Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.223
Peer-review started: July 31, 2014
First decision: September 28, 2014
Revised: October 14, 2014
Accepted: October 31, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 167 Days and 8 Hours
AIM: To investigate adenoviral transduction in mesenchymal stem cells (MSCs) and effects on stemness in vitro and function as a cell therapy in vivo.
METHODS: Bone marrow-derived adult and fetal MSC were isolated from an equine source and expanded in monolayer tissue culture. Polyethylenimine (PEI)-mediated transfection of pcDNA3-eGFP or adenoviral transduction of green fluorescent protein (GFP) was evaluated in fetal MSCs. Adenoviral-mediated transduction was chosen for subsequent experiments. All experiments were carried out at least in triplicate unless otherwise noted. Outcome assessment was obtained by flow cytometry or immunohystochemistry and included transduction efficiency, cell viability, stemness (i.e., cell proliferation, osteogenic and chondrogenic cell differentiation), and quantification of GFP expression. Fetal and adult MSCs were then transduced with an adenoviral vector containing the gene for the bone morphogenic protein 2 (BMP2). In vitro BMP2 expression was assessed by enzyme linked immunosorbent assay. In addition, MSC-mediated gene delivery of BMP2 was evaluated in vivo in an osteoinduction nude mouse quadriceps model. New bone formation was evaluated by microradiography and histology.
RESULTS: PEI provided greater transfection and viability in fetal MSCs than other commercial chemical reagents. Adenoviral transduction efficiency was superior to PEI-mediated transfection of GFP in fetal MSCs (81.3% ± 1.3% vs 35.0% ± 1.6%, P < 0.05) and was similar in adult MSCs (78.1% ± 1.9%). Adenoviral transduction provided significantly greater expression of GFP in fetal than adult MSCs (7.4 ± 0.1 vs 4.4 ± 0.3 millions of mean fluorescence intensity units, P < 0.01) as well as significantly greater in vitro BMP2 expression (0.16 pg/cell-day vs 0.10 pg/cell-day, P < 0.01). Fraction of fetal MSC GFP positive cells decreased significantly faster than adult MSCs (1.15% ± 0.05% vs 11.4% ± 2.1% GFP positive at 2 wk post-transduction, P < 0.05). Cell proliferation and osteogenic differentiation in vitro were not affected by Ad transduction in both fetal and adult MSCs, but fetal MSCs had reduced chondrogenic differentiation in vitro when compared to adult (P < 0.01). Chondrogenic differentiation was also significantly reduced in Ad-GFP transduced cells (P < 0.05). Ad-BMP2 transduced adult MSCs induced new bone formation in more thighs than Ad-BMP2 transduced fetal MSCs (83% vs 17% of the six treated thighs per group, P < 0.05) and resulted in increased femur midshaft diameter due to greater extent of periosteal new bone (1.57 ± 0.35 mm vs 1.27 ± 0.08 mm, P < 0.05).
CONCLUSION: Fetal MSCs may be genetically manipulated ex vivo with adenoviral vectors. Nonetheless, the abbreviated expression of the exogenous gene may limit their applications in vivo.
Core tip: Fetal mesenchymal stem cells (MSCs) can be genetically manipulated ex vivo with adenoviral vectors without major effects on stemness. Their greater expression of exogenous genes than adult MSCs is promising for MSC-mediated gene therapy. MSC-mediated bone morphogenic protein 2 gene delivery provides osteogenic induction in vivo, which could have clinical applications in bone regeneration. Nonetheless, the abbreviated expression of the exogenous gene provided via adenoviral transduction may limit their applications in vivo.
-
Citation: Santiago-Torres JE, Lovasz R, Bertone AL. Fetal
vs adult mesenchymal stem cells achieve greater gene expression, but less osteoinduction. World J Stem Cells 2015; 7(1): 223-234 - URL: https://www.wjgnet.com/1948-0210/full/v7/i1/223.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.223
The best-characterized source of human mesenchymal stem cells (MSC) to date is bone marrow (BM)[1]. Transplantation of BM-derived MSCs has been studied as a potential therapy for myocardial ischemic injury[2,3], autoimmune diseases[4], and musculoskeletal conditions such as osteoarthritis[5]. MSCs could be useful in the active regeneration of joint and cartilage tissue because of their multipotency and fast proliferation, as a cell therapy acting to replace lost lineages and to modify existing cells. However, in certain degenerative diseases, such as osteoarthritis, MSCs are depleted and have reduced ability to differentiate and proliferate[6]. As an alternative source of MSCs, young cells such as fetal (f) MSCs could be harvested from the umbilical cord, placental stroma, and the amnion[7-9]. Ethical concerns regarding the isolation of MSCs from fetal sources are minimized, as these tissues are normally discarded after birth[1].
Zhang et al[10] demonstrated that human fMSCs are a superior cellular candidate for bone tissue engineering applications. This related to their superior proliferation capacity, more robust osteogenic potential, and lower immunogenicity, as compared to MSC from postnatal sources[10]. Nevertheless, the permissiveness to gene delivery and the effect of genetic engineering on stemness in these young cells has not been extensively studied. Gene delivery to fMSCs may differ from adult (a) MSC due to immaturity of gene expression cellular pathways and cell surface receptors, such as the coxsackievirus adenovirus receptor required for adenoviral transduction[11]. To our knowledge, only two original research studies have evaluated gene delivery on fMSCs. Campagnoli et al[12] demonstrated that fMSCs could be retrovirally transduced with high efficiency without selection. Also, Chan et al[13] showed that human fMSCs retain their proliferative and differentiation capacities after stable lentiviral transduction. The paucity of studies addressing gene delivery in fMSCs, and the lack of studies on transient gene delivery in these young cells, highlights the need for more studies in these topics.
We chose to study the transient transduction characteristics of adenoviral vectors (Ad) in fMSC. Adenoviral vectors’ lack of insertional mutagenesis result in a desirable safety profile, which added to their capacity to achieve high levels of transgene expression[14], make them attractive for gene delivery particularly for bone regeneration. Our study addressed the effectiveness of Ad-mediated transduction of equine fetal bone marrow-derived MSCs. We hypothesized that Ad-mediated transduction will be efficient in fMSCs without affecting measures of cell stemness. In addition, Ad-transduced equine MSC-mediated bone morphogenic protein 2 (BMP2) gene delivery was studied to assess its ability to induce bone formation in a mouse model.
The use of animals in this study was carried out in accordance to regulations by The Ohio State University Institutional Animal Care and Use Committee (IACUC) in compliance with national guidelines. The IACUC reviewed and approved our Protocol 2009A0119.
Both bone marrow-derived aMSCs and fMSCs were isolated and expanded in monolayer tissue culture. Low passage MSCs were immunophenotyped by CD90 and CD34 surface expression by flow cytometry. Polyethylenimine (PEI)-mediated transfection of pcDNA3-egreen fluorescent protein (GFP) or Ad transduction of GFP were evaluated in fMSCs. Ad-mediated transduction was chosen for subsequent experiments. Outcome assessment included transduction efficiency, cell viability, stemness (i.e., cell proliferation, osteogenic and chondrogenic cell differentiation), and quantification and duration of GFP and BMP2 gene expression. All experiments were carried out at least in triplicate unless otherwise noted. In vitro BMP2 gene expression was assessed by ELISA. MSC-mediated BMP2 gene delivery was then evaluated in vivo in an osteoinduction nude mouse quadriceps model.
Adult MSCs were obtained from equine bone marrow of the sternum of adult horses (age range 2-5 years old) using methods described by Ishihara et al[15]. Fetal MSCs were isolated following a similar protocol from a 20-wk-old equine fetus. The mare was euthanized for terminal problems, while the fetus was apparently healthy. Isolated cells were then resuspended in 1 mL of 90% fetal bovine serum with 10% dimethyl sulfoxide for cryopreservation. Prior to each experiment, preserved cells were allowed to thaw at 37 °C, rinsed in Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY, United States), and were grown separately in T-75 flasks in DMEM supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, United States), 100 U/mL penicillin, and 100 ug/mL streptomycin (Gibco). Cells were incubated at 37 °C and 5% CO2. Medium was changed every 2-3 d. Cells were trypsinized when confluency was above 80% with Trypsin-EDTA (Gibco) after a washing step with Dulbecco’s phosphate buffered saline (PBS, Gibco). MSCs were evaluated by microscopy for homogenous elongated spindle-shaped morphology. Cells were also immunophenotyped by flow cytometry for expression of CD90 after incubation with phycoerythrin (PE)-conjugated anti-hCD90 antibody (561970, BD Biosciences) detected with a 585/40 nm optical filter. Lack of expression of CD34 was assessed with allophycocyanin (APC)-conjugated anti-hCD34 antibody (555824, BD Biosciences) detected with a 675/25 nm optical filter.
Escherishia coli DH5α were transformed with a plasmid consisting of a pcDNA3 backbone containing a gene for the enhanced GFP. The transformed cells were amplified in lysogeny broth medium at 37 °C overnight at 225 rpm in a shaker incubator. Isolation and purification of the plasmid was carried out with a Midiprep kit (K2100, LifeTechnologies) according to the given protocol. The concentration and purity of the plasmid were determined by the 260/280 ultraviolet absorbance method with a Nanodrop 2000 (Thermo Scientific). The purified plasmid was stored at -20 °C.
A 1 mg/mL transfection reagent solution was made from 50 mg of linear PEI (23966, Polysciences) in 50 mL of PBS adjusting the pH to 4.5 with HCl and dissolving at 70 °C, followed by filter sterilization and storage at 4 °C. On the day of transfection a stock solution was made with 100 μL of warm PBS, 5 μL of 1 mg/mL PEI, and 2 μL of the pcDNA3-eGFP plasmid, which is sufficient for transfection in one well in a 6-well plate. The transfection solution was upscaled as needed.
Replication-deficient, E1-A-deleted adenoviral vector encoding either a 1547 base-pair open reading frame segment of human BMP2 (Ad-BMP2) or GFP (Ad-GFP) under the control of the cytomegalovirus promoter were previously generated[16]. These were amplified in 80% confluent HEK 293A cells (R70507, Invitrogen) and titered by plaque assay following the methods described in the provided user guide by Life Technologies. Aliquots were stored at -80 °C.
A fluorescent Olympus 1X51 microscope was used to evaluate the PEI-mediated eGFP transfection and Ad-GFP transduction efficiency of MSCs in monolayers at various time points. Fluorescent and phase contrast images were merged using ImageJ.
Green fluorescence after gene delivery into aMSCs and fMSCs was assessed in an Accuri C6 BD Biosciences flow cytometer, excited by an argon 488 nm laser and detected by use of a 533/30 nm optical filter. The mean fluorescence intensity (MFI) data were collected from these cell populations. All data was analyzed with a minimum of 15000 events setting the respective negative control to less than 1% in BD CFlow software.
MSCs were seeded at a density of 6 × 104 cells/well in a 12-well plate to 75% confluency with 2 mL of DMEM per well. PEI, FuGENE 6 (Promega), and Lipofectamine (Invitrogen) were tested for GFP gene transfection in fMSCs. Transfection conditions were optimized for the three reagents. Transfection efficiency and viability were qualitatively assessed at 48 h post-transfection. In addition, transduction with Ad-GFP was tested at MOI of 0.5, 3, 15, 25, and 50 in fMSCs.
Adult MSCs or fMSCs were seeded at 1 × 105 cells in triplicate in T-25 flasks and transduced 24 h later or kept as control cells. Ad-GFP transduced cells (Ad-GFP-MSC) and control cells were evaluated by flow cytometry at 48 h, 1 wk, and 2 wk post-Ad-GFP transduction at MOI of 25. Outcome assessment included transduction efficiency, MFI, cell confluence to calculate cell proliferation, and cell viability by staining with 7-AAD (559925, BD Biosciences) detected with a 670 nm long pass filter. Cell morphology and GFP expression were documented with fluorescent microscopy.
Chondrogenic and osteogenic differentiation was carried out following Lonza’s protocol (AA-2501-16). The cultures were stained with Von Kossa stain and toluidine blue for evaluation of osteogenesis and chondrogenesis, respectively, of Ad-GFP-MSCs compared to control MSCs. Images were evaluated in ImageJ software.
MSCs at 80% confluency were transduced at MOI of 25 with Ad-BMP2 or used as control. On the following day, ascorbic acid was added to both, tranduced and control cells, in the DMEM to a final concentration of 50 pg/mL. At day 3 after transduction, samples from the media were evaluated for BMP2 expression using methods described for the BMP2 Quantikine ELISA Kit (BD Biosciences, DBP200). BMP2 expression per cell was estimated through quantification of BMP2 standard provided with the kit and using media from untransduced cells as background.
Ad-BMP2 MSCs and control cells were trypsinized at day 3 post-transduction and trypan blue exclusion count was performed to confirm viability of at least 90%. The cells were washed twice with PBS and resuspended in 0.9% NaCl to a concentration of 2 × 106 cells/0.1 mL. Thighs from twelve 6-wk-old mice (BALB/c Foxn1 nu/nu, Taconic Farms, Hudson, NY, United States) were randomly assigned to five groups: aMSC, Ad-BMP2-aMSC, fMSC, Ad-BMP2-fMSC, or saline. Anesthesia was induced with 5% isoflurane (IsoFlo, Abbott Animal Health, North Chicago, IL, United States) in 100% oxygen and maintained with 0%-2% isoflurane delivered by a precision vaporizer. Cranial thigh injection sites were disinfected with three alternating scrubs of povidone iodine and 70% isopropyl alcohol. Mice received one intramuscular injection in each thigh of 0.1 mL cells or saline using a 25 G needle. All mice recovered from anesthesia uneventfully. Three weeks after injection, mice were euthanized by CO2 followed by cervical dislocation. The hind limbs were harvested, and the skin was removed.
Digital microradiography (Model LX-60, Faxitron Bioptics, Tucson, AZ, United States) was performed separately for each experimental group. Lateromedial views of the hind limbs were obtained at 30 kV for 10 sand analyzed with ImageJ at 40% contrast. Outcome measurements included: number of mineralized bodies (i.e., ossicles), lateromedial area (mm2) of the ossicles, extent of periosteal new bone formation, and midshaft diameter (mm). Extent of periosteal new bone was scored as 0, 1, 2, 3, or 4 corresponding to no formation, new bone in less than 50% of the cranial aspect of the femur, more than 50% cranial, less than 50% involving both the cranial and the caudal aspects of the femur, or more than 50% cranial and caudal aspects involved, respectively.
Harvested limbs were fixed in 10% neutral buffered formalin for 4 mo. Samples were decalcified in 14% EDTA neutralized solution, overnight. The samples then underwent procedures for embedding, incising, and staining with toluidine blue or hematoxylin and eosin.
Data were reported as the mean ± SD. Group means were compared with two-tailed sample t test or One-way ANOVA. Relationships between categorical variables were tested by the chi-square method. Statistical analysis was performed using StatPlus. A value of P≤ 0.05 was deemed significant.
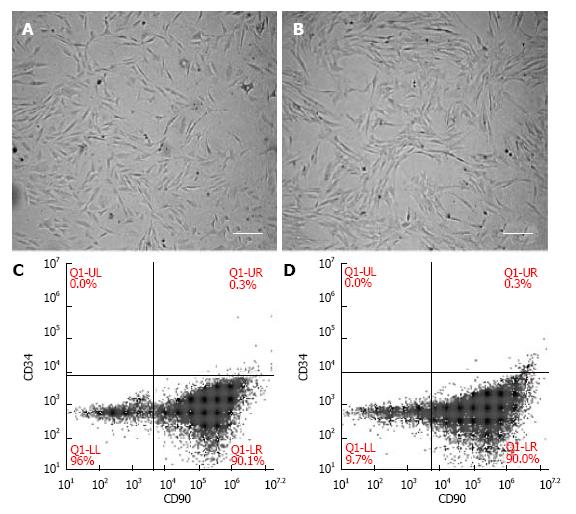
Cultured fMSCs and aMSCs displayed an elongated spindle-shaped population and maintained their morphology during subsequent passages (Figure 1A and B). MSCs were more than 90% positive for CD90 and negative for the hematopoietic stem cell marker CD34 (Figure 1C and D).
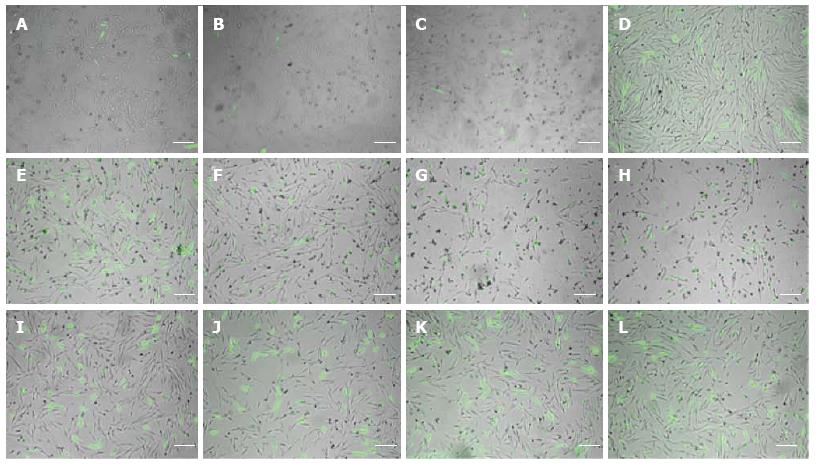
A qualitative analysis of fluorescent photomicrographs 48 h after pcDNA3-eGFP transfection revealed that FuGENE 6 resulted in the lowest transfection efficiency (Figure 2A-D) and Lipofectamine resulted in the lowest viability (Figure 2E-H), while PEI provided superior transfection efficiency (Figure 2I-L) in fMSCs than FuGENE 6 or Lipofectamine transfection reagents at 3:1, 3:2, 6:1, and 5:2 μL of reagent to μg of DNA ratio.
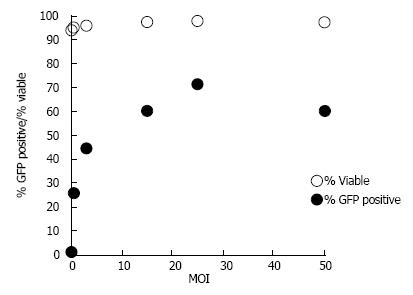
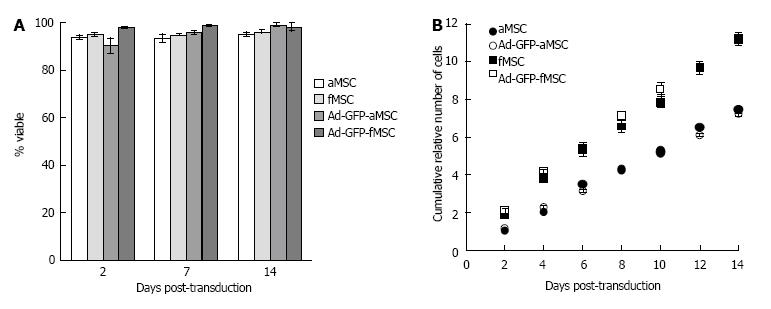
Optimized PEI-mediated transfection of pcDNA3-eGFP (5:2 μL of reagent/μg of DNA ratio) resulted in a maximum efficiency of 35.0% ± 1.6% as assessed by flow cytometry. No significant decrease in viability was observed at either of the tested MOIs when compared to control untreated cells (Figure 3). Ad transduction at MOI of 25 resulted in transduction efficiency above 70% with similar viability to control cells (Figure 3). Thus, Ad transduction at an MOI of 25 was selected for succeeding experiments.
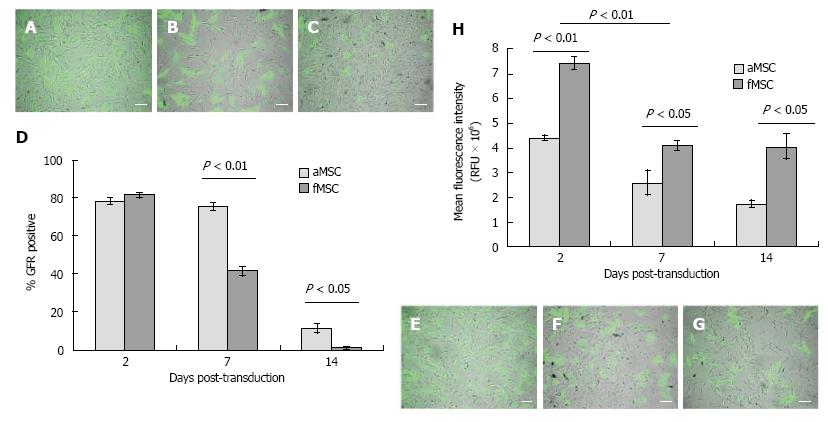
Ad-GFP transduction at MOI of 25 resulted in transfection efficiency of 81.3% ± 1.3% in fMSC, which was not statistically different from the efficiency of 78.1% ± 1.9% in aMSCs. There was a greater decrease of GFP positive cells in fMSC than aMSC within 2 wk post-transduction (Figure 4A-G). Mean fluorescence intensity (MFI) was 7.43 ± 0.08 × 106 and 4.40 ± 0.25 × 106 channel numbers in fMSCs and aMSCs, respectively, at 48 h post transduction (Figure 4H). Ad-GFP-fMSCs retained superior gene expression intensity compared to Ad-GFP-aMSCs at one and two weeks post transduction.
Cell viability of both fMSCs and aMSCs in culture was not significantly affected by exogenous administration of GFP gene via Ad-transduction at MOI of 25 when compared to control untransduced cells (Figure 5A). Number of cells was similar in both Ad-GFP-MSCs and control MSCs, with fMSCs showing a greater number of cells than aMSCs (Figure 5B), which in combination with results on viability reflect greater proliferation capacity of about 1.5 times faster proliferation than aMSCs (doubling time of 29 h vs 45 h). The latter, in combination with data on % GFP positive expression, suggests that Ad genome is gradually diluted out as cell division occurs.
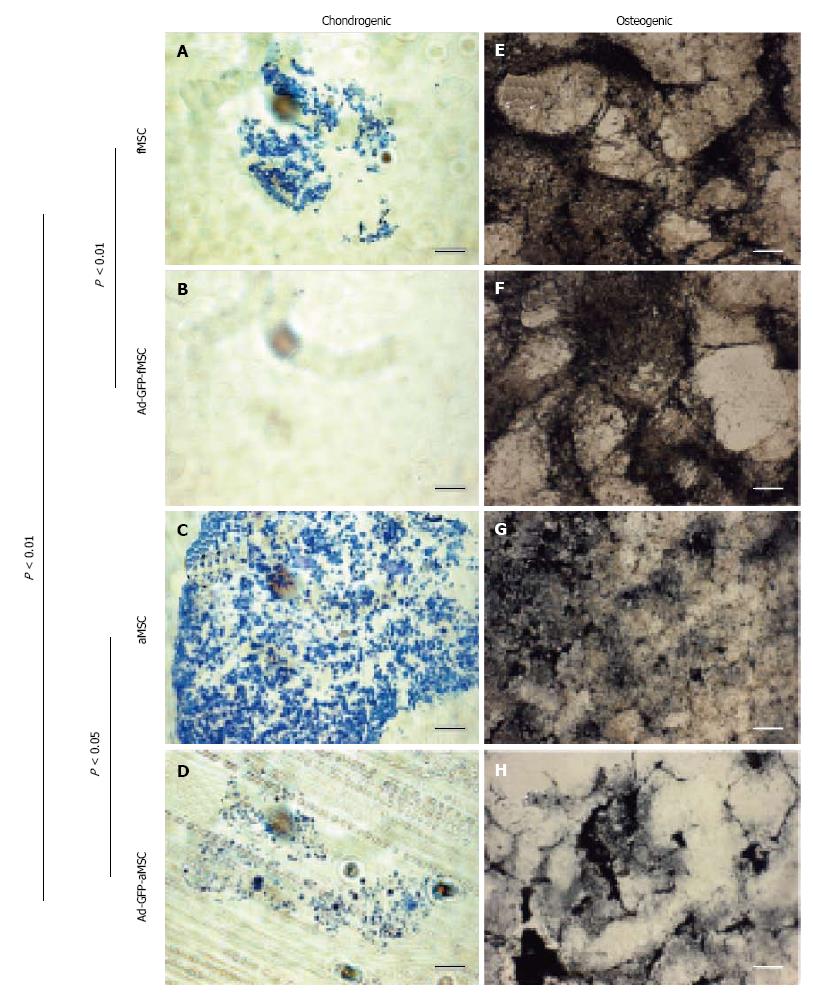
Chondrogenic differentiation in vitro was significantly less for fMSCs than aMSCs and decreased post Ad-GFP transduction as assessed by intensity and amount of toluidine blue staining in cell pellets (Figure 6A-D). Osteogenic differentiation was robust in transduced fMSC and aMSC, with no statistically significant difference between groups (Figure 6E-H).
Supernatants collected 3 d post-transduction from monolayer cultures used for osteoinduction were evaluated for BMP2 expression. It was determined that Ad-BMP2-fMSCs transduced at MOI of 25 released significantly more soluble BMP2 than Ad-BMP2-aMSCs (0.16 pg/day-cell vs 0.10 pg/day-cell; Table 1). Also, BMP2 expression per injection of 2 × 106 transduced MSC was estimated at 3.28 and 1.85 × 104 pg/dwith fMSCs and aMSCs, respectively (Table 1), consistent with the greater intensity of GFP expression in Ad-GFP-fMSCs than aMSCs.
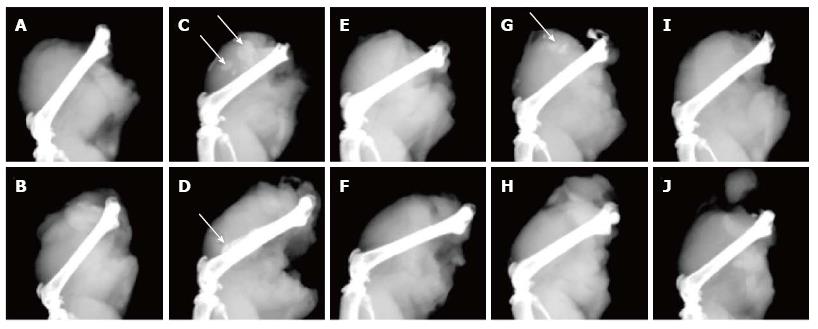
Digital microradiography of the harvested thigh specimens revealed intramuscular bone ossicle formation, new periosteal bone, and femur cortical bone thickening in treated mice with Ad-BMP2-MSCs, but not in saline-injection control group (Figure 7A and B). Five of six thighs treated with Ad-BMP2-aMSC resulted in new bone formation, which was significantly greater than saline, untransduced, and Ad-BMP2-fMSC groups (Table 2). The Ad-BMP2-aMSC group also had significantly greater femur midshaft diameter due to cortical thickening and new periosteal bone when compared to saline, untransduced, and Ad-BMP2-fMSC groups (Table 2). Bone ossicles were identified in two of the six thighs treated with Ad-BMP2-aMSCs (Table 3 and Figure 7C) and of the other four thighs in the Ad-BMP2-aMSC group, 3 showed femur cortical thickening and new periosteal bone (Table 4 and Figure 7D), while no changes were found in the untransduced aMSC group (Figure 7E-F). One of the six thighs treated with Ad-BMP2-fMSCs showed ossicle formation (Figure 7G-H), none resulted in periosteal changes, and no changes were noted in the untransduced fMSC group (Figure 7I-J).
| Treatment group | No.of thighs (n) | Ossicles (n) | Mean ossicle area(mm2± SD; range) |
| Ad-BMP2-aMSC | |||
| No ossicle | 4 | ||
| Ossicle | 2 | 6 | 0.78 ± 1.23 (0.03-3.20) |
| Ad-BMP2-fMSC | |||
| No ossicle | 5 | ||
| Ossicle | 1 | 3 | 0.45 ± 0.12 (0.32-0.47) |
| Extent of periosteal new bone | Thighs (n) | Midshaft diameter (mm) |
| 0 | 3 | 1.27 |
| 1 | 2 | 1.76 |
| 4 | 1 | 2.10 |
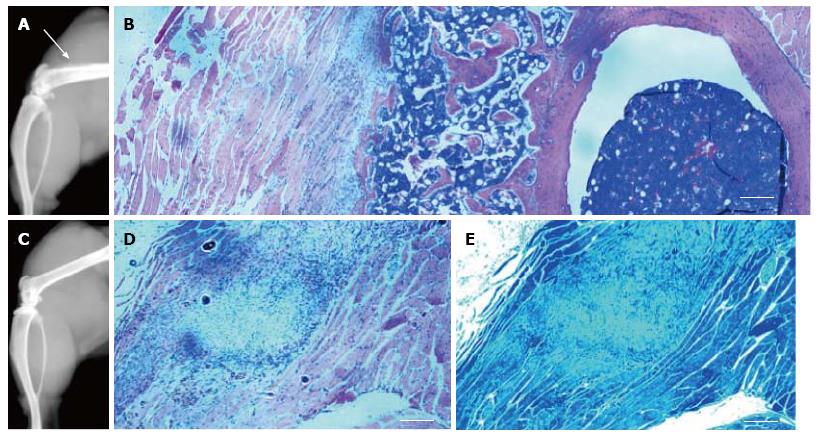
Histological examination confirmed new trabecular bone formation, normal in morphology, in the thighs of the Ad-BMP2-aMSC group (Figure 8A). This new trabecular bone contained highly cellular bone marrow (Figure 8B). A nodule of what appears to be our MSCs was identified in the untransduced aMSC group with no bone or cartilage formation (Figure 8C) as evaluated by H&E and toluidine blue stains, respectively (Figure 8D-E).
Our study confirmed that adenoviral vector-mediated transduction was efficient in fMSCs without affecting measures of MSC stemness. Chemical-mediated transfection was less efficient than Ad transduction, but PEI was superior and more effective in fMSCs than other commercial products. Fetal MSCs were not superior to aMSCs in osteoinduction in vivo. Both fMSCs and aMSCs were able to express BMP2 in vitro and to deliver BMP2 in vivo, which could have clinical applications in bone regeneration.
In the evaluation of chemical-mediated transfection, we found that PEI provided a significant level of transfection efficiency in fMSCs. Our results are in accordance with a study by Wang et al[17], which showed that genetic modification of human MSCs by PEI with a relatively low efficiency of 4% achieved biologically significant effects in vivo, with transfection efficiency proportional to cells in S phase[17]. Our analysis further supported the use of PEI as a transfection reagent in MSCs over other reagents. While we achieved around 35% transfection efficiency of pcDNA3-eGFP ratio, we found PEI to be relatively nontoxic in contrast to Lipofectamine and FuGENE 6. In conclusion, PEI offers an alternative to viral vector transduction and advantages over other chemical transfection reagents, especially when funding to support the use of viral vectors is not available.
Furthermore, the current study showed that Ad transduction provided superior efficiency to chemical transfection, including PEI. It has been shown that lentiviral and retroviral-mediated transduction are effective in fetal MSCs[12,13], however these are non transient and oncogenic such that their confirmed risk may be even greater when used in a stem cell. Our study was the first to test transient adenoviral mediated transduction in fetal MSCs. The present study confirmed that Ad transduction of fMSCs did not significantly affect measures of stemness, consistent with the previous studies addressing lentiviral and retroviral-mediated transduction of fMSCs. Viability was maintained above 90% for both fetal and adult MSCs, consistent with Ishihara et al[18] who reported equine aMSCs to not be sensitive to cytotoxic effects associated with Ad transduction at MOI of 100 or less. Our study also confirmed that fMSCs maintained a higher proliferative capacity than aMSCs post-transduction. This is again consistent with previous studies on viral vector-mediated stable transduction[12,13]. Also, our observed greater proliferative capacity of fMSCs than their adult counterpart is consistent with studies in human fMSCs[19]. These advantages of fMSCs would suggest advantages in vivo.
Ad-mediated transduction provided a similar efficiency in both fMSCs and aMSCs, but significantly greater intensity with greater decrease of the fraction of GFP positive cells in fMSCs compared to aMSCs. The greater proliferation of fMSC is sufficient to explain the greater reduction in GFP positive cells resulting in a gradual dilution of the adenoviral genome as cell division occurs[20-22]. This is due to the fact that Ad transduction does not result in DNA integration into the host genome; therefore the delivered gene will be expressed only as long as the viral genome is present[11,18].
Osteogenic differentiation capacity was not affected by Ad transduction in vitro. Our results are consistent with those of Zhang et al[10], who suggested that human fMSCs are a superior cellular candidate for bone tissue engineering applications than their adult counterpart because of their higher proliferation capacity, lower immunogenicity, and robust osteogenic potential as assessed via mineralization in vitro. On the other hand, the chondrogenic capacity of aMSCs was superior to fMSCs when inducing chondrogenesis with TGFβ3, and both decreased post-transduction with Ad-GFP. In fact, TGFβ3 was recently shown to induce chondrogenesis in aMSCs by phosphorylating SMAD3 but not in fMSCs[23], which explains in part the lower differentiation of fMSCs into chondrocytes found in the present study.
In the evaluation of osteogenesis in vivo, the present study showed that Ad-BMP2-aMSCs are capable of inducing regenerative bone formation more effectively than Ad-BMP2-fMSCs. Naïve fetal or adult MSCs alone were not osteoinductive in vivo. In fact, previous work by Zhang et al[10] have demonstrated that predifferentiation and a scaffold were necessary for osteoinduction by naïve MSCs. Although fMSCs showed higher BMP2 expression post-tranduction in vitro, fMSC resulted in minimal new bone formation in vivo. These findings may be related to the shorter expression of the transgene in fMSCs. Alternatively, initial high BMP2 expression and release from fMSCs could have caused a transient increase in number of osteoclasts derived from hematopoietic stem cells[24] but there was no evidence of bone remodeling in our histology to suggest this mechanism was at work. Based on our in vitro findings with fMSC, and recent work from our laboratory demonstrating superior outcomes of neocartilage growth with a mosaic of 90% naïve and 10% Ad-BMP2-transduced juvenile chondrocytes[25], it was anticipated that the dilution of BMP2 expressing cells in the rapidly proliferating fMSC population may be an advantage in vivo. In addition, Brady and colleagues recently demonstrated that recombinant BMP2 can induce chondrogenesis in pellet culture of fetal but not adult MSCs[23]. However, we were unable to demonstrate this potential as either enhanced endochondral ossification or chondrogenesis in vivo by fMSC, which could be due to difference in delivery method (recombinant BMP2 protein vs BMP2 gene transduction) or the fact that our study was in vivo. It is also possible that chondrogenesis would not be identified by microradiography and chondral ossicles may have evaded histologic sectioning, but, regardless, we saw minimal evidence of activity of fMSC in vivo.
In conclusion, the present study demonstrated adenoviral vectors as an effective method to transiently deliver genes into fMSCs. Nonetheless, the relative faster decrease in the fraction of cells expressing the exogenous gene when compared to transduced aMSCs may limit their applications. Our study also suggested fMSC-mediated BMP2 gene delivery and osteogenic induction in vivo was less robust than aMSCs at least in their capacity to induce bone formation. Both Ad-BMP2-aMSCs and Ad-BMP2-fMSCs were capable of expressing BMP2 in vitro and can be employed to deliver BMP2 for osteoinduction in vivo for the purpose of bone regeneration. Cell delivery may offer advantages over direct vector delivery. In addition, Ad vectors lack insertional mutagenesis, resulting in transient expression of the exogenous gene, which may be desirable over stable expression in applications involving bone morphogenic proteins to induce bone or cartilage regeneration. Further studies are necessary to elucidate differences in osteoinductive mechanisms of aMSCs and fMSCs in vivo.
We thank the following for technical assistance: Heather Strange for guidance with laboratory equipment and procedures, Hayam Hamzah Hussein for providing and guiding with the use of some of the MSCs and reagents, Juhnnel Vera for preparation of viral stocks, and Nathalie Reisbig for assistance with in vivo experiments.
In recent years, there have been hundreds of active clinical trials testing the use of mesenchymal stem cells (MSCs) in treating disorders such as myocardial infarction, osteoarthritis, spinal cord injury, and autoimmune diseases. Recent studies have demonstrated that fetal MSCs are a superior cellular candidate than adult MSCs for bone tissue engineering applications. While bone regeneration can be optimized with genetic engineering techniques, the permissiveness to gene delivery and the effect of genetic engineering on stemness in fetal MSCs has not been extensively studied.
Bone regeneration is a complex process that requires interplay of molecular, cellular, and metabolic factors. In the area of bone tissue regeneration and MSCs, the current hotspot is genetic engineering, microenvironment, and homing of MSCs to optimize bone healing.
Previous studies have found that fetal MSCs can be retrovirally and lentivirally transduced with high efficiency and retain their proliferative and differentiation capacities. The present study demonstrated that adenoviral vectors are an effective method to transiently deliver genes into fetal MSCs and bone morphogenic protein 2 (BMP2)-transduced MSCs can be employed for osteoinduction in vivo.
The study results suggest that fetal MSCs can be genetically engineered with adenoviral vectors to deliver BMP2 and induce osteoinduction, with promising applications in bone tissue regeneration.
MSCs are capable of self replicating and differentiating into mesenchymal tissues such as bone, cartilage, and adipose tissue. MSCs can be genetically engineered for applications in tissue regeneration. Adenoviral vectors can deliver genetic material into MSCs. These vectors lack insertional mutagenesis resulting in a desirable safety profile, which added to their capacity to achieve high levels of transgene expression make them attractive for gene delivery particularly for bone regeneration. BMP2 is a growth factor involved in signaling pathways for osteoinduction resulting in osteoblast differentiation of stem cells.
The authors have asked some interesting questions and they have compared the fetal vs adult MSCs for their ability to express transgenes via viral and non-viral vectors, and their differentiation capability. It is a very good study. Conclusions are supported by their data.
P- Reviewer: Guo ZS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Roubelakis MG. Therapeutic potential of fetal mesenchymal stem cells. Curr Stem Cell Res Ther. 2013;8:115-116. [PubMed] |
| 2. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2690] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 3. | Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol. 2013;25:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86-96; discussion 96-102, 170-174, 239-241. [PubMed] |
| 7. | In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [PubMed] |
| 8. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Jo CH, Kim OS, Park EY, Kim BJ, Lee JH, Kang SB, Lee JH, Han HS, Rhee SH, Yoon KS. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Zhang ZY, Teoh SH, Hui JH, Fisk NM, Choolani M, Chan JK. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009;90:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Campagnoli C, Bellantuono I, Kumar S, Fairbairn LJ, Roberts I, Fisk NM. High transduction efficiency of circulating first trimester fetal mesenchymal stem cells: potential targets for in utero ex vivo gene therapy. BJOG. 2002;109:952-954. [PubMed] |
| 13. | Chan J, O’Donoghue K, de la Fuente J, Roberts IA, Kumar S, Morgan JE, Fisk NM. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2005;23:93-102. [PubMed] |
| 14. | Castro MG, Candolfi M, Wilson TJ, Calinescu A, Paran C, Kamran N, Koschmann C, Moreno-Ayala MA, Assi H, Lowenstein PR. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin Biol Ther. 2014;14:1241-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ishihara A, Helbig HJ, Sanchez-Hodge RB, Wellman ML, Landrigan MD, Bertone AL. Performance of a gravitational marrow separator, multidirectional bone marrow aspiration needle, and repeated bone marrow collections on the production of concentrated bone marrow and separation of mesenchymal stem cells in horses. Am J Vet Res. 2013;74:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bertone AL, Pittman DD, Bouxsein ML, Li J, Clancy B, Seeherman HJ. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. J Orthop Res. 2004;22:1261-1270. [PubMed] |
| 17. | Wang W, Li W, Ou L, Flick E, Mark P, Nesselmann C, Lux CA, Gatzen HH, Kaminski A, Liebold A. Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. J Cell Mol Med. 2011;15:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ishihara A, Zachos TA, Bartlett JS, Bertone AL. Evaluation of permissiveness and cytotoxic effects in equine chondrocytes, synovial cells, and stem cells in response to infection with adenovirus 5 vectors for gene delivery. Am J Vet Res. 2006;67:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | O’Donoghue K, Chan J. Human fetal mesenchymal stem cells. Curr Stem Cell Res Ther. 2006;1:371-386. [PubMed] |
| 20. | Chen HH, Mack LM, Choi SY, Ontell M, Kochanek S, Clemens PR. DNA from both high-capacity and first-generation adenoviral vectors remains intact in skeletal muscle. Hum Gene Ther. 1999;10:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Fan X, Brun A, Segrén S, Jacobsen SE, Karlsson S. Efficient adenoviral vector transduction of human hematopoietic SCID-repopulating and long-term culture-initiating cells. Hum Gene Ther. 2000;11:1313-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Navarro V, Millecamps S, Geoffroy MC, Robert JJ, Valin A, Mallet J, Gal La Salle GL. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Ther. 1999;6:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Brady K, Dickinson SC, Guillot PV, Polak J, Blom AW, Kafienah W, Hollander AP. Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis. Stem Cells Dev. 2014;23:541-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I, Francetic I. The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop. 2014;38:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Ng VY, Jump SS, Santangelo KS, Russell DS, Bertone AL. Genetic engineering of juvenile human chondrocytes improves scaffold-free mosaic neocartilage grafts. Clin Orthop Relat Res. 2013;471:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |