INTRODUCTION
Pluripotency is the ability of a cell to differentiate into any cell type of the developing or adult animal or human. Stem cells that are pluripotent, while not being malignant, were first discovered in mice in 1981[1], sparking radical new research avenues such as in vitro studying of early embryo development, cell differentiation and genetically modified animals. For this latter application of pluripotent stem cells (PSCs), Martin Evans earned the Nobel Prize for Medicine in 2007[2]. Seventeen years later, James Thomson and colleagues succeeded in deriving human PSCs from human embryos issued from in vitro fertilization, generating human embryonic stem cells (hESCs)[3]. This feat provided a completely new source of cells for biomedical applications[4,5]. Recently, the field of pluripotency was again shaken by the breakthrough discovery of Kazutoshi Takahashi and Shinya Yamanaka, evidencing that a differentiated somatic cell was amenable to complete dedifferentiation into PSCs by the over-expression of only four transcription factors (TFs)[6,7]. This technique of generating induced pluripotent stem cells (iPSCs) has provided an unrivaled means to understand the production and maintenance of pluripotency, resolved the ethical issues of the destruction of human embryos connected to hESCs, and outlined a method to use PSCs in medicine in an autologous setting that is more practical than therapeutic cloning. We will review here the specific determinants of pluripotency, the requirement for PSC culture, the expected use of PSCs in cellular therapy, and the pitfalls that must be anticipated and avoided to bring PSCs safely to therapeutics.
INTRINSIC MOLECULAR DETERMINANTS OF PLURIPOTENCY
Gradually, the molecular mechanisms that underlie pluripotency are becoming unveiled. The determinants of pluripotency can be divided into two broad categories: intrinsic determinants; i.e. cell-autonomous factors, for example, TFs, and extrinsic determinants that are non-cell autonomous, for example, growth factors[8]. Strikingly, intrinsic determinants are largely shared between mouse and human PSCs, whereas extrinsic determinants are often radically different between these two species. This last point accounts for, at least in part, the extended period that elapsed between the identification of ESCs in mice and in humans. The core transcriptional circuitry, the major determinants of intrinsic pluripotency, is composed of the TFs OCT4, NANOG and SOX2[9]. These three TFs repeatedly co-occupy the promoters of their target genes, including themselves, thus inducing a positive regulatory loop of pluripotency. Paradoxically, the core pluripotency TFs not only occupy the promoters of genes involved in pluripotency, putting them in close association with RNA polymerase II, but also promoters of genes that are inactive in PSCs and linked to cell differentiation, such as PAX6, HAND1 or ISL1, by placing them in proximity to proteins of the polycomb group[10].
The fact that for differentiation genes the cognate promoters are simultaneously co-occupied by the core pluripotency TFs and the polycomb repressive complex 2 subunit SUZ12, leading to a repressive chromatin modification by trimethylation at histone H3 K27 (H3K27me3), indicates a link between pluripotency and the epigenome. Several lines of evidence suggest that PSCs are characterized by a very specific chromatin state[11]. Global gene expression analyses by whole-genome tiling arrays have shown widespread transcription in coding and non coding regions in ESCs, as opposed to differentiated cells in which the transcriptional landscape subsides as differentiation proceeds[12]. This distinct expression profile in PSCs is associated with a high expression of chromatin remodeling genes, such as TOP2A, DNMT3B, JARID2, SMARCA5, CBX1 or CBX5[13]. While a majority of promoters are occupied by nucleosomes with H3K4me3 modifications, typically associated with an open chromatin structure and active transcription, not all H3K4me3-modified promoters are transcriptionally active[14]. One explanation for this contradiction is the concomitant repressive modification by H3K27me3, hence forming ‘‘bivalent’’ modifications[15,16]. The bivalent H3K4me3/H3K27me3 modification can easily switch to a monovalent modification, chiefly H3K4me3, and therefore the bivalent mark was proposed to be an indicator of genes specially poised to initiate transcription during differentiation. Bivalent modifications were first found in ESCs, but were subsequently also found in fully differentiated cells, suggesting a mechanism that is general and not restricted to ESCs. Another explanation can be found in the recent findings that the most cell-type-specific histone modification pattern is observed at enhancers and not at promoters[17]. The mechanisms that are necessary to keep this chromatin state may involve the chromatin remodeling factor Chd1, since its ablation disrupts PSC differentiation capacities[18]. Hence, the global picture that emerges is that ESCs have an open chromatin largely devoid of heterochromatin, priming their genes for transcription at later stages of development, thereby accelerating the full transcription activation required by cell differentiation.
Niall Dillon’s group has reported that genes that are transcriptionally silent in ESCs are nonetheless subject to preinitiation complex assembly but are simultaneously targeted by the proteasome[19,20]. Their data suggested that the 26S proteasome promotes a dynamic turnover of TFs and Pol II, binding at tissue-specific gene domains in ESCs, which would restrict permissive transcriptional activity but keep the genes in a primed state for later activation. In line with the potential role of the proteasome machinery in the distinct transcription regulation of PSCs, we recently reported the overexpression of several genes involved in the canonical ubiquitin-proteasome pathway[13]. Significantly overexpressed in hESCs were genes coding for enzymes from the three (E1/E2/E3) ubiquitination classes; i.e. the E1 ubiquitin-activating enzyme UBE1C, the E2 ubiquitin-conjugating enzymes UBE2G1, UBE2V1 and UBE2V2, and the E3 ubiquitin protein ligases UBE3B and breast cancer 1, early onset (BRCA1), as well as four catalytic β proteasome subunits (PSMA2, PSMA3, PSMA4 and PSMA5), three regulatory subunits, the ATPase PSMC6, and the non- ATPase PSMD10 and PSMD11 from the proteasome machinery. This peculiar expression of the proteasome in PSCs was correlated with an acute sensitivity of hESCs to proteasome inhibitors.
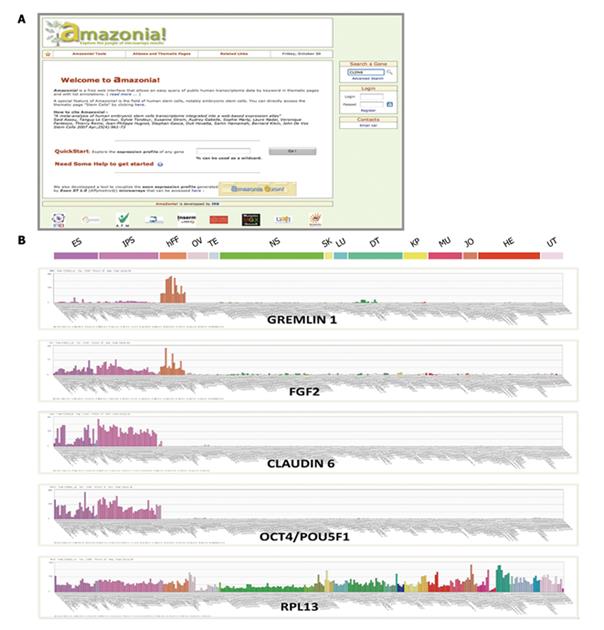
In addition, other genes are also overexpressed in PSCs, including numerous zinc finger TFs that could play a role in the intrinsic determination of the pluripotency state. We have re-analyzed a large panel of hESC transcriptome studies and have established a common list of genes involved in pluripotency[21]. Importantly, we have made the transcriptome of PSCs available through Amazonia!, a web-based atlas of human gene expression that compiles a selection of publicly available transcriptome datasets and is freely accessible through a user friendly interface to the research community. Using this interface, one can easily grasp the very specific expression pattern of the core pluripotency TFs in PSCs, as well as in the central nervous system, upper digestive, airway tract, etc. (Figure 1).
Figure 1 Visualization of gene expression in PSCs and comparison with somatic cells.
A: The Amazonia! web Atlas interface (http://www.amazonia.transcriptome.eu); B: Expression bar plots, generated with Amazonia!, for RPL13, a ubiquitously expressed gene, OCT4/POU5F1 and CLAUDIN 6 as highly PSC-specific genes, FGF2, a major human PSC growth factor expressed as an autocrine loop and by human fibroblast cells, and GREMLIN 1, an inhibitor of BMPs secreted by human fibroblast feeder cells. ES: Human embryonic stem cells; iPS cells: Induced pluripotent stem cells; hFF: Human foreskin fibroblasts; OV: Ovary & oocytes samples; TE: Testis; NS : Nervous system; SK: Skin; LU: Normal lung; DT: Digestive tract; KP: Kidney & prostate; HM: Heart & muscle; JO: Joint; HE: Normal hematological samples; UT: Uterus; PSCs: Pluripotent stem cells. Y-axis is the microarray signal value, obtained by MAS5 normalization with a TGT at 100 using Expression Console (Affymetrix, Santa-Clara, CA).
Another level of cell fate regulation that takes place in PSCs is micro RNA (miRNA). Certain miRNAs have a high expression in hESCs and are lost upon differentiation into embryoid bodies, such as the miR-302 and miR-371 clusters[22,23]. Conversely, miR-145 expression increases during PSC differentiation and directly represses OCT4, SOX2, and KLF4, thus blocking pluripotency by a negative feedback loop[24]. In addition, the pluripotency gene LIN28 was observed to hinder the biogenesis of some miRNAs, such as the processing of pri-let-7 miRNAs[25]. These findings explain the complete absence of mature miR-let-7 in ESCs.
EXTRINSIC MOLECULAR DETERMINANTS OF PLURIPOTENCY
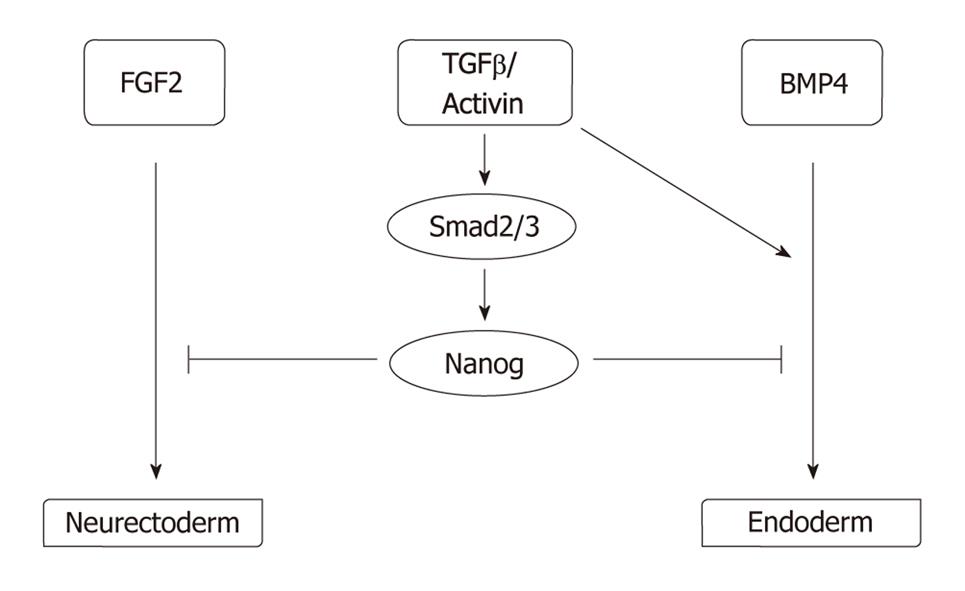
While intrinsic pluripotency determinants ensure that pluripotency is maintained, extracellular signals alter this undifferentiated state and drive the PSCs to differentiation. Hence, pluripotency is under tight control by extrinsic determinants; i.e. growth factors and other soluble factors, cell-to-cell contact, and the extracellular matrix and O2 level. As mentioned above, growth factor requirements vary widely between mice and humans. For maintenance of pluripotency, mice ESCs rely on leukemia inhibitory factor (LIF), via a signaling cascade involving the phosphorylation of STAT3[26], and on bone morphogenic proteins (BMPs), via the expression of Id proteins[27]. By contrast, hESCs are indifferent to the action of LIF[28-30], and are highly sensitive to the action of BMPs, which induce hESC differentiation[31]. Human pluripotency is favored by the action of FGF2[32]; in contrast, an autocrine FGF loop in mouse ESCs drives their differentiation unless the action of this loop is counterbalanced by LIF[33]. The debate remains open as to whether the differences between growth factor requirements in mice and humans are secondary to speciation or rather to a different origin of developmental stage as suggested by the identification of epiblast stem cells in mice whose growth is dependent upon activins and FGF2[34]. Other growth factors are important for human pluripotency, such as TGFβ and activins[35,36], neurotrophins[37], GABA[38], sphingosine-1-phosphate[39], WNTs[40], IGFs[41], and EGF family members such as Heregulin[41] or pleiotrophin[42]. The role of the TGFβ/Activin pathway is essential as it induces the expression of NANOG via SMAD2/3, which in turn counteracts the induction of neurectoderm by FGF2 or endoderm by BMP4 signaling[36,43] (Figure 2). Noggin, a BMP inhibitor, has also been described as promoting pluripotency in combination with a high concentration of FGF2[32].
Figure 2 Model explaining the role of the major human PSC growth factors identified to date, FGF2 and TGFβ/Activin, based on Vallier et al[36] and Xu et al[43].
The role of cell-to-cell contact (stem cell-stem cell or stem cell-feeder) is clearly demonstrated by the well-known difficulty to clone PSCs, due to a high apoptosis rate after enzymatic dissociation. This dependency can be reversed, at least partially, by the selective inhibition of the rho-associated kinase using the pharmacologic compound Y-27632[44,45]. Similarly, PSCs are tightly dependent on their attachment to a feeder layer or a synthetic matrix. This dependency could be due to anoikis, a subtype of apoptosis provoked by detachment of adherent cells from their matrix[46].
PSC cultured on plastic undergo rapid differentiation and apoptosis, exemplifying the need for these cells to be on an extracellular matrix. Historically, a feeder of irradiated or mitomycin-C treated murine embryonic fibroblasts (MEFs) was used to derive and maintain in culture the first ESC lines[1,3,47]. This technique is still widely used because of its low cost and high efficiency in PSC maintenance. MEFs can be replaced by human fibroblasts such as foreskin fibroblasts[48]. These feeder cells produce soluble factors, such as the BMP inhibitor GREMLIN 1[49], or the pluripotency promoting growth factor FGF2 (Figure 1B), but also numerous extracellular matrix components. In line with this observation, in vitro culture protocols have been developed that replace feeder cells by various purified or unpurified matrices such as laminin[50], collagen IV/fibronectin/laminin/vitronectin[38], vitronectin[51] or Matrigel, which is a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma, rich in extracellular matrix proteins[50]. It should be noted that, in many of these matrix conditions, hESC maintenance requires the use of either MEF- or foreskin fibroblast-conditioned medium or defined medium such as TeSR1 (see below), suggesting that some soluble proteins secreted by the feeder cells are necessary to compensate for the still incomplete synthetic matrices that have been tested.
Another important factor in hESC maintenance is the O2 level. Several papers have reported the role of low O2 (3%-5%) tension in preventing hESC differentiation[38,52,53]. These O2 conditions are similar to those required for early human embryo development. However, it should be conceded that most in vitro culture protocols maintain their hESCs under high O2 (20%) tension due to obvious technical and cost constraints.
DRIVING PSCs TOWARD CELLS OF MEDICAL VALUE: TUNING DIFFERENTIATION
As hESCs can differentiate into virtually any cell type, they could theoretically cure any illness resulting from the loss of functional cells. But one crucial issue is to determine which hESC-derived cell population will be most helpful. It is now clear that undifferentiated PSCs should not be used for cell repair, as PSCs are highly proliferating cells that can, upon injection, form non malignant tumors of undifferentiated and differentiated cells that should be called teratomas, as they are formed by the association of “somatic tissue and their immature (fetal) precursors derived from more than one of the three embryonic germ layers”[54]. The occurrence of teratomas is not systematic. But the risk of teratoma development is obviously not acceptable in any clinical application. For instance, the transplanting of low doses of undifferentiated murine ESCs (1000-2000 cells) into the striatum of a rat model of Parkinson disease resulted in a well oriented dopamine neuron differentiation and was associated with a clinical improvement in 14 of 19 animals that had been successfully grafted, but resulted in the growth of an undifferentiated cell population and the death of 5 of the 19 animals[55]. In line with these observations, Roy et al[56] treated a similar rodent model with hESCs differentiated into a cell population highly enriched in dopamine neurons obtained by successive culture steps, including the co-culture with fetal human astrocytes, and obtained significant improvement of the treated animals as compared to the sham-treated. While an important human dopamine neuron population was observed at the periphery of the injection site, in close contact with the rat glia, the center of the injection site was filled with immature nestin-positive and proliferating human neural precursors. These observations suggested that the cell preparation contained the appropriate dopamine neuron population, but still contained poorly differentiated and proliferating cells, whose developmental and tumor potential is not well known. This experiment gave reason for a word of caution against the injection of unpurified ESCs, even after in vitro differentiation. A similar observation was recently reported by Aubry et al[57] These authors injected into the quinolinate lesioned right striatum of immunocompetent rats a population of hESCs differentiated into DARPP32-expressing striatal neurons. After 2 mo, the animals manifested lethargy, weight loss and hemiparesis, caused by a massive outgrowth of the human neural progenitor injected into the striatum. The answer to this paramount problem raised by PSCs could be cell sorting after differentiation. Darabi et al[58] set up a protocol to regenerate muscle in dystrophic mdx mice using murine ESCs. The investigators determined that expression of PDGFβ-R, a marker of paraxial mesoderm, and absence of Flk-1, a marker of lateral plate mesoderm, identified a cell population of myogenic progenitors that could be purified by flow cytometry cell sorting. Without cell purification, the animals developed teratomas, formed by cells originating from the donor, containg keratinocytes and cartilage formation, at the injection site; but after cell purification, this major side effect was eliminated, strongly supporting the idea that undesired cells, including undifferentiated cells, must be eliminated by cell sorting before in vivo transplantation.
While there seems to be a consensus to exclude undifferentiated cells, the level of differentiation to be achieved for clinical use of PSCs is still an open question. Naturally, the answer to this question will be tissue-dependent, or maybe even disease-specific. To generate cardiomyocytes, some authors have proposed a very brief time of in vitro differentiation, as short as 48 h, reducing the differentiation step to a simple cardiac-commitment step using BMP2[59]. By contrast, most neuron differentiation protocols are multi-step, several weeks long protocols[56,57]. Hematopoietic differentiation also requires long and complex culture steps, usually including the overexpression of the HOXB4 TF[60,61]. Numerous pre-clinical trial studies have convincingly showed that PSCs can be differentiated into cells with the capacity for tissue repair, but there is still a long way to go before all the differentiation issues are solved[59,62-64]. A recent benchmark comparison of different sources of human hepatocytes transplanted into Alb-uPAtg(+/-)Rag2(-/-)Gama c(-/-) mice suggested that primary adult hepatocytes were the best source of cells for attaining a significant liver repopulation, while fetal hepatocytes ranked second best and hepatocytes derived from ESCs worked poorly[65]. Though a huge amount of work is still needed to improve our hepatic differentiating protocols for PSCs, this study clearly highlights the fact that current protocols mainly generate hepatocytes with a fetal phenotype; i.e. low expression of homeostasis and detoxification genes, persistence of β-feto protein, that are not best suited for liver regeneration[66,67]. Overall, the in vitro transformation of PSCs into cell drugs is still in its infancy stage and further work and testing in pre-clinical studies is needed to improve these protocols.
GOOD MANUFACTURING PRACTICE IN CLINICAL-GRADE CELL GENERATION AND DIFFERENTIATION
The use of differentiated PSCs suitable for human therapy will require the same rigorous manufacturing as for any cell therapy product. As pointed out above, many differentiation protocols are based on extensive manipulation, involving many successive reagents, co-culture steps and several weeks of incubation at 37 °C. Any constituent that will come in contact with the PSCs will have to meet the safety requirements of regulatory bodies. Several academic teams have already published defined or xeno-free media that could be used to develop clinical grade PSCs. The use for non-human materials bears a risk of transmitting pathogens. The elimination of animal serum is also an important step because hESCs cultured with animal products or animal cells express Neu5Gc, a nonhuman sialic acid that could be immunogenic if these cells were to be used for cellular therapy[68]. Henrik Semb’s group has described a protocol using 20% of human serum instead of fetal calf serum or knock-out serum replacement (KO-SR), and have derived a new hESC line in these conditions[69]. Ludwig et al[38] have proposed a fully defined medium for PSC culture, based on the analysis of the expression of cell-surface receptors of hESCs and the finding that some of the ligands of these receptors have a positive effect on pluripotency (FGF2, ClLi, GABA, pipecolic acid, and TGFβ). This medium was termed TeSR1 and, in combination with human laminin, collagen IV, fibronectin and vitronectin, was able to sustain the derivation of two new hESC lines, demonstrating that the maintenance of pluripotency was not restricted to culture-adapted subclones of hESCs[38]. A modification of this medium, consisting of the replacement of human albumin with its bovine counter part, the replacement of human FGF2 with zebrafish FGF2, and the use of Matrigel instead of the purified human matrices, has been commercialized and is now widely used as mTeSR1 world wide[70]. Other xeno free media have been documented, but all compare poorly with fibroblast feeder/KO-SR standard culture conditions when tested on hESCs that were mechanically derived and passaged[71].
Another issue is the feeder cell layer that imparts complexity in cell handling and a risk of pathogen transmission, either for human or murine feeder cells. Therefore, both on scientific and medical grounds, substitution of the co-culture system by a synthetic matrix would be preferable. As early as 2001, the team of Melissa Carpenter proposed Matrigel (see above) or laminin as a replacement for MEFs, but only if using MEF-conditioned medium[50]. Numerous other proposals have been made since, such as the use of MEF sodium deoxycholate extract, which does not solve the xenogeneic source of the matrix but resolves the practical conundrum of the co-culture system[72], human fibroblast extracts[73], a mix of human purified extracellular components[38], and recombinant vitronectin on its own[51].
A recent twist in the domain of xeno-free PSC culture was the ability of such culture conditions to generate iPSCs. Several recent publications have illustrated this technical possibility, such as the use of TeSR1 and Matrigel[74] or a human plasma-derived cell culture additive called F44, obtained through cold-ethanol industrial plasma fractionation[75].
MAJOR HURDLES TO OVERCOME
The use of PSCs for clinical applications raises several issues that must be carefully addressed. These difficulties are: (1) cell proliferation; (2) cell differentiation; (3) genetic integrity; (4) allogenicity; and (5) ethical issues.
As noted above, PSCs are characterized by an abbreviated G1 phase of the cell cycle, resulting in sustained cell proliferation. Therefore, the injection of undifferentiated PSCs carries the risk of inducing teratomas, which consist of the non-malignant proliferation of PSCs associated with multilineage and uncontrolled cell differentiation, both of which are unwanted and deleterious[54]. An open question is the extent of differentiation necessary to prevent any risk of teratomas at the site of injection and the relevant cell markers that can be used to sort the cells. Obviously, the response to this answer will be cell type-specific, but one can anticipate that the loss of one or several (signature) pluripotency markers may turn out to be mandatory.
Another issue is the type of differentiation that PSCs must attain to be of therapeutic value. As already underlined, insufficient differentiation exposes unwanted in situ cell proliferation and uncontrolled in situ cell differentiation. However, excessive differentiation carries the risk that the injected cell preparation will fail to integrate the organ that must be repaired. For example, it is plain that terminally differentiated neurons displaying a full-grown axon will not be able to connect themselves with the surrounding or distant cells of the nervous system, hence diminishing the regenerative purpose of the cell injection. Furthermore, inappropriate differentiation such as a fetal phenotype to treat adult patients will prevent effective functional improvements from being achieved. However, the capability of cells to acquire a functional phenotype after transplantation should not be disregarded. Kroon et al[63] by using hESCs differentiated in vitro into pancreatic-like cells (similar to fetal 6-9 wk pancreatic tissue), showed that these cells develop in vivo into endocrine cells similar to pancreatic islets and protect mice against hyperglycemia.
Another concern with PSCs, a concern also associated with some other stem cell types, is that culture conditions may select for abnormal cell clones that harbor chromosomal or other genetic abnormalities[76]. These abnormalities are not random, and several teams have described the recurrent gain of extra copies of the long arm of chromosome 17, and the short arm of chromosome 12 or chromosome 20[77-80]. These karyotypic changes are similar to that of testicular germ cell tumors and may therefore raise safety concerns[79]. In addition to these chromosomal abnormalities, other changes have been described, including microarray comparative genomic hybridization (CGH) and promoter methylation[81]. The high proliferation rate of PSCs, metabolic stress in large cell colonies, and enzymatic passaging may contribute to these genetic and epigenetic changes acquired over the long term in in vitro culture. IPSCs bring further worry on the subject because some of the barriers to cell reprogramming are the same that prevent malignant transformation, namely the p53/mdm2/p21 and the Ink4/p16/Arf pathways[82-86]. Indeed, alleviating these barriers by genetic means results in a marked increase in cell reprogramming, but at the expense of DNA integrity. The team of Maria Blasco has reported that, during reprogramming, the presence of pre-existing, but tolerated, DNA damage resulted in the activation of a DNA damage response and p53-dependent apoptosis. This response was abrogated by p53 downregulation, producing iPSCs carrying persistent DNA damage and chromosomal aberrations[82]. Consequently, it will be mandatory to screen human iPSCs for genetic alteration just after reprogramming, as well as after prolonged in vitro cell culture as noted above.
The matter of allogenicity is raised by the fact that the probability of a given hESC to be HLA compatible with a patient is exceedingly low. There are several ways to resolve HLA disparity between cell lines and patients. One way is to immunomodulate patients receiving HLA-incompatible cells. For certain organs, this has proven feasible, therefore it may be possible for hESCs that have been differentiated in certain cell types, but not for all. For example, complete HLA disparity precludes the injection of immune system cells into a patient, whatever the immunosuppressive drugs given. Alternatively, a limited collection of chosen hESC lines could cover, with an acceptable HLA compatibility, a majority of the population. About 150 hESC lines obtained consecutively would provide a beneficial match (defined as one HLA-A or one HLA-B mismatch only, HLA-DR being matched) or better for 37% of the general population in the United Kingdom or in Japan[87,88]. Furthermore, the selection of PSC lines homozygous for the HLA locus would lower the number of cell lines necessary for the bank. This could be obtained by parthenogenesis, which produces hESC lines that are homozygous for the HLA locus, except in the unlikely case where the meiotic recombination would take place in the middle of the locus. It has been estimated that, in the Japanese population, 55 randomly selected parthenogenetic hESCs could cover 80% of the patients with a match for HLA-A, HLA-B and HLA-DR[88]. By screening 24 000 individuals, it would be possible to select 50 HLA homozygotes for the HLA-A, HLA-B and HLA-DR loci, from which 50 iPSC lines could be derived, which would match more than 90% of the patients[89]. Alternatively, the iPSC technology is a way to generate autologous PSCs for each patient, paving the way to personalized regenerative medicine[90]. However, even when the hurdles to the generation and differentiation of human iPSCs in GMP conditions are solved, the problem will remain concerning the time scale necessary to generate, amplify and qualify autologous iPSCs, in contrast with the urgency for some diseases to be treated, such as heart infarct, and the considerable cost of such personalized medicine.
Finally, the use of human PSCs in research and in regenerative medicine has spurred countless debates on the ethics of research on human embryos[5]. It has been proposed that iPSCs could solve the ethics around PSCs as they are generated without the need to destroy a human embryo. However, in addition to the fact that this technology is still in its complete infancy stage, necessitating that the reprogramming technology becomes GMP compliant and virus integration free, some ethical issues have arisen, such as the theoretical possibility of generating a human being that would be of 100% iPSC origin by tetraploid embryo complementation, as suggested by rodent experiments[91,92], or the differentiation of iPSCs into gametes that could then be fertilized, generating a human embryo[93,94].
CONCLUSION
In early 2009, a cellular therapy protocol based on hESCs had been agreed on by the US Food and Drug Administration (FDA)[95]. This protocol, conducted by Geron, based in Menlo Park, California, was a phase I safety study for spinal-cord injury. What was to be the first PSC-derived treatment was delayed in September 2009 after animal data revealed microscopic cysts growing around the injury site. Hence, this promising stem cell category still awaits its first use in human therapeutics. The reasons for the discrepancy between the huge expectation for disease treatment and the effective use of these cells in a clinical setting are the technical hurdles listed above. Much research is still needed to effectively resolve these problems. The recent advent of the iPSC technology has considerably boosted the PSC field and will therefore contribute to accelerate the advent of applications for PSC in curing human diseases. However, it must not be forgotten that though iPSCs strongly resemble hESCs, there are differences[96]. Therefore, research on hESCs and human embryos is mandatory to define the similarities and dissimilarities between these two cell types before envisioning the use of human iPSCs in regenerative medicine.