Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.106194
Revised: April 20, 2025
Accepted: June 25, 2025
Published online: July 26, 2025
Processing time: 156 Days and 2.1 Hours
Insulin plays a crucial role in the metabolic priming and proliferation of neural stem cells (NSCs). However, insulin resistance (IR) is associated with impaired NSC proliferation and cognitive dysfunction, which are the hallmarks of psychiatric disorders (PDs). In addition to insulin, de novo lipogenesis (DNL) also plays an essential role in NSC proliferation and function as it supplies fatty acids for membrane phospholipid synthesis and cell signaling. However, enhanced DNL is associated with lipid/fatty acid accumulation, IR, and impaired NSC proliferation. Intriguingly, data from lipidomic studies suggest that DNL could be enhanced before the onset of classical symptoms in patients with PDs. Further, evidence suggests that patients with PDs may develop IR during childhood or before adolescence; therefore, DNL could be enhanced preceding the deve
Core Tip: Childhood insulin resistance (IR) is a potential risk factor for developing psychiatric disorders (PDs). Although insulin regulates neural stem cell (NSC) proliferation and function, IR is associated with impaired NSC proliferation and cognitive dysfunction, which are prominent features of PDs. While the mechanisms underlying IR and NSC dysfunction in PDs remain unclear, intracellular lipids/fatty acids synthesized via de novo lipogenesis could be the primary mediators. Since psychotropic drugs further deteriorate IR and stimulate de novo lipogenesis, prospects of various adjunctive therapies, especially stem cell therapy, in treating IR in schizophrenia and depression are discussed.
- Citation: Khan MM, Khan ZA, Khan MA, Pandey G. Childhood insulin resistance and neural stem cell dysfunction in psychiatric disorders: Role of de novo lipogenesis and treatment perspectives. World J Stem Cells 2025; 17(7): 106194
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/106194.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.106194
Psychiatric disorders (PDs) are complex brain disorders with heterogenous etiology[1]. Among various PDs, a high prevalence rate has been reported for schizophrenia, depression, bipolar disorder, anxiety, and attention-deficit hyperactivity disorder, among others[2]. Although PDs are diagnosed based on the appearance of psychiatric symptoms, brain abnormalities, including impaired neural stem cell (NSC) proliferation, synaptic dysfunction, and reduced grey and white matter volume of the cortex and hippocampus, usually develop before the onset of psychiatric symptoms[3-5]. In addition, cognitive dysfunction and metabolic abnormalities have been reported to precede the onset of psychiatric symptoms in patients with PDs[6]. Although various neurological disorders such as Alzheimer’s disease (AD) and Parkinson’s disease also display the aforementioned brain abnormalities and metabolic dysfunction[7,8], the extent of severity is high due to neuronal degeneration, and the illness onset is late; whereas, PDs could be diagnosed early, usually in adolescence or before, and show less severe brain pathologies[3-5].
Recent evidence suggests that childhood insulin resistance (IR) could be a potential risk factor for developing PDs[9-11]. Although animal studies have shown that insulin regulates NSC proliferation, cognitive behavior, and energy homeostasis[12-15], IR is associated with impaired NSC proliferation, cognitive dysfunction, and reduced energy production[15-18], which are the hallmarks of patients with PDs[9,10,19-21]. These findings suggest that reducing IR could be an effective strategy in improving cognitive function and global outcomes in patients with PDs.
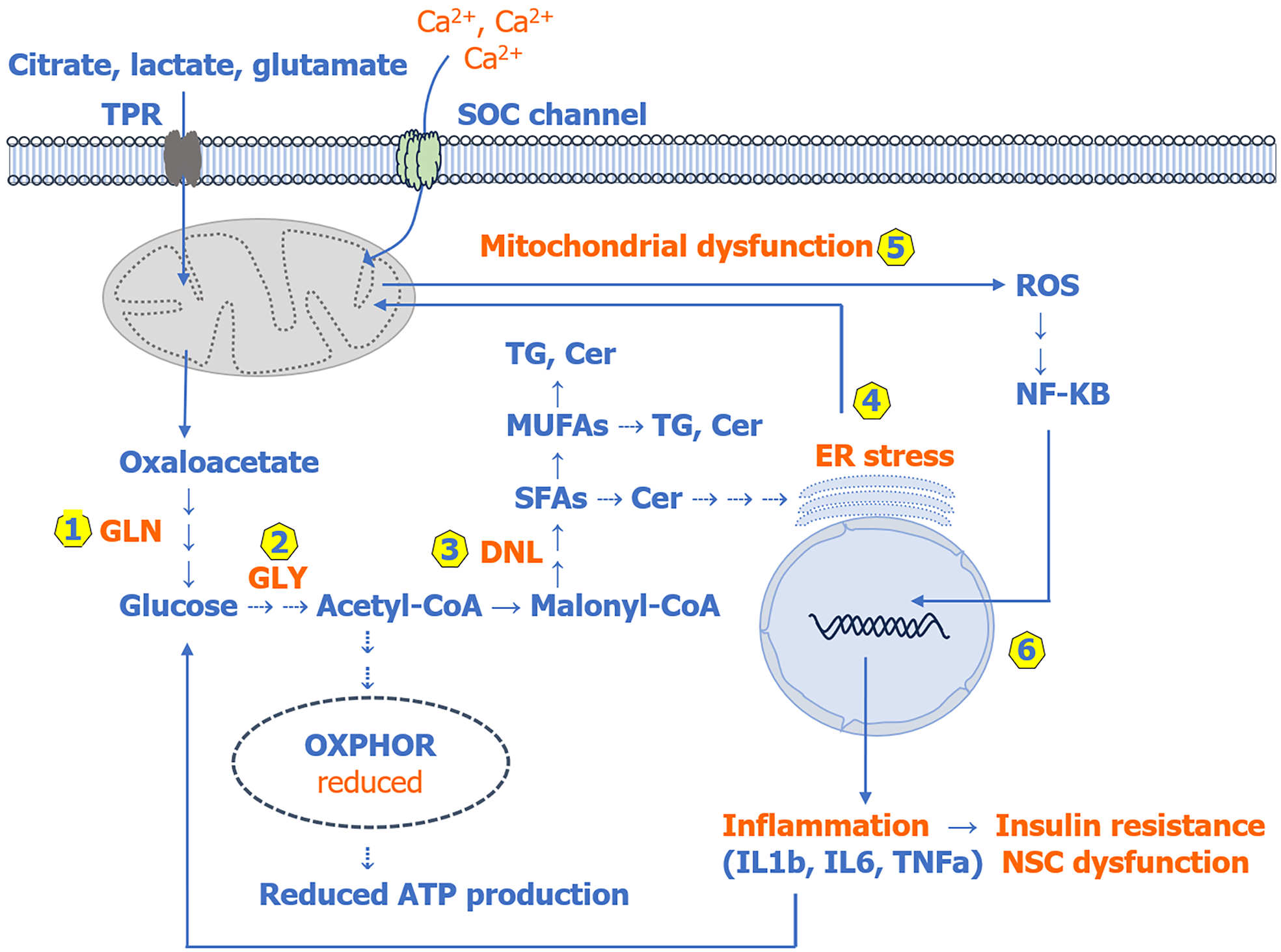
Although several mechanisms have been shown to induce IR, they seem to be triggered by endogenous lipids/fatty acids synthesized via de novo lipogenesis (DNL)[22-26]. As shown in Figure 1, DNL produces mainly saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs), which play an essential role in membrane phospholipid synthesis and cell signaling. However, enhanced DNL has been associated with elevated levels of SFAs and MUFAs and their increased incorporation into membrane phospholipids, and consequently, altered membrane fluidity, which has been reported in patients with PDs[25-29]. Several lines of evidence suggest that reduced membrane fluidity substantially increases the risk of developing IR and diabetes, which are the characteristic features of patients with PDs[6,9,10,24-26]. Further evidence suggests that the effect of SFAs and their ceramide derivatives (SFAs stimulate de novo ceramide biosynthesis as shown in Figure 2) on IR could be detrimental because they increase oxidative stress and inflammation by disrupting calcium (Ca2+) homeostasis, the endoplasmic reticulum (ER), and mitochondrial function[22,23,25,30,31]. The disrupted Ca2+ homeostasis, ER stress, mitochondrial dysfunction, and elevated oxidative stress coincide with the onset of cognitive and psychiatric symptoms in patients with PDs[32-35]. It should be mentioned that several studies, including our own, have reported that membrane polyunsaturated fatty acids are reduced, whereas SFAs and MUFAs and their ceramide derivatives are mostly increased and show a strong association with cognitive and psychiatric symptoms in patients with PDs[32,36-42].
DNL is an essential process, as genetic ablation of fatty acid synthase (FAS), the main enzyme of DNL, has been shown to induce embryonic and stem cell lethality[43-45]. However, enhanced DNL in adult animals has been associated with lipid/fatty acid accumulation, NSC dysfunction, metabolic abnormalities, and cognitive dysfunction[43,44,46]. Intri
In this review, recent findings from basic and clinical research are discussed, which suggest that IR in PDs is primarily triggered by endogenous lipids/fatty acids synthesized via DNL. Once IR develops, it may further stimulate DNL (Figures 1 and 2). Therefore, IR and enhanced DNL seem to be the two faces of the same coin and together could be the most likely cause of impaired NSC proliferation, cognitive dysfunction, and impaired energy homeostasis in patients with PDs. Since psychotropic drugs further deteriorate IR and stimulate DNL[32,33], prospects of various adjunctive drugs/therapies, including chemical, physical, and especially stem cell therapy, in treating IR and NSC dysfunction in patients with PDs, particularly schizophrenia and depression, are also discussed. The literature cited in this article was searched using PubMed, Scopus, and Google, and the articles published primarily in English and occasionally in French or German were included. References were selected mostly from those published within the last 5 years, while the older references were included only when deemed necessary.
Childhood or early life adversities play a major role in disposing individuals to develop metabolic, cognitive, and psychiatric diseases[1,10,11]. As mentioned above, longitudinal studies have reported that the great majority of patients with PDs can develop IR long before the onset of psychiatric symptoms, usually during childhood or before adolescence[10]. This suggests that IR could be an intrinsic trait in patients with PDs. In adult patients with schizophrenia, Steiner et al[47], while assessing IR and stress hormone levels, observed that IR and disrupted glucose homeostasis were present in a significantly high proportion of drug-naive patients with recent-onset psychosis. The authors suggested that this could be illness-related and not arise redundantly, due to pharmacotherapy, obesity, or hypothalamic-pituitary-adrenal axis activation; although, serum stress hormone levels could be elevated. Chouinard et al[9] reported that siblings or first-degree relatives of patients with psychosis may also display IR, which could be an intrinsic risk factor for psychosis. These findings have been confirmed in recent meta-analyses, which suggest that hormonal stress axis activation and lifestyle factors could also be the potential confounders associated with IR in PDs[48]. In support of this, reports suggest that plasma cortisol could be elevated in patients with psychosis[49], a condition that is associated with IR and ectopic fat deposition as a result of enhanced DNL[31,42,50].
Likewise, evidence suggests that a high proportion of patients with depression may develop IR before the diagnosis of classical symptoms[10,51]. In a longitudinal study, Perry et al[10] reported that insulin signaling is dysregulated from childhood or early adolescence in patients with depression. They also observed a positive association between IR and obesity at the age of 24 years, which is the time of diagnosis. Additionally, several studies have shown that IR is mostly deteriorated both in children and adolescents after treatment with both antidepressants and antipsychotic drugs[52]. This suggests that IR in PD could be an irreparable risk factor and requires more effective therapies/drugs.
Glucose is the main substrate for DNL, and evidence suggests that increased gluconeogenesis could be the major source of glucose for DNL in patients with PDs[24,31,33,53]. In support of this, several non-carbohydrate metabolic precursors, including citrate, lactate, glutamate, glutamine, serine, and others, which are used in gluconeogenesis, have been reported to be elevated in patients with PDs[54-57]. Since patients with PDs cannot utilize dietary glucose efficiently due to IR, elevated levels of these metabolic intermediates could be one of the potential causative factors associated with enhanced gluconeogenesis, hyperglycemia, and consequently increased DNL in PDs. An overview of gluconeogenesis, DNL, and other downstream pathways is given in Figure 1. Here, it is worth differentiating between lipogenesis and DNL; while lipogenesis is a general term used for lipid synthesis from fatty acids that are obtained either through the diet or synthesized via the de novo pathway from glucose, the term DNL is used for lipid synthesis from fatty acids produced only via the de novo pathway and is essential for the development and function of NSCs[24,25,46].
Although several mechanisms have been shown to induce IR, they appear to be triggered mostly by elevated endogenous lipids/fatty acids synthesized via DNL (Figure 1). Even high-fat diet-induced IR is triggered by lipids/fatty acids synthesized via DNL[22-25,31,33]. Since patients with PDs may develop IR during childhood or before adolescence, DNL could also be enhanced preceding the development of IR[9-11,24,51,53]. In support of this, several studies, including our own, have shown that the levels of SFAs, MUFAs, and their ceramide derivatives are increased in patients with recent onset PDs, which could be due to enhanced DNL[32,33,36-41]. In addition, the levels of ceramides are also elevated in the brain phospholipid from patients with PDs[42]. These findings, together with increased brain lactate levels, suggest that DNL could also be enhanced in the brain of patients with PDs[56,57].
While several studies have shown that both SFAs and MUFAs can induce IR, the effect of SFAs could be detrimental because they increase reactive oxygen species (ROS) and pro-inflammatory cytokine production[23,25,30,58]. On the other hand, elevated MUFAs usually do not increase the production of ROS and pro-inflammatory cytokines but can induce IR[22,59]. Therefore, one way or the other, patients with PDs will remain at risk of developing IR either due to elevated levels of SFAs or MUFAs. In addition, triglycerides (TG) and ceramide derivatives of SFAs and MUFAs, which are elevated in patients with PDs[42,48,55,60], can further increase oxidative stress, inflammation, and the severity of IR[31,61-63].
Regarding the mechanism(s) underlying lipid/fatty acid-induced IR, it has been shown that extracellular SFAs (especially palmitic acid) can trigger IR directly or indirectly by increasing pro-inflammatory cytokine production via activating the nuclear factor kappa B pathway and plasma membrane toll-like receptor 2/4. In addition, elevated intracellular SFAs (palmitic acid) can also induce IR by inhibiting sarcoplasmic ER Ca2+ pump and blocking Ca2+ release from the ER causing ER stress[30,31,33,63,64]. This can dramatically increase cytosolic Ca2+via entry through plasma membrane-bound store-operated Ca2+ channels (Figure 1). Although excess cytosolic Ca2+ is mainly sequestered and stored in the ER and mitochondria, during ER stress, cytosolic Ca2+ is diverted mostly towards mitochondria, causing mitochondrial dysfunction, a condition that leads to increased ROS production[30,31,64]. As mentioned before, recent studies have implicated ER stress and mitochondrial dysfunction in the pathophysiology of PDs, likely due to their well-established role in ROS and proinflammatory cytokine production. Elevated ROS increase the production of pro-inflammatory cytokines in various cells, including microglia, monocytes-macrophages, adipocytes, and liver, by activating the nuclear factor kappa B pathway[30,63,64]. While activated microglia play a key role in brain inflammation, activated monocytes-macrophages and adipocytes are the major players involved in the peripheral tissue inflammation through their secretion of various pro-inflammatory cytokines, including interleukin 1 beta (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α). These pro-inflammatory cytokines are reportedly elevated in patients with PDs[65,66]. Although treatment with antidepressants and antipsychotic drugs has been shown to reduce the production of some of these cytokines, others, including IL-1β, IL-6, IL-8, and TNF-α, are only transiently and marginally affected[65,66].
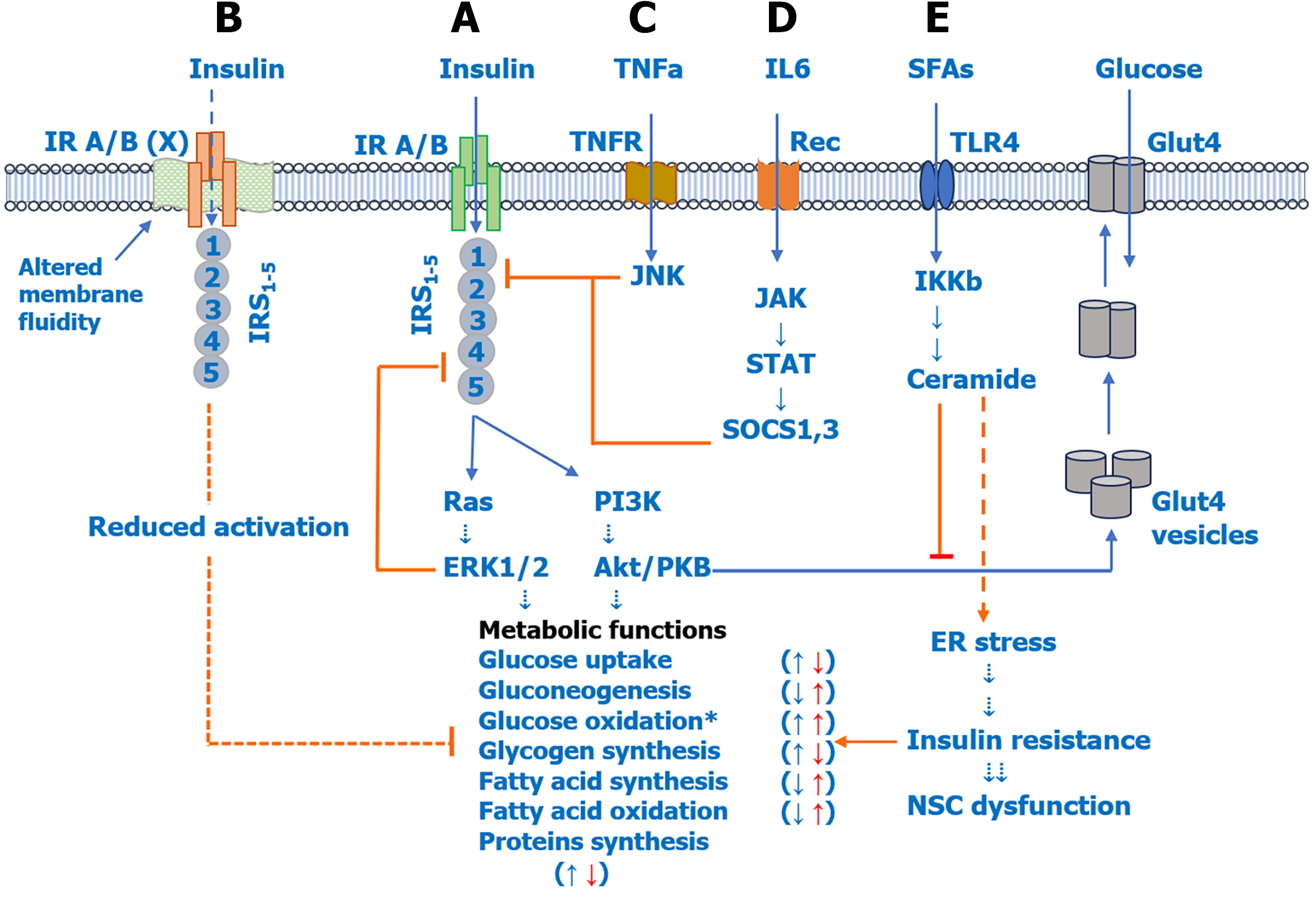
Figure 2 shows various signaling pathways activated by pro-inflammatory cytokines and SFAs and their role in IR and NSC dysfunction. As shown in Figure 2A, insulin performs its action by activating plasma membrane-bound insulin receptor A/B (IR A/B), IR substrate 1-5 (IRS1-5), and downstream rat sarcoma-extracellular-regulated kinase 1/2 and phosphoinositide 3 kinase-Akt/protein kinase B pathways, which have been shown to regulate various metabolic functions including glucose uptake, oxidation/glycolysis, gluconeogenesis, glycogen synthesis, fatty acid synthesis/oxidation, and protein synthesis; whereas, IR has been shown to have opposite effects[12,15,18]. Evidence suggests that reduced membrane fluidity (Figure 2B) can itself reduce IR A/B activity and function, leading to reduced insulin sensitivity[33,67]. Pro-inflammatory cytokines TNF-α (Figure 2C) and IL-6 (Figure 2D) induce IR by activating the c-Jun terminal kinase and Janus kinase-signal transducer and activator of transcription pathways, which inhibit IRS2 and IRS1 activation (phosphorylation), respectively. IL-6 increases the expression of suppressor of cytokine signaling proteins, which mediates inhibition of IRS1 and also IR. In addition to pro-inflammatory cytokines, SFAs can also induce IR indirectly by increasing de novo ceramide biosynthesis (Figure 2E). Ceramides can induce IR in two ways, namely by inducing ER stress and by inhibiting Akt/protein kinase B, which facilitates glucose uptake by increasing translocation of glucose transporter 4 to the plasma membrane[31,58,62].
In Table 1, we have summarized various markers/products of DNL, precursors of gluconeogenesis, and various metabolic parameters (abnormalities) associated with the mechanism(s) discussed above, which are also illustrated in Figures 1 and 2. Although IR may affect the development and function of stem cells residing in distinct tissues, including bone marrow, liver, adipose tissue, and the brain, below we discuss the influence of IR on NSCs only.
| Parameters | Psychosis | Depression |
| DNL markers | ||
| SFAs | Increased[32,36-39] | Increased[40,41] |
| MUFAs | Increased[32,36-39] | Increased[40,41] |
| PUFAs1 | Reduced[32,36-39] | Reduced[40,41] |
| Ceramides | Increased[42] | Increased[42] |
| Triglycerides | Increased[48,60] | Increased[55,61] |
| GLN substrates | ||
| Lactate | Increased[56] | Increased[57] |
| Citrate | Increased[33] | Unknown |
| Pyruvate | Increased[54] | Unknown |
| GluAA | Increased[4,54] | Increased[55] |
| Metabolic parameters | ||
| Blood glucose | Increased[9,10,47,48] | Increased[10,51] |
| IR/insulin level | Increased[9,10,47,48] | Increased[10,51] |
| Obesity (BMI) | Increased[6,47] | Increased[10,51] |
| Diabetes | Increased[9,10,47] | Increased[10,51] |
Over the years, it has been established that NSCs exist in several functionally distinct areas/neurogenic niches of the adult brain, including the subventricular zone around the lateral ventricle and subgranular in the dentate gyrus of the hippocampus[68]. In the adult brain, NSCs differentiate into three types of cells: Including neurons (via a process called neurogenesis), astrocytes, and oligodendrocytes (via a process known as gliogenesis). These three brain cell types are the major players that maintain structural organization and regulate functional plasticity of the brain throughout adult life[68-71]. IR affects both aspects of NSCs, that is, neurogenesis and gliogenesis.
Neurogenesis is a dynamic process in which NSCs produce an enormous number of new neurons daily in the adult brain[69]. New neurons mature primarily into interneurons and participate in cellular and synapse circuit formation and in adaptation to various behavioral and environmental cues[70,71]. Patients with PDs display learning and memory deficits, which suggest that neurogenesis could be impaired[72,73]. Indeed, several studies have reported reduced neurogenesis in functionally distinct areas of the brain of patients with PDs.
In schizophrenia, postmortem studies have documented reduced expression of the cell proliferation marker Ki67 in the anterior dentate gyrus of the hippocampus, suggesting that neurogenesis could be reduced[74,75]. Another study found a significant reduction in polysialylated neural cell adhesion molecule-positive staining in the hilus regions of the hippocampus but not in the dentate gyrus, suggesting that the density of immature neurons could be reduced in schizophrenia[76]. Furthermore, reduced density of mature neurons has also been reported in the left dentate gyrus and in the cingulate cortices in schizophrenia, but not in the cornu ammonis 1-4 regions or subiculum of the anterior hippocampus[77-79]. A recent study also reported reduced cortical neuron number and neuron density in the Brodmann area 24 of the medial prefrontal cortex in the schizophrenic brain[80]. Weissleder et al[81] reported reduced NSC proliferation in the subependymal zone, which supplies new neurons to cortical and subcortical areas. This suggests that cortical neuro
Insulin plays a crucial role in neurogenesis by stimulating the proliferation and maturation of NSCs with a concomitant improvement in cognitive behaviors and a decrease in the inflammatory cues in laboratory animals[12-14,16]. Insulin has been shown to mediate these effects both in vitro and in vivo by activating specific IR A/B and IRSs, which themselves have been reported to be highly expressed in the neurogenic niches of the adult brain[12,85]. In addition, insulin signaling also plays a crucial role in the self-renewal, proliferation, and differentiation of NSCs during development[12,85]. Conversely, IR or impaired insulin signaling has been shown to have a detrimental effect on neurogenesis, learning, and memory in laboratory animals[16,86], which could be the most likely scenario in patients with PDs[72,73].
Glial cells, especially astrocytes, act like ‘glue’ in the brain. They provide chemical and biological support to the neurons and regulate the formation of both inhibitory and excitatory synapses[87]. Astrocytes are identified in the brain by their high expression of a protein called glial fibrillary acid protein (GFAP). A significant proportion of NSCs express GFAP in the neurogenic niches of the brain[68,70]. This suggests that astrocytes develop from the same neuronal cell lineage as neurons. Over the years, several studies have analyzed astrocyte density and expression of astrocyte-specific markers in the brain of patients with PDs. The findings are briefly discussed below.
In schizophrenia, reduced astrocyte density has been observed in various anatomically and functionally distinct regions of the brain including the dentate gyrus, cingulate cortex, motor cortex, nucleus accumbens, basal nuclei, and substantia nigra[88,89]. It should be noted that a few studies have reported no change or an increase in astrocyte density in other regions, including the temporal and frontal cortex and amygdala[89]. Altogether, while it appears that astrocyte proliferation could be affected differentially in a region-dependent manner in schizophrenia, the findings in patients with depression are more consistent with regard to reduced astrocyte density. Using histological/immunohistological methods, several authors have reported reduced proliferation or loss of astrocytes in the prefrontal cortex, hippocampus, and other regions of the brain in patients with depression[82,84,88-92].
The density of oligodendrocytes is also significantly affected in the brain of patients with PDs[79,90,93,94]. Oligo
The evidence discussed above suggests that the proliferation and differentiation of NSCs into neurons and glial cells could be impaired in patients with PDs; however, the underlying mechanism(s) remain unclear. IR could be a potential mediator, as insulin alone or in association with growth factors (discussed below) has been shown to regulate the proliferation of NSCs, and IR itself is associated with reduced proliferation[100,101]. Regarding the role of DNL in NSC dysfunction, IR coincides with enhanced DNL, and evidence suggests that enhanced DNL leads to lipid/fatty acid accumulation, which could be associated with reduced neurogenesis and learning-memory deficit[43,44,46,102]. In support of this, mice expressing mutant FAS (the main enzyme of DNL) with increased activity had impaired proliferation of NSCs in the hippocampus along with cognitive deficit, most likely due to fatty acid accumulation in NSCs and development of lipogenic ER stress[43]. In another experiment, human embryonic stem cell-derived NSCs expressing mutant FAS showed reduced proliferation[43,46]. Further, it was shown that tissue-specific deletion of FAS in mice significantly reduced NSC proliferation and altered the polarity of apical and radial glia cell progenitors[102]. Together, the above findings suggest that while DNL is essential for neurogenesis, fatty acid accumulation as a result of enhanced DNL could be associated with reduced neurogenesis, reduced oligodendrocyte density, and cognitive abnormalities in patients with PDs.
Growth factors are master regulators of NSC proliferation, maturation, and survival. In addition, growth factors also regulate metabolic functions, including glucose and lipid metabolism and energy homeostasis, directly or in association with insulin[103,104]. In the last few years, several studies have reported reduced expression of over a dozen growth factors in patients with PDs. Among the notable growth factors, which are reduced, are brain-derived neurotrophic factor, insulin growth factor, fibroblast growth factor, epidermal growth factor, nerve growth factor, and others[105-108]. While these studies provide further evidence for impaired NSC proliferation and/or function in patients with PDs, IR/diabetes is reportedly associated with the reduced expression of many of these growth factors in otherwise mentally healthy individuals[109,110]. In cell culture studies, growth factor treatment has been shown to improve insulin sensitivity, hyperglycemia, and glucose tolerance[111-113].
Although psychotropic drugs have been shown to significantly restore circulating levels of various growth factors, levels of some growth factors either remain unaltered or only slightly increased. For instance, Zhang et al[114] reported that serum epidermal growth factor was significantly reduced in drug-naïve patients with psychosis and was not increased by antipsychotic treatment alone or by a combined treatment with electroconvulsive therapy. Moreover, as mentioned above, almost all antidepressants and antipsychotic drugs induce IR, obesity, and nonalcoholic fatty liver disease (NAFLD)[51,52,115-118], which are associated with the reduced growth factor expression[109,110]. Thus, additional research is needed to identify new effective treatments for reducing IR and increasing NSC proliferation and function in patients with PDs.
Psychotropic drugs are the first line of treatment for PDs; however, their long-term use has been shown to induce or deteriorate pre-existing IR, leading to the development of obesity, diabetes, and NAFLD[115-117]. The mechanism(s) underlying these metabolic abnormalities remain unclear; however, evidence suggests that enhanced DNL leading to lipid/fatty acid accumulation could be the most likely mechanism(s) involved[33,118]. In support of this, several studies, including our own, have shown that antipsychotic treatment increases the levels of erythrocyte SFAs, MUFAs, and plasma TG[32,38,119,120] and triggers the development of obesity and NAFLD in patients with psychosis[115-117,121]. Since SFA and MUFA composition of erythrocytes has been used to assess the extent of DNL in health and diseases, an increase in erythrocyte SFA and MUFA levels by treatment with psychotropic drugs suggests that DNL could be enhanced. Intriguingly, while no clinical trials have been conducted to directly target IR in PDs, several adjunctive drugs/therapies, which have shown promising success in reducing psychiatric symptoms in patients with PDs, also reduce IR and enhance NSC proliferation and function. Among these are chemical therapy, including anti-inflammatory agents and antioxidants, aerobic/physical therapy, and stem cell therapy, which are discussed below.
In the last two decades, several anti-inflammatory agents and antioxidants have been used to reduce inflammation and improve the therapeutic efficacy of psychotropic drugs in patients with PDs. As shown in Table 2, these agents include aspirin, N-acetylcysteine, minocycline, pregnanolone, estrogens, raloxifene, curcumin, pioglitazone, celecoxib, and w-3 polyunsaturated fatty acids. Addition of these agents to the clinically approved doses of antidepressants or antipsychotic drugs has been shown to reduce symptoms of psychosis and depression in patients with PDs[122-141]. However, in patients with depression, evidence suggests that some of these agents, including minocycline, estrogens, and raloxifene, may not be effective or may even worsen the symptoms; thus, further studies are needed to evaluate their safety in treating PDs.
| Drugs/therapies | Psychosis1 | Depression2 |
| Aspirin | Reduced[122] | Reduced[130] |
| N-acetylcysteine | Reduced[123] | Reduced[131] |
| Minocycline | Reduced[122] | No effect[132] |
| Pregnenolone | Reduced[122] | Reduced[133] |
| Estrogens | Reduced[122,124] | Deteriorated[134] |
| Raloxifene | Reduced[122] | No effect[135] |
| Curcumin | Reduced[125] | Reduced[136] |
| Pioglitazone | Reduced[126] | Reduced[137] |
| Celecoxib | Reduced[127] | Reduced[138] |
| ω3-PUFAs | Reduced[128] | Reduced[139] |
| Aerobic exercise | Reduced[129] | Reduced[140] |
| Resistance exercise | May reduce[129] | Reduced [141] |
| MSC therapy | Unknown | Unknown |
On the other hand, as shown in Table 3, the chemical agents discussed above also have potent antidiabetic properties as they reduce IR while improving cognitive behavior and increasing NSC proliferation/neurogenesis in laboratory animals[142-184]. Together, the above evidence suggests that therapeutic agents that regulate DNL and/or IR could be effective in enhancing the proliferation and function of NSCs in patients with PDs. Since patients with PDs may develop IR from the childhood or adolescence stage, early intervention with an appropriate adjunctive drug could be more effective in increasing NSC proliferation and treatment outcomes in patients with PDs.
| Drugs/therapies | Insulin resistance | Neurogenesis | Cognition |
| Aspirin | Reduced[142] | Increased[157] | Improved[157] |
| N-acetylcysteine | Reduced[143] | Increased[158] | Improved[172] |
| Minocycline | Reduced[144] | Increased[159] | Improved[173] |
| Pregnenolone | May reduce[145] | Increased[160] | Improved[174] |
| Estrogens | Reduced[146] | Increased[161] | Improved[175] |
| Raloxifene | Reduced[147] | Increased[162] | Improved[176] |
| Tamoxifen | Increased[148] | Increased[163] | Improved[177] |
| Curcumin | Reduced[149] | Increased[164] | Improved[164] |
| Pioglitazone | Reduced[150] | Increased[165] | Improved[178] |
| Celecoxib | Reduced[151] | May reduce[166] | Improved[179] |
| ω3-PUFAs | Reduced[152] | Increased[167] | Improved[180] |
| SIRT1-A | Reduced[153] | Increased[168] | Improved[181] |
| Aerobic exercise | Reduced[154] | Increased[169] | Improved[182] |
| Resistance exercise | May reduce[155] | Increased[170] | Improved[183] |
| MSC therapy | Reduced[156] | Increased[171] | Improved[184] |
Apart from the chemical agents discussed above, sirtuin 1 (SIRT1) agonists/activators (Table 3) have shown tremen
Emerging evidence suggests that aerobic exercise as an add-on treatment can significantly increase the effectiveness of psychotropic drugs to alleviate the symptoms of depression and psychosis in patients with PDs[129,140,141]. In patients with depression, aerobic exercise improves global cognitive function and reduces depression in older adults with mild cognitive impairment[182]. The mean effect of global cognitive function is increased with higher exercise frequency. In patients with schizophrenia, aerobic exercise has also been shown to improve various domains of cognition, including global cognition, working memory, social cognition, and attention/vigilance[183]. These cognition-enhancing effects of aerobic exercise could be due to increased neurogenesis/NSC proliferation, as well as reduced IR and DNL in patients with PDs[154,169,187-189].
In addition, recent evidence suggests that resistance exercise could also improve cognitive behavior, increase neurogenesis, and reduce IR in laboratory animals and/or human volunteers[155,170,183,189]. These pleiotropic effects of aerobic and resistance exercise are in support of the findings suggesting that patients with PDs may develop tissue hypoxia on or before the onset of psychiatric symptoms[190]. Intriguingly, chronic hypoxia has been shown to disrupt NSC proliferation and differentiation in laboratory animals, likely due to lipid/fatty acid accumulation as a consequence of enhanced DNL[191,192]. In conclusion, increased efficacy of psychotropic drugs when combined with resistance exercise in PDs could be a result of the cumulative effect of reduced IR, reduced DNL, and enhanced NSC proliferation.
Stem cells, especially those isolated from mesenchyme of bone marrow or human umbilical cord, have been used extensively in model animals and human subjects for ameliorating inflammation, IR/diabetes, and cognitive symptoms[193]. Mesenchymal stem cells (MSCs) are also referred to as multipotential stromal cells, mesenchymal stromal cells, or mesenchymal progenitor cells. They have been shown to differentiate into specific cell types under in vitro-specified conditions and in vivo after implantation[193]. MSCs can be isolated from distinct tissues, including umbilical cord, endometrial polyps, menstrual blood, bone marrow, and adipose tissue. At present, MSCs are being used in numerous clinical trials for various diseases[193]. Here, the outcomes of MSC therapy in model animals of diabetes and PDs are discussed.
Stem cell therapy reduces IR and lipid abnormalities: Emerging data from recent animal model and clinical studies suggest that MSC therapy can have multiple effects in a diabetic environment[193-195]. Along with regulating immune cell proliferation and function, MSCs can reduce peripheral IR, halt beta-cell destruction, preserve residual beta-cell mass, promote beta-cell regeneration and insulin production, support islet grafts, and reduce lipid accumulation and DNL[194,195].
In mice, MSC therapy has been shown to reduce IR and enhance glucose uptake by peripheral tissues such as skeletal muscle, liver, and adipose tissue, while restoring glycemic control and beta-cell function and reducing the risk of type 2 diabetes-related complications[195,196]. MSC therapy reduces peripheral tissue IR via phosphoinositide 3 kinase-dependent phosphorylation of IRS-1, which in turn increases glucose transporter 4 and IR expression on the cell membrane and reduces activation of stress-induced serine kinases, such as c-Jun terminal kinase 1 and extracellular-regulated kinase 1[197-199]. Also, several studies have shown that MSCs from different sources can differentiate into glucose-responsive insulin-producing beta cells in vitro and in vivo[194]. Further, MSCs secrete various trophic/growth factors such as vascular endothelial growth factor, fibroblast growth factor, angiopoietin-1, and hepatocyte growth factor, which can profoundly improve beta-cell function[200].
Regarding the influence of MSC therapy on lipid abnormalities, several studies have shown that infusion of MSCs, especially from umbilical cords, can significantly reduce hyperglycemia and elevate hepatic transaminases and lipid contents, including TGs, total cholesterol, and low-density lipoprotein cholesterol[195,201]. It can also significantly reduce liver injury, as suggested by reduced lipid accumulation and decreased hepatic steatosis. Regarding the mechanism, MSC therapy has been found to exert dual effects on lipid metabolism, with increased expression of fatty acid oxidation-related genes and reduced expression of lipogenesis-related genes mediated by the upregulated hepatocyte nuclear factor 4 alpha-carboxylesterase 2 pathway[195,201].
Stem cell therapy enhances NSC proliferation and cognitive outcome: Stem cells, including both MSCs and NSCs, have been extensively used in various animal model studies for improving cognition and reducing neurodegeneration[202-206]. In the amyloid-β (Aβ)-related animal model of AD, the Wnt signaling pathway has been suggested to impair neurogenesis. MSC infusion in Aβ-treated animals significantly increases NSC proliferation/neurogenesis in the dentate gyrus of the hippocampus at 2 weeks and 4 weeks[202]. Likewise, in another study, MSC infusion significantly reduced neurodegeneration and increased neurogenesis and synaptic function[203]. Similar results have been obtained with both human umbilical cord and adipose-derived MSCs, suggesting that intravenous infusion of human MSCs could be an effective approach for increasing neurogenesis and synaptic function in human patients. In another study, McGinley et al[204] implanted human NSCs in a murine model of AD and observed significant improvement in cognitive function along with reduced load of Aβ peptide/plaques[204].
Aging is a potential risk factor for developing PDs and is accompanied by a significant decline in NSC proliferation (neurogenesis) and cognitive function. Recent studies showed that MSCs and exosome implantation increased neurogenesis and improved cognitive function while reducing inflammation in aged animals[205]. Another study showed that MSC-derived exosome implantation significantly increased neurogenesis and improved cognitive function in mice treated with repeated injections of methamphetamine, a chemical that is used to induce psychosis in humans and animals[206]. These results suggest that MSC therapy could be effective in improving metabolic, neurogenic, and cognitive function in patients with PDs.
Outcome of stem cell therapy in an animal model of PDs: In a recent study, Gobshtis et al[171] transplanted bone marrow-derived MSCs in the intracerebroventricular region of a ketamine-induced murine model of schizophrenia. The authors observed that MSCs successfully engrafted and survived for up to 3 months following transplantation. The animals showed significant improvement in social novelty preference and pre-pulse inhibition with a concomitant increase in hippocampal neurogenesis. They also observed an independent aging effect on behavior and neurogenesis, which was attenuated by MSC treatment. These collective findings suggest that long-term effects during aging could be dependent on the self-renewal potential of NSCs.
In another study, You et al[207] intravenously infused human umbilical cord-derived MSCs (hUC-MSCs) with a potent immunomodulatory effect on an animal model of schizophrenia. The authors observed that neuroinflammation along with peripheral TNF-α elevation was associated with schizophrenia-relevant behaviors in amphetamine-sensitized mice, and hUC-MSC infusion significantly reduced schizophrenia-relevant behaviors and neuroinflammatory cues. They concluded that a single hUC-MSC infusion had long-term beneficial effects via regulatory T-cell induction and secretion of IL-10 in this mouse model of schizophrenia.
Tfilin et al[208] analyzed the therapeutic potential of MSCs in the rat Flinders sensitive line (FSL), an animal model for depression. The authors gave an intracerebroventricular injection of culture-expanded and 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate-labeled bone marrow-derived MSCs to FSL rats. They observed that MSC-transplanted FSL rats had significant improvement in behavioral performance. They also observed that neurogenesis was increased in the ipsilateral dentate gyrus and hippocampus, and correlated with behavioral performance. Although these findings suggest that MSCs may serve as a novel option for treating depression, clinical trials with MSCs in patients with depression remain to be conducted.
Insulin signaling is required for priming NSCs for glucose uptake, metabolism, and energy production. However, IR is associated with detrimental effects on NSC proliferation and function. Since patients with PDs may develop IR before adolescence, impaired insulin signaling could be a potential causative factor associated with reduced NSC proliferation, cognitive dysfunction, and reduced energy production in patients with PDs.
In addition to insulin, DNL also plays an essential role in NSC proliferation and function as it supplies SFAs and MUFAs for membrane phospholipid synthesis and cell signaling. Intriguingly, insulin signaling regulates DNL, whereas IR is associated with enhanced DNL. Evidence suggests that IR is primarily triggered by excess endogenous lipids/fatty acids (SFAs, MUFAs, TG, ceramides) synthesized via DNL. However, once IR develops, it can further stimulate DNL, leading to lipid/fatty acid accumulation, impaired NSC proliferation, cognitive dysfunction, and reduced energy production, which appears to be the most likely scenario in patients with PDs. Therefore, reducing IR could be a promising therapeutic option in PDs.
Although no specific clinical trials targeting IR have been performed in PDs, various adjunctive drugs/therapies, including chemical, physical/aerobic, and MSC therapy, which have been shown to improve cognitive and psychiatric symptoms in patients with PDs, also improve insulin sensitivity and metabolic profile in animal models of diabetes and PDs. Moreover, beneficial effects of these agents/therapies in model animals were correlated with increased NSC proliferation and improvement in cognitive behavior. Evidence suggests that these agents/therapies also reduce DNL and lipid/fatty acid accumulation, a leading cause of IR in PDs.
Intriguingly, similar to chemical/physical therapies, treatment with MSCs in rodents has been reported to reduce IR, enhance neurogenesis, and improve cognitive behavior with concomitant improvement in metabolic parameters without inducing any serious side effects. Thus, MSC therapy in PDs could be highly beneficial and further investigation is warranted. Since IR could be diagnosed during childhood or in the adolescent stage in patients with PDs, early intervention with an appropriate adjunctive therapy/drug alone or in combination may normalize cellular signaling(s) that trigger the development of IR and lipid abnormalities while disrupting neurogenesis and cognitive function in patients with PDs. For example, combining MSC therapy with aerobic/resistance exercise may be worth exploring. In this regard, animal model studies as well as human clinical trials are urgently warranted.
We sincerely acknowledge the facilities provided by the Department of Biotechnology, Era’s Lucknow Medical College and Hospital (Lucknow, India) and Faculty of Science, Era University (Lucknow, India). We also gratefully acknowledge the expert comments and language editing of this manuscript by Professor Dr. Darrell W Brann, Augusta University (Augusta, GA, United States).
| 1. | Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry. 2017;16:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 2. | GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 2639] [Article Influence: 879.7] [Reference Citation Analysis (0)] |
| 3. | Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2355] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 4. | Howes OD, Bukala BR, Beck K. Schizophrenia: from neurochemistry to circuits, symptoms and treatments. Nat Rev Neurol. 2024;20:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 5. | Marx W, Penninx BWJH, Solmi M, Furukawa TA, Firth J, Carvalho AF, Berk M. Major depressive disorder. Nat Rev Dis Primers. 2023;9:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 212] [Reference Citation Analysis (0)] |
| 6. | Penninx BWJH, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 7. | Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 1239] [Article Influence: 309.8] [Reference Citation Analysis (1)] |
| 8. | Pagonabarraga J, Bejr-Kasem H, Martinez-Horta S, Kulisevsky J. Parkinson disease psychosis: from phenomenology to neurobiological mechanisms. Nat Rev Neurol. 2024;20:135-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Chouinard VA, Henderson DC, Dalla Man C, Valeri L, Gray BE, Ryan KP, Cypess AM, Cobelli C, Cohen BM, Öngür D. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry. 2019;24:1513-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Perry BI, Stochl J, Upthegrove R, Zammit S, Wareham N, Langenberg C, Winpenny E, Dunger D, Jones PB, Khandaker GM. Longitudinal Trends in Childhood Insulin Levels and Body Mass Index and Associations With Risks of Psychosis and Depression in Young Adults. JAMA Psychiatry. 2021;78:416-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Alberry B, Silveira PP. Brain insulin signaling as a potential mediator of early life adversity effects on physical and mental health. Neurosci Biobehav Rev. 2023;153:105350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 12. | Ziegler AN, Levison SW, Wood TL. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 2015;11:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Ren Z, Xu J, Deng C, Zhang Z, Godoy-Parejo C, Xu F, Huang ECC, Wang J, Cai Z, Liu W, Hu G, Chen G. Insulin Directs Dichotomous Translational Regulation to Control Human Pluripotent Stem Cell Survival, Proliferation and Pluripotency. Int J Biol Sci. 2022;18:3562-3575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Adzovic L, Lynn AE, D'Angelo HM, Crockett AM, Kaercher RM, Royer SE, Hopp SC, Wenk GL. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation. 2015;12:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17:468-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Reagan LP, Cowan HB, Woodruff JL, Piroli GG, Erichsen JM, Evans AN, Burzynski HE, Maxwell ND, Loyo-Rosado FZ, Macht VA, Grillo CA. Hippocampal-specific insulin resistance elicits behavioral despair and hippocampal dendritic atrophy. Neurobiol Stress. 2021;15:100354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, Pothos EN, Kahn CR. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112:3463-3468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 18. | Burkart AM, Tan K, Warren L, Iovino S, Hughes KJ, Kahn CR, Patti ME. Insulin Resistance in Human iPS Cells Reduces Mitochondrial Size and Function. Sci Rep. 2016;6:22788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb Perspect Biol. 2016;8:a019026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front Neurosci. 2017;11:493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Katz Shroitman N, Yitzhaky A, Ben Shachar D, Gurwitz D, Hertzberg L. Meta-analysis of brain samples of individuals with schizophrenia detects down-regulation of multiple ATP synthase encoding genes in both females and males. J Psychiatr Res. 2023;158:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35:1686-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Button EB, Mitchell AS, Domingos MM, Chung JH, Bradley RM, Hashemi A, Marvyn PM, Patterson AC, Stark KD, Quadrilatero J, Duncan RE. Microglial cell activation increases saturated and decreases monounsaturated fatty acid content, but both lipid species are proinflammatory. Lipids. 2014;49:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 938] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 25. | Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 26. | Weijers RN. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr Diabetes Rev. 2012;8:390-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Yao JK, van Kammen DP. Red blood cell membrane dynamics in schizophrenia. I. Membrane fluidity. Schizophr Res. 1994;11:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Nakashima H, Ueda K, Yasugawa S, Katsuragi S, Kimura T, Miyakawa T. Erythrocyte deformability in schizophrenic patients. Psychiatry Clin Neurosci. 1996;50:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Barshtein G, Ponizovsky AM, Nechamkin Y, Ritsner M, Yedgar S, Bergelson LD. Aggregability of red blood cells of schizophrenia patients with negative syndrome is selectively enhanced. Schizophr Bull. 2004;30:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49:e291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 540] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 32. | Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Khan MM. Role of de novo lipogenesis in insulin resistance in first-episode psychosis and therapeutic options. Neurosci Biobehav Rev. 2022;143:104919. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2019;45:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 35. | Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 310] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 36. | Alqarni A, Mitchell TW, McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Berger M, Mossaheb N, Schlögelhofer M, Smesny S, Hickie IB, Berger GE, Chen EYH, de Haan L, Nieman DH, Nordentoft M, Riecher-Rössler A, Verma S, Thompson A, Yung AR, Amminger GP, Meyer BJ. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophr Res. 2020;226:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Dickens AM, Sen P, Kempton MJ, Barrantes-Vidal N, Iyegbe C, Nordentoft M, Pollak T, Riecher-Rössler A, Ruhrmann S, Sachs G, Bressan R, Krebs MO, Amminger GP, de Haan L, van der Gaag M, Valmaggia L, Hyötyläinen T; EU-GEI High Risk Study Group, Orešič M, McGuire P. Dysregulated Lipid Metabolism Precedes Onset of Psychosis. Biol Psychiatry. 2021;89:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Khan MM. Disrupted leptin-fatty acid biosynthesis is an early manifestation of metabolic abnormalities in schizophrenia. World J Psychiatry. 2022;12:827-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 39. | Li M, Gao Y, Wang D, Hu X, Jiang J, Qing Y, Yang X, Cui G, Wang P, Zhang J, Sun L, Wan C. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr Bull. 2022;48:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Wang L, Liu T, Guo J, Zhao T, Tang H, Jin K, Li L, Xue Y, Yang R, Chen J, Tang M. Abnormal erythrocyte fatty acid composition in first-diagnosed, drug-naïve patients with depression. J Affect Disord. 2022;318:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Liu T, Wang L, Guo J, Zhao T, Tang H, Dong F, Wang C, Chen J, Tang M. Erythrocyte Membrane Fatty Acid Composition as a Potential Biomarker for Depression. Int J Neuropsychopharmacol. 2023;26:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 42. | Bernal-Vega S, García-Juárez M, Camacho-Morales A. Contribution of ceramides metabolism in psychiatric disorders. J Neurochem. 2023;164:708-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 43. | Bowers M, Liang T, Gonzalez-Bohorquez D, Zocher S, Jaeger BN, Kovacs WJ, Röhrl C, Cramb KML, Winterer J, Kruse M, Dimitrieva S, Overall RW, Wegleiter T, Najmabadi H, Semenkovich CF, Kempermann G, Földy C, Jessberger S. FASN-Dependent Lipid Metabolism Links Neurogenic Stem/Progenitor Cell Activity to Learning and Memory Deficits. Cell Stem Cell. 2020;27:98-109.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 44. | Ardah MT, Parween S, Varghese DS, Emerald BS, Ansari SA. Saturated fatty acid alters embryonic cortical neurogenesis through modulation of gene expression in neural stem cells. J Nutr Biochem. 2018;62:230-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Guo J, Niu YJ, Shin KT, Kwon JW, Kim NH, Cui XS. Fatty acid synthase knockout impairs early embryonic development via induction of endoplasmic reticulum stress in pigs. J Cell Physiol. 2018;233:4225-4234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Araúzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 47. | Steiner J, Berger M, Guest PC, Dobrowolny H, Westphal S, Schiltz K, Sarnyai Z. Assessment of Insulin Resistance Among Drug-Naive Patients With First-Episode Schizophrenia in the Context of Hormonal Stress Axis Activation. JAMA Psychiatry. 2017;74:968-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Pillinger T, McCutcheon RA, Howes OD. Variability of glucose, insulin, and lipid disturbances in first-episode psychosis: a meta-analysis. Psychol Med. 2023;53:3150-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Dziurkowska E, Wesolowski M. Cortisol as a Biomarker of Mental Disorder Severity. J Clin Med. 2021;10:5204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 50. | García-Eguren G, Sala-Vila A, Giró O, Vega-Beyhart A, Hanzu FA. Long-term hypercortisolism induces lipogenesis promoting palmitic acid accumulation and inflammation in visceral adipose tissue compared with HFD-induced obesity. Am J Physiol Endocrinol Metab. 2020;318:E995-E1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Perry BI, Khandaker GM, Marwaha S, Thompson A, Zammit S, Singh SP, Upthegrove R. Insulin resistance and obesity, and their association with depression in relatively young people: findings from a large UK birth cohort. Psychol Med. 2020;50:556-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Sun JW, Hernández-Díaz S, Haneuse S, Bourgeois FT, Vine SM, Olfson M, Bateman BT, Huybrechts KF. Association of Selective Serotonin Reuptake Inhibitors With the Risk of Type 2 Diabetes in Children and Adolescents. JAMA Psychiatry. 2021;78:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 511] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 54. | De Luca V, Viggiano E, Messina G, Viggiano A, Borlido C, Viggiano A, Monda M. Peripheral amino Acid levels in schizophrenia and antipsychotic treatment. Psychiatry Investig. 2008;5:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Xu DR, Gao X, Zhao LB, Liu SD, Tang G, Zhou CJ, Chen Y. Association between triglyceride and depression: A systematic review and meta-analysis. PLoS One. 2024;19:e0311625. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Sullivan CR, Mielnik CA, Funk A, O'Donovan SM, Bentea E, Pletnikov M, Ramsey AJ, Wen Z, Rowland LM, McCullumsmith RE. Measurement of lactate levels in postmortem brain, iPSCs, and animal models of schizophrenia. Sci Rep. 2019;9:5087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Bradley KA, Mao X, Case JA, Kang G, Shungu DC, Gabbay V. Increased ventricular cerebrospinal fluid lactate in depressed adolescents. Eur Psychiatry. 2016;32:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Sánchez-Alegría K, Bastián-Eugenio CE, Vaca L, Arias C. Palmitic acid induces insulin resistance by a mechanism associated with energy metabolism and calcium entry in neuronal cells. FASEB J. 2021;35:e21712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Halvorsen B, Rustan AC, Madsen L, Reseland J, Berge RK, Sletnes P, Christiansen EN. Effects of long-chain monounsaturated and n-3 fatty acids on fatty acid oxidation and lipid composition in rats. Ann Nutr Metab. 2001;45:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry. 2017;211:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 61. | Shi YY, Zheng R, Cai JJ, Qian SZ. The association between triglyceride glucose index and depression: data from NHANES 2005-2018. BMC Psychiatry. 2021;21:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 62. | Chathoth S, Ismail MH, Alghamdi HM, Zakaria HM, Hassan KA, Alshomimi S, Vatte C, Cyrus C, Alsaif HS, Mostafa A, Shaaban H, Al Ali A. Insulin resistance induced by de novo pathway-generated C16-ceramide is associated with type 2 diabetes in an obese population. Lipids Health Dis. 2022;21:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 63. | Murphy CE, Walker AK, Weickert CS. Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Transl Psychiatry. 2021;11:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 64. | Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 65. | Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neurosci Biobehav Rev. 2017;77:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Gasparini A, Callegari C, Lucca G, Bellini A, Caselli I, Ielmini M. Inflammatory Biomarker and Response to Antidepressant in Major Depressive Disorder: a Systematic Review and Meta-Analysis. Psychopharmacol Bull. 2022;52:36-52. [PubMed] |
| 67. | De Meyts P. The Insulin Receptor and Its Signal Transduction Network. 2016 Apr 27. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] |
| 68. | Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1017] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 69. | Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1299] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 70. | Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 71. | Konefal S, Elliot M, Crespi B. The adaptive significance of adult neurogenesis: an integrative approach. Front Neuroanat. 2013;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Catalan A, Salazar de Pablo G, Aymerich C, Damiani S, Sordi V, Radua J, Oliver D, McGuire P, Giuliano AJ, Stone WS, Fusar-Poli P. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021;78:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 73. | Maalouf FT, Brent D, Clark L, Tavitian L, McHugh RM, Sahakian BJ, Phillips ML. Neurocognitive impairment in adolescent major depressive disorder: state vs. trait illness markers. J Affect Disord. 2011;133:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Allen KM, Fung SJ, Weickert CS. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust N Z J Psychiatry. 2016;50:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 469] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 76. | Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci U S A. 1995;92:2785-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 237] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 549] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 78. | Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 79. | Falkai P, Malchow B, Wetzestein K, Nowastowski V, Bernstein HG, Steiner J, Schneider-Axmann T, Kraus T, Hasan A, Bogerts B, Schmitz C, Schmitt A. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A Stereological Postmortem Study. Schizophr Bull. 2016;42 Suppl 1:S4-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 80. | Gaus R, Popal M, Heinsen H, Schmitt A, Falkai P, Hof PR, Schmitz C, Vollhardt A. Reduced cortical neuron number and neuron density in schizophrenia with focus on area 24: a post-mortem case-control study. Eur Arch Psychiatry Clin Neurosci. 2023;273:1209-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Weissleder C, North HF, Bitar M, Fullerton JM, Sager R, Barry G, Piper M, Halliday GM, Webster MJ, Shannon Weickert C. Reduced adult neurogenesis is associated with increased macrophages in the subependymal zone in schizophrenia. Mol Psychiatry. 2021;26:6880-6895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 82. | Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1042] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 83. | Xie XH, Xu SX, Yao L, Chen MM, Zhang H, Wang C, Nagy C, Liu Z. Altered in vivo early neurogenesis traits in patients with depression: Evidence from neuron-derived extracellular vesicles and electroconvulsive therapy. Brain Stimul. 2024;17:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 84. | Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 442] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 85. | Chidambaram S, Velloso FJ, Rothbard DE, Deshpande K, Cajuste Y, Snyder KM, Fajardo E, Fiser A, Tapinos N, Levison SW, Wood TL. Subventricular zone adult mouse neural stem cells require insulin receptor for self-renewal. Stem Cell Reports. 2022;17:1411-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 86. | Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64:3927-3936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 87. | Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 711] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 88. | Bernstein HG, Dobrowolny H, Keilhoff G, Bogerts B, Steiner J. Reduced Density of DISC1 Expressing Astrocytes in the Dentate Gyrus but not in the Subventricular Zone in Schizophrenia. Neuropsychopharmacology. 2018;43:457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Tarasov VV, Svistunov AA, Chubarev VN, Sologova SS, Mukhortova P, Levushkin D, Somasundaram SG, Kirkland CE, Bachurin SO, Aliev G. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front Pharmacol. 2019;10:1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 448] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 91. | Cobb JA, O'Neill K, Milner J, Mahajan GJ, Lawrence TJ, May WL, Miguel-Hidalgo J, Rajkowska G, Stockmeier CA. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 92. | O'Leary LA, Mechawar N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia. 2021;69:2077-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 93. | Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 94. | Vostrikov VM, Uranova NA. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr Res. 2020;215:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Patro N, Patro I, Tandon PN. Oligodendrocyte: Structure, Function and Pathology. In: Patro I, Seth P, Patro N, Tandon PN. The Biology of Glial Cells: Recent Advances. Singapore: Springer, 2022. [DOI] [Full Text] |
| 96. | Moore S, Meschkat M, Ruhwedel T, Trevisiol A, Tzvetanova ID, Battefeld A, Kusch K, Kole MHP, Strenzke N, Möbius W, de Hoz L, Nave KA. A role of oligodendrocytes in information processing. Nat Commun. 2020;11:5497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 97. | Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 98. | Klauser P, Baker ST, Cropley VL, Bousman C, Fornito A, Cocchi L, Fullerton JM, Rasser P, Schall U, Henskens F, Michie PT, Loughland C, Catts SV, Mowry B, Weickert TW, Shannon Weickert C, Carr V, Lenroot R, Pantelis C, Zalesky A. White Matter Disruptions in Schizophrenia Are Spatially Widespread and Topologically Converge on Brain Network Hubs. Schizophr Bull. 2017;43:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 99. | Sacchet MD, Gotlib IH. Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Sci Rep. 2017;7:2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 100. | de la Monte SM, Grammas P. Insulin Resistance and Oligodendrocyte/Microvascular Endothelial Cell Dysfunction as Mediators of White Matter Degeneration in Alzheimer’s Disease. In: Alzheimer’s Disease [Internet]. Brisbane (AU): Codon Publications; 2019. [PubMed] [DOI] [Full Text] |
| 101. | Long KLP, Breton JM, Barraza MK, Perloff OS, Kaufer D. Hormonal Regulation of Oligodendrogenesis I: Effects across the Lifespan. Biomolecules. 2021;11:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Gonzalez-Bohorquez D, Gallego López IM, Jaeger BN, Pfammatter S, Bowers M, Semenkovich CF, Jessberger S. FASN-dependent de novo lipogenesis is required for brain development. Proc Natl Acad Sci U S A. 2022;119:e2112040119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 104. | Thompson CB, Bielska AA. Growth factors stimulate anabolic metabolism by directing nutrient uptake. J Biol Chem. 2019;294:17883-17888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 106. | Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl). 2018;235:2195-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 107. | Deng Z, Deng S, Zhang MR, Tang MM. Fibroblast Growth Factors in Depression. Front Pharmacol. 2019;10:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Khan MM, Parikh V. Prospects for Neurotrophic Factor-Based Early Intervention in Schizophrenia: Lessons Learned from the Effects of Antipsychotic Drugs on Cognition, Neurogenesis, and Neurotrophic Factors. CNS Neurol Disord Drug Targets. 2023;22:289-303. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 109. | Kuang J, Zhang L, Xu Y, Xue J, Liang S, Xiao J. Reduced Insulin-Like Growth Factor 1 Is Associated with Insulin Resistance in Obese Prepubertal Boys. Biomed Res Int. 2021;2021:6680316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Davarpanah M, Shokri-Mashhadi N, Ziaei R, Saneei P. A systematic review and meta-analysis of association between brain-derived neurotrophic factor and type 2 diabetes and glycemic profile. Sci Rep. 2021;11:13773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, Wiegand SJ, Wong V. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 112. | Tsuchida A, Nakagawa T, Itakura Y, Ichihara J, Ogawa W, Kasuga M, Taiji M, Noguchi H. The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia. 2001;44:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Hong H, Cui ZZ, Zhu L, Fu SP, Rossi M, Cui YH, Zhu BM. Central IGF1 improves glucose tolerance and insulin sensitivity in mice. Nutr Diabetes. 2017;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Zhang X, Xiao W, Chen K, Zhao Y, Ye F, Tang X, Du X. Serum Epidermal Growth Factor is Low in Schizophrenia and Not Affected by Antipsychotics Alone or Combined With Electroconvulsive Therapy. Front Psychiatry. 2020;11:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Galiano Rus S, Ortiz García de la Foz V, Arias-Loste MT, Iruzubieta P, Gómez-Revuelta M, Juncal-Ruiz M, Crespo J, Crespo-Facorro B, Vázquez-Bourgon J. Elevated risk of liver steatosis in first-episode psychosis patients: Results from a 3-year prospective study. Schizophr Res. 2022;246:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Vancampfort D, Wampers M, Mitchell AJ, Correll CU, De Herdt A, Probst M, De Hert M. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 117. | Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, Probst M, Scheewe TW, De Hert M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014;44:2017-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 118. | Chen CH, Shyue SK, Hsu CP, Lee TS. Atypical Antipsychotic Drug Olanzapine Deregulates Hepatic Lipid Metabolism and Aortic Inflammation and Aggravates Atherosclerosis. Cell Physiol Biochem. 2018;50:1216-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Li N, Yang P, Tang M, Liu Y, Guo W, Lang B, Wang J, Wu H, Tang H, Yu Y, Wu X, Zeng C, Cao T, Cai H. Reduced erythrocyte membrane polyunsaturated fatty acid levels indicate diminished treatment response in patients with multi- versus first-episode schizophrenia. Schizophrenia (Heidelb). 2022;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 120. | Mocking RJ, Verburg HF, Westerink AM, Assies J, Vaz FM, Koeter MW, Ruhé HG, Schene AH. Fatty acid metabolism and its longitudinal relationship with the hypothalamic-pituitary-adrenal axis in major depression: Associations with prospective antidepressant response. Psychoneuroendocrinology. 2015;59:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Burschinski A, Schneider-Thoma J, Chiocchia V, Schestag K, Wang D, Siafis S, Bighelli I, Wu H, Hansen WP, Priller J, Davis JM, Salanti G, Leucht S. Metabolic side effects in persons with schizophrenia during mid- to long-term treatment with antipsychotics: a network meta-analysis of randomized controlled trials. World Psychiatry. 2023;22:116-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 122. | Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. 2019;53:742-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 123. | Yolland CO, Hanratty D, Neill E, Rossell SL, Berk M, Dean OM, Castle DJ, Tan EJ, Phillipou A, Harris AW, Barreiros AR, Hansen A, Siskind D. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry. 2020;54:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 124. | Jeppesen R, Christensen RHB, Pedersen EMJ, Nordentoft M, Hjorthøj C, Köhler-Forsberg O, Benros ME. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders - A comprehensive systematic review and meta-analysis. Brain Behav Immun. 2020;90:364-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 125. | Dinakaran D, Sreeraj VS, Venkatasubramanian G. Role of Curcumin in the Management of Schizophrenia: A Narrative Review. Indian J Psychol Med. 2022;44:107-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 126. | Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, Mortiere C, Lohr J, Hu Q, Davis JM. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophr Res. 2013;143:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Marini S, De Berardis D, Vellante F, Santacroce R, Orsolini L, Valchera A, Girinelli G, Carano A, Fornaro M, Gambi F, Martinotti G, Di Giannantonio M. Celecoxib Adjunctive Treatment to Antipsychotics in Schizophrenia: A Review of Randomized Clinical Add-On Trials. Mediators Inflamm. 2016;2016:3476240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Goh KK, Chen CY, Chen CH, Lu ML. Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials. J Psychopharmacol. 2021;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Bredin SSD, Kaufman KL, Chow MI, Lang DJ, Wu N, Kim DD, Warburton DER. Effects of Aerobic, Resistance, and Combined Exercise Training on Psychiatric Symptom Severity and Related Health Measures in Adults Living With Schizophrenia: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:753117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 130. | Berk M, Agustini B, Woods RL, Nelson MR, Shah RC, Reid CM, Storey E, Fitzgerald SM, Lockery JE, Wolfe R, Mohebbi M, Dodd S, Murray AM, Stocks N, Fitzgerald PB, Mazza C, McNeil JJ. Effects of aspirin on the long-term management of depression in older people: a double-blind randomised placebo-controlled trial. Mol Psychiatry. 2021;26:5161-5170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 131. | Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77:e457-e466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 132. | Hellmann-Regen J, Clemens V, Grözinger M, Kornhuber J, Reif A, Prvulovic D, Goya-Maldonado R, Wiltfang J, Gruber O, Schüle C, Padberg F, Ising M, Uhr M, Friede T, Huber C, Manook A, Baghai TC, Rupprecht R, Heuser I. Effect of Minocycline on Depressive Symptoms in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2230367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 133. | Brown ES, Park J, Marx CE, Hynan LS, Gardner C, Davila D, Nakamura A, Sunderajan P, Lo A, Holmes T. A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology. 2014;39:2867-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 134. | Wium-Andersen MK, Jørgensen TSH, Halvorsen AH, Hartsteen BH, Jørgensen MB, Osler M. Association of Hormone Therapy With Depression During Menopause in a Cohort of Danish Women. JAMA Netw Open. 2022;5:e2239491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 135. | Khorsand I, Kashef R, Ghazanfarpour M, Mansouri E, Dashti S, Khadivzadeh T. The Beneficial and Adverse Effects of Raloxifene in Menopausal Women: A Mini Review. J Menopausal Med. 2018;24:183-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Fusar-Poli L, Vozza L, Gabbiadini A, Vanella A, Concas I, Tinacci S, Petralia A, Signorelli MS, Aguglia E. Curcumin for depression: a meta-analysis. Crit Rev Food Sci Nutr. 2020;60:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 137. | Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 138. | Wang Z, Wu Q, Wang Q. Effect of celecoxib on improving depression: A systematic review and meta-analysis. World J Clin Cases. 2022;10:7872-7882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 139. | Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, Fan B, Lu C, McIntyre RS. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry. 2019;9:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 140. | Noetel M, Sanders T, Gallardo-Gómez D, Taylor P, Del Pozo Cruz B, van den Hoek D, Smith JJ, Mahoney J, Spathis J, Moresi M, Pagano R, Pagano L, Vasconcellos R, Arnott H, Varley B, Parker P, Biddle S, Lonsdale C. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384:e075847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 113] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 141. | O'Sullivan D, Gordon BR, Lyons M, Meyer JD, Herring MP. Effects of resistance exercise training on depressive symptoms among young adults: A randomized controlled trial. Psychiatry Res. 2023;326:115322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 142. | Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 143. | Pereira S, Shah A, George Fantus I, Joseph JW, Giacca A. Effect of N-acetyl-l-cysteine on insulin resistance caused by prolonged free fatty acid elevation. J Endocrinol. 2015;225:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Coker CR, White M, Singal A, Bingaman SS, Paul A, Arnold AC, Silberman Y. Minocycline Reduces Hypothalamic Microglia Activation and Improves Metabolic Dysfunction in High Fat Diet-Induced Obese Mice. Front Physiol. 2022;13:933706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 145. | Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 α-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y, Wu C, Allred K, Allred C, Sun Y, Guo S. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes. 2019;68:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 147. | Lasco A, Gaudio A, Morabito N, Previti M, Mileto A, Frisina N, Cucinotta D. Effects of a long-term treatment with raloxifene on insulin sensitivity in postmenopausal women. Diabetologia. 2004;47:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Klöting N, Kern M, Moruzzi M, Stumvoll M, Blüher M. Tamoxifen treatment causes early hepatic insulin resistance. Acta Diabetol. 2020;57:495-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Tian J, Feng B, Tian Z. The Effect of Curcumin on Lipid Profile and Glycemic Status of Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2022;2022:8278744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 150. | Qian X, Wang H, Yang G, Gao Z, Luo Y, Dong A, Zhang F, Xu M, Liu S, Yang X, Chen Y, Li G. Pioglitazone Improved Insulin Sensitivity and First Phase Insulin Secretion Among Obese and Lean People with Diabetes: A Multicenter Clamp Study. Diabetes Ther. 2018;9:815-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 151. | Tian F, Zhang YJ, Li Y, Xie Y. Celecoxib ameliorates non-alcoholic steatohepatitis in type 2 diabetic rats via suppression of the non-canonical Wnt signaling pathway expression. PLoS One. 2014;9:e83819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 152. | Baynes HW, Mideksa S, Ambachew S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte. 2018;7:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 153. | Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 577] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 154. | Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev Med. 2016;93:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 155. | Niemann MJ, Tucker LA, Bailey BW, Davidson LE. Strength Training and Insulin Resistance: The Mediating Role of Body Composition. J Diabetes Res. 2020;2020:7694825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 156. | Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res Ther. 2020;11:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Vergil Andrews JF, Selvaraj DB, Kumar A, Roshan SA, Anusuyadevi M, Kandasamy M. A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice. Brain Sci. 2023;13:1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 158. | Zhang Z, Dawson PA, Piper M, Simmons DG. Postnatal N-acetylcysteine administration rescues impaired social behaviors and neurogenesis in Slc13a4 haploinsufficient mice. EBioMedicine. 2019;43:435-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 159. | Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS. Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behav Brain Res. 2013;242:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 160. | Mayo W, Lemaire V, Malaterre J, Rodriguez JJ, Cayre M, Stewart MG, Kharouby M, Rougon G, Le Moal M, Piazza PV, Abrous DN. Pregnenolone sulfate enhances neurogenesis and PSA-NCAM in young and aged hippocampus. Neurobiol Aging. 2005;26:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 161. | Huang Y, Sun W, Gao F, Ma H, Yuan T, Liu Z, Liu H, Hu J, Bai J, Zhang X, Wang R. Brain-Derived Estrogen Regulates Neurogenesis, Learning and Memory with Aging in Female Rats. Biology (Basel). 2023;12:760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 162. | Khan MM, Wakade C, de Sevilla L, Brann DW. Selective estrogen receptor modulators (SERMs) enhance neurogenesis and spine density following focal cerebral ischemia. J Steroid Biochem Mol Biol. 2015;146:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 163. | Smith BM, Saulsbery AI, Sarchet P, Devasthali N, Einstein D, Kirby ED. Oral and Injected Tamoxifen Alter Adult Hippocampal Neurogenesis in Female and Male Mice. eNeuro. 2022;9:ENEURO.0422-ENEU21.2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 164. | Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immun. 2018;69:499-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 165. | Han Y, Wang J, Zhao Q, Xie X, Song R, Xiao Y, Kang X, Zhang L, Zhang Y, Peng C, You Z. Pioglitazone alleviates maternal sleep deprivation-induced cognitive deficits in male rat offspring by enhancing microglia-mediated neurogenesis. Brain Behav Immun. 2020;87:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 166. | Wang R, Tian S, Yang X, Liu J, Wang Y, Sun K. Celecoxib-induced inhibition of neurogenesis in fetal frontal cortex is attenuated by curcumin via Wnt/β-catenin pathway. Life Sci. 2017;185:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 167. | Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 168. | Wu X, Zhang Y, Wang J, Qin L, Li Y, He Q, Zhang T, Wang Y, Song L, Ji L, Long B, Wang Q. Role of SIRT1-mediated synaptic plasticity and neurogenesis: Sex-differences in antidepressant-like efficacy of catalpol. Phytomedicine. 2024;135:156120. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 169. | Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, Kainulainen H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594:1855-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 170. | Novaes Gomes FG, Fernandes J, Vannucci Campos D, Cassilhas RC, Viana GM, D'Almeida V, de Moraes Rêgo MK, Buainain PI, Cavalheiro EA, Arida RM. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology. 2014;50:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 171. | Gobshtis N, Tfilin M, Fraifeld VE, Turgeman G. Transplantation of mesenchymal stem cells causes long-term alleviation of schizophrenia-like behaviour coupled with increased neurogenesis. Mol Psychiatry. 2021;26:4448-4463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 172. | Skvarc DR, Dean OM, Byrne LK, Gray L, Lane S, Lewis M, Fernandes BS, Berk M, Marriott A. The effect of N-acetylcysteine (NAC) on human cognition - A systematic review. Neurosci Biobehav Rev. 2017;78:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 173. | Poggini S, Banqueri M, Ciano Albanese N, Golia MT, Ibáñez FG, Limatola C, Furhmann M, Lalowski M, Tremblay ME, Maggi L, Kaminska B, Branchi I. Minocycline treatment improves cognitive and functional plasticity in a preclinical mouse model of major depressive disorder. Behav Brain Res. 2023;441:114295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 174. | Wong P, Sze Y, Chang CC, Lee J, Zhang X. Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3β pathway. Transl Psychiatry. 2015;5:e528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 175. | Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol Rev. 2015;95:785-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 176. | Weickert TW, Weinberg D, Lenroot R, Catts SV, Wells R, Vercammen A, O'Donnell M, Galletly C, Liu D, Balzan R, Short B, Pellen D, Curtis J, Carr VJ, Kulkarni J, Schofield PR, Weickert CS. Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia. Mol Psychiatry. 2015;20:685-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 177. | Newhouse P, Albert K, Astur R, Johnson J, Naylor M, Dumas J. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacology. 2013;38:2632-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 178. | Seok H, Lee M, Shin E, Yun MR, Lee YH, Moon JH, Kim E, Lee PH, Lee BW, Kang ES, Lee HC, Cha BS. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep. 2019;9:4414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 179. | Small GW, Siddarth P, Silverman DH, Ercoli LM, Miller KJ, Lavretsky H, Bookheimer SY, Huang SC, Barrio JR, Phelps ME. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: randomized controlled study. Am J Geriatr Psychiatry. 2008;16:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Cutuli D, Pagani M, Caporali P, Galbusera A, Laricchiuta D, Foti F, Neri C, Spalletta G, Caltagirone C, Petrosini L, Gozzi A. Effects of Omega-3 Fatty Acid Supplementation on Cognitive Functions and Neural Substrates: A Voxel-Based Morphometry Study in Aged Mice. Front Aging Neurosci. 2016;8:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 181. | Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695-9707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 182. | Ren FF, Hillman CH, Wang WG, Li RH, Zhou WS, Liang WM, Yang Y, Chen FT, Chang YK. Effects of aerobic exercise on cognitive function in adults with major depressive disorder: A systematic review and meta-analysis. Int J Clin Health Psychol. 2024;24:100447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 183. | Dauwan M, Begemann MJ, Heringa SM, Sommer IE. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull. 2016;42:588-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 184. | Skok M, Deryabina O, Lykhmus O, Kalashnyk O, Uspenska K, Shuvalova N, Pokholenko I, Lushnikova I, Smozhanyk K, Skibo G, Kordyum V. Mesenchymal stem cell application for treatment of neuroinflammation-induced cognitive impairment in mice. Regen Med. 2022;17:533-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 185. | Fang X, Chen Y, Wang Y, Ren J, Zhang C. Depressive symptoms in schizophrenia patients: A possible relationship between SIRT1 and BDNF. Prog Neuropsychopharmacol Biol Psychiatry. 2019;95:109673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 186. | Luo XJ, Zhang C. Down-Regulation of SIRT1 Gene Expression in Major Depressive Disorder. Am J Psychiatry. 2016;173:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 187. | Ahn J, Kim M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2023;106:104855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 188. | Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, Elliott R, Nuechterlein KH, Yung AR. Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Bull. 2017;43:546-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 189. | Rabøl R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011;108:13705-13709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 190. | Huang X, Lu QL, Zhu XM, Zeng YB, Liu Y, Hu HY. Histogenous Hypoxia and Acid Retention in Schizophrenia: Changes in Venous Blood Gas Analysis and SOD in Acute and Stable Schizophrenia Patients. Front Psychiatry. 2021;12:792560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | Khuu MA, Pagan CM, Nallamothu T, Hevner RF, Hodge RD, Ramirez JM, Garcia AJ 3rd. Intermittent Hypoxia Disrupts Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. J Neurosci. 2019;39:1320-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 192. | Hazlehurst JM, Lim TR, Charlton C, Miller JJ, Gathercole LL, Cornfield T, Nikolaou N, Harris SE, Moolla A, Othonos N, Heather LC, Marjot T, Tyler DJ, Carr C, Hodson L, McKeating J, Tomlinson JW. Acute intermittent hypoxia drives hepatic de novo lipogenesis in humans and rodents. Metabol Open. 2022;14:100177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 193. | Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, Ganti L, Kaye AD, Mosel LI, Carroll CB, Viswanath O, Urits I. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia). 2022;14:37498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 194. | Zeinhom A, Fadallah SA, Mahmoud M. Human mesenchymal stem/stromal cell based-therapy in diabetes mellitus: experimental and clinical perspectives. Stem Cell Res Ther. 2024;15:384. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 195. | Ma Y, Wang L, Yang S, Liu D, Zeng Y, Lin L, Qiu L, Lu J, Chang J, Li Z. The tissue origin of human mesenchymal stem cells dictates their therapeutic efficacy on glucose and lipid metabolic disorders in type II diabetic mice. Stem Cell Res Ther. 2021;12:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 196. | Kotikalapudi N, Sampath SJP, Sinha SN, Bhonde R, Mungamuri SK, Venkatesan V. Human placental mesenchymal stromal cell therapy restores the cytokine efflux and insulin signaling in the skeletal muscle of obesity-induced type 2 diabetes rat model. Hum Cell. 2022;35:557-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 197. | Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 198. | Qi Y, Ma J, Li S, Liu W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. 2019;10:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 199. | Sanap A, Bhonde R, Joshi K. Conditioned medium of adipose derived Mesenchymal Stem Cells reverse insulin resistance through downregulation of stress induced serine kinases. Eur J Pharmacol. 2020;881:173215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 200. | Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H, Traktuev DO, March KL, Evans-Molina C. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32:1831-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 201. | Li B, Cheng Y, Yu S, Zang L, Yin Y, Liu J, Zhang L, Mu Y. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Nonalcoholic Fatty Liver Disease in Obese Type 2 Diabetic Mice. Stem Cells Int. 2019;2019:8628027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 202. | Oh SH, Kim HN, Park HJ, Shin JY, Lee PH. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer's Disease Model. Cell Transplant. 2015;24:1097-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 203. | Doshmanziari M, Shirian S, Kouchakian MR, Moniri SF, Jangnoo S, Mohammadi N, Zafari F. Mesenchymal stem cells act as stimulators of neurogenesis and synaptic function in a rat model of Alzheimer's disease. Heliyon. 2021;7:e07996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 204. | McGinley LM, Kashlan ON, Bruno ES, Chen KS, Hayes JM, Kashlan SR, Raykin J, Johe K, Murphy GG, Feldman EL. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer's disease. Sci Rep. 2018;8:14776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 205. | Zhang X, Hou X, Te L, Zhongsheng Z, Jiang J, Wu X. Mesenchymal stem cells and exosomes improve cognitive function in the aging brain by promoting neurogenesis. Front Aging Neurosci. 2022;14:1010562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 206. | Fallahi S, Zangbar HS, Farajdokht F, Rahbarghazi R, Ghiasi F, Mohaddes G. Mesenchymal stem cell-derived exosomes improve neurogenesis and cognitive function of mice with methamphetamine addiction: A novel treatment approach. CNS Neurosci Ther. 2024;30:e14719. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 207. | You MJ, Bang M, Park HS, Yang B, Jang KB, Yoo J, Hwang DY, Kim M, Kim B, Lee SH, Kwon MS. Human umbilical cord-derived mesenchymal stem cells alleviate schizophrenia-relevant behaviors in amphetamine-sensitized mice by inhibiting neuroinflammation. Transl Psychiatry. 2020;10:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 208. | Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15:1164-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |