INTRODUCTION
The microenvironment of mammalian bone marrow is composed of several different elements that support hematopoiesis and bone homeostasis[1,2]. It includes a heterogeneous population of cells: macrophages, fibroblasts, adipocytes, osteoprogenitors, endothelial cells and reticular cells. Among these, there are also non-hematopoietic stem cells that possess a multilineage potential[3,4]. These stem cells are commonly described as marrow stromal stem cells or mesenchymal stem cells (MSCs). Mesenchymal cells are primordial cells of mesodermal origin, able to give rise to skeletal muscle cells, vascular and urogenital systems and to connective tissues throughout the body (Figure 1)[5-7]. For this reason, the word “mesenchymal” should not be used to refer to stem cells that are also able to produce blood cells and which derive from a distinct stem cell population present in the bone marrow: the hemapoietic stem cells (HSCs)[5-7].
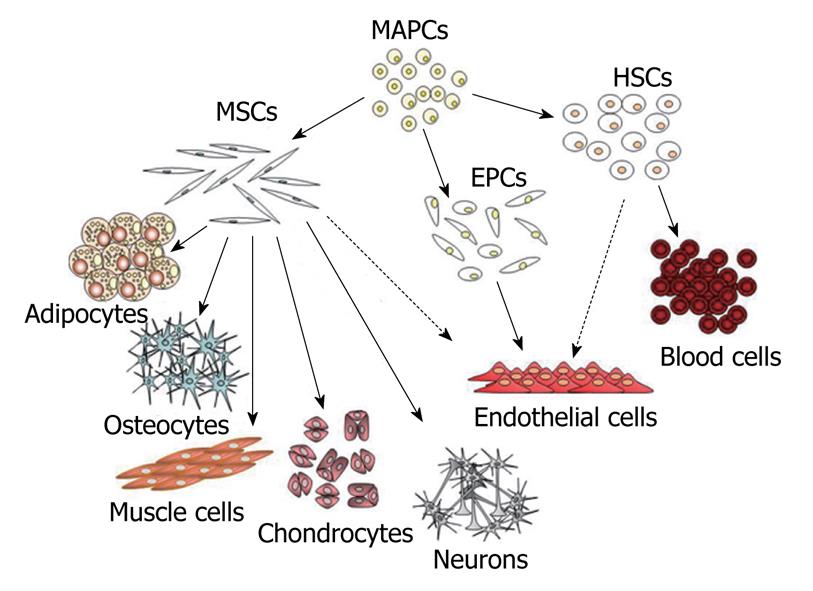
Figure 1 Diagram of mesenchymal stem cell hierarchy.
At top of diagram are indicated the MAPCs that posses a higher proliferative and differentiative potential compared to classical MSCs. These may represent a more primitive subset of stem cells that could be the common precursor of MSCs, HSCs and EPCs. In the diagram are indicated the mesenchymal and non-mesenchymal cell types that originate from these different classes of stem cells. The dashed lines indicate putative differentiation pathways. MSCs: Mesenchymal stem cells; MAPCs: Multipotent adult progenitor cells; HSCs: Hematopoietic stem cells; EPCs: Endothelial progenitor cells.
MSCs can be hence considered non-hematopoietic, multi-potent stem-like cells that are capable of differentiating into both mesenchymal and non-mesenchymal lineages. In fact, in addition to bone, cartilage, fat and myoblasts, MSCs have been demonstrated to be capable of differentiating into neurons and astrocytes, in vitro and in vivo[7-10].
MSCs are of interest because they can easily be isolated from a small aspirate of bone marrow and expanded through as many as 50 population doublings in about 10 wk. As such, these cells are currently being tested for their potential use in cell and gene therapy for a number of human diseases. Nevertheless, there are still some open questions about the origin, multipotentiality and anatomical localization of MSCs. As far as this latter point is concerned, it has been shown that MSCs can be isolated from different tissues other than bone marrow, although this remains the primary source for obtaining these stem cells. Indeed, MSCs have been successfully isolated from adipose tissue, liver, tendons, synovial membrane, amniotic fluid, placenta, umbilical cord and teeth[5-8].
Another hot issue is the lack of an unambiguous single marker to clearly define MSCs. In fact, at the present, MSCs are identified through a combination of physical, phenotypic and functional properties. The classical methodology utilized to identify MSCs is the colony forming unit assay, which recognizes adherent spindle-shaped cells that proliferate to form colonies and that can be induced to differentiate into adipocytes, osteocytes and chondrocytes[5-8].
Further obstacles in defining MSCs arise from the fact that different laboratories have employed different sources, extraction and cultivation methods. These variables are clearly responsible for the phenotype and function of the resulting cell populations. Whether these conditions selectively promote the expansion of different populations of MSCs or cause similar cell populations to acquire different phenotypes is still not clear[7]. For this reason, it is not possible to define the relationships between MSCs and other stem cell populations which are similar to MSCs but defined with a different nomenclature, such as bone marrow stromal stem cells, stromal precursor cells, recycling stem cells, marrow isolated adult multineage inducible stem cells (MIAMI cells) and multi-potent adult progenitor cells (MAPC) (Figure 1)[7,11,12]. MIAMI and MAPC stem cells display a higher proliferative and differentiative potential compared to classical MSCs. These cells have been suggested to represent a more primitive subset of stem cells, the possible common precursor of MSCs and HSCs[7,11,12]. If this is the case, then the relationship between these cell populations and the hemangioblasts, considered the mesodermal precursors of hematopoietic and endothelial cell lineages, has to be determined[6,13].
HOMING OF MSCS IN TUMOR TISSUES
Several researches have demonstrated that MSCs can efficiently home to injured tissues and actively participate to tissue repair. MSCs can repair injured tissues by secreting cytokines and growth factors that can restore tissue homeostasis, by reducing local inflammation and by differentiating in one or more of the cell types resident in the injured tissues[3,10,14].
Tumor microenvironment may be considered similar to that of an injured tissue. For this reason, tumor growth often appears associated with several types of stromal cells in a manner that overlaps wound healing and tissue repair processes. In tumors, bone marrow-derived MSCs, hematopoietic stem cells and endothelial progenitors cells are recruited to establish a supportive stroma, a phenomenon elicited by the release of endocrine and paracrine signals, as observed in injured tissues. Signaling molecules, such as CXCR4, CXCR12 and CCL12 are involved in tumor homing of MSCs[15]. The exact homing mechanism has not been fully elucidated, since several studies have addressed this issue by studying migratory properties of MSCs derived from in vitro cultures. During in vitro growth, MSCs often lose the expression of chemokine receptors and thus responsiveness to chemokines[16], a phenomenon that could bias data interpretation on the homing process. In fact, MSCs harvested after a few days of in vitro growth may express a different pattern of chemokine receptors compared with cells that have been cultivated for a longer time.
On the other hand, a complete analysis on the mobilization to tumor regions of endogenous bone marrow resident MSCs is still underway. There are, however, several pieces of evidence showing a major contribution of MSCs to the formation of tumor stroma. For example, Haniffa and co-workers suggest that mesenchymal fibroblasts within solid tumors (also called carcinoma-associated fibroblasts) originate from bone marrow MSCs[17]. Moreover, other investigators show that, in pancreatic tumors, about 25% of myofibroblasts derive from bone marrow cells[18,19].
CONTRIBUTION OF MSCS TO TUMOR GROWTH
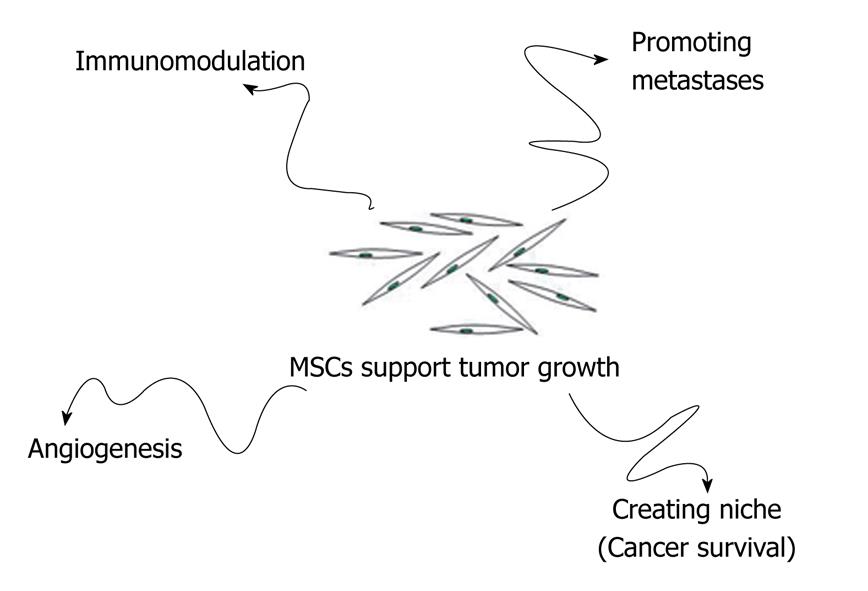
MSCs that reach tumor stroma may contribute to tumor growth in several ways: (1) by promoting angiogenesis; (2) by creating a niche to support cancer stem cells survival; (3) by modulating the organism’s immune response against cancer cells; and (4) by promoting formation of metastasis (Figure 2).
Figure 2 MSCs may contribute to tumor growth in several ways: Promoting angiogenesis; Creating a niche to support cancer stem cells survival; Modulating the organism’s immune response against cancer cells; Promoting formation of metastasis.
Promotion of angiogenesis
MSCs can contribute to tumor vessel formation either by producing growth factors acting on tumor cells, endothelial cells and smooth muscle cells or by producing angiogenic growth factors (VEGF, PDGF, bFGF, SDF-1). MSC-derived angiogenic factors can promote proliferation, survival and migration of endothelial cells, thus contributing to vessel formation. Similarly, MSCs appear able to promote smooth muscle cell proliferation and migration[20-22]. MSCs can also contribute to vessel formation by differentiating in endothelial cells, smooth muscle cells and pericytes. The latter are elongated cells of mesodermal origin that partially surround the endothelial cells of small vessels and are involved in the maintenance of microvascular homeostasis. In vitro, MSCs can differentiate in endothelial cells following treatment with VEGF and are also capable of forming vessel-like structures when cultured on tri-dimensional Matrigel[23-26]. Annabi and co-workers show that subcutaneous co-injection of MSCs and malignant glioma cells in nude mice results in formation of highly vascularized tumors. In these tumors, MSCs differentiate into endothelial-like cells and localize at the lumen of vascular structures[27]. These investigators suggest that MSCs could be recruited at the sites of active tumor neovascularization through paracrine regulation of their angiogenic properties. The ability of MSCs to differentiate in smooth muscle cells has been demonstrated at least in ischemia models. In a dog model of cardiac ischemia, intramyocardial injection of MSCs results in differentiation into smooth muscle cells and endothelial cells with increased vascularity and improved cardiac function[28].
Niche for cancer cell survival
Osteoblasts derived from MSCs are key components of the hematopoietic stem cell niche, a specialized microenvironment that provides cytokine and cell contact signals to regulate stem cell physiology[2,29]. On this premise, a tantalizing hypothesis arises from studies of Dazzi and co-workers[30] that show that MSCs are able to inhibit proliferation of hematopoietic and non-hematopoietic tumors in vitro. This phenomenon is associated with a reduction of apoptosis even in stress conditions, such as low concentration of survival factors. Nevertheless, when tumor cells and MSCs are co-injected into immunodeficient mice, cancer cells grew much faster as compared to the group receiving only tumor cells. To explain this discrepancy between in vitro and in vivo data, the authors suggest that MSCs have the ability to form a cancer stem cell niche, in which tumor cells can preserve the potential to proliferate, are protected from apoptosis and can thus sustain the malignant process[30].
Drug resistance in acute myelogenous leukemia cells is induced by the attachment of very late antigen (VLA)-4 of leukemic cells to fibronectin on bone-marrow stromal cells. VLA-4-positive cells acquire resistance to loss of anchorage or drug-induced apoptosis through the PI-3K/AKT/Bcl-2 signaling pathway, which is activated by the interaction of VLA-4 and fibronectin[31].
Immune response modulation
Immunosuppressive activity of MSCs can promote tumor growth by impairing rejection of cancer cell by the immune system. Jorgensen’s group investigated whether in vivo injection of MSCs could display side effects related to systemic immunosuppression, thus favoring tumor growth[32]. Using the murine B16 melanoma model, they showed that subcutaneous injection of melanoma cells leads to tumor growth in allogeneic recipients only when MSCs are co-injected[32].
Promotion of metastasis formation
MSCs may also promote the metastatic process. In fact, several reports point out that MSCs are recruited in large number to the stroma of developing tumors[33]. Karnoub and co-workers characterized the role of MSCs in tumorigenesis, thus demonstrating that these stem cells may promote breast cancer metastasis[34]. They investigated, in detail, the functional consequences of the interaction between MSCs and mammary carcinoma cells in a mouse xenograft model. Human breast cancer cells were mixed with MSCs and injected subcutaneously into immunocompromised mice. MSCs appear to accelerate tumor growth in mice along with a significantly increased presence of micro- and macroscopic lung metastases. Explants of primary tumors and lung metastases were then expanded in vitro and re-injected subcutaneously into host mice, in order to evaluate their respective metastatic power. Under these conditions, lung metastasis-derived cells show the same metastatic potential of the primitive tumor-derived cells. The authors thus suggest that the MSC-induced metastatic power reflects a reversibly induced trait of breast cancer cells, and that the ability of these cells to metastasize is a consequence of their “education” by MSCs, rather than a clonal selection of rare variants with higher metastatic power[34]. These findings indicate that MSCs supply local-acting paracrine signals that induce breast cancer cells to metastasize. On the other hand, breast cancer cells stimulate the secretion of the chemokine CCL5 from MSCs, which then acts in a paracrine way on cancer cells to enhance their motility, invasion and metastasis[34].
In the investigations described above, MSCs migrating from bone marrow to primary tumors can promote the acquisition of metastatic properties to cancer cells while, complementarily, MSCs residing in bone marrow may render this organ a privileged site for metastases. To elucidate this process, the molecular mechanisms of MSC interaction with breast cancer cells have been addressed, and the role of stromal cell-derived factor 1 α (SDF-1α) has received great attention. SDF-1α belongs to the chemokine family and is constitutively produced in bone marrow stroma cells (including MSCs). The SDF-1 protein has two isoforms (α and β) which both interact with CXCR4 receptors. Breast cancer cells expressing CXCR4 may migrate toward organs with high levels of SDF-1α, such as bone marrow[35]. Bone metastases have been reported also in the absence of a clinically evident primary breast cancer, thus suggesting a migration of cancer cells during the early phases of the disease. These cancer cells can be supported by the bone marrow microenvironment and are likely to survive chemotherapy.
MSCs possess the potential to give rise to a wide variety of differentiated cell types, and the stem cell hypothesis of cancer onset postulates that they may also be the origin of an interesting range of cancers, such as osteosarcoma, chondrosarcoma, rhabdomyosarcoma, liposarcoma, fibrosarcoma, etc[36]. Indeed, MSCs can undergo spontaneous transformation following in vitro culture. Rubio and co-workers show that, although MSCs can be managed safely during the standard in vitro expansion period (6-8 wk), human MSCs can be subjected to spontaneous transformation following long-term in vitro culture (4-5 mo)[37]. Another study demonstrates that MSCs transduced with the telomerase (TERT) gene undergo neoplastic transformation. One of the TERT-transduced clones at population doubling 256 showed loss of contact inhibition, anchorage independence and 100% tumorigenicity when injected into immuno-deficient mice (tumor take in 10 out of 10 animals)[38]. Another interesting study analyzes the relationship between MSC transformation and cancer during aging of the individual[39]. Peripheral and bone marrow-derived stem cells are long-lived and are candidate cells for the “cancer-initiating cell”. Based on the facts that MSCs can undergo neoplastic transformation and that tumors in aged C57BL/6 mice are frequently fibrosarcomas, Li and co-workers used a genetically tagged bone marrow (BM) transplant model to show that aged mice develop MSC-derived fibrosarcomas[39]. They also show that, MSCs transform spontaneously in culture with aging and that, when placed into a mouse model, they replicate the naturally occurring fibrosarcomas of the aged mice, showing similarly altered gene expression changes and, specifically, p53 mutation. Of great interest, spontaneously transformed MSCs contribute directly to the tumor formation, tumor vasculature, and tumor adipose tissue, recruit additional host BM-derived cells (BMDC) to the area, and fuse with the host BMDC[39].
MSCs AS VEHICLE FOR ANTICANCER DRUGS
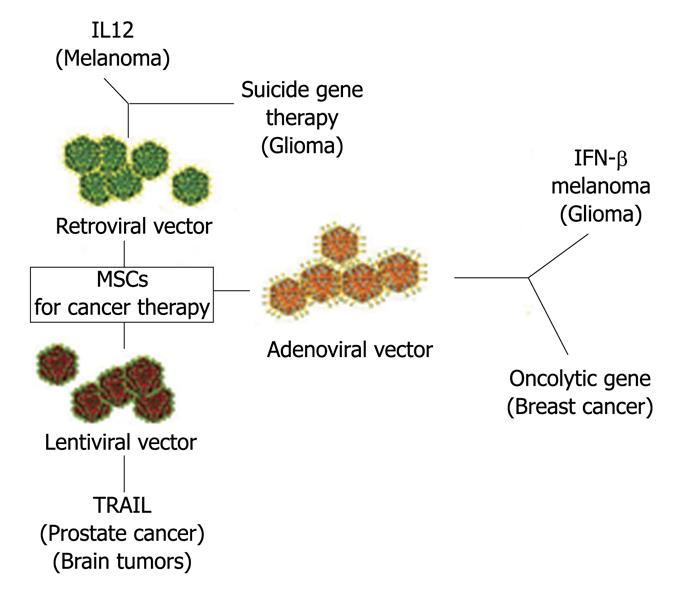
MSCs are attractive as a vehicle for the expression of therapeutic proteins by gene transfer, since they are easy in harvesting, isolation and expansion, transducibility with viral vectors, systemic or local delivery and are refractory to host immune response. As MSCs have the capacity to home to the tumor microenvironment, they may thus be promising tools for the selective delivery of antineoplastic drugs to tumors (Figure 3).
Figure 3 Mesenchymal stem cells as vehicle for anticancer drug.
In the Figure are indicated the vectors used to engineer MSCs and the “therapeutical genes”. Target cancer cells are in parenthesis. MSCs: Mesenchymal stem cells; IL12: Interleukin-12; IFN-γ: Interferon-gamma; TRAIL: Tumor necrosis (TNF)-related apoptosis-inducing ligand.
A pioneering study has been carried out by Studeny and collaborators[40]. They demonstrate that, for the purpose of anticancer therapy, MSCs can produce biological agents locally at tumor sites. In detail, they show that MSCs with forced expression of IFN-β inhibit the growth of malignant cells in vivo. Importantly, this effect requires the physical integration of MSCs into the tumors and cannot be achieved by systemically delivered IFN-β or by IFN-β produced by MSCs at sites distant from the tumor[40]. In detail, these investigators transduced MSCs with an adenoviral expression vector carrying the human IFN-β gene. Co-culture of IFN-β-expressing MSCs (MSC-IFN-β), cells with A375SM melanoma cells or MDA 231 breast carcinoma cells inhibited tumor cell growth as compared with the growth of tumor cells cultured alone. Intravenous injection of MSC-IFN-β cells into mice with established MDA 231 or A375SM pulmonary metastases led to incorporation of MSCs in the tumor architecture and to prolonged survival, when compared with untreated control mice[40,41]. This research is the proof of principle that MSCs may serve as a valid platform for selective delivery of biological agents in tumors.
Another interesting application of MSCs has been demonstrated for the treatment of malignant gliomas. Patients with malignant glioma often have a poor prognosis, partly related to the inability to deliver efficiently therapeutic agents to the tumor, due to the presence of the blood brain barrier. Nakamizo et al[42] hypothesized that MSCs may have a strong tropism for brain tumors and thus could be used as delivery vehicles for malignant glioma therapy. To test this, they isolated MSCs from bone marrow of healthy volunteers. These cells were fluorescently labeled and then injected into the carotid artery of mice bearing intracranial xenografts of a human malignant glioma. Interestingly, homing of these labeled MSCs occurred efficiently within the brain tumors, regardless of whether the cells were injected into the ipsilateral or contralateral carotid artery. In contrast, intracarotid injections of fibroblasts or U87 malignant glioma cells resulted in a widespread distribution of delivered cells without tumor specificity. In order to evaluate the potential of MSCs to deliver a therapeutic agent, these investigators engineered stem cells to release IFN-β. In vivo experiments showed that treatment of intracranial U87 xenografts with MSC-IFN-β significantly increases animal survival when compared with controls.
Another approach to the treatment of tumors was to use MSCs as a vehicle to deliver oncolytic viruses to tumor sites. Natural and genetically modified oncolytic viruses, such as replicative adenoviruses, have been systematically tested as anticancer therapeutics. Unfortunately, clinical trials have shown limited antitumor efficacy, partly due to insufficient viral delivery to tumor sites. Komarova and colleagues infected MSCs with an adenovirus, genetically modified for coxsackie and adenovirus receptor-independent infection (Ad5/3), which replicates in the carrier cells[43]. MSCs loaded with Ad5/3 cause total cell killing when co-cultured with a cancer cell line. In an animal model of ovarian cancer, MSC-based delivery of Ad5/3 increases the survival of tumor-bearing mice compared with direct viral injection. Tumor imaging analyses confirmed a decrease in tumor burden in animals treated with oncolytic virus delivered by MSC carriers compared with the direct injection of the adenovirus[43].
A similar approach was used by Curiel and collaborators for the treatment of breast cancer[44]. MSCs were transduced with conditionally replicating adenoviruses (CRAds) and a SCID mouse xenograft model was used to examine the effects of systemically injected CRAd-loaded MSCs or CRAd alone on the growth of pulmonary metastases of breast cancer in vivo. The intravenous injection of CRAd-loaded MSCs homed to the tumor site and led to extended mouse survival compared to mice treated with CRAd alone[44].
Retroviral vectors have also been used to obtain MSCs expressing therapeutic molecules. Pavlovic’s group evaluated the anti-tumor activity of human MSCs, stably transduced with a retroviral vector expressing the cytokine Interleukin-12 (IL-12) in a mouse melanoma model. MSCs expressing IL-12 significantly reduced the formation of lung metastases of B16F10 melanoma cells[45]. The therapeutic effectiveness of IL-12-expressing MSCs is in part mediated by CD8(+) T cells, while natural killer cells and CD4(+) T cells appear to play a minor role[45].
Suicide gene therapy has been tested for the treatment of invasive tumors. In this context, treatments have been developed using retroviral vectors expressing the thymidine kinase of Herpes Simplex Virus combined with the prodrug ganciclovir. Fo a safe and effective procedure, it is essential that the vector has a tumor tracking property to selectively and effectively attack invasive or metastatic lesions with minimal adverse effects. Uchibori and co-workers developed MSCs that locally express the HSV-tk-expressing retroviral vectors (VPMSCs)[46]. Systemic delivery of modified MSCs results in enhanced transgene expression in the 9L malignant glioma tumors in mice. VPMSC administration was accompanied by a significant suppression of tumor growth[46].
Other authors have introduced suicide genes into MSCs to produce a tumor-specific prodrug-converting cellular vehicle for targeted chemotherapy. Kucerova and collaborators used retroviral transduction to introduce the fusion gene cytosine deaminase/uracil phosphoribosyltransferase into adipose derived MSCs[47]. Cytosine deaminase (CD) converts cytosine to uracil and ammonia, providing an important mechanism for pyrimidine salvage in microbes. Because this activity is not found in mammalian cells, CD is being explored for use in suicide gene therapy, due to its ability to also convert the antifungal agent 5-fluorocytosine (5FC) to the potent antimetabolite drug, 5-fluorouracil (5FU). In the cell, 5FU is metabolized by endogenous enzymes to 5FdUMP, an irreversible inhibitor of thymidylate synthetase, thereby restricting the production of dTMP and downstream phosphorylated products. Depletion of dTTP pools results in inhibition of DNA synthesis and, consequently, to apoptosis[48].
Several reports demonstrate that lentiviral transduction is more efficient than onco-retroviral transduction and improves engraftment of MSCs without affecting their stem cell properties. Sasportas and co-workers showed that human MSCs are efficiently transduced with lentiviral vectors and migrate efficiently to tumor sites in a mouse model of human glioblastoma[49]. These authors then evaluated the effectiveness of MSCs transduced with lentiviruses expressing the TNF-related apoptosis inducing ligand (TRAIL). The ability of TRAIL to selectively target tumor cells, while remaining harmless to most normal cells, makes this an attractive candidate for an apoptotic therapy in tumor treatment. TRAIL signals via two pro-apoptotic death receptors (DR4 and DR5), inducing a caspase-8-dependent apoptotic cascade in tumor cells. The Sasportas group developed MSCs producing a secretable form of TRAIL. These cells were able to induce caspase-3-mediated apoptosis in GBM8 cells, a CD133-positive primary brain tumor cell line in vitro, and, when implanted into established GBM8 tumors, resulting in a significant increase in survival of mice bearing malignant gliomas[49].
In another study, researchers produced MSCs expressing TNFα-Tumstatin gene following lentivirus gene transduction. The effects of this approach have been evaluated on human prostate cancer cells by co-culturing them with engineered MSCs in vitro. The antitumor effects of these cells were then determined in PC3 prostate cancer cells in vivo. The results showed that engineered MSCs both inhibited proliferation and induced apoptosis in prostate cancer cells and xenograft tumors[50].
CONCLUSION
At this point, it appears clear why MSCs are acquiring such relevance in cancer cell biology as well as in antineoplastic pharmacology. There are , however, some concerns, mainly in the therapeutic approach. The key questions raised are: (1) How specific would the MSC targeting capability towards cancer cells be? In other words, are we sure that the use of such toxic substances by these “armed” MSCs is safe enough and delivered only to tumor cells? (2) How dangerous could such a targeted cancer cure using MSCs be for the patient, if we consider their elevated susceptibility to undergo spontaneous cell transformation?
Stemness is a characteristic which appears often associated with cancer onset and progression. It is well known that many recent findings support the notion that cells whose properties reproduce those of stem cells appear essential for both development and continuation of a substantial number of human cancers. Eradication of the stem cell compartment of a tumor is thus considered a prerequisite for a complete cancer remission or even total cure of the disease. For this reason, it should be unquestionable that the cancer propensity of MSC is really under control when these cells are used in cancer therapy. Once again, we realize that we are dealing with a double-edged sword and that only a strong further commitment by the scientific community is the real weapon against cancer.
Peer reviewer: Chie-Pein Chen, MD, PhD, Division of High Risk Pregnancy, Mackay Memorial Hospital, 92 Sec 2, Zhong-Shan North Road, Taipei 104, Taiwan, China
S- Editor Li LF L- Editor Hughes D E- Editor Yang C