Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.495
Peer-review started: August 11, 2014
First decision: October 14, 2014
Revised: October 22, 2014
Accepted: October 31, 2014
Article in press: November 3, 2014
Published online: March 26, 2015
Processing time: 221 Days and 3 Hours
Within the epidermis and dermis of the skin, cells secrete and are surrounded by the extracellular matrix (ECM), which provides structural and biochemical support. The ECM of the epidermis is the basement membrane, and collagen and other dermal components constitute the ECM of the dermis. There is significant variation in the composition of the ECM of the epidermis and dermis, which can affect “cell to cell” and “cell to ECM” interactions. These interactions, in turn, can influence biological responses, aging, and wound healing; abnormal ECM signaling likely contributes to skin diseases. Thus, strategies for manipulating cell-ECM interactions are critical for treating wounds and a variety of skin diseases. Many of these strategies focus on epidermal stem cells, which reside in a unique niche in which the ECM is the most important component; interactions between the ECM and epidermal stem cells play a major role in regulating stem cell fate. As they constitute a major portion of the ECM, it is likely that integrins and type IV collagens are important in stem cell regulation and maintenance. In this review, we highlight recent research-including our previous work-exploring the role that the ECM and its associated components play in shaping the epidermal stem cell niche.
Core tip: Epidermal stem cells reside in a unique niche within the skin, which is shaped by interactions between stem cell-associated integrins and components of the extracellular matrix. Here, we review literature evaluating the role that integrins play in epidermal stem cell maintenance and proliferation, and highlight methods that have been used to enrich for epidermal stem cells. We stress that by understanding the epidermal stem cell niche, new regenerative medicine applications can be developed.
- Citation: Choi HR, Byun SY, Kwon SH, Park KC. Niche interactions in epidermal stem cells. World J Stem Cells 2015; 7(2): 495-501
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/495.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.495
The skin has epithelial and mesenchymal components[1]. Normally, tissues consist of differentiated cells and a small number of stem cells localized to specific niches[2]. Stem cell niches are specific microenvironments that determine stem cell number and affect stem cell mobility[3]. Furthermore, the complex nature of stem cell niches allows for the formation of distinct structures specialized for different stem cell types[4]. Epidermis is multilayered and is continually renewed and replaced by new cells, a process that results from the action of stem cells. It is known that the extracellular matrix (ECM) is a key component of the stem cell niche in the skin, and biophysical properties of the ECM (such as stiffness) also can affect cell fate determination[4]. As epidermal stem cells express high levels of integrins, these cells are able to interact directly with the ECM[1,4]. Thus, the interaction between stem cell-associated integrins and the ECM may be important in shaping the epidermal stem cell niche. In addition to the ECM and integrins, growth factors or mechanobiological factors can also affect stem cell fate[4,5]. Furthermore, low oxygen tensions (hypoxia) are necessary to maintain undifferentiated stem cell phenotypes, and also influence proliferation and stem cell fate[6]. However, among all of these factors, the ECM seems to play the largest role in shaping stem cell niches and stem cell behavior, and may thus have a major underlying role in physiological and pathological conditions.
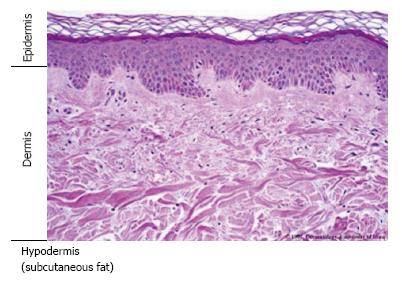
Skin is composed of the epidermis, dermis, and hypodermis (Figure 1). Multilayered epidermis serves as the outer layer of the skin. Additional structures such as hair follicles and sebaceous glands are also found within the skin. Stem cell proliferation and differentiation are vital for the maintenance of the epidermis, and different pools of skin stem cells are located in the hair follicle (the bulge) and the interfollicular epidermis[7,8]. Histologically, the lower layer of the epidermis is attached to the basement membrane (BM), which separates the epidermis from the underlying connective tissue layer known as the dermis.
The BM of the epidermis contains distinct subtypes of laminin, type IV collagen, nidogen, and perlecan (a heparan sulfate proteoglycan); immunohistochemical staining and gene expression studies have demonstrated that there is considerable regional variation in the BM[1]. The mechanical support provided by the BM is determined primarily by its type IV collagen scaffold; however, laminin is essential for the initial assembly of the BM in vivo[9]. Integrins also demonstrate variable expression in different regions of the epidermis[10], and these transmembrane proteins themselves have different kinds of combination (i.e., α2β1, α3β1, and α6β4). Integrins are the major receptors for ECM proteins, and interact with growth factors and cytokine receptors[4]. Growth factors and morphogens can also directly bind to the ECM; this suggests that the ECM might integrate different types of signals in biological microenvironments. As a result, clinical applications of stem cells have emphasized the relationship between epidermal stem cells and the ECM[4]. Recent research has sought to understand how the ECM and integrins cooperate with one another to form the architecture of the epidermal stem cell niche, and how they maintain the balance between stem cell renewal and differentiation[4]. It is possible that variations within the ECM may create distinct environments that can potentially affect the properties of different stem cell populations[1].
Stem cells are localized to “niches”-specific sites that regulate how stem cells participate in tissue maintenance and repair. In a niche, stem cell proliferation is regulated and these cells are protected from depletion[11]. Interestingly, evidence suggests that cancer-a common cause of death-is also a stem cell-based disease. Cancer stem cells (CSCs) are a subset of cancer cells that demonstrate stem cell-like abilities -abilities that may induce tumor growth and tumor recurrence. CSCs also require a special “CSC niche” to regulate their stemness and proliferation, and as with endogenous stem cells, this niche prevents CSCs from being depleted[12]. Thus, the stem cell niche is a key factor in the maintenance of stem cells.
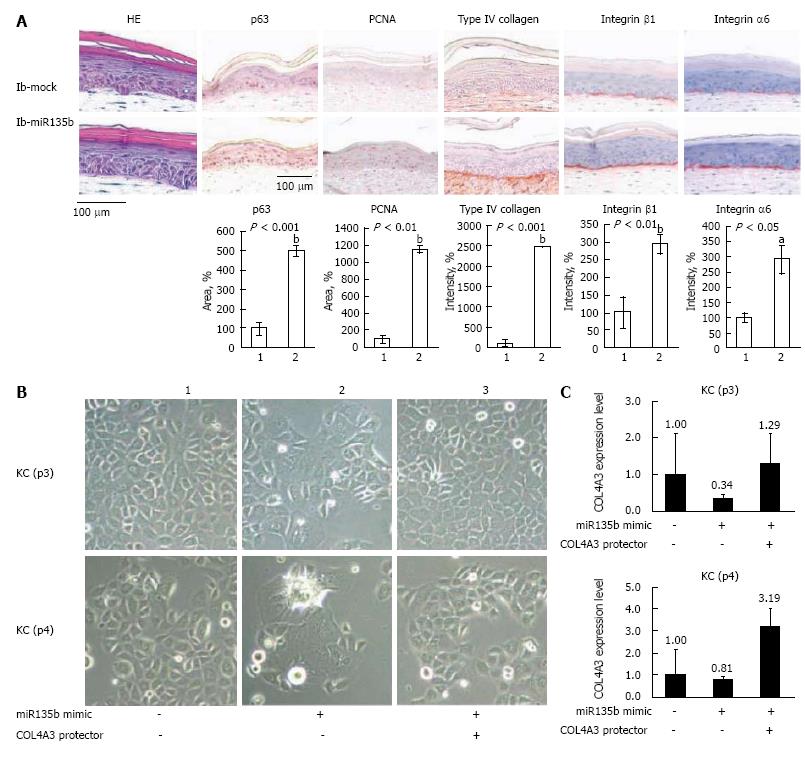
In the skin, an increased level of integrins expression is considered to be a marker of epidermal stem cells[13]. Although the expression levels of integrins in stem cells are only 2-3-fold higher than in stem cell progeny, this is sufficient to make stem cells more adhesive to ECM proteins[14]. Based on these findings, we developed a simple method to isolate epidermal stem cells based on integrin expression. As epidermal stem cells express high levels of integrins, type IV collagen is a good candidate to bind epidermal stem cells[14]. To purify epidermal stem cells, three keratinocyte populations were first characterized by their ability to bind type IV collagen: rapidly adhering (RA), slowly adhering (SA), and non-adhering (NA) cells. The expression levels of p63 and c-Myc were also compared between cells in these three populations. It was concluded that RA cells could be easily isolated from the epidermis, and that these cells were likely epidermal stem cells[15]. This research suggests that integrins may play an important role in the regulation of epidermal stem cells. Type IV collagen has not only been used to isolate epidermal stem cells, but has also been suggested to be directly involved in maintaining epidermal stem cells. Very recently, we reported that inhibition of miR135b could increase the proliferative potential of normal human keratinocytes[16]. Our data demonstrated that type IV collagen is a target of miR135b, and that the inhibition of miR135b may improve the epidermal stem cell microenvironment and increase the proliferative potential of basal cells (Figure 2). These findings suggest that restoration of type IV collagen to the basement membrane is important for stem cell maintenance in skin. Microarray analysis also revealed differences in the expression of ECM proteins between hair follicle stem cells and other epidermal stem cells[17]. These results suggest that integrins and type IV collagen are not the only proteins involved in determining the epidermal stem cell niche; ECM proteins are also important in shaping the niche within the interfollicular epidermis. Although no specific markers are available for the identification of epidermal stem cells, p63 is commonly regarded as a potential epidermal stem cell marker, as p63-/- mice fail to develop stratified squamous epithelia[18].
An effective response of the body to injury depends on regenerative processes that maintain proper cell numbers and replace damaged cells. This “reparative” potential is determined by the presence of stem and progenitor cells, which respond to exogenous cues such as tissue damage. Therapeutic applications of stem cells address the biological constraints of these cells, the stem cell niche, and the location to which these cells are summoned to respond. Understanding the role of the stem cell niche is essential for the application of stem cells in regenerative medicine[19].
A major concern in regenerative medicine is obtaining an adequate number of stem cells to treat an injury or disease. For practical applications, one of the most important problems is the small number of stem cells. In order to overcome this challenge, researchers often expand the number of stem cells by in vitro culture; however, most stem cells begin to lose their stem cell character during this process. In many cases, replicating the niche environment in culture is critical to maintain stem cell characteristics[20]. Similarly, “mimicking the native niche” has been shown to be important in tissue engineering of human corneal limbal crypts[21]. As wound healing is a critical process that re-establishes the epithelial barrier following disease or injury, poor wound healing increases infection risk, causes patient morbidity, and may lead to the scar formation. If there is a defect in the stem cell niche, wound repair could be delayed by the abnormal response of stem cells to injury[5]. Thus, understanding how to recreate the stem cell niche is key to regenerative medicine applications.
Integrins are a complicated group of proteins that function as transmembrane receptors; these proteins either connect cells to one another, or to the ECM. Such connections trigger signal transduction pathways, which result in biological responses such as cell proliferation or cell motility. Fibronectin, vitronectin, collagen, and laminin are all ligands for integrins. However, other proteins can mediate cell-cell or cell-ECM interactions; these include cadherins, the immunoglobulin superfamily of cell adhesion molecules, namely, selectins, and syndecans.
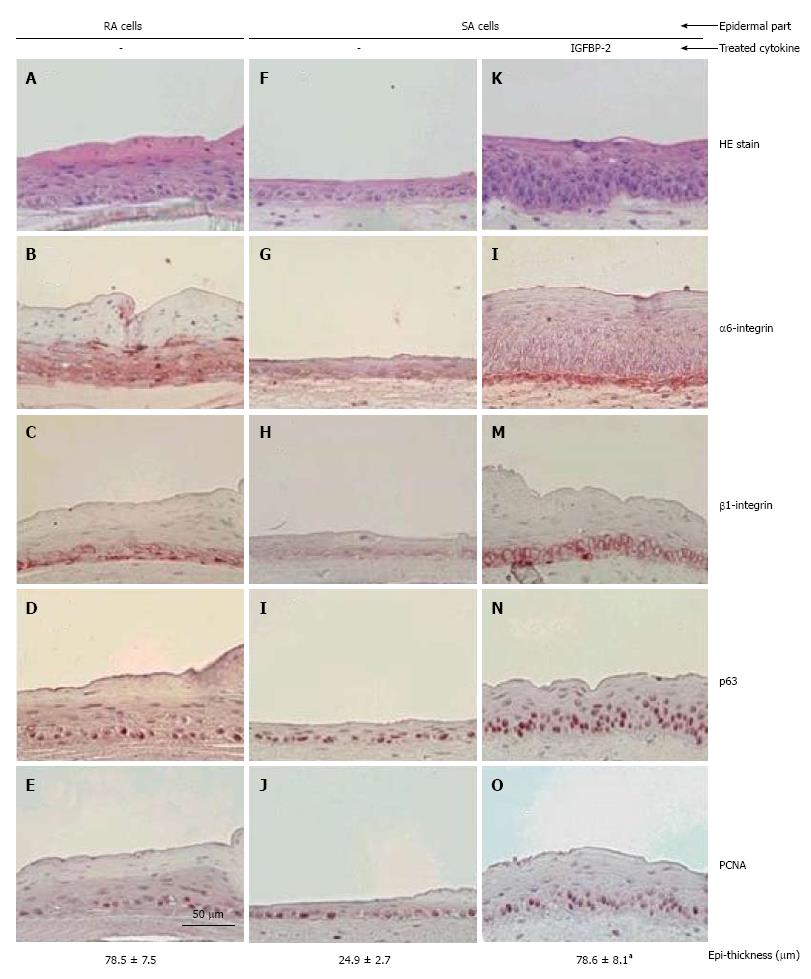
Stem cells, whether multipotent or tissue-specific, embryonic or adult, are controlled by intrinsic transcriptional regulation and extrinsic signals[22]. The extrinsic signals originate from the local microenvironment in which stem cells reside. It is well known that the ECM is an important component of the epidermal stem cell niche (see above). In the skin, high integrin levels can distinguish epidermal stem cells[14], and now integrin expression can be used to enrich for stem cells in mixed cell populations[23,24]. In our reports, on skin equivalents (SEs), addition of IGFBP-2 to culture media increased the expression levels of both α6 integrins and β1 integrins in cultured keratinocytes, which are associated with an increased number of epidermal stem cells (p63 positive cells)[25] (Figure 3). Furthermore, it has been reported that activation of β1 integrin decreases terminal differentiation[26]. Signaling studies have demonstrated that the Erk/MAP kinase pathway, which acts downstream of β1 integrins, provides a signal which inhibits differentiation[26,27]. Collectively, these findings are consistent with previous reports that integrins are related to stem cell maintenance.
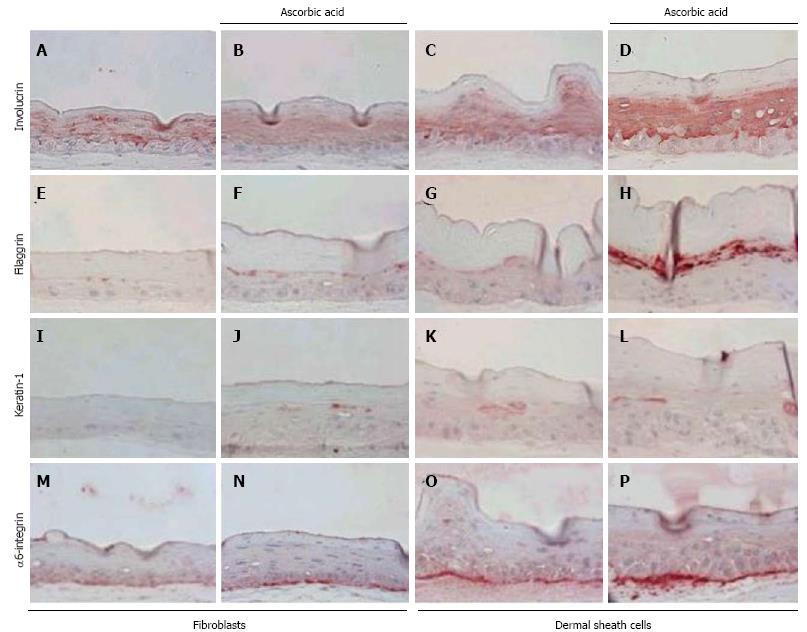
Few studies have dissected how epidermal integrin expression and ECM gene expression are regulated. In our previous study, we reported that when hair follicle dermal sheath cells were used to construct SEs, this resulted in increased epidermal thickness and increased expression of α6 integrins, compared to SEs generated from fibroblasts[28] (Figure 4). Building upon this study, it was found that IGFBP-2 is a major factor produced by dermal sheath cells, which regulates the regenerative capacity of the skin; IGFBP-2 plays an important role in maintaining stem cell characteristics in human epidermal keratinocytes[25]. These findings suggest that levels of integrins, which can be regulated by IGFBP-2, are vital for the maintenance of epidermal stem cell characteristics in the skin.
As it is well known that fetal wounds heal rapidly, we also evaluated the regenerative potential of-and expression of integrins in-neonatal keratinocytes and fibroblasts. When neonatal keratinocytes were used for epidermis reconstruction, expression of p63 was increased compared to adult keratinocytes; levels of integrins were also increased in this model[28]. Recently, we also demonstrated that when neonatal fibroblasts were used to make a dermal substitute, both integrin and p63 expression increased (personal communication). Collectively, these results demonstrate that neonatal cells maintain a higher regenerative potential compared to adult cells. This may result from the fact that neonatal cells have the potential to express more integrins, which favors the environment of the epidermal stem cell niche.
Many ECM genes are up regulated in epidermal stem cells. These proteins act as extrinsic signals that may affect stem cell fate. In this review, we have presented a general overview of ECM interactions, and discussed our previous reports demonstrating the important role that integrins and type IV collagen play in shaping the epidermal stem cell niche.
P- Reviewer: Latif N, Rentala S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3. [PubMed] |
| 2. | Pajonk F, Vlashi E. Characterization of the stem cell niche and its importance in radiobiological response. Semin Radiat Oncol. 2013;23:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. [PubMed] |
| 4. | Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013;28:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 7. | Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Breitkreutz D, Koxholt I, Thiemann K, Nischt R. Skin basement membrane: the foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int. 2013;2013:179784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 490] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1285] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 12. | Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol. 2014;35:3945-3951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Watt FM, Jones PH. Expression and function of the keratinocyte integrins. Dev Suppl. 1993;185-192. [PubMed] |
| 14. | Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 598] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 15. | Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci. 2004;61:2774-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Choi HR, Nam KM, Park SJ, Kim DS, Huh CH, Park WY, Park KC. Suppression of miR135b increases the proliferative potential of normal human keratinocytes. J Invest Dermatol. 2014;134:1161-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1000] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 18. | Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1739] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 19. | Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Katano T, Ootani A, Mizoshita T, Tanida S, Tsukamoto H, Ozeki K, Ebi M, Mori Y, Kataoka H, Kamiya T. Establishment of a long-term three-dimensional primary culture of mouse glandular stomach epithelial cells within the stem cell niche. Biochem Biophys Res Commun. 2013;432:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Levis HJ, Massie I, Dziasko MA, Kaasi A, Daniels JT. Rapid tissue engineering of biomimetic human corneal limbal crypts with 3D niche architecture. Biomaterials. 2013;34:8860-8868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993-997. [PubMed] |
| 24. | Wagers AJ, Weissman IL. Differential expression of alpha2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1+ hematopoietic stem cells. Stem Cells. 2006;24:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Kim DS, Cho HJ, Yang SK, Shin JW, Huh CH, Park KC. Insulin-like growth factor-binding protein contributes to the proliferation of less proliferative cells in forming skin equivalents. Tissue Eng Part A. 2009;15:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Evans RD, Perkins VC, Henry A, Stephens PE, Robinson MK, Watt FM. A tumor-associated beta 1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol. 2003;160:589-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA. 1999;96:6728-6733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Cho HJ, Bae IH, Chung HJ, Kim DS, Kwon SB, Cho YJ, Youn SW, Park KC. Effects of hair follicle dermal sheath cells in the reconstruction of skin equivalents. J Dermatol Sci. 2004;35:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |