Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.75
Peer-review started: August 22, 2014
First decision: September 28, 2014
Revised: October 2, 2014
Accepted: October 28, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 145 Days and 9.5 Hours
The discovery of endogenous neural stem cells (eNSCs) in the adult mammalian brain with their ability to self-renew and differentiate into functional neurons, astrocytes and oligodendrocytes has raised the hope for novel therapies of neurological diseases. Experimentally, those eNSCs can be mobilized in vivo, enhancing regeneration and accelerating functional recovery after, e.g., focal cerebral ischemia, thus constituting a most promising approach in stem cell research. In order to translate those current experimental approaches into a clinical setting in the future, non-invasive imaging methods are required to monitor eNSC activation in a longitudinal and intra-individual manner. As yet, imaging protocols to assess eNSC mobilization non-invasively in the live brain remain scarce, but considerable progress has been made in this field in recent years. This review summarizes and discusses the current imaging modalities suitable to monitor eNSCs in individual experimental animals over time, including optical imaging, magnetic resonance tomography and-spectroscopy, as well as positron emission tomography (PET). Special emphasis is put on the potential of each imaging method for a possible clinical translation, and on the specificity of the signal obtained. PET-imaging with the radiotracer 3’-deoxy-3’-[18F]fluoro-L-thymidine in particular constitutes a modality with excellent potential for clinical translation but low specificity; however, concomitant imaging of neuroinflammation is feasible and increases its specificity. The non-invasive imaging strategies presented here allow for the exploitation of novel treatment strategies based upon the regenerative potential of eNSCs, and will help to facilitate a translation into the clinical setting.
Core tip: Endogenous neural stem cells (eNSCs) in the adult mammalian brain can be mobilized by, e.g., pharmacological methods to facilitate regeneration and enhance functional recovery in neurological disease. In order to translate experimental approaches into the clinical setting, non-invasive imaging of eNSCs is required to monitor their fate in vivo. This review summarizes current imaging modalities suitable to monitor eNSCs in individual experimental animals over time, including optical imaging, magnetic resonance tomography and-spectroscopy, as well as Positron-Emission-Tomography, placing emphasis on the specificity of the signal obtained, as well as on their potential for clinical translation.
-
Citation: Rueger MA, Schroeter M.
In vivo imaging of endogenous neural stem cells in the adult brain. World J Stem Cells 2015; 7(1): 75-83 - URL: https://www.wjgnet.com/1948-0210/full/v7/i1/75.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.75
The discovery of neural stem cells (NSCs) in the adult brain and their ability to self-renew and differentiate into tissue-appropriate, functional cell types has raised intense scientific interest and the hope for radical new therapies of neurological diseases. Altman et al[1] first detected the ability of the adult mammalian brain to generate new neurons, and their work was followed up by Kaplan et al[2]. Using radioactive thymidine to label all dividing cells and histological examination of postmortem brains, they found labeled neurons. This proved that new neurons in the adult brain can be generated following cell division. After those pioneer studies focusing on neurogenesis, later studies aimed to detect the immature precursor cells capable of differentiating into all three cell fates of the central nervous system (CNS): neurons, astrocytes and oligodendrocytes. Several biomarkers have been suggested to identify those undifferentiated stem cells in the brain, the first one being the intermediate filament nestin (neuroepithelial stem cell intermediate filament[3]). Subsequently, NSCs were characterized in the developing and in the postnatal/adult mammalian brain[4-11]. More biomarkers were consecutively identified including Sox2, Shh pathway components, PDGF, EGFR, GFAP, Hes3, Hes5, Musashi, and CD133[5,12-22]. However, since no single marker has yet been identified to unambiguously distinguish NSCs from somatic cells, co-stainings are usually required to comprehensively characterize them. It is now well accepted that endogenous NSCs (eNSCs) persist in the adult mammalian brain in at least two distinct regions, the subventricular zone, lining the lateral ventricles, and the dentate gyrus of the hippocampus[23,24].
Insults to the CNS such as cerebral ischemia or neurodegenerative disease result in a mobilization of eNSCs and their migration towards the compromised areas[25-29], constituting a physiological regenerative response of the brain. However, in most cases the intrinsic regenerative response of eNSCs is obviously not sufficient to lead to functional recovery. Experimentally, it has been shown that eNSCs can be mobilized pharmacologically for therapeutic purposes. Early studies showed that activating the tyrosine kinase receptors for fibroblast growth factor 2 and epithelial growth factor on eNSCs by introducing those growth factors into the lateral cerebral ventricle of experimental animals stimulates the proliferation of eNSCs in vivo[30,31]. Ligands for these receptors also show benefit in animal models of cerebral ischemia, where growth factor-induced eNSC mobilization is associated with enhanced functional recovery from motor deficits[32,33]. Recently, several other drugs have been identified to mobilize eNSCs in the naïve and in the injured rodent brain, including Notch-ligands[13], angiopoietin 2[14], the neural cell adhesion molecule mimetic peptide FG Loop[34], and aromatic-turmerone[35]. Enhancing this (physiological) mobilization of eNSCs by pharmacological intervention leads to an improvement of neurological function after, e.g., cerebral ischemia[13,32,33]. To convey these regenerative effects, it seems that the differentiation of eNSCs into mature neurons that functionally integrate into the damaged circuitry-previously thought to be required for regeneration-only plays a minor role[36]. Rather, eNSCs secrete trophic factors supporting neuroprotection such as glial-derived neurotrophic factor, vascular endothelial growth factor, or Shh[13,37]. Other regenerative processes induced by NSCs include remyelination, angiogenesis, remodeling, and immunomodulation[38,39]. Since treatments based on the transplantation of stem cells harbor several disadvantages including poor long-term cell survival, a lack of integration into the host circuitry, immune reactions against the transplants, and limited availability of appropriate cells[40], mobilizing the endogenous neural stem cell niche for therapeutic purposes constitutes a most promising approach in stem cell research.
Developing strategies to mobilize the endogenous NSC niche in vivo, with the aim to later translate them into clinical applications, creates the need for in vivo imaging technology to monitor those interventions. Imaging techniques should be non-invasive, so they can be applied repetitively in the same individual in a longitudinal fashion, and thus track quantity and localization of endogenous NSC over any period of time. While considerable progress has been made in recent years to track transplanted, pre-labeled cells[41-47], the detection of endogenous NSCs in the living brain remains elusive. Current approaches to image eNSCs in vivo include (1) the use of transgenic animals whose eNSCs exhibit certain imaging properties; (2) labeling eNSCs in vivo by injecting a labeling substance into the brain; or (3) imaging some intrinsic and putative unique property of eNSCs with a tailored imaging assay.
Transgenic animals expressing a fluorescent or bioluminescent protein under the control of a stem cell-characteristic promoter such as nestin or doublecortin render their eNSCs detectable by optical imaging techniques[48-52]. Under ideal conditions, optical imaging can specifically detect clusters of about 10³ cells in vivo, but its sensitivity is limited by the relatively poor spatial resolution with shallow tissue penetration[53]. Moreover, the need for transgenic mice limits the potential applications of optical imaging and prohibits its translation into a clinical setting.
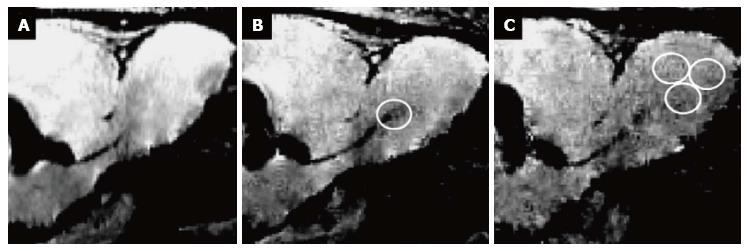
In the attempt to (specifically) label eNSCs in vivo, various methods have been suggested. Labels can be micro-injected directly into (or close to) the neurogenic niches, or they can be coupled to a vector in order to specifically target certain cells. Direct injection of a paramagnetic label such as small particles of iron oxide allows for cell detection using magnetic resonance imaging (MRI) with excellent spatial resolution on the single cell level (Figure 1)[54-59]. However, this type of labeling is neither specific for a certain cell type, nor does it reflect cell viability, as only the particles themselves are visualized[53]. Moreover, directly injecting iron oxide in e.g., the lateral ventricles of the brain results in image distortion in the region of injection, allowing only for the detection of cells migrating away from the injection site[54,57,58]. A more specific in vivo labeling approach is achieved by attaching an imaging label to a retro-or lentiviral vector, thus targeting proliferating cells in particular. This has been shown to be effective for optical imaging techniques after introduction of firefly luciferase[60,61] or channelrhodopsin-2[62]. Alternatively, ferritin can be introduced into proliferating (stem) cells using viral vectors, rendering them detectable for MR-imaging[63,64]. Recent progress in this field has also been made by the development of a monoclonal antibody binding to neural precursor cells, coupled to magnetic glyconanoparticles allowing for their detection by MRI[65].While these approaches are quite promising to track eNSCs in individual experimental animals, a major disadvantage of those in vivo- labeling approaches is the lack of applicability in human beings due to the invasiveness of the labeling procedure.
Truly non-invasive imaging techniques, utilizing methods that could be translated from the bench to the bedside, are rare. Manganas and colleagues investigated the spectrum of electromagnetic energy from established neurogenic niches using proton magnetic resonance spectroscopy (1H-MRS). They observed a prominent peak in the spectrum at the frequency of 1.28 parts per million (ppm) in the hippocampus of the adult mammalian brain in vivo, which was not observed in other regions of the brain[66]. Later studies investigating this phenomenon have shown that it is not specific to eNSCs or neurogenesis, but closely related to apoptosis and quiescence[67,68]. Since apoptosis is a major selection process during neurogenesis, 1H-MRS may be used as an indirect method to detect neurogenesis within the known neurogenic niches and under physiological conditions. Disorders of the CNS associated with increased apoptosis of neural- or other non-neural cells, however, cannot be studied with this approach. Besides, MRS offers quite a low spatial resolution, failing to notice small clusters of cells and leading to the low sensitivity of this method.
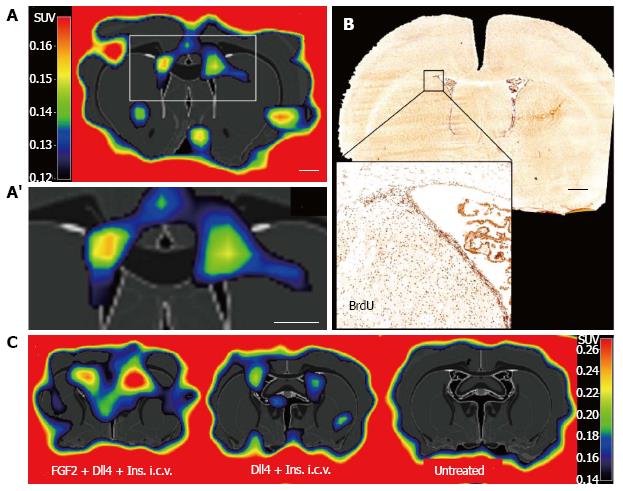
We took advantage of the proliferative activity of eNSCs to develop an imaging assay using positron emission tomography (PET). Extensive studies in neurooncology have established the radiotracer 3’-deoxy-3’-[18F]fluoro-L-thymidine ([18F]FLT) to label proliferating cells in the adult rodent and human brain, allowing for non-invasive imaging of tumor cell proliferation with PET[69-72]. [18F]FLT is a thymidine analogue that is incorporated into the DNA of dividing cells-similar to bromodeoxyuridine (BrdU) well established for their immunohistochemical detection- where it is irreversibly trapped, its positron-emitting properties allowing for its non-invasive detection[73]. No evidence of toxicity or other complications have been reported following intravenous injection of [18F]FLT[74]. [18F]FLT-PET detects ceasing glioma cell proliferation as soon as 3 d after initiation of an anti-proliferative treatment, as we have previously shown in mice and humans[69,72]. Besides tumor cells, proliferating NSCs incorporate [18F]FLT both in vitro as well as in vivo after its systemic (intravenous) injection into adult rats[75]. Thus, [18F]FLT labels proliferating eNSCs in the neurogenic niches of the healthy rodent brain with high sensitivity (Figure 2A), corresponding to BrdU-accumulation (Figure 2B). Moreover, [18F]FLT-PET quantifies eNSC mobilization mediated by pharmacological stimulation (Figure 2C). The resulting PET-signal can be quantified to reflect the extent of eNSC mobilization[75]. Using a high-resolution PET-scanner and optimizing image reconstruction, the detection level can be as low as -104 cells. However, the PET-signal is not specific to endogenous NSC, since other proliferating cells are labeled as well. To increase specificity of this imaging assay, multi-modal imaging protocols can be applied as detailed below.
Stroke is the third leading cause of death and the leading cause of adult disability in the Western world[76]. Since rescue of affected neurons can only be achieved by re-perfusion within a very narrow time window, treatment is mainly confined to the amelioration of neurological deficits and the prevention of further events. Especially in the subacute and chronic phase, i.e. days to months after stroke, therapeutic options are limited to physiotherapy, ergotherapy and logopedia to rehabilitate impaired neurological functions. However, from the pathophysiological point of view, evolution of ischemic damage is not limited to the minutes after vessel occlusion. After the disruption of blood flow below a threshold has led to rapid necrotic cell death within a localized region, the surrounding tissue that has been spared in this initial phase consecutively also undergoes relevant, but less rapid changes which aim at encapsulating the necrotic tissue, clearing of debris, and facilitating regeneration. These processes - often referred to as neuroinflammation- involve the rapid activation of glial cells (microglia, astrocytes) as well as recruitment of hematogenous cells (granulocytes, T-cells, monocytes/macrophages) from the blood stream[77-82].
While neuroinflammation on the one hand contributes to the evolution of secondary damage to the surrounding tissue by the excessive production of reactive oxygen species and pro-inflammatory cytokines secreted by the immune cells, it also has beneficial effects on the prevention of secondary tissue damage[83]. Besides the containment of necrotic tissue, another most relevant beneficial aspect of stroke-induced neuroinflammation is the induction of a strong regenerative response, leading to a robust expansion of eNSCs[84]. Quality, extent and timing of neuroinflammatory processes determine whether manipulating that particular response after stroke will be deleterious or therapeutically beneficial. The activation of resident microglia can under some circumstances be neurotoxic[85], under others neuroprotective[86], depending on the specific activating conditions[87]. Interestingly, differentially activated microglia also have opposing effects on NSC[88]. eNSC are attracted to the site of the lesion by various inflammation-associated cytokines such as stromal cell-derived factor-1[89-91], tumor necrosis factor-alpha, and interferon-γ[84,92].This mobilization of eNSCs has been shown for various ischemia models in experimental animals, including transient global ischemia[28], transient focal ischemia[26,36], or permanent focal ischemia[93]. However, without any further pharmacological mobilization, the vast majority of newly generated neuroblasts in ischemic stroke models die by the time they have reached the peri-infarct area[36]. Moreover, neurogenesis after stroke seems to play even less a role in humans than it does in rodent models[94]. Those recent findings highlight the importance of developing novel therapeutic approaches to additionally mobilize and invigorate eNSCs after stroke, either by pharmacological[34,35] or by non-pharmacological treatments[95].
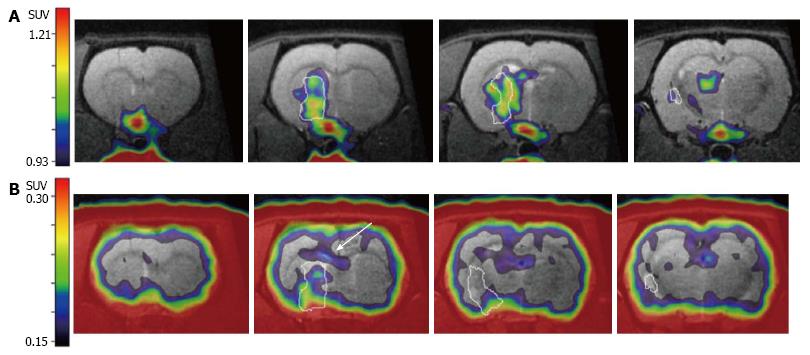
In order to advance those experimental therapeutic approaches into the clinical setting, non-invasive imaging assays have to be established that reliably monitor eNSC activation in the rodent and human brain under the pathophysiological condition of stroke. In this context, the post-ischemic neuroinflammatory processes introduced above resemble a major impediment, since immune cells proliferate in the ischemic brain just as eNSCs do, and [18F]FLT-PET does not differentiate between stem cell- and immune cell-derived proliferation. An elegant way to circumvent this problem consists of an imaging assay specific to (neuro-)inflammatory cells that does not visualize eNSCs. The radiotracer [11C]PK11195 fulfils this requirement by selectively binding to the translocator protein-18 kDa expressed on inflammatory cells, specifically visualizing post-ischemic cellular neuroinflammatory processes[96]. Since [11C]PK11195 is radiolabeled with the isotope 11-C with a half-life of -20 min, sequential PET-imaging with [18F]FLT is possible by waiting for -5 half-lives or 100 min between scans. During this time, the animal remains anesthetized within the PET-scanner, avoiding any movement, and enabling an exact co-registration of the imaging data[97]. This co-registered imaging data on [11C]PK11195- and [18F]FLT-accumulation allows for a conclusive differentiation between cell proliferation from eNSCs and immune cells (Figure 3).
Therefore, PET is a promising tool to image eNSCs in a non-invasive fashion in neurological disorders that can be translated from bench to bedside. However, to further advance the imaging technology in this direction, complementary verification of imaging parameters with immunohistochemical analyses are still needed, and the actual translation of such strategies to a clinical environment has yet a long way to go. However, the non-invasive imaging strategies presented here will help to facilitate translation into the clinical setting, and allow for the exploitation of novel treatment strategies based upon the regenerative potential of eNSCs.
P- Reviewer: Li SC, Perron M S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2478] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 2. | Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429-1441. [PubMed] |
| 3. | Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2434] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 4. | Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 551] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2818] [Cited by in RCA: 2971] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 6. | Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310-3328. [PubMed] |
| 7. | Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 970] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 8. | Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1394] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 9. | Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1074] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3870] [Cited by in RCA: 3834] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 11. | Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 276] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 566] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 13. | Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 751] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 14. | Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, Walbridge S, Munasinghe J, Koretsky AP, Lonser RR. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci USA. 2009;106:13570-13575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | D’Amour KA, Gage FH. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11866-11872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 18. | Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 411] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Temple S, Raff MC. Differentiation of a bipotential glial progenitor cell in a single cell microculture. Nature. 1985;313:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 188] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720-14725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1312] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 22. | Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002-5012. [PubMed] |
| 23. | Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1963] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 25. | Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci USA. 2003;100:9023-9027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 26. | Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 834] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 27. | Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 740] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 28. | Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768-7778. [PubMed] |
| 29. | Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820-5829. [PubMed] |
| 31. | Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1077] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 34. | Klein R, Blaschke S, Neumaier B, Endepols H, Graf R, Keuters M, Hucklenbroich J, Albrechtsen M, Rees S, Fink GR. The synthetic NCAM mimetic peptide FGL mobilizes neural stem cells in vitro and in vivo. Stem Cell Rev. 2014;10:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Hucklenbroich JKR, Neumaier B, Graf R, Fink GR, Schroeter M, Rueger MA. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Research & Therapy. 2014;5:100. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963-970. [PubMed] |
| 37. | Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143-S145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 41. | Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267-16272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 526] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 42. | Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Li SC, Tachiki LM, Luo J, Dethlefs BA, Chen Z, Loudon WG. A biological global positioning system: considerations for tracking stem cell behaviors in the whole body. Stem Cell Rev. 2010;6:317-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 44. | Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, Williams SC. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Okada S, Ishii K, Yamane J, Iwanami A, Ikegami T, Katoh H, Iwamoto Y, Nakamura M, Miyoshi H, Okano HJ. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19:1839-1841. [PubMed] |
| 46. | Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 47. | Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Zhang L, Arniego P, Ho KL, Chopp M. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003;53:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Couillard-Despres S, Finkl R, Winner B, Ploetz S, Wiedermann D, Aigner R, Bogdahn U, Winkler J, Hoehn M, Aigner L. In vivo optical imaging of neurogenesis: watching new neurons in the intact brain. Mol Imaging. 2008;7:28-34. [PubMed] |
| 49. | Couillard-Despres S, Quehl E, Altendorfer K, Karl C, Ploetz S, Bogdahn U, Winkler J, Aigner L. Human in vitro reporter model of neuronal development and early differentiation processes. BMC Neurosci. 2008;9:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 799] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 51. | Karl C, Couillard-Despres S, Prang P, Munding M, Kilb W, Brigadski T, Plötz S, Mages W, Luhmann H, Winkler J. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem. 2005;92:264-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 53. | Couillard-Despres S, Vreys R, Aigner L, Van der Linden A. In vivo monitoring of adult neurogenesis in health and disease. Front Neurosci. 2011;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Granot D, Scheinost D, Markakis EA, Papademetris X, Shapiro EM. Serial monitoring of endogenous neuroblast migration by cellular MRI. Neuroimage. 2011;57:817-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Nieman BJ, Shyu JY, Rodriguez JJ, Garcia AD, Joyner AL, Turnbull DH. In vivo MRI of neural cell migration dynamics in the mouse brain. Neuroimage. 2010;50:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Panizzo RA, Kyrtatos PG, Price AN, Gadian DG, Ferretti P, Lythgoe MF. In vivo magnetic resonance imaging of endogenous neuroblasts labelled with a ferumoxide-polycation complex. Neuroimage. 2009;44:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Shapiro EM, Gonzalez-Perez O, Manuel García-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Sumner JP, Shapiro EM, Maric D, Conroy R, Koretsky AP. In vivo labeling of adult neural progenitors for MRI with micron sized particles of iron oxide: quantification of labeled cell phenotype. Neuroimage. 2009;44:671-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Vreys R, Vande Velde G, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, Baekelandt V, Van der Linden A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: validation of various MPIO labeling strategies. Neuroimage. 2010;49:2094-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Vandeputte C, Reumers V, Aelvoet SA, Thiry I, De Swaef S, Van den Haute C, Pascual-Brazo J, Farr TD, Vande Velde G, Hoehn M. Bioluminescence imaging of stroke-induced endogenous neural stem cell response. Neurobiol Dis. 2014;69:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Reumers V, Deroose CM, Krylyshkina O, Nuyts J, Geraerts M, Mortelmans L, Gijsbers R, Van den Haute C, Debyser Z, Baekelandt V. Noninvasive and quantitative monitoring of adult neuronal stem cell migration in mouse brain using bioluminescence imaging. Stem Cells. 2008;26:2382-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 567] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 63. | Vande Velde G, Rangarajan JR, Toelen J, Dresselaers T, Ibrahimi A, Krylychkina O, Vreys R, Van der Linden A, Maes F, Debyser Z. Evaluation of the specificity and sensitivity of ferritin as an MRI reporter gene in the mouse brain using lentiviral and adeno-associated viral vectors. Gene Ther. 2011;18:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Vande Velde G, Raman Rangarajan J, Vreys R, Guglielmetti C, Dresselaers T, Verhoye M, Van der Linden A, Debyser Z, Baekelandt V, Maes F. Quantitative evaluation of MRI-based tracking of ferritin-labeled endogenous neural stem cell progeny in rodent brain. Neuroimage. 2012;62:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Elvira G, Garcia I, Benito M, Gallo J, Desco M, Penades S, Garcia-Sanz JA, Silva A. Live imaging of mouse endogenous neural progenitors migrating in response to an induced tumor. PloS one. 2012;7:e44466. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 67. | Ramm P, Couillard-Despres S, Plötz S, Rivera FJ, Krampert M, Lehner B, Kremer W, Bogdahn U, Kalbitzer HR, Aigner L. A nuclear magnetic resonance biomarker for neural progenitor cells: is it all neurogenesis? Stem Cells. 2009;27:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Loewenbrück KF, Fuchs B, Hermann A, Brandt M, Werner A, Kirsch M, Schwarz S, Schwarz J, Schiller J, Storch A. Proton MR spectroscopy of neural stem cells: does the proton-NMR peak at 1.28 ppm function as a biomarker for cell type or state? Rejuvenation Res. 2011;14:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Galldiks N, Kracht LW, Burghaus L, Ullrich RT, Backes H, Brunn A, Heiss WD, Jacobs AH. Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol Imaging. 2010;9:40-46. [PubMed] |
| 70. | Jacobs AH, Rueger MA. Winkeler A, Li H, Vollmar S, Waerzeggers Y, Rueckriem B, Kummer C, Dittmar C, Klein M, Heneka MT, Herrlinger U, Fraefel C, Graf R, Wienhard K, Heiss WD. Imaging-Guided Gene Therapy of Experimental Gliomas. Cancer Res. 2007;67:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G, Galldiks N, Klein JC, Sobesky J, Hilker R. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46:1948-1958. [PubMed] |
| 72. | Rueger MA, Ameli M, Li H, Winkeler A, Rueckriem B, Vollmar S, Galldiks N, Hesselmann V, Fraefel C, Wienhard K. [18F]FLT PET for non-invasive monitoring of early response to gene therapy in experimental gliomas. Mol Imaging Biol. 2011;13:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 849] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 74. | Turcotte E, Wiens LW, Grierson JR, Peterson LM, Wener MH, Vesselle H. Toxicology evaluation of radiotracer doses of 3'-deoxy-3'-[18F]fluorothymidine (18F-FLT) for human PET imaging: Laboratory analysis of serial blood samples and comparison to previously investigated therapeutic FLT doses. BMC nuclear medicine. 2007;7:3. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Rueger MA, Backes H, Walberer M, Neumaier B, Ullrich R, Simard ML, Emig B, Fink GR, Hoehn M, Graf R. Noninvasive imaging of endogenous neural stem cell mobilization in vivo using positron emission tomography. J Neurosci. 2010;30:6454-6460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | World Health Organization. Annex table 2. Deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002. Swiss: Geneva 2004; . |
| 77. | Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, Obrenovitch TP, Contreras TJ. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 325] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 79. | Schroeter M, Jander S, Huitinga I, Stoll G. CD8+ phagocytes in focal ischemia of the rat brain: predominant origin from hematogenous macrophages and targeting to areas of pannecrosis. Acta Neuropathol. 2001;101:440-448. [PubMed] |
| 80. | Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 212] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Schroeter M, Jander S, Witte OW, Stoll G. Heterogeneity of the microglial response in photochemically induced focal ischemia of the rat cerebral cortex. Neuroscience. 1999;89:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 919] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 83. | Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87-113. [PubMed] |
| 84. | Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182-3191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 85. | Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3304] [Cited by in RCA: 3113] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 86. | Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 711] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 87. | Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2477] [Cited by in RCA: 2753] [Article Influence: 161.9] [Reference Citation Analysis (0)] |
| 88. | Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 695] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 89. | Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117-18122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 851] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 90. | Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 91. | Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 92. | Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 93. | Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Huttner HB, Bergmann O, Salehpour M, Rácz A, Tatarishvili J, Lindgren E, Csonka T, Csiba L, Hortobágyi T, Méhes G. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci. 2014;17:801-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 95. | Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, Fink GR, Graf R, Schroeter M. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7:e43776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 96. | Schroeter M, Dennin MA, Walberer M, Backes H, Neumaier B, Fink GR, Graf R. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J Cereb Blood Flow Metab. 2009;29:1216-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | Rueger MA, Muesken S, Walberer M, Jantzen SU, Schnakenburg K, Backes H, Graf R, Neumaier B, Hoehn M, Fink GR. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience. 2012;215:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |