Published online Nov 26, 2014. doi: 10.4252/wjsc.v6.i5.579
Revised: August 24, 2014
Accepted: August 30, 2014
Published online: November 26, 2014
Processing time: 65 Days and 15.5 Hours
Liver cancer is an aggressive disease with a high mortality rate. Management of liver cancer is strongly dependent on the tumor stage and underlying liver disease. Unfortunately, most cases are discovered when the cancer is already advanced, missing the opportunity for surgical resection. Thus, an improved understanding of the mechanisms responsible for liver cancer initiation and progression will facilitate the detection of more reliable tumor markers and the development of new small molecules for targeted therapy of liver cancer. Recently, there is increasing evidence for the “cancer stem cell hypothesis”, which postulates that liver cancer originates from the malignant transformation of liver stem/progenitor cells (liver cancer stem cells). This cancer stem cell model has important significance for understanding the basic biology of liver cancer and has profound importance for the development of new strategies for cancer prevention and treatment. In this review, we highlight recent advances in the role of liver stem cells in hepatocarcinogenesis. Our review of the literature shows that identification of the cellular origin and the signaling pathways involved is challenging issues in liver cancer with pivotal implications in therapeutic perspectives. Although the dedifferentiation of mature hepatocytes/cholangiocytes in hepatocarcinogenesis cannot be excluded, neoplastic transformation of a stem cell subpopulation more easily explains hepatocarcinogenesis. Elimination of liver cancer stem cells in liver cancer could result in the degeneration of downstream cells, which makes them potential targets for liver cancer therapies. Therefore, liver stem cells could represent a new target for therapeutic approaches to liver cancer in the near future.
Core tip: Liver cancer is an aggressive disease with a high mortality rate. However, the concept of liver cancer origin is controversial. Recently, there is increasing evidence for the “cancer stem cell hypothesis”, which proposes that liver cancer originates from the malignant transformation of liver stem/progenitor cells (liver cancer stem cells). This cancer stem cell model has important significance for understanding the basic biology of liver cancer and has profound importance for the development of new strategies for cancer prevention and treatment. This review discusses current knowledge concerning the role of liver stem cells in the hepatocarcinogenesis of primary liver cancer.
- Citation: Xu LB, Liu C. Role of liver stem cells in hepatocarcinogenesis. World J Stem Cells 2014; 6(5): 579-590
- URL: https://www.wjgnet.com/1948-0210/full/v6/i5/579.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i5.579
Liver cancer is one of the most common tumors and represents the second leading cause of cancer-related death worldwide. Its incidence continues to increase while the prognosis remains gloomy[1]. Management of liver cancer is strongly dependent on the tumor stage and underlying liver disease. Unfortunately, most cases are discovered when the cancer is already advanced, missing the opportunity for surgical resection. For patients with unresectable or metastatic disease, however, no systemic treatment has been found to prolong survival in randomized studies and no systemic chemotherapy provides a sustained remission[2]. Although Llovet et al[3] showed that sorafenib, an oral multikinase inhibitor, prolonged the median survival and the time to progression in patients with advanced hepatocellular carcinoma (HCC), most of the recent phase III trials of multi-targeted tyrosine kinase inhibitors (TKIs) have obtained disappointing results[4-6]. Thus, an improved understanding of the mechanisms responsible for liver cancer initiation and progression will facilitate the detection of more reliable tumor markers and the development of new small molecules for targeted therapy of liver cancer[3].
Primary liver cancer (PLC) is a form of liver cancer that begins in the liver. The molecular mechanism associated with initiation and progression of PLC remains obscure. HCC is the most common type of PLC, representing more than 80% of the cases of PLC. Cholangiocellular carcinoma (CCC), the second most common PLC, accounts for approximately 15% of PLC cases worldwide[7]. Combined HCC and cholangiocarcinoma (cHCC-CC) is an uncommon subtype of PLC that displays components of both HCC and CCC and now accounts for 0.4% to 14.2% of all PLC cases, with significant variations from country to country[8-10]. Although all three subtypes of PLC begin in the liver, they show very different biological characteristics that have remained unexplained until now.
Stem cells are undifferentiated biological cells with the capacity to undergo extended self-renewal through mitotic division (to produce more stem cells) and to differentiate into mature cells. There are two broad types of stem cells in mammals: embryonic stem (ES) cells that are found in the inner cell mass of blastocysts, and adult stem cells that are found in various adult tissues. In adult organisms, stem cells are responsible for tissue renewal and repair, replenishing aged or damaged tissues[11]. Fifty-six years ago, Wilson and Leduc suggested that liver stem cells (LSCs) are present in the adult liver[12]. Later, accumulating evidence suggested that LSCs play a pivotal role in the initiation and progression of PLC. This review summarizes and discusses current knowledge regarding the role of LSCs in the hepatocarcinogenesis of PLC.
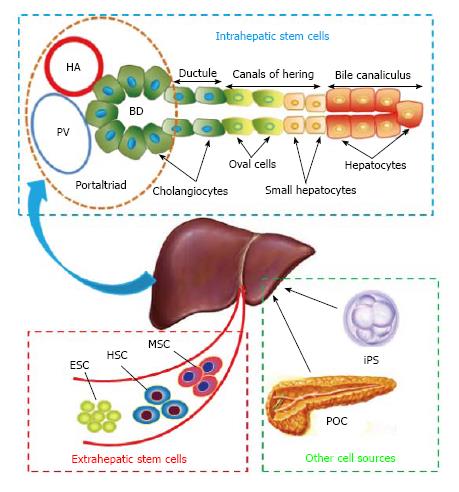
The liver is known to comprise two epithelial cell lineages, hepatocytes and cholangiocytes, which are known to originate from hepatoblasts during embryonic development. LSCs are bi-potential stem cells that are able to differentiate towards the hepatocyte and the biliary lineages. Under normal physiologic conditions, LSCs are quiescent stem cells with a low proliferating rate, representing a reserve compartment[13]. Upon acute injury, the mature hepatocytes and cholangiocytes, which can be considered conceptually as unipotent stem cells, acquire unexpected plasticity by direct dedifferentiation into LSCs, compensating for the loss[14,15]. However, when the mature epithelial cells of the liver are continuously damaged or in cases of severe cell loss, LSCs are activated as a consequence and contribute to liver regeneration[13]. There are two possible sources of liver stem cells: endogenous or intrahepatic LSCs and exogenous or extrahepatic LSCs (Figure 1)[13,16].
Included in the intrahepatic LSC compartment are the adult liver stem/progenitor cells (referred to as oval cells), which are present in great numbers but with a short term proliferation capacity. In 1956, the term oval cell was first assigned by Farber[17], who observed a population of nonparenchymal cells in the portal area of the rat liver after being fed ethionine, and described them as small oval cells with scanty, lightly basophilic cytoplasm and pale blue-staining nuclei. Over the past several decades, oval cells have been shown to be localized within the canals of Hering (the most peripheral branches of the intrahepatic biliary tree)[18,19], interlobular bile ducts[20], or in the periductular/intraportal zone of the liver[21]. These cells are called into action when hepatocytes/cholangiocytes are insufficient or unable to respond. Numerous investigators have concluded that oval cell activation was the first step in liver regeneration in response to certain types of injury[18,22,23].
In addition, it has been reported that mature hepatocytes have the capacity to dedifferentiate into LSCs through a transient oval cell-like stage both in vitro and in vivo, which indicates that mature hepatocytes are direct contributors to the LSC pool[14]. Moreover, some investigators observed that liver regeneration also can proceed from a novel cell type, the small hepatocyte-like progenitor cells (SHPCs), which are phenotypically distinct from fully differentiated hepatocytes/cholangiocytes and oval cells[24,25]. However, some other researchers suggest that SHPCs may represent an intermediate cell type between mature hepatic parenchymal cells and oval cells rather than a distinct stem/progenitor cell population[26,27]. Thus, further studies are required to better understand this phenomenon.
Extrahepatic LSCs comprise ES cells and bone marrow stem cells (BMSCs), which are usually present in small numbers but have a long-term proliferation capacity. These cells have been reported to be capable of self-renewal, giving rise to oval cells and mature, fully functioning liver cells both in vitro and in vivo[22,28,29].
ES cells, continuously growing pluripotent stem cells derived from the inner cell mass of blastocysts, are capable of indefinite continuous culture and can generate all cell types in the body. Utilizing liver-specific marker staining and subsequent functional analysis, Jones et al[30] proved that murine ES cells can differentiate into hepatocytes. Using immunohistochemical assays and reverse transcription-polymerase chain reaction tests for hepatocyte-specific proteins and mRNAs, Kuai et al[31] confirmed that mouse ES cells can differentiate into functioning hepatocytes in the presence of hepatocyte growth factor and nerve growth factor-β. Similarly, increasing evidence shows that human ES cells can be progressively differentiated into definitive endoderm, LSCs, and hepatocytes/cholangiocytes[32,33]. Recently, several newly developed techniques have been reported to facilitate the in vitro maturation of human ES cell-derived hepatocyte-like cells[34-36].
BMSCs mainly contain two types of multipotent stem cells: hematopoietic stem cells (HSCs), which give rise to the three classes of mature blood cells; and mesenchymal stem cells (MSCs), which can differentiate into a variety of cell types such as osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells), and adipocytes (fat cells)[37,38]. Both HSCs[39] and MSCs[40,41] have been shown to differentiate/transdifferentiate into oval cells and mature hepatic parenchymal cells, although these phenomena occur weakly and infrequently[42]. In addition, MSCs can be found in nearly all tissues, and various lines of experimental evidence have shown that non-bone marrow-derived MSCs such as adipose-derived MSCs (AD-MSCs)[43], umbilical cord-derived MSCs[44,45], and peripheral blood-derived MSCs[46] also can give rise to oval cells and mature liver parenchymal cells[47].
Strikingly, LSCs also can be transdifferentiated from non-hepatic sources such as pancreatic cells and induced pluripotent stem cells. Rao and Reddy first reported that massive depletion of the acinar cell pool causes a change in the oval and ductular cells that result in transdifferentiation into hepatocytes. Pancreatic hepatocytes exhibit all the morphological and functional properties of liver parenchymal cells. The cells that generate hepatocytes have been thought to be pancreatic oval cells[48]. The results of the studies by Shen et al[49] and Marek et al[50] demonstrated that a rat pancreatic cell line, AR42J-B13, can be transdifferentiated into functional hepatocytes in vitro, expressing albumin and functional cytochrome P450s, in response to treatment with dexamethasone.
Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from adult cells[51]. Yu et al[52] reported that liver organogenesis transcription factors (Hnf1β and Foxa3) are sufficient to reprogram mouse embryonic fibroblasts into induced hepatic stem cells. These reprogrammed cells can be stably expanded in vitro and possess the potential for bidirectional differentiation into both hepatocyte and biliary lineages. However, pluripotent stem cells readily form a teratoma when injected into immunodeficient mice, which is considered a major obstacle to their clinical application[53]. On this basis, Zhu et al[54] reported the generation of human fibroblast-derived hepatocytes that can proliferate extensively and function similarly to adult hepatocytes by cut short reprogramming to pluripotency to generate an induced multipotent progenitor cell from which hepatocytes can be efficiently differentiated.
Several cell types in the liver, i.e., hepatocytes, cholangiocytes, and LSCs, have the longevity that is needed to be the cellular origin of PLC[19]. Determining the identity of the founder cells for PLC is more problematic and difficult. Therefore, unveiling the mechanisms by which these cells are activated to proliferate and differentiate during liver regeneration is important for the development of new therapies to treat liver diseases.
It is well known that different tumor cells can show distinct morphological and physiological features, such as cellular morphology, gene expression (including the expression of cell surface markers, growth factors and hormonal receptors), metabolism, proliferation, and immunogenic, angiogenic, and metastatic potential. This heterogeneity occurs both within tumors (intra-tumor heterogeneity) and between tumors (inter-tumor heterogeneity)[55]. In 1937, Furth et al[56] first demonstrated that a single malignant white blood cell is capable of producing leukemia. Afterwards, the cancer stem cell (CSC) hypothesis was proposed to explain the tumor heterogeneity phenomenon[57,58]. This model postulates that most cancer cells have only a limited proliferative potential. However, a small subset of tumor cells has the ability to self-renew and is able to generate diverse tumor cells. These cells are defined as cancer stem cells (CSCs) to reflect their stem cell-like properties: indefinite potential for self-renewal and pluripotency. This theory assumes that only CSCs have the ability to initiate new tumors, both at primary and metastatic sites. Thus, this theory indicates that only elimination of all CSCs is fundamental to eradicate tumors[57].
Over the past few years, there is a growing realization that many cancers contain a small population of CSCs. However, the cellular origin of PLC is controversial and whether PLC contains cells that possess properties of CSCs requires further exploration. Numerous observations indicate that any proliferative cell in the liver can be susceptible to neoplastic transformation. In the past, it has been considered that HCC is derived from dedifferentiation of hepatocytes and CCC originates from the dedifferentiation of intrahepatic biliary epithelial cells. In contrast, cHCC-CC is thought to be derived from transformed LSCs[59,60]. More recently, due to the rapid progress of stem cell research, it is widely accepted that cancer is a disease of stem cells, as these are the only cells that persist in the tissue for a sufficient length of time to acquire the requisite number of genetic changes for neoplastic development[61].
Previous studies reported by Steinberg et al[62] have shown that transfection of an active Ha-ras proto-oncogene into oval cells can lead to their malignant transformation. By using hepatitis B virus X (HBx) transgenic mice and a drug-induced liver injury model, Wang et al[63] found that HBx may enable malignant transformation and the acquisition of tumorigenic potential in LSCs, suggesting that liver cancer cells are of LSC origin. The results of Chiba et al[64,65] implied that disruption of the self-renewal of LSCs generates a CSC population and highlight the important role of LSCs in hepatocarcinogenesis. A study by You et al[66] showed that inactivation of the tumor suppressor gene Tg737 results in the malignant transformation of fetal LSCs by promoting cell-cycle progression and differentiation arrest. In a clinical study, Ward et al[67] concluded that PLC in children often arises from the malignant transformation of LSCs at an early stage. In a similar study, Ishikawa et al[68] considered that CCC may derive from the oncogenic transformation of normal LSCs. Collectively, extensive animal modeling and clinical studies have demonstrated that PLC is a disease derived from maturation arrest of LSCs[61].
This theory has been confirmed by the discovery of putative CSCs in the liver. Analysis of the cells in PLC supports the presence of cells with functional properties of somatic CSCs (e.g., immortality, resistance to therapy, and efficient transplantability), which indicates that PLC derives from liver CSCs (LCSCs)[61]. Suetsugu et al[69] isolated CD133+ cells from human HCC cell lines and demonstrated that these cells possess cancer stem/progenitor cell-like properties. Ma et al[70,71] and Yin et al[72] also identified a CSC population in HCC characterized by a CD133 phenotype, suggesting that CD133 might be one of the markers for HCC cancer stem-like cells. Side population (SP) cells are a sub-population of cells that are distinct from the main population and exhibits distinguishing stem cell-like characteristics. In a study of SP cells in different hepatoma cell lines, Chiba et al[73] concluded that SP cells in hepatoma cell lines possess extreme tumorigenic potential, which suggests that a minor population of liver cancer cells harbors LCSC-like properties. A variety of recent studies of hepatoma cell lines and clinical samples suggest that epithelial cell adhesion molecule (EpCAM)[74-76], CD13[77-80], CD24[81-83], CD44[84,85], CD90[86,87], intercellular adhesion molecule-1 (ICAM-1)[88], α2δ1 subunit of voltage-gated calcium channels[89], and OV6[90] may serve as putative LCSC markers. The CSC theory emphasizes the role of LSCs in the hepatocarcinogenesis of PLC. Although the aforementioned proteins and/or molecules have been postulated as putative LCSC markers, no definitive markers have yet been identified directly and widely recognized. Moreover, no LCSCs have been isolated[61]. Therefore, additional studies are needed to obtain a definitive molecular marker of LCSCs and to isolate LCSCs from PLC cell lines, animal models, and clinical samples.
Based on the studies mentioned above, we can scientifically conclude that PLC may derive from neoplastic transformation of LSCs. However, the underlying molecular mechanisms are poorly understood. Studies investigating cancer and CSCs show that several key genes and regulatory signaling pathways are oncogenic, such as Bmi1, Wnt, Notch, Hedgehog, and transforming growth factor-β (TGF-β), and therefore are potentially involved in the malignant transformation of LSCs[91]. Here, current knowledge of these pathways is discussed.
Polycomb group (PcG) proteins are a family of transcriptional repressors that epigenetically remodel chromatin and participate in the establishment and maintenance of cell fates. These proteins play a central role in hematopoiesis, stem cell self-renewal, cellular proliferation and neoplastic development. To date, four distinct PcG-encoded protein complexes have been purified from different species: Polycomb repressive complex 1 (PRC1), PRC2, Pho repressive complex (PhoRC), and Polycomb repressive deubiquitinase (PR-DUB)[92].
Bmi1, encoded by the BMI1 gene (B cell-specific Moloney murine leukemia virus integration site 1), is the most important core subunit of the PRC1 complex, which plays a pivotal role in the self-renewal of both normal stem cells and CSCs. Increasing evidence indicates that Bmi1 protein is elevated in many human malignancies including PLC and has a vital effect on tumorigenesis, cancer progression, and the malignant transformation of stem cells. Therefore, Bmi1 was identified as an important stem cell factor and a proto-oncogene[93].
In PLC, a number of studies have shown that Bmi1 contributes to the maintenance of tumor-initiating SP cells[94] and can cooperate with other oncogenic signals to promote hepatic carcinogenesis in vivo[95]. Our empirical results suggest that Bmi1 is highly expressed in patients with PLC and correlates positively with the proliferation and invasiveness of human hepatoma cells[96,97]. Furthermore, Chiba et al[64,65] observed that forced expression of Bmi1 promotes the self-renewal of LSCs, and the transplantation of such cells that have been clonally expanded from single LSC produces tumors that exhibit the histologic features of cHCC-CC. The above results indicate that Bmi1 plays a crucial role in the oncogenic transformation of LSCs and therefore drives cancer initiation.
The Wnt signaling pathways are ancient and evolutionarily conserved pathways that transmit signals from outside of a cell through cell surface receptors to the inside of the cell and regulate cell-to-cell interactions. Wnt signaling is one of the most well studied molecular pathways during the human life span and involves a large number of proteins that are required for basic developmental processes such as embryonic development, cell fate determination, cell proliferation, cell migration, and cell polarity, in a variety of species and organs[98].
Three major categories of Wnt signaling pathways are recognized: the canonical Wnt pathway in which the cytoplasmic protein β-catenin is a key mediator, the noncanonical planar cell polarity pathway (β-catenin independent), and the noncanonical Wnt/calcium pathway. Activation of the canonical Wnt/β-catenin pathway causes an accumulation of β-catenin in the cytoplasm and its eventual translocation into the nucleus to act as a transcriptional coactivator of transcription factors. Without Wnt signaling, β-catenin would not accumulate in the cytoplasm because it would be degraded by a destruction complex[99]. Ever since its initial discovery, Wnt signaling has had an association with cancer[100]. There is substantial evidence to suggest that dysregulation of Wnt signaling is critical for the initiation and progression of PLC[101,102].
Wnt signaling pathways, particularly the canonical Wnt/β-catenin pathway, are also involved in the self-renewal and maintenance of embryonic and adult stem cells, and as recent findings demonstrated, in CSCs. Functional characterization of LCSCs has revealed that Wnt/β-catenin pathways were critical for inducing the stem cell properties of hepatoma cells and in promoting self-renewal, tumorigenicity, and chemoresistance[103]. In the aforementioned HBx-mediated tumorigenic effects, Wang et al[63] suggest that HBx may enable LSCs with tumorigenic potential via activation of the Wnt/β-catenin signaling pathway. As shown in several in vivo and in vitro experiments, the Wnt/β-catenin signaling pathway contributes to the activation of normal and tumorigenic LSCs[104]. Moreover, Chiba et al[64] demonstrated that Wnt/β-catenin signaling activation strongly enhances the self-renewal capability of LSCs and generates a CSC population as an early event, thereby contributing to the initiation of PLC.
Notch signaling is a complex, highly conserved signal transduction pathway in multicellular organisms. In mammalian cells, the pathway is initiated when Notch ligands (Jagged-1, Jagged-2, and Delta-like 1, 3, and 4) bind to the epidermal growth factor (EGF)-like receptors Notch1-4. Signaling is processed by the enzyme γ-secretase, which results in the subsequent activation of downstream target genes[105,106]. The Notch signaling pathway functions as a major regulator of cell-fate decisions during embryonic development and adult life, and it is crucial for the regulation of self-renewing tissues. Accordingly, dysregulation of Notch signaling underlies a wide range of human disorders from developmental syndromes to adult-onset diseases and cancer[105,107].
Like other solid tumors, misregulation of the Notch pathway in PLC has been described as both oncogenic and tumor suppressive, depending on the cellular context[108]. Qi et al[109] reported that overexpression of Notch1 inhibits the growth of HCC cells by inducing cell cycle arrest and apoptosis. In 2009, the same authors showed that Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in HCC cells[110]. In addition, Viatour et al[111] demonstrated that activation of the Notch pathway serves as a negative feedback mechanism to slow HCC growth during tumor progression. At odds with these findings, however, some recent studies have provided strong evidence in favor of the pro-oncogenic activity of Notch in PLC. For example, Wang et al[112] showed that aberrantly high expression of Notch1 is significantly associated with metastatic disease parameters in HCC patients, and shRNA-mediated silencing of Notch1 reverses HCC tumor metastasis in a mouse model. In human HCC cell lines, Gao et al[113] demonstrated that Notch1 activation contributes to tumor cell growth. In accordance, we have shown that Notch1 is overexpressed in human intrahepatic CCC and is associated with its proliferation, invasiveness and sensitivity to 5-fluorouracil in vitro[114]. Taken together, these data highlight the concept that the Notch pathway plays an essential yet controversial role in PLC, presumably depending on the tumor cell type, local inflammatory microenvironment and the status of other signaling pathways[115,116].
The aforementioned hypothesis was further supported by recent studies examining Notch signaling in the regulation of stem cell and in the development of LSC-driven PLC[117,118]. Utilizing a genetically engineered mouse model and comparative functional genomics, Strazzaboscoet al[115], Villanueva et al[119] and Razumilava et al[120] observed that liver-specific Notch activation in mice recapitulates different stages of human hepatocarcinogenesis and results in HCC, including histological features associated with stem cell expansion. They also confirmed that Notch1 is a bona fide oncogene in experimental liver cancer. Using a transgenic mouse model, Zender et al[116] proved that stable overexpression of Notch 1 in bipotential LSCs causes the formation of intrahepatic CCCs. Dill et al[121] and Cardinale et al[122] also reported that liver-specific expression of the intracellular domain of Notch2 (N2ICD) in mice is sufficient to induce HCC formation, while DENN2ICD (diethylnitrosamine-induced HCCs with constitutive Notch2 signaling) mice develop large hepatic cysts, dysplasia of the biliary epithelium, and eventually CCC. These studies also suggested that the LSC compartment is the most likely candidate for oncogenic events[115,116,119-122].
Nevertheless, these newly published studies raise one question: how can one pathway, Notch signaling, contribute to two different subtypes of PLC: HCC and CCC? Of note, the balance between Notch/Wnt signaling has been proposed to be crucial for the determination of the LSC cell fate in liver disease. Activation of Notch signaling in LSCs leads to biliary specification; in contrast, Wnt signaling activation inhibits default-activated Notch signaling via Numb (a target of canonical Wnt signaling), allowing LSCs to escape the biliary cell fate and acquire a hepatocellular specification[123-125]. Therefore, based on previous studies and to the best of our knowledge[123-126], we propose that the balance between Notch/Wnt signaling pathways determines the oncogenic transformation of LSCs into HCC, CCC, or cHCC-CC phenotype. The predominance of Notch over the Wnt signaling in LSCs leads to the CCC phenotype, and activation of Wnt signaling likely prevents activation of the Notch pathway and thus leads to the HCC phenotype. When the comparison is balanced between the two signaling pathways, the cell has a higher probability of entering the cHCC-CC phenotype. In summary, the role of such a pleiotropic pathway in liver regeneration and liver diseases seems to be highly context dependent. Additional research is required to clearly establish the effects of the Notch signaling pathway during hepatocarcinogenesis.
The Hedgehog signaling pathway is one of the key regulators of embryonic development. Mammals have three Hedgehog homologues, Sonic (SHH), Indian (IHH), and Desert (DHH), of which Sonic is the best studied. Like the Wnt and Notch pathways, the Hedgehog signaling pathway also plays significant roles in stem cell self-renewal[127] and cancer cell proliferation[128,129].
Sicklick et al[130] showed that Hedgehog signaling is conserved in hepatic progenitors from fetal development through adulthood and is essential for the maintenance of LSC survival. In a study reported by Jeng et al[131], the SHH pathway is activated in CD133+ mouse liver cancer cells that harbor stem cell features. In human CCC tissues and cell lines, El Khatib and colleagues[132] demonstrated that inhibition of Hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis in CCC. Chen et al[133] showed that enhanced Hedgehog signaling activity may be responsible for the invasion and chemoresistance of hepatoma subpopulations. In a fibrosis-associated hepatocarcinogenesis model, Philips et al[134] further established that Hedgehog signaling pathway activation promotes hepatocarcinogenesis while inhibiting Hedgehog signaling safely reverses this process even in advanced HCC.
TGF-βsignaling pathway
The TGF-β signaling pathway is involved in various cellular functions in both the developing embryo and the adult organism including cell growth, cell differentiation, apoptosis, and cellular homeostasis. The pathway is activated upon binding of TGF-β to its receptors, TGF-β receptor I (TGFBR1) and TGFBR2, resulting in the translocation of Smad proteins to the nucleus where they act as transcription factors and participate in the regulation of target gene expression[135,136].
The role of TGF-β in tumors is rather complicated. In healthy tissue, it acts as a tumor suppressor controlling the cell cycle and inducing apoptosis. During carcinogenesis, TGF-β acts as a potent inducer of cell motility, invasion and metastasis. In liver cancer, TGF-β has been shown to have both tumor-promoting and tumor-suppressing effects, and its expression is decreased in early but increased in later stages of carcinogenesis. Although the underlying molecular mechanisms remain largely undefined, it had been speculated that the dual role of TGF-β signaling in liver cancer results from its effect on the tumor microenvironment[135,136].
It has long been known that TGF-β signaling is vitally involved in stem cell renewal and lineage specification, including in LSCs[137]. Recently, TGF-β signaling has also been linked to the malignant transformation of LSCs in hepatocarcinogenesis. Nishimura et al[138] reported that TGF-β treatment increases the percentage of SP cells in a hepatoma cell line. Yuan et al[139] reported that HCC cells with aberrantly high expression of TGF-β signaling that are positive for Oct4 (octamer-binding transcription factor 4) are likely cancer progenitor cells with the potential to give rise to HCC. Using several experimental approaches, Wu et al[140] confirmed that long-term treatment of oval cells with TGF-β impaired their LSC potential but granted them tumor-initiating cell (TIC) properties including the expression of TIC markers, increased self-renewal capacity, stronger chemoresistance, and tumorigenicity in nude mice. In opposition to these findings, however, Tang et al[141,142] showed that activation of the interleukin-6 (IL-6) signaling pathway induces neoplastic transformation of LSCs along with inactivation of the TGF-β signaling pathway. Lin et al[143] suggested that disruption of TGF-β signaling is an important molecular event in the transformation of normal LSCs to cancer progenitor/stem cells. These data suggest an important but contradictory role for TGF-β signaling in LSC-driven hepatocarcinogenesis, potentially due to the interaction with other signaling pathways.
Interestingly, CSCs can potentially transdifferentiate into cell types other than the original type from which the tumor arose. Several recent studies have shown that CSCs also can transdifferentiate into functional vascular endothelial cells that line the tumor vasculature, mediating tumor growth and metastasis[144-146]. In 2010, Wang et al[147] and Ricci-Vitiani et al[148] provided strong evidence that a proportion of the endothelial cells that contribute to blood vessels in glioblastoma originate from the tumor itself, having differentiated from tumor stem-like cells. Wang et al[147] also demonstrated that blocking VEGF (vascular endothelial growth factor) or silencing VEGFR2 (VEGF receptor 2) inhibits the maturation of tumor endothelial progenitors into endothelium but not the transdifferentiation of tumor stem-like cells into endothelial progenitors, whereas γ-secretase inhibition or Notch1 silencing blocks the transition into endothelial progenitors. Subsequently, multiple studies have confirmed the presence of tumor-derived endothelial cells in several other malignancies, such as renal[149,150], ovarian[151], and breast cancers[152,153], which suggests that this is a general phenomenon in CSCs.
Similarly, Marfels et al[154] found that chemoresistant hepatoma cells show increased pluripotent capacities and the ability to transdifferentiate into functional endothelial like cells both in vitro and in vivo. These tumor-derived endothelial cells possess increased angiogenesis and drug resistance capability (including chemotherapeutics and angiogenesis inhibitors) compared with normal endothelial cells[155,156]. Taken together, these data may provide new perspectives on the biology of CSCs and reveal new insights into the mechanisms of resistance to anti-angiogenesis therapy.
Our review of the literature shows that identification of the cellular origin and the signaling pathways involved is challenging issues in PLC with pivotal implications in the therapeutic perspectives. Although dedifferentiation of mature hepatocytes/cholangiocytes in hepatocarcinogenesis cannot be excluded, neoplastic transformation of a stem cell subpopulation more easily explains hepatocarcinogenesis. Elimination of LCSCs in PLC could result in the degeneration of downstream cells, making them potential targets for liver cancer therapies. Therefore, LSCs could represent a new target for therapeutic approaches to PLC in the near future. However, though LSCs have a bright future, their efficient therapeutic applications will demand further scientific advances.
P- Reviewer: Bellanti F, Hann HW, Morioka D S- Editor: Song XX L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;Jan 4; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 2. | Germano D, Daniele B. Systemic therapy of hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol. 2014;20:3087-3099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10258] [Article Influence: 603.4] [Reference Citation Analysis (2)] |
| 4. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 484] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 7. | Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Willekens I, Hoorens A, Geers C, Op de Beeck B, Vandenbroucke F, de Mey J. Combined hepatocellular and cholangiocellular carcinoma presenting with radiological characteristics of focal nodular hyperplasia. World J Gastroenterol. 2009;15:3940-3943. [PubMed] |
| 10. | Yu XH, Xu LB, Zeng H, Zhang R, Wang J, Liu C. Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:620-625. [PubMed] |
| 11. | Cheng X, O’Neill HC. Oncogenesis and cancer stem cells: current opinions and future directions. J Cell Mol Med. 2009;13:4377-4384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | WILSON JW, LEDUC EH. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958;76:441-449. [PubMed] |
| 13. | Gaudio E, Carpino G, Cardinale V, Franchitto A, Onori P, Alvaro D. New insights into liver stem cells. Dig Liver Dis. 2009;41:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Wong PP, Sjeklocha L, Steer CJ, Sahin MB. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology. 2012;55:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Rodrigo-Torres D, Affò S, Coll M, Morales-Ibanez O, Millán C, Blaya D, Alvarez-Guaita A, Rentero C, Lozano JJ, Maestro MA. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;Feb 20; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Navarro-Alvarez N, Soto-Gutierrez A, Kobayashi N. Hepatic stem cells and liver development. Methods Mol Biol. 2010;640:181-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3’-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. [PubMed] |
| 18. | Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 485] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell? Hepatology. 1999;30:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] |
| 23. | Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology. 2006;43:S89-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, Alison MR, Forbes SJ. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Dan YY, Yeoh GC. Liver stem cells: a scientific and clinical perspective. J Gastroenterol Hepatol. 2008;23:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Rehman K, Iqbal MJ, Zahra N, Akash MS. Liver stem cells: from preface to advancements. Curr Stem Cell Res Ther. 2014;9:10-21. [PubMed] |
| 30. | Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res. 2002;272:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl. 2003;9:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Magner NL, Jung Y, Wu J, Nolta JA, Zern MA, Zhou P. Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cells. 2013;31:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Subramanian K, Owens DJ, Raju R, Firpo M, O’Brien TD, Verfaillie CM, Hu WS. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014;23:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Farzaneh Z, Pakzad M, Vosough M, Pournasr B, Baharvand H. Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem Cell Biol. 2014;142:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Park Y, Chen Y, Ordovas L, Verfaillie CM. Hepatic differentiation of human embryonic stem cells on microcarriers. J Biotechnol. 2014;174:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Ghodsizadeh A, Hosseinkhani H, Piryaei A, Pournasr B, Najarasl M, Hiraoka Y, Baharvand H. Galactosylated collagen matrix enhanced in vitro maturation of human embryonic stem cell-derived hepatocyte-like cells. Biotechnol Lett. 2014;36:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Bonnet D. Biology of human bone marrow stem cells. Clin Exp Med. 2003;3:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Popp FC, Piso P, Schlitt HJ, Dahlke MH. Therapeutic potential of bone marrow stem cells for liver diseases. Curr Stem Cell Res Ther. 2006;1:411-418. [PubMed] |
| 39. | Khurana S, Mukhopadhyay A. In vitro transdifferentiation of adult hematopoietic stem cells: an alternative source of engraftable hepatocytes. J Hepatol. 2008;49:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Ishii K, Yoshida Y, Akechi Y, Sakabe T, Nishio R, Ikeda R, Terabayashi K, Matsumi Y, Gonda K, Okamoto H. Hepatic differentiation of human bone marrow-derived mesenchymal stem cells by tetracycline-regulated hepatocyte nuclear factor 3beta. Hepatology. 2008;48:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11:360-371. [PubMed] |
| 42. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [PubMed] |
| 43. | Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 44. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 659] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 45. | An SY, Han J, Lim HJ, Park SY, Kim JH, Do BR, Kim JH. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell. 2014;46:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Cesselli D, Beltrami AP, Rigo S, Bergamin N, D’Aurizio F, Verardo R, Piazza S, Klaric E, Fanin R, Toffoletto B. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Laurson J, Selden C, Hodgson HJ. Hepatocyte progenitors in man and in rodents--multiple pathways, multiple candidates. Int J Exp Pathol. 2005;86:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6:151-156. [PubMed] |
| 49. | Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Marek CJ, Cameron GA, Elrick LJ, Hawksworth GM, Wright MC. Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor cell line in vitro. Biochem J. 2003;370:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 52. | Yu B, He ZY, You P, Han QW, Xiang D, Chen F, Wang MJ, Liu CC, Lin XW, Borjigin U. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell. 2013;13:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 53. | Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 54. | Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 55. | Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 863] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 56. | Furth J, Kahn M. The transmission of leukemia of mice with a single cell. Am J Cancer. 1937;31:276-282. |
| 57. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6911] [Article Influence: 288.0] [Reference Citation Analysis (0)] |
| 58. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1450] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 59. | Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298-304. [PubMed] |
| 60. | Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Steinberg P, Frank H, Odenthal M, Dienes HP, Seidel A. Role of the Ha-ras gene in the malignant transformation of rat liver oval cells. Int J Cancer. 1997;71:680-685. [PubMed] |
| 63. | Wang C, Yang W, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology. 2012;55:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, Nakanuma Y. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007;133:937-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 65. | Chiba T, Seki A, Aoki R, Ichikawa H, Negishi M, Miyagi S, Oguro H, Saraya A, Kamiya A, Nakauchi H. Bmi1 promotes hepatic stem cell expansion and tumorigenicity in both Ink4a/Arf-dependent and -independent manners in mice. Hepatology. 2010;52:1111-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | You N, Liu W, Zhong X, Ji R, Zhang M, You H, Dou K, Tao K. Tg737 inhibition results in malignant transformation in fetal liver stem/progenitor cells by promoting cell-cycle progression and differentiation arrest. Mol Carcinog. 2012;51:659-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Ward SC, Thung SN, Lim KH, Tran TT, Hong TK, Hoang PL, Jang JJ, Park YN, Abe K. Hepatic progenitor cells in liver cancers from Asian children. Liver Int. 2010;30:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Ishikawa K, Sasaki A, Haraguchi N, Yoshikawa Y, Mori M. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 70. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 71. | Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 605] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 72. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 73. | Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 74. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 957] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 75. | Kimura O, Takahashi T, Ishii N, Inoue Y, Ueno Y, Kogure T, Fukushima K, Shiina M, Yamagiwa Y, Kondo Y. Characterization of the epithelial cell adhesion molecule (EpCAM)+ cell population in hepatocellular carcinoma cell lines. Cancer Sci. 2010;101:2145-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Zeng Z, Ren J, O’Neil M, Zhao J, Bridges B, Cox J, Abdulkarim B, Schmitt TM, Kumer SC, Weinman SA. Impact of stem cell marker expression on recurrence of TACE-treated hepatocellular carcinoma post liver transplantation. BMC Cancer. 2012;12:584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326-3339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 78. | Christ B, Stock P, Dollinger MM. CD13: Waving the flag for a novel cancer stem cell target. Hepatology. 2011;53:1388-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Kim HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012;19 Suppl 3:S539-S548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Nagano H, Ishii H, Marubashi S, Haraguchi N, Eguchi H, Doki Y, Mori M. Novel therapeutic target for cancer stem cells in hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 82. | Liu AY, Cai Y, Mao Y, Lin Y, Zheng H, Wu T, Huang Y, Fang X, Lin S, Feng Q. Twist2 promotes self-renewal of liver cancer stem-like cells by regulating CD24. Carcinogenesis. 2014;35:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Yang Y, Hou J, Lin Z, Zhuo H, Chen D, Zhang X, Chen Y, Sun B. Attenuated Listeria monocytogenes as a cancer vaccine vector for the delivery of CD24, a biomarker for hepatic cancer stem cells. Cell Mol Immunol. 2014;11:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 85. | Iwahashi S, Utsunomiya T, Shimada M, Saito Y, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Bando Y. High expression of cancer stem cell markers in cholangiolocellular carcinoma. Surg Today. 2013;43:654-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 926] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 87. | Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 88. | Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, Wang J, Zhang D, Cheng S, Liu S. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology. 2013;144:1031-1041.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 89. | Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, Chen Y, Cheng H, Lu F, Fang W. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel α2δ1 subunit. Cancer Cell. 2013;23:541-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 90. | Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, Yan HX, Fu J, Chen Y, Zhang HL. OV6⁺ tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Kitisin K, Pishvaian MJ, Johnson LB, Mishra L. Liver stem cells and molecular signaling pathways in hepatocellular carcinoma. Gastrointest Cancer Res. 2007;1:S13-S21. [PubMed] |
| 92. | Lanzuolo C, Lo Sardo F, Diamantini A, Orlando V. PcG complexes set the stage for epigenetic inheritance of gene silencing in early S phase before replication. PLoS Genet. 2011;7:e1002370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Jiang L, Li J, Song L. Bmi-1, stem cells and cancer. Acta Biochim Biophys Sin (Shanghai). 2009;41:527-534. [PubMed] |
| 94. | Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, Yonemitsu Y, Yokosuka O, Taniguchi H, Nakauchi H. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742-7749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 95. | Xu CR, Lee S, Ho C, Bommi P, Huang SA, Cheung ST, Dimri GP, Chen X. Bmi1 functions as an oncogene independent of Ink4A/Arf repression in hepatic carcinogenesis. Mol Cancer Res. 2009;7:1937-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Zhang R, Xu LB, Yue XJ, Yu XH, Wang J, Liu C. Bmi1 gene silencing inhibits the proliferation and invasiveness of human hepatocellular carcinoma cells and increases their sensitivity to 5-fluorouracil. Oncol Rep. 2013;29:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Zhang R, Xu LB, Zeng H, Yu XH, Wang J, Liu C. Elevated expression of Bmi1 in hepatocellular carcinoma with bile duct tumor thrombi. Hepatogastroenterology. 2013;60:2042-2047. [PubMed] |
| 98. | Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68-75. [PubMed] |
| 99. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2765] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 100. | Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670-2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 101. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 102. | Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 103. | Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 104. | Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 105. | Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 106. | Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2980] [Cited by in RCA: 2802] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 107. | Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 108. | Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 639] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 109. | Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323-8329. [PubMed] |
| 110. | Wang C, Qi R, Li N, Wang Z, An H, Zhang Q, Yu Y, Cao X. Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. J Biol Chem. 2009;284:16183-16190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 111. | Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963-1976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 112. | Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu X, Sun J, Yang S, Poon RT, Fan ST. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int J Cancer. 2012;131:E163-E172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 113. | Gao J, Dong Y, Zhang B, Xiong Y, Xu W, Cheng Y, Dai M, Yu Z, Xu H, Zheng G. Notch1 activation contributes to tumor cell growth and proliferation in human hepatocellular carcinoma HepG2 and SMMC7721 cells. Int J Oncol. 2012;41:1773-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Wu WR, Zhang R, Shi XD, Zhu MS, Xu LB, Zeng H, Liu C. Notch1 is overexpressed in human intrahepatic cholangiocarcinoma and is associated with its proliferation, invasiveness and sensitivity to 5-fluorouracil in vitro. Oncol Rep. 2014;31:2515-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: guilty in association! Gastroenterology. 2012;143:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 117. | Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 118. | Grudzien P, Lo S, Albain KS, Robinson P, Rajan P, Strack PR, Golde TE, Miele L, Foreman KE. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer Res. 2010;30:3853-3867. [PubMed] |
| 119. | Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S, Stanger BZ. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660-1669.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 120. | Razumilava N, Gores GJ. Notch-driven carcinogenesis: the merging of hepatocellular cancer and cholangiocarcinoma into a common molecular liver cancer subtype. J Hepatol. 2013;58:1244-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, Tchorz JS. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2013;57:1607-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 122. | Cardinale V, Carpino G, Reid LM, Gaudio E, Alvaro D. Notch2 signaling and undifferentiated liver cancers: evidence of hepatic stem/progenitor cell origin. Hepatology. 2013;58:1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 124. | Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 606] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 125. | Strazzabosco M, Fabris L. The balance between Notch/Wnt signaling regulates progenitor cells’ commitment during liver repair: mystery solved? J Hepatol. 2013;58:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Shu J, Wu C, Wu Y, Li Z, Shao S, Zhao W, Tang X, Yang H, Shen L, Zuo X. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013;153:963-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 127. | Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int J Mol Med. 2006;18:1019-1023. [PubMed] |
| 128. | Marquardt JU, Factor VM, Thorgeirsson SS. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol. 2010;53:568-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 129. | McMillan R, Matsui W. Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res. 2012;18:4883-4888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 130. | Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859-G870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 131. | Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau HI, Chang FY, Tsai HH. Activation of the sonic hedgehog signaling pathway occurs in the CD133 positive cells of mouse liver cancer Hepa 1-6 cells. Onco Targets Ther. 2013;6:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 132. | El Khatib M, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, Wilkens L, Plentz RR. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 133. | Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55:838-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 134. | Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn WK. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 135. | Bogaerts E, Heindryckx F, Vandewynckel YP, Van Grunsven LA, Van Vlierberghe H. The roles of transforming growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours (Review). Int J Oncol. 2014;44:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 136. | Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci. 2011;7:517-535. [PubMed] |
| 137. | Majumdar A, Curley SA, Wu X, Brown P, Hwang JP, Shetty K, Yao ZX, He AR, Li S, Katz L. Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 138. | Nishimura T, Azuma T, Yokoyama A, Ochiai H, Saito H, Hibi T. New mechanism of transforming growth factor-beta signaling in hepatoma: Dramatic up-regulation of tumor initiating cells and epidermal growth factor receptor expression. Hepatol Res. 2009;39:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 139. | Yuan F, Zhou W, Zou C, Zhang Z, Hu H, Dai Z, Zhang Y. Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem. 2010;343:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 140. | Wu K, Ding J, Chen C, Sun W, Ning BF, Wen W, Huang L, Han T, Yang W, Wang C. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology. 2012;56:2255-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 141. | Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 2008;105:2445-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 142. | Shackel NA, McCaughan GW, Warner FJ. Hepatocellular carcinoma development requires hepatic stem cells with altered transforming growth factor and interleukin-6 signaling. Hepatology. 2008;47:2134-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 143. | Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 144. | Bautch VL. Cancer: Tumour stem cells switch sides. Nature. 2010;468:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Hutchinson E. Stem cells: Tumour stem cells generate vasculature. Nat Rev Cancer. 2011;11:4. [PubMed] |
| 146. | Hutchinson E. Cancer: tumour stem cells generate vasculature. Nat Rev Neurosci. 2011;12:3. [PubMed] |
| 147. | Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 910] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 148. | Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1019] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 149. | Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 150. | Bussolati B, Dekel B, Azzarone B, Camussi G. Human renal cancer stem cells. Cancer Lett. 2013;338:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 151. | Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 152. | Bussolati B, Grange C, Sapino A, Camussi G. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med. 2009;13:309-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 153. | Tang W, Yu F, Yao H, Cui X, Jiao Y, Lin L, Chen J, Yin D, Song E, Liu Q. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene. 2014;33:2629-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 154. | Marfels C, Hoehn M, Wagner E, Günther M. Characterization of in vivo chemoresistant human hepatocellular carcinoma cells with transendothelial differentiation capacities. BMC Cancer. 2013;13:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Dudley AC, Klagsbrun M. Tumor endothelial cells join the resistance. Clin Cancer Res. 2009;15:4787-4789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 156. | Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15:4838-4846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |