Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.408
Peer-review started: July 12, 2014
First decision: September 24, 2014
Revised: October 1, 2014
Accepted: November 7, 2014
Article in press: November 10, 2014
Published online: March 26, 2015
Processing time: 251 Days and 10.1 Hours
Multipotent mesenchymal stromal cells [also referred to as mesenchymal stem cells (MSCs)] are a heterogeneous subset of stromal cells. They can be isolated from bone marrow and many other types of tissue. MSCs are currently being tested for therapeutic purposes (i.e., improving hematopoietic stem cell engraftment, managing inflammatory diseases and regenerating damaged organs). Their tropism for tumors and inflamed sites and their context-dependent potential for producing trophic and immunomodulatory factors raises the question as to whether MSCs promote cancer and/or infection. This article reviews the effect of MSCs on tumor establishment, growth and metastasis and also susceptibility to infection and its progression. Data published to date shows a paradoxical effect regarding MSCs, which seems to depend on isolation and expansion, cells source and dose and the route and timing of administration. Cancer and infection may thus be adverse or therapeutic effects arising form MSC administration.
Core tip: Mesenchymal stem cells (MSCs) derived from different origins have recently received much attention as potential therapeutic. However, such cells also appear to have essential functions in building and supporting tumor microenvironments. Here, we review the effect of MSCs on tumor establishment, as also susceptibility to infection and its progression. The literature reveals incongruity regarding the impact of MSCs on the development of cancer and infection; such paradoxical effect might be attributed to differences in isolation and expansion conditions, the source and dose of the cells, the administration route and its timing and host characteristics. MSCs immunomodulatory potential seems to be the leading mechanism responsible for such effects.
- Citation: Arango-Rodriguez ML, Ezquer F, Ezquer M, Conget P. Could cancer and infection be adverse effects of mesenchymal stromal cell therapy? World J Stem Cells 2015; 7(2): 408-417
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/408.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.408
Multipotent mesenchymal stromal cells, also referred to as mesenchymal stem cells (MSCs), were described for the first time half a century ago[1]. Such cells are distributed throughout the stroma of several organs in vivo whilst MSCs adhere to plastic in vitro and proliferate when stimulated by fetal bovine serum[1]. MSCs differentiate into mesodermal cells in vitro and in vivo (i.e., adipocytes, chondrocytes, osteocytes and myocytes)[2]. They can also cross the germ line barrier and produce cells from endo- and ectodermal lineages, such property being known as cell plasticity[3].
MSCs are an ideal tool for cell therapy because they are easily procured from live donors[4] and can be efficiently expanded ex vivo[5]. The receptors do not need to have been conditioned before cell administration transplant[6], as in total bone marrow or hematopoietic stem cell transplant. Once administered intravenously, they are able to home onto and engraft into damaged tissue where they could become differentiated into tissue-specific cells, release trophic factors, promote neovascularization, manage oxidative stress and fibrosis, or trigger an anti-inflammatory response[7-11].
MSCs from the same individual (autologous) were administered into a human for the first time in 1995[12]; MSCs were safely allogeneically transplanted seven years later[13]. More than 350 clinical trials involving the use of MSCs are currently under way (http://www.clinicaltrials.gov) and no serious adverse events have been reported to date. Nevertheless, MSCs biosafety is still a major concern, particularly regarding the development of adverse event-related cancer and infection.
Like any other cell, when MSCs are manipulated in the long-term they might have chromosomal aberrations and produce tumors in healthy animals[14]; this has mainly been reported regarding mouse cells, which require extensive cultures for producing a significant number of hematopoietic-free MSCs[14]. For instance, it has been shown that intravenously administered NOC/SCID bone marrow-derived MSCs embolize within the lung capillaries, expand and invade the lung parenchyma and form tumor nodules[15]. These lesions rarely contain lung epithelial cells but they have the characteristics of cartilage and immature bone resembling well-differentiated osteosarcoma.
No transformation has been proven so far for human MSCs when expanded properly ex vivo (i.e., non-exhausted and not forced to cell crisis)[14]. The Canadian Critical Care Trials Group has recently published a meta-analysis of randomized, non-randomized, controlled and uncontrolled, phase I and phase II clinical trials[16]; no association between autologous or allogeneic MSCs administration and tumor formation was reported in the 36 studies reviewed by them. Nonetheless, longer follow-up is required to draw a final conclusion regarding human MSCs’ tumorigenic potential.
Human bone marrow-derived MSCs have increased the growth of ERAα positive breast cancer cell lines (T47D, BT474 and ZR-75-1) in an in vitro three-dimensional tumor environment, but have had no effect on an ERAα negative cell line (MDA-MB-231)[17]; however, the growth rate of another ERAα negative cell line (MDA-MB-468) was high in the presence of human MSCs. Another study has shown that both human fetal MSCs and human adipose-derived MSCs transplanted subcutaneously into BALB/c-nu/nu mice alone or together with tumor cell lines F6 or SW480 (ratio 1:1 or 1:10), favored the growth of these tumor cell lines[18].
Tumor cells obtained from primary breast cancer grown in the presence of human bone marrow-derived MSCs (ratio 1:1) and tested in secondary mice have been seen to have greater tumor-producing ability than cells obtained from primary tumors and grown in the absence of MSCs[19]. Besides, tumor incidence and/or size[18,20,21] as well as tumor vascularity[22] have all increased when breast, lung, colon or prostate tumor cells have been co-injected with human adipose-derived or bone marrow-derived MSCs. The same has been proven for osteosarcoma, melanoma and glioma tumor cells[23]. Another interesting observation concerned adipose tissue implant adjacent to lung cancer or Kaposi sarcoma xenografts resulting in a substantial increase in tumor size along with the appearance of stromal cells from the implant; adipose-derived MSCs can thus promote tumor growth[24].
MSCs’ innate tropism for established tumors has been widely reported[24], yet the mechanism behind it still remains to be fully elucidated[25]. The explanation advanced to date is that tumors behave as unresolved wounds as their stroma closely resemble healing granulation tissue and they produce cytokines, chemokines and other chemoattractants[26] and MSCs chemotactic properties are similar to those of leukocytes[27,28]. MSCs tropism for tumors has been successfully exploited for the delivery of antitumor agents in animal models of lung and breast cancer and melanoma and glioma[25].
Breast cancer cells co-cultured with human bone marrow-derived MSCs (ratio 1:1) up-regulate the expression of oncogenes and proto-oncogenes associated with tissue invasion, angiogenesis and apoptosis (i.e., N-cadherin, vimentin, Twist, Snail and E-cadherin)[29]. Such molecular changes have been accompanied by morphological and growth alterations, these being features of a more metastatic phenotype. It has been seen that 0.5 × 105 breast cancer cells co-injected subcutaneously with 1.3 × 106 human bone marrow-derived MSCs have significantly increased lung metastasis rate in NOD/SCID mice. This effect was lost when bone marrow-derived MSCs were injected separately from tumor cells[20]. On the other hand, it has been shown that bone marrow-derived MSCs facilitate cancer cells [MCF-7, T47D low invasive cell lines and stromal cell-derived factor 1 (SDF-1)null MDA-MB-231 highly aggressive ones] homing into bone marrow and have modified the metastatic niche through trophic factor secretion (SDF-1 and CXCR4) and improved neovasculogenesis in a xenogeneic mouse model[30].
It has been shown that human bone marrow-derived MSCs interfere in vitro with small cell lung cancer (A549), esophageal cancer (Eca-109), Kaposi’s sarcoma and leukemic cell line proliferation kinetics[31]. The foregoing was observed when 0.5 × 105 tumor cells were co-cultivated with 0.5 × 105 human bone marrow-derived MSCs but also when they were exposed to MSCs-conditioned medium; cells were arrested during the cell cycle G1 phase in both cases by the downregulation of cyclin D2 and induction of apoptosis[32,33]. MSCs from other sources, including human fetal skin-derived MSCs and adipose-derived MSCs, have also inhibited the growth of human liver cancer cell lines[34], breast cancer (MCF-7)[35] and primary leukemia cells by reducing their proliferation, colony formation and oncogene expression[22]. The intravenous injection of 4 × 106 human bone marrow-derived MSCs into Kaposi’s sarcoma-bearing nude mice has inhibited tumor cell growth[36]. A similar effect has been observed in an animal model of hepatocellular carcinoma and pancreatic tumors as altering cell cycle progression has led to decreased cell proliferation[22,37]; the same has happened with melanoma due to increased apoptosis of capillaries[38] and rat colon carcinoma growth has been inhibited when rat MSCs (the MPC1cE cell line) were co-implanted with tumor cells in a 1:1 or 1:10 ratio[39].
Human fetal skin-derived MSCs (Z3 cell line) have also delayed liver tumor growth and decreased tumor size when injected with the same number of cells from the H7402 cell line in SCID mice[34]. Injecting human adipose-derived MSCs (1 × 103 cells/mm3) into established pancreatic cancer xenografts has led to apoptosis and the abrogation of tumor growth in female Swiss nude (athymic) mice[37].
The role of MSCs in cancer thus remains paradoxical. Evidence to date has suggested that they are pro- as well as anti-tumorigenic[40-42] such discrepancy seems to depend on isolation and expansion conditions, cell source and dose, the administration route and the tumor model used.
MSCs can be recruited into inflamed sites secondary to microbial infection where they promote potent immune-suppressive activity[43,44]. For instance, it has been shown that administering MSCs (1.25 × 105 cells/kg) to animals infected by Trypanosoma cruzi (T. cruzi, protozoa) or Mycobacterium tuberculosis (Mtb, bacteria) has worsened the natural course of infection. Activated macrophages play an essential role in host defense against T. cruzi as they can destroy intracellular parasites via interferon (INF)-γ- and tumor necrosis factor (TNF)-α-stimulated nitric oxide (NO) production[45]. It has been shown that mice bone marrow-derived MSCs switch macrophages to an anti-inflammatory profile, thereby suppressing inflammatory cytokine production and enhancing interleukin (IL)-10 production[46]. An immune response to Mtb depends on IFN-γ-producing T-lymphocytes activating macrophages to produce NO[47,48]. Bone marrow-derived MSCs (2.5 × 105/kg) infusion into animals, which are normally resistant to this infection [transforming growth factor β (TGF-β) RIIDN transgenic mice], has resulted in making them susceptible to disease. Furthermore, it has been observed that donor MSCs have been recruited to the periphery of live bacteria-containing granuloma and have induced regulatory T-cell differentiation, thus resulting in immunosuppression.
A recent study aimed to prove the safety and feasibility of autologous bone marrow-derived MSCs infusion (1 × 106 cells/kg, two doses) into kidney allograft recipients, showing that three out of six enrolled patients developed an opportunistic viral infection[49].
Regarding fungal infection, the intravenous administration of an IL-17-producing sub-population of bone marrow derived-MSCs (1 × 106 cells) significantly reduced the fungal burden of kidneys in immunocompetent mice, which had suffered invasive candidiasis[50].
Both un-stimulated and IFN-γ stimulated human MSCs can inhibit the growth of Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, as well as the growth of Gram-positive pathogens such as Staphylococcus aureus, Staphylococcus epidermidis, group B Streptococci and Enterococcus faecium[46,51]. MSCs’ antimicrobial effect depends on whether they have been stimulated[51]; while cathelicidin LL-37 antimicrobial peptide is critical to un-stimulated human MSCs, tryptophan-catabolizing enzyme heme oxygenase-1 and indoleamine-2,3-dioxygenase (IDO) are needed in IFN-γ-stimulated human MSCs. Tryptophan depletion and toxic kynurenine accumulation leads to the inhibition of bacterial growth in the latter case. Differences between human and murine MSCs antibacterial activity have also been reported; murine MSCs do not produce cathelicidin LL-37 and cannot express IDO, even after stimulation with a combination of cytokines, but they do produce lipocalin 2 (an antimicrobial molecule)[52].
Little data has been published concerning the impact of MSCs on viral pathogens. One study has reported that IFN-γ-stimulated human MSCs have reduced intracellular replication of cytomegalovirus and herpes simplex virus type 1 in vitro[46], such effect being attributed to IDO activity[46].
Together with MSCs’ direct antimicrobial effect, it has been shown that they play an important role in the complex network of host immune response against pathogens, particularly regarding the dynamic coordination of the immune system’s pro- and anti-inflammatory components[53].
MSCs’ antimicrobial activity observed in vitro has been clearly supported by animal models of experimental infection, such as polymicrobial sepsis[42], lipopolysaccharide (LPS) administration[54] and pulmonary respiratory distress syndrome[55]. Regardless of MSCs source, administration route (intravenously cf intraperitoneally) or strategy (prophylaxis cf therapy), their administration leads to reduced pathogen burden and significantly improved survival rate[41,42,53,56].
It has been shown that administering 2.5 × 105 mouse bone marrow-derived MSCs led to decreased mortality, controlled multi-organ dysfunction/injury and reduced pulmonary and systemic inflammation in a clinically relevant model of polymicrobial sepsis where infection was settled after the inoculation of Gram-negative and Gram-positive organisms[42]. An endotoxemic rat model (involving intravenous LPS injection) has been used to demonstrate that administering 2.5 × 105 human adipose-derived MSCs decreased inflammatory cytokine level in serum and the lungs, reduced inflammatory changes in the lungs, prevented apoptosis in the kidneys and reduced multi-organ injury[54]. A pulmonary respiratory distress syndrome model (induced by intratracheal endotoxin administration) has been used to show that the intrapulmonary delivery of mouse bone marrow-derived MSCs has down-regulated an LPS-induced inflammatory response and reduced lung injury, while direct lung injury by toxins or pneumonitis led to severe pulmonary edema and inflammation[57,58].
MSCs' antimicrobial effect has also been demonstrated in the blood, peritoneum, liver and spleen, using a Gram-negative pneumonia model involving immunocompetent mice[42,44,56].
As MSCs might lessen the development of infection, they have been used recently in a clinical study aimed at treating patients suffering acute respiratory distress syndrome (NCT01902082); one intravenous dose of 1 × 106 cells/kg allogeneic adipose-derived MSCs proved to represent a safe and feasible therapeutic tool for this infection[59].
MSCs have been seen as an innovative therapeutic tool for preventing or treating graft-versus-host disease (GvHD) following allogeneic hematopoietic stem cell transplant (HSCT)[60,61] owing to their immunosuppressive properties, such as not eliciting immunological responses from alloreactive T-lymphocytes and/or other immunological effector cells. However, it is not known whether using immunosuppressive MSCs may inadvertently inhibit antimicrobial immune responses and ultimately result in an increased risk of infection in allogeneic HSCT recipients[62], considering that infection is one of the major complications following an HSCT contributing to high morbidity and mortality indexes[63,64]. One open randomized clinical trial has demonstrated that acute grade II-IV and chronic GVHD incidence in 10 patients receiving a median 3.4 × 105/kg MSCs dose from a human leukocyte antigen-identical sibling donor was lower than in 15 patients who did not receive MSCs (11% cf 53% and 14% cf 29%, respectively)[65]. Unfortunately, this did not mean a lower risk for infectious complications. Early and mid-phase severe infection incidence was even higher in patients who had received a co-transplant of hematopoietic stem cells and MSCs compared to a control group, which did not receive MSCs, although differences were not statistically significant [4/10 (40%) cf 5/15 (33%)]. Patients receiving MSCs suffered from cytomegalovirus (CMV) interstitial pneumonia and bacterial and/or fungal infection whereas this was only seen in two of the patients who did not receive MSCs[65]; no patient treated with MSCs died because of infectious complications, whereas this happened in two control group patients who did not receive MSCs. This raises the question of whether infection severity is lower when MSCs are co-transplanted with a graft.
By contrast, another two non-randomized clinical trials, involving 20 patients[66] and 14 pediatric patients[67], showed that co-transplanting MSCs did not result in higher infection incidence and severity when compared to historical controls.
On the other hand, multivariate analysis regarding a retrospective cohort study of 691 HSCT patients showed that GVHD grade II-IV, CMV infection and having received human bone marrow-derived MSCs were factors which were associated with overall pneumonia-related deaths[68].
Thus, the role of MSCs in infection is paradoxical. Evidence reported to date suggests that there may be pro- as well as anti-microbial effects[40-42] and this seems to depend on isolation and expansion conditions, cell origin and dose and administration route and timing.
Although the cancer stem cell (CSC) concept was first introduced in hematological malignancies (chronic and acute leukemia)[69], it has been identified during recent years in a variety of solid tumors such as glioblastomas, medulloblastomas and carcinomas[70]. It has been demonstrated that MSCs interact with CSC in human cancer and regulate their own self-renewal through cytokine networks involving IL-6 and CXCL7[19]. CSC-produced IL-6 interacts with IL6R/gp130 expressed on MSCs to produce CXCL7; this molecule interacts with CSCs through the CXCR2 receptor where it induces the synthesis of others cytokines (i.e., IL-8, IL-6, CXCL6, and CXCL5)[19]. These cytokines trigger CSC self-renewal and enhance their invasive properties while IL-6 mediates chemotaxis, which may facilitate MSCs homing to primary tumor growth sites. It has been shown that MSCs administered subcutaneously in mice having had a breast tumor xenograft became recruited to the tumors and produced IL-6 and IL-8, which accelerated their growth by regulating the CSC population[20].
Most malignancies have an epithelial origin, and cancer progression is often associated with epithelial-to-mesenchymal transition (EMT)[71]; this is a physiological process, which is recognized as being crucial for embryogenesis and wound healing. It involves epithelial cell conversion to mesenchymal cells through the disruption of cell-cell junctions and the reorganization of the actin cytoskeleton; EMT has gained much attention recently due to its role in converting benign lesions into invasive and metastatic tumors[72]. It is governed by complex networks, which are influenced by signals from the neoplastic microenvironment, such as collagen, cytokines and TGFβ, epidermal growth factor, fibroblast growth factor (FGF), hepatocyte growth factor (HGF) and platelet-derived growth factor[71-73]. Interestingly, all the aforementioned factors are secreted by MSCs[9].
Vasculogenesis plays a critical role in tumor growth[74]; MSCs could contribute towards tumor vasculogenesis because they act as pericytes but may also differentiate into endothelial cells and secrete provasculogenic factors[75-77], thereby allowing blood vessel formation[75]. Vascular endothelial growth factor (VEGF) and FGF-2 are the two main MSCs-secreted vasculogenic factors involved in tumor neovascularization[76]. VEGF is known to regulate MSCs mobilization and recruitment to neovascularization sites and directs MSCs differentiation to vascular cell[78,79]. VEGF expression in MSCs can be enhanced by hypoxia, a common phenomenon in tumor tissue[80] whilst FGF-2 is a potent mitogen which is produced and secreted by endothelial cells and MSCs[81]. This factor has been implicated in cell proliferation and endothelial cell migration during tumor growth[81]; conversely, MSCs appear to reduce vascular density due to endothelial cell cytotoxicity in certain conditions[38].
MSCs suppress both innate and adaptive immune responses[82,83]; they inhibit CD4+ and CD8+ T-cell proliferation[84] by producing a wide range of mediators, including TGFβ1, HGF, insulin-like growth factor, prostaglandin E2, NO, heme oxigenase-1 and IDO[85-89]. MSCs also inhibit monocyte and hematopoietic progenitor proliferation and differentiation into mature dendritic cells[32,90]. Other MSCs-induced effects regarding dendritic cells would be a loss of their ability to stimulate allo-responses[91], acquiring a regulatory phenotype due to the production of large amounts of IL-10[91] and changing dendritic cells’ cytokine secretion profile by MSCs-derived PGE2[91]. MSCs alter the natural killer (NK) cell phenotype besides suppressing their proliferation and cytokine secretion[92]; this requires cell-to-cell contact and soluble factors (TGFβ1 and PGE2). Hence, MSCs could promote an anti-inflammatory response within a tumor, thereby allowing its enlargement[93]. Systemically administered MSCs have promoted immune-tolerance in damaged organs, irrespective of whether donor cells home into them[7,8,94]. It is expected that MSCs would worsen the immune-destruction of tumor cells and thus facilitate tumor growth and metastasis. Conversely, increased macrophage and granulocyte infiltration in MSCs-injected tumors has been shown, suggesting that allogeneic MSCs immunogenicity might contribute towards their antitumor effect[32,39].
Changes in MSCs microenvironment, together with changes in transformed cells, would also seem to contribute towards carcinogenesis[95].
MSCs can participate in host defense through the secretion of antimicrobial peptides (cathelicidin LL-37[51] and lipocalin 2[52]), which can directly inhibit bacterial growth or kill the pathogens. The secretion of these soluble peptides improves resident phagocyte ability to clear bacteria through the up-regulation of pathways associated with monocyte/macrophage, phagocytosis, NK cell activity and antigen presentation[42] while MSCs antifungal activity means an increased amount of TH17 cells in the blood, thereby promoting TH1-type immune responses and restraining TH2-type ones[50].
MSCs induce a marked decrease in Toll-like receptor 2 expression, which plays a fundamental role in pathogen recognition and activation of innate immunity[96]. MSCs induce a marked increase in macrophage susceptibility to infection by parasites and bacteria. The mechanisms so involved appear to be linked to the production of inflammatory cytokines TNF-α, IL-12p70 and IFN-γ which drive NO production[43]. MSCs switch activated macrophages into regulatory ones producing low levels of pro-inflammatory cytokines. MSCs could modify an immune response against microorganisms by inducing apoptosis and cell-cycle arrest of T-cells by producing NO, TGFβ or IDO[91]. MSCs can inhibit cellular immune responses and promote regulatory T-lymphocyte production, thereby establishing T-cell tolerance for microorganisms[97-100].
In vitro and in vivo studies have demonstrated MSCs’ pro- and anti-cancer and pro- and anti-infection effects nevertheless, most clinical trials have reported that MSCs-based therapy appears safe and has not been associated with serve adverse events. Together, due to MSCs’ context-dependent potential to produce immune-modulatory factors they seem to be an ideal therapeutic tool for both cancer and infections.
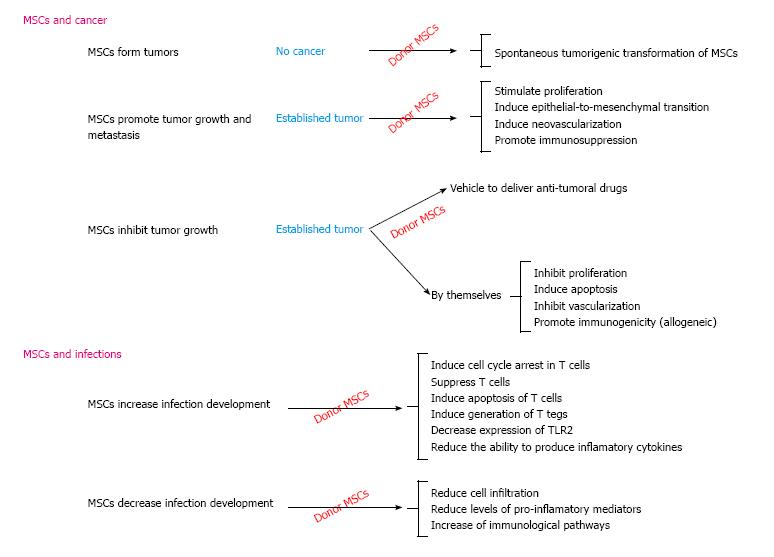
The pertinent literature reveals incongruity regarding the impact of MSCs on the development of cancer and infection (Figure 1); such paradoxical effect might be attributed to differences in isolation and expansion conditions, the source and dose of the cells being used, the administration route and its timing and host characteristics. MSCs immunomodulatory potential seems to be the leading mechanism responsible for such effects. Until conclusive data becomes available, cancer and infection will still be seen as adverse effects and therapeutic targets for using MSCs-based therapy.
We thank Mr. Jason Garry for English editing of the manuscript.
P- Reviewer: Kerkis I S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1276] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 2. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15195] [Article Influence: 584.4] [Reference Citation Analysis (0)] |
| 3. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 4. | Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226:507-520. [PubMed] |
| 6. | Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Ezquer F, Ezquer M, Simon V, Conget P. The antidiabetic effect of MSCs is not impaired by insulin prophylaxis and is not improved by a second dose of cells. PLoS One. 2011;6:e16566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ezquer F, Ezquer M, Simon V, Pardo F, Yañez A, Carpio D, Conget P. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [PubMed] |
| 10. | Ishikane S, Hosoda H, Yamahara K, Akitake Y, Kyoungsook J, Mishima K, Iwasaki K, Fujiwara M, Miyazato M, Kangawa K. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557-564. [PubMed] |
| 13. | Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932-8937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1202] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 14. | Rubio D, Garcia S, De la Cueva T, Paz MF, Lloyd AC, Bernad A, Garcia-Castro J. Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Exp Cell Res. 2008;314:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 847] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 17. | Sasser AK, Mundy BL, Smith KM, Studebaker AW, Axel AE, Haidet AM, Fernandez SA, Hall BM. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255-264. [PubMed] |
| 18. | Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 20. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [PubMed] |
| 21. | Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 23. | Bian ZY, Fan QM, Li G, Xu WT, Tang TT. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101:2554-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259-5266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 429] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 26. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. [PubMed] |
| 27. | Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1672] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 29. | Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010;124:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS, Rameshwar P. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One. 2008;3:e2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Tian LL, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 33. | Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 34. | Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235-1247. [PubMed] |
| 37. | Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, Touil I, André M, Grolleau JL, Péron JM. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197-4205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 39. | Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248-255. [PubMed] |
| 40. | Alagesan S, Griffin MD. Autologous and allogeneic mesenchymal stem cells in organ transplantation: what do we know about their safety and efficacy? Curr Opin Organ Transplant. 2014;19:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Hall SR, Tsoyi K, Ith B, Padera RF, Lederer JA, Wang Z, Liu X, Perrella MA. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 43. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 44. | Raghuvanshi S, Sharma P, Singh S, Van Kaer L, Das G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:21653-21658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Romano PS, Cueto JA, Casassa AF, Vanrell MC, Gottlieb RA, Colombo MI. Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay. IUBMB Life. 2012;64:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Meisel R, Brockers S, Heseler K, Degistirici O, Bülle H, Woite C, Stuhlsatz S, Schwippert W, Jäger M, Sorg R. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648-654. [PubMed] |
| 47. | MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3127] [Cited by in RCA: 3072] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 48. | Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol. 2000;3:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 50. | Yang R, Liu Y, Kelk P, Qu C, Akiyama K, Chen C, Atsuta I, Chen W, Zhou Y, Shi S. A subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans effect. Cell Res. 2013;23:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229-2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 52. | Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 53. | Balan A, Lucchini G, Schmidt S, Schneider A, Tramsen L, Kuci S, Meisel R, Bader P, Lehrnbecher T. Mesenchymal stromal cells in the antimicrobial host response of hematopoietic stem cell recipients with graft-versus-host disease-friends or foes? Leukemia. 2014;28:1941-1948. [PubMed] |
| 54. | Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee W, Choi S. The therapeutic effect of human adult stem cells derived from adipose tissue in endotoxemic rat model. Int J Med Sci. 2013;10:8-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Curley GF, Scott JA, Laffey JG. Therapeutic potential and mechanisms of action of mesenchymal stromal cells for acute respiratory distress syndrome. Curr Stem Cell Res Ther. 2014;9:319-329. [PubMed] |
| 56. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 57. | Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. [PubMed] |
| 59. | Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 60. | Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012;18:822-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Kim EJ, Kim N, Cho SG. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med. 2013;45:e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1334] [Article Influence: 74.1] [Reference Citation Analysis (1)] |
| 63. | Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med. 2003;54:491-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Sparrelid E, Hägglund H, Remberger M, Ringdén O, Lönnqvist B, Ljungman P, Andersson J. Bacteraemia during the aplastic phase after allogeneic bone marrow transplantation is associated with early death from invasive fungal infection. Bone Marrow Transplant. 1998;22:795-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, Yu Z, Li B, Xu C, Li Y. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 66. | Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen JF, Hafraoui K, Lejeune M, Gothot A. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 67. | Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-2767. [PubMed] |
| 68. | Forslöw U, Blennow O, LeBlanc K, Ringdén O, Gustafsson B, Mattsson J, Remberger M. Treatment with mesenchymal stromal cells is a risk factor for pneumonia-related death after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2012;89:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4858] [Article Influence: 173.5] [Reference Citation Analysis (1)] |
| 70. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [PubMed] |
| 71. | Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12:1383-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 72. | Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991-5995; discussion 5995. [PubMed] |
| 73. | Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Hiratsuka S. Vasculogenensis, angiogenesis and special features of tumor blood vessels. Front Biosci (Landmark Ed). 2011;16:1413-1427. [PubMed] |
| 75. | Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 76. | Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 621] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 77. | Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 79. | Gyöngyösi M, Posa A, Pavo N, Hemetsberger R, Kvakan H, Steiner-Böker S, Petrási Z, Manczur F, Pavo IJ, Edes IF. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thromb Haemost. 2010;104:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 81. | Delli-Bovi P, Curatola AM, Newman KM, Sato Y, Moscatelli D, Hewick RM, Rifkin DB, Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988;8:2933-2941. [PubMed] |
| 82. | Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013;2:22-38. [PubMed] |
| 83. | Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 84. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 832] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 85. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1730] [Cited by in RCA: 1642] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 86. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 87. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 88. | Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691-3694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 89. | Gieseke F, Schütt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007;110:2197-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 91. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3276] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 92. | Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 683] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 93. | Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 94. | Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 95. | Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J Surg Oncol. 2011;103:468-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 96. | Mo IF, Yip KH, Chan WK, Law HK, Lau YL, Chan GC. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Niedbala W, Besnard AG, Jiang HR, Alves-Filho JC, Fukada SY, Nascimento D, Mitani A, Pushparaj P, Alqahtani MH, Liew FY. Nitric oxide-induced regulatory T cells inhibit Th17 but not Th1 cell differentiation and function. J Immunol. 2013;191:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 98. | Yuan J, Zhang G, Yang X, Liu K, Wang F. Transplantation of allograft transforming growth factor-β1 transfected CD103⁺ lamina propria dendritic cells could effectively induce antigen-specific regulatory T cells in vivo. Transplant Proc. 2013;45:3408-3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Curran TA, Jalili RB, Farrokhi A, Ghahary A. IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells. Immunobiology. 2014;219:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res. 2014;324:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |