Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.137
Peer-review started: July 28, 2014
First decision: September 16, 2014
Revised: October 14, 2014
Accepted: October 28, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 170 Days and 3.2 Hours
Recent advances in stem cell biology have shed light on how normal stem and progenitor cells can evolve to acquire malignant characteristics during tumorigenesis. The cancer counterparts of normal stem and progenitor cells might be occurred through alterations of stem cell fates including an increase in self-renewal capability and a decrease in differentiation and/or apoptosis. This oncogenic evolution of cancer stem and progenitor cells, which often associates with aggressive phenotypes of the tumorigenic cells, is controlled in part by dysregulated epigenetic mechanisms including aberrant DNA methylation leading to abnormal epigenetic memory. Epigenetic therapy by targeting DNA methyltransferases (DNMT) 1, DNMT3A and DNMT3B via 5-Azacytidine (Aza) and 5-Aza-2’-deoxycytidine (Aza-dC) has proved to be successful toward treatment of hematologic neoplasms especially for patients with myelodysplastic syndrome. In this review, I summarize the current knowledge of mechanisms underlying the inhibition of DNA methylation by Aza and Aza-dC, and of their apoptotic- and differentiation-inducing effects on cancer stem and progenitor cells in leukemia, medulloblastoma, glioblastoma, neuroblastoma, prostate cancer, pancreatic cancer and testicular germ cell tumors. Since cancer stem and progenitor cells are implicated in cancer aggressiveness such as tumor formation, progression, metastasis and recurrence, I propose that effective therapeutic strategies might be achieved through eradication of cancer stem and progenitor cells by targeting the DNA methylation machineries to interfere their “malignant memory”.
Core tip: Several types of cancers can be developed from genetic and/or epigenetic instabilities of stem and progenitor cells. Epigenetic abnormality involving dysregulation of DNA methylation has been reported to implicate in cancer aggressiveness. Inhibition of DNA methyltransferase activity by DNA methylation inhibitors has shown promising results toward treatment of myelodysplastic syndrome, a disease associated with leukemic stem cells. This review summarizes evidences which are pertinent to the antitumorigenic potential of DNA methylation inhibitors 5-Azacytidine and 5-Aza-2’-deoxycytidine on cancer stem and progenitor cells including those of leukemia and other solid tumors.
- Citation: Wongtrakoongate P. Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries. World J Stem Cells 2015; 7(1): 137-148
- URL: https://www.wjgnet.com/1948-0210/full/v7/i1/137.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.137
The study of stem cell biology, especially that of hematopoiesis, has provided an insight into the biology of cancers[1,2]. In particular, normal stem cells and cancer cells share one of their characteristics, which is an ability to proliferate without differentiation, the property known as self-renewal. Three concepts of cancer biology are proposed based on the study of hematopoietic stem cells and their tumorigenic counterparts as the paradigm: (1) normal stem cells and cancer cells might possess similar mechanisms to control their proliferation or self-renewal; (2) cancer cells can be derived by differentiation of normal stem cells via mutation of transit-amplifying cells or progenitors; and (3) there might be cancer stem cells inside tumors where the tumorigenesis occurred with normal stem cells[3]. Leukemia, which is the cancers of hematopoietic system, has provided the best evidence that normal stem cells are the target of oncogenic evolution, and that leukemogenesis is driven by cancer stem cells. For example, the tumor initiating cells of myelodysplastic syndrome (MDS), which can develop into acute myeloid leukemia (AML), are cancer stem cells, at least those with genetic deletion of chromosome 5q[4]. Cancer stem and progenitor cells have been suggested to involve with relapse in many types of cancers[5-13]. This tumorigenic recurrence of cancer stem and progenitor cells, which often associates with more aggressive phenotypes compared with differentiated cancer cells, can occur via accumulation of genetic mutations and/or epigenetic defects leading to selective advantage and hence the oncogenic evolution of cancer stem and progenitor cells.
Gene expression must be tightly regulated by both genetic and epigenetic mechanisms in non-cancerous normal cells, thereby avoiding malignant transformation. Both genetic- and epigenetic-dependent mechanisms ensure that expression of genes, whose function is involved in cell proliferation, apoptosis, self-renewal and differentiation, is normally controlled in temporal- and spatial-dependent manners. Deregulated epigenetic mechanisms have been shown to implicate in tumorigenesis. One epigenetic mechanism, which has been prominently focused in cancer research, is DNA methylation which also represents one of the most active fields of epigenetic research.
DNA methylation is one of the most common defects in epigenetic regulation observed in tumorigenesis. Earlier studies have suggested that cancer cells possess global DNA hypomethylation, which results in chromosomal instability[14]. However, in the past decade most cancer epigenetics studies have paid attention to regulatory region-associated DNA hypermethylation[15]. DNA hypermethylation of CpG dinucleotides has been well-documented in many types of cancer such as leukemia and colon cancer, and has been proposed as a hallmark of tumorigenesis[16]. Specifically, DNA hypermethylation at promoters of tumor suppressor- and differentiation-associated genes has been frequently reported. The DNA hypermethylation of those genes then leads to a reduced gene expression, which in turn provides a selective advantage to cancer cells. Thus, down-regulation of the genes by DNA hypermethylation might result in the emergence of cancer stem and progenitor cells[17,18].
DNA methylation has been widely accepted to mediate malignant memory of cancer cells due to its stable inheritance during cell division. Gene regulatory network specific to cancer stem and progenitor cells might play a direct role in dysregulation of DNA methylation. For example, the pluripotency-associated transcription factors OCT4 and NANOG have been shown to directly up-regulate expression of the maintenance DNA methyltransferase (DNMT) DNMT1 in mesenchymal stem cells[19]. In fact, maintenance of DNA methylation has been reported to be critical for cancer stem cell properties in leukemic, colon and lung cancer stem cells[20-22], which might be required to suppress expression of differentiation- and apoptosis-related genes. For instance, p16 and MLH1 are frequently hypermethylated in certain types of cancer. Of interesting is MLH1, which is a member of DNA mismatch repair pathway. Thus the epigenetic instability of MLH1 might be a cause of genetic instability of cancer stem and progenitor cells. The epigenetic defects of other genes involved in different pathways related to cell migration, hormone receptors, and cell cycle control, have also been described in tumorigenesis[18]. Different patterns of DNA hypermethylation have also been found in different types of cancers, which might be explained by cellular function of the cells in respective tissues[16]. In addition, the pattern of DNA hypermethylation might be influenced by an interaction between DNMTs and polycomb repressive complex 2 (PRC2) protein subunits[23], since different tumors also have different patterns of PRC2 binding[17,24,25]. Epigenetic therapy based on DNA methylation inhibitors including 5-Azacytidine (Aza) and 5-Aza-2’-deoxycytidine (Aza-dC) has promised a therapeutic regimen to patients with DNA hypermethylation-induced cancer diseases[15]. Therefore, understanding how DNMTs transfer a methyl group to genomic DNA, how they function in cancer cells, and how the drugs inhibit DNMT activity is crucial for development of superior anti-cancer therapy.
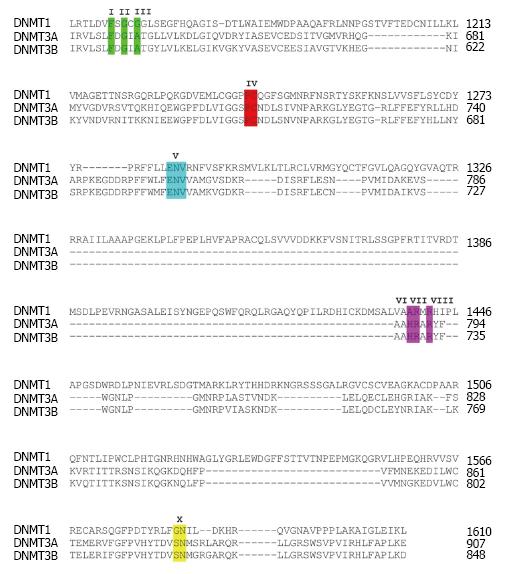
DNA methylation is responsible for multiple chromatin-related processes including regulation gene expression by adding a methyl group from S-adenosyl methionine (SAM) to the C5 position of a cytosine base mostly within CpG dinucleotide context at gene regulatory regions such as promoters and CpG islands. The CpG methylation is catalyzed by DNMTs. Five DNMT family proteins, i.e., DNMT1, DNMT2, DNMT3A, DNMT3B and DNMT3L, have been identified in mammalian genomes[26]. Among these, DNMT2 does not function as a methyltransferase for DNA but as a RNA methyltransferase[27,28]. In addition, DNMT3L does not possess the methyltransferase catalytic domain[29], leaving only DNMT1, DNMT3A and DNMT3B that are functional DNA methyltransferases[30]. Two mechanisms underlying DNA methylation have been reported. The first mechanism is maintenance DNA methylation pattern, of which all three DNMTs have the ability to copy DNA methylation pattern from DNA template to daughter DNA strand[31-37]. In one study, Sharma et al[34] have proposed a homeostatic inheritance system for maintenance of DNA methylation, whereby high levels of 5-methylcytosine stabilize DNMT3A and DNMT3B and prevent them from proteolysis[34]. The second mechanism is de novo DNA methylation which can only be performed by DNMT3A and DNMT3B. Apart from repressing gene expression via methylation at gene promoters, DNA methylation also plays important roles in many epigenetic phenomena involved in development for example genomic imprinting, X chromosome inactivation and higher-order chromatin organization[38,39]. Hence, apart from cancers, dysregulation of DNA methylation are also implicated in pathogenesis of many diseases.
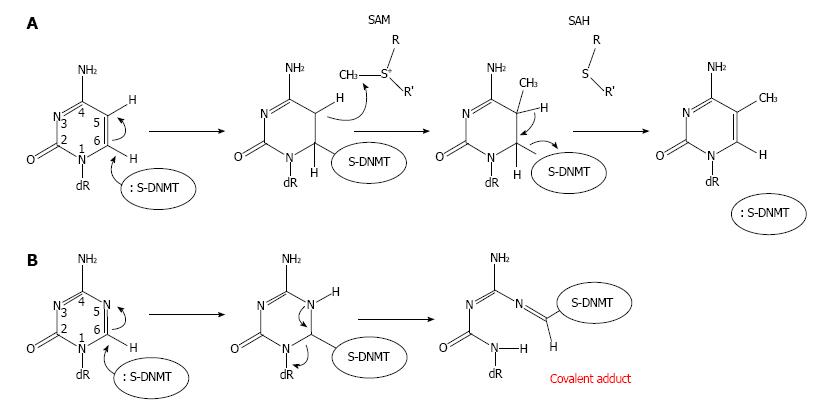
Several DNMT domains play important role in the methylation catalysis. Region IV harbors a prolyl-cysteinyl dipeptide that functions as the active site (Figure 1). Regions I and X share amino acid residues, which are critical for binding of SAM. Region V contains glutamyl/asparaginyl residue, which functions to protonate nitrogen 3 (N3) of targeted cytosine during the methyltransferase reaction. The enzymatic mechanism of DNA methylation begins with flipping cytosine out of the DNA helix upon binding with a DNMT. The conformational change of the cytosine nitrogenous base ensures that carbon 5 (C5) of the base aligns at the catalytic pocket with a bound SAM, and that C6 is at close proximity to a cysteine residue at the active site of the enzymes. The base flipping leads to formation of a covalent bond between C6 of the cytosine in DNA and the cysteine residue mediated by nucleophilic attack of sulfhydryl group of the cysteine to the C6 resulting in a change from C5 = C6 double bond into C5-C6 single bond (Figure 2A). The chemical bonding between C6 and cysteine then leads to an increase in electron flow toward C5 position of cytosine. At the same time, a methyl group from SAM is transferred to C5 generating C5-CH3 of the cytosine and S-adenosyl-homocysteine as the products. The proton at C5 position is then abstracted due to its weak bonding with C5 resulting in β-elimination at the C5-C6 single bond and in the reformation of the C5 = C6 double bond, which return a single electron to the cysteine of DNMT and discharge to the enzyme[30]. The C5-cytosine is now become methylated giving rise to methylcytosine.
The ability of Aza and Aza-dC to induce not only cell death but also differentiation had encouraged the exploitation of the compounds for treatment of cancers associated with stem/progenitor cells such as leukemia[40,41]. Unlike Aza which can be incorporated into both DNA and RNA, Aza-dC is only incorporated into DNA and is therefore a specific inhibitor for DNA but not RNA methyltransferases[42,43]. Consequently, the difference between the two epigenetic drugs results in differential cellular responses and causes distinct changes in the gene expression patterns of cells treated with Aza-dC and Aza[44,45].
Intracellular uptake of Aza-dC is mediated by the human concentrative nucleoside transporter 1 HCNT1, also known as SLC28A1[46]. Once inside cells, Aza-dC is then phosphorylated by deoxycytidine kinase yielding Aza-dCMP. Aza-dCMP is further phosphorylated to Aza-dCDP and subsequent Aza-dCTP, the active substrate form for DNA replication, by dCMP kinase and diphosphokinase, respectively. Hence the irreversible inhibition of Aza-dC on DNA methylation takes place only if Aza-dCTP is used as a substrate by DNA polymerases during DNA replication at S phase of the cell cycle, but not from free Aza-dC itself. Within DNA and with the presence of DNMTs, which bind to genomic DNA, the Aza-dC base can make a covalent bond with a DNMT enzyme (Figure 2B), disrupting the function of the enzyme. Mechanistically, because the nitrogen atom at position 5 (N5) of Aza-dC has a higher electronegativity than C5 of cytosine, the incorporated Aza-dC can covalently trap the DNMT enzyme. A single bond between N5-C6 is formed upon the nucleophilic attack of sulfhydryl group to the C6. However, unlike C5 in cytosine, the N5 can abstract a proton, and hence stably form a single bond to the proton with a strong affinity due to its high electronegativity. The DNMT is therefore trapped with the incorporated Aza-dC through a covalent bond between C6 and the cysteine residue leading to a DNA-DNMT adduct. Thus only in the presence of DNMTs can Aza-dC function to inhibit DNA methylation activity[47]. At chromatin level, gene reactivation activity of Aza-dC has been mechanistically demonstrated by Jones et al[31]. The authors devised a novel technique called AcceSssIble[48]. They find that approximately 2% of chromatin regions, which are demethylated after Aza-dC treatment in a colon cancer cell line, govern a feature of open chromatin[48] possibly associated with deposition of the histone variant H2A.Z[49]. Importantly, they show that of all genes reactivated by Aza-dC treatment, 90% of them possess DNA demethylation and chromatin accessibility supporting the role of the DNA methylation inhibitor as a gene reactivator. Interestingly, many reactivated genes are also tumor suppressor genes[48].
Several reports have demonstrated that depletion of DNMT1, DNMT3A or DNMT3B can counteract the cytotoxic and apoptotic effect of Aza-dC on treated cancer cells, confirming the essential role of DNMTs in mediating the Aza-dC response[47,50-52]. In contrast to the apoptotic response mediated by DNMTs, whether DNMTs also mediate stem cell differentiation induced by Aza-dC is not well understood. Epigenetic therapy by induced differentiation offers an option for treating malignancies, in which cancer tissues contain both cancer stem/progenitor cells and differentiated cancer cells, such as chronic leukemia, teratocarcinoma, and other solid tumors. Thus elucidating how cell differentiation can be triggered by Aza-dC or Aza could have an enormous impact on epigenetic therapy targeting cancer stem and progenitor cells.
Leukemia is considered to be the prototypical model for development of epigenetic therapy. MDS together with AML have been identified for being diseases of cancer stem and progenitor cells[4,53], which might contribute to relapse and resistance of chemotherapies. In fact, the DNMT inhibitor Aza is the first epigenetic drug which is approved by the Food and Drug Administration for treatment of MDS. Aza and Aza-dC have initially been shown to induce differentiation of Friend erythroleukemia cells[54]. In this study, the authors show that Aza-dC has a greater effect on reduction of cell proliferation compared with Aza when used at the same concentration, which might be associated with an increase in iron uptake and heme biosynthesis[55]. Later on, Pinto et al[56] have reported the first evidence of Aza-dC as a differentiation inducing agent of primary cells derived from patients with AML[56]. The differentiation inducing effect of Aza and Aza-dC has proven them to be promising therapeutic approaches aiming at induction of leukemic stem and progenitor cells toward non-malignant differentiated cells[57,58].
The induction ability of myeloid differentiation by Aza-dC in myeloid leukemic cells correlates with an up-regulation of CD15, myeloperoxidase, lysozyme and the tumor suppressor p15[59]. Several mechanisms have been proposed to underline Aza- and Aza-dC-induced differentiation of myeloid leukemia. For example, Aza-dC facilitates tumor necrosis factor alpha-induced monocytic differentiation of two AML cell lines NB4 and U937 by demethylation of DIF2 (also known as IER3) promoter and thus up-regulation of the gene[60]. Similarly, an upstream regulatory element within PU.1 promoter has been shown to be demethylated by Aza leading to an up-regulation of PU.1 and its target genes[61]. Co-treatment of CD34+ cells derived from MDS patients by Aza and granulocyte colony-stimulating factor led to myeloid differentiation[61]. Another study has shown that Aza-dC induces expression of olfactomedin 4 (OLFM4), of which an over-expression led to apoptosis and differentiation HL60 cell line[62]. Treatment of myeloid leukemia cells with Aza-dC also led to an induction of differentiation-associated genes including the erythroid-lineage transcription factor GATA1, which inhibits proliferation of the cancer cells[20]. A link between DNA hypermethylation and AML1/ETO-mediated repression of microRNA expression has been suggested as a means to inhibit apoptosis and differentiation of leukemic stem and progenitor cells[63,64]. In AML and chronic myeloid leukemia cell lines, Aza treatment reactivated expression of microRNA-193a, which suppresses translation of oncogenes including c-kit. Furthermore, an ectopic over-expression of microRNA-193a in primary cells of AML blasts led to apoptosis and differentiation of the cells[63]. Remarkably, Tsai et al[65] have recently shown that application of Aza or Aza-dC at low and non-acute toxic doses has successfully reduced stem cell characteristics of leukemia and also other cancer stem and progenitor cell lines and of primary tumors, without adverse effects on normal human bone marrow cells[65]. The low concentrations of the epigenetic drugs can demethylate both CpG island- and non-CpG island-containing promoters, which up-regulate expression of tumor suppressor- and differentiation-associated genes.
Recently, a failure of Aza to eradicate cancer stem and progenitor cells in patients with MDS and AML has been reported[66]. However, it has yet to be determined the mechanisms underlying this failure in order to improve new strategies of Aza treatment, and to design new drugs and clinical regimes that can completely diminish the cancer stem and progenitor cells of MDS and AML, either through an induction of cell death or differentiation. Interestingly, tranylcypromine, an inhibitor targeting histone H3 lysine 4 demethylase LSD1 (also known as KDM1A) which is pivotal for maintaining the stem cell state of AML cells[67], shows a promising result when combined with retinoic acid to induce myeloid differentiation of AML cells[68]. Therefore, combination therapies between DNA methylation inhibitors, preferentially with low doses[65], and other molecules which induce apoptosis and/or differentiation of cancer stem and progenitor cells of MDS and AML cells might offer an alternative direction to target the malignant cells in MDS and AML patients.
Although the effect of DNMT inhibitors on cytotoxicity and differentiation of lymphoblastic leukemia is less studied, a recent finding has suggested the role of Aza-dC in induction of apoptosis and differentiation of B-cell acute lymphoblastic leukemia (B-ALL) cell line NALM-6 and T-cell acute lymphoblastic leukemia (T-ALL) cell line CCRF-CEM[69]. The apoptotic induction by Aza-dC in B-ALL cells might be due to reactivation of HES5 gene, which has been shown to be hypermethylated in primary B-ALL cells[70]. Further investigations are needed to ascertain whether Aza and Aza-dC induces differentiation of primary B-ALL and T-ALL cells, and to elucidate mechanisms responsible for the eradication of cancer stem and progenitor cells of B-ALL and T-ALL by DNMT inhibitors.
Medulloblastoma is the most common malignant brain tumor found in children, and is a disease of cancer stem cells which possess activity of cell signaling pathways resemble to that of embryonic stages of neural progenitors[71]. Recent genome-wide DNA methylation analyses have suggested that DNA methylation plays a major role in the pathogenesis of medulloblastoma by repression of genes involved in developmental regulation thus avoiding cellular differentiation[72,73]. In addition, DNA methylation might also be important for medulloblastoma to avoid cell death, since an inhibition of DNA methylation sensitized medulloblastoma cell lines to undergo apoptosis induced by interferon gamma and TNF-related apoptosis-inducingligand (TRAIL)[74]. Treatment of medulloblastoma cell lines by Aza-dC has been shown to induce expression of SPINT2, which is an inhibitor of hepatocyte growth factor signaling and is hypermethylated in a number of patients with medulloblastoma[75]. Interestingly, an ectopic expression of SPINT2 led to a decrease in proliferation, colony survival and cell migration of medulloblastoma cell lines. Moreover, mice transplanted with SPINT2 over-expressing medulloblastoma cells show a prolonged survival compared with controls suggesting that SPINT2 functions as a tumor suppressor in vivo[75]. Similar to SPINT2, expression of CRABP2, which encodes a retinoic acid binding protein, is silenced by DNA methylation in medulloblastoma. Treatment of the cancer cells by Aza-dC restored expression of CRABP2, which in turn allowed neuronal differentiation of medulloblastoma cells induced by retinoic acid[76]. Co-treatment of Aza-dC and the histone deacetylase (HDAC) inhibitor valproic acid also resulted in a reduction of medulloblastoma formation in Patched mutant mouse models, and extended survival of the mice[77]. Thus further preclinical studies on combined therapies using DNA methylation inhibitors and HDAC inhibitors might be worthwhile for treatment of patients with medulloblastoma.
While medulloblastoma is the most prevalent brain tumor in children, glioblastoma is the most common and aggressive brain tumor found in adults. The role of DNA methylation in repression of genes involved in differentiation seems to be similar between glioblastoma and medulloblastoma. For example, DNA hypermethylation has been reported at CpG island upstream of CRABP2. Inhibition of DNA methylation by Aza-dC treatment in primary glioblastoma resulted in activation of CRABP2 expression and differentiation of the cells[76,78]. However, in contrast to medulloblastoma[74], treatment of glioblastoma stem-like cells by Aza-dC failed to sensitize the cells toward TRAIL-induced apoptosis, although expression of caspase-8 was induced by Aza-dC[79]. A connection of DNA methylation with p53 pathway has been described, in which Aza-dC treatment led to demethylation at p53 promoter. As the result, p53 then directly binds to the promoter region of the G protein-coupled formylpeptide receptor, and represses its expression leading to differentiation of glioblastoma cells[80]. Aza-dC has also been reported to induce expression of microRNA-137, which is involved in anti-proliferation and differentiation of glioblastoma stem cells[81].
Neuroblastoma has been proposed to develop from undifferentiated neural crest progenitor cells, which possess the potency to differentiate into peripheral neural and mesenchymal lineages[82]. It is a pediatric cancer, which might be explained by the embryonic origin of neural crest progenitor cells. DNA methylation has been reported to epigenetically silence expression of caspase-8, which is a mediator of apoptosis in neuroblastoma[83]. Co-treatment of Aza-dC and either chemotherapeutic drugs cisplatin or etoposide induced cell death possibly through caspase-8 reactivation[84]. In addition, as mentioned above for medulloblastoma[74], the same study also shows that a combined treatment of Aza-dC and interferon gamma led to apoptotic induction via TRAIL pathway in neuroblastoma cell lines[74]. It would be of interest to further investigate whether Aza-dC and interferon gamma might synergistically and extensively induce differentiation of the cancer progenitor cells as neuroblastoma have been shown to differentiate by Aza-dC treatment[85,86].
Androgen receptor (AR) is an important survival factor in prostate cancer. However, in some cases of prostate cancer, AR can function as a tumor suppressor gene[87]. The inhibitory role of AR in aggressiveness of prostate cancer is supported by loss of AR expression via promoter DNA methylation[88-91]. Interestingly, expression of AR is also reduced in prostate cancer stem cells particularly with high CD133 expression level[92]. Treatment of prostate cancer stem cells with Aza-dC led to an up-regulation of AR and a decrease in proliferation and tumor formation. Moreover, Aza-dC treatment also induced differentiation of the cancer stem cells, which is correlated with a reduced expression of stem cell-associated genes OCT4 and NANOG[92]. Thus, this study suggests that the inhibition of DNA methylation might be clinically translated for patients having prostate cancers with androgen-independent feature.
Pancreatic cancer stem cells have been shown to be sensitive to Aza-dC, which reduces tumor sphere formation and induces apoptosis of the cancer stem cells through activation of caspase-3 pathway[93]. The tumor suppressor activity of Aza-dC on pancreatic cancer stem cells is due to its ability to induce expression of microRNA-34, which in turn suppresses expression of the growth factor VEGF-B[93]. In addition to the apoptotic induction effect, Aza-dC has also been demonstrated to induce differentiation of the pancreatic cancer progenitor cell lines MIA PaCa-2[94] and PANC1[95]. Further investigations on how Aza-dC induces differentiation of pancreatic cancer stem and progenitor cells at molecular level are important toward its clinical translation.
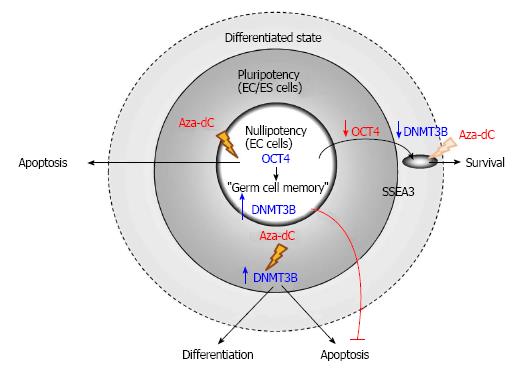
Germ cell tumors (GCTs) with stem cell characteristics comprise of seminoma and non-seminoma, which belong to type II testicular GCTs. Seminoma and non-seminoma have been proposed to develop from carcinoma in situ, also known as intratubular germ-cell neoplasia unclassified lesion or testicular intratubular neoplasia, which is the malignant counterpart of gonocytes[96,97]. Although the human seminoma-like cell line TCam-2 is resistant to Aza-dC even applied at 10 μmol/L[98], human embryonal carcinoma (EC) cells, the stem cells of teratocarcinoma, are extremely sensitive to the epigenetic drug of which only 10 nmol/L is sufficient to reduce viability of EC cells. This cytoxicity of Aza-dC on human EC cells is mediated by DNMT3B, which is a marker of human pluripotent stem cells[99] and is highly expressed in human EC cells[51]. Subsequently, DNMT3B has been reported to play a significant role in activation of DNA damage response and expression of p53 target genes induced by Aza-dC in human pluripotent EC cell line NTERA2[100].
Albeit having the cytotoxic effect on the cancer stem cells, Aza-dC fails to induce apoptosis of differentiated cells derived from human nullipotent EC cells, which can be isolated from teratocarcinoma more frequently than their pluripotent counterparts[52]. Although human nullipotent EC cells undergo apoptosis induced by Aza-dC, they do not differentiate. On the other hand, Aza-dC induces both differentiation and apoptosis of human pluripotent EC cells and also human embryonic stem (ES) cells. The ability to induce apoptosis of Aza-dC depends on DNMT3B in human pluripotent EC cells but not in their nullipotent counterparts (Figure 3). Similarly, the ability to induce differentiation of Aza-dC in human ES cells also depends on DNMT3B, supporting the role of DNMT3B in facilitating differentiation of human ES cells[101]. The failure of Aza-dC to induce differentiation of human nullipotent EC cells suggests that mechanism(s) other than DNA methylation is responsible for maintaining the stem cell state of nullipotent EC cells[102], which might still possess a remnant of germ cell memory[103]. Therefore, to effectively eradicate teratocarcinoma comprising of EC cells and their associated teratoma tissues, combined therapies between Aza-dC and agents that target the differentiated cancer cells should be considered.
Progresses in an understanding of normal stem cell biology have revolutionized the concept of cancer stem and progenitor cells, which might be the key toward success in treatment of a number of cancer diseases by targeting at cancer stem and progenitor cells. Aza and Aza-dC, although being the drugs currently used for treatment of hematologic malignancy, have held a great promise for treatment of solid tumors[65]. Future studies on mechanisms underlying differentiation and/or apoptosis induced by Aza and Aza-dC on cancer stem and progenitor cells are required for effective treatment of the epigenetic drugs. Several questions emerge including; (1) Do gene regulatory networks, which are specifically associated with cancer counterparts of stem and progenitor cells, up-regulate expression of DNMTs? (2) Which signaling pathways and their downstream target genes are functionally perturbed by Aza and Aza-dC treatment? (3) Are cell death pathways other than apoptosis involved in the anti-proliferation effect? (4) Are Aza and Aza-dC effective to eradicate cancer stem and progenitor cells in xenograft tumors? (5) In case of relapse, do recurrent cancer stem and progenitor cells evolve a distinct “malignant memory” at epigenomic levels compared with the cancer cells isolated before admission? and, if so, (6) Does the oncogenic evolution lead to more aggressive phenotypes such as a reduced differentiation potential of cancer stem and progenitor cells ? Treatments with Aza and Aza-dC can lead to certain toxicity and side effects to hematopoietic, nervous as well as metabolic systems[104-107]. Nevertheless, they have been recognized as having lower toxicity compared to traditional chemotherapy. In fact, low-dose treatment of Aza-dC in patients with MDS[108] and solid tumors[109] resulted in complete response of the patients. Thus using low-dose DNA methylation inhibitors may provide a safe therapeutic choice[65] especially for elderly patients[107,110]. Whether long-termed treatments of low-dose Aza and Aza-dC would perturb fates of stem and progenitor cells without side effects and toxicity in patients have yet to be elucidated. Addressing these questions will not only provide an understanding of cancer stem cell biology but also shed light on development of novel clinical regimes.
The author is grateful to the Thai Commission on Higher Education for the Strategic Frontier Research scholarship on stem cell biology.
P- Reviewer: Kondo Y, Scatena R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 2. | Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1671] [Article Influence: 151.9] [Reference Citation Analysis (0)] |
| 3. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6906] [Article Influence: 287.8] [Reference Citation Analysis (0)] |
| 4. | Woll PS, Kjällquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S, Erlandsson R, Ngara M, Anderson K, Deng Q. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25:794-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Hsu DM, Agarwal S, Benham A, Coarfa C, Trahan DN, Chen Z, Stowers PN, Courtney AN, Lakoma A, Barbieri E. G-CSF receptor positive neuroblastoma subpopulations are enriched in chemotherapy-resistant or relapsed tumors and are highly tumorigenic. Cancer Res. 2013;73:4134-4146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Jain S, Ward MM, O’Loughlin J, Boeck M, Wiener N, Chuang E, Cigler T, Moore A, Donovan D, Lam C. Incremental increase in VEGFR1⁺ hematopoietic progenitor cells and VEGFR2⁺ endothelial progenitor cells predicts relapse and lack of tumor response in breast cancer patients. Breast Cancer Res Treat. 2012;132:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Fotovati A, Abu-Ali S, Wang PS, Deleyrolle LP, Lee C, Triscott J, Chen JY, Franciosi S, Nakamura Y, Sugita Y. YB-1 bridges neural stem cells and brain tumor-initiating cells via its roles in differentiation and cell growth. Cancer Res. 2011;71:5569-5578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Schemionek M, Elling C, Steidl U, Bäumer N, Hamilton A, Spieker T, Göthert JR, Stehling M, Wagers A, Huettner CS. BCR-ABL enhances differentiation of long-term repopulating hematopoietic stem cells. Blood. 2010;115:3185-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Gilbert DC, McIntyre A, Summersgill B, Missiaglia E, Goddard NC, Chandler I, Huddart RA, Shipley J. Minimum regions of genomic imbalance in stage I testicular embryonal carcinoma and association of 22q loss with relapse. Genes Chromosomes Cancer. 2011;50:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Mego M. Cancer stem cell in relapsed testicular germ cell cancer: embryonic or somatic? Int J Androl. 2006;29:627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Pogribny IP, Beland FA. DNA hypomethylation in the origin and pathogenesis of human diseases. Cell Mol Life Sci. 2009;66:2249-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Yang X, Lay F, Han H, Jones PA. Targeting DNA methylation for epigenetic therapy. Trends Pharmacol Sci. 2010;31:536-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16 Spec No 1:R50-R59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 841] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 18. | Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3299] [Cited by in RCA: 3396] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 19. | Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 20. | Bröske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Morita R, Hirohashi Y, Suzuki H, Takahashi A, Tamura Y, Kanaseki T, Asanuma H, Inoda S, Kondo T, Hashino S. DNA methyltransferase 1 is essential for initiation of the colon cancers. Exp Mol Pathol. 2013;94:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu HS, Hung SC. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1627] [Cited by in RCA: 1665] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 24. | Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, Weisenberger DJ, Campan M, Young J, Jacobs I. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 792] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 25. | Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 895] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 26. | Siedlecki P, Zielenkiewicz P. Mammalian DNA methyltransferases. Acta Biochim Pol. 2006;53:245-256. [PubMed] |
| 27. | Raddatz G, Guzzardo PM, Olova N, Fantappié MR, Rampp M, Schaefer M, Reik W, Hannon GJ, Lyko F. Dnmt2-dependent methylomes lack defined DNA methylation patterns. Proc Natl Acad Sci USA. 2013;110:8627-8631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 772] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 29. | Aapola U, Kawasaki K, Scott HS, Ollila J, Vihinen M, Heino M, Shintani A, Kawasaki K, Minoshima S, Krohn K. Isolation and initial characterization of a novel zinc finger gene, DNMT3L, on 21q22.3, related to the cytosine-5-methyltransferase 3 gene family. Genomics. 2000;65:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Santi DV, Garrett CE, Barr PJ. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983;33:9-10. [PubMed] |
| 31. | Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 32. | Bachman KE, Rountree MR, Baylin SB. Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J Biol Chem. 2001;276:32282-32287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 325] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 33. | Jeong S, Liang G, Sharma S, Lin JC, Choi SH, Han H, Yoo CB, Egger G, Yang AS, Jones PA. Selective anchoring of DNA methyltransferases 3A and 3B to nucleosomes containing methylated DNA. Mol Cell Biol. 2009;29:5366-5376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Sharma S, De Carvalho DD, Jeong S, Jones PA, Liang G. Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance. PLoS Genet. 2011;7:e1001286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003;23:5594-5605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 566] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 36. | Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 920] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 37. | Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, Laird PW, Jones PA. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol. 2002;22:480-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 410] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 38. | Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2022] [Cited by in RCA: 2177] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 39. | Li G, Reinberg D. Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev. 2011;21:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 40. | Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 871] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 41. | Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1365] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 42. | Li LH, Olin EJ, Buskirk HH, Reineke LM. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970;30:2760-2769. [PubMed] |
| 43. | Lu LJ, Randerath K. Mechanism of 5-azacytidine-induced transfer RNA cytosine-5-methyltransferase deficiency. Cancer Res. 1980;40:2701-2705. [PubMed] |
| 44. | Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N, Krushel L, Aukerman SL, Heise C, MacBeth KJ. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5:e9001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 45. | Qiu X, Hother C, Ralfkiær UM, Søgaard A, Lu Q, Workman CT, Liang G, Jones PA, Grønbæk K. Equitoxic doses of 5-azacytidine and 5-aza-2’deoxycytidine induce diverse immediate and overlapping heritable changes in the transcriptome. PLoS One. 2010;5:e12994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Rius M, Stresemann C, Keller D, Brom M, Schirrmacher E, Keppler D, Lyko F. Human concentrative nucleoside transporter 1-mediated uptake of 5-azacytidine enhances DNA demethylation. Mol Cancer Ther. 2009;8:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2’-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;91:11797-11801. [PubMed] |
| 48. | Pandiyan K, You JS, Yang X, Dai C, Zhou XJ, Baylin SB, Jones PA, Liang G. Functional DNA demethylation is accompanied by chromatin accessibility. Nucleic Acids Res. 2013;41:3973-3985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Yang X, Noushmehr H, Han H, Andreu-Vieyra C, Liang G, Jones PA. Gene reactivation by 5-aza-2’-deoxycytidine-induced demethylation requires SRCAP-mediated H2A.Z insertion to establish nucleosome depleted regions. PLoS Genet. 2012;8:e1002604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Oka M, Meacham AM, Hamazaki T, Rodić N, Chang LJ, Terada N. De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2’-deoxycytidine. Oncogene. 2005;24:3091-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Beyrouthy MJ, Garner KM, Hever MP, Freemantle SJ, Eastman A, Dmitrovsky E, Spinella MJ. High DNA methyltransferase 3B expression mediates 5-aza-deoxycytidine hypersensitivity in testicular germ cell tumors. Cancer Res. 2009;69:9360-9366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Wongtrakoongate P, Li J, Andrews PW. Aza-deoxycytidine induces apoptosis or differentiation via DNMT3B and targets embryonal carcinoma cells but not their differentiated derivatives. Br J Cancer. 2014;110:2131-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4849] [Article Influence: 173.2] [Reference Citation Analysis (1)] |
| 54. | Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2’-deoxycytidine. J Biol Chem. 1982;257:2041-2048. [PubMed] |
| 55. | Ning B, Liu G, Liu Y, Su X, Anderson GJ, Zheng X, Chang Y, Guo M, Liu Y, Zhao Y. 5-aza-2’-deoxycytidine activates iron uptake and heme biosynthesis by increasing c-Myc nuclear localization and binding to the E-boxes of transferrin receptor 1 (TfR1) and ferrochelatase (Fech) genes. J Biol Chem. 2011;286:37196-37206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Pinto A, Attadia V, Fusco A, Ferrara F, Spada OA, Di Fiore PP. 5-Aza-2’-deoxycytidine induces terminal differentiation of leukemic blasts from patients with acute myeloid leukemias. Blood. 1984;64:922-929. [PubMed] |
| 57. | Zagonel V, Lo Re G, Marotta G, Babare R, Sardeo G, Gattei V, De Angelis V, Monfardini S, Pinto A. 5-Aza-2’-deoxycytidine (Decitabine) induces trilineage response in unfavourable myelodysplastic syndromes. Leukemia. 1993;7 Suppl 1:30-35. [PubMed] |
| 58. | Pinto A, Zagonel V, Attadia V, Bullian PL, Gattei V, Carbone A, Monfardini S, Colombatti A. 5-Aza-2’-deoxycytidine as a differentiation inducer in acute myeloid leukaemias and myelodysplastic syndromes of the elderly. Bone Marrow Transplant. 1989;4 Suppl 3:28-32. [PubMed] |
| 59. | Guo Y, Engelhardt M, Wider D, Abdelkarim M, Lübbert M. Effects of 5-aza-2’-deoxycytidine on proliferation, differentiation and p15/INK4b regulation of human hematopoietic progenitor cells. Leukemia. 2006;20:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Laurenzana A, Petruccelli LA, Pettersson F, Figueroa ME, Melnick A, Baldwin AS, Paoletti F, Miller WH. Inhibition of DNA methyltransferase activates tumor necrosis factor alpha-induced monocytic differentiation in acute myeloid leukemia cells. Cancer Res. 2009;69:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Curik N, Burda P, Vargova K, Pospisil V, Belickova M, Vlckova P, Savvulidi F, Necas E, Hajkova H, Haskovec C. 5-azacitidine in aggressive myelodysplastic syndromes regulates chromatin structure at PU.1 gene and cell differentiation capacity. Leukemia. 2012;26:1804-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Liu W, Lee HW, Liu Y, Wang R, Rodgers GP. Olfactomedin 4 is a novel target gene of retinoic acids and 5-aza-2’-deoxycytidine involved in human myeloid leukemia cell growth, differentiation, and apoptosis. Blood. 2010;116:4938-4947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Gao XN, Lin J, Li YH, Gao L, Wang XR, Wang W, Kang HY, Yan GT, Wang LL, Yu L. MicroRNA-193a represses c-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene. 2011;30:3416-3428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Li Y, Gao L, Luo X, Wang L, Gao X, Wang W, Sun J, Dou L, Li J, Xu C. Epigenetic silencing of microRNA-193a contributes to leukemogenesis in t(8; 21) acute myeloid leukemia by activating the PTEN/PI3K signal pathway. Blood. 2013;121:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 66. | Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, Aztberger A, Schuh A, Grimwade D, Ivey A. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013;27:1028-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y, Ciceri F, Blaser JG, Greystoke BF, Jordan AM. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell. 2012;21:473-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 464] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 68. | Schenk T, Chen WC, Göllner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012;18:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 526] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 69. | Soleymani Fard S, Jeddi Tehrani M, Ardekani AM. Prostaglandin E2 induces growth inhibition, apoptosis and differentiation in T and B cell-derived acute lymphoblastic leukemia cell lines (CCRF-CEM and Nalm-6). Prostaglandins Leukot Essent Fatty Acids. 2012;87:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Kuang SQ, Fang Z, Zweidler-McKay PA, Yang H, Wei Y, Gonzalez-Cervantes EA, Boumber Y, Garcia-Manero G. Epigenetic inactivation of Notch-Hes pathway in human B-cell acute lymphoblastic leukemia. PLoS One. 2013;8:e61807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Manoranjan B, Venugopal C, McFarlane N, Doble BW, Dunn SE, Scheinemann K, Singh SK. Medulloblastoma stem cells: where development and cancer cross pathways. Pediatr Res. 2012;71:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Hovestadt V, Jones DT, Picelli S, Wang W, Kool M, Northcott PA, Sultan M, Stachurski K, Ryzhova M, Warnatz HJ. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature. 2014;510:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 73. | Diede SJ, Guenthoer J, Geng LN, Mahoney SE, Marotta M, Olson JM, Tanaka H, Tapscott SJ. DNA methylation of developmental genes in pediatric medulloblastomas identified by denaturation analysis of methylation differences. Proc Natl Acad Sci USA. 2010;107:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Fulda S, Debatin KM. 5-Aza-2’-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25:5125-5133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Kongkham PN, Northcott PA, Ra YS, Nakahara Y, Mainprize TG, Croul SE, Smith CA, Taylor MD, Rutka JT. An epigenetic genome-wide screen identifies SPINT2 as a novel tumor suppressor gene in pediatric medulloblastoma. Cancer Res. 2008;68:9945-9953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Fu YS, Wang Q, Ma JX, Yang XH, Wu ML, Zhang KL, Kong QY, Chen XY, Sun Y, Chen NN. CRABP-II methylation: a critical determinant of retinoic acid resistance of medulloblastoma cells. Mol Oncol. 2012;6:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Ecke I, Petry F, Rosenberger A, Tauber S, Mönkemeyer S, Hess I, Dullin C, Kimmina S, Pirngruber J, Johnsen SA. Antitumor effects of a combined 5-aza-2’deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res. 2009;69:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Campos B, Warta R, Chaisaingmongkol J, Geiselhart L, Popanda O, Hartmann C, von Deimling A, Unterberg A, Plass C, Schmezer P. Epigenetically mediated downregulation of the differentiation-promoting chaperon protein CRABP2 in astrocytic gliomas. Int J Cancer. 2012;131:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Capper D, Gaiser T, Hartmann C, Habel A, Mueller W, Herold-Mende C, von Deimling A, Siegelin MD. Stem-cell-like glioma cells are resistant to TRAIL/Apo2L and exhibit down-regulation of caspase-8 by promoter methylation. Acta Neuropathol. 2009;117:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Huang J, Chen K, Huang J, Gong W, Dunlop NM, Howard OM, Bian X, Gao Y, Wang JM. Regulation of the leucocyte chemoattractant receptor FPR in glioblastoma cells by cell differentiation. Carcinogenesis. 2009;30:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 81. | Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 725] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 82. | Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 612] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 83. | Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 558] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 84. | Charlet J, Schnekenburger M, Brown KW, Diederich M. DNA demethylation increases sensitivity of neuroblastoma cells to chemotherapeutic drugs. Biochem Pharmacol. 2012;83:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Bartolucci S, Estenoz M, Longo A, Santoro B, Momparler RL, Rossi M, Augusti-Tocco G. 5-Aza-2’-deoxycytidine as inducer of differentiation and growth inhibition in mouse neuroblastoma cells. Cell Differ Dev. 1989;27:47-55. [PubMed] |
| 86. | Bartolucci S, Estenoz M, de Franciscis V, Carpinelli P, Colucci GL, Augusti Tocco G, Rossi M. Effect of cytidine analogs on cell growth and differentiation on a human neuroblastoma line. Cell Biophys. 1989;15:67-77. [PubMed] |
| 87. | Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. 2014;33:3225-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 88. | Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, Meisner LF, Chang C, Herman JG, Isaacs WB, Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58:5310-5314. [PubMed] |
| 89. | Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60:3623-3630. [PubMed] |
| 90. | Takahashi S, Inaguma S, Sakakibara M, Cho YM, Suzuki S, Ikeda Y, Cui L, Shirai T. DNA methylation in the androgen receptor gene promoter region in rat prostate cancers. Prostate. 2002;52:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto Si, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Tian J, Lee SO, Liang L, Luo J, Huang CK, Li L, Niu Y, Chang C. Targeting the unique methylation pattern of androgen receptor (AR) promoter in prostate stem/progenitor cells with 5-aza-2’-deoxycytidine (5-AZA) leads to suppressed prostate tumorigenesis. J Biol Chem. 2012;287:39954-39966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6:e24099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 94. | Yamada T, Ohwada S, Saitoh F, Adachi M, Morishita Y, Hozumi M. Induction of Ley antigen by 5-aza-2’-deoxycytidine in association with differentiation and apoptosis in human pancreatic cancer cells. Anticancer Res. 1996;16:735-740. [PubMed] |
| 95. | Lefebvre B, Belaich S, Longue J, Vandewalle B, Oberholzer J, Gmyr V, Pattou F, Kerr-Conte J. 5’-AZA induces Ngn3 expression and endocrine differentiation in the PANC-1 human ductal cell line. Biochem Biophys Res Commun. 2010;391:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Rajpert-De Meyts E, Jørgensen N, Brøndum-Nielsen K, Müller J, Skakkebaek NE. Developmental arrest of germ cells in the pathogenesis of germ cell neoplasia. APMIS. 1998;106:198-204; discussion 204-206. [PubMed] |
| 97. | Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 464] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 98. | Nettersheim D, Gillis A, Biermann K, Looijenga LH, Schorle H. The seminoma cell line TCam-2 is sensitive to HDAC inhibitor depsipeptide but tolerates various other chemotherapeutic drugs and loss of NANOG expression. Genes Chromosomes Cancer. 2011;50:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, Hartung O, Rho J, Ince TA. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 100. | Biswal BK, Beyrouthy MJ, Hever-Jardine MP, Armstrong D, Tomlinson CR, Christensen BC, Marsit CJ, Spinella MJ. Acute hypersensitivity of pluripotent testicular cancer-derived embryonal carcinoma to low-dose 5-aza deoxycytidine is associated with global DNA Damage-associated p53 activation, anti-pluripotency and DNA demethylation. PLoS One. 2012;7:e53003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Wongtrakoongate P, Li J, Andrews PW. DNMT3B inhibits the re-expression of genes associated with induced pluripotency. Exp Cell Res. 2014;321:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Sutiwisesak R, Kitiyanant N, Kotchabhakdi N, Felsenfeld G, Andrews PW, Wongtrakoongate P. Induced pluripotency enables differentiation of human nullipotent embryonal carcinoma cells N2102Ep. Biochim Biophys Acta. 2014;1843:2611-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Wongtrakoongate P, Jones M, Gokhale PJ, Andrews PW. STELLA facilitates differentiation of germ cell and endodermal lineages of human embryonic stem cells. PLoS One. 2013;8:e56893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Momparler RL, Bouffard DY, Momparler LF, Dionne J, Belanger K, Ayoub J. Pilot phase I-II study on 5-aza-2’-deoxycytidine (Decitabine) in patients with metastatic lung cancer. Anticancer Drugs. 1997;8:358-368. [PubMed] |
| 105. | Wijermans P, Lübbert M, Verhoef G, Bosly A, Ravoet C, Andre M, Ferrant A. Low-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18:956-962. [PubMed] |
| 106. | Oki Y, Kondo Y, Yamamoto K, Ogura M, Kasai M, Kobayashi Y, Watanabe T, Uike N, Ohyashiki K, Okamoto S. Phase I/II study of decitabine in patients with myelodysplastic syndrome: a multi-center study in Japan. Cancer Sci. 2012;103:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 107. | Lübbert M, Rüter BH, Claus R, Schmoor C, Schmid M, Germing U, Kuendgen A, Rethwisch V, Ganser A, Platzbecker U. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 108. | Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, Faderl S, Bueso-Ramos C, Ravandi F, Estrov Z. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 529] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 109. | Fan H, Lu X, Wang X, Liu Y, Guo B, Zhang Y, Zhang W, Nie J, Feng K, Chen M. Low-dose decitabine-based chemoimmunotherapy for patients with refractory advanced solid tumors: a phase I/II report. J Immunol Res. 2014;2014:371087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Khan C, Pathe N, Fazal S, Lister J, Rossetti JM. Azacitidine in the management of patients with myelodysplastic syndromes. Ther Adv Hematol. 2012;3:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |