rather than cultured on culture inserts. Currently, no unique marker profile for ligaments exists; thus, tendon-like cells are commonly distinguished using a combination of markers, including scleraxis, tenomodulin, tenascin-C, collagen Iand collagen III[6,14].
Here, we present analyses on cell compatibility for primary ACL-derived TCs and bone-marrow derived MSCs seeded on collagen scaffolds. We investigated the phenotype of these cells using real time real time polymerase chain reaction (RT-PCR) and biochemical analysis in order to judge the suitability of cell-seeded collagen scaffolds for the Ligamys surgery application to “boost” the healing process and possibly to improve the surgery’s outcome. To the best of our knowledge, no studies have been previously published with this aim.
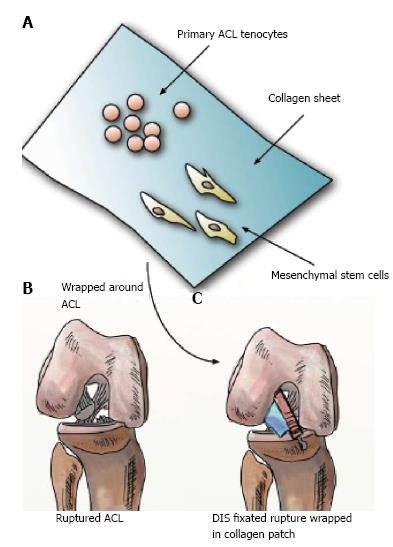
Two types of commercially available and CE label approved bio-resorbable collagen scaffolds were tested in the experiments: Novocart© (NC) produced by Tetec AG (Reutlingen, Germany), a B. Braun AG Company, and Chondro-Gide© (CG), produced by Geistlich Pharma AG (Wolhusen, Switzerland). NC is a biphasic collagen-chondroitin sulfate matrix of bovine origin; CG represents a porcine collagen bilayer matrix. Both scaffolds were specifically developed for human chondrocytes and are used clinically in connection with autologous chondrocyte transplantation and autologous matrix-induced chondrogenesis for cartilage regeneration. Here, we investigated these scaffolds for application in ACL repair (Figures 1 and 2). The NC and CG scaffolds comprise a porous sponge and an adhering compact membrane to protect the cells and the lesion. Cells were seeded on the porous side as intended by its application. The sponge itself consists of fibers, building a three-dimensional structure with interconnecting pores.
Prior to cell seeding, the scaffolds were cut in equally sized samples using an 8 mm sterile biopsy punch (Kai Medical, Polymed Inc., Glattbrugg, Switzerland) and then soaked in high glucose Dulbecco’s Modified Eagle’s Medium (HG-DMEM), (Gibco, Life Technologies, Zug, Switzerland) with 10% fetal calf serum (FCS) and 1 × penicillin-streptomycin (P/S) (all Sigma-Aldrich, Buchs, Switzerland). The patches were seeded with 40000 cells; in addition, material only controls were also cultured under the same culture conditions.
Primary ACL derived TCs were isolated from ACL tissue obtained from full-knee prosthesis surgery (ethical approval was obtained from the local committee: KEK registration 22-12-13). Primary TCs were isolated by cutting the tissue in smaller pieces of approximately 4 mm3 pieces, then washed in phosphate buffered saline (PBS) twice and digested overnight in collagenase 2 (Worthington, London, United Kingdom) at 37 °C, under constant shaking at 10 RPM. The released cells were filtered with a 100 μm cell strainer (BD Falcon, Switzerland) and seeded at a density of 1000 cells/cm2 for monolayer expansion in HG-DMEM + 10% FCS. With written consent human bone marrow was obtained from patients ages 55-84 undergoing hip or spine surgery. The procedure was approved by the Ethics Office of the Canton of Bern (KEK # 187/10), all patients gave their informed consent prior to their inclusion in the study. Human MSCs were amplified from the mononuclear cell fraction after density gradient centrifugation (Histopaque-1077, Sigma) by selection for plastic adherence for 2-3 passages. The MSCs were expanded using α-Minimal Essential Medium (α-MEM) with 10% FCS, 100 μg/mL penicillin, 100 UI/mL streptomycin, and 5 ng/mL bFGF-2[17].
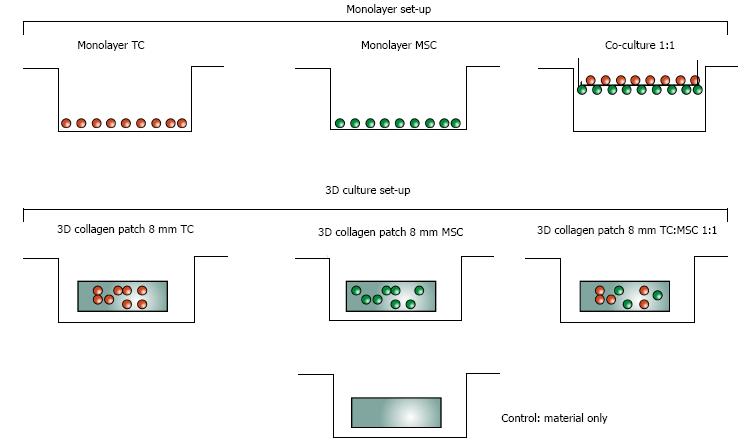
Scaffolds that were seeded contained either a single cell type-human MSCs or human TCs- or a combination of both cell types (Figure 3). For the samples containing co-cultures, the two cell types were mixed in a ratio of 1:1 prior to seeding; recent literature demonstrates that this provides the most effective ratio[16]. All patches were placed in 12 well plates, and the samples were incubated for approximately one hour prior to adding 2 mL medium to each well, in order to give the cells time to adhere to the patch.
In addition to the scaffold samples, we also grew monolayer cell cultures as well as co-cultures using translucent inserts (Falcon, 6 well cell culture inserts, 0.4 μm PET high density pores, Falcon™, BD Biosciences, Allschwil) in 6 well plates (Falcon™). The single cell population monolayer controls served as reference samples for the real-time PCR results, while the inserts allowed us to evaluate changes in MSCs gene expression in co-culture. Separating the MSCs and TCs cultures with inserts made it possible to attribute the relative gene expression results of cells in co-culture to the correct cell type. The pore size was chosen to facilitate cell-cell contact between the two cell types, without allowing for cell migration between the two cell populations. This setup allowed us to observe whether cell-cell contact permits differentiation of MSCs into TCs phenotype.
The co-culture for the gene expression analysis was set up as follows: culture inserts were seeded on the bottom with 100000 MSCs. Two hundred microlitre HG-DMEM medium with 10% FCS and 1 × P/S was added to the layer of MSCs. The inserts, bottom side facing up, were placed on a petri dish, covered with a beaker and placed in a 37 °C incubator for 1 h. Within this timeframe, the MSCs adhered to the insert, as has been shown in previous studies by our group[18]. Subsequently, the other side of the insert was also seeded with the same number of TCs.
Patches were incubated at 37 °C on a shaker for 5 h with resazurin red solution to measure mitochondrial activity as described in[19]. Resazurin is a nontoxic, cell-permeable compound that is blue in color and virtually non-fluorescent. Upon entry into cells, resazurin is reduced to resorufin, which emits very bright red fluorescence. The amount of fluorescence or absorbance is proportional to the number of living cells (the linearity of transformation was evaluated based on primary cells) and corresponds to the cell’s metabolic activity. The amount of fluorescence was monitored with a Softmax® M5 Pro Multi wavelength fluorescence reader (Molecular Devices, distributed by Bucher Biotec, Switzerland) at excitation 547 nm and emission wavelength 582 nm.
For biochemical analysis, dry-weight of each sample was measured and then each sample was digested with papain. We used the dimethylmethylene blue (DMMB) dye method to analyze the digested samples for sulphated glycosaminoglycans (GAGs), using bovine chondroitin sulfate as standard. Total collagen content was determined based on the hydroxy-proline content after acid hydrolysis and reaction with p-dimethyl-amino-benz-aldehyde and chloramine-T[20,21].
All samples were frozen down on day 0 and day 7 of each experiment, except for two samples collected during the pilot experiment, which were collected on day 8 (NC patch with TCs and NC patch with MSCs). RNA extraction was performed with TRI reagent [Molecular Research Center (MRC), Cincinnati, MA, United States], polyacrylcarrier (MRC), 100% molecular grade ethanol, 1-bromo-3-chloropropane (BCP, Sigma Aldrich), GenElute miniprep kit, and the AMP-D1 DNase I Kit which contains both DNase I and 10 × DNase Digest Buffer (all Sigma Aldrich). The same process was followed for all samples, except that the scaffold samples were subject to an additional step, since those samples had been snap frozen in liquid nitrogen on day 7 of the experiments and placed in -80 °C without any RNA protective solution. In order to perform RNA extraction on those samples, the scaffold samples were first ground using a mortar pre-cooled with liquid nitrogen, then immediately placed in a solution containing 1 mL TRI and 5 μL polyacrylcarrier. The samples were then centrifuged at 16000 g to separate the scaffold debris from the solution. cDNA synthesis was performed with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Cressier, Switzerland). For each sample, 20 μL reaction mix was set up containing 10 μL RNA template (about 0.5-1 μg total RNA), 4 μL 5 × iScript Reaction Mix, 1 μL iScript reverse transcriptase and 5 μL nuclease-free water. After the incubation, the cDNA samples were diluted with 40 μL 1 × TE Buffer.
We evaluated the relative gene expression for the genes we were interested in using human primers (all Microsynth, Balgach, Switzerland; see Table 1 for a detailed list of primers). Separate forward and reverse primers were used to set up the reaction mix, which also contained iQ SYBR Green Supermix2X (Bio-Rad) and RNAse free water. We tested ligament fibroblast markers including scleraxis, tenomodulin, tenascin-C, collagen I and III[6,22]. In addition, we tested for gene expression of two matrix metalloproteinases (MMPs, enzymes which regulate matrix turnover) MMP3 and 13, aggrecan and collagen type II. MMP3 (stromelysin 1) and MMP13 (collagenase 3) break down collagen and proteoglycans quickly and are known to play a role in ligament remodeling[23]. Collagen II was used to test that the cells did not differentiate into chondrocyte-like cells. There has been some evidence of increased amounts of proteoglycans in pathological tendons; therefore, gene expression of aggrecan was also evaluated[22]. To calculate relative gene expression, the Livak method (2-∆∆Ct)[24] was used, which makes use of both a calibrator sample and a normalizing reference gene; we applied 18S (ribosomal RNA) as the reference gene for all samples[25]. Relative gene expression levels for samples were calibrated to day 0 reference samples. Day 7 TCs samples were compared relative to TCs at day 0; day 7 MSCs samples were compared relative to MSCs at day 0 (i.e., expression = 1.0).
| Abbreviation | Gene name | Forward | Reverse |
| 18S | 18S Ribosomal RNA | CGA TGC GGC GGC GTT ATT C | TCT GTC AAT CCT GTC CGT GTC C |
| HsACAN | Core protein | CAT CAC TGC AGC TGT CAC | AGC AGC ACT ACC TCC TTC |
| Hs_col1 | Collagen 1 A2 | GTG GCA GTG ATG GAA GTG | CAC CAG TAA GGC CGT TTG |
| Hs_col2 | Collagen 2 A1 | AGCAAGAGCAAGGAGAAG | GGGAGCCAGATTGTCATC |
| Hs_col3 | Collagen 3 A1 | GTG AGC CTG GTA AGA ATG G | CCT GGA ACA CCT GGA ATA C |
| Hs_TNC | Tenascin C | CAC GCT GAG GTT GAT GTT C | GTT GAT GGT CGC TGG ATT G |
| Hs_SCX | Scleraxis A | CAC CAA CAG CGT GAA CAC | GCA GCG TCT CAA TCT TGG A |
| Hs_TNMD | Tenomodulin | ACA AGC AAG TGA GGA AGA A | GAC GGC AGT AAA TAC AAC AAT |
| Hs_MMP13 | Collagenase 3 | AGT GGT GGT GAT GAA GAT | CTA AGG TGT TAT CGT CAA GTT |
| Hs_MMP3 | Matrix metallopeptidase 3 | CAA GGC ATA GAG ACA ACA TAG A | GCA CAG CAA CAG TAG GAT |
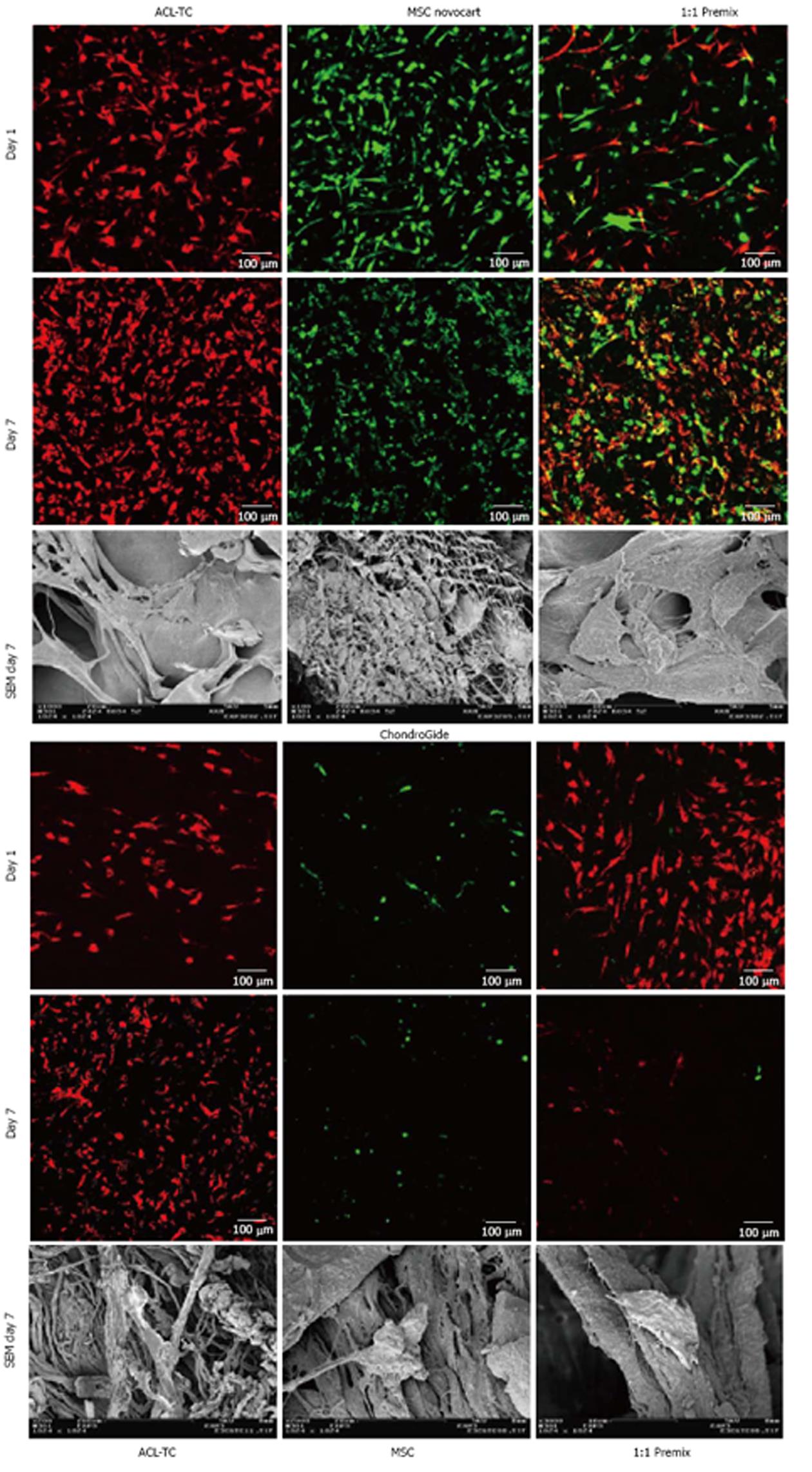
Confocal laser scanning microscopy (cLSM) was used to confirm visually that cells are able to adhere and proliferate on the scaffolds in the first two experiments. Samples for the cLSM were stained using Vybrant membrane tracker dyes DiO (DiO = green, for MSCs) and DiL (DiL = red, for TCs) (both Molecular Probes, Life Technologies, Basel, Switzerland); these dyes are lipophilic carbocyanines, which integrate into the membrane[26]. In addition, we also imaged the cells on both sides of the culture inserts. This allowed us to confirm that the cells adhered to both sides of the culture insert and could therefore be evaluated with real-time PCR.
Further, additional samples were prepared during one experiment to be processed with scanning electron microscopy (SEM), as this method permits visualization of 3D structures on a very small scale. This technique also allowed us to see how the cells attach to the scaffold, as well as provided a more detailed visualization of the scaffolds themselves. On day 7, the samples were first washed with PBS to remove traces of serum, then fixed with 2.5% glutar-aldehyde. On the day of SEM sample preparation, the specimen were again washed with PBS prior to following a dehydration protocol using an alcohol series of increasing concentration, i.e., 50%, 70%, 80%, 90% and 2 × 100%, with incubation periods of 10 min. Subsequently, the specimen were critical point–dried in a Bal-Tec CPD 030 (BalTec inc., Balzers, Liechtenstein) and sputtered with approximately 10 nm (30 mA for 30 s at 50 mm working distance), producing a 10 nm gold layer in a SCD 004 (BalTec inc.). Samples were then stored in an exsiccator until examination at 5 kV accelerating voltage in a Zeiss scanning electron microscope DSM 982 (Carl Zeiss, Jena, Germany).
Data were analyzed using the Kruskal-Wallis test unless otherwise noted, followed by Dunn’s multiple comparisons test using the PRISM software package (GraphPad, La Jolla, United States, version 6.0e). RT-PCR data was tested using a t-test statistic and mean gene expression was tested if significantly different from a hypothetical value of 1.0. A significance value of P < 0.05 was specified to be significant. Where applicable groups were tested in 2-way ANOVA for treatment and culture time, multiple comparisons were then performed using Bonferroni’s multiple testing. The statistics was checked by a biostatistics expert.
The experiments were meant to provide initial insight into whether NC and CG collagen patches, which are currently being used in cartilage repair, are suitable for ACL repair. Thus, adherence and proliferation of cells was tested, as well as the collagen deposition and differentiation potential of cells.
CLSM and SEM imaging and the metabolic cell activity assay confirmed that both cell types, TCs and MSCs, adhere on the substrate and seem to proliferate on both patch types (Figure 4). The images seem to show, however, that MSCs decreased in cell number after 7 d.
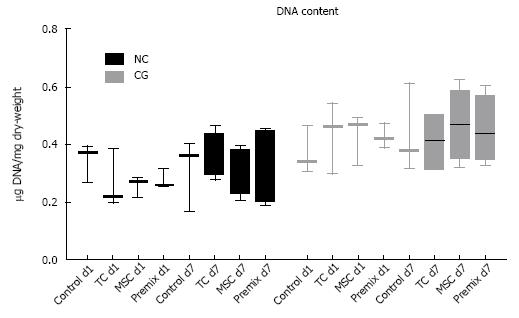
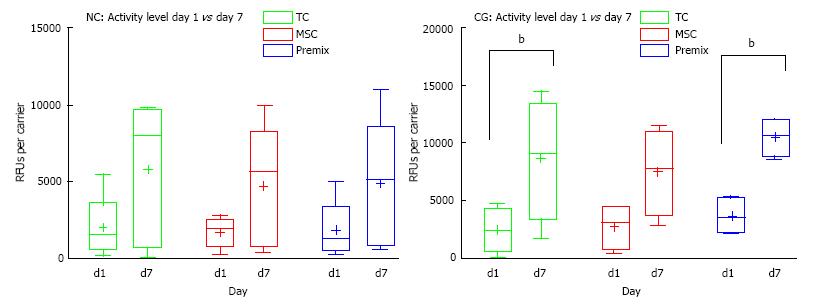
Noteworthy, DNA content (Hoechst) of collagen scaffolds contained a relatively high amount of porcine/bovine DNA (Figure 5), which was confirmed by DNAse digestion (data not shown). There was no significant change in DNA content among the groups over the 7-d culture period. The metabolic cell activity assay (see Figure 6) revealed increased cell activity on day 7 vs day 1 for all cell treatment groups, and a slightly higher absolute level of cell activity for all CG samples versus NC patches on a given day. The relative increase in cell activity level from day 1 to day 7 in both patch types was similar (Figure 6).
The non-parametric Kruskal-Wallis tests showed that cell type did not have a significant impact on the relative change in metabolic activity for either of the two patch types (Figure 6). Furthermore, we found no significant differences between NC and CG samples in terms of the relative changes in metabolic cell activity between the two patch types. While both patch types also showed an increase in absolute cell activity, as measured in RFUs, from day 1 to day 7 for every cell type, this trend was not significant for NC patches, but significant at an overall level for CG patches (P = 0.0298, n = 4). Dunn’s multiple comparisons test for CG however revealed no significant P values for individual treatment groups (TCs, MSCs, premix) with different treatment lengths (Figure 6). However, after testing for 2-way ANOVA for time and treatment we found a significant effect (P = 0.0001) of culture time and found that activity increased over time for TCs and premixes (P = 0.0237 and P = 0.0120, respectively) (Figure 6).
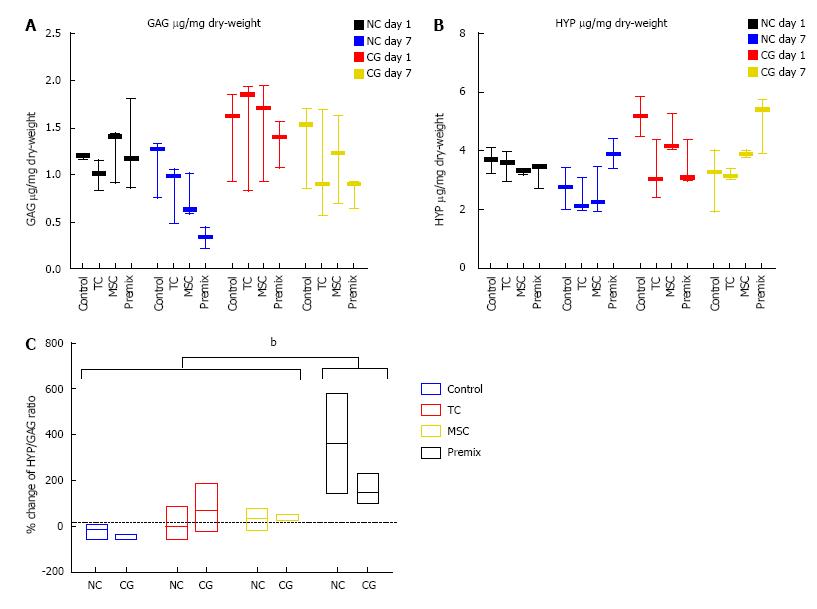
Previous studies suggested that pathologic tendons exhibit higher GAG content[22]. We, therefore, measured GAG content on day 1 and day 7 (n = 3). In all samples, there was a decrease in the amount of GAG from day 1 to day 7 (μg per mg of dry-weight, corrected for controls using day 7 sample with no cells as a baseline for all samples). No significant difference was found between the two patch types or cell types in terms of the relative changes of GAG measured (Figure 7A).
Hydroxy-proline (HYP) levels were measured in order to assess collagen deposition of the different cell types on the scaffold (Figure 7B). Data revealed no significant change in HYP over time. In line with the other analyses, the different cell types were not found to have a significant effect on the percentage change of HYP measured, neither for NC patches nor for CG patches. Also, the Kruskal-Wallis test did not reveal a significant difference between the two patch types. However, when the ratio of HYP/GAG was considered, the 2-way ANOVA for scaffold and cell treatment showed a significant relative change for the Premix group (P = 0.005) (Figure 7C).
Both cell types (TCs and MSCs) were analyzed individually with regards to the effect of different substrates (NC patch, CG patch, inserts or just well plates) on the relative gene expression for the genes of interest. A summary of the relative gene expression measurements is shown in Figure 8.
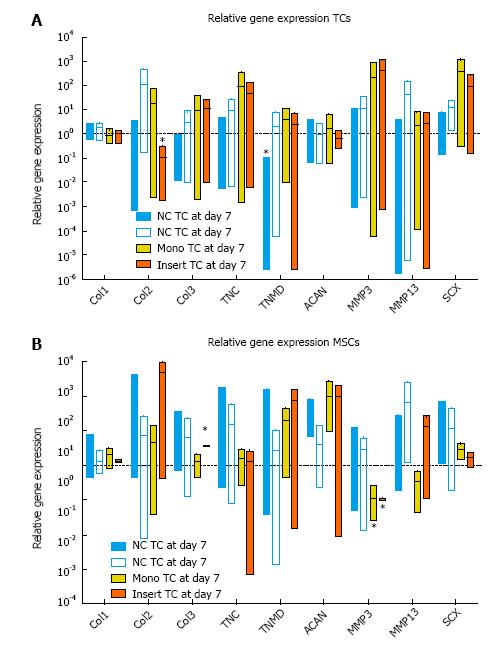
Relative gene expression revealed a significant down-regulation of TNMD for TCs grown on NC scaffolds (P < 0.0001) and for col2 in TCs grown on culture inserts (P = 0.0121) relative to day 0 controls (Figure 8A). Monolayer MSCs significantly down-regulated MMP3 (P = 0.0065) after 7 d of culture, whereas MSCs grown on culture inserts significantly up-regulated col3 (P = 0.006) and down-regulated MMP3 (P = 0.0065).
In line with expectations, both patch types showed a slight up-regulation of collagen I gene expression for TCs in comparison to TCs grown in monolayers (Figure 8). Scleraxis expression trended up on patches vs d0 but was in line with monolayer results (Figure 8). At the same time, both patch types did not result in a significant up-regulation of collagen II or aggrecan, compared to monolayer (Figure 8). These factors suggest that the cells did not differentiate into cells of a chondrocyte phenotype. Based on the non-parametric Kruskal-Wallis test statistic, the differences in relative gene expression between TCs cultured on NC or CG patches or in monolayers are not significant, which is in line with our initial hypothesis. We did observe significant changes of relative gene expression when tested with one-sample t-test. While gene expression levels for TCs on NC patches were not vastly different from those cultured on CG or in monolayers, TCs grown on NC significantly expressed less tenomodulin D (TNMD) and do seem to express slightly lower levels for the ligament markers collagen type 3 (col3), tenascin- C (TNC) and scleraxis (SCX) relative to those two substrates (see Figure 8A).
At first sight, especially when considering these data in light of the metabolic cell activity results, it may seem that the CG patches provide a better environment for TCs. The metabolic cell activity measurements showed that cells grown on CG patches vs NC patches exhibited a slightly higher (yet statistically insignificant) absolute cell activity on any given measurement day, as well as a somewhat higher relative increase in cell activity from day 1 to day 7 of the experiments. However, this aspect needs to be carefully examined further.
It is possible that the culture time was not long enough to clearly discern significant differences between patch types. MSCs and TCs were cultured for up to 28 d and checked for cell adherence with the use of CLSM and the cell tracker dyes. Studies related to osteogenesis show that osteogenic differentiation of MSCs can take up to four weeks, with an initial early differentiation phase taking place between day 5 and day 14[27]. Conversely, it has also been shown that MSCs can show signs of differentiation into tenogenic phenotype after as little as seven days[28]. It is also possible that the cells on the NC scaffolds first partially de-differentiated. The down-regulation of tenomodulin (TNMD) has been observed for cells that were cultured two-dimensionally[29]. Since we observed a stronger down-regulation for cells cultured on NC than in monolayers, this may be interpreted as an indication of de-differentiation. This de-differentiation has been previously observed in monolayers as well as in organ cultures[15].
Furthermore, scleraxis has been shown to modulate TNMD expression, such that the up-regulation of TNMD occurred several days after an up-regulation in scleraxis was observed[30].
It is known that the structure of scaffolds can impact the cell morphology; ACL fibroblasts for instance orient parallel to collagen fibers. Cell shape on the other hand can impact cell differentiation[30,31]. In fact, both patches were primarily constructed for use with chondrocytes. The manufacturer of NC patches clearly states that the patches are meant to induce a spherical morphology, which describes a chondrocyte phenotype. However, with SEM imaging we observed flat networks covering the NC patches. This observation was in line with the gene expression results, which demonstrate that the cells do not up-regulate genes, which are typical for chondrocyte phenotype. It would be interesting to re-evaluate all variables in experiments running for 14 or 21 d, to test whether this still holds true.
MSCs cultured on patches showed a trend towards up-regulation of ligament markers versus MSCs monolayers, particularly on NC scaffolds. For instance, collagen III tended to be up-regulated on NC, with a more inconsistent expression for MSCs on CG (Figure 8). Scleraxis tended to be higher expressed on both patches compared to MSCs cultured in monolayers or on inserts (Figure 8). These results seem to indicate that MSCs are developing into a TCs phenotype on the patches. MSCs cultured with TCs, however, consistently up-regulated col 3 expression, a sign for early differentiation of the MSCs towards a more TC-like phenotype. Again, additional time might be required in order to draw more conclusive insights about the potential for MSCs to differentiate on the various substrates. However, interpreting the results of the RT-PCR data demonstrating a significant increase of col 3 in presence of TCs on culture inserts together with the significantly increased HYP/GAG ratio of “premix” of TCs and MSCs on the 3D patches (Figure 7C) could be interpreted as an early differentiation of MSCs shutting down gene expression of GAG and increasing collagen expression. A study related to tenogenic differentiation of rat MSCs showed that a significant change in expression levels for some ligament markers could already be detected after 7 d (tenascin-C as well as scleraxis); however, other markers were not up-regulated until a few days later (collagen I and III)[28].
There were some inherent limitations in the cell types used in our study. Cells had to be obtained from patients whom may already exhibit certain morbidities. TCs were provided from patients undergoing knee surgeries– either total knee arthroplasties or ACL reconstruction. Patient number 67, for instance, underwent ACL reconstruction due to a traumatic rupture, where the stumps were intra-operatively removed prior to graft insertion. It is possible that the cells cultured from these stumps may naturally exhibit slightly different characteristics, a topic that should be explored further. Moreover, it is also possible that the age of the donor plays a role in terms of the differentiation potential of the MSCs.
In this respect, it is also worth mentioning the existence of native ACL-derived autochthonous stem cells (ACL-SC), which already may exist in the tissue[32]. Zhang et al[32] described the isolation and characterization of such plastic-adherent cells, which possess stem cell characteristic surface markers from healthy tissue samples. However, it is unknown whether these cells might still be present in more degenerated human tissue as analyzed in this study. An additional aspect that should be considered is the donor characteristics of cells (Table 2). In four out of five trials we had male MSC donors and in all five experiments, we had female TC donors (Table 2). The gender and age of the donors may have a hidden effect on the outcomes, including proliferation rates or differentiation ability. However, current evidence of the impact of gender and age is inconclusive[33,34].
| MSCs donor characteristics | TCs donor characteristics | ||||||||||
| Experiment | ID | Passage | Donor gender | Donor age | Source | Experiment | ID | Passage | Donor gender | Donor age | Source |
| 1 | 62 | 1 | m | 75 | Lumbar | 1 | 66 | 1 | f | 62 | ACL |
| 2 | 62 | 2 | m | 75 | Lumbar | 2 | 66 | 2 | f | 62 | ACL |
| 3 | 65 | 1 | f | 84 | Lower limbs | 3 | 67 | 1 | f | 38 | ACL |
| 4 | 47 | 2 | m | 64 | Thoracal | 4 | 67 | 2 | f | 38 | ACL |
| 5 | 48 | 2 | m | 55 | Thoracal | 5 | 70 | 1 | f | 74 | ACL |
Confocal laser scanning microscopy (CLSM) images of CG patches showed less MSCs than TCs, both in the single cell culture of MSCs as well as when cultured together with TCs. The MSCs appear in a rounded shape in the CLSM images, as opposed to the TCs, which have a more elongated appearance. This is in line with the SEM results for the CG patches, on which we found rather roundish morphologies.
There seems a conflict in the data that the cell density on CG patches was decreasing for MSCs (Figure 4 lower panel) but DNA content (Figure 5) and cell activity (Figure 6) showed a steady-state and a trend of increased activity, respectively. We interpret this as a donor-specific phenomenon in the CLSM data.
GAG and HYP values were also corrected for control values, as we registered significant amounts of initial GAG and HYP in our controls (Figure 7A and 7B), which was expected considering that collagen patches consist primarily of collagen 1 and 3. Correcting for the controls, we see a decrease in GAG per mg of dry-weight. This suggests that the MSCs are changing in phenotype and possibly produce less GAG over time if in direct 1:1 co-culture. Interestingly, comparing the relative change of the HYP/GAG ratio of day 7 relative to day 1 showed a significant effect of “premix” cells but there was no effect of the differences between the two scaffolds (Figure 7). The effect of cells was highly significant in the 2-way ANOVA (P = 0.005, Figure 7). Thus, premix of cells shifted the ratio between HYP/GAG towards lower production of GAG and slight increase of HYP.
In conclusion, the results of these 3D cell culture experiments demonstrate the potential of collagen patches for ACL repair[4]. Using biochemical and imaging methods, we were able to demonstrate that primary TCs and MSCs can both be grown on the NC and CG patches, which were originally designed to induce a chondrogenic phenotype. Both TCs and MSCs adhere to and proliferate on the patches. Moreover, MSCs seem to show a tenogenic differentiation potential on these scaffolds as indicated by the significant increase of collagen type 3. Future work might consider the importance of alternative stimuli to accelerate healing such as the application of platelet rich plasma (PRP) or platelet rich fibrin (PRF). The problem with ACL-derived TCs and MSCs, which have to be expanded first ex vivo on plastics, is their clinical approval. With PRP on the other hand are unsolved issues about the reproducibility and the ideal dose of platelet concentrations[35,36].
Further studies should be performed to evaluate additional aspects. First of all, it would be beneficial to run the experiments with a longer culture time. This would permit a more in depth analysis of the induced cell morphologies on the different patches, which could alter the survivability of the cells as well as the differentiation potential. Additionally, it would allow us to observe whether this changes the gene expression profile of the cells. It is also likely that longer culturing duration would lead to more pronounced differences between cell and patch types and be able to further support the trends we have observed so far.
Furthermore, studies should also be performed using mechanical loading. It has been shown that mechanical strain induces MSCs to differentiate into a TCs phenotype[37,38]. It would be interesting to see whether the cells respond differently on the two patch types, subjected to equal parameters. Also, the mechanical properties of a scaffold are known to impact cell differentiation. In fact, several prior attempts at ligament or tendon repair have resulted in ectopic bone formation[30]. Thus, further experiments testing the impact of the mechanical properties would be helpful in evaluating the full potential of these patches. However, in these studies, it would be crucial also to look at bone-specific markers such as osteocalcin[30].
In conclusion, while certain aspects should be examined in more depth, the use of collagen scaffolds for ACL regeneration is promising[4]. The usage of a biological ACL repair model including collagen patches in a non-loading repair model seems a genius option to improve healing. What remains to be shown is whether mechanical loading improves the healing process or whether platelet rich fibrin PRF or PRP might be another option to be included at the site of rupture for the healing or whether it might be even hindering the healing process as has been recently proposed[35,36,39,40].
Rachel Horovitz collected cell culture data and provided input for the manuscript. Elena Calandriello assisted in biochemical assays. The imaging part of this study was performed with the facility of the Microscopy Imaging Center (MIC), University of Bern.
Mesenchymal stem cells (MSCs) and anterior cruciate ligament (ACL)-derived tenocyte (TCs) are both potential source cells to be applied for the cell seeding of the dynamic intraligamentary stabilization for the fixation of ruptured ACLs. Current treatment options for non-fusions of ruptured ACLs are very limited.
ACL injury is a common disease and the repair of ACL remains a challenge due to its limited self-regenerative ability. Despite of the progression of reconstructive techniques for ACL repair, an ideal biological scaffold for ACL reconstruction is needed to improve clinical outcome, especially in the case of non-unions.
In this study, the authors present novel data on the regenerative technique to use commercially Food and Drug Administration (FDA)-approved collagen 1 and 3 scaffolds along with ACL-derived TCs and MSCs. They provide microscopy imaging data, biochemical data, and gene expression data of human derived primary bone-marrow derived MSCs and ACL-derived tenocytes. There was not much known how cell therapy could accelerate and improve healing for ACL ruptures in combination with a recently developed dynamic anchoring technique called Ligamys©. The combination of regenerative approaches involving TCs and MSCs transplantation along with commercially available FDA-approved collagen patches seems a straightforward approach, as the membrane is highly biocompatible and does not carry the mechanical force.
Cyto-compatibility for the collagen patches is a tool to explain good clinical outcome.
This paper compared two different collagen patches seeding with either bone marrow mesenchymal stem cells or primary tenocytes for possible anterior cruciate ligament repair. Chongrogide is composed of porcine collagen I and III as a bilayer membrane. Novocart is a biphasic, three-dimensional collagen-based matrix from bovine. Both collagen sponges have been used for cartilage repair. ACL injury is a common disease and the repair of ACL remains a challenge due to its limited self regenerative ability. Despite of the progression of reconstructive techniques for ACL repair, an ideal biological scaffold for ACL reconstruction is needed. Thus, this study is of importance and experiments strongly suggest that the patches of collagen may be useful for anterior cruciate ligament repair.
P- Reviewer: Chandra D, Li XD, Minana MD, Matsui Y S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Voigt C, Schönaich M, Lill H. Anterior cruciate ligament reconstruction: state of the art. Eur J Trauma. 2006;32:332-339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Siegel L, Vandenakker-Albanese C, Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clin J Sport Med. 2012;22:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Markatos K, Kaseta MK, Lallos SN, Korres DS, Efstathopoulos N. The anatomy of the ACL and its importance in ACL reconstruction. Eur J Orthop Surg Traumatol. 2013;23:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Saka T. Principles of postoperative anterior cruciate ligament rehabilitation. World J Orthop. 2014;5:450-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 8. | Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis Prevalence Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Numbers-Needed-to-Treat Analysis. J Athl Train. 2014;49:806-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Murray MM, Vavken P, Fleming BC. The ACL Handbook. NY: Springer 2013; . |
| 10. | Vavken P, Murray MM. The potential for primary repair of the ACL. Sports Med Arthrosc. 2011;19:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Kohl S, Evangelopoulos DS, Ahmad SS, Kohlhof H, Herrmann G, Bonel H, Eggli S. A novel technique, dynamic intraligamentary stabilization creates optimal conditions for primary ACL healing: a preliminary biomechanical study. Knee. 2014;21:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Eggli S, Kohlhof H, Zumstein M, Henle P, Hartel M, Evangelopoulos DS, Bonel H, Kohl S. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2014;Mar 21; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Kohl S, Evangelopoulos DS, Kohlhof H, Hartel M, Bonel H, Henle P, von Rechenberg B, Eggli S. Anterior crucial ligament rupture: self-healing through dynamic intraligamentary stabilization technique. Knee Surg Sports Traumatol Arthrosc. 2013;21:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Milz S, Ockert B, Putz R. [Tenocytes and the extracellular matrix : a reciprocal relationship]. Orthopade. 2009;38:1071-1079. [PubMed] |
| 15. | Lui PP, Rui YF, Ni M, Chan KM. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. 2011;5:e144-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Canseco JA, Kojima K, Penvose AR, Ross JD, Obokata H, Gomoll AH, Vacanti CA. Effect on ligament marker expression by direct-contact co-culture of mesenchymal stem cells and anterior cruciate ligament cells. Tissue Eng Part A. 2012;18:2549-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 362] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 18. | Gantenbein-Ritter B, Benneker LM, Alini M, Grad S. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 2011;20:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Xiao J, Zhang Y, Wang J, Yu W, Wang W, Ma X. Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Appl Biochem Biotechnol. 2010;162:1996-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-177. [PubMed] |
| 21. | Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Attia E, Bohnert K, Brown H, Bhargava M, Hannafin JA. Characterization of total and active matrix metalloproteinases-1, -3, and -13 synthesized and secreted by anterior cruciate ligament fibroblasts in three-dimensional collagen gels. Tissue Eng Part A. 2014;20:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133700] [Article Influence: 5570.8] [Reference Citation Analysis (1)] |
| 25. | Goidin D, Mamessier A, Staquet M-J, Schmitt D, Berthier-Vergnes O. Ribosomal 18S RNA Prevails over Glyceraldehyde-3-Phosphate Dehydrogenase and [beta]-Actin Genes as Internal Standard for Quantitative Comparison of mRNA Levels in Invasive and Noninvasive Human Melanoma Cell Subpopulations. Anal Biochem. 2001;295:17-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Pawley J. Handbook of biological confocal microscopy. NY: Springer 2010; . |
| 27. | Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13-27. [PubMed] |
| 28. | Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, Wildemann B. Characterization of tendon cell cultures of the human rotator cuff. Eur Cell Mater. 2010;20:84-97. [PubMed] |
| 30. | Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33:2137-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Seiler C, Gazdhar A, Reyes M, Benneker LM, Geiser T, Siebenrock KA, Gantenbein-Ritter B. Time-lapse microscopy and classification of 2D human mesenchymal stem cells based on cell shape picks up myogenic from osteogenic and adipogenic differentiation. J Tissue Eng Regen Med. 2014;8:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Zhang J, Pan T, Im HJ, Fu FH, Wang JH. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011;9:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Fossett E, Khan WS, Longo UG, Smitham PJ. Effect of age and gender on cell proliferation and cell surface characterization of synovial fat pad derived mesenchymal stem cells. J Orthop Res. 2012;30:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Rust PA, Kalsi P, Briggs TW, Cannon SR, Blunn GW. Will mesenchymal stem cells differentiate into osteoblasts on allograft? Clin Orthop Relat Res. 2007;457:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Yoshida R, Cheng M, Murray MM. Increasing platelet concentration in platelet-rich plasma inhibits anterior cruciate ligament cell function in three-dimensional culture. J Orthop Res. 2014;32:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Fleming BC, Proffen BL, Vavken P, Shalvoy MR, Machan JT, Murray MM. Increased platelet concentration does not improve functional graft healing in bio-enhanced ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;Mar 18; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Pietschmann MF, Frankewycz B, Schmitz P, Docheva D, Sievers B, Jansson V, Schieker M, Müller PE. Comparison of tenocytes and mesenchymal stem cells seeded on biodegradable scaffolds in a full-size tendon defect model. J Mater Sci Mater Med. 2013;24:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11:124-132. [PubMed] |
| 39. | Hutchinson ID, Rodeo SA, Perrone GS, Murray MM. Can Platelet-Rich Plasma Enhance Anterior Cruciate Ligament and Meniscal Repair? J Knee Surg. 2015;28:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |