Published online May 26, 2011. doi: 10.4252/wjsc.v3.i5.43
Revised: January 5, 2011
Accepted: January 12, 2011
Published online: May 26, 2011
AIM: To expand hematopoietic/progenitor stem cells (HS/PCs) from umbilical cord blood (UCB) and prepare the HS/PC product, and analyze preclinical transplantation and safety of HS/PC product.
METHODS: Human bone marrow-derived mesenchymal stem cells (MSCs) were used as feeder cells to expand HS/PCs from UCB in a serum-free culture system. The proliferation potential of HS/PCs was analyzed. The expanded HS/PCs were suspended in the L-15 medium to prepare the HS/PC product. The contamination of bacteria, fungi and mycoplasmas, the infection of exogenous virus, the concentration of bacterial endotoxin, and the SCF residual in HS/PC product were determined. Finally, cells from the HS/PC product with or without bone marrow-derived mesenchymal stem cells (BM-MSCs) were transplanted into the irradiated NOD/SCID mice to determine the in vivo engraftment potential.
RESULTS: After co-culture for 10 d, the total nuclear cells (TNCs) increased 125-fold, and CD34+ cells increased 43-fold. The granulocyte-macrophage colony- forming cells (GM-CFCs) and erythroid colony-forming cells (E-CFCs) increased 3.3- and 4.7-fold respectively. The expanded cells were collected and prepared as the expanded product of HS/PCs by re-suspending cells in L-15 medium. For preclinical safety, the HS/PC product was analysed for contamination by bacteria, fungi and mycoplasmas, the bacterial endotoxin concentration and the SCF content. The results showed that the HS/PC product contained no bacteria, fungi or mycoplasmas. The bacterial endotoxin concentration was less than the detection limit of 6 EU/mL, and residual SCF was 75 pg/mL. Based on clinical safety, the HS/PC product was qualified for clinical transplantation. Finally, the HS/PC product was transplanted the irradiated mice where it resulted in rapid engraftment of hematopoietic cells.
CONCLUSION: HSPC product prepared from UCB in the serum-free culture system with hMSCs as feeder cells should be clinically safe and effective for clinical transplantation.
- Citation: Guo CJ, Gao Y, Hou D, Shi DY, Tong XM, Shen D, Xi YM, Wang JF. Preclinical transplantation and safety of HS/PCs expanded from human umbilical cord blood. World J Stem Cells 2011; 3(5): 43-52
- URL: https://www.wjgnet.com/1948-0210/full/v3/i5/43.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i5.43
Umbilical cord blood transplantation (UCBT) has been used in treatment of hematologic malignancies, aplastic anemia, hemoglobinopathies, and severe combined immunodeficiency[1]. Compared with bone marrow and peripheral blood, umbilical cord blood (UCB) has the advantage of convenient collection, immature cellular immunity and no strict matching requirement in transplantation[2]. Thus, hematopoietic/progenitor stem cells (HS/PCs) from UCB have potential clinical value. However, the HS/PCs in a stock of UCB are low in quantity, limiting their clinical application. Therefore, HS/PCs from UCB should be ex vivo expanded to meet the needs for clinical transplantation. In vivo, mesenchymal stem cell (MSCs) in bone marrow supply an appropriate scaffold for hematopoiesis and a complex network of cytokines, adhesion molecules, and extracellular matrix proteins that regulate survival, proliferation, growth and differentiation of HS/PCs. Bone marrow-derived mesenchymal stem cells (BM-MSCs) can be used as a feeder layer to support the ex vivo proliferation of HS/PCs. In co-culture systems, BM-MSCs are generally considered to provide signal transduction to promote the expansion of HS/PCs through two major mechanisms. First, BM-MSCs secrete various soluble factors, such as growth factors and cytokines that stimulate the proliferation and differentiation of HS/PCs, and make the niche an appropriate environment for the proliferation and maintenance of HS/PCs. Secondly, co-culture with BM-MSCs provides the direct cell-cell contact, which plays an important role in HS/PC proliferation and differentiation. Previous studies demonstrated that co-culture with BM-MSCs resulted in the increase of ex vivo proliferation, the regulation of differentiation, the decrease of apoptosis[3-7], and the decrease of allostimulatory capacity of HS/PCs in transplantation[8]. Culture of CD34+ cells culture with microencapsulated stromal cells effectively stimulated the total cell population and maintenance of primitive progenitor cells. The transplantation of their progeny was able to shorten the time to engraftment by bridging the pancytopenic period and support functional hematopoietic repopulation[9,10].
Great attention should be paid to the preclinical safety of any ex vivo expanded cell product before clinical transplantation. It was reported that the static amplification of BM-MSCs is prone to contamination[11]. Any carelessness during culture may result in the contamination of the expanded cell product, including possible contamination by bacteria, fungi, mycoplasmas and exogenous viruses[12]. Bacterial contamination remains one of the major risks associated with blood product transfusion and is known to cause severe transplantation-related infections[13]. Contamination of a cellular product may lead to transplantation failure or death of transplanted patient. This notion is supported by reports from the French Hemovigilance system demonstrating that between 1994 and 1998 transplantation associated bacterial infection was responsible for 22% of fatal cases associated with transplantation[14]. Most Drug Administrations have, therefore, taken notice of this problem and defined that there should be no exogenous factor contamination in cellular products[15-17]. Given all the considerationsmentioned above, cellular products should be examined carefully to reduce the possibility of contamination before clinical transplantation.
In this paper, human BM-MSCs were used as feeder layer to support the expansion of HS/PCs from UCB in a serum-free culture system (the serum-free co-culture system). The proliferation potential of HS/PCs was analyzed. The expanded HS/PCs were suspended in L-15 medium to prepare the HS/PC product. Possible contamination by bacteria, fungi and mycoplasmas, the infection of exogenous virus, the concentration of bacterial endotoxin, and the SCF residual in HS/PC product were analysed. Finally, cells from the HS/PC product, with or without BM-MSCs, were transplanted into irradiated NOD/SCID mice to determine their in vivo engraftment potential.
Bone marrow (BM) was provided by the First Affiliated Hospital, Zhejiang University, and was approved by the Ethics Committee of Hospital. All donors had given informed consent before collection of bone marrow. The collected BM was washed three times with phosphate buffered saline (PBS). The cell pellet was re-suspended in α-MEM (Gbico, Hangzhou, China), and then loaded on Ficoll density gradient fractionation columns (density = 1.077 g/L, Dingguo, Hangzhou, China). Cells were centrifuged at 300 ×g for 25 min. The mononuclear cells (MNCs) in the interface were collected and washed three times with PBS. MNCs were re-suspended in MesenCult® 05401 medium (StemCell, Hangzhou, China), the cell density adjusted to 5 × l06/mL, and cells seeded in the 25-culture flask. After culture of 3 d in 100% humidified 5% CO2 at air 37oC, the cells were demi-replaced with fresh medium and cultured for another 3 d. Then, the medium was totally replaced with fresh medium every 3 d. At 90% confluence, the cells were detached by trypsin-EDTA (Gibco) and seeded into the 175-culture flask at a density of 1.4 × 106 cells as passage 1. The cells in passage 2 or 3 were used for co-culture with HS/PCs from UCB.
UCBs were obtained from normal full-term deliveries in the Second Peoples’ Hospital, Zhejiang University, as approved by the Ethics Committee of Hospital. Parturient women gave written consent for the use of UCB for research purposes. Collected UCB was heparinized to a final heparin concentration of 20 U/mL.
MNCs in UCB were isolated using Ficoll density gradient centrifugation. The red blood cells (RBCs) were depleted by a 3% gelatin (Sigma, Hangzhou, China) sedimentation method. The leukocyte-rich fraction was washed with PBS to remove the gelatin, and then loaded on Ficoll density gradient fractionation columns (density = 1.077g/L, Dingguo). Cells were centrifuged at 300 ×g for 25 min. MNCs at the interface were collected and washed three times with PBS, and then re-suspended in serum-free Stem Span H3000 (StemCell) medium.
MNCs from UCB were seeded at a density of 1 × 105 cells/mL in the 175-culture flask with the confluent BM-MSCs. The medium for MNC culture was Stem Span H3000 containing SCF (50 ng/mL, Peprotecb, Beijing, China), TPO (10 U/Ml, Sansheng, Shenyang, China) and G-CSF (50 ng/mL, Jiuyuan, Hangzhou, China). After culture in 100% humidified 5% CO2 at air 37°C for 10 d, the non-adherent cells in the culture medium were collected and analyzed for cell growth, flow cytometry characteristics and colony-forming units. The co-culture system with 10% FBS and three cytokines (see above) with- and without- BM-MSCs were used as Control 1 culture system and Control 2 culture system respectively.
The MNCs from UCB and the cells expanded in co-culture system for 10 d were analysed for cell surface CD34 antigen by the FACScalibur flow cytometer (Becton Dickinson, Beijing, China). The collected cells were diluted to 1 × 106 cells/mL with PBS containing 1%BSA. 10 μL of antigens was added to 100 μL samples and the cells were incubated for 30 min at 37°C. After washing once with PBS, the cells were re-suspended in 0.5 mL PBS and stained with mouse anti-human CD34-FITC and IgG1-FITC (Immunotech, Shanghai, China).
Granulocyte-macrophage colony-forming cells (GM-CFCs) and erythroid colony- forming cells (E-CFCs) were quantified in semisolid culture as follows: (1) GM-CFCs: cells were seeded at a density of 1 × 105 cells/mL in 2 mL of IMDM (Gibco) containing 2.7% methylcellulose and 100 ng/mL GM-CSF (Peprotech), and incubated at 37°C with 5% CO2 for 14 d; and (2) E-CFCs: cells were seeded at the same density in 2 mL of IMDM containing 2.7% methylcellulose, 1 × 10-4M 2-mercaptoethanol(Sigma,Shanghai, China), 3% glutamine (Sigma), 2 U/mL EPO (Peprotech), and incubated at 37°C with 5% CO2 for 14 d. The colonies were counted by three persons in two different laboratories to reduce errors.
The expanded HS/PCs from UCB were collected and washed three times with PBS, re-suspended with L-15 medium containing 10% dimethyl sulfoxide (DMSO, Gaylord, Shanghai, China) at a density of 1 × 107 cells/mL, and then transferred into the 5 mL cryovial as the HS/PC product. The pre-clinical safety of the HS/PC product was evaluated according to the Pharmacopoeia of the People’s Republic of China (PC, 2005 Edition).
A direct inoculation method was used to determine the possible contamination of the HS/PC products with bacteria, fungi and mycoplasmas. The HS/PC product was centrifuged at 300 ×g for 10 min, and the supernatant was collected and cultured in Thioglycollate Medium (Sanyao, Beijing, China) for more than 14 d at 35°C to determine possible contamination by bacteria, in Modified Martin Broth Medium (Sanyao) for more than 14 d at 25°C to determine possible contamination by fungi, and in Mycoplasma Broth Base Medium (Sanyao) for 21 d at 37°C to determine possible contamination by mycoplasmas.
Possible infection by exogenous virus was determined by in vitro culture, innoculation in SPF mice (Zheda, Hangzhou, China) and inoculation in avian embryos (Yibang, Hangzhou, China). For the in vitro culture, the cell suspension of HS/PC product was seeded at a density of 1 × 107 cells per well in a 6-well plate with confluent human fibroblast cells for 7 d at 37°C with 5% CO2. Then, the human fibroblast cells were collected and seeded at a density of 5 × 105 cells per well in the new 6-well plates to culture for 14 d. The morphology of human fibroblast cells was detected. For SPF mouse inoculation, a cell suspension with 1 × 107 of expanded HS/PCs was transplanted into the brain and cavum peritonaei of SPF mice. L-15 medium was used as control. At the 21th d, the physiological reaction of mice was examined. For avian embyro inoculation, a cell suspension with 1 × 107 of expanded HS/PCs and injected into the allantoic cavity of 9-11 d old avian embyros and the yolk sac of 5-6 d old avian embyros. 5 avian embyros were used for each group. After 5 d, the survival of the avian embyros was determined.
The bacterial endotoxin test (BET) method was used to detect the bacterial endotoxin concentration. Briefly, 4 solutions were prepared as follows: A solution used for the sample test [dilution of test sample no more than maximum valid dilution (MVD)], B solution used for the interfering test (control standard endotoxin (CSE, China National Biotec Group, Beijing, China) diluted with A solution), C solution used for the positive control [CSE diluted with tachypleus amebocyte lysate (TAL) Reagent Water (Zhanjiang A & C Biological LTD, Zhanjiang, China)], and D solution used for the negative control TAL Reagent Water(Zhanjiang).
According to PC (2005 edition, p365-368), the determining limitation (L) and MVD of the sample were calculated by the formulas: L = K/M and MVD = CL/λ [C: the concentration of sample. L is expressed in EU/mL,.λ: the sensitivity of TAL]. To recheck the TAL sensibility, CSE was diluted with TAL Reagent Water to 0.25, 0.125, 0.063 and 0.032 EU/mL according to TAL (λ = 0.125 EU/mL). 0.1mL of diluted solution, with 2 replicates per concentration, was placed into a depyrogenated ampoule (Zhanjiang). D solution and C solution were used as negative and positive controls respectively. The ampoules with solution were incubated at 37°C for 60 min. Then, the ampoules were reversed and the formation of gel was observed. A durable gel was determined as positive, a non-durable gel was determined as negative.
For the interfering test on the HS/PC product, CSE was diluted with HS/PC product at 48 dilutions in a series of solutions: 0.25, 0.125, 0.063 and 0.032 EU/mL with 2 replicates per concentration. TAL Reagent Water was used as the negative control. The ampoules with solution were incubated at 37°C for 60 min and the formation of gel in each sample was determined as above.
A Human Stem Cell Factor ELISA kit (B & D, Shanghai, China) was used, per manufacturers’ instructions, to detect any residual stem cell factor (SCF) in HS/PC product. Briefly, the wells in an ImmunoModule plate were coated with a mouse anti-human SCF mAb overnight. Then, the wells were rinsed with PBS and blocked with 1% BSA for 2-4 h. The wells were rinsed and dried, and then the plate was stored at 4°C. After an overnight incubation (4°C), the wells were rinsed three times with the wash buffer. 100 μL standards and 100 μL supernatant from the expanded HP/SC product were applied to each well respectively and incubated for 1.5 h at 37°C. Then, the wells were rinsed 5 times with the wash buffer and 100 μL biotinylated SCF per well was applied and incubated for 1 h at 37°C. After the wells were rinsed 5 times with wash buffer, 100 μL horse radish peroxidase (HRP) per well was applied and incubated for 30 min at 37°C. The wells were rinsed 5 times with the wash buffer and incubated with 100 μL tetramethyl benzidine (TMB) substrate per well at 37°C. After 5-20 min, the reaction was stopped with100 μL stop solution per well, and the results were read at 450 nm on a Dynatech Plate Reader (Biotech Instrument, Shanghai, China).
The transplantation of the HS/PC product expanded from UCB in the serum-free co-culture system and the combination transplantation of HS/PCs expanded from UCB and BM-MSCs harvested from serum-free co-culture system was performed as previously described[18], with slight modifications. Briefly, 8-wk-old male NOD/SCID mice were obtained from the Beijing Institute for Experimental Animals, CAS. All animals were handled under sterile conditions and maintained under microisolators in the animal facility located at The Center of Experimental Animals, Zhejiang University. All animal experiments were approved by the Institutional Animal Care and Use Committee and Animal Ethics Committee of Zhejiang University. At 24 h before transplantation, 100 μL peripheral blood was collected from NOD/SCID mice by cutting the tail and diluted up to 250 μL with PBS/1% HSA to detect the baseline of WBCs using Advia 120 (Bayer, Leverkusen, Germany). Then, mice were irradiated with a 60Coγ in a split dose with a 4 h interval between doses. Each dose consisted of 15 min at 14.5 r/min. The expanded cells were transplanted by tail-vein injection into the irradiated NOD/SCID mice. Group A (n = 22 mice) was transplanted with cells from the HS/PC product, Group B (n = 25 mice) was transplanted with cells from the HS/PC product and BM-MSCs from serum-free co-culture system, and Group C (n = 18 mice) was injected with the same volume (200 μL) of PBS/1% HAS (irradiation control). In the Group A, the number of transplanted cells for each irradiated mouse was 8.5 × 105 cells in 200 μL suspension. In the Group B, the 200 μL of transplanted cells were composed of 6.0 × 105 cells from the HS/PC product and 2.5 × 105 cells of BM-MSCs from serum-free co-culture system. Starting on 3 d after transplantation and at subsequent regular intervals, 100 μL peripheral blood was collected by retro orbital sampling from each mouse. The recovery of WBCs in the peripheral blood was analyzed as above. The surviving recipients were killed 30 d after transplantation, and the bone marrow (from the femurs and tibiae) was harvested. Real-time PCR (RT-PCR) analysis using human-specific Alu sequence primers was performed for cells harvested from the bone marrow of recipients as described previously[18]. The GAPDH mRNA was used as a housekeeper gene to normalize the threshold cycle measurements for the Alu product. Result were shown as the concentration ratio of PCR product to that of GAPDH mRNA. We also detected human CD45+ cells in the bone marrow of transplanted mice using flow cytometry.
Results are expressed as mean ± SE, and statistical comparisons were performed using the one-way analysis of variance (ANOVA). A P-value of less than 0.05 was considered significantly different.
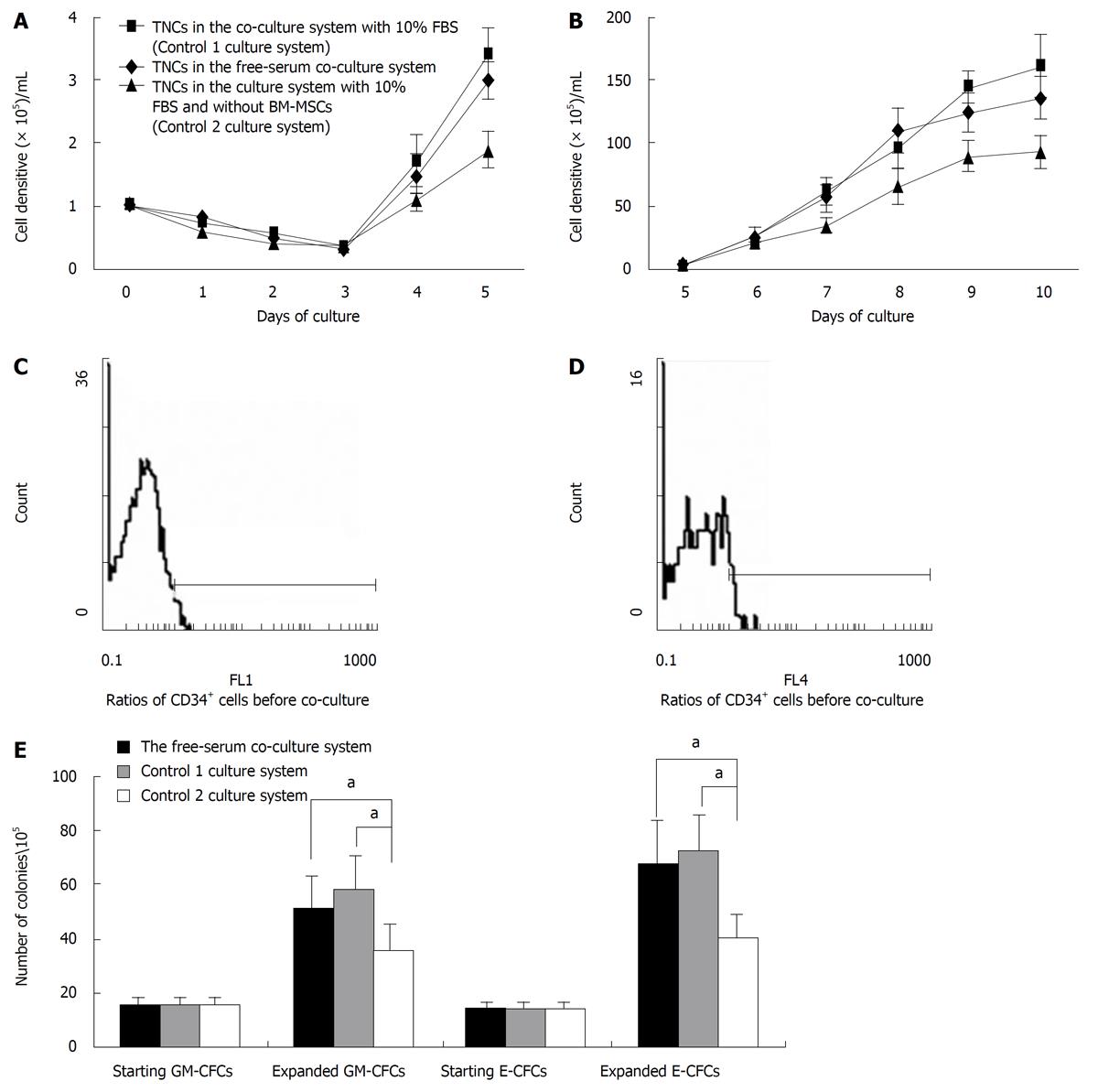
MNCs from human UCB were cultured in the serum-free co-culture system for 10 d. The co-culture system with serum (Control 1 culture system) and the culture system without BM-MSCs (Control 2 culture system) were used as controls. The ex vivo proliferation kinetics of non-adherent cells in three culture systems are shown in Figure 1A (day 0 to day 5) and 1B (day 5 to day 10). In the serum-free co-culture system, the total nuclear cells (TNCs) decreased gradually until the 4th d. Then, TNCs increased and grew during the lag period. TNCs in Control 1 culture system showed similar growth kinetics to TNCs in the serum-free co-culture system. After 10 d of culture, there was no significant difference in proliferation potentials between these two systems (1.25 ± 0.42 × 107 cells/mL for the serum-free co-culture system and 1.60 ± 0.70 × 107 cells/mL for the Control 1 culture system, P > 0.05). The proliferation potential of TNCs in the Control 2 culture system was significantly lower than in the serum-free co-culture system (0.93 ±0.31 × 107 cells/mL for the Control 2 culture system, P < 0.05).
The MNCs from UCB and the cells expanded in the three culture systems for 10 d were evaluated for cell surface CD34 antigen by the flow cytometry. In pre-expansion, the ratio of CD34+ cells in the MNCs was 1.8% ± 0.3% (Figure 1C). After 10 d of culture, the ratios of CD34+ cells in the serum-free co-culture system, the Control 1 culture system and the Control 2 culture system were 0.62% ± 0.19% (Figure 1D), 0.49% ± 0.15% and 0.23% ± 0.09% respectively (data not shown). According to the calculation of total CD34+ cells/mL, CD34+ cells increased 43.3-fold in the serum-free co-culture system, 43.5-fold in the Control 1 culture system and 11.8-fold in the Control 2 culture system.
Granulocyte-macrophage colony-forming cell (GM-CFC) and erythroid colony-forming cell (E-CFC) assays were performed with non-adherent cells at the beginning and the termination of culture. As shown in Figure 1E, the serum-free co-culture system and the Control 1 culture system showed greater increase in GM-CFCs and E-CFCs than the Control 2 culture system (P < 0.05). The mean number of GM-CFCs and E-CFCs in the starting MNC cell fraction was 15.6 ± 3.2/105 and 14.3 ± 2.5/105, respectively. After expansion, there were 51.2 ± 12.3/105 GM-CFCs and 67.6 ± 15.5/105 E-CFCs in the serum-free co-culture system, 57.4 ± 13.4/105 GM-CFCs and 71.8 ± 14.1/105 E-CFCs in the Control 1 culture system, and 35.2 ± 9.7/105 GM-CFCs and 40.4 ± 8.6/105 E-CFCs in the Control 2 culture system. In the serum-free co-culture system, GM-CFCs increased 3.3-fold and E-CFCs increased 4.7-fold.
The expanded HS/PCs from UCB were collected and re-suspended at a density of 1 × 107 cells/mL in L-15 medium to prepare the HS/PC product. The direct inoculation method was used to determine possible contamination of the HS/PC product by bacteria, fungi and mycoplasmas. After inoculation of the supernatant from the HS/PC product in Thioglycollate Medium and Modified Martin Broth Medium for more than 14 d and in Mycoplasma Broth Base Medium for 21 d to detect possible contamination by bacteria, fungi and mycoplasmas respectively. Results showed no contamination of the HS/PC product.
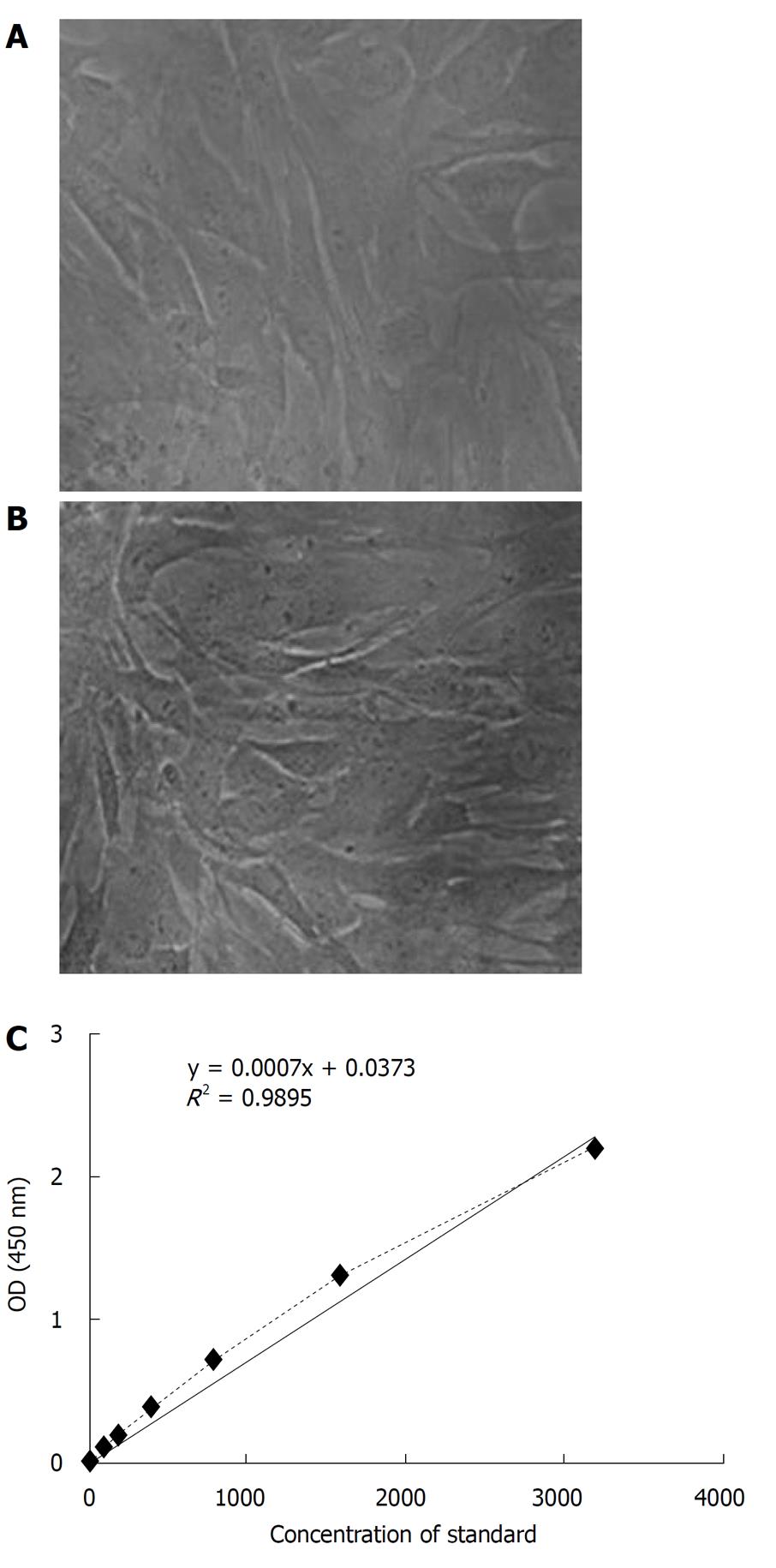
To determine possible infection of the HS/PC product by exogenous viruses, the cell suspension of HS/PC product was evaluated by in vitro culture with the human fibroblast cells. Figure 2A and B shows that the cell suspension of the HS/PC product had no effect on the cellular morphology and growth potential of human fibroblast cells. Innoculation experiments on SPF mice inoculation and on avian embyros failed to detect any abnormalities according to the Pharmacopoeia of the People’s Republic of China (PC, 2005 Edition, p363-364), the HS/PC product was determined to contain no infection of exogenous virus.
The BET method was used to detect the possible bacterial endotoxin concentration. The determining limitation (L) was calculated using the formula L = K/M and found to be 6 EU/mL, so MVD was 48. Then, the recheck of TAL sensitivity was calculated using λc = lg-1 (ΣX/4), determined to be 0.204. Because λc was between 2.0-0.5λ (0.25-0.06 EU/mL), TAL sensitivity was qualified as per Table 1. For the interfering test of the HS/PC product, ES was determined between 2.0-0.5λ (0.25-0.06 EU/mL) when tested with 0.125 EU/mL TAL. There was no interference of the HS/PC product with 48 dilutions as per Table 2. Therefore, the bacterial endotoxin concentration of HS/PC product did not exceed the test limitation (6 EU/mL, see Table 3).
| Tube | C solution(EU/mL) | D solution | Ending concentration | × (lg Ending con) | |||
| 0.25 | 0.125 | 0.063 | 0.032 | ||||
| 1 | + | + | - | - | - | 0.125 | -0.9208 |
| 2 | + | + | - | - | - | 0.125 | -0.9208 |
| 3 | + | + | + | - | - | 0.060 | -1.2000 |
| 4 | + | + | - | - | - | 0.125 | -0.9208 |
| TAL (0.125EU/mL) | Bacterial endotoxin concentration (EU/mL) | Negative control | E (EU/mL) | |||
| 0.25 | 0.125 | 0.063 | 0.032 | |||
| A solution | + + | + + | - - | - - | - - | 0.125 |
| B solution | + + | - - | - - | - - | - - | 0.25 |
| C solution | + + | - - | - - | - - | - - | 0.25 |
| Group | Result | |
| A solution | - | - |
| B solution | + | + |
| C solution | + | + |
| D solution | - | - |
Residual SCF in the HS/PC product was detected by ELISA. A standard curve was constructed by plotting the mean absorbance for each standard against the concentration and a best fitting curve was drawn (Figure 2C). From this standard curev, the residual SCF in the HS/PC product was calculated as 75 pg/mL. This was greatly reduced in comparison with the 50 ng/mL of exogenous SCF added in the serum-free co-culture system.
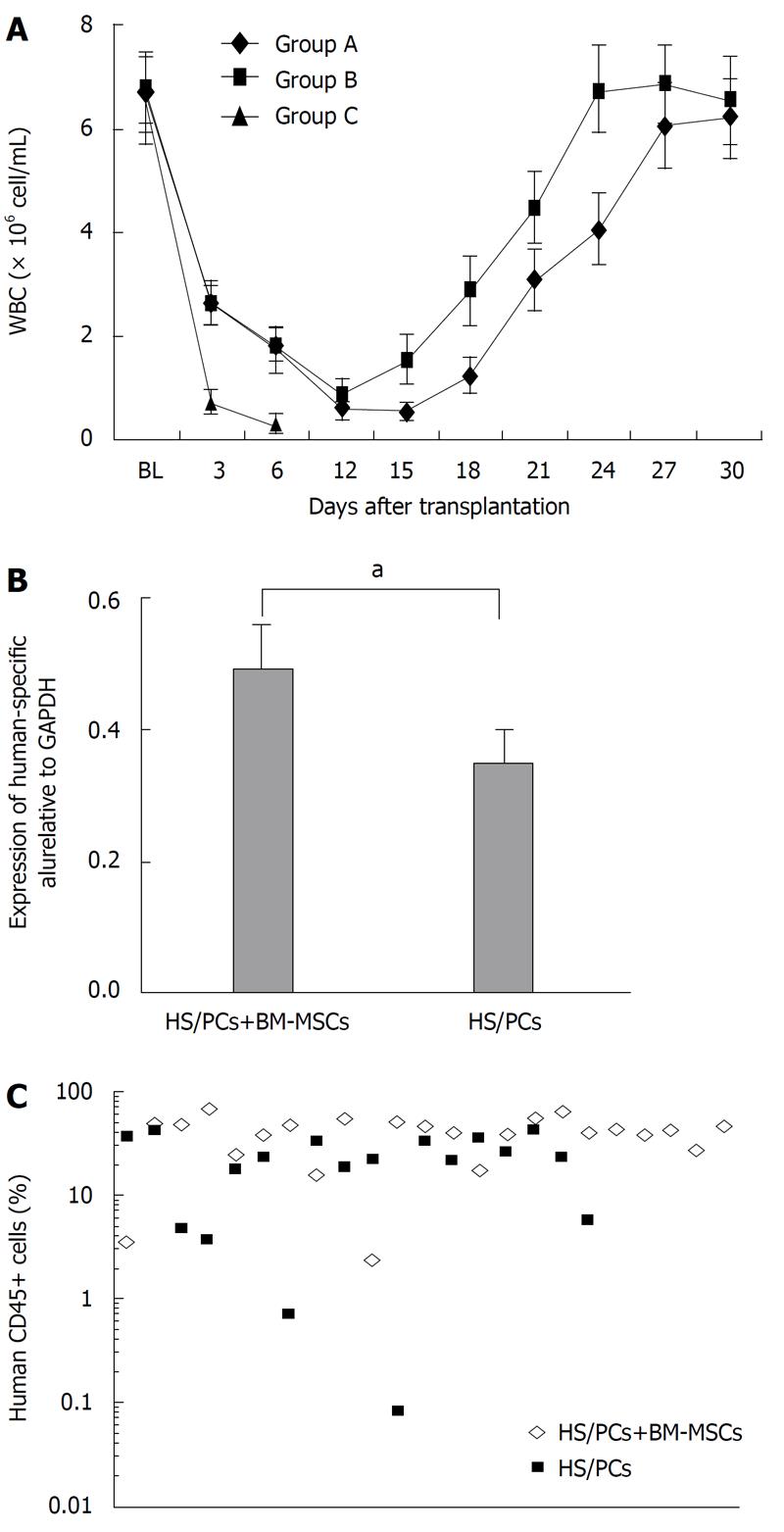
The in vivo engraftment potential of cells from the HS/PC product in NOD/SCID mice was determined with three transplantation treatments, as described in Materials and Methods. All mice in the control group (Group C) were dead within 12 d. In transplantation Group A, two mice died at the 13th d, and two mice died on the 15th and 17th d after transplantation, respectively. In the transplantation Group B, two mice died on the 20th and 23th d after transplantation,respectively. The numbers of white blood cells (WBCs) in mice after transplantation are shown in Figure 3A. The number of WBCs in mice transplanted only with cells from the HS/PC product (Group A) decreased until the 18th d after transplantation. Then, WBCs increased and reached baseline pretreatment levels at 27-29 d after transplantation. WBCs in mice transplanted with the combination of HS/PCs and BM-MSCs (Group B) showed similar growth dynamics to Group A. However, the recovery of hematopoiesis was faster than that of Group A. WBCs in mice of Group B started increasing on the 15th d and reached baseline pretreatment levels on the 24th d after transplantation.
Thirty days after transplantation, mice were killed and bone marrow was collected to determine the homing of human cells in the bone marrow. RT-PCR analysis demonstrated the presence of human hematopoietic cells in the bone marrow of NOD/SCID mice transplanted with cells from the HS/PC product and and with the combination of expanded HS/PCs and BM-MSCs. However, the expression level of the human Alu gene and the percentage of human CD45+ cells in the bone marrow of mice transplanted with the combination of expanded HS/PCs and BM-MSCs was significantly higher than that of mice transplanted with only HS/PCs from UCB (P < 0.05) (Figure 3B and C). As a negative control, the expression of human the Alu gene in normal mouse bone marrow was not detected (data not shown).
Co-culture with BM-MSCs can promote the expansion of HS/PCs. BM-MSCs provide biological support for HS/PCs by constitutively secreting cytokines important for HS/PC differentiation, including interleukin (IL)-1, IL-6, IL-7, IL-8, IL-11, IL-12, IL-14, IL-15, IL-27, leukemia inhibitory factor (LIF), Flt-3 ligand, SCF, G-CSF and GM-CSF[19]. Another important characteristic of BM-MSCs that relates to hematopoiesis is cell-to-cell contact. BM-MSCs express surface molecules that can interact with cells of the hematopoietic lineage, including Intercellular Adhesion Molecule (ICAM)-1 (CD54), ICAM-2 (CD102), Vascular Cell Adhesion Molecule (VCAM-1, CD106), Lymphocyte Function-associated Antigen 3 (LFA-3, CD58), Activated Leukocyte Cellular Adhesion Molecule (ALCAM, CD166), Hyaluronate Receptor (HCAM, CD44) and integrins such as Very Late Antigen (VLA, CD49)[20]. Through interaction with adhesion molecules, the HS/PCs can adhere to the BM-MSCs, and direct contact is essential for the expansion of HS/PCs. The study by Kawad et al[4] showed that not only the effectiveness of in vitro expansion by direct contact of HS/PCs with BM-MSCs is higher than the no contact control, but also the area of direct contact has a positive correlation with the effectiveness of HS/PC expansion. This suggested that there might be an essential ligand-accepter relationship between the cellular membrane surfaces of BM-MSCs and HS/PCs. Only after contact between the cells, can the proliferation and differentiation of HS/PCs be regulated by the ligand-accepter effect. Nowadays, when HS/PCs are cultured and expanded in vitro, BM-MSCs are always used as a feeder layer combining with cytokines to promote the efficiency of HS/PC amplification. Here, the expansion levels of HS/PCs from UCB in two co-culture systems with BM-MSCs as the feeder layer were higher than that in the culture system without BM-MSCs, confirming that BM-MSCs supported the expansion of HS/PCs through providing a hematopoirtic microenvironment. In determining the proliferation potential of HS/PCs, we analyzed only the expansion level of cells suspended in the medium, although there was the evidence of HS/PC-derived cobblestone-forming hematopoietic cells beneath the BM-MSC layer[21]. However, the expansion levels of TNCs, CD34+ cells and colony-forming cells suspended in the medium were enough to demonstrate the capacity of BM-MSCs for supporting the ex vivo expansion of HS/PCs from UCB.
Bovine serum or equine serum is always added during in vitro expansion of HS/PCs. Therefore, there may be residual serum albumin (SA) in cellular products. SA is a sensitinogen to humans which may affect the human immune system. There are two main ways to solve this problem: (1) use of SFM (serum-free medium); and (2) limiting residual bovine serum. We use BM-MSCs as a feeder layer to co-culture MNCs from UCB in a serum-free culture system supplemented with three cytokines. Ous results showed that the difference in CD34+ cells and colony-forming cell expansion potential between the two co-culture systems with and without serum was not statistically significant although the number of TNCs expanded in the serum-free co-culture system was less than that in the co-culture system with serum. However, the degree of expansion of TNCs, CD34+ and colony-forming cells in the serum-free co-culture system were higher than in the culture system without BM-MSCs. We can infer that BM-MSCs support the ex vivo expansion of HS/PCs, especially the ex vivo expansion of hematopoietic stem cells (HSCs). Therefore, the serum-free co-culture system with BM-MSCs can be recommended for culture of HS/PCs from UCB for clinical transplantation.
BM-MSCs have become the attractive for clinical use due to their multi-differentiation potential, easy isolation and culture as well as for their high ex vivo expansive potential[20,22]. Co-transplantation of BM-MSCs is capable of enhancing engraftment of HS/PCs in a fetal sheep model[23-25], suggesting that co-transplantation of BM-MSCs and HS/PCs from human UCB results in the acceleration of short-, medium- and long-term engraftments of HS/PCs. Our experiments on transplantation in NOD/SCID mice indicated the reconstituting ability of expanded HS/PCs in the irradiated mice. The co-transplantation of expanded HS/PCs with BM-MSCs from the serum-free co-culture system promoted greater engraftment of HS/PCs in recipients in comparison to the transplantation of HS/PCs expanded alone. It is thought that BM-MSCs enhance engraftment and support hematopoiesis by mechanisms that may promote either homing or proliferation of HS/PCs through release of cytokines[20]. It is well known that stromal derived factor (SDF)-1 has significant importance in the homing of HS/PCs to their niche in the bone marrow. It was demonstrated that SDF-1 effects the recruitment of CD34+ cells to the marrow in a NOD/SCID model of human hematopoiesis[19]. Thus, BM-MSCs have a role in homing both by inducing the expression of SDF-1 via secretion of stromal cell factor (SCF), and directly by secretion of SDF-1. Primary BM-MSCs derived from adult sources promote the engraftment of UCB-derived CD34+ cells to a similar degree as culture-expanded BM-MSCs, indicating that the biological properties of primary BM-MSCs are preserved during expansion. This important for the safety of culture-expanded cells in potential therapeutic application[26].
A series of studies indicates that BM-MSCs escape recognition by alloreactive cells, and are immune privileged[27]. BM-MSCs generally express only HLA class I. Despite a few studies suggesting that BM-MSCs could be induced by IFN-γ to up-regulate the expression of HLA class II, neither the BM-MSCs that expressed HLA class I only, nor the BM-MSCs that expressed both HLA class I and class II induced by IFN-γ, showed immunogenic potential. Furthermore, both cell types could inhibit T cell immune responses, and the up-regulation of HLA class II by IFN-γ did not elicit a proliferative response of T cells[27,28]. BM-MSCs have been shown both in vivo[29,30] and in vitro[31,32] to suppress T cell activation. Additionally, BM-MSCs have been shown to suppress the proliferation of activated T cells induced by allo-antigens in the mixed lymphocyte reaction (MLR)[31] or induced by mitogens such as phytohemagglutinin[33], or concanavalin A[27], as well as the activation of T cells by CD3 and CD28 antibody stimulation[34]. Several studies have shown similar effects when using BM-MSCs that are autologous or allogeneic to the responder cells, indicating a genetically unrestricted suppression[19]. Having low immunogenicity, BM-MSCs have no requirement for HLA matching and should, therefore, be considered for co-transplantation with HS/PCs in therapeutic applications.
Patients with hematologic malignancies must undergo a flushing dose of radiotherapy or chemotherapy before HSCT treatment, in order to eliminate cancer cells that could otherwise cause the killing of bone marrow cells and inhibition of the activity of immune cells. Therefore, the contamination of the expanded cellular product could result in serious post-transplantation infection in recipients. Bacterial endotoxin,released by Gram-negative bacteria, is and exogenous pyrogen that can activate heterophil granulocytes to release an endogenous pyrogen which will cause fever by acting upon the body’s temperature regulating center[35]. The expanded cellular product must be rigorously controlled before transplantation to make sure that it is free from xenogeneic cell pathogens and exogenous contaminants from isolation and culture of cells which could jeopardize the patient. The HS/PC product of the serum-free co-culture system was tested for the possibility of contamination by exogenous pathogens and other material according to the PC and China Biological Regulations. The results showed that there no contamination or infection was present in the HS/PC products. In the culture system used, three cytokines were added to the medium. TPO and G-CSF used in the culture system were commercial products approved for clinical application. Because the SCF used in the culture system was only approved for laboratory application, the content of residual SCF in the HS/PC product was determined. The results showed that the levels of endotoxin and residual SCF l in the HS/PC products were below the allowed limits. These safety standards will be applied in the preparation of HS/PC products for clinical transplantation.
Umbilical cord blood transplantation (UCBT) has been used in treatment of hematologic malignancies, aplastic anemia, hemoglobinopathies, and severe combined immunodeficiency and so on. However, the low quantity of hematopoietic/progenitor stem cells (HS/PCs) in UCB may lead to the failure of transplantation, and the contamination of the cellular product could even cause death of transplanted patient. Therefore, for the clinical application of USB it is important to prepare sufficient HS/PCs from UCB that are contamination-free, according to clinical standards.
In recent years, co-culture HS/PCs with BM-MSCs has been used to expand HS/PCs. bone marrow-mesenchymal stem cells (BM-MSCs) can secrete various soluble factors and provide the direct cell-cell contact, which are important to the proliferation and differentiation of HS/PCs. However, the cell product harvested from co-culture system is prone to be contaminated. So, before transplantation, it should be carefully determined whether there is any contamination of the HS/PC product by bacteria, fungi, mycoplasmas or exogenous viruses, and whether the transplantation of the HS/PC product is effective for therapeutic applications.
In this study, the authors used the co-culture system to improve the proliferation and differentiation of HS/PCs. Through the stringent control of the co-culture system, such as in the quality of both the HS/PCs and BM-MSCs, the amount of the cytokines and the time of co-culture, the TNCs and CD34+ cells were greatly increased. This HS/PC product is safe for clinical transplantation, without any contamination by bacteria, fungi and mycoplasmas, and with concentrations of bacterial endotoxin and residual SCF residual which are too low to be harmful. Because of the high quality of our product, the rapid engraftment of hematopoietic cells can be effectively established, as demonstrated by the transplantation experiments in mice.
This research has finally established an efficient and safe way to expand HS/PCs, which can effectively achieve the rapid engraftment of hematopoietic cells. Using this method, the authors have produced the HS/PC product, and its high quality has been acknowledged by the National Institutes for Food and Drug Control. So, this research provides both the theoretical and experimental foundation for the clinical application of this HS/PC product.
This research provides a procedure for effectively expansion of HS/PCs, and confirms that HS/PC product is clinically safe for clinical transplantation. The HS/PC product is efficient in the rapid engraftment of hematopoietic cells.
Peer reviewer: Kristbjorn Orri Gudmundsson, PhD, Laboratory of Cancer Prevention, Hematopoiesis and Stem Cell Biology Section, 1050 Boyles Street, Building 560, Room 12-03, NCI-Frederick, Frederick, MD 21702-1201, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Ma WH
| 1. | Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28:1197-1205. [PubMed] |
| 2. | Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 719] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | da Silva CL, Gonçalves R, dos Santos F, Andrade PZ, Almeida-Porada G, Cabral JM. Dynamic cell-cell interactions between cord blood haematopoietic progenitors and the cellular niche are essential for the expansion of CD34+, CD34+CD38- and early lymphoid CD7+ cells. J Tissue Eng Regen Med. 2010;4:149-158. [PubMed] |
| 4. | Kawada H, Ando K, Tsuji T, Shimakura Y, Nakamura Y, Chargui J, Hagihara M, Itagaki H, Shimizu T, Inokuchi S. Rapid ex vivo expansion of human umbilical cord hematopoietic progenitors using a novel culture system. Exp Hematol. 1999;27:904-915. [PubMed] |
| 5. | Wang JF, Wang LJ, Harrintong J, McNiece I K. Effects of bone marrow-derived mesenchymal stem cell layer on expansion and differentiation of hematopoietic stem cells from cord blood. J of Zhejiang Univer (Sci Ed). 2003;30:93-97. [PubMed] |
| 6. | Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E, Daum-Woods K, Goetchius GW, Fu P, Welniak LA, Murphy WJ, Laughlin MJ. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(-) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Koh SH, Choi HS, Park ES, Kang HJ, Ahn HS, Shin HY. Co-culture of human CD34+ cells with mesenchymal stem cells increases the survival of CD34+ cells against the 5-aza-deoxycytidine- or trichostatin A-induced cell death. Biochem Biophys Res Commun. 2005;329:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, Stoltz JF, Eljaafari A. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol. 2007;35:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Fujimoto N, Fujita S, Tsuji T, Toguchida J, Ida K, Suginami H, Iwata H. Microencapsulated feeder cells as a source of soluble factors for expansion of CD34(+) hematopoietic stem cells. Biomaterials. 2007;28:4795-4805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 10. | Fei XM, Wu YJ, Chang Z, Miao KR, Tang YH, Zhou XY, Wang LX, Pan QQ, Wang CY. Co-culture of cord blood CD34(+) cells with human BM mesenchymal stromal cells enhances short-term engraftment of cord blood cells in NOD/SCID mice. Cytotherapy. 2007;9:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Chen X, Xu H, Wan C, McCaigue M, Li G. Bioreactor expansion of human adult bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Goldman M, Sher G, Blajchman M. Bacterial contamination of cellular blood products: the Canadian perspective. Transfus Sci. 2000;23:17-19. [PubMed] |
| 13. | Ribault S, Faucon A, Grave L, Nannini P, Faure IB. Detection of bacteria in red blood cell concentrates by the Scansystem method. J Clin Microbiol. 2005;43:2251-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, Hervé P. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002;42:1356-1364. [PubMed] |
| 15. | WHO Expert Committee On Biological Standardization. World Health Organ Tech Rep Ser. 1998;878:i-vi, 1-101. [PubMed] |
| 16. | FDA. Points to consider in the characterization of cell lines used to produce biologicals. 1993;. |
| 17. | Points to consider in the manufacture and testing of monoclonal antibody products for human use (1997). U.S. Food and Drug Administration Center for Biologics Evaluation and Research. J Immunother. 1997;20:214-243. [PubMed] |
| 18. | Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH, McNiece IK, Wang JF. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16:579-585. [PubMed] |
| 19. | Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Fibbe WE, Noort WA. Mesenchymal stem cells and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003;996:235-244. [PubMed] |
| 21. | Xie CG, Wang JF, Xiang Y, Jia BB, Qiu LY, Wang LJ, Wang GZ, Huang GP. Marrow mesenchymal stem cells transduced with TPO/FL genes as support for ex vivo expansion of hematopoietic stem/progenitor cells. Cell Mol Life Sci. 2005;62:2495-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226:507-520. [PubMed] |
| 23. | Anklesaria P, Kase K, Glowacki J, Holland CA, Sakakeeny MA, Wright JA, FitzGerald TJ, Lee CY, Greenberger JS. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci U S A. 1987;84:7681-7685. [PubMed] |
| 24. | Almeida-Porada G, Flake AW, Glimp HA, Zanjani ED. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27:1569-1575. [PubMed] |
| 25. | Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620-3627. [PubMed] |
| 26. | in 't Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31:881-889. [PubMed] |
| 27. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [PubMed] |
| 28. | Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 29. | Kim DW, Chung YJ, Kim TG, Kim YL, Oh IH. Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood. 2004;103:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 2029] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 31. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [PubMed] |
| 32. | Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761-7767. [PubMed] |
| 33. | Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, Mao N. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 35. | National Pharmacopoeia Community. 3rd ed. Appendix, Edition. . : Pharmacopoeia of the People's Republic of China 2005; 360-368. |