Published online May 26, 2012. doi: 10.4252/wjsc.v4.i5.35
Revised: November 8, 2011
Accepted: November 15, 2011
Published online: May 26, 2012
AIM: To compare the efficacy of cell-free derivatives from Bone marrow derived human mesenchymal stem cells (hMSCs) in wound therapy.
METHODS: hMSCs have been shown to play an important role in wound therapy. The present study sought to compare efficacy of hMSCs and cell-free derivatives of hMSCs, which may be clinically more relevant as they are easier to prepare, formulate and transport. hMSCs were isolated from human bone marrow and cultured. Multi lineage differentiation of hMSCs was performed to confirm their identity. The ability of hMSCs to migrate was evaluated using in vitro and in vivo migration assays. Cell lysates and conditioned medium concentrate was prepared from hMSCs (see Methods for details). Wounds were induced in mice and wound areas were measure before and after cell and cell-free derivative treatment. RNA and proteins were extracted from the skin and cytokine levels were measured.
RESULTS: Co-culture of hMSCs with keratinocytes resulted in increased expression of CXCL-12 (SDF1) and ENA78 (CXCL-5) in the conditioned media indicating that the hMSCs can respond to signals from keratinocytes. Accelerated wound closure was observed when hMSCs were injected near the site of excisional wounds in athymic as well as NOD/SCID mice. Interestingly, cell-free lysates prepared from hMSCs were also effective in inducing accelerated wound closure and increased expression of SDF1 and CXCL-5 at the wound bed. Additionally, concentrated media from hMSCs as well as an emulsion containing lysates prepared from hMSCs was also found to be more effective in rapid re-epithelialization than fibroblasts or vehicle-alone control. Use of cell-free derivatives may help replace expensive wound care approaches including use of growth factors, epidermal/dermal substitutes, synthetic membranes, cytokines, and matrix components, and most importantly avoid transmission of pathogens from human and animal products.
CONCLUSION: These results encourage development of derivatives of hMSCs for wound care and re-epithelialization applications.
- Citation: Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells 2012; 4(5): 35-43
- URL: https://www.wjgnet.com/1948-0210/full/v4/i5/35.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i5.35
Wound healing is a coordinated process comprising an inflammatory reaction, a proliferative process leading to tissue restoration, angiogenesis and formation of extracellular matrix accompanied by scar tissue remodeling. Cellular participants as well as multiple growth factors and cytokines released by the cells at the wound site regulate these processes and finally result in wound closure. Deregulated healing processes may delay repair and may eventually lead to chronic wounds, such as those observed in diabetics, that are expensive and difficult to heal and may also result in excessive fibrosis, which leads to keloid formation[1-8]. Treatment of chronic wounds remains difficult despite increased understanding of underlying biological principles, significant developments including use of recombinant growth factors, bioengineered skin equivalents and overall improvement in standards of wound care[9-15]. Clinical trials using bone marrow derived mesenchymal stem cells in myocardial infarctions and graft vs host disease have recently been launched[16-18], and encourage investigation of bone marrow derived (BMD)-human mesenchymal stem cells (hMSCs) for use in other areas of regenerative medicine including chronic wound healing.
The bone marrow harbors two major types of stem cells, the hematopoietic stem cell and mesenchymal stromal/stem cell, termed BMD-hMSCs. HMSCs give rise to cells of muscle, bone, fat, and cartilage lineage[19,20]. Like true stem cells, hMSCs have the capacity for self-renewal and differentiation, and hold promise for clinical applications in regenerative medicine[21-24]. hMSCs migrate to various locations, including sites of hematopoiesis such as the bone marrow, sites of inflammation and sites of injury, suggesting that they may play a role in the recovery process following injury. We and others have demonstrated that bone marrow derived hMSCs differentiate into myofibroblast like cells[25]. In wound healing, myofibroblasts are responsible for generation of mechanical forces that allow proper granulation, tissue contraction and wound healing[26,27]. Matrix contraction depends on both α-smooth muscle actin (α-SMA) expression within cellular stress fibers, and assembly of large focal adhesions linking myofibroblasts to the matrix[28]. It is possible that local induction of myofibroblasts using either hMSCs or cell-free derivatives obtained from hMSCs may accelerate wound healing. Previous reports have demonstrated efficacy of murine bone marrow derived hMSCs in vitro and in vivo models including models of chronic wounds[23-36]. Herein, we present evidence that human BMD-hMSCs and their cell-free derivatives such as lysates and concentrated conditioned medium are effective in wound healing process.
Unprocessed bone marrow (36 × 106 cells/ml) was purchased from Lonza (Walkersville, MD). A Ficoll gradient was used for separation of peripheral blood mononuclear cells (PBMNCs). Isolated PBMNCs were plated in T75 cm2 tissue culture flasks with MesenCult media (Stem Cell Technologies, Vancouver) containing hMSC stimulatory supplements and fetal bovine serum (FBS) for hMSCs. Once cultures were established, several clones were isolated and expanded in culture in the same medium. Established cultures were grown in minimum essential media (α-MEM) containing 10% FBS and penicillin/streptomycin. The cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Cells were subcultured every 4 to 5 d and aliquots from passage 2 to 8 were frozen in liquid nitrogen for use. Cell surface markers expressed on these cells were determined by flow cytometry using FITC labeled Abs (BD Biosciences, San Jose, CA) and included Stro1, CD105, CD90, HLA-ABC and CD44 while they were negative for CD45, and CD11b[25].
Several of the passages from 2 to 8 were tested for their ability to differentiate in culture, cytokine production and migration towards keratinocytes and were found to be comparable (data not shown). For the sake of consistency, passage 5 cells were used for all studies reported here.
Expanded cultures of hMSCs were analyzed for myogenic, osteogenic and adipogenic differentiation in vitro to determine multipotency according to standard conditions as described before[19,25].
Migration assays were carried out as described previously[25]. Briefly, Falcon tissue culture plates with 24 wells along with Falcon cell culture inserts were used for the migration assay. Conditioned media (CM) from keratinocytes (collected after overnight culture in fresh growth medium) or keratinocytes (1 × 104) were plated in the bottom chamber and incubated overnight at 37 °C and 5% CO2. Next day, the insert was placed aseptically in the well with flanges resting in the notches on the top edge of each well. Naive hMSCs (2 × 104) were plated on the top chamber. Following overnight incubation (18h) the assay was terminated and hMSCs that had migrated through the membrane (8 μm pore size) were stained (after removal of cells remaining on top with a wet Q-tip) using crystal violet prepared in methanol and formaldehyde.
Fluorescent dye (CFDASE) labeled 5 × 105 hMSCs were transplanted locally at the periphery of wounded skin subcutaneously. Saline (100 μL) was injected subcutaneously near the wounds as a control. After 48 h wound areas were excised and immediately snap frozen using 2-methylbutane and liquid nitrogen in OCT cryo embedding compound. Thin sections were cut and placed onto glass slides for staining with DAPI and fluorescence microscopy.
The hMSC cell lysates were prepared using 5 × 106 cells for each animal. Cells grown in cultures were washed in phosphate buffer saline (PBS) collected by brief trypsinization and centrifugation in an Eppendorf centrifuge (5000 r/min 30 s) to collect the cell pellet. Cell pellet (in a 0.5 mL Eppendorf centrifuge tube placed in ice), re-suspended in ice cold PBS, was sonicated using six bursts for 30 s at half max setting (Vibra Cell, Sonics and Materials, Danbury, CT). The sonicated cells were centrifuged at 10 000 r/min for 1 min. The lysate (supernatant) was injected (100 μL) once near excision an wound in the nu/nu (n = 5) and NOD/SCID (n = 8) mice. Lysate was evaluated to ensure absence of viable cells by plating it in the tissue culture plate containing complete growth medium. The lysate was also analyzed for cytokine content by multiplex and Elisa as described later.
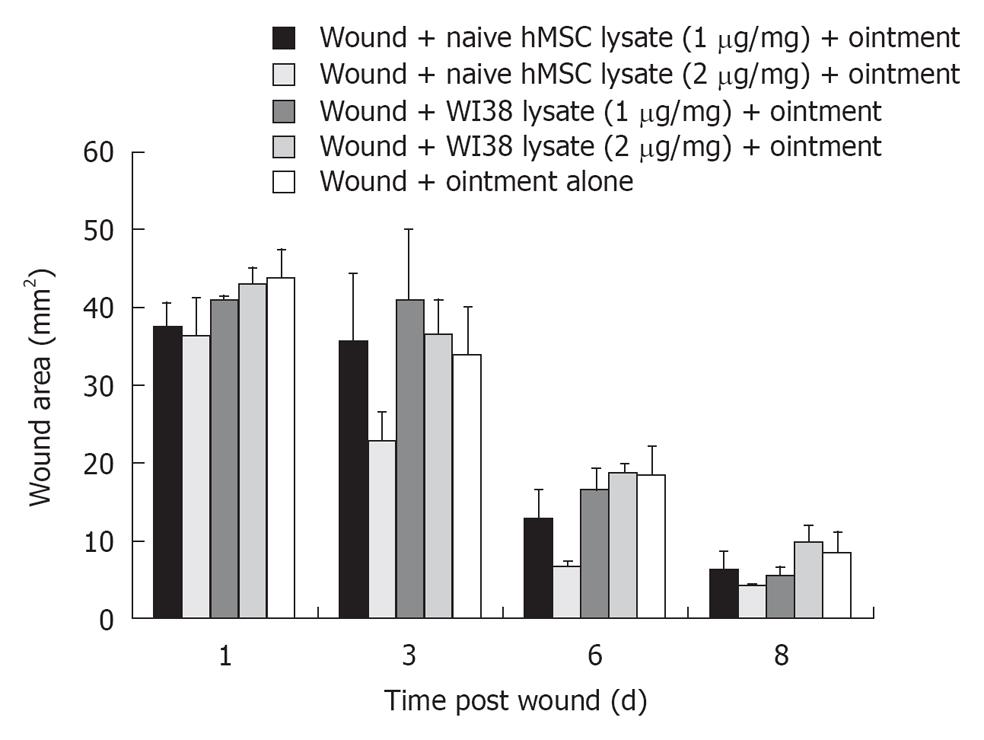
For topical application, protein concentration in the hMSC cell lysate was detected using standard Bradford method. Cell lysate (1-2 μg total protein/mg) was admixed with mineral oil based hydrophilic ointment (Fougera; NY) and applied as an emulsion on the wound surface.
HMSCs were cultured to 60%-70% confluence under standard culture conditions as described earlier. Conditioned medium from hMSCs (5 flasks per experiment with 2 × 107 cells per flask) was collected and further concentrated (50 times) by Amicon ultra centrifugal filter unit with approximately 5 kDa cut-off (Amicon Ultra-15; Millipore, MA) following manufacturer’s instructions. Conditioned medium concentrate (100 μL final concentrate resulting from 5 flasks of hMSC culture medium) from hMSC [hMSC (CMC)] was injected once in the periphery of each wound. Saline (100 μL) was injected in the periphery of wounds as a control and served as the naturally healing group. An aliquot was also analyzed for cytokine content by multiplex and Elisa as described later.
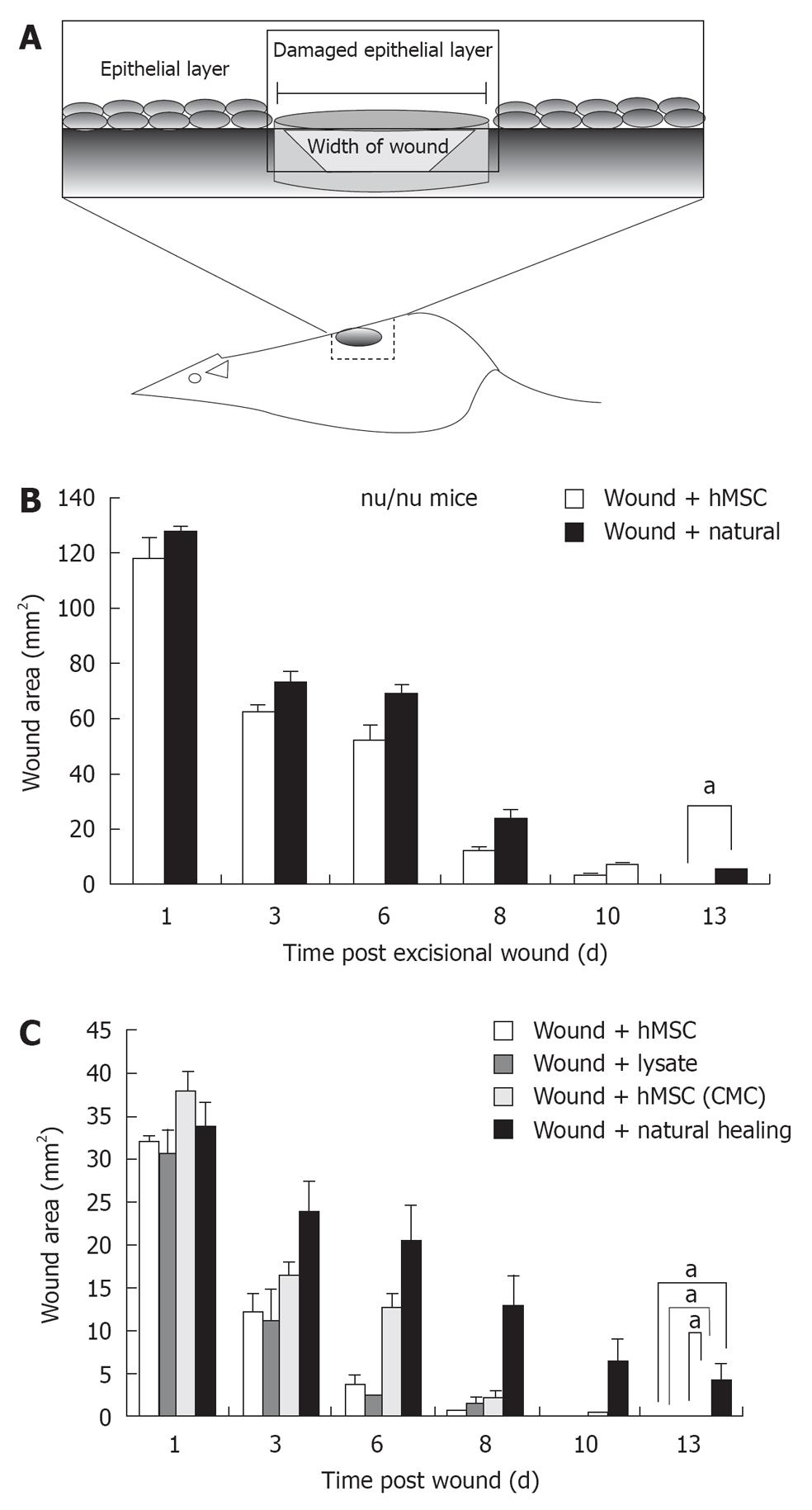
Mice (strain: male nu/nu, and NODSCID mice; age: 4-5 wk from Taconic Farms, NY) were anesthetized with ketamine/xylazine and the skin surface was sterilized with alcohol wipes. The NODSCID (non obsese diabetic/severe combined immunodeficient model for chronic wound) mice were shaved to expose skin for wounding. Excisional wounds (approximate area 30 to 50 mm2) were made in the back of each mouse. For deep wounds, similar cuts were made aseptically in the back of mice but the wounds measured approximately 120-140 mm2. All wounds were covered with transparent adhesive bandage for 48 h post wounding. 5 × 105 hMSCs (including fluorescently labeled) were injected subcutaneously in the periphery of each wound in experimental groups. Saline (100 μL) was injected subcutaneously near the wounds in a control group. Measurement of wound healing was carried out using area of ellipse formula (0.5 × length of Major axis) (0.5 × length of Minor axis) (π)[37]. Wound bearing animals were housed individually during the course of the experiment.
The cytokine profile of each sample was analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA) and by Elisa assay for CXCL5 and SDF-1 which was performed on cell-free supernatants from conditioned medium using the Quantikine human ENA-78 ELISA kit (R and D Systems; MN) according to the manufacturer’s instructions. All samples were assayed in triplicate. CM was harvested from cultured cells (hMSCs co-cultured with keratinocytes and alone, cultured for 48 h) and filtered through cellulose acetate membrane with 0.45 μm pore size (Corning; NY). hMSCs and keratinocytes were co-cultured (1:5) in serum-free keratinocyte growth medium (Promocell; Germany), cells alone (hMSCs and keratinocytes) were grown as controls.
Skin sections within close proximity of the wounded area were peeled out after euthanasia from mice (nu/nu age: 4-5 wk from Taconic Farms, NY) injected with hMSCs or lysates prepared from hMSCs along with naturally healing and normal mice. The resected section was immediately dipped in LN2 and transferred to a ceramic mortar filled with LN2 where skin sections were ground with a cold pestle until turned into amorphous powder. The powder was scraped in to a pre-chilled Falcon tube in a dry ice containing TRIzol (Invitrogen; Carlsbad CA, USA) reagent (1 mL/40-100 mg of tissue weight). The tube was vortexed vigorously and transferred into a pre-cleaned homogenizer and homogenized with 20 up and 20 down strokes. The homogenized solution was incubated for 5min at room temperature followed by addition of molecular biology grade chloroform (Sigma, 400 μL/1.5 mL of TRIzol Reagent) and mixed. The solution was incubated for an additional 5 min at room temperature and centrifuged (Eppendorf table top centrifuge) at 12 000 ×g (15-17 min at 4 °C). The upper aqueous phase was collected in a new sterile Falcon tube and Isopropyl alcohol was added (1:1), mixed thoroughly and incubated (10 min at RT) followed by centrifugation (12 000 ×g for 10-15 min at 4 °C). Supernatant was aspirated carefully without disturbing the pellet. The RNA pellet was washed with 500 μL of 70% ethanol prepared in RNase free water (GIBCO, Invitrogen))and centrifuged (7000 ×g for 5 min at 4 °C ). The RNA pellet was air dried (20 min) and then resuspended in 40 μL RNase free water and stored at -80 °C until used.
RT-PCR analysis was carried out using superscript one step RT-PCR (Invitrogen, Carlsbad, CA) kit to determine mRNA expression levels of SDF-1 and CXCL5 in hMSC and hMSC lysate injected skin (wounded), naturally healing wounded skin and normal skin.
Primer sequences (F: Forward, R: Reverse) for SDF-1, CXCL5 and GAPDH (internal control) were F-5'-GAGAGCCACATCGCCAGAG-3', R-5'-TTTCGGGTCAATGCACACTTG-3', F-5'-TTCATGAGAAGGCAATGCTG-3' R-5'-CCCAGGCTCAGACGTAAGAA-3' and F-5'-ACCACAGTCCATGCCATCAC-3', R-5'-TCCACCACCCTGTTGCTGTA-3' respectively. PCR conditions were 94 °C for 15 s, 50 °C for 30 s, 72 °C for 1 min, and 30 cycles for each target. A final elongation step was carried out at 72 °C for 7 min. The PCR product was subjected to agarose gel electrophoresis and photographed using a Geldoc imager (Bio-Rad XRS).
For immunohistochemical analysis, animals (nu/nu age: 4-5 wk from Taconic Farms, NY) were euthanized; wound areas were excised and immediately fixed for 24 h before processing through a graded series of alcohols and embedded in paraffin wax. Thin sections (4 microns) were cut and placed onto glass slides for staining. Primary antibody was optimized using Ventana Medical Systems Discovery XT automated immunostainer. Antigen retrieval was performed using CC1 (Cell Conditioning Solution, Ventana medical systems, Cat # 950-124). Primary antibody was applied and incubated at 37 °C for 1 h. Universal secondary antibody (Ventana medical systems, Cat # 760-4205) was applied for 12 min followed by chromogenic detection kit DABMap (Ventana Medical Systems, Cat # 760-124). Slides were counterstained with Hematoxylin and dehydrated and cleared before cover slipping from Xylene.
Wound measurements were carried out by the described method and graphs plotted to show wound closure over time. The graphs show mean ± SE. The number of animals used in each experiment is indicated in the figure legends. Students t-test was performed to determine significance of difference between groups (P < 0.05 was considered significant).
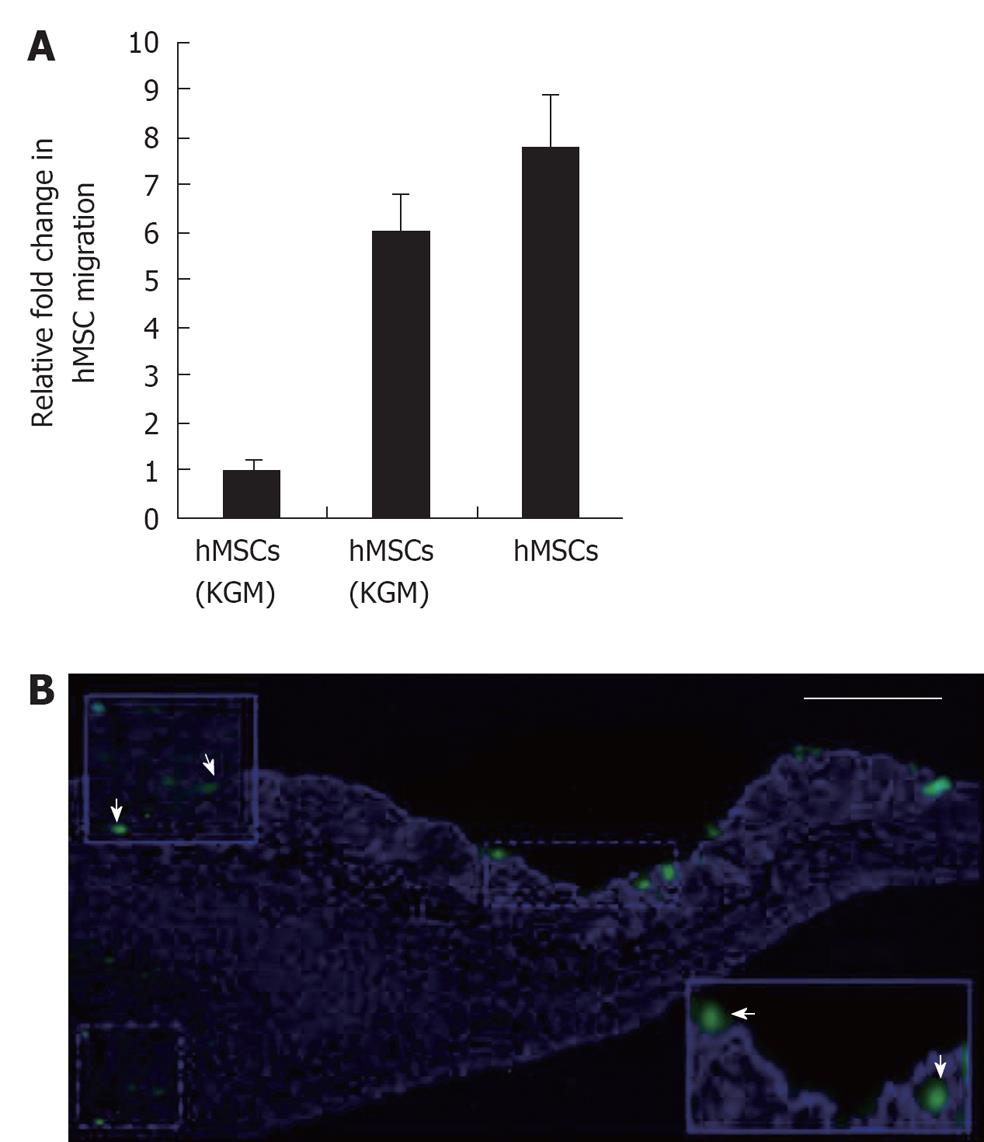
The hMSCs were found to migrate toward keratinocytes as well as to KCM in greater numbers than to control medium (Figure 1A). Thus, exposure to secreted factors such as cytokines present in KCM may “prime” hMSCs to respond and migrate towards keratinocytes. To examine whether such migration could be observed (albeit at microscopic level), fluorescently labeled hMSCs were injected near wound sites. Following incubation for 2 d, skin sections were processed for microscopy and revealed that fluorescent dye labeled hMSCs appeared to migrate towards the repair site in the wounded skin (Figure 1B) indicating that they may participate directly in wound healing. Cells of human origin could be discriminated from mouse cells by their larger nuclei (DAPI staining) and fluorescent label.
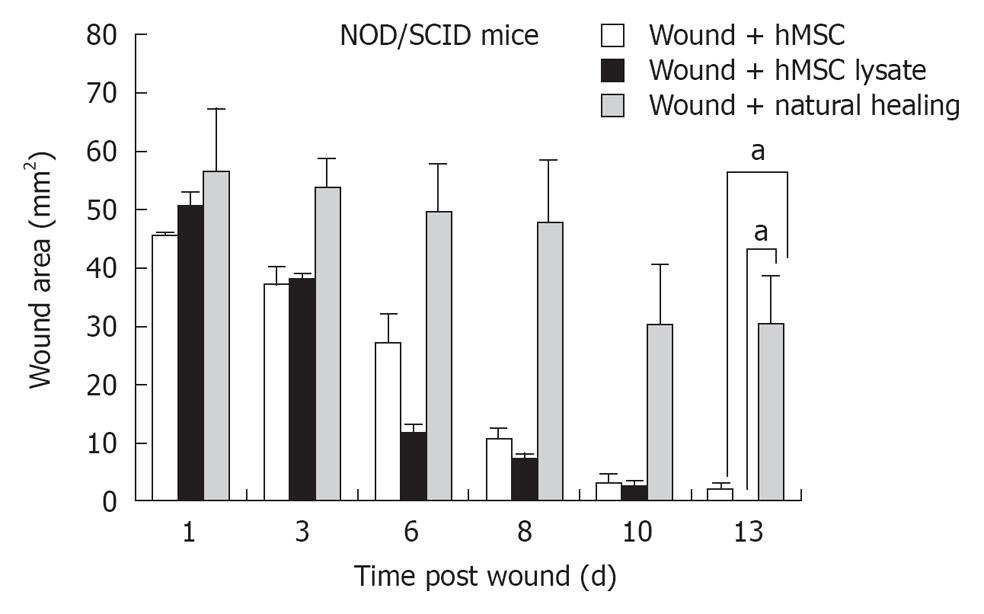
To evaluate effect of hMSCs and derivatives on wound healing, wound area following administration of 5 × 105 hMSCs or saline control was measured over 15 d in the nude mouse model (acute wound model) and 25 d in the NOD/SCID model (chronic wound model) (Figure 2A). Quantification of the wound area indicated that mice administered hMSCs showed accelerated wound healing in both models, compared with saline-treated control. In the nude mouse model, healing was completed between 6-8 d for the hMSC treated group, (Figure 2B). Further, in the nude mouse model, animals treated with hMSCs or lysates from hMSCs, or concentrated conditioned medium from cultured hMSCs (CMC) completed wound healing in 6-8 d (Figure 2C). Animals allowed to heal naturally took 13-14 d in the nude model. In the chronic wound model, natural healing takes 24 d (Figure 3 for NOD/SCID model). Again, we observed accelerated wound healing when hMSCs or lysates from hMSCs were administered. Although natural wound healing occurred in both models, hMSCs or products derived from hMSCs clearly accelerated wound healing in both nu/nu and NOD/SCID models.
To examine whether a clinically relevant lysate preparation in oil based hydrophilic ointment was effective in wound healing, a mixture was prepared containing either 1 or 2 μg/mg of ointment base and applied to the wound site topically. The hMSCs cell lysate (1-2 μg lysate protein/mg of vehicle) when admixed with oil based hydrophilic ointment and applied topically at the wound site resulted in faster rate of wound closure as compared with fibroblast WI38 lysate (1-2 μg/mg) and ointment alone (Figure 4). The lysate preparation at 2 μg lysate protein/mg ointment was significantly better than wound plus ointment base alone in wound repair. Lysate preparations from WI38, human fibroblast controls, were not as effective as lysate preparations from hMSCs.
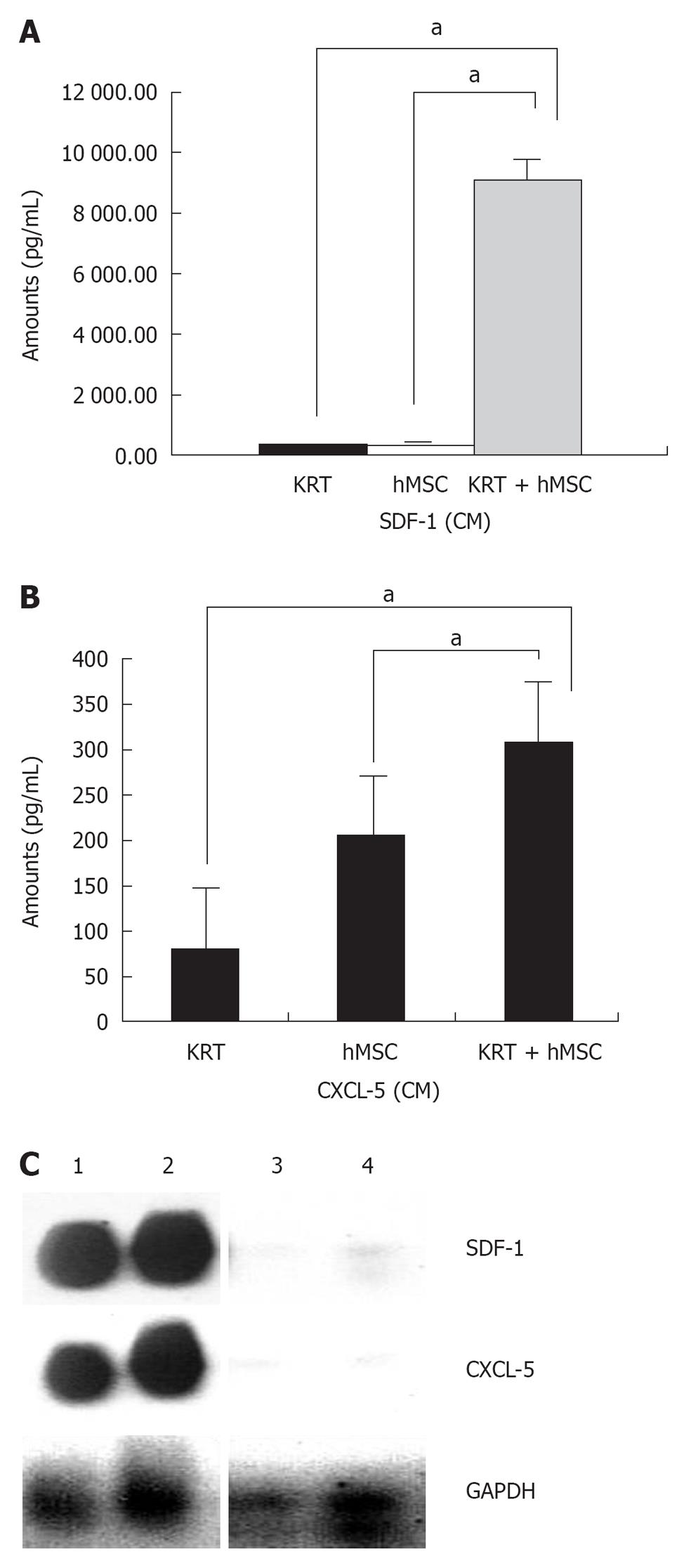
Cytokine profiling revealed that various cytokines were (i.e., IL8, IL6, MCP-1, G-CSF, MIP-1α and VEGF) secreted by hMSCs and present in the CM, lysate and hMSC concentrated conditioned medium. Among the cytokines measured, greatest increase in levels of SDF-1 and CXCL5 were observed when hMSCs and keratinocytes were co-cultured together in vitro (Figure 5A and B). This indicated that hMSCs and keratinocytes may cooperate in wound healing by acting in a synergistic manner to produce predominantly high levels of important cytokines such as SDF-1 (CXCL12) and CXCL5, identified biologically and statistically significant in our dataset (not shown); although other factors are clearly involved.
RT-PCR analysis of RNA isolated from normal and healing wound shows that levels of CXCL5 and SDF-1 were increased significantly in the healing wound treated with hMSCs as compared to normal skin (Figure 5C) in agreement with the cytokine protein profile shown in Figure 5A and B.
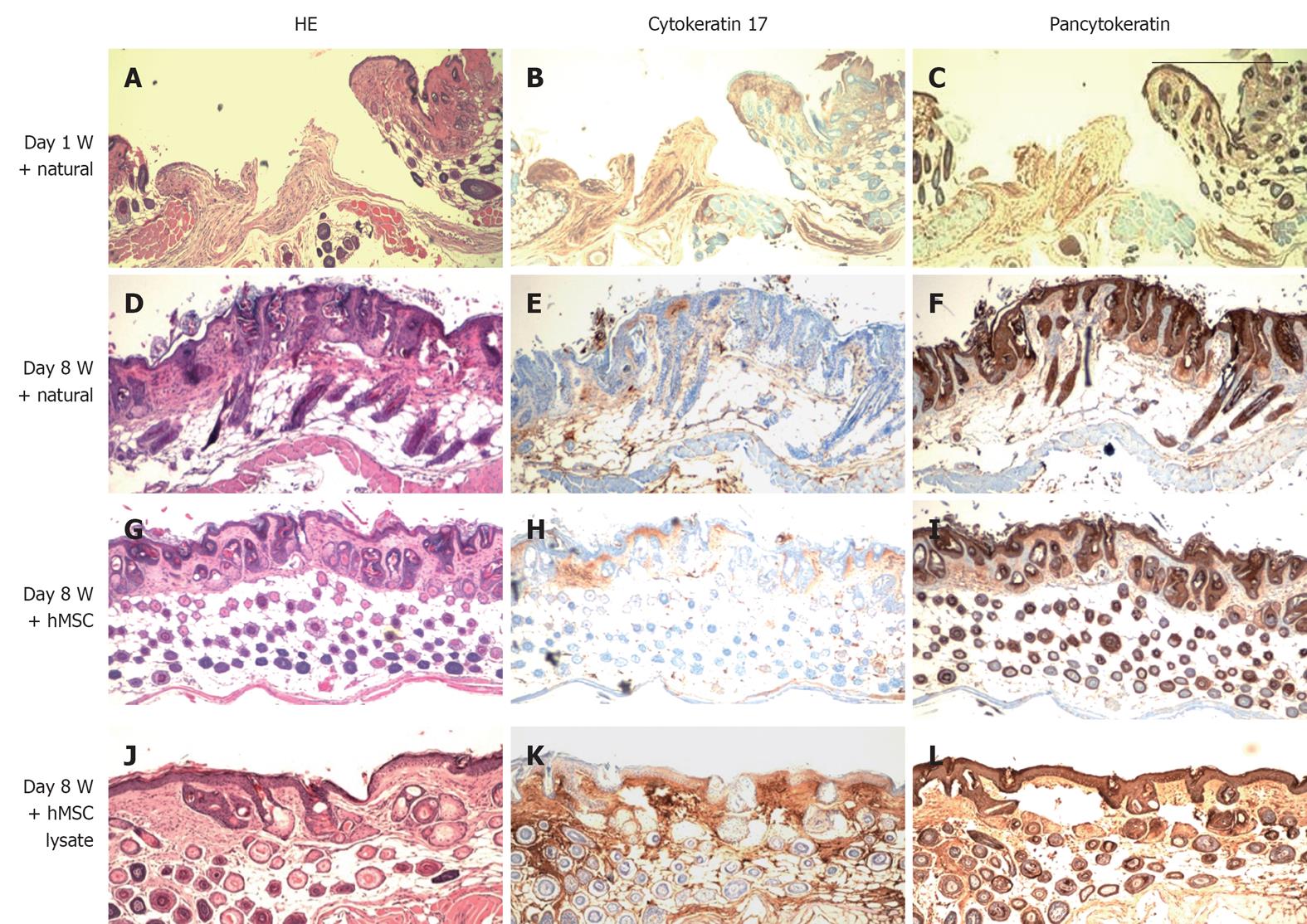
In addition to wound closure as a measure of wound healing we were interested in determining whether we could observe histological differences in wound healing between control (natural wound healing) wounds treated with hMSCs or derivatives of hMSCs. HE stains and immunohistochemical staining indicated that administration of hMSCs and lysates from hMSCs near wound sites led to improved regeneration of the skin structure as compared with sections prepared from animals either untreated or treated with control WI38 cells(data not shown). Figure 6 shows restoration of both dermis and epidermis in skins of mice treated with hMSCs as well as lysates as compared with controls (Figure 6A-L). A large number of pancytokeratin positive cells was observed in the dermis of hMSC treated wounds (Figure 6I) indicating that administration of hMSCs at the wound site may have induced increased proliferation of keratinocytes.
In the present study, we demonstrate that hMSCs and cell-free derivatives of hMSCs can be successfully used to treat wounds using athymic and NOD/SCID mouse models. These models represent the acute and chronic wound models respectively. Moreover, as they are incapable of mounting an immune response against human cells, wound healing can be studied using human MSCs and derivatives. We also demonstrate that hMSCs respond to the cytokine signals from human keratinocytes, an abundant cell type in skin. HMSCs migrate towards keratinocytes as well as toward conditioned medium from human keratinocytes. Accelerated wound healing was observed when hMSCs were transplanted locally near the site of incisional/excisional wounds as well as deep wounds in athymic (Nu/Nu) mice and in NOD/SCID (non obese diabetic SCID, representing chronic diabetic wound) mice when compared with healing in presence of normal human fetal lung fibroblast WI38 cells or saline control. Accelerated wound healing was also observed when concentrates prepared from conditioned medium of hMSCs were applied to wound site. IHC analysis on day 8 revealed comparable repair of the wound site in all groups examined with matured epidermis, although pancytokeratin staining was variable, it was clear that administration of hMSCs or derivatives was effective in wound healing. Long term follow up of wound healing revealed that in the human MSC treated animals there was little evidence of residual scarring (data not shown), although natural wound healing as well as healing in presence of lysates or concentrates was accompanied by scar formation (data not shown), underscoring the importance of myofibroblasts in prevention of scarring. More importantly, long-term follow up did not show any adverse reaction to administration of hMSCs in the animals, pointing to the safety of these cells.
Impaired recruitment of hMSCs and therefore reduced number of myofibroblasts can impede wound closure as myofibroblasts have been shown to be key cells in wound contraction leading to wound closure. By administering hMSCs at wound site we ensured that an adequate number of myofibroblasts was available and contributed to proper wound closure. The high contractile force generated by myofibroblasts is beneficial for physiological tissue remodeling but detrimental for tissue function when it becomes excessive, such as in hypertrophic scars, in virtually all fibrotic diseases and during stroma reaction to tumors[25,27-29].
During the granulation phase of wound healing, mesenchymal cells are maximally activated in the granulation tissue leading to cell proliferation and synthesis of copious amounts of extracellular matrix. Epithelial cells in turn proliferate and form a layer over the newly formed matrix of mesenchymal cells in granulation tissue, leading to wound closure. Keratinocytes stimulate fibroblasts to synthesize growth factors, which in turn stimulate keratinocyte proliferation in a reciprocal manner. Of particular interest is the observed increase in CXCL5 (also known as ENA78, a known stimulator of keratinocytes) and SDF-1 (CXCL12) secretion from co-cultured human hMSCs and keratinocytes as compared with levels secreted by individual cells. Importantly, interaction of keratinocytes and hMSCs results in increased expression of CXCL5 in the healing wound. Although our data points to the involvement of CXCL5 and SDF-1, there are clearly other factors that play an equally important role in the process of wound healing. We have previously shown SDF1 produced by hMSCs acts in an autocrine manner to activate and stimulate migration, signal transduction in hMSCs via the Jak/Stat pathway[38]. These stimulatory signals further increase cytokine production and cross talk with neighboring cells including keratinocytes, as observed in the coculture experiments. CXCL5 has been shown to be a potent stimulator of keratinocytes. From our results it appears that hMSCs (and their derivatives) cooperate with keratinocytes to induce accelerated wound healing. In addition to the cytokine mediated dialog with keratinocytes, the hMSCs and derivatives appear to participate in important areas of wound repair possibly by local generation of myofibroblasts and formation of a matrix of appropriate tensile strength upon which new layers of dermis and epidermis are formed. We are currently investigating these possibilities. These results together suggest that hMSCs and derivatives will be useful for regenerative purposes particularly for healing of chronic wounds. One caveat in the use of mouse models of wound healing is the fact that wounds in mice heal by contraction, unlike human wounds. However, we have used excisional rather than incisional wounds in mice, more closely resembling the human wound. Indeed, for testing the efficacy of biologics such as mesenchymal stem cells in preclinical in vivo models, mice have been used successfully by others[33-36]. Paracrine factors secreted by hMSCs that play an important role in wound healing and angiogenesis have been described by others previously[39,40].
The endogenous pathway for wound healing involves recruitment of MSC from bone marrow at the wound site followed by keratinocyte-MSC interaction, and increased local cytokine production, resulting in formation of myofibroblasts from MSC that may cause haemostasis, inflammation and wound closure. Our studies indicate that cell-free derivatives of human MSCs are useful for wound healing purposes and can circumvent the need for intact cells which are cumbersome to prepare, formulate and transport.
Wound healing is a coordinated process comprising of an inflammatory reaction, a proliferative process leading to tissue restoration, angiogenesis and formation of extracellular matrix accompanied by scar tissue remodeling. Cellular participants as well as multiple growth factors and cytokines released by the cells at the wound site regulate these processes and finally result in wound closure. Deregulated healing process may delay repair and may eventually lead to chronic wounds, such as are found in diabetics, that are expensive and difficult to heal and may also result in excessive fibrosis leading to keloid formation. Treatment of chronic wounds remains difficult despite increased understanding of underlying biological principles, significant developments including use of recombinant growth factors, bioengineered skin equivalents and overall improvement in standards of wound care. Clinical trials using bone marrow derived mesenchymal stem cells in myocardial infarctions and graft vs host disease have recently been launched, and encourage investigation of bone marrow derived human mesenchymal stem cells (hMSCs) for use in other areas of regenerative medicine including chronic wound healing. The present study sought to compare efficacy of hMSCs and cell-free derivatives of hMSCs, which may be clinically more relevant as they are easier to prepare, formulate and transport.
Bone marrow derived mesenchymal stem cells have been shown to play an important role in wound therapy. The present study sought to compare the efficacy of hMSCs and cell-free derivatives of hMSCs, which may be clinically more relevant as they are easier to prepare, formulate and transport.
Co-culture of hMSCs with keratinocytes resulted in increased expression of CXCL-12 (SDF-1) and ENA78 (CXCL-5) in the conditioned media (CM) indicating that the hMSCs can respond to signals from keratinocytes. Accelerated wound closure was observed when hMSCs were injected near the site of excisional wounds in athymic as well as NOD/SCID mice. Interestingly, cell-free lysates prepared from hMSCs were also effective in inducing accelerated wound closure and increased expression of SDF-1 and CXCL-5 at the wound bed. Additionally, concentrated media from hMSCs as well as an emulsion containing lysates prepared from hMSCs was also found to be effective in rapid re-epithelialization compared with fibroblast or vehicle alone control.
Use of cell-free derivatives may help replace expensive wound care approaches including use of growth factors, epidermal/dermal substitutes, synthetic membranes, cytokines, and matrix components, and avoid transmission of pathogens from human and animal products.
BMD-hMSC: Bone marrow derived mesenchymal stem cells are pluripotent adult stem cells isolated from human bone marrow; Cell-Free Derivatives: Extracts from hMSCs were referred to as cell-free derivatives; Concentrate: CM derived from hMSCs; Lysate: cell lysate derived from hMSCs.
This research is important since it clarifies role of some humoral factors involved in wound healing. The result is significant for understanding the mechanism of wound healing and finding new therapies in wound treatment. The hypothesis including migration of hMSCs to keratinocytes is very interesting. The structure of the manuscript is quite reader-friendly.
Peer reviewer: Shihori Tanabe, PhD, Senior Researcher, In Concurrent with Director for Planning and Coordination’s, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, 1-18-1, Kami-yoga, Setagaya-ku, Tokyo, 158-8501, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 4181] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 2. | Fowler E. Chronic wounds: an overview. Chronic wound care: a clinical source book for healthcare professionals. Pennsylvania: Health Management Publications Inc 1990; 12-18. |
| 3. | Rees RS, Hirshberg JA. Wound care centers: costs, care, and strategies. Adv Wound Care. 1999;12:4-7. |
| 4. | Callam M. Prevalence of chronic leg ulceration and severe chronic venous disease in Western countries. Phlebology. 1992;7:6-12 http://www.sign.ac.uk/guidelines/fulltext/120/references.html. |
| 5. | Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674-86; discussion 686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Sibbald RG, Cameron J. Dermatological aspects of wound care. Chronic wound care: a clinical source book for healthcare professionals. 3rd ed. Wayne: Health Management Publications 2001; 273-285. |
| 8. | Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol. 2007;25:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1651] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 10. | Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005;13:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Bansal C, Scott R, Stewart D, Cockerell CJ. Decubitus ulcers: a review of the literature. Int J Dermatol. 2005;44:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1145] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 14. | Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 828] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 15. | Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 16. | Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552-556. [PubMed] |
| 17. | Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 18. | Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 19. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15196] [Article Influence: 584.5] [Reference Citation Analysis (0)] |
| 20. | Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004;29:1971-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 23. | Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697-702; discussion 702-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331-4339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 724] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 26. | Gabbiani G. The cellular derivation and the life span of the myofibroblast. Pathol Res Pract. 1996;192:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Helary C, Ovtracht L, Coulomb B, Godeau G, Giraud-Guille MM. Dense fibrillar collagen matrices: a model to study myofibroblast behaviour during wound healing. Biomaterials. 2006;27:4443-4452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21-29. [PubMed] |
| 29. | Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Fukuda K, Fujita J. Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice. Kidney Int. 2005;68:1940-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 691] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 32. | Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1193] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 33. | Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Badillo AT, Redden RA, Zhang L, Doolin EJ, Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007;329:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581-2587. [PubMed] |
| 37. | Wysocki AB. Wound measurement. Int J Dermatol. 1996;35:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 889] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 40. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1177] [Article Influence: 69.2] [Reference Citation Analysis (0)] |