Published online Nov 26, 2011. doi: 10.4252/wjsc.v3.i11.104
Revised: September 30, 2011
Accepted: October 5, 2011
Published online: November 26, 2011
AIM: To study the immunophenotype of hematopoietic progenitor cells from cord blood (CB) grafts (n = 39) in comparison with adult apheresis grafts (AG, n = 229) and pre-apheresis peripheral blood (PAPB) samples (n = 908) using flow cytometry analysis.
METHODS: First, we performed a qualitative analysis of CD34+ cell sub-populations in both CB and PAPB grafts using the standardized ISHAGE protocol and a wide panel of 20 monoclonal antibodies. Next, we studied some parameters, such as the age of mothers and the weight of newborns, which can influence the quality and the quantity of CD34+ cells from CB.
RESULTS: We found that the percentage of apoptotic cells was high in CB in comparison to PAPB (PAPB: 4.6% ± 2.6% vs CB: 53.4% ± 5.2%, P < 0.001). In CB, the weight of newborn and the age of the mother have the influence on CD34+ cells. The follow-up of Ag CD133 in the ISHAGE double platform protocol in association with CD45, CD34 and the 7’AAD shows an equal rate between the two cell populations CD133+CD45+CD34+ high and CD34+CD45+ high with a higher percentage. So, is the inclusion of Ac CD133 necessary in the present panel included in the ISHAGE method? Last part, we showed a significant presence of interferon γ in CB in comparison to PAPB, the annexin showing the high number of apoptotic cells in CB.
CONCLUSION: This study demonstrates that many different obstetric factors must be taken into account when processing and cryo-banking umbilical CB units for transplantation.
- Citation: Azouna NB, Berraeis L, Regaya Z, Jenhani F. Immunophenotyping of hematopoietic progenitor cells: Comparison between cord blood and adult mobilized blood grafts. World J Stem Cells 2011; 3(11): 104-112
- URL: https://www.wjgnet.com/1948-0210/full/v3/i11/104.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i11.104
The global rise in the use of umbilical cord blood (UCB) as a transplant source has been amazing; over 20 000 transplants have already taken place alone[1-3]. It has become a real alternative to bone marrow (BM) and peripheral blood as a source of adult stem cells to treat multiple diseases.
UCB has become such a popular adult stem cell source for many reasons, not least because over 130 million births per annum worldwide represent the largest, easily available stem cell source. It also allows for storage of units from ethnic minorities not easily possible within BM registries[2,3]. This potentially allows for an increase in the rate of matched unrelated donor allogenic transplants[3]. It has also been found that there is a lower risk of graft versus host disease (GvHD) when transplanting UCB when compared to BM[3-6].
Although a valuable source of hematopoietic stem cells (HSCs), in order to bank UCB units suitable for transplantation effectively, samples need to be characterized and obstetric factors which impact upon UCB quality should be further examined. In this study, we compared two different parts of UCB: before placenta delivery (CB) and after placenta delivery (PlaB). For this comparison we used four different physiological parameters that pertain to either the baby or the mother and we compared levels of HSC CD34+. The four different parameters were: number of pregnancies of mothers, mother’s age at delivery, newborn weight and newborn’s sex. Previous studies show that some patterns have already emerged. Birth weight impacted on HSC concentrations, especially mid-stage HSC[6-8]. When looking at mother’s age, a previous study demonstrated that HSC concentration is greatly reduced as age increases[7]. Infant gender has previously been found to have an impact on HSC of UCB samples and newborn boys appear to have fewer stem cells than girls[8,9] whereas other works showed that the newborn’s sex was not found to be significant to influence HSC in UCB. The number of pregnancies was also studied and seems to have an impact on HSC concentrations in UCB[7,8].
The principle aim of this study was to optimize UCB separation and cryopreservation by the characterization of these cellular groups. Several physiological factors were examined in order to determine the most suitable method. However, some of these findings appeared themselves to be of particular interest. In the last part of this work, variable levels of immaturity were detected on pre-apheresis peripheral blood (PAPB) and UCB populations using CD34, CD133 and CD45 antigens. In parallel, we analyzed some antigens to compare between these two HSC sources.
PAPB samples (n = 190) were collected from patients from the Hematology Department at Aziza Othmana Hospital, the National Center of Bone Marrow Transplantation, Salah Azaiez Hospital, the Military Hospital and the National Blood center (Tunis, Tunisia). These patients, suffering from various conditions including: 34 acute myeloid leukemia, 24 acute lymphoblastic leukemia, 5 chronic myelocytic leukemia, 32 Medullar Aplasis, 31 multiple myelomas, 4 Diffuse Large Cell B Lymphomas, 13 Fanconi disease, 4 Gaucher disease, 6 Drepanocytosis, 2 β-Thalassemic, 24 Hodgkin’s diseases, 6 Non Hodgkin’s diseases, 1 mantle cell lymphoma and 1 Kahler’s disease were destined for autologous or allogenic HSC transplantation performed at the National Centre of Bone Marrow Transplantation. Blood samples were taken in tubes containing EDTA as anticoagulant (Vacutainer®, Becton-Dickinson). These patients consisted of 89 women and 98 men whose mean age was 39.5 years. We noted that these PAPB samples are collected from patients after treatment with conditioning factors such as as G-CSF.
Apheresis graft (AG) samples were collected in the same patients (n = 189) who were studied in PAPB.
Cord blood (CB) and PlaB samples were collected from women (n = 39) who had delivered at the Wassila Bourguiba maternity centre in Tunis and whose mean age (± SE) was 28.4 ± 4.4 years. After the umbilical cord was clamped off from the newborn, the CB was collected by aspiration using a syringe needle in a sterile tube containing EDTA as anticoagulant (Vacutainer®, Becton-Dickinson). Two collection methods were used by blood punction: (1) from the maternal end of the severed cord after vaginal delivery of the infant while the placenta was still in uterus (CB sample); and (2) from placenta-umbilical cord junction after placenta delivery (PlaB sample).
Numbers of CD34+ cells were derived from either the flow cytometry assessed per cent CD34+ cells within the nucleated cells and/or the white blood cell count from a hematology cell analyzer. All the samples were processed within 1 h of collection.
Four-color flow cytometric analysis was performed with Cell Quest Pro (Becton Dickinson), as follows. Whole blood cells were stained with appropriate amounts of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin-chlorophyll-a protein- and allophycocyanin (APC) conjugated to the following monoclonal antibodies at 4°C for 20 min: mouse anti-human- anti-CD34-FITC, anti-CD45-FITC, anti-CD133- APC, anti-CD19-PE, anti-CD38-FITC, anti-CD11c-FITC, anti-CD25-FITC, anti-CD117-PE, anti-HLA-DR-FITC, - anti-CD7-FITC, anti-CD33-PE, anti-CD20-FITC, anti-CD15-FITC, anti-CD56-PE and anti- CD10-PE.
All McAbss were obtained from Becton Dickinson. The intracellular-antigens were: anti-TdT-PE, anti-IL2-PE, anti-IL4-PE and anti-interferon (IFN)α-FITC. The cell viability marker 7’AAD was used to exclude dead or apoptotic cells or DNA colorants to mark the cells and exclude the fragments.
Red blood cells were then lysed with FACS lysing solution (Becton-Dickinson, San Diego, CA). The cells were washed twice and suspended in phosphate-buffered saline. Analysis was performed on cells within the mononuclear light scatter and side scatter and on CD34-positive cells by CD34-positive staining and side scatter. A total of at least 100 000 events were analyzed for each sample.
The total number of CD34+ cells from PAPB, AG or CB samples was measured by direct flow cytometry. The results were expressed as %CD34+ cells and the number of CD34+ cells/μL.
Means were compared statistically using the STATGRAPHICS Centurion XVI program, version 16.0.08. A one-way analysis of variance and Newman-Keuls multiple range test were carried out to determine any significant difference between group values at P < 0.05. Values are mean ± SE.
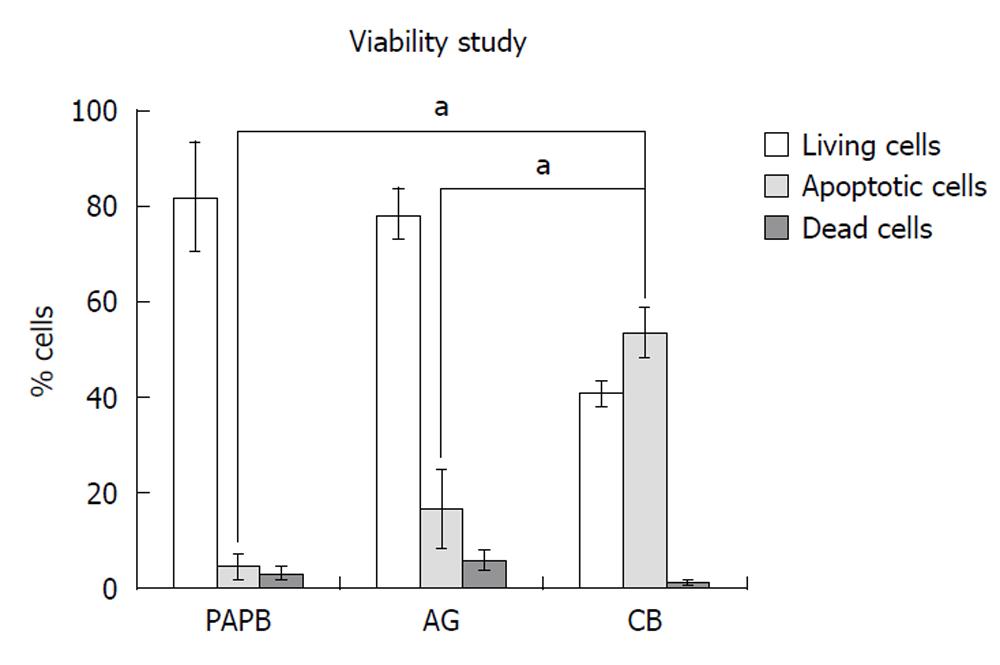
The cell viability determined by the 7’AAD staining of each biological sample (Figure 1) allowed us to distinguish three cell populations: (1) living cells (7’AADneg), (2) dead cells (7’AADpos); and (3) apoptotic cells (7’AADlow).
Thus, for PAPB samples mean percentage of living cells was 82.0% ± 11.9% that of dead cells 13.4% ± 12.4% and that of apoptotic cells 4.6% ± 2.6%. For AG samples, this percentage was 49% ± 14.4%, 33.5% ± 13.4% and 18.3% ± 8.4% respectively. For CB samples, this percentage was 40.7% ± 2.7%, 1.1% ± 0.37% and 53.4% ± 5.2%, respectively (P = 0.0003).
From all the 190 PAPB samples, the mean CD34+ cell counts in PAPB was 80.5 ± 5.8 × 106 per kg. Forty-five per cent of patients had counts less than 50 cells/μL, 25% between 50 and 100 cells/μL, 18 % between 100 and 200 cells/μL and 12% above 200 cells/μL.
From 189 AG, mean CD34+ cell count was 5 ± 0.3 × 106 per kg in collected grafts. Only one aphaeresis was performed in 52% of the patients, with a mean of (2500 ± 181.9 CD34+ cells/μL), while 36% of the patients were collected after two aphaeresis (1365.4 ± 128.5 CD34+ cells/μL), and only 10% of patients had more than 2 aphaeresis (978 ± 217 CD34+ cells/μL).
From 39 CB samples, mean CD34+ cell count was 16.0 ± 3.5/μL, while from PlaB samples this mean was 14.57 ± 2.9/μL. No Statistical significance between CB and PlaB samples for CD34+ was found (P = 0.31).
The most important feature with CB samples and PlaB samples was a much wider variation rate of CD34+ cell counts compared to PAPB and AG samples. This finding led us to investigate several factors that could affect CB and PlaB CD34+ cell content, including those related to the mother such as age, parity, blood group and newborn’s sex.
We used a panel of 20 different McAbs to determine precisely the different subpopulations of CD34+ cells present in PAPB, AG, CB and PlaB samples.
We compared three sources of CD34+ cells (PAPB, AG and CB), the samples were gated on 100% living CD45+CD34+ cells.
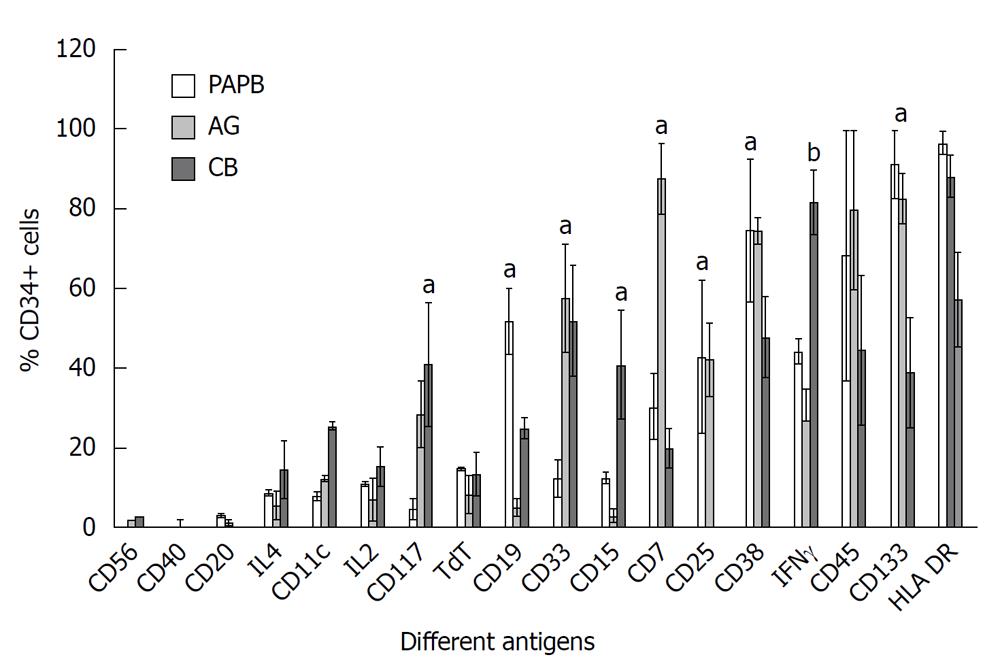
Some antigens were strongly expressed on CD34+ cells in PAPB in comparison to CB such as CD133+ (91.3% ± 8.6% in PAPB vs 39% ± 13.8%, P = 0.04) and CD38 (74.8% ± 17.9% in PAPB vs 47.8% ± 10.1%, P < 0.05). Other antigens were also strongly expressed but little statistical significance in expression was found such as: HLA-DR+ (96.7% ± 3% in PAPB vs 57.3% ± 11.8% in CB) and CD45+ (68.5% ± 31.4% in PAPB vs 44.6% ± 18.7% in CB, P > 0.05) (Figure 2).
The intracellular antigen IFNγ appeared in CB in a higher percentage than PAPB and AG (81.7% ± 8.1% in CB vs 27.1% ± 7.7% in AG and 44.3% ± 3% in PAPB, P < 0.0001). Also the same statistical result was found for CD33+ antigen: (52% ± 13.8% in CB and 57.7% ± 13.6% in AG vs only 12.5% ± 4.6%, P = 0.01) (Figure 2).
Other antigens were moderately expressed like CD25 with a higher percentage in PAPB in comparison to CB (43% ± 19.1% in PAPB and 42.2% ± 9.1% in AG vs 0% in CB, P = 0.01), CD19 (51.9% ± 8.3% in PAPB vs 5.14% ± 2.2% in AG and 25% ± 2.5% in CB, P < 0.05) and CD7 with a low occurrence in CB (30.4% ± 8.2% in PAPB and 87.8% ± 8.9% in AG vs 20% ± 5% in CB, P = 0.01) whereas CD15 presented a high percentage in CB (41% ± 13.8% in CB vs 3% ± 1.6% in AG and 12.5% ± 1.3%, P = 0.01), also CD33 presented a statistically significant level in CB (52% ± 13.8%) and AG (57.7% ± 13.6%) compared with the low percentage in PAPB (12.5% ± 4.6%, P = 0.01).
Finally, a few antigens were practically absent in the three different CD34+ sources such as: CD11c, CD20, CD10 and CD56 (Figure 2).
Comparable results were found for CD117 antigen which was expressed in higher proportions in CB than in PAPB with mean values of (41.0% ± 15.4% in CB vs 4.84% ± 2.7%, P = 0.01) (Figure 2).
Analysis of the factors that could influence CD34+ cell content in CB and placental blood: Number of pregnancies, mother’s age, newborn’s weight and newborn’s gender
In this study, many parameters that can impact on CD34+ cells from CB were analyzed. For every parameter we compared two different parts of the umbilical cord: CB is the part near to the newborn and PlaB is part of placenta after delivery.
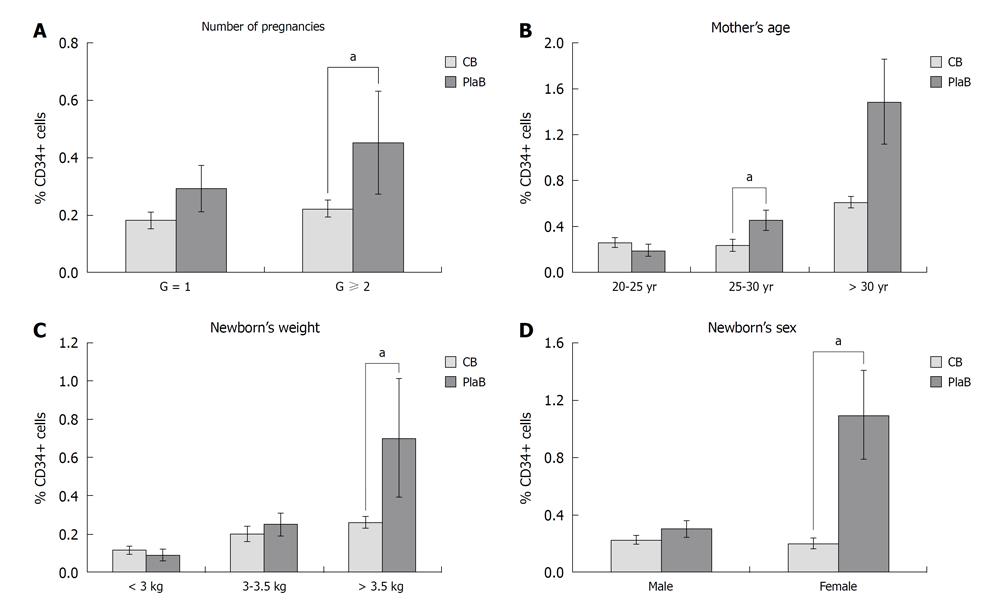
The number of pregnancies ranged from 1 to 3. This parameter appears to have an impact on CB CD34+ cell counts. Thus, CBs from multiparous women (G > 2) displayed higher CD34+ cell counts than that from primiparous women (G = 1) (0.22% ± 0.03% vs 0.18% ± 0.03%). Figure 3A illustrates the relationship between the number of pregnancies and CD34+ cell counts in CB and in PlaB. This difference was statistically clear in multiparous women (0.45% ± 0.18% vs 0.29% ± 0.08%, P = 0.02) (Figure 3A).
The relationship between mother’s age at delivery and CD34+ cell counts was also examined (Figure 3B). The age range of the 39 women studied was between 19-39 years with a mean age of 28.26 ± 4.4 years.
Mothers under 25 years presented (CB: 0.21% ± 0.04% vs PlaB: 0.18% ± 0.05%, P = 0.35), and over 30 years presented (CB: 0.26% ± 0.05% vs PlaB: 0.84% ± 0.56%, P = 0.1), these results were not statistically significant. Those mothers aged between 25-30 years tended to have lower CD34+ cell counts (CB: 0.2% ± 0.05% vs PlaB: 0.45% ± 0.09%, P = 0.02) with a significant difference.
Newborn’s weight appeared to influence CD34+ counts in CB samples. Thus, CD34+ counts were higher with newborns above 3.5 kg body weight than with newborns beneath 3 kg body weight (mean values 0.26% ± 0.03% vs 0.11% ± 0.02%). The difference between the UCB sites (CB and PlaB) was statistically significant only for body weight superior to 3.5 kg (0.7% ± 0.31%) (P = 0.05) (Figure 3C).
Newborn’s gender presented a significant difference: female CD34+% samples differed between PlaB and CB (CB: 0.19% ± 0.04% vs PlaB: 0.74% ± 0.36%, P = 0.02) whereas the male samples did not show a significant difference between the two sources (Figure 3D).
Another parameter was studied: mother’s blood group, but it had no statistically significant influence on CD34+ cell counts.
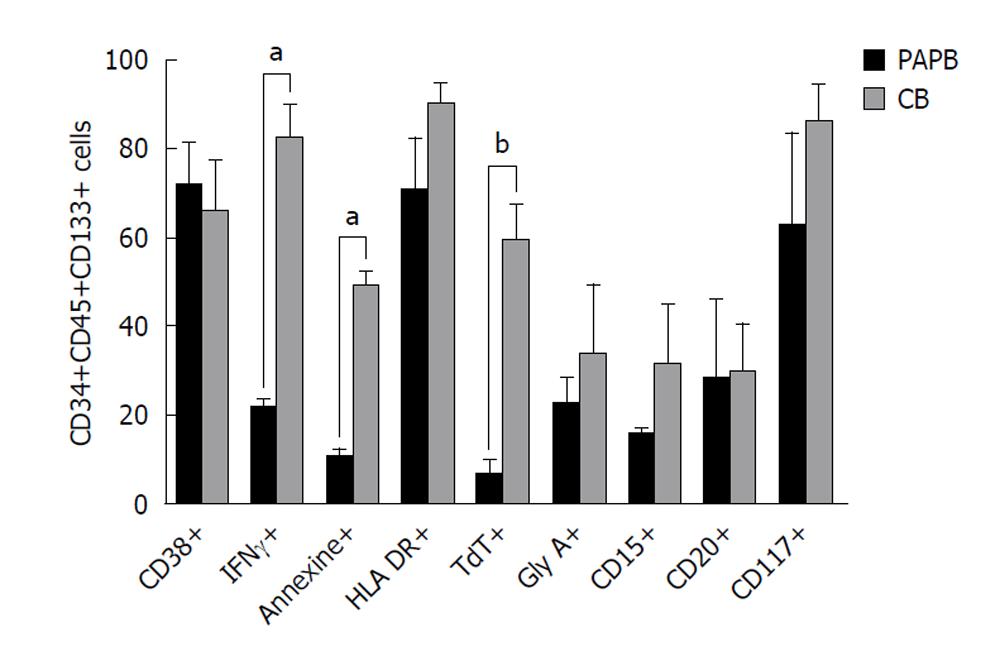
In this part of study, we were interested in the CD34+CD45+CD133+ cell population. We analyzed different antigen expression with the objective of determining the phenotype and studying the level of immaturity between PAPB and CB.
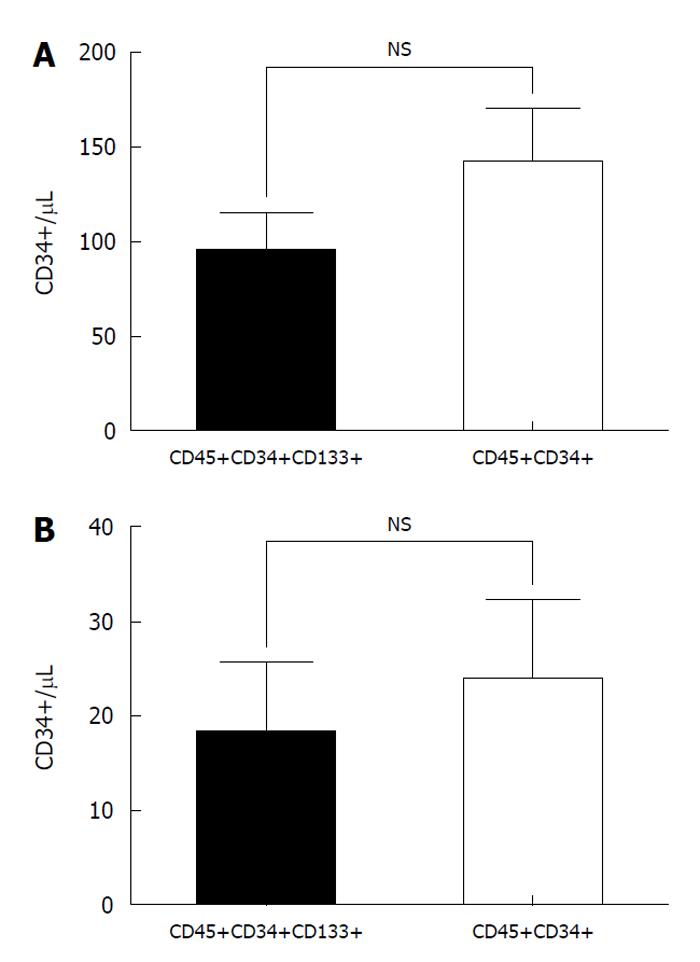
Firstly, we studied the presence of CD133 and used the panel of Abs to detect HSCs with the antigen CD34. We showed that the number of positive cells CD133+, CD45+, CD34+, 7’AAD- was equivalent to the number of CD45+ CD34+ cells. This result for PAPB: (CD133+CD45+CD34+; 94.7 ± 18.9 cells/μL vs CD45+CD34+; 142.1 ± 25.5 cells/μL, P > 0.05) (Figure 4A).
In the case of CB, the values were lower than PAPB, but the same result is obtained: equivalent numbers of positive cells (CD133+CD45+CD34+; 18.0 ± 7.7 cells/μL vs CD45+CD34+; 23.7 ± 8.6 cells/μL, P > 0.05) (Figure 4B).
Secondly, different antigens were studied: CD38, IFNγ, Annexin, HLA-DR, TdT, GlyA, CD15, CD20 and CD117. We showed that the intracellular marker IFNγ was present in a higher percentage in CB samples comparedto PAPB (81.7% ± 7.5% vs 21.2% ± 1.7%, P < 0.05).
Also, we found a similar, statistically significant result with TdT antigen (58.7% ± 8.1% in CB vs 6.2% ± 3.3% in PAPB, P < 0.01).
Our data, using Annexin, show that CB contains a significantly higher percentage apoptotic cells compared to PAPB CD34+CD45+CD133+ cells. This result is in accordance with our results of viability with 7’AAD. Here, we found the same high percentage of apoptotic cells in CB (48.8% ± 3.3% in CB vs 10.2% ± 1.9%, P < 0.05) (Figure 5).
Reserachers have long been interested in determining the best immunophenotyping technique for identifying and counting HSC CD34+.
Our work focused on the qualitative and quantitative study of HSC CD34+ cells in three samples groups: PAPB, AG and CB. The use of the viability marker 7’AAD allowed us to conduct our study using flow cytometry. Thus, each time we observed three cell populations: living cells 7’-AADneg, apoptotic cells 7’-AADdim and dead cells7’-AADpos.
Our results showed that the viability of HSC CD34+ cells, in AG samples, was reduced in comparison with PAPB (33.5% of cells 7’AADpos for AG vs 13.5% only for PAPB). The number of apoptotic cells is higher in CB (59%). Our results are similar to those described by Shim et al[10] in 2006 who confirmed, using Annexin V (as an indicator of apoptosis), the very high level of apoptotic cells in CB. This apoptosis increases even more with cryopreservation. Several studies made by different authors inform us that 7’AAD is a very important marker in the qualitative and quantitative study of HSC CD34+[11-17].
Keeney et al[18] have shown that the use of 7’AAD evaluates negatively the presence of cells CD34+/μL per sample, sometimes at 50%, suggesting that these cells are not viable and so not useful to graft; this has allowed for its integration into the routine enumerating protocol of HSC CD34+.
The qualitative analysis of HSC CD34+ in three samples types (PAPB, AG and CB) revealed differentiation markers expressed at a lower level in CB: CD133 (91.3% ± 8.6% in PAPB vs 39.0% ± 13.8% in CB): this was shown to be significant (P = 0.04).
According to the literature, the lower rate of antigen HLA-DR expression in CB has also been observed by other authors. It has, been suggested that this is an advantage in comparison with other sources of HSC CD34+ (BM) in engraftment capacity with a reduction of GvHD[19] reactions.
In our study, antigen CD133 appeared with a higher frequency in PAPB and AG than CB (91.3% ± 8.6% in PAPB vs 39.0% ± 13.8% in CB, P = 0.04). Our results agreed with those suggested by other authors. Indeed, in 2007, Tura et al[20] noted an expression percentage of phenotype CD34+ CD133+ of about 79% in PAPB, of 53% in CB and only 13% in BM. This significant difference between CB and PAPB seems to be related to the injection of growth factors like G-CSF.
The antigen CD45 is one of the most important factors in the detection of CSH CD34+ by means of different qualitative and quantitative protocols. This is why it was always included in our study panels. However, it was expressed with a weak intensity in comparison with other antigens (CD34, CD133) less than 102 on the logarithmic scale in FL1 and with variable frequencies according to the biological sample under examination: (68.56% ± 31.4% in PAPB, 80% ± 20% in AG) and a lesser rate in the case of CB: (44.66% ± 18.7%). However, this difference was not statistically significant in our study. CD45 is a surface marker restricted to the hematopoietic lineage in mature PB, with the exception of erythroid cells and platelets which causes the loss of this antigen during maturation. Ogata et al[21], suggest edthat cells (CD45+ CD34- CD38- Lin-) are probably less mature than cells (CD45+ CD34+ CD38- Lin-). Other authors[22-26] studied the co-expression of antigens CD34 and CD45 in different sources of HSC CD34+ and they noted that the ratio of CD34+ CD45+ cells appeared to be similar between CB and normal PB at 30% but it was higher in BM (60%). The level was higher still in PAPB. Indeed, we think that the level of CD34+ CD45+cells depends on conditioning and on the protocol used for this conditioning: (chemotherapy and G-CSF: 35%, chemotherapy only 20%).
The immunophenotyping of HSC CD34+ has allowed us to follow the expression frequency of the antigen CD33. Indeed, the latter was present at a lower level in PAPB (12.5% ± 4.6%) compared with CB (52.0% ± 13.8%, P = 0.01). It appeared also more highly expressed in AG samples (57.7% ± 13.6%). In 1997, the results of Sakabe et al[27] proved that CD34+ cells, in PAPB, which do not express antigens CD33, HLA-DR or CD38, contain all types of progenitors including CFU-Mix. On the contrary, the population (CD34+ CD33+) contains a large number of CFU-GM, unlike cells CD34+ CD38- which contain few CFU-GM cells.
In this study, the antigen CD38 was strongly expressed on CD34+ cells in PAPB compared to CB (74.8% ± 17.9% in PAPB vs 47.8% ± 10.1%, P < 0.05). Many researchers showed that the expression of CD38, multifunctional membrane co-enzyme, is a negative regulator in HSC. This phenomenon is still a potential research topic. The results of the percentage of cell population (CD34+ CD38-) and (CD34+ CD38+) seem contradictory from one team to another[28]. Many authors[2,3,29], note that (CD34+ CD38-) CB cells can proliferate rapidly in response to cytokine stimulation compared to those of BM.
In summary, our data shows that UCB contains a significantly higher percentage of these CD34+CD38- primitive progenitors compared to PAPB CD34+ cells. This suggests that CD34+ stem cell populations taken from UCB have higher engraftment capacity than CD34+cells from conditioned PB since they contain a higher percentage of pluripotent stem cells. This is in accordance with the successful clinical use of UCB even when a low number of cells was transplanted[2,3].
Recently, McKenzie et al[30] confirmed that the expression of antigen CD38 is not continuous in the hematopoietic hierarchy but has a high expression rate in cells involved in cell-cell interactions: CD34+ CD38+ cells have only the capacity for cell repopulation and producing progenitors in the short term. The expression of the marker CD38, beyond a certain level, becomes non-reversible and is associated with differentiation and the repopulation capacity.
Concerning Ag CD117 or c-kit, we mention in our results a low expression percentage in PAPB (4.84% ± 2.7%) compared to higher rates in CB (41.0% ± 15.4%, P = 0.01). Our results confirm the works of Sakabe et al[27] who found a 20% frequency of CD34+ cells expressing Ag c-kit in PAPB, clearly lower than the cells of BM or those deriving from CB. Moreover, three cellular fractions have been identified: c-kithigh, c-kitlow and c-kit-. The two populations CD34+ c-kithigh and CD34+c-kitlow are most abundant, at about 70%, enriched mainly by the BFU-E; while the CD34+c-kit population is the least abundant, enriched by the CFU-GM.
In 2006, Machaliński et al[31] had the idea of following the expression potential of Ag CD117 according to the age of the donors: they reported that the Ag CD117 seemed significantly higher among the donors 35 years or younger compared with thoseover 35 years.
The multiparous factor of the mothers appears to have an impact on CB CD34+ cell counts. Our CB data from multiparous women (G > 2) displayed higher CD34+ cell counts than that from primiparous women (G = 1) (0.22% ± 0.03% vs 0.18% ± 0.03%).
Indeed, recently, different authors have suggested that this parameter has an impact on CD34+ cells from CB. Gajkowska et al[32] reported that the incidence of mononuclear cells, including that of CD34+ cells, decreases significantly (P= 0.0001) with the increase of the number of gestations (from 1 to 7).
Concerning the newborn’s weight, our results showed that CD34+ counts were higher with newborns above 3.5 kg body weight than with newborns below 3 kg (mean values 0.26% ± 0.03% vs 0.11% ± 0.02%). The difference between the two sites of CB was statistically significant only for body weight over 3.5 kg (0.7% ± 0.31%, P = 0.05). Our results confirm those suggested by McGuckin et al[9].
The mother’s age at the time of delivery indicated that the younger the mother, the higher the incidence of CD34+ cells. In fact, mothers between 25-30 years tended to have lower CD34+ cell counts (CB: 0.2% ± 0.05% vs PlaB: 0.45% ± 0.09%, P = 0.02) with a significant difference between the two sites of CB samples. We notice that similar results were described by McGuckin et al[9].
The newborn’s sex seems to influence the number of CSH CD34+ cells in CB. We have noticed in our series of samples a significant difference in females between the PlaB and CB (CB: 0.19% ± 0.04% vs PlaB: 0.74% ± 0.36%, P = 0.02) whereas there was not a significant difference in males between the two sources of CD34+% cells. McGuckin et al[9] made the same observations as ours with additionally, the presence, among female babies, of a higher concentration of precocious cells (CD45+dim/CD34-/CD133+) and late cells (CD45+/CD34+/CD133+).
A great debate concerning the concentration of CSH CD34+ cells in the placenta and the umbilical cord presents a potential research topic. The difference in blood sampling site in the CB shows, in a curious way, a clear variation of the percentage in CSH CD34+. However, it is important to point out that the sampling in the cord is made before the delivery of the placenta. Through the different parameters mentioned in this work, our researches compared the variation of the rate of CSH CD34+ only before and after delivery.
The mother’s blood group is one parameter studied in our work, but our data showed a statistical difference. Galan et al[33] also described the possible effects of blood groups on the proliferation and the capacity for self-renewal of CD34+ CB cells. Indeed, the results of this study suggested that, following culture in the presence of growth factors, the proliferation of CD34+ cells with the phenotype O+ 23.2% (extremes from 21% to 25%) for the group (O) may be more important than the one perceived with the phenotypes A: 21% (from 15% to 33%) and B: 19% (from 12% to 30%) which seemed to be independent of the culture conditions.
There are other parameters we have not dealt with in our work such as the age of gestation. According to one study, the percentage of CD34+ cells, in relation to CD45+ cells or mononuclear cells, is inversely proportional to the age of gestation[9]. The frequency of these cells is significantly higher among the premature newborn than among the babies born at term[7]. The sharp change in the incidence of CD34+ cells seems to occur in the 40th gestation week with a low incidence before and a high incidence after[8]. Thus, do the best units of CB valid for a future graft, come from the eldest among the newborn and those born of mothers who are younger and have had fewer pregnancies? Can other parameters such as the placenta weight and vaginal delivery, have an effect on the incidence of CSH CD34+ of CB?
Finally, in the population (CD45+CD34+CD133+), we showed the highest percentage of IFNγ in CB comparedto PAPB (P < 0.0001). The follow-up of the antigen CD133 by flow cytometry, in association with CD45, CD34 and 7’AAD shows that the total number, of positive cells (CD133+ CD45+CD34+7’AAD-) is equivalent to the total number of (CD45+CD34+7’AAD-) cells. This equality of incidence of these two cellular populations implies that in order to detect the (CD34+CD133+high) cells, the CD34+ cells should be targeted. Several studies have targeted the CD34+CD133+ cellular population. The same result was found in PAPB and CB.
The proportion of CD34+ cells which co-express the marker CD133 appears in a decreasing way in PAPB, CB and BM[24].
Other authors reported that after conditioning with G-CSF, the predominance of doubly positive populations (CD34+CD133+) is observed in a quantity similar to that of cells CD34+ with a low frequency of GvHD[22].
UCB raised great hope as an alternative source of transplantable hematopoietic stem/progenitor cell (HSPC) to adult BM or PAPB. CB HSPC has been used successfully in over 2000 HSC transplantations in a range of malignant and non-malignant diseases. In spite of the high level of apoptotic cells in CB shown in this study, it still offers a new source of cellular therapy.
Recently, cells expressing the CD133 antigen were considered a potent substitute to CD34+ cells. Phenotypic and functional studies revealed CB CD133+ populations contain higher levels of early HSPC than CB CD34+ harvested populations.
In summary, the simple maneuver of placing the new-born on the maternal abdomen after delivery and the method to collect the blood before or after cord clamping may significantly increase the influence of other parameters like the newborn’s weight or the mother’s age and therefore the absolute amount of blood progenitors required for engraftment success of UCB transplant without any harmful effects to the newborn.
We showed also that the population CD34+CD45+ was similar to the CD34+CD45+CD133+ positive cells in PAPB and CB. Our data also showed that in CD34+CD133+ cells there was an increased level of cells positive for IFNγ from CB compared to PAPB. In earlier studies, IFNγ was shown to act as an anti-proliferative and pro-inflammatory cytokine.
Studies of Immunophenotyping hematopoietic progenitor cells from cord blood (CB) and some parameters, such as the age of mothers and the weight of newborns, which can influence the quality and the quantity of CD34+ cells from CB: a comparison with adult mobilized blood grafts
CSH CD34+ is a sort of natural biological material and it has been processed into many forms for medical us in celltherapy. The research hotspot is to demonstrate that many different obstetric factors must be taken into account when processing and cryo-banking umbilical CB (UCB) units for transplantation.
In the previous application of CB CSH CD34+ in celltherapy in human, medicine particularly in the hematopoietic reconstructions it was found that the simple maneuver of placing the new-born on the maternal abdomen after delivery and the method to collect the blood before or after cord clamping may significantly increase the influence of other parameters like the newborn`s weight or the mother`s age and therefore the absolute amount of blood progenitors required for engraftment success of UCB transplant without any harmful effects to the newborn.
The study results suggest that the CB CSH CD34 + is a potential therapeutic material that could be used as a transplant source. It has become a real alternative to bone marrow (BM) and peripheral blood (PB) as a source of adult stem cells to treat multiple diseases.
The most important feature with CB samples and placenta delivery (PlaB) samples was a much wider variation rate of CD34+ cell counts compared to pre-apheresis peripheral blood (PAPB) and apheresis grafts samples. This finding led us to investigate several factors that could affect CB and PlaB CD34+ cell content, including those related to the mother such as age, parity, blood group and newborn’s sex.
This is a descriptive study in which authors analyze and optimize UCB separation and cryopreservation by the characterization of these cellular groups. Several physiological factors were examined in order to determine the most suitable method by flow cytometry using monoclonal antibodies. The results are interesting and suggest that the UCB raised great hope as an alternative source of transplantable hematopoietic stem/progenitor cell (HSPC) to adult BM or PAPB. CB HSPC and a potential therapeutic substance that could be used in medicine regenerative.
Peer reviewer: Pranela Rameshwar, PhD, Professor, Department of Medicine-Hematology/Oncology, UMDNJ-New Jersey Medical School, MSB, Room E-579, 185 South Orange Avenue, Newark, NJ 07103, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Barrett J, Jiang YZ. Allogeneic immunotherapy for malignant diseases. New York/Basel: Marcel Dekker 2000; . [DOI] [Full Text] |
| 2. | Belvedere O, Feruglio C, Malangone W, Bonora ML, Donini A, Dorotea L, Tonutti E, Rinaldi C, Pittino M, Baccarani M. Phenotypic characterization of immunomagnetically purified umbilical cord blood CD34+ cells. Blood Cells Mol Dis. 1999;25:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Bender JG, Unverzagt K, Walker DE, Lee W, Smith S, Williams S, Van Epps DE. Phenotypic analysis and characterization of CD34+ cells from normal human bone marrow, cord blood, peripheral blood, and mobilized peripheral blood from patients undergoing autologous stem cell transplantation. Clin Immunol Immunopathol. 1994;70:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Kim DK, Fujiki Y, Fukushima T, Ema H, Shibuya A, Nakauchi H. Comparison of hematopoietic activities of human bone marrow and umbilical cord blood CD34 positive and negative cells. Stem Cells. 1999;17:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Lang P, Bader P, Schumm M, Feuchtinger T, Einsele H, Führer M, Weinstock C, Handgretinger R, Kuci S, Martin D. Transplantation of a combination of CD133+ and CD34+ selected progenitor cells from alternative donors. Br J Haematol. 2004;124:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Li K, Yau FW, Fok TF, So KW, Li CK, Yuen PM. Haematopoietic stem and progenitor cells in human term and preterm neonatal blood. Vox Sang. 2001;80:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Meister B, Tötsch M, Mayr A, Widschwendter M, Huter O, Sperl W. Identification of CD34+ cord blood cells and their subpopulations in preterm and term neonates using three-color flow cytometry. Biol Neonate. 1994;66:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Opie TM, Shields LE, Andrews RG. Cell-surface antigen expression in early and term gestation fetal hematopoietic progenitor cells. Stem Cells. 1998;16:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | McGuckin CP, Basford C, Hanger K, Habibollah S, Forraz N. Cord blood revelations: the importance of being a first born girl, big, on time and to a young mother! Early Hum Dev. 2007;83:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Shim JS, Cho B, Kim M, Park GS, Shin JC, Hwang HK, Kim TG, Oh IH. Early apoptosis in CD34+ cells as a potential heterogeneity in quality of cryopreserved umbilical cord blood. Br J Haematol. 2006;135:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Brocklebank AM, Sparrow RL. Enumeration of CD34+ cells in cord blood: a variation on a single-platform flow cytometric method based on the ISHAGE gating strategy. Cytometry. 2001;46:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Gratama JW, Kraan J, Keeney M, Sutherland DR, Granger V, Barnett D. Validation of the single-platform ISHAGE method for CD34(+) hematopoietic stem and progenitor cell enumeration in an international multicenter study. Cytotherapy. 2003;5:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Levering WH, Preijers FW, van Wieringen WN, Kraan J, van Beers WA, Sintnicolaas K, van Rhenen DJ, Gratama JW. Flow cytometric CD34+ stem cell enumeration: lessons from nine years' external quality assessment within the Benelux countries. Cytometry B Clin Cytom. 2007;72:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rivadeneyra-Espínoza L, Pérez-Romano B, González-Flores A, Guzmán-García MO, Carvajal-Armora F, Ruiz-Argüelles A. Instrument- and protocol-dependent variation in the enumeration of CD34+ cells by flow cytometry. Transfusion. 2006;46:530-536. [PubMed] |
| 15. | Moretti S, Dabusti M, Castagnari B, Tieghi A, Ferrari L, Campioni D, Punturieri M, Dominici M, Castoldi GL, Lanza F. Comparison of single and dual platform methodologies for the estimation of CD34+ hematopoietic progenitor cells: correlation with colony assay. Int J Biol Markers. 2002;17:259-267. [PubMed] |
| 16. | Piedras-Ross J, León-Rodríguez E, Sánchez-Guerrero S, López-Karpovitch X. Comparison of single- and dual-platform approaches to enumerate CD34(+) cells in bone marrow and mobilized peripheral blood stem cells. Arch Med Res. 2003;34:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | TILL JE, McCULLOCH EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3171] [Cited by in RCA: 2643] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 18. | Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61-70. [PubMed] |
| 19. | Fasouliotis SJ, Schenker JG. Human umbilical cord blood banking and transplantation: a state of the art. Eur J Obstet Gynecol Reprod Biol. 2000;90:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources. J Transl Med. 2007;5:37. [PubMed] |
| 21. | Ogata K, Satoh C, Tachibana M, Hyodo H, Tamura H, Dan K, Kimura T, Sonoda Y, Tsuji T. Identification and hematopoietic potential of CD45- clonal cells with very immature phenotype (CD45-CD34-CD38-Lin-) in patients with myelodysplastic syndromes. Stem Cells. 2005;23:619-630. [PubMed] |
| 22. | Bidri M, Arock M. Differentiation of haematopoietic cells: role of cytokines and expression of membrane markers. Rev Fr Allergol. 1996;36:859-878. |
| 23. | Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(-)Lin(-) and CD34(+)Lin(-) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95:2813-2820. [PubMed] |
| 24. | Götze KS, Schiemann M, Marz S, Jacobs VR, Debus G, Peschel C, Oostendorp RA. CD133-enriched CD34(-) (CD33/CD38/CD71)(-) cord blood cells acquire CD34 prior to cell division and hematopoietic activity is exclusively associated with CD34 expression. Exp Hematol. 2007;35:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Lefrère F, Zohar S, Beaudier S, Audat F, Ribeil JA, Ghez D, Varet B, Cavazzana-Calvo M, Dal Cortivo L, Letestu R. Evaluation of an algorithm based on peripheral blood hematopoietic progenitor cell and CD34+ cell concentrations to optimize peripheral blood progenitor cell collection by apheresis. Transfusion. 2007;47:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Yu J, Leisenring W, Bensinger WI, Holmberg LA, Rowley SD. The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 1999;39:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Sakabe H, Ohmizono Y, Tanimukai S, Kimura T, Mori KJ, Abe T, Sonoda Y. Functional differences between subpopulations of mobilized peripheral blood-derived CD34+ cells expressing different levels of HLA-DR, CD33, CD38 and c-kit antigens. Stem Cells. 1997;15:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86:3745-3753. [PubMed] |
| 29. | Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102-110. [PubMed] |
| 30. | McKenzie JL, Gan OI, Doedens M, Dick JE. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp Hematol. 2007;35:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Machaliński B, Paczkowska E, Hałasa M, Pabisiak K, Walczak M, Sieńko J, Kozik W, Ostrowski M, Syrenicz A, Sulikowski T. Expression of stem cell markers on mononuclear cells derived from heparinized cadaveric organ donors before and after disconnection from the respirator. Transplant Proc. 2006;38:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Gajkowska A, Oldak T, Jastrzewska M, Machaj EK, Walewski J, Kraszewska E, Pojda Z. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells in leukapheresis product and bone marrow for clinical transplantation: a comparison of three methods. Folia Histochem Cytobiol. 2006;44:53-60. [PubMed] |
| 33. | Galan I, Santolaya-Forgas J, Leon JD, Uhlmann RA, Montenegro D, Hume R, Mari G. Effect of the ABO blood group on the proliferative and clonogenic capacity of umbilical cord stem cells. Transfus Apher Sci. 2006;35:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |