Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.124
Revised: June 5, 2013
Accepted: July 4, 2013
Published online: October 26, 2013
Processing time: 231 Days and 3.1 Hours
Stem cells, including embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells and amniotic fluid stem cells have the potential to be expanded and differentiated into various cell types in the body. Efficient differentiation of stem cells with the desired tissue-specific function is critical for stem cell-based cell therapy, tissue engineering, drug discovery and disease modeling. Bioreactors provide a great platform to regulate the stem cell microenvironment, known as “niches”, to impact stem cell fate decision. The niche factors include the regulatory factors such as oxygen, extracellular matrix (synthetic and decellularized), paracrine/autocrine signaling and physical forces (i.e., mechanical force, electrical force and flow shear). The use of novel bioreactors with precise control and recapitulation of niche factors through modulating reactor operation parameters can enable efficient stem cell expansion and differentiation. Recently, the development of microfluidic devices and microbioreactors also provides powerful tools to manipulate the stem cell microenvironment by adjusting flow rate and cytokine gradients. In general, bioreactor engineering can be used to better modulate stem cell niches critical for stem cell expansion, differentiation and applications as novel cell-based biomedicines. This paper reviews important factors that can be more precisely controlled in bioreactors and their effects on stem cell engineering.
Core tip: Stem cells are promising cell sources for cell therapy, tissue engineering, drug discovery and disease modeling due to their ability of self-renewal and immense capability of lineage-specific differentiation. Bioreactor systems with engineered stem cell microenvironments, called “niches”, play an important role in deriving functional cell populations from stem cells. Some important factors and their effects on stem cell engineering in bioreactors are reviewed in this paper. The understanding of bioreactor regulation of stem cell niches is of great interest in developing novel biomedicines.
- Citation: Liu M, Liu N, Zang R, Li Y, Yang ST. Engineering stem cell niches in bioreactors. World J Stem Cells 2013; 5(4): 124-135
- URL: https://www.wjgnet.com/1948-0210/full/v5/i4/124.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i4.124
Stem cells are promising cell sources for cell therapy, tissue engineering, drug discovery and disease modeling due to their ability of self-renewal and immense capability of lineage-specific differentiation[1-3]. Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the potential to be expanded indefinitely and differentiated into all cell types in the body[4,5]. In addition, adult stem cells, such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and neural stem cells (NSCs), also have limited lifespan and the restricted lineage differentiation, which may be extended by fetal stem cells such as amniotic fluid stem cells (AFSC)[6]. Although stem cells open a new era for tissue regeneration, tissue modeling, and drug discovery, efficient differentiation of stem cells with the desired function is still the major challenge in stem cell engineering to fulfill their potential in biomedical applications.
Stem cells have been differentiated into a variety of cell types; however, the function of stem cell-derived tissue specific cells have not been well understood[2]. For example, evidences indicated that the human PSC-derived cardiomyocytes more resemble embryonic cardiomyocytes rather than mature cardiomyocytes[7]. Human ESC-derived erythroid progenitors expressed mostly fetal haemoglobin but have limited ability to activate mature haemoglobin[8]. In the example of MSCs, trophic factor secretion by the cells, the most important characteristics of MSC therapy, is highly dependent on the culture systems[9]. In addition, the efficiency of the lineage specific differentiations remain as the major challenge in stem cell bioprocessing because most current protocols resulted in low purity of the target lineages[10]. To date, all in vitro studies have indicated that the recapitulation of stem cell microenvironment is critical to regulate stem cell fate decision and the functionality of the differentiated tissue-specific cells.
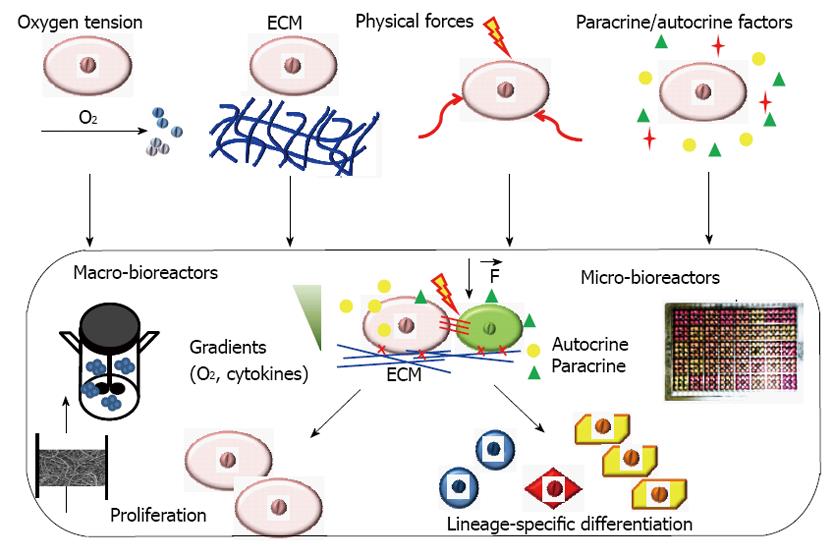
Bioreactor systems with engineered stem cell microenvironments, called “niches”, play an important role in deriving functional cell populations from stem cells (Figure 1)[11]. These bioreactor systems with tightly controlled and highly reproducible environmental factors can be used to regulate stem cell differentiation and tissue formation[12,13]. The critical culture parameters that have been widely studied in a bioreactor system for engineering stem cell niches include oxygen tension, 3-D scaffolds (synthetic or decellularized), physical forces such as hydrodynamic forces, mechanical strain, flow shear and electrical stimulation (Figure 1). Recently, microfluidic devices and microbioreactors have been designed and used for high throughput regulation of microenvironmental factors to better understand stem cell niches and for drug screening[14,15]. Paracrine/autocrine factors can also be revealed using microfluidic devices and microbioreactors to better control stem cell differentiation. While the design of novel bioreactor affects all types of stem cells, the key factors and the optimal values differ for different process objectives and different tissue-specific cell types. Some important factors and their effects on stem cell engineering in bioreactors are summarized in Table 1 and reviewed in this paper. The understanding of bioreactor regulation of stem cell niches is of great interests in developing novel biomedicines.
| Factors | Effects on stem cell niches | Ref. |
| Oxygen tension | Hypoxia promotes the proliferation of NSC, HSC, and MSC, inhibits spontaneous differentiation of humn PSC, and promotes iPSC reprogramming and growth | [21-24,33] |
| Hypoxia promotes lineage specific differentiation from NSC, MSC and human PSC | [27,29,32] | |
| Scaffold/ substrate cues | Higher cell proliferation rates under higher mechanical stresses; Substrate stiffness directs stem cell differentiation; Control of cell shape via substrate size directs human MSC differentiation | [38,41,44] |
| 3-D fibrous matrix promoted neural differentiation of ESC, silk scaffold promoted bone tissue formation from MSCs, honeycombs for cardiac tissue formation | [42,43,47,50] | |
| Enhanced MSC proliferation in collagen scaffolds in a radial-flow bioreactor | [51] | |
| Decellularized ECMs | Decellularized bone matrix in a perfusion bioreactor promoted human PSC differentiation into bone tissue; Decellularized cardiac matrix promoted human PSC differentiation into cardiac lineage. | [55,58,59] |
| Human PSC-derived ECM supported PSC proliferation | [56] | |
| Mechanical forces | Mechanical stimulation significantly improved the function of engineered ligaments | [64] |
| Mechanical compression enhanced MSC differentiation | [66] | |
| Dynamic compression with deformational loading and hydrostatic pressure improved cartilage tissue engineering; | [61] | |
| Hydrodynamic shear, cyclic flexure, and cyclic stretch accelerated heart valve tissue formation | [68] | |
| Pulsatile flow and circumferential stretch improved the engineered blood vessels | [119] | |
| Electrical stimulation | Induced cellular tension and promoted cellular and functional properties of engineered cardiac tissue | [71,72] |
| Electrical stimulation enhanced neural differentiation | [69,70] | |
| Flow shear force | Lower flow (shear) rates enhanced MSC proliferation and higher flow (shear) rate increased osteogenic differentiation; Parallel flow and transverse flow affected osteogenic differentiation of human MSCs | [80,81] |
| Perfusion improved tissue architecture of engineered cardiac muscle and increased matrix synthesis in engineered chondrocytes | [73,74] | |
| Agitation preserved Oct-4 expressing cells during PSC differentiation | [82,83] |
Oxygen is an important microenvironmental factor for stem cell differentiation via its role in regulating cell metabolism[16,17]. Traditionally, in vitro cell cultures are performed in incubators supplied with air and 5% CO2, in which the oxygen concentration is about 20%. However, the mean oxygen concentration in vivo is around 3% and varies with different tissue regions (i.e., 12% for arterial blood while 1.5% for the brain)[18,19]. In embryos where stem cells are abundant relative to adult tissues, the oxygen tension is at a lower level than those in mature somatic tissues. Bioreactors allow in vitro cultures at various scales to more exquisitely replicate oxygen levels in normal physiological conditions that cannot be easily done with traditional static cultures.
Reduced oxygen tension can maintain stem cells at primitive stage as well as regulating lineage-specific differentiation. Extensive studies have shown that hypoxia (0.9%-1% oxygen) maintains the primitive property of HSCs and their self-renewal ability[20,21]. Human MSCs grown in hypoxia (2% O2) had higher colony-forming unit activity and expressed higher levels of stem cell genes than those cultured at 20% O2[22]. For human ESCs, 5% O2 reduced the frequency of spontaneous differentiation through the up-regulation of hypoxia inducing factors (HIF)[23]. Hypoxia (5%-6% O2) also enhanced the efficiency of reprogramming to generate iPSCs, although the optimal O2 concentration and duration of hypoxic culture in the reprogramming process still require further investigations[24]. In the example of NSC differentiation, mild hypoxia (2.5%-5% O2) enhanced human NSC differentiation into neuronal and oligodendrocyte cells, while under normoxia (20% O2) human NSC showed the preferential commitment to astrocyte lineage[25]. The beneficial effects of hypoxia may be due to the active mitochondria activity and better cell survival during differentiation. Similar to NSCs, oligodendrocyte progenitor cells were sensitive to oxygen[26], because low oxygen was found to suppress bone morphogenetic proteins (BMP) signaling[27]. For human ESC-derived neurospheres, hypoxic preconditioning promoted neuronal differentiation and up-regulated HIF-1α, HIF-2α and the genes of erythropoietin, vascular endothelial growth factor (VEGF), and Bcl-2 family members[28]. All these studies demonstrated the important roles of oxygen in stem cell proliferation and differentiation.
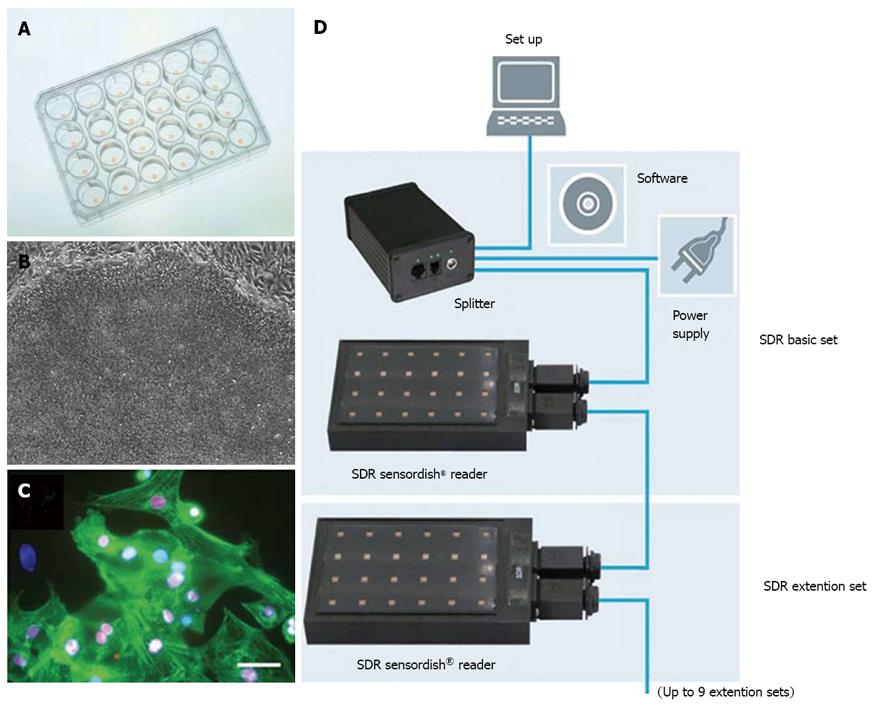
Bioreactors allow the precise control of oxygen tension through the sensors and the feedback system. Lovett et al[29]. demonstrated enhanced chondrogenic differentiation of human MSCs at 5% O2 and adipogenic differentiation at 20% O2 in a modular bioreactor with well characterized oxygen gradients[29]. Neural progenitors were also prepared in bioreactors with controlled oxygen for clinical trials[30]. Agarose-encapsulated murine ESC aggregates cultured in a perfused stirred tank bioreactor were directed to cardiomyocyte differentiation at 4% O2[31]. Similarly, a study with size-controlled embryoid bodies (EBs) derived from human ESCs showed that 4% O2 resulted in the up-regulation of cardiac genes compared to normoxia in a spinner bioreactor[32]. Continuous production of iPSCs was also assessed in an acoustic perfused bioreactor at different oxygen tensions, which showed that a significant increase in iPSC growth at the low oxygen tension of 5% due to the preferential glycolytic metabolism over mitochondria oxidation[33]. It has to be pointed out that the oxygen gradient could exist around the local cell organizations. Even that the medium was exposed to normoxia, different oxygen transfer and consumption rates in the culture system might expose the stem cells to the local hypoxia environment. Therefore, using a biosensor to measure the in situ oxygen level, as shown in Figure 2, would be useful to fully understand the effect of oxygen in stem cell fate decision.
Biophysical cues provided by a tissue scaffold as synthetic extracellular matrix (ECM) play an important role in engineering stem cells in the bioreactor microenvironment. A wide variety of natural and synthetic materials, including Matrigel, collagen, alginate, hyaluronic acid, poly (ethylene terephthalate) (PET) matrix, poly(ethylene glycol) hydrogels and self-assembling peptide gels, have been utilized for culturing and engineering stem cells[12,34-37]. The material properties including surface topology and stiffness or tensile strength have profound effects on cell adhesion, proliferation, and the differentiated cellular function[38]. For example, substrates with high stiffness directed stem cells into osteoblasts, medium stiffness promoted myogenesis and low stiffness promoted neurogenesis[39]. The scaffolds also provide 3-D biophysical cues that regulate cell organization and morphogenesis[40,41]. Neural differentiation from ESCs was promoted in 3-D fibrous matrices compared to 2-D culture[42,43]. It was believed that the biomechanical forces from the scaffold caused cytoskeletal tension that affected cell shape and signal transduction, which in turn regulated stem cell lineage commitment[44]. The structures of the 3-D scaffolds, including pore size and porosity, affect mass transport and must be compatible with perfusion bioreactors for the development of thick and compact tissue grafts.
The optimal scaffold design for perfused bioreactor cultures should be guided by the structural and mechanical properties of native tissues. Hydrogels with tunable molecular, mechanical and degradation properties have been applied in human ESC cultures[45] and the engineering of soft tissues such as cartilage[35]. For engineering bone and cardiac muscle, a scaffold must provide good balance in pore curvature for cell attachment, pore size for cell migration, and hierarchical structures including orientation, anisotropy and channels for vascular conduits[46]. Mechanically strong, highly porous and mineralized silk scaffold can promote the bone tissue formation from MSCs[47]. The pore size of the scaffold has been shown to affect the type of the engineered bone tissue with the formation of flat bone in small pore, trabecular bone in large pore and transient bone in a gradient of pore structures[48]. In contrast, soft, highly porous and channeled elastomer scaffold has been preferentially used for the engineering of vascularized cardiac tissue[49]. For example, an accordion-like elastomer scaffold with structural and mechanical anisotropy has been designed and used for cardiac tissue engineering[50], which induced the alignment and coupling of neonatal heart myocytes and generated direction-dependent contractions close to native heart tissues. Bioreactors can also enhance the mass transfer in the scaffolds through perfusion and other flow pattern to promote the stem cell proliferation or differentiation. For example, a radial-flow bioreactor was used to support 3-D expansion of human MSCs on a large collagen scaffold, which enhanced colony-forming unit activity of MSCs[51]. ESCs differentiated on 3-D porous tantalum-based scaffolds inside spinner bioreactors showed significant enhancement in hematopoiesis compared to cells cultured under static conditions[52]. Thus, the use of biomimetic scaffolds in the bioreactors is critical to recapitulate in vivo-like stem cell microenvironment to promote the desired lineage and the tissue-specific function.
Compared to synthetic ECMs, decellularized ECMs contain a mixture of adhesion ECM proteins, such as fibronectin and collagen, and the sequestered growth factors, such as fibroblast growth factor (FGF)-2, which can modulate the activities of receptor-ligand binding[53,54]. These interactions constitute a dynamic and reciprocal relationship among the cells, growth factors and ECMs which cannot be readily reproduced by synthetic ECMs[55,56]. For example, human PSC-derived ECM supported PSC propagation with the deposited growth factors acting in transforming growth factor (TGF)-β/Nodal pathways[56]. Decellularized ECMs from the heart was repopulated in a perfusion bioreactor and the cells displayed pumping function[57]. Decellularized cardiac matrices also preserved their signaling capacity and induced human ESC differentiation toward the cardiac lineage[58]. Decelluarized bone matrix in a perfusion bioreactor supported human ESC differentiation into stable bone-like tissue[59]. Studies using the decellularized scaffolds supplied by nature will lead to the design of synthetic ECMs which mimic the native ECMs to be engineered in bioreactors.
Mechanical forces affect cellular differentiation pathways and are important to the control of tissue morphogenesis from stem cells[60]. Mechanical stimulations affect cells in various ways: compression and shear can cause cellular deformation, pressure and shear force result in mechanical stress, and tension causes cell elongation and the deformation of cell nucleus[46]. Therefore, mechanical signals play significant roles in the development and function of various tissues, including bone, cartilage, skeletal muscle and cardiovascular tissues. It is well-recognized that the mechanical forces and the magnitudes that govern tissue development and remodeling in vivo would also enhance tissue development and function in vitro.
Specialized bioreactors have been developed to study the effects of mechanical stimulation on stem cell differentiation. Deformational loading and hydrostatic pressure are the primary factors of cell microenvironment for articular cartilage, and have been employed in cartilage tissue engineering[12,61]. Mechanical stimulation such as longitudinal strain accelerated the bone morphogenesis from MSCs[62]. Zhang et al[63] developed a biaxial rotating bioreactor (BXR) to generate tissue engineered bone grafts from human fetal MSC and showed that higher cellularity, confluence, and more robust osteogenic differentiation were achieved in the BXR as compared to spinner flasks, perfusion and rotating wall bioreactors. Human ligaments were engineered from human MSCs in a bioreactor by combining dynamic tension and torsion mimicking forces in vivo, which significantly improved the ligament function[64]. Using MSCs and fibroblasts seeded in tendon constructs in a bioreactor with cyclic mechanical load, tendons with comparable ultimate tensile stress and elastic modulus to fresh tendons were obtained[65]. Different cell types, such as MSCs and human EB-derived mesenchymal progenitors, may respond differently to the same mechanical force in a mechanical compression bioreactor[66]. MSCs in hydrogels responded to mechanical stimulation in the absence of TGF-β1 by upregulating chondrogenic genes while human EB-derived cells required the presence of TGF-β1. Biomimetic perfusion systems have also been developed for engineering small-diameter blood vessel grafts. For example, Niklason et al[67]. obtained the engineered vessels from smooth muscle cells cultured in fibrous scaffolds under pulsatile flow in a perfusion bioreactor[67]. Cyclic flexure and laminar flow were also applied in the bioreactor, which synergistically accelerated the tissue formation of heart valve with significantly increased collagen contents and tissue stiffness[68]. Thus, novel design of bioreactors can be and has been successfully used to recreate physiological loading environment and control stem cell differentiation.
Besides mechanical forces, electrical stimulation is also an important microenvironmental factor for mediating stem cell differentiation. Electrical stimulation regulates the action potentials of excitable cells and is especially useful for differentiation into neural and cardiac lineages. Mild electrical stimulation strongly influenced ESCs to assume a neuronal fate by the induction of calcium ion influx[69]. The application of a lateral current through the single-walled carbon nanotube/laminin composites stimulated the generation of neuronal action potentials during NSC differentiation[70]. The application of direct current electrical fields to human ESC-derived EBs promoted cardiomyocyte differentiation by regulating the generation of reactive oxygen species[71]. Cardiac-like electrical stimulation was also applied to generate excitation-contraction to induce cellular tension on cells cultured on scaffolds[72]. Similar to the application of mechanical stretch, the electrically stimulated cells underwent electromechanical coupling and conducted electrical pacing signals over macroscopic distances with synchronously beating at the frequency of stimulation.
Flow shear force generated by perfusion or agitation can improve mass transfer in the scaffolds and thus provides better control on nutrient delivery. For example, perfusion improved tissue architecture of engineered cardiac muscle[73], increased cellularity and matrix synthesis in chondrocyte cultures[74], and enhanced chondrogenesis of human ESC-derived MSCs[75]. Perfusion also improved cellularity and bone matrix components of engineered constructs using human adipose-derived MSCs[76]. Grayson et al[77] engineered anatomically shaped human bone grafts using human MSCs in a bioreactor with continuous perfusion and found that the bone matrix architecture and density correlated with the interstitial flow pattern and intensity. In addition, perfusion was found to give better pO2 control and more uniform cell distribution within 3-D scaffolds[78], and facilitated long-term ESC culture to reach a high cell density[79]. MSC proliferation was enhanced in highly porous matrices at different flow rates (0.1-1.5 mL/min), and the higher flow rate of 1.5 mL/min upregulated osteogenic differentiation[80]. Different perfusion flow configurations like parallel flow and transverse flow were found to affect osteogenic differentiation of human MSCs due to the regulation of ECM and FGF-2 secretion by different flow patterns[81]. Flow shear induced by agitation preserved Oct-4 expressing cells during PSC differentiation[82,83]. It was suggested that shear stress modulated gene expression through mechano-transduction to induce autocrine or paracrine signaling to suppress spontaneous differentiation[84,85]. These findings underscore the importance of reciprocal interactions of flow shear force and cell signaling in 3-D cellular organizations.
Microfabrication techniques, especially soft lithography, can be used to control features at a micrometer scale between 1 and 1000 μm in microdevices[86], which provide controllable microenvironments for engineering stem cell differentiation as well as for cytotoxicity screening in high-throughput assays (Table 2)[14,87]. Via laminar flow within microchannels, microfluidic systems can be used to generate biochemical gradients and deliver cytokines at well-defined concentrations[88,89]. Microfluidic devices also can be used to regulate molecular and biomechanical signals, and to study their effects on cell morphogenesis, migration, proliferation and cell-cell interaction. Microbioreactors, or microfluidic-based cell culture arrays, allow the spatial and temporal control of stem cell microenvironment compared to large scale bioreactors. With microfluidic devices and microbioreactors, stem cell differentiation can be regulated by controlling substrate size and stiffness, cytokine gradients, flow rate and autocrine/paracrine signaling[90].
| Microfluidic devices and microbioreactors | Stem cell type | Applications | Ref. |
| Gradient-generating microfluidic device | NSC | Proliferation and astrocyte differentiation | [91] |
| MEMS automated microfluidic device | AFSC | Adipogenic and osteogenic differentiation | [92] |
| 3-D hydrogel incorporated microfluidics | NSC | 3-D differentiation into neuronal and oligodendrocyte differentiation | [93] |
| Micro-grooved PDMS sheets with cyclic strain | MSC | MSC proliferation and differentiation | [94] |
| Microfluidic device with logarithmical flow rate | Mouse ESC | ESC adhesion and proliferation | [95] |
| A microfluidic chip which creates arbitrary culture media formulations | MSC | Proliferation, osteogenic | [96] |
| differentiation and motility | |||
| A microscaffold cell chip with precisely controlled microenvironment | Retinal stem cells | Decrease apoptosis during the retinal differentiation | [98,99] |
| Microbioreactor array for 2-D and 3-D hydrogel cultures | Human ESC | Adjust flow rate and evaluate vascular differentiation | [100] |
| Microbioreactor arrays for drug screening | ESC, MSC | Incorporate 3D culture, biomaterials, etc. to screen drugs in a high-throughput manner | [103] |
| Compartmentalizing microfluidic devices | Cancer stem cells | Understanding of cell migration and cancer invasion | [104] |
| Microbioreactor array with 3-D fibrous matrix | Mouse ESC | High-through cell-based assay for drug screening | [14,87,102,106] |
| Microbioreactor array with full factorial design of growth factor combinations | Human ESC | Screening exogenous and paracrine factors in human ESC differentiation into mesoderm cells | [15] |
| Microfluidics with patterning and temporal analysis | PSC | Reveal paracrine/autocrine signaling for PSC self-renewal | [110] |
| Microbioreactor array with 3-D cell culture setting | EBs derived from human ESC or iPSC | PSC mesoderm differentiation, with controlled cytokine gradients. | [101] |
| Microfluidics with varying flow rates | PSC | Reveal paracrine/autocrine signaling during PSC self-renewal | [108,115] |
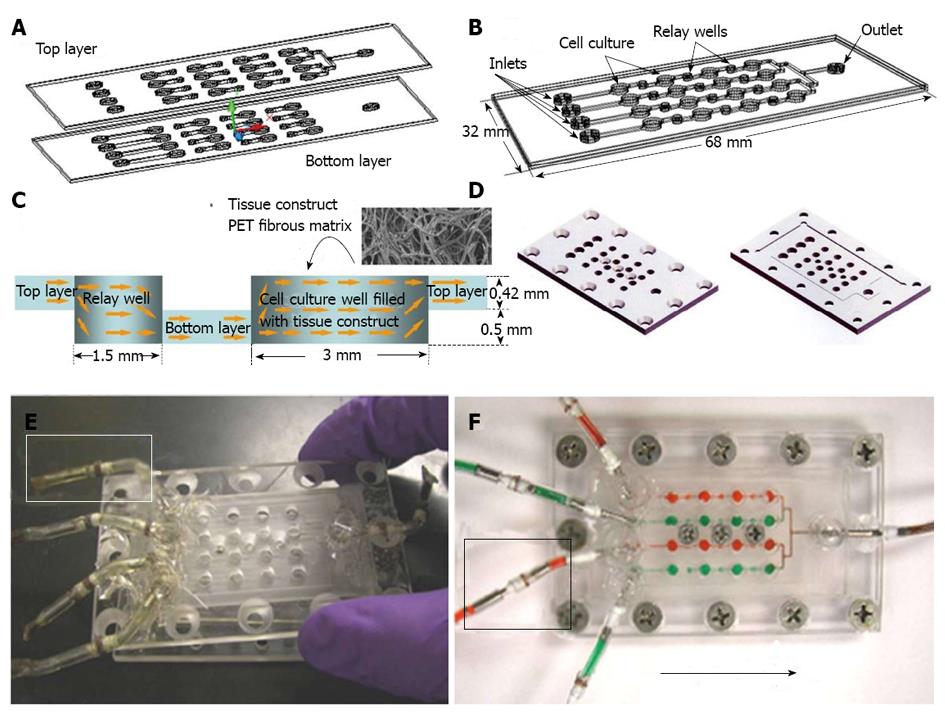
Microfluidics and microbioreactors have been studied recently for the specific lineage differentiation from various stem cell types. Spatial control of the proliferation and differentiation of NSCs was achieved by controlling gradients of growth factors in microfluidic devices[91]. An automatic microfluidic system for adipogenic and osteogenic differentiation of human AFSCs has been developed using micro-electro-mechanical-systems (MEMS) technology[92]. Neuronal and oligodendrocytic differentiation of NSC was significantly enhanced by 3-D microenvironments in microfluidic channels, suggesting that mimicking in vivo microenvironment should promote NSC differentiation[93]. Microdevices have also been incorporated with cyclic strain; for example, micro-grooved polydimethylsiloxane (PDMS) sheets have been used to study the mechanosensing properties of MSCs[94]. Kim et al[95] designed microfluidic arrays for perfused ESC culture over a logarithmic range of flow rates and observed the changes of colony sizes and shapes in response to flow rate[95]. A microfluidic chip was used to automatically analyze proliferation, motility and osteogenic differentiation of MSC in a range of cell culture regimes including medium formulation, seeding density and feeding schedules[96]. Besides controlling soluble and chemical niches in both 2D and 3D stem cell cultures, microdevices have been applied to study the synergistic effect of soluble/chemical factors and biomaterials in a miniaturized high-throughput manner[12]. Synthetic biomaterial arrays were incorporated with microfluidics to test the interactions of stem cells with a variety of extracellular signals[97]. A microscaffold cell chip, made of poly-methyl-methacrylate bonded to a perforated poly-carbonate membrane, has been used to study the single spheroid behavior of retinal stem cells with the precisely controlled microenvironment[98,99]. Using a microarray, thousands of polymeric materials and their effects on the differentiation of human ESCs and MSCs could be evaluated. A microfluidic platform coupling with an array of microbioreactors (Figure 3) has been applied for high throughput studies of human ESC differentiation into mesoderm cells[100]. The microfluidic platform allows quantitative assessments of human ESC characteristics in both 2-D and 3-D microenvironments and can be used to determine the effects of cytokine gradients (i.e., BMP-4, Activin A and Wnt3a) on cell differentiation[101]. These microbioreactors can serve as the high-throughput platform for screening cytokines, tissue scaffolds, and environmental factors, and thus can be used in developing and optimizing bioprocesses for culturing and engineering stem cells[102].
Microfluidics and microbioreactors have also been studied recently for the drug screening based on stem cell proliferation and differentiation[103]. The migratory behavior of cancer stem cells were evaluated in the compartmentalizing microfluidics by combining gradient generators, fluid handling, micro-electrodes and live cell imaging, which can be used for drug screening and disease diagnosis[104]. Microbiroeactors with 3-D fibrous matrices (Figure 4) were also developed to assess the cytotoxicity of drug and medium supplements[14,87,101,105]. This 3-D model more resembled the in vivo microenvironment and thus the cell response to the drugs more reflected the response that could occur in vivo. For example, various Chinese herb medicines were tested in the 3-D microbioreactors seeded with mouse ESCs and cancer cells[106], in which the sensitivity was greatly enhanced compared to 2-D screening platform. Thus the microfluidics and microbioreactors recreate the stem cell microenvironment while still allow the high throughput analysis, significantly improving the reliability of the screening outcomes.
Soluble growth factor secreted by stem cells is a critical niche factor for efficient stem cell differentiation. For examples, MSCs secreted various trophic factors to modulate the immune response and cell survival[107]. PSCs have also been shown to self-regulate the expansion and differentiation through autocrine/paracrine signaling such as Wnt, lefty, and Activin A[108-112]. For hematopoietic differentiation, the secreted VEGF, stem cell factor (SCF) and anti-apoptotic factors from ESCs stimulated the formation of colony-forming cells[113]. Autocrine and paracrine signaling through transforming growth factor (TGF)-β regulates Smad crosstalk and controls the survival and repopulation ability of HSCs[114]. The identified paracrine/autocrine signaling can be used to improve the stem cell differentiation by enhancing the positive signaling and inhibiting the negative feedback signaling.
Continuous flow microbioreactor arrays revealed the effect of paracrine-dependent mechanism during human ESC differentiation into early mesoderm[15]. The negative feedback loop to the mesoderm progenitors could be overcome by adding the glycogen synthase kinase (GSK)-3β inhibitor CHIR99021 and the positive induction signals were enhanced by supplementing FGF-2. Endogenous Activin A may also be secreted during human PSC differentiation into early mesoderm cells, which requires the inhibition of this signaling to promote the mesodermal specification[101]. Autocrine/paracrine signaling also supported PSC self-renewal. By increasing the flow rate to wash out the endogenous secreted factors, PSC spontaneous differentiation occurred[108,115]. While microfluidics has been explored to study the autocrine/paracrine signaling of adherent stem cells[108,110], its use in suspension stem cells is also possible in combination with other approaches such as pathway inhibition and fed-batch dilutio[110,116]. Cell communication and spatial ligand distribution in 3-D suspended cells are determined by cell organization, cell density and ligand diffusivity. The aggregate models have been shown to up-regulate factor secretion and provide spatial effect compared to monolayer cultures due to the enhanced cell-cell and cell-ECM interactions[117,118]. For example, EB derived from human ESCs and iPSCs were seeded into the multi-wells which prevented the EB washout[101]. The revealing mechanism of autocrine/paracrine signaling enables the novel design of differentiation protocols and potential translation into large scale bioprocesses. Thus, microfluidics and microbioreactors can be used as powerful tools to better understand the autocrine/paracrine factors that could lead to more efficient stem cell engineering and differentiation.
Efficient stem cell differentiation into functional tissue-specific cells is one of the major challenges in stem cell engineering. Regulating the stem cell niches in bioreactors provides a platform to precisely control stem cell fate decision. The complexity of stem cell niches can be recreated in bioreactors by modulating the regulatory factors such as oxygen tension, the scaffolding materials and configurations, and by providing various stimulation forces. Microfluidics and microbioreactors increased the throughput of screening various microenvironmental factors and improved the understanding of local paracrine and autocrine signaling. Further studies are still required to recapitulate the in vivo stimuli and integrate various niche factors to interrogate the interactions among cells, ECMs, autocrine/paracrine signaling and physical forces. Overall, bioreactors provide the bridge from the fundamental mechanism to the enabling technology for stem cell-derived biomedicines and therapeutics.
P- Reviewer Eric G S- Editor Zhai HH L- Editor A E- Editor Wu HL
| 1. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [PubMed] |
| 2. | Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Fisher MB, Mauck RL. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng Part B Rev. 2013;19:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] |
| 5. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] |
| 6. | Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570-582. [PubMed] |
| 7. | Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, Zaike Y, Tsuchida E, Nakahata T, Nakauchi H. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087-13092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Placzek MR, Chung IM, Macedo HM, Ismail S, Mortera Blanco T, Lim M, Cha JM, Fauzi I, Kang Y, Yeo DC. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6:209-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8:252-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 329] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394-398. [PubMed] |
| 14. | Wen Y, Zhang X, Yang ST. Microplate-reader compatible perfusion microbioreactor array for modular tissue culture and cytotoxicity assays. Biotechnol Prog. 2010;26:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Titmarsh DM, Hudson JE, Hidalgo A, Elefanty AG, Stanley EG, Wolvetang EJ, Cooper-White JJ. Microbioreactor arrays for full factorial screening of exogenous and paracrine factors in human embryonic stem cell differentiation. PLoS One. 2012;7:e52405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 17. | Ma T, Grayson WL, Fröhlich M, Vunjak-Novakovic G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98:6859-6864. [PubMed] |
| 19. | Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999;91:1-10. [PubMed] |
| 20. | Ivanovic Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Dello Sbarba P. Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34(+) murine bone marrow cells. Exp Hematol. 2002;30:67-73. [PubMed] |
| 21. | Ivanović Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482-1488. [PubMed] |
| 22. | Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331-339. [PubMed] |
| 23. | Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 24. | Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 567] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 25. | Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5:e8575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291-2301. [PubMed] |
| 27. | Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci. 2007;35:424-435. [PubMed] |
| 28. | Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Lovett M, Rockwood D, Baryshyan A, Kaplan DL. Simple modular bioreactors for tissue engineering: a system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng Part C Methods. 2010;16:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Baghbaderani BA, Mukhida K, Hong M, Mendez I, Behie LA. A review of bioreactor protocols for human neural precursor cell expansion in preparation for clinical trials. Curr Stem Cell Res Ther. 2011;6:229-254. [PubMed] |
| 31. | Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452-461. [PubMed] |
| 32. | Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Baptista RP, Fluri DA, Zandstra PW. High density continuous production of murine pluripotent cells in an acoustic perfused bioreactor at different oxygen concentrations. Biotechnol Bioeng. 2013;110:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215-228. [PubMed] |
| 35. | Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199-214. [PubMed] |
| 36. | Ng R, Zang R, Yang KK, Liu N, Yang ST. Three-dimensional fibrous scaffolds with microstructures and nanotextures for tissue engineering. RSC Advances. 2012;2:10110-10124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Li Y, Gautam A, Yang J, Qiu L, Melkoumian Z, Weber J, Telukuntla L, Srivastava R, Whiteley EM, Brandenberger R. Differentiation of oligodendrocyte progenitor cells from human embryonic stem cells on vitronectin-derived synthetic Peptide acrylate surface. Stem Cells Dev. 2013;22:1497-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2247] [Cited by in RCA: 1898] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 39. | Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139-1143. [PubMed] |
| 40. | Li Y, Yang ST. Effects of three-dimensional scaffolods on cell organization and tissue development. Biotechnol Bioprocess Eng. 2001;6:311-325. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47-55. [PubMed] |
| 42. | Zang R, Yang ST. Multiwall carbon nanotube-coated polyethylene terephthalate fibrous matrices for enhanced neuronal differentiation of mouse embryonic stem cells. J Mater Chem B. 2013;1:646-653. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Liu N, Ouyang A, Li Y, Yang ST. Three-dimensional neural differentiation of embryonic stem cells with ACM induction in microfibrous matrices in bioreactors. Biotechnol Prog. 2013;29:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483-495. [PubMed] |
| 45. | Elisseeff J, Ferran A, Hwang S, Varghese S, Zhang Z. The role of biomaterials in stem cell differentiation: applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295-303. [PubMed] |
| 46. | Freytes DO, Wan LQ, Vunjak-Novakovic G. Geometry and force control of cell function. J Cell Biochem. 2009;108:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112-122. [PubMed] |
| 48. | Uebersax L, Hagenmüller H, Hofmann S, Gruenblatt E, Müller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12:3417-3429. [PubMed] |
| 49. | Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077-2091. [PubMed] |
| 50. | Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003-1010. [PubMed] |
| 51. | Katayama A, Arano T, Sato T, Ikada Y, Yoshinari M. Radial-flow bioreactor enables uniform proliferation of human mesenchymal stem cells throughout a three-dimensional scaffold. Tissue Eng Part C Methods. 2013;19:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978-5989. [PubMed] |
| 53. | Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2554] [Cited by in RCA: 2443] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 54. | Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587-3593. [PubMed] |
| 55. | Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 56. | Hughes C, Radan L, Chang WY, Stanford WL, Betts DH, Postovit LM, Lajoie GA. Mass spectrometry-based proteomic analysis of the matrix microenvironment in pluripotent stem cell culture. Mol Cell Proteomics. 2012;11:1924-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1823] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 58. | Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571-7580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci USA. 2012;109:8705-8709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008;103:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35-49. [PubMed] |
| 62. | van Griensven M, Diederichs S, Roeker S, Boehm S, Peterbauer A, Wolbank S, Riechers D, Stahl F, Kasper C. Mechanical Strain Using 2D and 3D Bioreactors Induces Osteogenesis: Implications for Bone Tissue Engineering. Adv Biochem Eng Biotechnol. 2009;112:95-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Zhang ZY, Teoh SH, Teo EY, Khoon Chong MS, Shin CW, Tien FT, Choolani MA, Chan JK. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials. 2010;31:8684-8695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1-9. [PubMed] |
| 65. | Angelidis IK, Thorfinn J, Connolly ID, Lindsey D, Pham HM, Chang J. Tissue engineering of flexor tendons: the effect of a tissue bioreactor on adipoderived stem cell-seeded and fibroblast-seeded tendon constructs. J Hand Surg Am. 2010;35:1466-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730-2738. [PubMed] |
| 67. | Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489-493. [PubMed] |
| 68. | Engelmayr GC, Sales VL, Mayer JE, Sacks MS. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues. Biomaterials. 2006;27:6083-6095. [PubMed] |
| 69. | Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, Kitamura N, Kawano M. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562-570. [PubMed] |
| 70. | Kam NW, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009;9:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res. 2009;315:3611-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 72. | Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129-18134. [PubMed] |
| 73. | Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175-188. [PubMed] |
| 74. | Davisson T, Sah RL, Ratcliffe A. Perfusion increases cell content and matrix synthesis in chondrocyte three-dimensional cultures. Tissue Eng. 2002;8:807-816. [PubMed] |
| 75. | Tiğli RS, Cannizaro C, Gümüşderelioğlu M, Kaplan DL. Chondrogenesis in perfusion bioreactors using porous silk scaffolds and hESC-derived MSCs. J Biomed Mater Res A. 2011;96:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Fröhlich M, Grayson WL, Marolt D, Gimble JM, Kregar-Velikonja N, Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA. 2010;107:3299-3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 78. | Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005;21:1269-1280. [PubMed] |
| 79. | Ouyang A, Yang ST. A two-stage perfusion fibrous bed bioreactor system for mass production of embryonic stem cells. Expert Opin Biol Ther. 2008;8:895-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96:584-595. [PubMed] |
| 81. | Kim J, Ma T. Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol Bioeng. 2012;109:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Shafa M, Krawetz R, Zhang Y, Rattner JB, Godollei A, Duff HJ, Rancourt DE. Impact of stirred suspension bioreactor culture on the differentiation of murine embryonic stem cells into cardiomyocytes. BMC Cell Biol. 2011;12:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Taiani JT, Krawetz RJ, Zur Nieden NI, Elizabeth Wu Y, Kallos MS, Matyas JR, Rancourt DE. Reduced differentiation efficiency of murine embryonic stem cells in stirred suspension bioreactors. Stem Cells Dev. 2010;19:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123-4133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, McDevitt TC. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363-2376. [PubMed] |
| 87. | Wen Y, Yang ST. The future of microfluidic assays in drug development. Expert Opin Drug Discov. 2008;3:1237-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48:5406-5415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1104] [Cited by in RCA: 866] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 89. | Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275-302. [PubMed] |
| 90. | Lesher-Perez SC, Frampton JP, Takayama S. Microfluidic systems: a new toolbox for pluripotent stem cells. Biotechnol J. 2013;8:180-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401-406. [PubMed] |
| 92. | Wu HW, Lin XZ, Hwang SM, Lee GB. The culture and differentiation of amniotic stem cells using a microfluidic system. Biomed Microdevices. 2009;11:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Han S, Yang K, Shin Y, Lee JS, Kamm RD, Chung S, Cho SW. Three-dimensional extracellular matrix-mediated neural stem cell differentiation in a microfluidic device. Lab Chip. 2012;12:2305-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA. 2006;103:16095-16100. [PubMed] |
| 95. | Kim L, Vahey MD, Lee HY, Voldman J. Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip. 2006;6:394-406. [PubMed] |
| 96. | Gómez-Sjöberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR. Versatile, fully automated, microfluidic cell culture system. Anal Chem. 2007;79:8557-8563. [PubMed] |
| 97. | Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1052] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 98. | Rieke M, Gottwald E, Weibezahn KF, Layer PG. Tissue reconstruction in 3D-spheroids from rodent retina in a motion-free, bioreactor-based microstructure. Lab Chip. 2008;8:2206-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Altmann B, Giselbrecht S, Rieke M, Welle A, Scharnweber T, Weibezahn KF, Gottwald E. Chip-based Tissue Engineering in Micro-Bioreactors. Methods in Bioengineering, 3D Tissue Engineering. Boston: Artech House 2010; 83-99. |
| 100. | Cimetta E, Figallo E, Cannizzaro C, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor arrays for controlling cellular environments: design principles for human embryonic stem cell applications. Methods. 2009;47:81-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Cimetta E, Sirabella D, Yeager K, Davidson K, Simon J, Moon RT, Vunjak-Novakovic G. Microfluidic bioreactor for dynamic regulation of early mesodermal commitment in human pluripotent stem cells. Lab Chip. 2013;13:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Zhang X, Yang ST. High-throughput 3-D cell-based proliferation and cytotoxicity assays for drug screening and bioprocess development. J Biotechnol. 2011;151:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JM. High-throughput cellular microarray platforms: applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 2009;27:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 104. | Huang Y, Agrawal B, Sun D, Kuo JS, Williams JC. Microfluidics-based devices: New tools for studying cancer and cancer stem cell migration. Biomicrofluidics. 2011;5:13412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 105. | Zang R, Li D, Tang IC, Wang J, Yang ST. Cell-based assays in high-throughput screening for drug discovery. Int J Biotechnol Wellness Ind. 2012;1:31-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Li D, Zang R, Yang ST, Wang J, Wang X. Cell-based high-throughput proliferation and cytotoxicity assays for screening traditional Chinese herbal medicines. Process Biochem. 2013;48:517-524. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Lee RH, Oh JY, Choi H, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem. 2011;112:3073-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 108. | Moledina F, Clarke G, Oskooei A, Onishi K, Günther A, Zandstra PW. Predictive microfluidic control of regulatory ligand trajectories in individual pluripotent cells. Proc Natl Acad Sci USA. 2012;109:3264-3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1-70. [PubMed] |
| 110. | Przybyla L, Voldman J. Probing embryonic stem cell autocrine and paracrine signaling using microfluidics. Annu Rev Anal Chem (Palo Alto Calif). 2012;5:293-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008;105:4329-4334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 112. | Giuffrida D, Rogers IM, Nagy A, Calogero AE, Brown TJ, Casper RF. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009;42:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Guo Y, Graham-Evans B, Broxmeyer HE. Murine embryonic stem cells secrete cytokines/growth modulators that enhance cell survival/anti-apoptosis and stimulate colony formation of murine hematopoietic progenitor cells. Stem Cells. 2006;24:850-856. [PubMed] |
| 114. | Ruscetti FW, Akel S, Bartelmez SH. Autocrine transforming growth factor-beta regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24:5751-5763. [PubMed] |
| 115. | Titmarsh D, Hidalgo A, Turner J, Wolvetang E, Cooper-White J. Optimization of flowrate for expansion of human embryonic stem cells in perfusion microbioreactors. Biotechnol Bioeng. 2011;108:2894-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, Boitano AE, Ito C, Zandstra PW. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 117. | Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33:2041-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 118. | Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA. 2010;107:13724-13729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 761] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 119. | Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25-35. [PubMed] |
| 120. | Xu C, Police S, Hassanipour M, Li Y, Chen Y, Priest C, O’Sullivan C, Laflamme MA, Zhu WZ, Van Biber B. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |