INTRODUCTION
The musculoskeletal (MSK) system is comprised of the bones, joints, tendons, ligaments and other connective tissues within the body of an organism to provide form, support, stability and movement. The structural and functional integrity of these distinctive parts is crucial for organ functioning, as well as for its integration with other systems. The functioning of the MSK system influences complex inter-system connectivity, including from interactions between physical activity and the environment to interpersonal relationships and social integration. The status of the MSK impacts metabolic activities and overall health in individuals of all ages, as well as functional independence and cognitive preservation in the elderly[1].
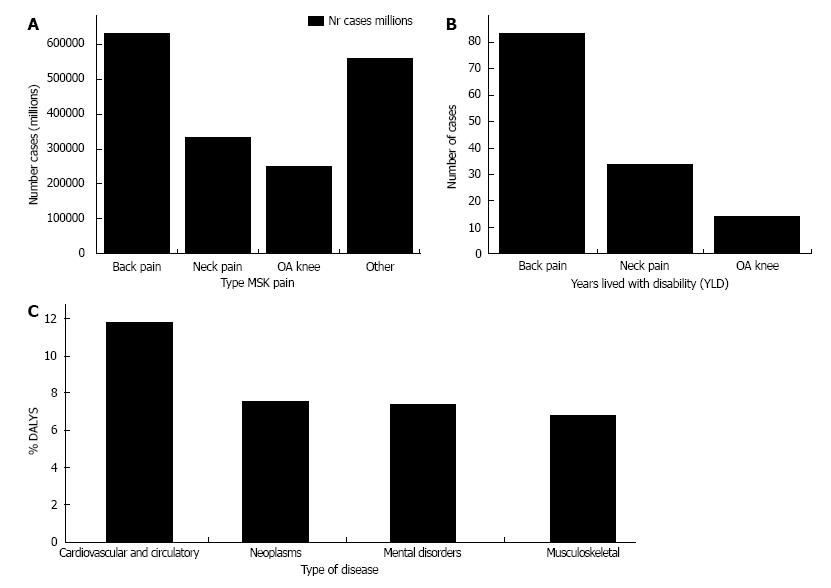
MSK diseases are a group of various acute and chronic traumatic, degenerative, tumoral or congenital conditions affecting one or several components of the MSK system. More than 150 MSK diseases or symptoms have been identified, comprising the most frequent cause of physical disability in the developed world. The global burden of MSK disease is dramatically rising, mainly due to the ageing of the population and incidence of musculoskeletal trauma[2]. Pain and loss of function are major symptoms associated with MSK disease (Figure 1), which impact quality of life and result in increasing health expenditures that are a major concern at individual, familial and societal levels.
Figure 1 Prevalence of musculoskeletal pain.
A: Number of persons affected by musculoskeletal pain (millions); B: Global number of years lived with disability (YLD; millions); C: Causes of disability-adjusted life years (DALY). Data are as of 2010, updated from the Source Global Burden of Disease 2010 Study[75]. OA: Osteoarthritis; MSK: Musculoskeletal.
Although current therapies only partially address MSK diseases, regenerative medicine (RM) provides the potential for efficient prevention, early detection, and treatment[3]. The use of stem cell therapies and stem cell-based tissue engineering offer clinically relevant solutions for the treatment of bone and cartilage defects, osteoarthritis (OA), inflammatory arthritis and tendon injuries[4]. Moreover, RM provides an opportunity to design therapies that are noninvasive, avoid the use of inert implants, and shorten rehabilitation time[5]. Although the primary goal of RM is complete structural and functional restoration of MSK tissues and organs, it may not be a realistic one due to the heterogeneous nature of MSK diseases, which include a wide diversity of associated pathologies and co-morbidities. As an example, different stages of OA and the complex situations generated by associated metabolic conditions present as distinct clinical entities. Such cases can be regarded as different forms of the same disease, which should be considered when designing RM therapies rather than aiming for a “one-size-fits-all” solution.
A clearly defined therapeutic goal backed by realistic assessment of individual necessities could help increase clinical applicability of RM interventions. It is known that MSK-related pain ranges from merely a symptom to a disease that solicits specific therapeutic intervention, such as with neuropathic pain in advanced stages of OA, or in OA associated with diabetes mellitus, complex regional pain or entrapment syndromes. Pain treatment should be regarded as a distinct therapeutic goal for functional and structural rehabilitation of the MSK system. This article briefly describes principal types of MSK-related pain and reviews stem cell-based therapies that are specifically designed for its treatment.
PRINCIPAL TYPES OF MSK-RELATED PAIN
Acute pain
Two of the most common causes of acute pain are MSK trauma or inflammation. Trauma can be isolated or encountered during the onset or flare-up of chronic conditions (such as inflammatory arthritis, OA, or tendonitis). The intensity and duration of acute pain varies, and usually correlates with the intensity and duration of the producing agent. Acute pain has been defined by the International Association for the Study of Pain as “the normal, predicted physiologic response to an adverse chemical, thermal, or mechanical stimulus[...]associated with surgery, trauma, or acute illness[5].” However, in its severe forms, acute pain needs to be regarded as potentially life threatening[6,7].
Acute pain is the result of tissue-specific nociceptor activation, sensitization and hyperalgesia. Nociceptors are the sensory receptors activated by injury that have different reactivity levels, and are tissue-and stimuli-dependent. Primary hyperalgesia from trauma or acute inflammationis produced by nociceptor sensitization. Secondary hyperalgesia refers to the increased responsiveness of central nervous system neurons to otherwise normal or subliminal peripheral afferent input, termed central sensitization, resulting in increased pain responses to stimuli that are outside the area of the initial injury[8]. Acute pain is normally self-limited, and can resolve completely or transform into chronic pain and/or generate immediate or delayed complications ranging from circulation and infection to complex post-traumatic syndromes.
Chronic pain
Chronic pain in the MSK system can occur due to unresolved acute pain, or arise as a distinct phenomenon. Degenerative diseases, tumors of articular cartilage, tendon, ligament or intervertebral discs, typically produce chronic pain of progressive intensity that alternates between periods of spontaneous relief and activation. MSK-related chronic pain particularly impacts the quality of life of the individual and family, as it is commonly associated with loss of mobility, which is the primary impediment to living independently[8]. Moreover, chronic pain is involved in functional decline, muscle weakness and increased propensity to fall in the elderly[9]. Individuals of all ages experiencing severe disabling chronic pain report poorer self-rated health, mental well-being and social functioning, and have high levels of depression and work loss[10].
Chronic pain results from the persistent activation of nociceptors that produce increased release of neurotransmitters in the central neurons located in the dorsal horn of the spinal cord. At the same time, neuroinflammatory peptides, such as substance P and calcitonin gene-related peptide, are released from the nerve endings at the site of injury and/or tissue inflammation and potentiate the inflammatory response[11].
Neuropathic pain
Both mechanisms of peripheral and central sensitization have been involved in the appearance of a distinct form of chronic pain associated with MSK diseases, neuropathic pain. Neuropathic pain is the direct consequence of a lesion in the somatosensory nervous system[12], involving tissue-specific nerve endings, medullary dorsal horn neurons, and the peripheral or central neurotransmitter balance. Neuropathic pain is a consequence of disproportionately enhanced intensity from painful stimuli (hyperalgesia) or of altered modality, such as hyperpathia or allodynia (pain produced by otherwise non painful stimulation). MSK-related neuropathic pain can also result from nerve entrapment, complex regional pain syndrome, joint pain associated with diabetes mellitus, and sensory nerve injury in advanced stages of OA[13].
The assessment and discrimination of neuropathic pain remains difficult and subjective, as it is currently diagnosed from the description of pain characteristics (e.g., “burning”, “aching”, “lancinating”). However, new methods have been described that are designed to measure peripheral and central pain in subjects with OA, which could allow for objective MSK pain evaluation in clinical settings in the near future. Quantitative sensory testing[14] and assessment of pain threshold using algometers have been used to demonstrate pain sensitization in OA patients, which is likely due to the increased firing of peripheral nociceptors[15]. In addition, brain neuroimaging tools, such as functional magnetic resonance imaging or fluorodeoxyglucose positron emission tomography, have been used to observe the activation of brain pain centers in hip and knee OA patients[16,17].
Pain in the MSK system has distinct characteristics depending on the tissue of origin, such as the various modalities of tissue-specific pain receptor activation. For example, muscle-, tendon- or joint-specific pain receptor activation generates pain of different intensities, duration and therapeutic responsiveness. Improved knowledge about the molecular pathways involved in the generation of different tissue-specific types of pain is important for the design of targeted therapies[18].
MOLECULAR MECHANISMS OF MSK PAIN
Inflammation plays an important role in the occurrence of acute and chronic MSK pain. Persistent aberrant inflammation is related on a molecular level to MSK pain produced by peripheral or central sensitization. The proinflammatory cytokines interleukin (IL)-1, IL-2 and IL-6[19]are associated with different intensities of MSK pain[20].Tumor necrosis factor (TNF)-α, a key molecule involved in inflammatory processes in various acute and chronic MSK diseases[21], is also a pain mediator[22] that maybe involved in the development of neuropathic pain of MSK origin[23]. IL-17 has been demonstrated as involved in the development of neuropathic pain in rodent models[24], and may contribute to neuropathic pain from rheumatoid arthritis as it is present in the synovial fluid of patients and IL-17 knockout mice do not develop collagen-induced arthritis[25]. Proinflammatory cytokines are also released by microglia (the immune cells of the central nervous system)in response to trauma, infection, inflammation and ischemia, thereby contributing to the immune response as well as to alteration of neuronal function. Neuropathic pain has been shown to result from microglial activation via adenosine 5’-triphosphate receptors or persistent stimulation of p38 mitogen-activated protein kinase[26].
OA pain can effectively be interrupted by local anesthetic treatment or after total joint replacement, suggesting that structures responsible for pain are in contact with the intra-articular milieu[27]. Synovial tissue is one of the joint components that generate pain via several mechanisms. The extensive network of free nerve endings in synovial tissue could be implicated in both inflammatory and neuropathic pain. Increased synovial mononuclear infiltration and overexpression of inflammatory mediators, such as TNF-α and IL-1β, are implicated in pain production during early stages of OA[28]. During late stages of OA, the local nerve endings in fibrotic tissue[29] or within lymphoid aggregates[30], as well as the increased expression of neuromediators such as substance P and calcitonin gene-related peptide, could contribute to generation of neuropathic pain[31]. Subchondral bone marrow and marrow cavities of osteophytes contain perivascular and free nerve fibers as well as nerve trunks that could be involved in generating neuropathic pain in advanced stages of OA[32,33].
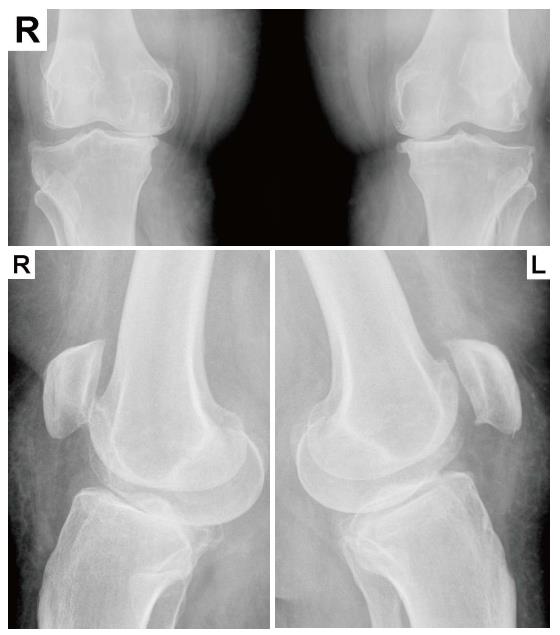
Indeed, OA progression has been shown to correlate with pain. A community-based population study found that pain was a predictor of structural and radiographic changes in hip OA; patients with hip pain were more likely to exhibit minimum joint space narrowing and undergo total replacement[34]. In addition, a lesser degree of knee pain was weakly associated with loss of knee cartilage volume as assessed by quantitative magnetic resonance imaging, but not with Western Ontario and McMaster Osteoarthritis Index scores, joint space width, or levels of urinary biomarkers of cartilage degradation[35]. However, pain intensity did not correlate with the severity of rotator cuff tear sin terms of size, retraction, or superior humeral head migration[36,37]. In our own experience, severe pain in patients with knee OA and diabetes mellitus is not correlated with radiologic and magnetic resonance imaging findings (unpublished data) even in the absence of diabetic polyneuropathy or peripheral vascular disease, and advanced stage OA (Kelgren-Lawrence 3) can be detected by radiologic examination in some asymptomatic patients (Figure 2).These findings may be explained by individual differences in pain thresholds, psychological and emotional states, or the presence of comorbidities.
Figure 2 Radiologic evidence of asymptomatic Kelgren-Lawrence grade 3 knee osteoarthritis.
Bilateral knee anteroposterior (top) and profile (bottom) plain X-rays from a 57-year-old asymptomatic female patient taken during emergency care after a traffic injury.
STEM CELLS FOR PAIN TREATMENT
Advancements in stem cell research may provide more effective on-pharmacologic treatment methods for various types of MSK-related pain. Such therapies could reduce or eliminate the need for repetitive drug administration and have longer-lasting effects, thus improving patient quality of life and reducing healthcare costs. To this end, several stem cell types are envisaged as having a potential for immediate clinical application: mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and genetically modified stem cells.
MSCs for the treatment of MSK diseases
MSCs or mesenchymal stromal cells are phenotypically heterogeneous populations of adult progenitors of mesodermal origin[38]. Initially thought to reside only within bone marrow stroma[39], MSC populations are found in a large variety of mesenchymal (fat[40], muscle[41], trabecular bone[42]) or non-mesenchymal (dental pulp[43], umbilical cord blood[44]) tissues. MSCs of various origins have been shown to proliferate into clinically relevant amounts of cells and to differentiate into several specific mesenchymal lineages such as osteoblasts, chondrocytes, tenocytes, and adipose tissue. MSCs are currently being tested in several RM strategies of cell therapy and tissue engineering for the repair/regeneration of MSK tissues. Based on their capability of engraftment, in vivo differentiation and integration within the host, MSCs were initially sought for structural repair of damaged or diseased tissue[45,46]. More recently, MSCs have been shown to exert cytokine-mediated paracrine effects contributing to tissue repair not only by direct participation, but also by means of recruiting local cells to induce repair[47].
The immunomodulatory properties of MSCs contribute to the restoration of tissue homeostasis when used as a cell therapy[38]. Moreover, MSCs are considered immune privileged as they express low major compatibility class I molecules but not class II, making them suitable as a source for allogeneic cells[48]. However, recent reports describe the generation of antibodies against, and immune rejection of, allogeneic-infused MSCs. Although the clinical manifestations are benign, it is not known whether the immunogenicity influences their therapeutic effect[49]. MSCs are known to modulate allogeneic immune responses by inhibiting maturation of dendritic cells and the proliferation and differentiation of B and T lymphocytes[50]. MSCs act by decreasing inflammatory cytokines, such as IL-1β and TNF-α, and by enhancing the amount of anti-inflammatory cytokines released by T cells[51]. Autologous or allogeneic MSCs have already been used in several clinical trials for the treatment of various MSK diseases, such as bone defects and non-unions, cartilage defects, osteoarthritis, and tendon suture augmentation[52,53].
MSCs for the treatment of MSK pain
The immunomodulatory properties of MSCs together with their natural capability of homing to the site of injury and inflammation make them attractive for the treatment of post-traumatic or inflammatory pain, and they are currently being used in preclinical and clinical trials for the treatment of various types of MSK-related pain. Proinflammatory cytokines in the serum or synovial fluid has been associated with OA-related pain in animal models[54]; IL-6 and TNF-α in synovial fluid[55], and TNF-α and IL-1β in vastus lateralis muscle of late stage OA subjects correlate with the degree of experienced pain or muscle atrophy[56]. It was suggested that the increased intensity of pain in those with degenerative disc disease compared to those with herniated discs is due to increased levels of TNF-α and IL-8 in the nucleus pulposus[57].
The use of MSCs as an anti-inflammatory agent is considered an attractive and convenient method for pain relief. In a pilot study, low back pain and disability produced by degenerative disc disease was successfully treated by local MSC infusion, to a degree comparable with surgery for spinal fusion[58]. Encouraging interim results have emerged from an ongoing phase 2 trial testing injections of allogeneic MSCs into degenerated lumbar vertebral discs, indicating significant pain reduction and functional improvement when compared to patients treated with hyaluronic acid[59]. Successful use of MSCs has been demonstrated for OA treatment in animal models[60], and for prevention of post-traumatic arthritis[61]. The alteration of intra-articular and serum cytokine levels reported by these studies may provide a basis for a method of pain treatment in both degenerating and traumatized joints.
A systematic review including eight completed studies and a total of 844 procedures of local autologous MSC delivery for the treatment of OA in human indicates that the procedure is safe, with noreported major adverse effects related to implanted cells[62]. Pain assessed by visual analogue score and functional outcome in 50 knee OA patients were significantly improved by treatment with MSC concentrate (buffy coat) compared with arthroscopic debridement only[63]. Functional improvement after 24 mo as measured by the American Orthopaedic Foot and Ankle Society’s ankle-hindfoot scale was reported after surgical debridement of talar dome defects and local delivery of concentrated bone marrow nucleated cells supported by collagen powder or hyaluronic acid membrane as scaffolds[64]. Core decompression and local delivery of bone marrow auto grafts improved Harris Hip scores and significantly reduced the need for arthroplasty when the procedure was performed in patients before the collapse of the joint surface[65]. Percutaneous delivery of culture-expanded autologous MSCs into OA knee joints resulted in improvement of visual analogue scores, physical therapy assessments, as well as increased cartilage volume[66]. The same group conducted a safety study on 227 clinical cases receiving intra-articular delivery of cultured MSCs for the symptomatic treatment of various OA joints, and reported three cases of self-limiting cell-related complications as the only safety issues[67]. No malignant transformations were detected during the two years of follow-up.
A large variability remains among reported methods for MSC isolation, purification and culture expansion. The development of MSC-based therapies requires concerted efforts for the establishment of optimized and standardized protocols for these methods, as well as for cryopreservation and storage of MSCs, which will significantly improve their clinical translation and provide new opportunities for cell-based pain treatment for MSK diseases[68].
MSCs for the treatment of MSK-related neuropathic pain
Although neuropathic pain is a potentially disabling disease that dramatically decreases quality of life, the complexity of the involved molecular pathways makes it difficult to treat. Neuropathic pain from MSK conditions is often underdiagnosed and undertreated[69], and when diagnosed, is treated with pharmacologic agents such as antidepressants, opioid analgesics and tramadol, which have a risk for addiction as well as of inducing other side-effects that can interfere with a normal, active life[70].
Neuropathic pain has been associated with neuro- inflammation and IL-17 signaling in a mouse model of peripheral nerve injury[24]. Inhibition of TNF-α-induced astrocyte and microglia activation can attenuate neuropathic pain, and IL-6 inhibition was shown to prevent vincristine-induced allodynia in rodents[71]. In humans, increased biochemical markers of inflammation were found in patients with painful diabetic neuropathy[72], and neuropathic pain correlates with joint inflammation in knee OA[13]. In addition, increased levels of IL-1β and IL-6 were found in cerebrospinal fluid of patients with complex regional pain syndrome[73].
MSCs have been investigated as anti-inflammatory agents for the treatment of MSK-related neuropathic pain. In a mouse model of streptozotocin-induced diabetes, toll-like receptor 3-primed, immunesuppressive human MSCs[74] attenuated neuropathic pain behaviors[75]. Systemically delivered human MSCs also exhibit long-lasting improvement of neuropathic pain in a spared-nerve injury mouse model, observed as decreases in IL-1β and IL-17, and increases in anti-inflammatory IL-10 and a marker of alternatively activated macrophages in the spinal cord[76]. These increases in anti-inflammatory cytokines were detected for up to 90 d, demonstrating the lasting effect from a single injection of MSCs.
MSCs have also been examined as neuroprotective agents in neuropathic treatment. Intravenous infusion of bone marrow mononucleated cells reversed neuropathic pain in rats produced by chronic constriction of the sciatic nerve, though the underlying mechanism of action could not be clarified[77]. Bone marrow MSCs injected into lumbar dorsal root ganglia of mice with sciatic nerve injury migrated to ganglia on the ipsilateral side and prevented mechanical and thermal allodynia by attenuating injury-induced neuropeptide expression[78]. In preclinical studies, MSCs were found to have a role in neuroprotection and axonal growth stimulation[79]. There are several clinical trials underway assessing the safety and efficacy of allogeneic MSCs for the treatment of spinal cord injury[80]. The potential for MSCs to reverse somatosensory system dysfunction in MSK-related neuropathic pain is still a matter of further investigation.
iPSCs for the treatment of MSK pain
The generation of iPSC populations from adult somatic cells by forced expression of several transcription factors represents a major scientific breakthrough and provides a new tool for RM. Patient-specific iPSCs could be used as autologous cell sources for any RM application by avoiding immune rejection and improving therapeutic specificity[81]. iPSC technology is being developed for RM application, with the first clinical trial underway in Japan for the treatment of age-related macular degeneration[82]. However, several important problems still need to be addressed, such as the method of delivery, the efficiency of reprogramming factors, and the genomic stability and epigenetic memory of reprogrammed cells. iPSCs will provide a major contribution to understanding molecular disturbances involved in the production of persistent, recurrent and MSK-related neuropathic pain through the generation of patient-specific disease models. Moreover, patient-specific iPSC-based models of complex neurosensory mechanisms that culminate in the generation of pain may enable the development of new drugs for patient and disease-targeted therapies.
Genetically modified stem cells
Stem cell-mediated gene therapy is regarded as a promising method of gene delivery that could provide long-term therapeutic impact[83]. Due to their lifespan and homing capabilities, MSCs are appealing tools for sustained gene delivery in various therapeutic scenarios. MSCs can be engineered to express a variety of proteins both in vivo and in vitro for use in a variety of genetic or acquired diseases. The expression of these therapeutic genes could last the lifetime of the patient, thus dramatically improving quality of life and reducing healthcare costs. MSC-mediated gene therapy for chronic pain management is therefore an appealing avenue for research. In vitro, expression of the human preproenkephalin gene by MSCs increased the production of the opioid peptide, met-enkephalin, which could serve as a safe supply for the treatment of opioid-sensitive pain[84]. Furthermore, hybrids produced by the fusion of opioid-producing chromaffin cells and MSCs express a catecholamine-producing phenotype, which could be used to generate a clinically significant population of therapeutic cells[85].
ESTABLISHING ENDPOINTS FOR THE TREATMENT OF MSK DISEASES
MSK-related pain is a widespread, potentially disabling condition for which effective treatments are lacking[86]. MSK pain can evolve from a symptom to a difficult-to-treat condition. Mechanisms of central sensitization are known to contribute to the maintenance of a local inflammatory microenvironment that aggravates pain and affects regional structure and function. Its roles in the abolishment of biomechanical stimuli necessary for normal tissue maintenance and repair have not yet been fully evaluated. A correlation between muscular atrophy and/or sarcopenia and MSK degeneration has been demonstrated[87], and MSK pain-related stiffness and muscular atrophy consistently decrease quality of life and interact with reparatory mechanisms. MSK system loss-of-function could be at least partially prevented if pain as a symptom or a disease could be more effectively treated. However, the molecular mechanisms implicated in pain-mediated MSK disease progression remain to be elucidated[88]. The identification of reliable biomarkers for diagnostic and targeted therapy is a stringent clinical necessity in MSK pain treatment.
RM and the use of stem cells show promise for several chronic non-life-threatening yet disabling conditions such as MSK-related pain. Several ongoing clinical trials are testing various forms of autologous or allogeneic, freshly isolated or cultured MSCs for the treatment of OA[89], tendon[90] and intervertebral disc degeneration[91]. It is therefore possible that therapeutic goals for MSK diseases can be re-established with the availability of more tangible results. Possible strategies such as elimination of local nociceptive stimuli, reversing the mechanism of central sensitization or somatosensory neuroprotection using paracrine effects fromlocal, regional or systemic MSC delivery could prevail over the attempts of recomposing MSK structure.
The large majority of current clinical trials focusing on tissue repair/regeneration have pain reduction as secondary endpoint. From a clinical perspective, it is important to evaluate the long-term safety and efficacy of pain control in disabling MSK disorders. Not only will this allow for accelerated acceptance of such therapies among clinicians and patients, but it will also likely contribute to structural rehabilitation generated by intrinsic mechanisms that resume in the absence of pain. The discovery of pain-related biomarkers will offer a tool for quantitative evaluation of pain and its correlation with degree of structural impairment in various MSK conditions. Furthermore, validation of these biomarkers will provide important therapeutic targets as well as methods for disease evaluation and treatment follow-up.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Stefancu Oana for her kind assistance in retrieving radiographic files.