Published online Jan 26, 2013. doi: 10.4252/wjsc.v5.i1.9
Revised: September 19, 2012
Accepted: December 20, 2012
Published online: January 26, 2013
AIM: To investigate the proteome changes of stem cells due to ciclopirox olamine (CPX) treatment compared to control and retinoic acid treated cells.
METHODS: Stem cells (SCs) are cells, which have the ability to continuously divide and differentiate into various other kinds of cells. Murine embryonic stem cells (ESCs) and multipotent adult germline stem cells (maGSCs) were treated with CPX, which has been shown to have an antiproliferative effect on stem cells, and compared to stem cells treated with retinoic acid (RA), which is known to have a differentiating effect on stem cells. Classical proteomic techniques like 2-D gel electrophoresis and differential in-gel electrophoresis (DIGE) were used to generate 2D protein maps from stem cells treated with RA or CPX as well as from non-treated stem cells. The resulting 2D gels were scanned and the digitalized images were collated with the help of Delta 2D software. The differentially expressed proteins were analyzed by a MALDI-TOF-TOF mass spectrometer, and the identified proteins were investigated and categorized using bioinformatics.
RESULTS: Treatment of stem cells with CPX, a synthetic antifungal clinically used to treat superficial mycoses, resulted in an antiproliferative effect in vitro, without impairment of pluripotency. To understand the mechanisms induced by CPX treatments which results in arrest of cell cycle without any marked effect on pluripotency, a comparative proteomics study was conducted. The obtained data revealed that the CPX impact on cell proliferation was accompanied with a significant alteration in stem cell proteome. By peptide mass fingerprinting and tandem mass spectrometry combined with searches of protein sequence databases, a set of 316 proteins was identified, corresponding to a library of 125 non-redundant proteins. With proteomic analysis of ESCs and maGSCs treated with CPX and RA, we could identify more than 90 single proteins, which were differently expressed in both cell lines. We could highlight, that CPX treatment of stem cells, with subsequent proliferation inhibition, resulted in an alteration of the expression of 56 proteins compared to non-treated cells, and 54 proteins compared to RA treated cells. Bioinformatics analysis of the regulated proteins demonstrated their involvement in various biological processes. To our interest, a number of proteins have potential roles in the regulation of cell proliferation either directly or indirectly. Furthermore the classification of the altered polypeptides according to their main known/postulated functions revealed that the majority of these proteins are involved in molecular functions like nucleotide binding and metal ion binding, and biological processes like nucleotide biosynthetic processes, gene expression, embryonic development, regulation of transcription, cell cycle processes, RNA and mRNA processing. Proteins, which are involved in nucleotide biosynthetic process and proteolysis, were downregulated in CPX treated cells compared to control, as well as in RA treated cells, which may explain the cell cycle arrest. Moreover, proteins which were involved in cell death, positive regulation of biosynthetic process, response to organic substance, glycolysis, anti-apoptosis, and phosphorylation were downregulated in RA treated cells compared to control and CPX treated cells.
CONCLUSION: The CPX treatment of SCs results in downregulation of nucleotide binding proteins and leads to cell cycle stop without impairment of pluripotency.
- Citation: Dihazi GH, Bibi A, Jahn O, Nolte J, Mueller GA, Engel W, Dihazi H. Impact of the antiproliferative agent ciclopirox olamine treatment on stem cells proteome. World J Stem Cells 2013; 5(1): 9-25
- URL: https://www.wjgnet.com/1948-0210/full/v5/i1/9.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i1.9
Stem cells (SCs) are cells, which are found in all multicellular organisms, which can continuously divide and differentiate into various specialized cell types and can also self-renew to produce more stem cells[1]. The therapeutic use of embryonic stem cells (ESCs) has been constrained by problems caused by immune rejection in the patient as well as ethical issues associated with the use of embryos[2]. Spermatogonial stem cells (SSCs) are self-renewing single cells located in the periphery of the seminiferous tubules whose continuous division maintain spermatogenesis throughout the life of a male individual[3]. SSCs were isolated from murine testis and cultured for the first time in 2006[4]. The pluripotency and plasticity of these cultured cells, named multipotent adult germline stem cells (maGSCs), were proven to be similar to ESCs. The ESC-like nature of maGSCs was confirmed on the microRNA level[5], on the transcriptome level[6] and on the proteome level[7]. In a recent study, we investigated the effects of retinoic acid (RA) treatment on the protein expression profiles of maGSCs and ESCs[8]. The study revealed the important role of Eif5a and its hypusination for stem cell differentiation and proliferation.
Eif5a is a universal translation elongation factor which is highly conserved in all cells. Eif5a has been shown to be associated with translation, viability and proliferation processes[9-12]. It is the only eukaryotic protein known to have the unusual amino acid hypusine. Hypusine is essential to the function of Eif5a and is involved in protein biosynthesis by promoting the formation of the first peptide bond and translation elongation[13]. The activation of Eif5a is controlled by a unique post-translational modification called hypusination. It occurs in two steps which are controlled by two different enzymes[14,15], which inactivation can lead to hypusination inhibition. Ciclopirox olamine (CPX), the ethanolamine salt of 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one, is a hypusination inhibitor that controls the second step of the modification, which is catalyzed by deoxyhypusine hydroxylase[14].
CPX, a synthetic antifungal agent, has been used topically to treat fungal and yeast infection of skin or mucosa for more than 20 years[16-19]. Apart from its antimycotic activity, CPX is also effective against both gram-positive and gram-negative bacteria[20]. CPX might also serve as an alternative to recombinant vascular endothelial growth factor (VEGF) treatment or to VEGF gene therapy for therapeutic angiogenesis[21]. The effect of CPX on several Saccharomyces cerevisiae mutants has been screened and tested, and it was suggested that CPX may exert its effect by disrupting DNA repair, DNA replication, cell division signals and a defect in mitotic spindle function. Furthermore CPX can influence the regulation of many processes, including signal transduction, transcription, cell division, and development[22]. Recent studies demonstrated CPX as a potential anti-cancer agent for the treatment of malignancies, including leukemia and myeloma[23-25]. However, the mechanism of CPX as a drug in angiogenesis and tumor treatment is poorly understood. CPX works as an inhibitor of the iron-dependent enzymes due to its role as a chelator of intracellular iron[22,23]. Other studies reported the inhibition of HIV-1 gene expression by CPX[26], the importance of Eif5a in embryogenesis and cell differentiation[27], in hepatocellular carcinoma[28] and in diabetes[29]. CPX has also been used as an inhibitor of hypusination.
In a recent study, the effect of CPX on the cellular viability and proliferation of ESCs and maGSCs was investigated. CPX treatment of the stem cells resulted in an antiproliferative effect on ESCs and maGSCs in vitro, but did not affect the cell pluripotency[8]. The inhibitory effect of CPX on cell differentiation was reversible and was not associated to apoptosis. The ESCs were found to be more sensitive to CPX than the maGSCs.
The aim of this study was to investigate the proteome changes of ESCs and maGSCs accompanying the treatment with CPX and subsequent inhibition of hypusination using classical proteomic techniques like 2-DE, DIGE and MS. 2D protein maps were generated from control cells and cells treated either with RA or CPX. The resulting protein maps were compared to each other and the differentially expressed proteins were investigated using bioinformatics. We could highlight that a treatment with CPX, involving proliferation inhibition, resulted in an alteration of the expression of 56 proteins compared to non-treated cells, and 54 proteins compared to RA treated cells. The majority of these proteins are involved in nucleotide binding and nucleotide biosynthetic processes, metal binding, DNA binding, and other processes which have been linked to CPX.
The derivation and culture of maGSCs 129/Sv was described previously[4]. In brief, testes from adult mice were isolated and digested using collagenase. Single cell suspension was derived after trypsin digestion followed by the culture of the testis suspension cells on a mouse embryonic fibroblasts (MEFs) feeder layer in the presence of GDNF. After appearance of morphological ES-like cells, the colonies were picked and expanded in standard ES cell conditions. In this case, the maGSC line was generated without genetic selection, only by morphological criteria. The ESC R1 line was derived from the 129/Sv mouse[30]. To maintain maGSCs and ESCs in an undifferentiated state, the cells were cultured under standard ESC culture conditions: DMEM (PAN, Aidenbach, Germany) supplemented with 20% fetal calf serum (PAN, Aidenbach, Germany), 2 mmol/L L-glutamine (PAN, Aidenbach, Germany), 50 mmol/L β-mercaptoethanol (Gibco/Invitrogen, Eggenstein, Germany), 1 × non-essential amino acids (Gibco/Invitrogen), sodium pyruvate (Gibco/Invitrogen), and penicillin/streptomycin (PAN, Aidenbach, Germany). ESCs and maGSCs were cultured on a feeder layer of mitomycin C-inactivated MEFs in the presence of 1000 U/mL recombinant mouse leukemia inhibitory factor (LIF) (Chemicon, Temecula, United States). ESCs were isolated as described previously, and male ESC lines were identified and selected by PCR amplification of Sry gene-specific sequences[31,32]. In order to differentiate maGSCs and male ESCs, the cells were plated on gelatin-coated dishes and culture medium was supplemented with 1 μmol/L RA (Sigma-Aldrich, Steinheim, Germany) instead of LIF. Cells were cultured for 48 h before they were lysed and the proteins were extracted. For examining the effect of CPX on the proteome level, ESCs and maGSCs were treated with culture medium supplemented with 2 μmol/L CPX for 72 h.
The protein extraction for 2-DE was performed as described previously[7]. Briefly, 75% confluent cultures were trypsinized and washed three times with PBS. The cells were harvested by centrifugation at 200 ×g for 10 min, the pellet was treated with 0.3-0.5 mL lysis buffer [9.5 mol/L urea, 2% CHAPS (w/v), 2% ampholytes (w/v), 1% DTT]. Ampholytes and DTT were added shortly before use. After adding the lysis buffer, the samples were incubated for 30 min at 4 °C. For removing the cell debris, sample centrifugation was carried out at 13 000 ×g and 4 °C for 45 min. The supernatant was recentrifuged at 13 000 ×g and 4 °C for an additional 45 min to get maximal purity. The resulting samples were used immediately or stored at -80 °C until use.
To reduce the salt contamination and to enrich the proteins, methanol-chloroform-precipitation according to Wessel et al[33] was performed. Briefly, 0.4 mL of methanol (100%) was added to 0.1 mL aliquots of protein samples and mixed together. 0.1 mL chloroform was added to the samples and the mixture was vortexed. Subsequently 0.3 mL water was added and the solution was vortexed and centrifuged at 13 000 ×g for 1 min. The aqueous layer was removed, and another 0.4 mL methanol (100%) was added to the rest of the chloroform and the interphase with the precipitated proteins. The sample was mixed and centrifuged for 2 min at 13 000 ×g and the supernatant was removed. The pellet was vacuum dried and dissolved in lysis buffer.
Total protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, United States) according to Bradford[34]. BSA (Sigma, Steinheim, Germany) was used as a standard.
IPG strips (11 cm, pI 5-8) were passively rehydrated in 185 μL solution containing 150 μg protein in a rehydration buffer (8 mol/L urea, 1% CHAPS, 1% DTT, 0.2% ampholytes, and a trace of bromophenol blue) for 12 h. The IEF step was performed on the PROTEAN® IEF Cell (Bio-Rad, Hercules, CA, United States). Temperature-controlled at 20 °C, the voltage was set to 500 V for 1 h, increased to 1000 V for 1 h, 2000 V for 1 h and left at 8000 V until a total of 50 000 Vhours was reached. Prior to SDS-PAGE, the IPG strips were reduced for 20 min at room temperature in SDS equilibration buffer containing 6 mol/L urea, 30% glycerol, 2% SDS 0.05 mol/L Tris-HCl, and 2% DTT on a rocking table. The strips were subsequently alkylated in the same solution with 2.5% iodoacetamide substituted for DTT, and a trace of bromophenol blue. For the SDS-PAGE, 12% BisTris Criterion precast gels (Bio-Rad, Hercules, CA, United States) were used according to manufacturer’s instructions. The gels were run at 150 V for 10 min followed by 200 V until the bromophenol blue dye front had reached the bottom of the gel.
For image analysis, 2-DE gels were fixed in a solution containing 50% methanol and 12% acetic acid overnight and fluorescent stained with Flamingo fluorescent gel stain (Bio-Rad, Hercules, CA, United States) for minimum 5 h. After staining, gels were scanned at 50 μm resolution on a Fuji FLA-5100 scanner. The digitalized images were analyzed using Delta 2D 3.4 (Decodon, Braunschweig, Germany). For protein visualization, 2-DE gels were additionally stained with colloidal Coomassie blue, Roti-Blue (Roth, Karlsruhe, Germany) overnight.
Protein extraction and methanol-chloroform-precipitation were performed as described above. The resulting pellet was dissolved in labeling buffer (30 mmol/L Tris-HCl pH 8.5, 9.5 mol/L urea, 2% CHAPS), centrifuged (5 min, 13 000 ×g), and the protein concentration of the supernatant was determined as described above. The preparation of the CyDyes as well as the labeling reaction was followed according to the manufacturer’s protocol (GE Healthcare).
To avoid the dye-specific protein labeling, every pair of protein samples from two independent cell extract preparations were processed in duplicate while swapping the dyes. Thereby four replicate gels were obtained, allowing to monitor regulation factors down to twofold changes[35]. An internal standard consisting of a mixture of the samples under investigation was labeled with Cy2 and included on all gels to facilitate gel matching, thereby eliminating artifacts from experimental variation. The three differentially labeled fractions were pooled. Rehydration buffer (8 mol/L urea, 1% CHAPS, 13 mmol/L DTT and 1% ampholytes 3-10) was added to make up the volume to 185 μL prior to IEF. The 2-DE was performed as described above. The CyDye-labeled gels were scanned at 50 μm resolution on a Fuji FLA5100 scanner (Fuji Photo, Kanagawa, Japan) with laser excitation light at 473 nm and long pass emission filter 510LP (Cy2), 532 nm and long pass emission filter 575LP (Cy3), and 635 nm and long pass emission filter 665LP (Cy5). Fluorescent images were acquired in 16-bit TIFF files format. Spot matching across gels and normalization based on the internal standard was performed with Delta2D software (Decodon, Greifswald, Germany). To analyze the significance of protein regulation, a Student’s t-test was performed, and statistical significance was assumed for p values less than 0.01. For protein visualization, the 2-DE gels were post-stained with colloidal Coomassie blue (Roti-Blue) overnight. Differentially regulated proteins were excised and processed for identification by mass spectrometry.
Manually excised gel plugs were subjected to an automated platform for the identification of gel-separated proteins[36] as described in the framework of recent DIGE-based[37] and large-scale proteome studies[38]. An Ultraflex MALDI-TOF-TOF mass spectrometer (Bruker Daltonik) was used to acquire both PMF and fragment ion spectra, resulting in confident protein identifications based on peptide mass and sequence information. Database searches in the Swiss-Prot primary sequence database restricted to the taxonomy mus musculus were performed using the MASCOT Software 2.2 (Matrix Science). Carboxamidomethylation of Cys residues was specified as fixed and oxidation of Met as variable modifications. One trypsin missed cleavage was allowed. Mass tolerances were set to 100 ppm for PMF searches and to 100 ppm (precursor ions) and 0.7 Da (fragment ions) for MS/MS ion searches. The minimal requirement for accepting a protein as identified was at least one peptide sequence match above identity threshold in addition to at least 20 % sequence coverage in the PMF.
The classification of the identified proteins according to their main known/postulated functions was carried out using DAVID bioinformatics[39,40]. This classification together with the official gene symbol was used to investigate and categorize the gene ontology (GO)-annotations (biological processes and molecular functions).
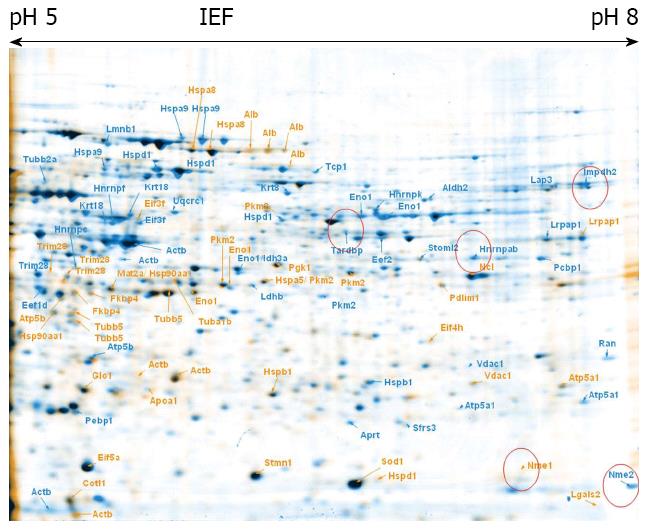
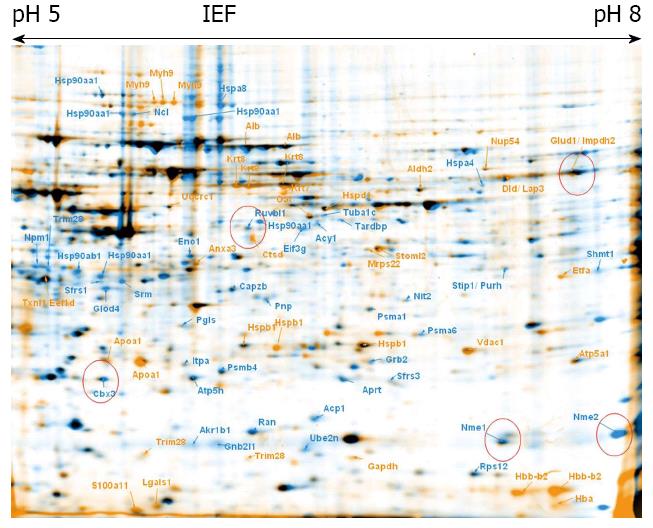
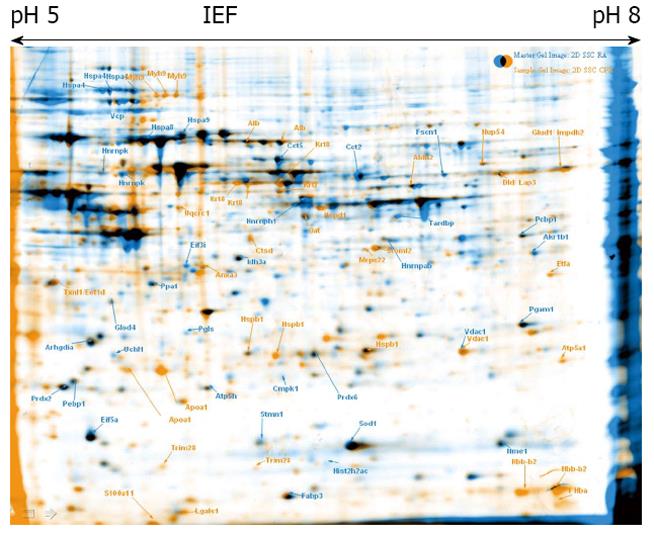
To explore proteome changes caused by CPX treatment, we treated ESCs as well as maGSCs with CPX for 72 h. In parallel, both cell types, ESC and maGSC, were treated with RA for 48 h. 2-DE was performed from these four different samples, as well as from the corresponding non-treated cells (Figures 1, 2, 3 and 4). Proteins, which were found to be differentially expressed, were excised and subjected to in-gel-digestion and mass spectrometry analyses. A total of 316 spots were identified, which resulted in 125 non-redundant proteins (Table 1). For further interpretation of the data, only proteins, which were regulated in the same direction in ESCs and concurrently in maGSCs, were taken into consideration.
| Protein name | Gene name | Swiss-prot | Nominal mass | CPI | PMF-score | PMF sequence coverage | MS/MS-score | MS/MS-sequence coverage |
| Low molecular weight phosphotyrosine protein phosphatase | Acp1 | PPAC_MOUSE | 18 636 | 6.4 | 96 | 65 | 80 | 24 |
| Actin, cytoplasmic 1 | Actb | ACTB_MOUSE | 42 052 | 5.2 | 170 | 70 | 312 | 15 |
| Aminoacylase-1 | Acy1 | ACY1_MOUSE | 45 980 | 5.9 | 167 | 56 | 44 | 5 |
| Aldose reductase | Akr1b1 | ALDR_MOUSE | 36 052 | 6.9 | 128 | 43 | 136 | 10 |
| Aldehyde dehydrogenase, mitochondrial | Aldh2 | ALDH2_MOUSE | 57 015 | 8.6 | 221 | 54 | 131 | 7 |
| Annexin A3 | Anxa3 | ANXA3_MOUSE | 36 520 | 5.2 | 84 | 47 | 111 | 14 |
| Adenine phosphoribosyltransferase | Aprt | APT_MOUSE | 19 883 | 6.4 | 88 | 67 | 216 | 27 |
| Rho GDP-dissociation inhibitor 1 | Arhgdia | GDIR1_MOUSE | 23 450 | 5 | 123 | 54 | 66 | 11 |
| ATP synthase subunit α, mitochondrial | Atp5a1 | ATPA_MOUSE | 59 830 | 9.7 | 100 | 28 | 53 | 4 |
| ATP synthase subunit β, mitochondrial | Atp5b | ATPB_MOUSE | 56 265 | 5.1 | 90 | 30 | 167 | 10 |
| ATP synthase subunit d, mitochondrial | Atp5h | ATP5H_MOUSE | 18 795 | 5.4 | 122 | 70 | 169 | 36 |
| F-actin-capping protein subunit α-2 | Capza2 | CAZA2_MOUSE | 33 118 | 5.5 | 148 | 69 | 19 | 9 |
| F-actin-capping protein subunit β | Capzb | CAPZB_MOUSE | 31 611 | 5.4 | 117 | 61 | 129 | 8 |
| Chromobox protein homolog 3 | Cbx3 | CBX3_MOUSE | 21 013 | 5 | 38 | 36 | 67 | 6 |
| T-complex protein 1 subunit β | Cct2 | TCPB_MOUSE | 57 783 | 6 | 248 | 61 | 75 | 9 |
| T-complex protein 1 subunit epsilon | Cct5 | TCPE_MOUSE | 60 042 | 5.7 | 186 | 60 | 138 | 6 |
| Cofilin-1 | Cfl1 | COF1_MOUSE | 18 776 | 9.1 | 95 | 45 | 87 | 13 |
| UMP-CMP kinase | Cmpk1 | KCY_MOUSE | 22 379 | 5.6 | 74 | 52 | 29 | 10 |
| Coactosin-like protein | Cotl1 | COTL1_MOUSE | 16 048 | 5.1 | 86 | 60 | 116 | 14 |
| Cathepsin D | Ctsd | CATD_MOUSE | 45 381 | 6.9 | 160 | 41 | 95 | 4 |
| Dihydrolipoyl dehydrogenase, mitochondrial | Dld | DLDH_MOUSE | 54 751 | 9 | 112 | 48 | 81 | 2 |
| Elongation factor 1-α1 | Eef1a1 | EF1A1_MOUSE | 50 424 | 9.7 | 68 | 34 | 115 | 8 |
| Elongation factor 1-δ | Eef1d | EF1D_MOUSE | 31 388 | 4.8 | 86 | 54 | 79 | 9 |
| Elongation factor 2 | Eef2 | EF2_MOUSE | 96 222 | 6.4 | 52 | 26 | 29 | 1 |
| Eukaryotic translation initiation factor 3 subunit F | Eif3f | EIF3F_MOUSE | 38 090 | 5.2 | 109 | 45 | 106 | 14 |
| Eukaryotic translation initiation factor 3 subunit G | Eif3g | EIF3G_MOUSE | 35 901 | 5.6 | 54 | 35 | 23 | 7 |
| Eukaryotic translation initiation factor 3 subunit I | Eif3i | EIF3I_MOUSE | 36 837 | 5.3 | 228 | 78 | 89 | 16 |
| Eukaryotic translation initiation factor 4H | Eif4h | IF4H_MOUSE | 27 381 | 7.5 | 83 | 51 | 65 | 8 |
| Eukaryotic translation initiation factor 5A-1 | Eif5a | IF5A1_MOUSE | 17 049 | 4.9 | 115 | 58 | 170 | 22 |
| α-enolase | Eno1 | ENOA_MOUSE | 47 453 | 6.4 | 183 | 64 | 170 | 13 |
| Electron transfer flavoprotein subunit α, mitochondrial | Etfa | ETFA_MOUSE | 35 330 | 9.5 | 138 | 59 | 100 | 9 |
| Fatty acid-binding protein, heart | Fabp3 | FABPH_MOUSE | 14 810 | 6.1 | 86 | 77 | 212 | 39 |
| Peptidyl-prolyl cis-trans isomerase FKBP4 | Fkbp4 | FKBP4_MOUSE | 51 939 | 5.4 | 122 | 38 | 168 | 9 |
| Fascin | Fscn1 | FSCN1_MOUSE | 55 215 | 6.5 | 129 | 45 | 26 | 6 |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | G3P_MOUSE | 36 072 | 9.2 | 62 | 38 | 40 | 8 |
| Lactoylglutathione lyase | Glo1 | LGUL_MOUSE | 20 967 | 5.1 | 134 | 66 | 114 | 20 |
| Glyoxalase domain-containing protein 4 | Glod4 | GLOD4_MOUSE | 33 581 | 5.2 | 167 | 69 | 115 | 13 |
| Glutamate dehydrogenase 1, mitochondrial | Glud1 | DHE3_MOUSE | 61 640 | 8.8 | 70 | 37 | 60 | 5 |
| Guanine nucleotide-binding protein subunit β-2-like 1 | Gnb2l1 | GBLP_MOUSE | 35 511 | 8.9 | 116 | 55 | 20 | 5 |
| Growth factor receptor-bound protein 2 | Grb2 | GRB2_MOUSE | 25 336 | 5.9 | 73 | 54 | 36 | 17 |
| Histone H2B type 1-B | Hist1h2bb | H2B1B_MOUSE | 13 944 | 10.8 | 52 | 41 | 93 | 19 |
| Histone H2A type 2-C | Hist2h2ac | H2A2C_MOUSE | 13 980 | 11.4 | 50 | 55 | 67 | 12 |
| Heterogeneous nuclear ribonucleoprotein A/B | Hnrnpab | ROAA_MOUSE | 30 926 | 8.7 | 83 | 30 | 107 | 5 |
| Heterogeneous nuclear ribonucleoproteins C1/C2 | Hnrnpc | HNRPC_MOUSE | 34 421 | 4.8 | 57 | 32 | 57 | 6 |
| Heterogeneous nuclear ribonucleoprotein F | Hnrnpf | HNRPF_MOUSE | 46 043 | 5.2 | 163 | 56 | 207 | 12 |
| Heterogeneous nuclear ribonucleoprotein H | Hnrnph1 | HNRH1_MOUSE | 49 454 | 5.9 | 166 | 61 | 134 | 15 |
| Heterogeneous nuclear ribonucleoprotein K | Hnrnpk | HNRPK_MOUSE | 51 230 | 5.3 | 144 | 46 | 251 | 11 |
| Heat shock protein HSP 90-α | Hsp90aa1 | HS90A_MOUSE | 85 134 | 4.8 | 131 | 31 | 130 | 5 |
| Heat shock protein HSP 90-β | Hsp90ab1 | HS90B_MOUSE | 83 615 | 4.8 | 62 | 25 | 78 | 6 |
| Heat shock 70 kDa protein 4 | Hspa4 | HSP74_MOUSE | 94 872 | 5 | 242 | 54 | 102 | 3 |
| 78 kDa glucose-regulated protein | Hspa5 | GRP78_MOUSE | 72 492 | 4.9 | 78 | 25 | 122 | 5 |
| Heat shock cognate 71 kDa protein | Hspa8 | HSP7C_MOUSE | 71 055 | 5.2 | 234 | 58 | 154 | 4 |
| Stress-70 protein, mitochondrial | Hspa9 | GRP75_MOUSE | 73 768 | 5.8 | 219 | 50 | 272 | 7 |
| Heat shock protein β-1 | Hspb1 | HSPB1_MOUSE | 23 057 | 6.1 | 144 | 55 | 344 | 24 |
| 60 kDa heat shock protein, mitochondrial | Hspd1 | CH60_MOUSE | 61 088 | 5.8 | 334 | 69 | 232 | 10 |
| Isocitrate dehydrogenase (NAD) subunit α, mitochondrial | Idh3a | IDH3A_MOUSE | 40 069 | 6.3 | 70 | 31 | 158 | 12 |
| Inosine-5'-monophosphate dehydrogenase 2 | Impdh2 | IMDH2_MOUSE | 56 179 | 7 | 173 | 50 | 107 | 7 |
| Inosine triphosphate pyrophosphatase | Itpa | ITPA_MOUSE | 22 225 | 5.5 | 84 | 72 | 128 | 15 |
| Keratin, type I cytoskeletal 18 | Krt18 | K1C18_MOUSE | 47 509 | 5.1 | 199 | 65 | 58 | 9 |
| Keratin, type II cytoskeletal 7 | Krt7 | K2C7_MOUSE | 50 678 | 5.6 | 137 | 52 | 55 | 4 |
| Keratin, type II cytoskeletal 8 | Krt8 | K2C8_MOUSE | 54 531 | 5.6 | 245 | 55 | 237 | 9 |
| Cytosol aminopeptidase | Lap3 | AMPL_MOUSE | 56 505 | 8.7 | 126 | 47 | 58 | 5 |
| L-lactate dehydrogenase B chain | Ldhb | LDHB_MOUSE | 36 834 | 5.6 | 84 | 46 | 30 | 6 |
| Galectin-1 | Lgals1 | LEG1_MOUSE | 15 198 | 5.2 | 109 | 70 | 172 | 25 |
| Galectin-2 | Lgals2 | LEG2_MOUSE | 14 984 | 7.9 | 120 | 88 | 60 | 14 |
| Lamin-B1 | Lmnb1 | LMNB1_MOUSE | 66 973 | 5 | 265 | 60 | 134 | 5 |
| α-2-macroglobulin receptor-associated protein | Lrpap1 | AMRP_MOUSE | 42 189 | 7.9 | 139 | 49 | 177 | 14 |
| S-adenosylmethionine synthase isoform type-2 | Mat2a | METK2_MOUSE | 44 003 | 6 | 73 | 41 | 59 | 3 |
| 28S ribosomal protein S22, mitochondrial | Mrps22 | RT22_MOUSE | 41 281 | 9.2 | 112 | 45 | 97 | 9 |
| Myosin-9 | Myh9 | MYH9_MOUSE | 227 429 | 5.4 | 73 | 15 | 103 | 2 |
| Nucleolin | Ncl | NUCL_MOUSE | 76 734 | 4.5 | 113 | 26 | 179 | 7 |
| Omega-amidase NIT2 | Nit2 | NIT2_MOUSE | 30 825 | 6.5 | 112 | 59 | 75 | 9 |
| Nucleoside diphosphate kinase A | Nme1 | NDKA_MOUSE | 17 311 | 7.7 | 125 | 72 | 223 | 30 |
| Nucleoside diphosphate kinase B | Nme2 | NDKB_MOUSE | 17 466 | 7.8 | 160 | 84 | 287 | 30 |
| Nucleophosmin | Npm1 | NPM_MOUSE | 32 711 | 4.5 | 53 | 33 | 136 | 10 |
| Nuclear pore complex protein Nup54 | Nup54 | NUP54_MOUSE | 55 812 | 6.6 | 55 | 21 | 23 | 3 |
| Nuclear pore glycoprotein p62 | Nup62 | NUP62_MOUSE | 53 336 | 5.1 | 13 | 43 | 5 | |
| Ornithine aminotransferase, mitochondrial | Oat | OAT_MOUSE | 48 723 | 6.2 | 174 | 64 | 125 | 9 |
| Poly(rC)-binding protein 1 | Pcbp1 | PCBP1_MOUSE | 37 987 | 6.8 | 175 | 69 | 115 | 12 |
| Protein disulfide-isomerase A3 | Pdia3 | PDIA3_MOUSE | 57 099 | 5.8 | 254 | 55 | 90 | 5 |
| Protein disulfide-isomerase A6 | Pdia6 | PDIA6_MOUSE | 48 469 | 4.9 | 75 | 40 | 47 | 2 |
| PDZ and LIM domain protein 1 | Pdlim1 | PDLI1_MOUSE | 36 208 | 6.4 | 200 | 73 | 56 | 7 |
| Phosphatidylethanolamine-binding protein 1 | Pebp1 | PEBP1_MOUSE | 20 988 | 5.1 | 130 | 79 | 107 | 11 |
| Phosphoglycerate mutase 1 | Pgam1 | PGAM1_MOUSE | 28 928 | 6.8 | 157 | 66 | 192 | 21 |
| Phosphoglycerate kinase 1 | Pgk1 | PGK1_MOUSE | 44 921 | 9 | 136 | 52 | 128 | 7 |
| 6-phosphogluconolactonase | Pgls | 6PGL_MOUSE | 27 465 | 5.5 | 102 | 49 | 148 | 17 |
| Pyruvate kinase isozymes M1/M2 | Pkm2 | KPYM_MOUSE | 58 378 | 7.9 | 178 | 49 | 106 | 9 |
| Purine nucleoside phosphorylase | Pnp | PNPH_MOUSE | 32 541 | 5.8 | 119 | 67 | 138 | 13 |
| Inorganic pyrophosphatase | Ppa1 | IPYR_MOUSE | 33 102 | 5.3 | 126 | 66 | 26 | 7 |
| Peroxiredoxin-2 | Prdx2 | PRDX2_MOUSE | 21 936 | 5.1 | 103 | 62 | 285 | 22 |
| Peroxiredoxin-6 | Prdx6 | PRDX6_MOUSE | 24 969 | 5.6 | 156 | 67 | 101 | 17 |
| Proteasome subunit α type-1 | Psma1 | PSA1_MOUSE | 29 813 | 6 | 71 | 52 | 140 | 17 |
| Proteasome subunit α type-6 | Psma6 | PSA6_MOUSE | 27 811 | 6.4 | 72 | 38 | 108 | 10 |
| Proteasome subunit β type-3 | Psmb3 | PSB3_MOUSE | 23 235 | 6.2 | 110 | 51 | 187 | 30 |
| Proteasome subunit β type-4 | Psmb4 | PSB4_MOUSE | 29 211 | 5.3 | 60 | 42 | 109 | 10 |
| 26S protease regulatory subunit 7 | Psmc2 | PRS7_MOUSE | 49 016 | 5.6 | 166 | 60 | 72 | 8 |
| 26S protease regulatory subunit 6B | Psmc4 | PRS6B_MOUSE | 47 366 | 5 | 144 | 55 | 109 | 9 |
| GTP-binding nuclear protein Ran | Ran | RAN_MOUSE | 24 579 | 7.8 | 124 | 51 | 139 | 11 |
| 40S ribosomal protein S12 | Rps12 | RS12_MOUSE | 14 858 | 7.7 | 77 | 62 | 95 | 11 |
| RuvB-like 1 | Ruvbl1 | RUVB1_MOUSE | 50 524 | 6 | 61 | 35 | 106 | 10 |
| Protein S100-A11 | S100a11 | S10AB_MOUSE | 11 247 | 5.1 | 36 | 147 | 27 | |
| Splicing factor, arginine/serine-rich 1 | Sfrs1 | SFRS1_MOUSE | 27 842 | 10.8 | 80 | 43 | 156 | 18 |
| Splicing factor, arginine/serine-rich 3 | Sfrs3 | SFRS3_MOUSE | 19 546 | 12.3 | 87 | 14 | ||
| Serine hydroxymethyltransferase, cytosolic | Shmt1 | GLYC_MOUSE | 53 065 | 6.5 | 98 | 43 | 19 | 2 |
| Superoxide dismutase [Cu-Zn] | Sod1 | SODC_MOUSE | 16 104 | 6 | 83 | 45 | 126 | 31 |
| Spermidine synthase | Srm | SPEE_MOUSE | 34 543 | 5.2 | 141 | 73 | 129 | 15 |
| Stress-induced-phosphoprotein 1 | Stip1 | STIP1_MOUSE | 63 170 | 6.4 | 184 | 55 | 89 | 4 |
| Stathmin | Stmn1 | STMN1_MOUSE | 17 264 | 5.7 | 28 | 24 | 69 | 8 |
| Stomatin-like protein 2 | Stoml2 | STML2_MOUSE | 38 475 | 9.5 | 144 | 61 | 165 | 15 |
| TAR DNA-binding protein 43 | Tardbp | TADBP_MOUSE | 44 918 | 6.3 | 68 | 30 | 107 | 7 |
| T-complex protein 1 subunit α | Tcp1 | TCPA_MOUSE | 60 867 | 5.8 | 61 | 27 | 28 | 4 |
| Transcription intermediary factor 1-β | Trim28 | TIF1B_MOUSE | 90 558 | 5.4 | 10 | 139 | 4 | |
| Tubulin α-1B chain | Tuba1b | TBA1B_MOUSE | 50 804 | 4.8 | 128 | 39 | 152 | 9 |
| Tubulin α-1C chain | Tuba1c | TBA1C_MOUSE | 50 562 | 4.8 | 53 | 24 | 52 | 6 |
| Tubulin β-2A chain | Tubb2a | TBB2A_MOUSE | 50 274 | 4.6 | 126 | 55 | 111 | 11 |
| Tubulin β-2C chain | Tubb2c | TBB2C_MOUSE | 50 255 | 4.6 | 150 | 56 | 49 | 8 |
| Tubulin β-5 chain | Tubb5 | TBB5_MOUSE | 50 095 | 4.6 | 169 | 57 | 237 | 9 |
| Thioredoxin | Txn | THIO_MOUSE | 12 010 | 4.6 | 63 | 67 | 92 | 22 |
| Thioredoxin-like protein 1 | Txnl1 | TXNL1_MOUSE | 32 616 | 4.7 | 144 | 78 | 39 | 2 |
| Ubiquitin-conjugating enzyme E2 N | Ube2n | UBE2N_MOUSE | 17 184 | 6.2 | 119 | 71 | 20 | 6 |
| Ubiquitin carboxyl-terminal hydrolase isozyme L1 | Uchl1 | UCHL1_MOUSE | 25 165 | 5 | 77 | 64 | 16 | 8 |
| Cytochrome b-c1 complex subunit 1, mitochondrial | Uqcrc1 | QCR1_MOUSE | 53 420 | 5.7 | 95 | 40 | 46 | 6 |
| Transitional endoplasmic reticulum ATPase | Vcp | TERA_MOUSE | 89 950 | 5 | 310 | 61 | 40 | 5 |
| Voltage-dependent anion-selective channel protein 1 | Vdac1 | VDAC1_MOUSE | 32 502 | 9.2 | 159 | 57 | 80 | 24 |
| Vimentin | Vim | VIME_MOUSE | 53 712 | 4.9 | 218 | 64 | 47 | 8 |
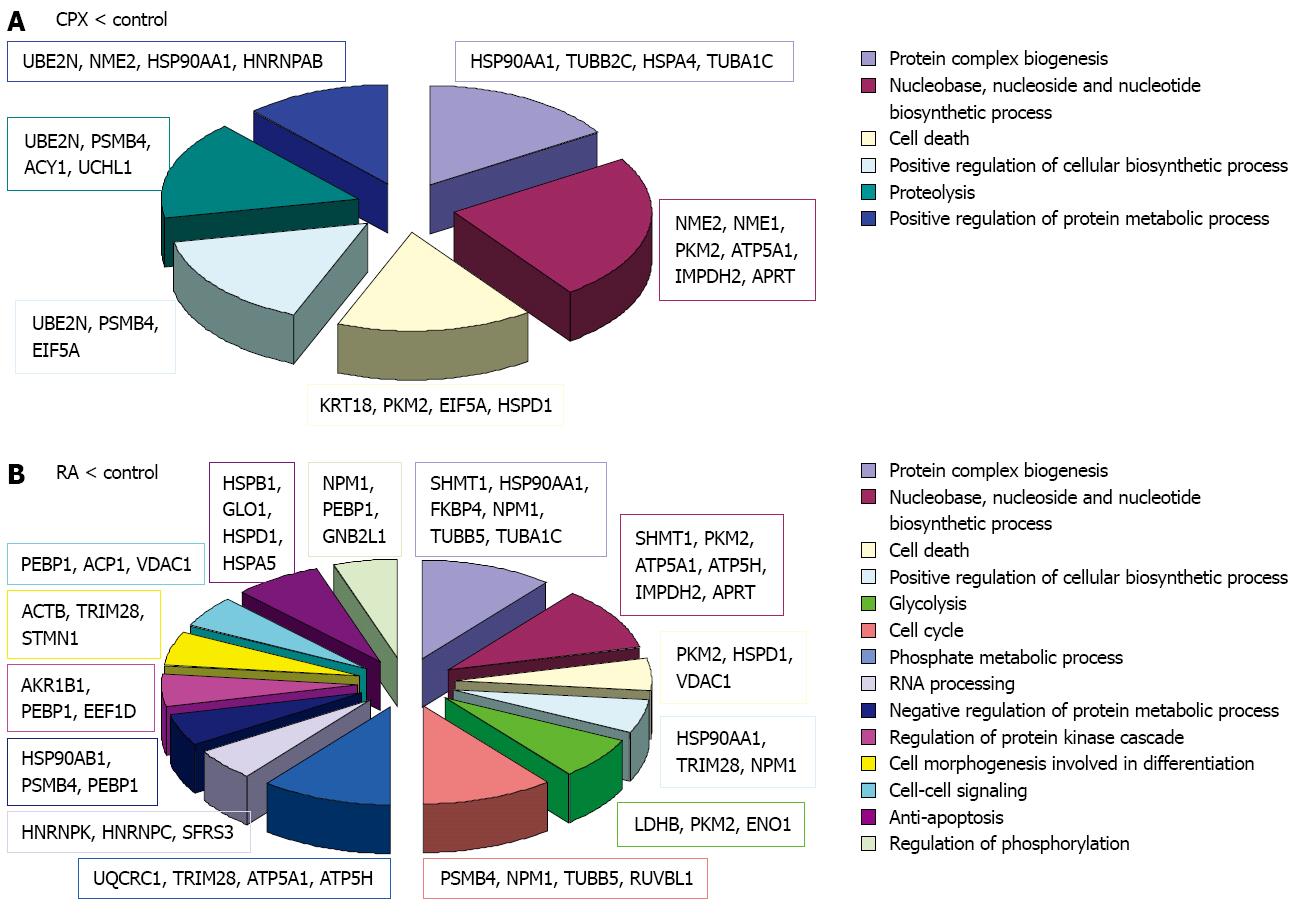
The identified proteins were classified using DAVID bioinformatics[39,40] focusing on its information considering the GO (Gene Ontology) annotations. The terms corresponding to the molecular function and biological process were regarded (Figures 5, 6 and 7).
Examination of all of the proteins, which expression was altered either by CPX or RA treatment, was performed regarding their involvement in biological processes. We found that seven proteins are involved in regulation of transcription. Among these proteins Ube2n, Tardbp, Cbx3 and Hnrnpab were downregulated in CPX treated cells compared to control, whereas Nup62 was upregulated in CPX treated cells compared to control (Table 2). Two other proteins Trim28 and Ruvbl1 were downregulated in RA treated cells compared to control. Detailed information is given in Table 2 and the protein expression regulation folds are given in Tables 3, 4, 5 and 6.
| Biological process | Proteins | CPX > RA | CPX < RA | CPX > c | CPX < c | RA > c | RA < c |
| Monosaccharide metabolic/catabolic processes | 5 | Pgls | Eno1 | Eno1 | Eno1 | ||
| Gapdh | Pkm2 | Ldhb | |||||
| Eno1 | Pkm2 | ||||||
| Pkm2 | |||||||
| Nucleobase, nucleoside, nucleotide, and nucleic acid biosynthetic processes | 7 | Atp5a1 | Aprt | Atp5a1 | Aprt | Nme2 | Aprt |
| Nme2 | Atp5a1 | Nme1 | Atp5a1 | ||||
| Nme1 | Impdh2 | Atp5h | |||||
| Nme2 | Impdh2 | ||||||
| Nme1 | |||||||
| Pnp | |||||||
| RNA and mRNA processing | 6 | Sfrs1 | Hnrnpk | Hnrnpc | |||
| Tardbp | Sfrs3 | ||||||
| Pcbp1 | |||||||
| Regulation of transcription | 7 | Ruvbl1 | Nup62 | Ube2n | Trim28 | ||
| Trim28 | Tardbp | Ruvbl1 | |||||
| Cbx3 | |||||||
| Hnrnpab | |||||||
| Embryonic development | 5 | Sfrs1 | Psmc4 | Atp5a1 | Eno1 | Myh9 | Eno1 |
| Eno1 | Eno1 | Myh9 | Atp5a1 | Psmc4 | |||
| Gene expression | 16 | Trim28 | Rps12 | Eif3f | Eef1a1 | Sfrs3 | |
| Sfrs1 | Eif3i | Eif5a | Eif3i | ||||
| Ruvbl1 | Eef1d | Hnrnpc | |||||
| Cbx3 | Ruvbl1 | ||||||
| Hnrnpk | Eef1d | ||||||
| Hnrnpab | Trim28 | ||||||
| Tardbp | |||||||
| Pcbp1 | |||||||
| Cell cycle processes | 6 | Ruvbl1 | Myh9 | Krt7 | Npm1 | ||
| Myh9 | Tubb5 | ||||||
| Stmn1 | |||||||
| Ruvbl1 | |||||||
| Cell morphogenesis involved in differentiation | 4 | Trim28 | Myh9 | Uchl1 | Myh9 | Stmn1 | |
| Hnrnpab | Trim28 | ||||||
| Regulation of cell proliferation | 4 | Nup62 | Nme2 | Nme2 | Npm1 | ||
| Pnp | |||||||
| Regulation of signal transduction | 4 | Nup62 | Ube2n | Npm1 | |||
| Hspa5 |
| k/CPX | ||
| ESC | maGSC | |
| Acp1 | 1.29 | 6.01 |
| Acy11 | 1.38 | 2.81 |
| Akr1b1 | 2.07 | 13.44 |
| Aprt1 | 4.80 | 3.60 |
| Atp5a11 | 3.50 | 1.21 |
| Capzb | 3.04 | 2.35 |
| Cbx31 | 1.72 | 2.12 |
| Cct21 | 12.00 | 1.28 |
| Cct51 | 1.06 | 2.02 |
| Eef1a11 | 2.47 | 1.74 |
| Eef1d1 | 1.46 | 2.03 |
| Eif5a1 | 1.31 | 2.07 |
| Eno11 | 3.56 | 1.60 |
| Fscn1 | 3.31 | 1.49 |
| Glod4 | 3.35 | 1.60 |
| Gnb2l1 | 2.61 | 12.92 |
| Hist1h2bb | 2.31 | 2.10 |
| Hist2h2ac | 17.33 | 67.90 |
| Hnrnpab1 | 2.41 | 3.36 |
| Hnrnpk1 | 2.00 | 1.17 |
| Hsp90aa11 | 1.19 | 6.79 |
| Hsp90aa11 | 1.84 | 3.02 |
| Hspa41 | 3.28 | 1.51 |
| Hspa4 | 1.13 | 3.14 |
| Hspa8 | 1.74 | 7.17 |
| Hspd1 | 1.67 | 3.07 |
| Impdh21 | 2.59 | 2.13 |
| Impdh21 | > 100 | 27.94 |
| Krt181 | 2.31 | 1.44 |
| Lgals2 | 2.82 | 8.45 |
| Ncl1 | 1.33 | 2.61 |
| Nit2 | 1.24 | 2.13 |
| Nme11 | 6.25 | 1.56 |
| Nme21 | 4.77 | 4.51 |
| Pcbp11 | 2.21 | 1.64 |
| Pkm21 | 3.75 | 3.27 |
| Pnp1 | 1.20 | 2.62 |
| Psmb41 | 1.01 | 2.32 |
| Ruvbl11 | 1.02 | 2.14 |
| Srm | 1.64 | 3.63 |
| Shmt11 | > 100 | > 100 |
| Tardbp1 | 1.38 | 3.52 |
| Tcp1 | 1.47 | 3.76 |
| Tuba1c1 | 1.87 | 3.11 |
| Tubb2c1 | 1.38 | 3.41 |
| Ube2n1 | 1.31 | 6.45 |
| Uchl11 | 2.66 | 1.66 |
| Label | RA/k | |
| ESC | maGSC | |
| Acp1 | 0.61 | 0.50 |
| Actb1 | 0.53 | 0.13 |
| Acy11 | 0.13 | 0.70 |
| Akr1b1 | 0.43 | 0.11 |
| Aprt1 | 0.46 | 0.39 |
| Atp5a11 | 0.76 | 0.38 |
| Atp5h1 | 0.69 | 0.40 |
| Cbx3 | 1.01 | 0.47 |
| Cotl11 | 0.50 | 0.44 |
| Eef1d1 | 0.70 | 0.15 |
| Eif3i1 | 0.09 | 0.92 |
| Eno11 | 0.24 | 0.04 |
| Eno11 | 0.55 | 0.22 |
| Fabp3 | 0.45 | |
| Fkbp41 | 0.90 | 0.40 |
| Glo11 | 0.74 | 0.41 |
| Glod4 | 0.82 | 0.30 |
| Impdh21 | 0.76 | 0.35 |
| Impdh21 | 0.54 | 0.20 |
| Gnb2l11 | 0.66 | 0.15 |
| Hnrnpc1 | 0.76 | 0.43 |
| Hsp90aa1 | 0.75 | 0.08 |
| Hsp90aa1 | 0.49 | 0.06 |
| Hsp90aa1 | 0.76 | 0.12 |
| Hspa51 | 0.32 | 0.22 |
| Hspa81 | 0.69 | 0.50 |
| Hspb11 | 0.36 | 0.47 |
| Hspb11 | 0.46 | 0.88 |
| Hspb11 | 0.90 | 0.41 |
| Hspd11 | 0.16 | 0.67 |
| Hspd11 | 0.34 | 0.95 |
| Itpa | 0.57 | 0.07 |
| Ldhb1 | 0.42 | 0.43 |
| Lgals2 | 0.29 | 0.03 |
| Ncl1 | 0.26 | 0.71 |
| Npm11 | 0.46 | 0.04 |
| Pebp11 | 0.89 | 0.42 |
| Pkm21 | 0.38 | 0.15 |
| Pkm21 | 0.32 | 0.65 |
| Pkm21 | 0.42 | 0.76 |
| Pkm21 | 0.21 | 0.43 |
| Psmb41 | 0.62 | 0.43 |
| Ruvbl11 | 0.63 | 0.22 |
| Sfrs31 | 0.41 | 0.46 |
| Shmt11 | 0.01 | 0.00 |
| Srm | 0.68 | 0.24 |
| Trim281 | 0.23 | 0.11 |
| Trim281 | 0.40 | 0.37 |
| Tuba1c1 | 0.27 | 0.71 |
| Tubb51 | 0.70 | 0.25 |
| Uqcrc11 | 0.24 | 0.22 |
| Vdac11 | 0.30 | 0.52 |
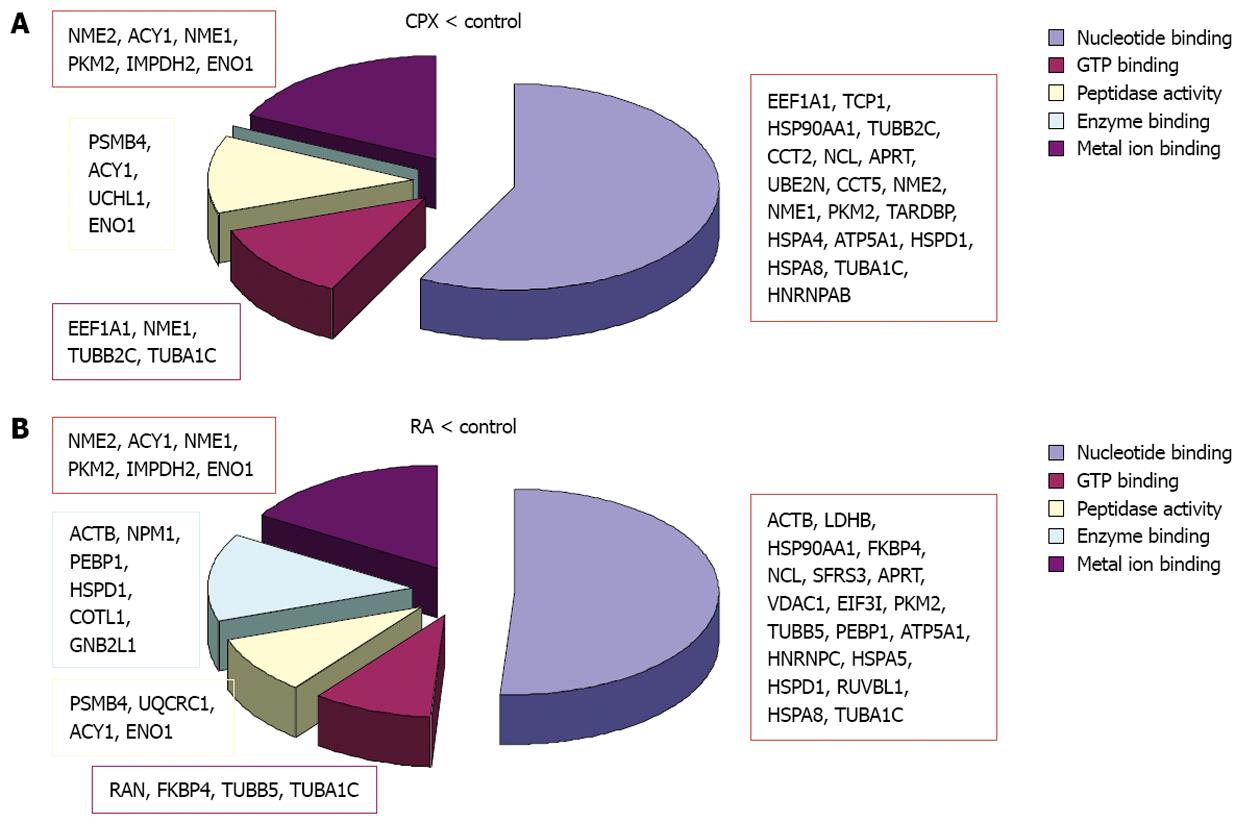
When we looked at the molecular function of the regulated proteins, we observed that a major part of the proteins are involved in nucleotide binding (Table 7). Approximately half of these proteins were downregulated and the other half was upregulated upon CPX treatment compared to RA treated cells. About 13 proteins are involved in metal ion binding, of these five proteins (Acy1, Uqcrc1, Sfrs1, Trim28, Glo1) are involved in transition metal ion binding, like Fe3+, which is known to be important in case of CPX, as CPX works as an inhibitor of the iron-dependent enzymes due to its role as a chelator of intracellular iron. Three of the proteins involved in transition metal ion binding (Sfrs1, Trim28 and Glo1), were up-regulated upon CPX treatment compared to RA-treated cells.
| Molecular function | Proteins | CPX > RA | CPX < RA | CPX > c | CPX < c | RA > c | RA < c |
| Nucleotide binding | 41 | Hsp90ab1 | Atp5b | Tubb2a | Cct2 | Cct2 | Ldhb |
| Fkbp4 | Cct2 | Etfa | Tardbp | Hspa4 | Fkbp4 | ||
| Tubb5 | Tardbp | Hspa9 | Hspa4 | Myh9 | Tubb5 | ||
| Hspa5 | Hspa4 | Actb | Tuba1c | Nme2 | Hnrnpc | ||
| Tubb1b | Actb | Myh9 | Hspa8 | Vcp | Hspa5 | ||
| Gapdh | Hsp90aa1 | Vdac1 | Hnrnpab | Psmc4 | Tuba1c | ||
| Etfa | Ncl | Atp5a1 | Eef1a1 | Nme1 | Hspa8 | ||
| Hspa9 | Aprt | Hspd1 | Tcp1 | Actb | |||
| Actb | Nme2 | Hsp90aa1 | Hsp90aa1 | ||||
| Hsp90aa1 | Vcp | Ncl | Ncl | ||||
| Sfrs1 | Psmc4 | Aprt | Sfrs3 | ||||
| Vdac1 | Nme1 | Ube2n | Aprt | ||||
| Pkm2 | Psmc2 | Nme2 | Vdac1 | ||||
| Atp5a1 | Cct5 | Pkm2 | |||||
| Ruvbl1 | Nme1 | Pebp1 | |||||
| Hspd1 | Pkm2 | Atp5a1 | |||||
| Atp5a1 | Ruvbl1 | ||||||
| Ruvbl1 | Hspd1 | ||||||
| Hspd1 | |||||||
| GTP binding | 8 | Fkbp4 | Nme1 | Tubb2a | Eef1a1 | Nme1 | Fkbp4 |
| Tubb5 | Nme1 | Tubb5 | |||||
| Tuba1b | Tuba1c | Tuba1c | |||||
| ATPase activity | 8 | Atp5a1 | Vcp | Atp5a1 | Atp5a1 | Vcp | Atp5a1 |
| Psmc4 | Myh9 | Hspa8 | Psmc4 | Atp5h | |||
| Atp5b | Myh9 | Hspa8 | |||||
| Psmc2 | |||||||
| Enzyme binding | 8 | Actb | Actb | Actb | Gnb2l1 | Vim | Actb |
| Npm1 | Gnb2l1 | Hspd1 | Pebp1 | ||||
| Hspd1 | Hspa9 | Hspd1 | |||||
| Cotl1 | Cotl1 | ||||||
| Hspa9 | |||||||
| Cofactor binding | 5 | Gapdh | Etfa | Shmt1 | Ldhb | ||
| Etfa | Shmt1 | ||||||
| Peptidase activity | 6 | Ctsd | Uchl1 | Ctsd | Psmb4 | Psmb4 | |
| Eno1 | Eno1 | Acy1 | Uqcrc1 | ||||
| Uchl1 | Acy1 | ||||||
| Eno1 | Eno1 | ||||||
| Metal ion binding | 13 | Trim28 | Atp5b | Acy1 | Pdia6 | Acy1 | |
| Sfrs1 | Pdia6 | Nme2 | Nme2 | Uqcrc1 | |||
| Pkm2 | Nme2 | Nme1 | Nme1 | Trim28 Pkm2 | |||
| Glo1 | Nme1 | Pkm2 | Glo1 | ||||
| Eno1 | Eno1 | Impdh2 | Impdh2 | ||||
| Eno1 | Eno1 |
Overall, it could be observed that most of the proteins of interest were downregulated in either CPX or RA treated cells compared to control.
About 56 of the 125 identified proteins showed different expression as a reaction to CPX treatment compared to control. Of these, 14 proteins were upregulated as a reaction to CPX treatment (Table 3), whereas 44 proteins were downregulated (Table 4). The expression of 52 proteins was found to be altered in both cell types, ESCs and maGSCs, under RA treatment compared to control (Tables 5 and 6). Of these proteins, 11 were upregulated and 41 were downregulated as a reaction to RA treatment.
In both experiments the majority of the regulated proteins were downregulated as a reaction to one of the treatments. Although mainly different proteins were regulated, bioinformatics analysis revealed that the downregulated proteins in both experiments are primarily involved in the same molecular functions (Figure 5). The downregulated proteins upon CPX treatment are mainly involved in nucleotide binding, GTP binding, peptidase activity and metal ion binding, particularly magnesium ion binding. The proteins which were downregulated upon RA treatment are involved in transition metal ion binding instead of magnesium ion binding, and furthermore involved in enzyme binding. Proteins, which were upregulated upon CPX treatment, are mainly involved in nucleotide binding, whereas proteins which were upregulated upon RA treatment are involved in nucleotide and metal ion binding.
When we look at the involvement of the regulated proteins in biological processes, more differences were observed (Figure 6). Both treatments showed downregulation of proteins involved in protein complex biogenesis, nucleotide biosynthetic process, cell death and positive regulation of biosynthetic process. Additionally, proteins involved in proteolysis and positive regulation of protein metabolic process were downregulated in SCs upon CPX treatment. Proteins which were downregulated in SCs upon RA treatment are, among others, involved in cell cycle, RNA processing, glycolysis and negative regulation of protein metabolic process.
Proteins which were upregulated in SCs upon CPX treatment are involved in nucleotide binding, regulation of cell death and protein transport, whereas proteins which were upregulated upon RA treatment are involved in nucleotide binding, metal ion binding and proteolysis.
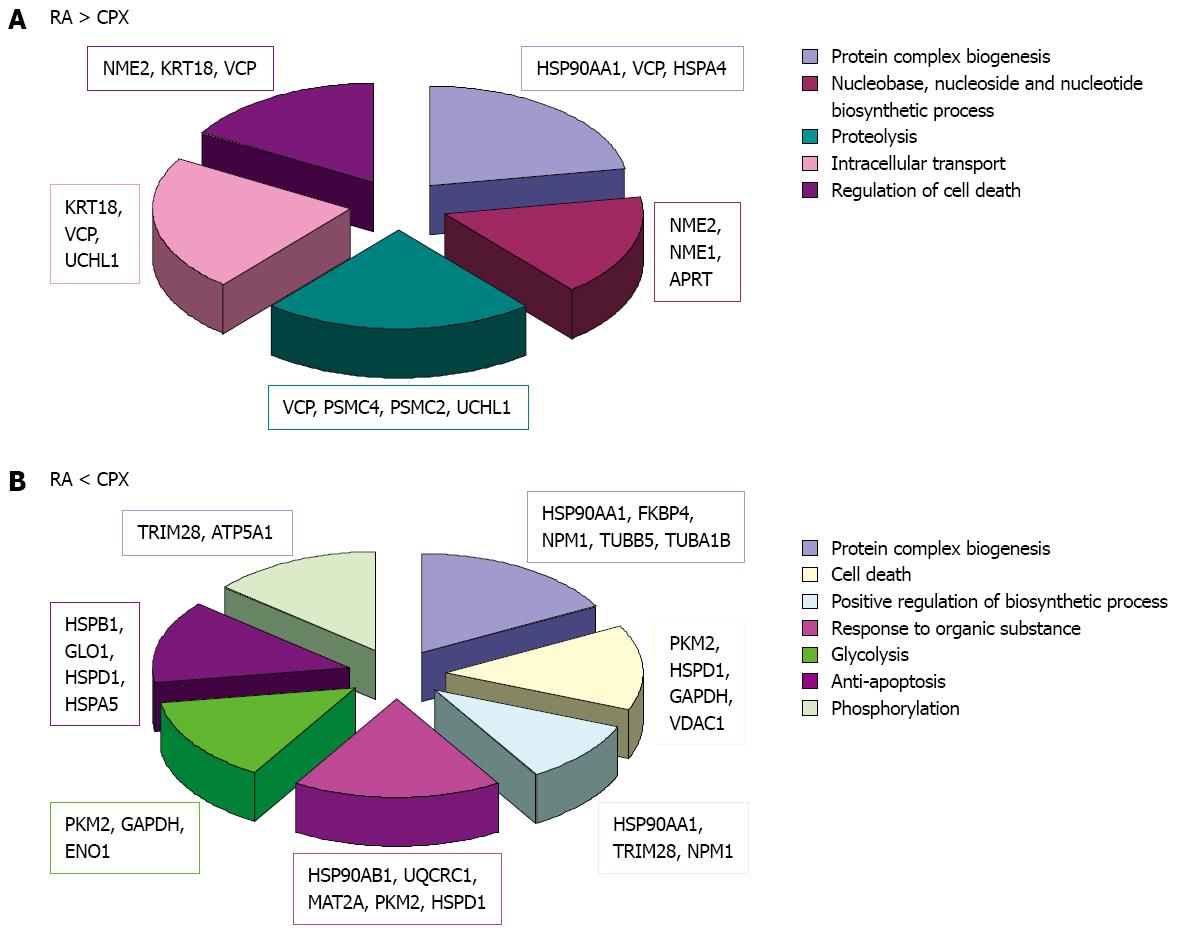
When the proteins in RA treated SCs were compared to CPX treated SCs, we observed that 54 proteins are differently regulated (Tables 8 and 9). Of these proteins, 31 were upregulated and 26 downregulated upon CPX treatment. In some cases, different forms of one protein, e.g., Actb, Eno1, and Hsp90aa1 were observed and showed different regulation.
| RA/CPX | ||
| ESC | maGSC | |
| Actb*1 | 0.12 | 0.10 |
| Actb1 | 0.14 | 0.15 |
| Atp5a11 | 0.43 | 0.48 |
| Cotl1 | 0.19 | 0.67 |
| Ctsd | 0.95 | 0.16 |
| Eif3i | 0.04 | 0.87 |
| Eno1*1 | 0.13 | 0.03 |
| Eno11 | 0.57 | 0.40 |
| Etfa | 0.68 | 0.16 |
| Fkbp41 | 0.46 | 0.43 |
| Gapdh1 | 0.35 | 0.59 |
| Glo1 | 0.31 | 0.66 |
| Glod4 | 0.85 | 0.36 |
| Hsp90aa1 | 0.28 | 0.38 |
| Hsp90aa1 | 0.43 | 0.13 |
| Hsp90ab1 | 0.41 | 0.26 |
| Hspa5*1 | 0.17 | 0.13 |
| Hspa9*1 | 0.50 | 0.28 |
| Hspb1*1 | 0.29 | 0.04 |
| Hspb1*1 | 0.20 | 0.42 |
| Hspb11 | 0.82 | 0.50 |
| Hspd11 | 0.49 | 0.74 |
| Hspd11 | 0.42 | 0.33 |
| Itpa | 0.21 | 0.08 |
| Mat2a1 | 0.41 | 0.14 |
| Npm11 | 0.28 | 0.18 |
| Nup62 | 0.57 | 0.26 |
| Pgls1 | 0.40 | 0.68 |
| Pkm21 | 0.22 | 0.25 |
| Pkm21 | 0.29 | 0.86 |
| Prdx6 | 0.46 | 0.94 |
| Ruvbl11 | 0.64 | 0.42 |
| S100a11*1 | 2 | 0.17 |
| Sfrs11 | 0.70 | 0.30 |
| Trim281 | 0.44 | 0.08 |
| Trim281 | 0.42 | 0.44 |
| Trim281 | 0.33 | 0.28 |
| Tuba1b*1 | 0.42 | 0.65 |
| Tubb51 | 0.42 | 0.54 |
| Tubb51 | 0.43 | 0.58 |
| Vdac1*1 | 0.17 | 0.09 |
| RA/CPX | ||
| ESC | maGSC | |
| Actb1 | 2.17 | 1.10 |
| Aldh2 | 2.61 | 2.17 |
| Aldh2 | 2.43 | 1.21 |
| Aprt1 | 2.20 | 1.42 |
| Atp5b | 1.15 | 2.17 |
| Capzb | 2.79 | 2.07 |
| Cct2*1 | 13.70 | 2.77 |
| Eno11 | 2.48 | 2.02 |
| Eno11 | 2.71 | 1.51 |
| Fscn1 | 2.20 | 2.14 |
| Gnb2l1 | 1.81 | 2.24 |
| Hist1h2bb | 7.53 | 1.62 |
| Hist2h2ac | 3.89 | 211.81 |
| Hnrnpk | 2.37 | 1.58 |
| Hsp90aa1*1 | 2.69 | 6.36 |
| Hsp90aa1 | 6.36 | 4.33 |
| Hspa4*1 | 12.98 | 3.72 |
| Hspa4 | 1.35 | 3.42 |
| Krt7 | > 100 | 1.14 |
| Krt18*1 | 4.04 | 1.76 |
| Ncl | 1.80 | 3.48 |
| Nme11 | 2.63 | 1.57 |
| Nme2*1 | 5.72 | 11.15 |
| Pdia6 | 1.74 | 6.33 |
| Psmc21 | 2.87 | 1.20 |
| Psmc4*1 | 3.06 | 2.26 |
| Rps12*1 | 2 | 2.05 |
| Tardbp | 1.11 | 3.85 |
| Uchl11 | 2.02 | 1.10 |
| Vcp1 | 8.94 | 2.57 |
The bioinformatics analysis of these proteins, focussing on biological processes, showed involvement of the proteins in different categories (Figure 7). Proteins which were downregulated in CPX treated cells are involved in processes like protein complex biogenesis, nucleotide biosynthetic process, proteolysis, intracellular transport and regulation of cell death. Proteins which were downregulated as a reaction to RA treatment are involved in protein complex biogenesis, cell death, positive regulation of biosynthetic process, response to organic substance, glycolysis, anti-apoptosis and phosphorylation.
To get a better focus on proteins, which may play a key role in proliferation, we also focussed on proteins, which showed contrary regulation upon CPX treatment and RA treatment compared to control. This resulted in 15 proteins, of which eight were upregulated upon CPX treatment and concurrently downregulated upon RA treatment compared to control, and seven proteins, which were downregulated upon CPX treatment and concurrently upregulated upon RA treatment compared to control (proteins marked by asterisk in Tables 8 and 9).
Bioinformatics analysis of the proteins, which were downregulated upon CPX treatment along with upregulated upon RA treatment were primarily involved in metabolic processes (Nme2, Hsp90aa1, Psmc4, Rps12, Cct2 and Eno1) like protein folding (Hsp90aa1, Cct2), whereas proteins, which were upregulated upon CPX-treatment and concurrently downregulated upon RA-treatment were additionally involved in developmental processes (Psmc4, Eno1) and transport/localization (Vdac1, Hspa9).
Analysis of the molecular function of the differently regulated proteins upon CPX and RA treatment showed their important role in nucleotide binding (Nme2, Hsp90aa1, Psmc4, Hspa4, Cct2, Actb, Pkm2, Hspa5, Vdac1 and Hspa9) and metal ion binding (Pkm2, S100a11, Eno1).
CPX is a synthetic antifungal drug, which is currently used for the treatment of superficial mycoses[41]. Since two decades CPX has also been used as an antitumor agent[42]. It has been shown that CPX can be used to treat solid tumors due to its strong antiangiogenic activity[43,23]. CPX might inhibit the cell proliferation and work as an antitumor agent due to its iron chelating function, as iron is essential for cell proliferation and function[24]. In a recent study, we investigated the effect of CPX on the cellular viability and proliferation of SCs. The study demonstrated that in contrast to RA, CPX treatment resulted in a reversible antiproliferative effect[8]. The present study was conducted to understand the anti-proliferative effect of CPX on stem cells in terms of proteins and molecular processes which are involved in its mode of action.
With proteomic analysis of ESCs and maGSCs treated with CPX and RA, we could identify more than 90 single proteins which were differently expressed in both cell lines. Bioinformatics analysis of the regulated proteins demonstrated their involvement in various biological processes. To our interest, a number of proteins have potential roles in the regulation of cell proliferation either directly or indirectly.
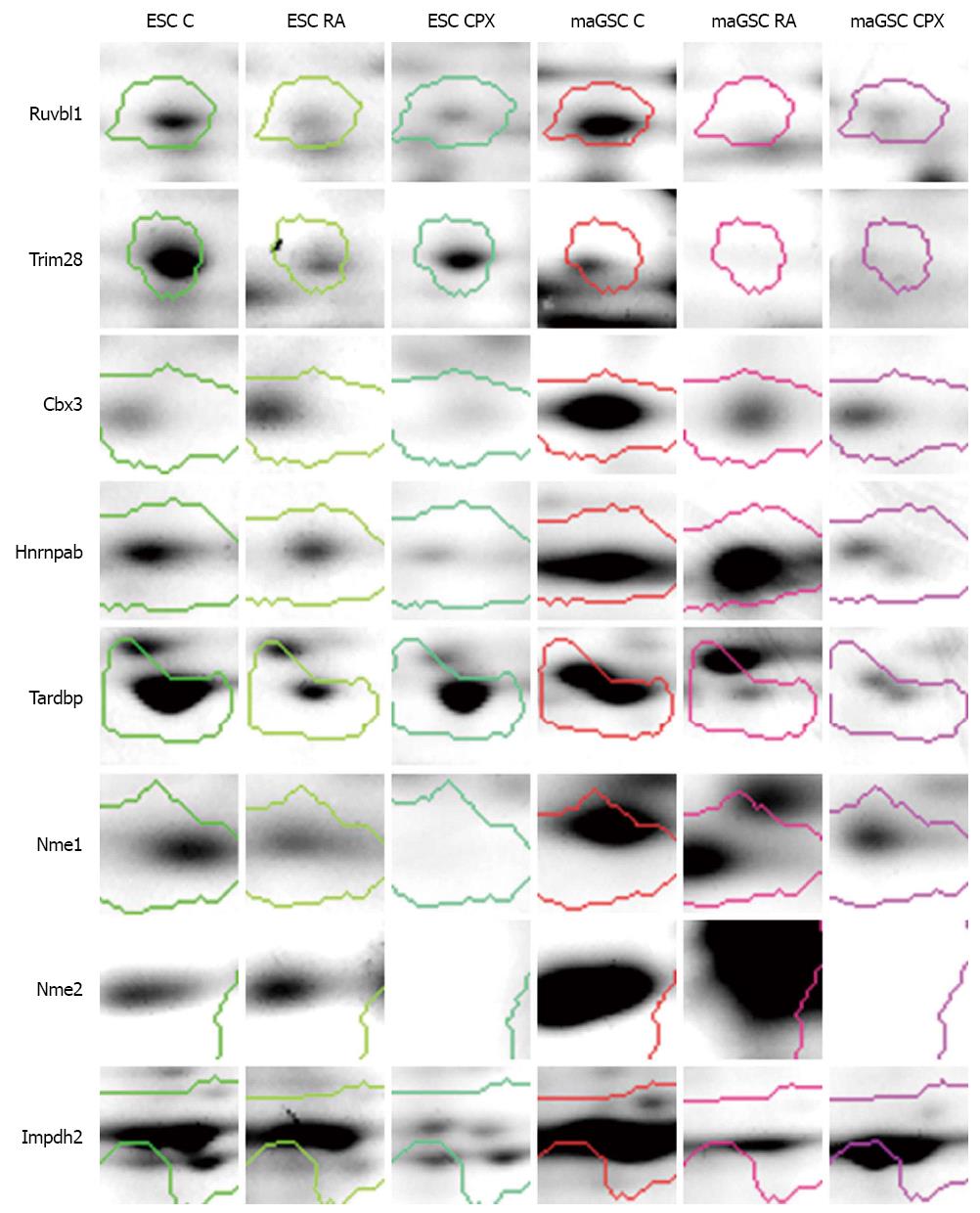
One of the possible mechanisms of CPX action on cell proliferation is through controlling the progression of the cell cycle[44]. We identified a number of proteins which are involved in cell cycle processes. Ruvbl1 is one of the differentially regulated proteins which is involved in cell cycle processes, gene expression and transcription regulation. It was found to be downregulated in CPX and RA treated cells compared to control (Figure 8). Ruvbl1 is an evolutionarily highly conserved eukaryotic protein belonging to the AAA+ family of ATPase’s[45]. It plays an important role in various cell cycle processes such as chromatin remodeling[46], gene activation[47], transcriptional regulation, DNA repair and transcription factor c-Myc[48]. It also controls Wnt signaling pathway through transcription-associated protein β-catenin[49,50]. Another protein, which was higher expressed in CPX treated cells compared to RA treated cells, is Trim28. Trim28 is involved in regulation of transcription and silencing gene expression through its ability to bind to DNA through interaction with a KRAB-ZFP protein. Other proteins, like Cbx3, Tardbp, and Hnrnpab, which are important in gene expression and regulation of transcription, were downregulated due to treatment with CPX. Tardpb is a DNA and RNA-binding protein, which regulates transcription and splicing. It is also involved in the regulation of CFTR (Cystic fibrosis transmembrane conductance regulator), microRNA biogenesis, apoptosis and cell division. It can repress HIV-1 transcription by binding to the HIV-1 long terminal repeat. Cbx3 seems to be involved in transcriptional silencing in heterochromatin-like complexes. It recognizes and binds histone H3 tails methylated at K9, which leads to epigenetic repression. It is suggested that these proteins, which are involved in cell cycle processes, transcription regulation and gene expression, might be potential candidates for cell proliferation regulation and their repression through down-regulation might result in cell cycle stop without impact on stem cell pluripotency.
Proteins, which are involved in nucleotide biosynthetic process and proteolysis, were downregulated in CPX treated cells compared to control, as well as in RA treated cells (Figures 6A and 7A). Nucleoside diphosphatase kinases A and B (Nme1 and Nme2) are some of the proteins which are involved in nucleotide biosynthetic process. These proteins are known to be involved in the synthesis of nucleoside triphosphatases[51] as well as in cell proliferation[52], differentiation[53] and development[54], signal transduction, G protein-coupled receptor endocytosis and gene expression. Nme1 was downregulated in CPX treated cells compared to control and RA treated cells (Figure 8). This may explain the slowdown of the proliferation of CPX treated SCs. Impdh2 is a rate limiting enzyme in the de novo synthesis of guanine nucleotides and is therefore involved in the regulation of cell growth and differentiation[55-58]. It may have a role in the development of malignancy and the growth progression of some tumors. Impdh2 was downregulated in CPX treated cells compared to control (Figure 8).
Proteins which were involved in cell death, positive regulation of biosynthetic process, response to organic substance, glycolysis, anti-apoptosis, and phosphorylation were downregulated in RA treated cells compared to control and CPX treated cells (Figures 6B and 7B).
Analysis of the molecular function of the differently expressed proteins demonstrated a potential involvement of some of these in metal ion binding, mainly iron binding. Cazzola et al[59] in 1990 established that iron is essential for proliferation, DNA synthesis and repair and mitochondrial electron transport. Therefore, it is assumed that CPX can stop the cell proliferation by regulating the expression of iron binding proteins.
The present study could give some insights into the mode of action of CPX in terms of expression regulation of various proteins. It not only shed light on the previously discussed roles of CPX, but could also provide some further insight into their mechanism. We could identify some potential candidates which can effect the cell proliferation directly or indirectly through other cellular processes. By understanding the mode of action of CPX, this study may provide new aspect that will help in the future strategy to improve therapeutic intervention in the treatment with CPX.
We thank Elke Brunst-Knoblich for technical assistance.
Ciclopirox olamine (CPX), a synthetic antifungal agent used in the treatment of fungal and yeast infection of skin or mucosa. Apart from its antimycotic activity, CPX is also effective against both gram-positive and gram-negative bacteria. CPX might also serve as an alternative agent for therapeutic angiogenesis. CPX was also shown to have an antiproliferative effect on stem cells without affecting their pluripotency.
Although CPX is used as therapeutic for different aspect the mechanism of action is still not clear. In this study, the authors investigated the impact of CPX on stem cell proteome and identified cellular mechanisms that may explain the way of action of CPX. The authors provided evidence that CPX is involved in expression regulation of nucleotide binding proteins resulting in cell cycle arrest.
It is postulated that the CPX works as an inhibitor of the iron-dependent enzymes due to its potential role as a chelator of intracellular iron. The present study could give some insights into the mode of action of CPX in terms of expression regulation of various proteins especially nucleotide-binding proteins. It not only shed light on the previously discussed roles of CPX, but could also provide some further insight into their mechanism. We could also identify some potential candidates, which can effect the cell proliferation directly or indirectly through other cellular processes.
By understanding the mode of action of CPX, this study may provide new aspects that will be helpful in the future strategy for therapeutic intervention in the treatment with CPX.
Multipotent adult germline stem cells (maGSCs) are spermatogonial stem cells isolated from murine testis. CPX, the ethanolamine salt of 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one, is a synthetic antifungal agent and is a hypusination inhibitor that controls the second step of the modification, which is catalyzed by deoxyhypusine hydroxylase. The hypusine is the result of a post-translational modification catalyzed by two enzymes: deoxyhypusine synthase and deoxyhypusine hydroxylase.
This is a descriptive study in which the authors analyzed the proteome changes of embryonic stem cells and maGSCs accompanying the treatment with CPX and subsequent inhibition of hypusination using classical proteomic techniques like 2-DE, differential in-gel electrophoresis and mass spectrometry. The results are interesting and we could highlight that a treatment with CPX resulted in an alteration of the expression of 56 proteins compared to non-treated cells, and 54 proteins compared to retinoic acid treated cells. The majority of these proteins are involved in nucleotide binding and nucleotide biosynthetic processes, metal binding, DNA binding, and other processes which have been linked to CPX.
P- Reviewers Marchal JA, Tanabe S, Zaminy A S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 539] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Stem cells. Lancet. 2005;366:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 982] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 4. | Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Zovoilis A, Nolte J, Drusenheimer N, Zechner U, Hada H, Guan K, Hasenfuss G, Nayernia K, Engel W. Multipotent adult germline stem cells and embryonic stem cells have similar microRNA profiles. Mol Hum Reprod. 2008;14:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Meyer S, Nolte J, Opitz L, Salinas-Riester G, Engel W. Pluripotent embryonic stem cells and multipotent adult germline stem cells reveal similar transcriptomes including pluripotency-related genes. Mol Hum Reprod. 2010;16:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Dihazi H, Dihazi GH, Nolte J, Meyer S, Jahn O, Müller GA, Engel W. Multipotent adult germline stem cells and embryonic stem cells: comparative proteomic approach. J Proteome Res. 2009;8:5497-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Dihazi H, Dihazi GH, Jahn O, Meyer S, Nolte J, Asif AR, Mueller GA, Engel W. Multipotent adult germline stem cells and embryonic stem cells functional proteomics revealed an important role of eukaryotic initiation factor 5A (Eif5a) in stem cell differentiation. J Proteome Res. 2011;10:1962-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105-3114. [PubMed] |
| 10. | Park MH, Lee YB, Joe YA. Hypusine is essential for eukaryotic cell proliferation. Biol Signals. 1997;6:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Jasiulionis MG, Luchessi AD, Moreira AG, Souza PP, Suenaga AP, Correa M, Costa CA, Curi R, Costa-Neto CM. Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination impairs melanoma growth. Cell Biochem Funct. 2007;25:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Jakus J, Wolff EC, Park MH, Folk JE. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268:13151-13159. [PubMed] |
| 13. | Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, Brassat U, Priemer M, Hauber I, Wilhelm T. Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood. 2007;109:1701-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Park MH, Wolff EC, Smit-McBride Z, Hershey JW, Folk JE. Comparison of the activities of variant forms of eIF-4D. The requirement for hypusine or deoxyhypusine. J Biol Chem. 1991;266:7988-7994. [PubMed] |
| 16. | Dittmar W, Lohaus G. HOE 296, a new antimycotic compound with a broad antimicrobial spectrum. Laboratory results. Arzneimittelforschung. 1973;23:670-674. [PubMed] |
| 17. | Jue SG, Dawson GW, Brogden RN. Ciclopirox olamine 1% cream. A preliminary review of its antimicrobial activity and therapeutic use. Drugs. 1985;29:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Abrams BB, Hänel H, Hoehler T. Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol. 1991;9:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Subissi A, Monti D, Togni G, Mailland F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70:2133-2152. [PubMed] |
| 20. | Dittmar W, Grau W, Raether W, Schrinner E, Wagner WH. [Microbiological laboratory studies with ciclopiroxolamine (author’s transl)]. Arzneimittelforschung. 1981;31:1317-1322. [PubMed] |
| 21. | Linden T, Katschinski DM, Eckhardt K, Scheid A, Pagel H, Wenger RH. The antimycotic ciclopirox olamine induces HIF-1alpha stability, VEGF expression, and angiogenesis. FASEB J. 2003;17:761-763. [PubMed] |
| 22. | Leem SH, Park JE, Kim IS, Chae JY, Sugino A, Sunwoo Y. The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae. Mol Cells. 2003;15:55-61. [PubMed] |
| 23. | Eberhard Y, McDermott SP, Wang X, Gronda M, Venugopal A, Wood TE, Hurren R, Datti A, Batey RA, Wrana J. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood. 2009;114:3064-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Zhou H, Shen T, Luo Y, Liu L, Chen W, Xu B, Han X, Pang J, Rivera CA, Huang S. The antitumor activity of the fungicide ciclopirox. Int J Cancer. 2010;127:2467-2477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Weir SJ, Patton L, Castle K, Rajewski L, Kasper J, Schimmer AD. The repositioning of the anti-fungal agent ciclopirox olamine as a novel therapeutic agent for the treatment of haematologic malignancy. J Clin Pharm Ther. 2011;36:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D’Alliessi Gandolfi D, Park MH, Pe’ery T, Mathews MB. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Parreiras-e-Silva LT, Luchessi AD, Reis RI, Oliver C, Jamur MC, Ramos RG, Oliveira EB, Curi R, Costa-Neto CM. Evidences of a role for eukaryotic translation initiation factor 5A (eIF5A) in mouse embryogenesis and cell differentiation. J Cell Physiol. 2010;225:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, Dong S, Guan XY, Poon RT, Luk JM. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127:968-976. [PubMed] |
| 29. | Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424-8428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1832] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 31. | Bächner D, Manca A, Steinbach P, Wöhrle D, Just W, Vogel W, Hameister H, Poustka A. Enhanced expression of the murine FMR1 gene during germ cell proliferation suggests a special function in both the male and the female gonad. Hum Mol Genet. 1993;2:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Cheng J, Dutra A, Takesono A, Garrett-Beal L, Schwartzberg PL. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis. 2004;39:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3088] [Cited by in RCA: 3187] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 34. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157317] [Article Influence: 3210.6] [Reference Citation Analysis (0)] |
| 35. | Karp NA, Griffin JL, Lilley KS. Application of partial least squares discriminant analysis to two-dimensional difference gel studies in expression proteomics. Proteomics. 2005;5:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Jahn O, Hesse D, Reinelt M, Kratzin HD. Technical innovations for the automated identification of gel-separated proteins by MALDI-TOF mass spectrometry. Anal Bioanal Chem. 2006;386:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, Orfaniotou F, Dhaunchak A, Brinkmann BG, Möbius W. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci. 2007;27:7717-7730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Reumann S, Babujee L, Ma C, Wienkoop S, Siemsen T, Antonicelli GE, Rasche N, Lüder F, Weckwerth W, Jahn O. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways, and defense mechanisms. Plant Cell. 2007;19:3170-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 39. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [PubMed] |
| 40. | Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10350] [Cited by in RCA: 11164] [Article Influence: 656.7] [Reference Citation Analysis (0)] |
| 41. | Sehgal VN. Ciclopirox: a new topical pyrodonium antimycotic agent. A double-blind study in superficial dermatomycoses. Br J Dermatol. 1976;95:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Bohn M, Kraemer KT. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol. 2000;43:S57-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer. 2002;100:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2982] [Cited by in RCA: 2821] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 45. | Neuwald C, Heinschink A, Müller MM. Evaluation of a new screening assay, protein C pathway (PCP) test, for identification of defects in the protein C pathway. Thromb Haemost. 1999;81:992-994. [PubMed] |
| 46. | Jónsson ZO, Jha S, Wohlschlegel JA, Dutta A. Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol Cell. 2004;16:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Bauer A, Chauvet S, Huber O, Usseglio F, Rothbächer U, Aragnol D, Kemler R, Pradel J. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 2000;19:6121-6130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Wood LJ, Mukherjee M, Dolde CE, Xu Y, Maher JF, Bunton TE, Williams JB, Resar LM. HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol. 2000;20:5490-5502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1180] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 50. | Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606-1609. [PubMed] |
| 51. | Parks RE, Brown PR, Cheng YC, Agarwal KC, Kong CM, Agarwal RP, Parks CC. Purine metabolism in primitive erythrocytes. Comp Biochem Physiol B. 1973;45:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Cipollini G, Berti A, Fiore L, Rainaldi G, Basolo F, Merlo G, Bevilacqua G, Caligo MA. Down-regulation of the nm23.h1 gene inhibits cell proliferation. Int J Cancer. 1997;73:297-302. [PubMed] |
| 53. | Rosengard AM, Krutzsch HC, Shearn A, Biggs JR, Barker E, Margulies IM, King CR, Liotta LA, Steeg PS. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989;342:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 393] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | Lakso M, Steeg PS, Westphal H. Embryonic expression of nm23 during mouse organogenesis. Cell Growth Differ. 1992;3:873-879. [PubMed] |
| 55. | Knight RD, Mangum J, Lucas DL, Cooney DA, Khan EC, Wright DG. Inosine monophosphate dehydrogenase and myeloid cell maturation. Blood. 1987;69:634-639. [PubMed] |
| 56. | Itoh O, Kuroiwa S, Atsumi S, Umezawa K, Takeuchi T, Hori M. Induction by the guanosine analogue oxanosine of reversion toward the normal phenotype of K-ras-transformed rat kidney cells. Cancer Res. 1989;49:996-1000. [PubMed] |
| 57. | Jackson RC, Weber G, Morris HP. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975;256:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 290] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Zimmermann A, Gu JJ, Spychala J, Mitchell BS. Inosine monophosphate dehydrogenase expression: transcriptional regulation of the type I and type II genes. Adv Enzyme Regul. 1996;36:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |