Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.105371
Revised: March 25, 2025
Accepted: June 20, 2025
Published online: July 26, 2025
Processing time: 185 Days and 18.4 Hours
Inflammatory bowel disease (IBD) is a persistent gastrointestinal ailment driven by a range of immunological and pathophysiological factors, and often exposes patients to persistent pain and a greater risk of tumor development. In clinical settings, sulfasalazine is among the most common treatments used to manage IBD, but such treatment can result in a range of side effects in addition to leading to relatively poor efficacy. In certain refractory cases, patients must undergo surgical resection of affected tissues, underscoring the need to devise safer and more efficacious forms of alternative treatment. Mesenchymal stem cells (MSCs) have recently been shown to exhibit been shown to exhibit robust immunomodulatory activity and potential for differentiation such that they may be an effective tool for treating IBD. Acupuncture has also shown promise as an efficacious treatment option for IBD, performing better than drug-based treatments in certain clinical trials. Acupuncture is capable of enhancing endogenous MSC proliferation and homing, enabling these cells to more effectively migrate toward target lesion sites and to promote tissue repair. In light of these findings, this review was formulated to survey the potential therapeutic advantages of combining MSCs and acupuncture when attempting to treat IBD.
Core Tip: This paper analyzes and summarizes previous studies and suggests that acupuncture combined with stem cell transplantation may be more effective than monotherapy in the treatment of inflammatory bowel disease and ensure safety. A systematic description of the possible mechanisms of action is provided to support the feasibility of this method.
- Citation: Ma WG, Si YX, Zhang YL, Gao WF, Dong YG, Li YQ, Xu ZF, Xi Q, Li ZZ. Combining acupuncture and mesenchymal stem cell therapy offers promise as a treatment for inflammatory bowel disease. World J Stem Cells 2025; 17(7): 105371
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/105371.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.105371
Inflammatory bowel disease (IBD) is an inflammatory form of gastrointestinal disease that can be further categorized into Crohn’s disease (CD) and ulcerative colitis (UC) based on the histological findings in affected patients[1]. IBD patho
Acupuncture offers significant utility as an approach to treating a growing number of diseases and disorders. Contemporary clinical acupuncture modalities primarily comprise manual acupuncture (MA) and electroacupuncture (EA), each with distinct operational frameworks and therapeutic advantages. MA involves the selection of disease-specific acupoints, followed by rapid needle insertion using techniques such as tube-assisted or rotational insertion to minimize discomfort. Skilled acupuncturists then manipulate needles through lifting-thrusting or twirling methods to elicit the Deqi sensation - characterized by localized soreness, numbness, or distension - adjusted to patient tolerance. This modality relies on periodic interventions to restore physiological balance, making it particularly suitable for chronic conditions requiring individualized modulation of immune and visceral functions. In contrast, EA enhances traditional needle stimulation through programmable electrical pulses, enabling precise neuromodulation. After achieving target needle depth, electrodes are attached to deliver frequency-specific stimulation, with intensity calibrated to induce subtle muscle contractions without pain. EA’s standardized parameters and quantifiable output not only improve reproducibility in research settings but also amplify therapeutic efficacy in scenarios demanding targeted anti-inflammatory or neuroregulatory effects, such as chronic pain management and synergistic applications with stem cell therapies. Consequently, while MA excels in holistic functional regulation, EA’s technological precision establishes it as the preferred modality for mechanistically driven translational studies.
In several preclinical and clinical analyses, acupuncture has been found to exert analgesic, anti-inflammatory, and immunomodulatory effects[14-18], with a steadily growing number of studies exploring this therapeutic modality. Acupuncture is minimally invasive and associated with a lower potential for side effects as compared to conventional pharmacological interventions, in addition to being readily administered and associated with significant therapeutic efficacy in clinical trials[19-22]. In these trials, acupuncture has outperformed oral 5-aminosalicylic acid as a means of treating IBD[23], indicating that it is a feasible alternative therapy for the management of IBD. Acupuncture can also have effects on the function of both endogenous and exogenous MSC populations[24]. In one study, the combination of EA and tropomyosin receptor kinase B-transduced MSCs was associated with significantly better functional recovery following ischemic stroke[25], while in a separate study, combination treatment strategies led to better improvement in axonal regeneration, spinal cord conduction, and overall functional recovery following spinal cord injury[26], highlighting the promise of combining acupuncture and MSC therapy as an approach to managing these conditions. This review offers an overview of the therapeutic utility of combining acupuncture and MSC therapy as a means of managing IBD, with a particular focus on the potential synergistic interactions between these two therapeutic modalities, thereby providing a foundation for additional basic research and translational work in the clinic.
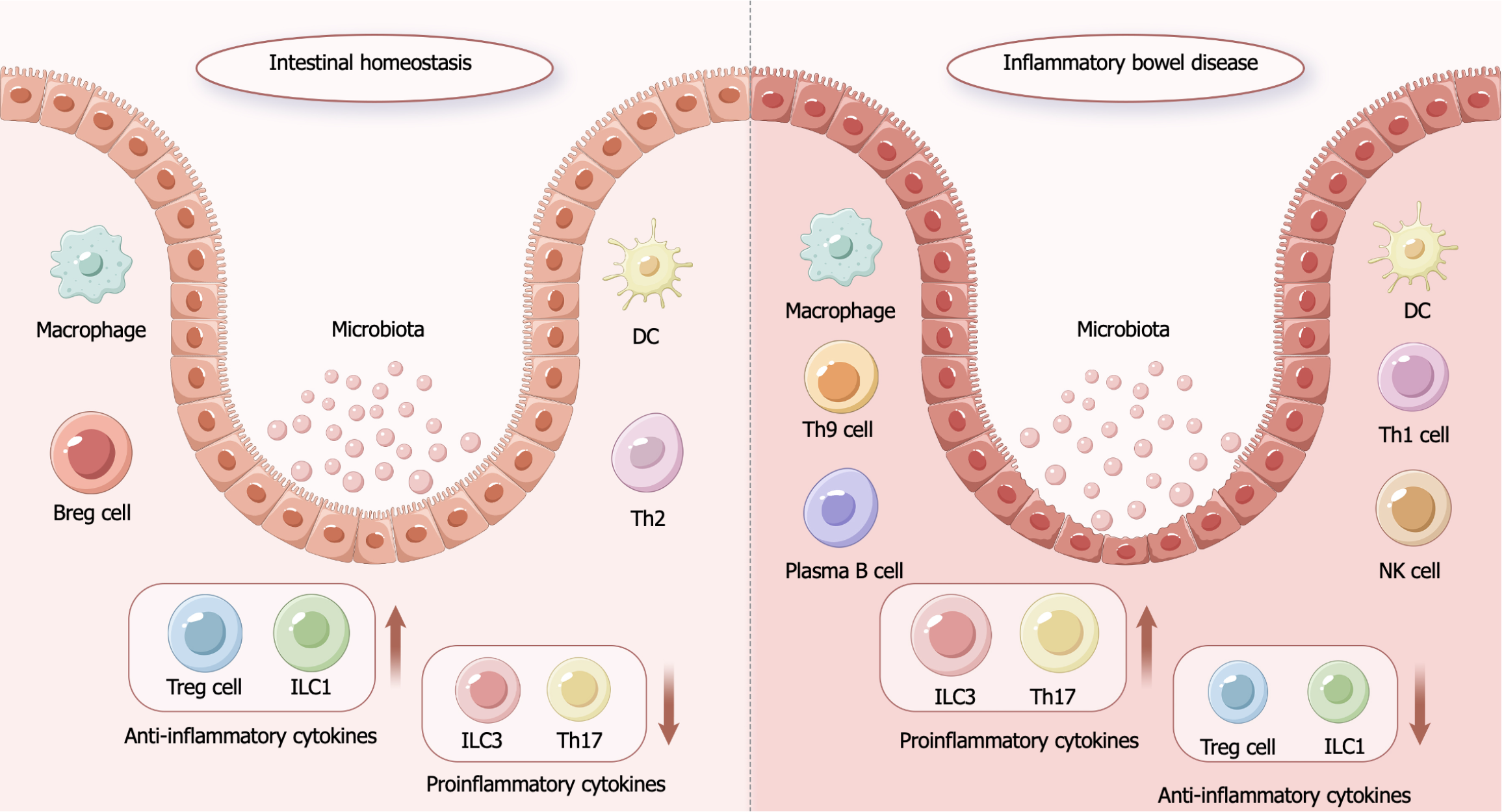
IBD is an autoimmune disease in which patients suffer from chronic intestinal inflammation and tissue damage due to the inappropriate activation of deleterious immune responses and associated immune cell infiltration[2] (Figure 1). While the precise pathogenesis of this disease is not fully understood, genetic, environmental, microecological, and immune factors are all believed to contribute to its etiology. The colonic lesions of patients with IBD present with elevated pro-inflammatory cytokine and chemokine levels[27], supporting a possible link between these factors and IBD progression. While early studies primarily focused on the roles that T helper type 1 cell (Th1) and Th2 populations play in IBD pathogenesis[28], Th17 cells have more recently been demonstrated to be critical for IBD onset[29], and interleukin (IL)-1 family cytokines are also central regulators of this process. IL-1β expression associated with macrophages and other innate immune cell populations is increased in the colonic mucosa of IBD patients[30]. Consistently, IL-18 levels are markedly elevated in the intestinal mucosa of patients with CD, leading to enhanced Th1 responses together with the disruption of normal T cell-derived cytokine secretion[31], altering the levels of factors including IL-6[32], IL-10[33], and IL-33[34], all of which are involved in IBD-related inflammatory responses. Chemokines are key mediators that guide the chemotactic migration of immune cells, with chemokines including CXC motif chemokine ligand 3 (CXCL3), CXCL6, and C-C motif chemokine ligand 28 being present at high levels in the colon in patients with IBD[35-37]. In addition to their modulatory effects on inflammatory and reparative responses, these chemokines are also vital for the maintenance of overall immunological homeostasis[38].
Oxidative stress is also a key determinant of IBD pathogenesis[39]. The IBD-associated infiltration of the intestinal wall by inflammatory immune cells leads to the production of abundant reactive oxygen species (ROS) by neutrophils and macrophages[40]. Such ROS exposure adversely affects the normal homeostatic balance between oxidative and antioxidant responses in the intestines, culminating in the disruption of the tight junctions that exist between cells of the intestinal epithelium, thus impairing overall epithelial barrier function. Consistent with such a model, both IBD patients and animal models of colitis present with higher ROS levels in their colonic mucosa relative to healthy controls[41,42]. In early studies, IBD patients were also found to exhibit lower levels of the key antioxidant enzyme superoxide dismutase (SOD), consistent with greater oxidative stress[43]. IBD patients also reportedly present with lower serum total antioxidant capacity as compared to healthy controls[44], reaffirming the link between IBD development and the loss of systemic redox homeostasis. These reports thus underscore the central role that oxidative stress plays in the onset and/or progression of IBD.
MSCs are a widely distributed stem cell type that can be isolated from tissues including adipose tissue, bone marrow, endometrial polyps, menstrual blood, and even umbilical cord tissues. In addition to their accessibility, MSCs exhibit advantages including an excellent capacity for self-renewal and strong differentiation potential[45-48]. MSCs are capable of differentiating into a range of cell populations including both adipose and bone tissue cells[49]. MSCs also present with robust anti-inflammatory and immunomodulatory activities such that they are capable of suppressing the symptoms of various forms of inflammatory diseases, including IBD[50]. These beneficial effects of MSCs are mediated through their ability to modulate oxidative stress-related signaling, to suppress inflammation, and to promote tissue repair in a manner that can ultimately help curtail disease-related inflammation and oxidative stress. MSCs are also capable of protecting the intestinal nervous system and promoting intestinal barrier repair[51]. MSCs are thus ideal tools for the management of inflammatory diseases including IBD, as they exhibit a multifaceted array of therapeutic effects.
The nuclear factor-kappa B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathways interact with one another to coordinate key transcriptional responses to inflammation and oxidative stress[52,53]. These factors are vital mediators of antioxidant processes (Table 1). The presence of H2O2 at micromolar concentrations, for instance, can activate NF-κB, while the antioxidant N-acetylcysteine can block this activation, emphasizing the close relationship between the activation of NF-κB signaling and oxidative stress[43]. Nrf2 is also closely related to oxidative stress, promoting the upregulation of heme oxygenase-1 (HO-1) and other factors that can respond to the loss of normal redox homeostasis[54]. Human umbilical cord MSC-derived exosome treatment was shown to protect against inflammation and oxidative stress induced by lipopolysaccharide and H2O2 treatment through the inhibition of Nrf2/NF-κB/nod-like receptor protein 3 (NLRP3) pathway activity within microglia[55]. These exosomes were found to upregulate Nrf2 while suppressing the phosphorylation of NF-κB p65 and the activation of the NLRP3 inflammasome in response to lipopolysaccharide. In a separate study, MSC transplantation in mice was found to help improve spinal cord injury outcomes, with the NF-κB pathway being related to the reparative mechanisms engaged following MSC transplantation[56]. In another study, knocking down Nrf2 was shown to weaken the antioxidant capacity of exosomes derived from MSCs in vitro and in vivo in mouse models of skin injury, supporting an important role for Nrf2 in this antioxidant pathway[57]. These findings indicate that MSCs are able to help alleviate oxidative stress in the context of disease through the regulation of the Nrf2 and NF-κB pathways.
| Ref. | Model | Cell type | Method | Mechanism indicators | Pathway |
| Che et al[55] | LPS-treated mice | hUC-MSC | Tail vein injection | TNF-α↓; IL-6↓; ROS↓; CD86↓; CD206↑; NLRP3↓; ASC↓; caspase-1↓ | Nrf2/NF-κB/NLRP3 |
| Cao et al[56] | SCI mice | hUC-MSC | Injected along the spinal cord | TNF-α↓; PTBP1↓ | TNF-α/NF-κB |
| Stavely et al[58] | IBD mice | BM-MSC | Enema | IL-6↓; TNF↓; IL-1α↓; IL-1b↓; IFNG↓; CXCL2↓; TBX21↓; ITGAM↓; NOS2↓; ARG1↓; GATA3↓; MRC1↓; HMOX1↓; FOXP3↑; CD45↓ | - |
| Xian et al[146] | SE mice | MSC-Exo | Intraventricular injection (in vivo), mix culture (in vitro) | GFAP↓; C3↓; CD81↓; Ki67↓; TNF-α↓; IL-1β↓ | Nrf2/NF-κB |
| Xiao et al[147] | ALI mice | BM-MSC | Tail vein injection | Ikbkb↓ | NF-κB/hedgehog |
| Zhang et al[148] | IBD mice and CIA mice | hUC-MSC | Tail vein injection | Tregs↑; Th17↓ | ETS2/AURKA/NF-κB/Fas/MCP-1 |
| Li et al[149] | ALI mice | BM-MSC | In vitro MSC isolation | TNF-α↓; IL-6↓; IL-10↓; HO-1↑; GPX-1↑ | Nrf2/ARE and NF-κB |
| Song et al[150] | ADR induced nephropathy mice | BM-MSC | Tail vein injection | MDA↓; IL-10↓; IFN-γ↓; TNF-α↓; IL-12↓; COX-2↓ | NF-κB |
| Han et al[151] | SAH rats | BM-MSC | Tail vein injection | TGFβ↑; iNOS↓; CD16↓; CD11b↓; IL-1β↓; p-AMPK↑ | AMPK/NF-κB |
| Wang et al[152] | SCI rats | MSC-Exo | Intrathecal injection | TNF-α↓; IL-1β↓; IL-6; Bcl2↓; Bax↓; caspase-3↓ | miR-146b/TLR4/NF-κB p65 |
The antioxidant benefits of MSCs were also recently shown to contribute to the neuroprotective benefits of these cells[58], as demonstrated by their ability to protect against oxidative stress and neuronal damage in mouse intestinal organotypic cultures. These antioxidant effects were also validated in a mouse model of IBD, underscoring the direct ability of MSCs to shield intestinal neurons from the damaging effects of oxidative stress. While the authors of that study did not clarify the mechanisms underlying these therapeutic benefits, their findings nonetheless attest to the ability of MSCs to potentially counteract the oxidative stress characteristic of IBD development.
Cytokines secreted by Th1, Th2, and Th17 cells are thought to be key mediators of IBD pathogenesis[28,29], including tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-2, IL-17, IL-10, and transforming growth factor-beta. The presence of elevated intestinal levels of these factors can contribute to inflammatory and autoimmune response induction[59,60], thereby compromising intestinal integrity. Through their robust immunoregulatory effects, MSCs can migrate to sites of intestinal damage and restore local homeostasis, leading to the repair of IBD-related pathological changes. Several studies have demonstrated the ability of MSCs to employ a range of mechanisms to exert their anti-inflammatory effects (Table 1). For example, MSCs can inhibit effector T cell activity while inducing more robust regulatory T cell (Treg) responses[61,62], interacting with dendritic cells (DCs)[63], and interacting with natural killer (NK) cells[64], thereby shaping immune response characteristics.
MSCs can suppress CD3, TNF-α, and IL-2 activity by secreting prostaglandin E2 (PGE2) and thereby suppressing inflammation and related immune activity, contributing to the relief of colitis[65]. MSCs derived from adipose tissue have been shown to suppress the activation of Th1 cells, while MSC-derived IL-10 was found to induce Tregs involved in the therapeutic benefits of such treatment in the context of IBD[50]. MSCs are also capable of limiting the proliferation and Th17 differentiation of CD4 T cells, resulting in reduced IL-17, IL-22, IFN-γ, and TNF-α production. MSCs can inhibit the activation of Th1 cells while favoring higher levels of IL-4 release and inducing the function of Th2 cells[66], consistent with a model wherein these cells may achieve a net anti-inflammatory effect by respectively suppressing and enhancing pro-inflammatory and anti-inflammatory activities.
MSCs are capable of interacting with DCs[63]. For instance, one report found that MSCs were able to induce DC differentiation into regulatory DCs as a means of protecting against pathological outcomes in a dextran sodium sulfate (DSS)-induced mouse model of colitis, leading to improved colon length, body weight, and survival[60]. MSC-derived IL-10 and transforming growth factor-beta may act on DCs, thereby suppressing their maturation and indirectly altering T cell responses, resulting in a shift in the DC activity profile towards one favoring anti-inflammatory effects[67].
Interactions between MSCs and NK cells, which are key cytotoxic effector cells often linked to antitumor immunity, are also important immunomodulatory processes. NK cells achieve their cytotoxic effects by producing inflammatory factors including TNF-α and IFN-γ, playing a role in IBD pathogenesis[64]. MSCs are able to down-regulate the release of these pro-inflammatory factors from NK cells, thereby modulating the extent of the immune response. In addition to this, MSCs can also reduce the proliferation of NK cells in a manner that does not require contact between the cells; this process is inhibited by down-regulation of IFN-γ, which is affected by the release of various soluble factors, such as indoleamine 2,3-dioxygenase (IDO) and PGE2[64,68,69]. It is noteworthy that these factors involved in the interaction between MSC and NK cells are not only involved in modulation of anti-inflammatory effects mediated by MSC and NK cell crosstalk but are also able to regulate macrophages and increase the proportion of Tregs, thus ameliorating inflammation caused by factors such as PGE2, IDO, and nitric oxide[70,71]. It has been found that adipose-derived MSCs could modulate the M1 macrophage population by promoting the secretion of PGE2, thereby reducing inflammation in mice with colitis[72]. Furthermore, a further study on MSCs in mice showed that secretion of PGE2 increased the number of Tregs, leading to the alleviation of DSS-induced colitis[73], while another investigation demonstrated that human umbilical cord-derived MSC-mediated regulation of PGE2 and IDO levels increased Treg differentiation and enhanced the therapeutic effects of human umbilical cord blood MSC on DSS-induced colitis[74].
The ability of MSCs to home to injured tissues in response to chemokines including CXCL12, CXCL4, and CXCL7 is closely related to their therapeutic benefits[75,76], with MSCs ultimately initiating their reparative programs after undergoing appropriate migration to sites of tissue damage.
MSCs can promote intestinal epithelial cell (IEC) proliferation while suppressing the apoptotic death of these cells, thereby helping mice recover normal intestinal structural and functional characteristics[77]. In one report focused on radiation-induced intestinal injury in mice that underwent human umbilical cord neural stem cell transplantation, these MSCs were able to engage in adipogenic and osteogenic differentiation, restore intestinal integrity, and promote functional recovery. In another study, bone marrow MSC treatment was able to suppress apoptotic cell death in a mouse model of necrotizing enterocolitis, significantly improving intestinal healing[78]. Studies have shown that conditioned medium derived from MSCs can promote IEC proliferation in the context of DSS- or trinitrobenzene sulfonic acid-induced colitis through the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling[79]. Another report yielded similar results[9], with both in vitro and in vivo data indicating that DSS-induced colitis model animals experienced better colonic integrity and function following human embryo-derived MSC, with these effects being mediated by the insulin-like growth factor 1 (IGF-1)/PI3K/AKT pathway. The ability of MSCs to promote the proliferation of IECs is thus an important mechanism involved in the restoration of intestinal function and morphology. The miR-181a-containing exosomes derived from MSCs can also reportedly promote zonula occludens-1 (ZO-1) and claudin-1 upregulation, ultimately helping to restore the function and morphology of the intestinal barrier[80]. SOD3-transduced MSCs have recently been shown to target TNF-α as a means of restoring reductions in tight junction-associated protein levels in the TNF-α-induced intestinal epithelium, thereby facilitating intestinal repair. ZO-1 is important as a mediator of intestinal repair[81,82], supporting the maintenance of tight junctions between IECs. Claudin-1 is similarly important for the preservation and restoration of the intestinal barrier, inducing the proliferation and differentiation of the colonic epithelium[83,84], thereby preserving intestinal barrier integrity. ZO-1 and claudin-1 are thus key tight junction proteins relevant to colonic mucosal repair. In another study, MSCs were similarly found to effectively control tight junction proteins and to suppress apoptotic death in the context of colitis, contributing to improvements in intestinal morphology and function[64]. These results suggest that MSCs can effectively help repair damage to the intestinal barrier associated with the pathogenesis of IBD.
Acupuncture exhibits robust anti-inflammatory activity (Table 2), downregulating inflammatory cytokine levels and inducing anti-inflammatory factor production in a manner similar to the effects of MSC treatment, thus allowing for the acupuncture-mediated relief of IBD. One meta-analysis of the acupuncture-based treatment of colitis determined that acupuncture was capable of downregulating TNF-α and IL-8 while promoting IL-10 upregulation, ultimately limiting inflammatory activity[85]. EA is capable of suppressing the activation of macrophages and the expression of IL-1β and inducible nitric oxide synthase, thus achieving anti-inflammatory effects[86]. Acupuncture can also modulate the Th17 to Treg ratio in IBD, decreasing CD3/CD8/IL-17-positive Th17 cell counts in splenic lymph nodes while increasing CD4/CD25/FoxP3-positive Treg numbers in mice[87]. Acupuncture can similarly help maintain appropriate Th1/Th2 and M1/M2 ratios[88,89]. ST36 EA can suppress NLRP3 and caspase-1 activation in a DSS-induced murine model of colitis, protecting against NLRP3/IL-1β pathway-associated inflammatory activity[90]. In contrast to its immunomodulatory effects in IBD.
| Ref. | Model | Intervention | Acupoints | Acupoint stimulation parameters | Mechanism indicators | Pathway |
| Zhang et al[86] | IBD mice | EA | Bilateral: BL25 | EA: 2 Hz, 1 mA, 30 minutes, 7 days | IL-1β↓; iNOS↓; CD68↓ | - |
| Song et al[90] | UC mice | EA | ST36 | EA1: 100 Hz, 1 mA, 30 minutes, 7 days; EA2: 10 Hz, 1 mA, 30 minutes, 7 days | IL-1β↓; IL-6↓; IL-12↓; TNF-α↓; IL-17↓; IL-10↑ | - |
| Zhou et al[95] | PTSD mice | EA | Pretreatment | EA: 2/15 Hz, 1 mA, 30 minutes, 7 days | BDNF↓; AMPK↓; HO-1↓ | AMPK/Nrf2; Keap1/Nrf2 |
| Yu et al[96] | ALI rabbits | EA | Bilateral: ST36; BL13 | EA1: 2/15 Hz, ≤ 1 mA, 15 minutes, 5 days; EA2: 2/15 Hz, ≤ 1 mA, 6 hours, 1 days | MDA↓; SOD↑; GPX↑; TNF-α↓; IL-6↓; HO-1↑ | Nrf2/ARE |
| Dai et al[153] | SCI mice | EA | Bilateral: ST36; SP6 | EA: 60 Hz for 1.05 seconds and 2 Hz for 2.85 seconds, alternately; ≤ 5 μA | ApoE↑; TNF-α↓; IL-6↓; IL-1β↓; IL-10↑; TGF-β↑ | Nrf2-HO-1; Nrf2/NQO1/HO-1 |
| Li et al[154] | CIRI rats | EA | GV20, SP6, bilateral: ST36 | EA: 2/15 Hz, 5 mA or 10 mA, 30 minutes, 5 days | TNF-α↓; HO-1↑; iNOS↓ | Nrf2/HO-1 |
| Wang et al[155] | VD mice | Acupuncture | GV20, bilateral: ST36 | 10 minutes needle retention | TNF-α↓; TLR4↓; IL-6↓ | MyD88/NF-κB |
| Lou et al[156] | I/R rats | EA | Pretreatment: ST36; SP6 | EA: 2/15 Hz, 1 mA | TNF-α↓; IL-1↓; IL-6↓; MPO↓; TLR4↓; p-NF-κB↓ | TLR4/NF-κB |
| Wu et al[157] | UC rats | EA and HPM | EA: Bilateral ST25. HPM: (Qihai)RN6; bilateral (Tianshu)ST25 | EA: 2 Hz, 4 mA, 20 minutes, 14 days; HPM: Moxa cones (refined mugwort floss) | IL-1β↓; IL-6↓ | - |
| Liu et al[158] | UC mice | EA | Bilateral: ST36 | EA: 2 Hz, 1 mA, 15 minutes, 7 days | IFN-γ↓; IL-6↓; TNF-α↓ | TLR4/MyD88 |
Acupuncture exhibits significant antioxidant activity (Table 2). SOD, glutathione peroxidase (GPX), and catalase (CAT) are all endogenous antioxidant mediators, while malondialdehyde (MDA) concentrations are positively correlated with oxidative stress levels[91,92]. In one meta-analysis, acupuncture was shown to significantly decrease levels of MDA while increasing SOD, GPX, and CAT levels[93]. In a rat model of acute pancreatitis, EA treatment was associated with decreased histopathological scores in colon tissue together with significant decreases in myeloperoxidase and MDA levels, and a significant increase in serum IL-10 levels[94]. Experimental studies suggest that EA pretreatment can enhance hippocampal Nrf2, HO-1, and brain-derived neurotrophic factor expression in rats subjected to enhanced single prolonged stress-induced post-traumatic stress disorder, together with AMP-activated protein kinase phosphorylation, indicating that EA may exert its antioxidant effects via the Kelch-like ECH-associated protein 1/Nrf2 pathway[95]. In another study, EA treatment of the Zusanli (ST36) acupoint was able to reduce blood TNF-α and IL-6 levels while increasing SOD, GPX, and CAT levels and significantly increasing HO-1 and Nrf2 levels, consistent with the ability of EA to activate the Nrf2/antioxidant response element pathway to protect against oxidative stress-related tissue damage[96]. Acupuncture can also exert its antioxidant effects via the p38 mitogen-activated protein kinases/NF-κB pathway. The acupuncture treatment of the Zusanli (ST36), Baihui (GV20), and Taixi (KI3) acupoints, for example, can reduce p38 mitogen-activated protein kinases levels to suppress inflammation in the central nervous system[97]. Acupuncture at Zusanli (ST36) can also reportedly suppress nuclear NF-κB translocation and TP53 expression in a multiple cerebral infarction model system, while acupuncture at the Baihui (GV20), Yintang (EX-HN3), and Shuigou (GV26) acupoints was sufficient to reduce the deposition of β-secretase 1 controlled by NF-κB in APP/PS1 transgenic mice[98]. Acupuncture is also capable of exerting antioxidant effects to limit ROS biogenesis and prevent the apoptotic death of neurons[99].
The effects of acupuncture in the context of IBD are not solely related to the modulation of pro- and anti-inflammatory mediator production, as it can also markedly affect intestinal barrier integrity and tissue function through the control of cellular apoptosis and the promotion of epithelial cell regeneration. Signaling via the PI3K/AKT axis is important for IEC proliferation, and activation of this pathway can improve intestinal integrity[9,79]. Acupuncture has been shown to activate PI3K/AKT signaling, controlling the Bcl-2/Bax and thereby inhibiting apoptotic cell death owing to the enhancement of anti-apoptotic signaling[100]. EA can also promote IGF-1 release and IGF-1/PI3K/AKT pathway acti
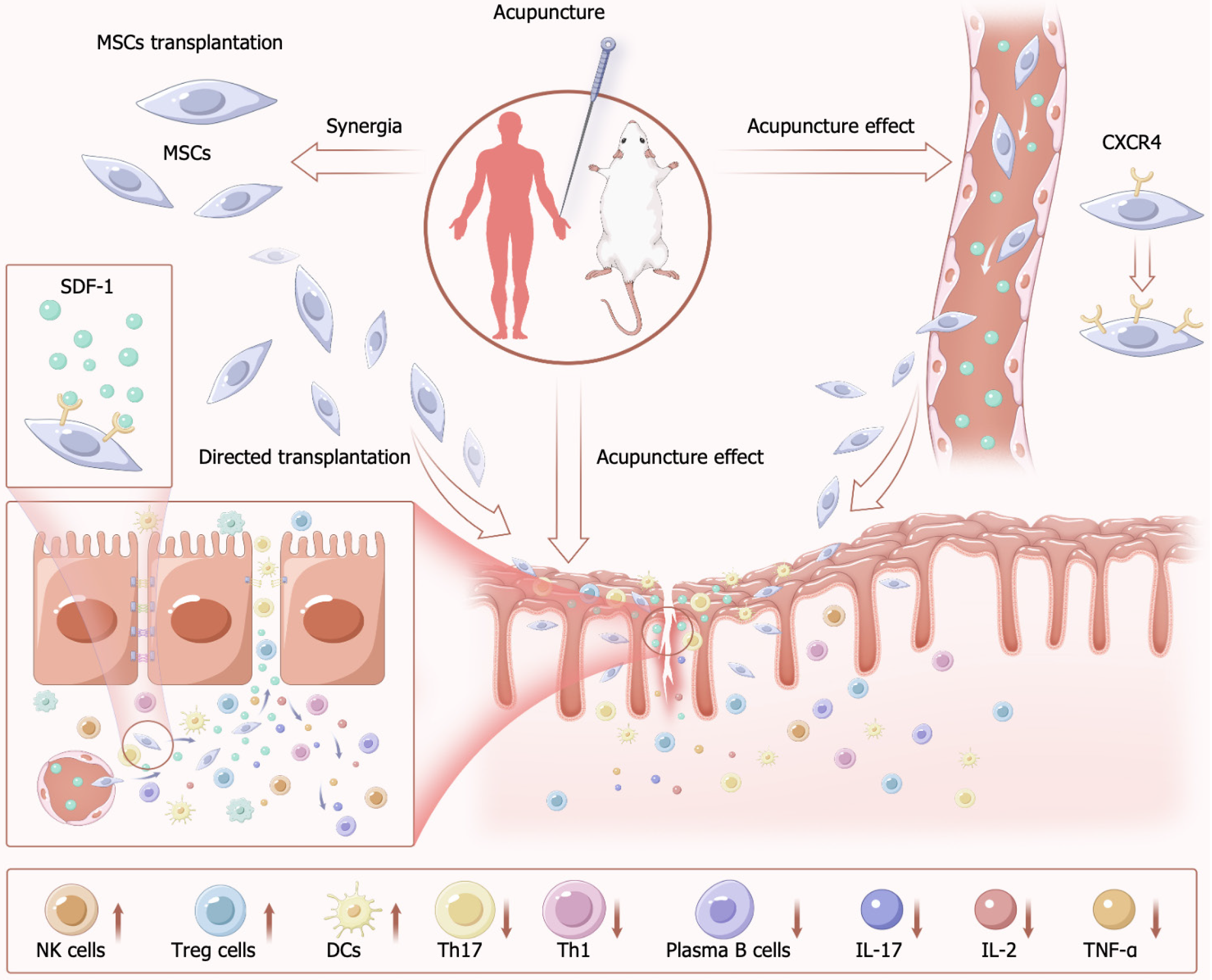
The synergistic effects of combined acupuncture and MSCs therapy in IBD may involve multi-level immunomodulation and tissue repair. Acupuncture activates the vagus nerve-cholinergic anti-inflammatory pathway, suppressing the release of pro-inflammatory cytokines (e.g., IL-6, TNF-α) in the spleen and mesenteric lymph nodes while enhancing MSC proliferation. This process further modulates MSC secretion of anti-inflammatory mediators such as IL-10, thereby restoring intestinal immune homeostasis.
Furthermore, the combination therapy exhibits dual antioxidant potentiation. Acupuncture stimulation significantly reduces myeloperoxidase and MDA levels, whereas MSCs mitigate oxidative stress by inhibiting the Nrf2/NF-κB/NLRP3 signaling pathway. Concurrently, acupuncture activates the PI3K/AKT axis to promote intestinal barrier integrity restoration. Upon homing to damaged intestinal mucosa, MSCs initiate reparative functions by stimulating IEC proliferation. These findings collectively demonstrate that the multi-targeted reparative capacities of acupuncture combined with MSC therapy ensure structural reconstruction of the intestinal barrier.
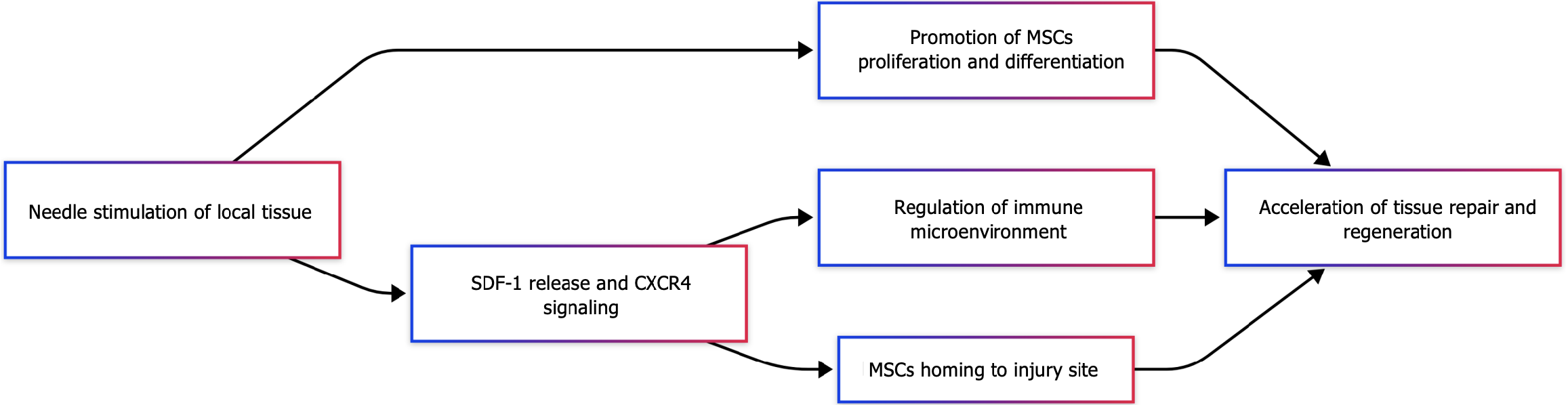
The ability of MSCs to home to sites of damage represents a unique therapeutic advantage, and can be broadly classified into both systemic and non-systemic forms of homing[105]. In cases of systemic homing, administered or endogenous MSCs accumulate in the bloodstream and then progress through the stages of tethering and rolling, activation, arrest, transmigration or diapedesis, and migration, thereby ultimately migrating into sites of local tissue injury. In cases of non-systemic migration, MSCS transplanted into target tissues can be guided to injured sites through the effects of chemokines and other chemotactic stimuli[106]. These homing effects are crucial to the efficacy of MSC therapy, and some imaging studies have suggested that approaches that entail systemic MSC administration are associated with lower levels of homing efficiency[107,108], underscoring the need for further efforts to enhance homing efficiency as a means of achieving more efficacious MSC therapy. Current studies have sought to improve MSC homing through strategies including magnetic guidance[109], genetic modification[110], cell surface engineering[111], in vitro culture[112], and target tissue modification[113]. The majority of these strategies primarily aim to bolster systemic homing activity, but some, including magnetic guidance, instead focus on the improvement of non-systemic homing. Despite the promising results yielded by these studies, there remains a persistent need to design safer and more effective means of improving MSC homing efficiency. Current evidence suggests that the expression of stroma cell-derived factor (SDF)-1 on endothelial cells is vital for the activation of MSC homing[114]. SDF-1 is a C-X-C chemokine receptor type 4 (CXCR4) ligand, and this receptor is expressed on MSC surfaces[115-117]. MSCs additionally express the chemokine receptors CC Chemokine receptor 1 (CCR1), CCR4, CCR7, CCR9, CCR10, CXCR5, and CXCR6[116], although their functional roles will require further clarification. In one study, the co-expression of CXCR4 and CXCR7 was observed on bone marrow-derived MSCs (BMSCs), with these receptors cooperating together to promote the migration of these BMSCs[118], supporting a potential role for multiple chemokine receptors in this migratory process.
Acupuncture can activate signaling via the SDF-1/CXCR4 axis. One study found that in a mouse model of myocardial infarction[119], EA treatment of the neiguan (PC6) and xinshu (BL15) acupoints resulted in CXCR4 upregulation and corresponding improvements in myocardial pathogenesis. This supports the ability of EA to promote the mobilization of stem cells and to protect against myocardial damage via this SDF-1/CXCR4 pathway. Separately, the EA treatment of a rat model of myocardial infarction was shown to induce significant CXCR4 and SDF-1 upregulation[120], suggesting that this SDF-1/CXCR4 axis is an important mechanism through which acupuncture can promote tissue repair (Figure 2).
Combining EA and BMSC therapy through efforts to target the SDF-1/CXCR4 pathway can effectively treat intrauterine adhesions in a rat model system, leading to significant reductions in levels of endometrial fibrosis and pro-inflammatory cytokine levels as compared to the BMSC monotherapy or model groups[121]. This indicates that acupuncture can be combined with BMSC treatment as a means of improving BMSC transplantation efficiency through this SDF-1/CXCR4 pathway. Consistent with such a model[122], combined EA and MSC treatment was associated with better outcomes than either therapy alone in a rat ischemia/reperfusion (I/R) model, with corresponding increases in SDF-1 and CXCR4 mRNA levels.
Current research highlights three primary mechanisms through which acupuncture exerts its therapeutic effects in synergy with MSC therapy: (1) Acupuncture promotes MSCs to secrete growth factors, such as epidermal growth factor, which modulate cellular proliferation in peri-lesional tissues; (2) The combinatorial therapy amplifies SDF-1 expression in damaged tissues, thereby enhancing MSC chemotaxis. Experimental evidence demonstrates that EA adjuvant therapy upregulates both SDF-1 and its receptor CXCR4, establishing a chemotactic gradient that directs MSC migration to injury sites. This targeted homing mechanism is critical for localized tissue regeneration and functional recovery; and (3) Acupuncture synergizes with MSC therapy to suppress pro-inflammatory signaling pathways. Both EA and MSC transplantation independently reduce NF-κB activation and downstream inflammatory mediators, including IL-6 and TNF-α. Notably, their combined use results in a more pronounced anti-inflammatory response, as evidenced by significantly attenuated cytokine levels and histopathological inflammation in preclinical models.
The use of acupuncture together with MSC transplantation has, to date, been used to manage central nervous system diseases including traumatic brain injury, spinal cord injury, and stroke[25,123,124], in addition to having been used to manage endometrial lesions and intestinal I/R injury[121,122]. This combination treatment strategy has been demon
In addition to promoting exogenous MSC differentiation, it can promote endogenous MSC proliferation. Studies in mice, rats, humans, and horses have demonstrated that macrophages and MSCs are released into the peripheral blood following acupuncture-mediated stimulation of certain acupoints including LI-11, LI-4, Du-14, and Du-20[129]. Acupu
Studies published to date have demonstrated the ability of combining acupuncture and MSC therapy to effectively treat IBD, with acupuncture facilitating the enhancement of MSC proliferation, differentiation, and transplantation. Acupuncture induces the local release of SDF-1 at sites of injury and into the bloodstream, inducing CXCR4 expression on MSCs and thereby improving their homing and differentiation abilities. In addition to their individual benefits, combining MSCs with acupuncture may lead to superior improvements in the intestinal environment through the ability of acupuncture to stimulate and mobilize endogenous MSCs and other cells with reparative functions (Figure 3). Acupu
MSCs have emerged as increasingly promising tools for IBD treatment given their robust immunomodulatory, anti-inflammatory, and reparative functions. MSCs have been demonstrated to offer strong benefits when used to treat animal models of IBD[50,60,74], and there are several clinical cases highlighting the benefits of MSC-based IBD treatment, underscoring the safety and feasibility of using this as a short-term treatment strategy[132]. However, the limited migratory activity of MSCs is an important issue that can hamper their efficacy, particularly when they are delivered systemically. While there have been marked advances in efforts to improve MSC transplantation, many challenges remain, especially with respect to safety[106].
A review of the current literature revealed that acupuncture, as a form of traditional Chinese medicine, can achieve marked benefits when treating IBD[19-23], while also promoting the differentiation, proliferation, and transplantation of MSCs. Acupuncture has been performed in China for more than 3000 years[133], and it continues to be a versatile, effective, widely applicable therapeutic modality. A growing number of studies have probed the mechanisms through which acupuncture functions, with several reports demonstrating the analgesic, anti-inflammatory, and antidepressant effects of acupuncture treatment[134-137]. Acupuncture has achieved marked efficacy in both preclinical and clinical research efforts.
MSCs have been demonstrated to exert their therapeutic benefits when used to treat IBD as a result of their antioxidant, anti-inflammatory, and neurotrophic activities together with their ability to repair the intestinal barrier, and the same is also true with respect to the ability of acupuncture to treat IBD[85,97,99]. Combining MSCs with acupuncture may thus provide a synergistic means of managing IBD. A review of the literature further revealed that EA stimulation can directly influence MSC differentiation and trigger endogenous MSC release in vivo[24]. Notably, EA can enhance MSC homing via the SDF-1/CXCR4 axis[122], indicating that acupuncture is a feasible approach to improving the efficiency of MSC transplantation. Additional information of interest found during the literature review when compiling this article included the observation that in certain malignancies, direct MSC transplantation can lead to negative effects, with MSCs associated with a potential risk of tumor differentiation or metastatic disease[138,139], thus potentially endangering patients. Acupuncture has been shown to enhance the cytotoxicity of NK and CD8 T cells in diseases characterized by immunosuppression, highlighting the unique bidirectional regulatory and immunomodulatory effects of this traditional treatment strategy[140,141]. This suggests that acupuncture, given its ability to reduce immunosuppression, may exert antitumor effects. This also raises the question of whether acupuncture is capable of counteracting the ability of MSCs to partially promote tumor growth and enhance MSC homing, allowing for combination treatment. Further work aimed at answering this question should be prioritized in the future.
Jin et al[142] recently demonstrated that the area postrema (AP) and caudal nucleus of the solitary tract (NST) transmit information related to inflammatory responses to the brain, with caudal NST silencing in mice contributing to unrestrained inflammation whereas neuronal activation was able to effectively regulate inflammatory activity. This suggests that the NST and AP in the brain are closely related to inflammation. Efforts to target the gastric and zusanli (ST36) neuronal cell groups have previously indicated that neurons labeled following ST36 acupuncture can project to the NST and AP[143], suggesting that acupuncture may help treat autoimmune diseases like IBD through its ability to impact this body-brain circuit. This provides additional support for a model in which acupuncture may lead to better efficacy in the context of stem cell therapy. Acupuncture is widely regarded as safe[20,144,145], making it an ideal component of therapeutic regimens for some patients who are unable to tolerate the side effects of certain drugs or are concerned about treatment-related risks. Combining acupuncture and MSCs is thus an attractive approach to IBD treatment. However, there remains a pressing need for additional studies seeking to clarify precisely how acupuncture can simultaneously regulate MSCs and local damage in the context of treatment.
MSCs have emerged as increasingly promising tools for IBD treatment given their robust immunomodulatory, anti-inflammatory, and reparative functions. MSCs have been demonstrated to offer strong benefits when used to treat animal models of IBD[50,61,76], and there are several clinical cases highlighting the benefits of MSC-based IBD treatment, underscoring the safety and feasibility of using this as a short-term treatment strategy[136]. However, the limited migratory activity of MSCs is an important issue that can hamper their efficacy, particularly when they are delivered systemically. While there have been marked advances in efforts to improve MSC transplantation, many challenges remain, especially with respect to safety[110].
The authors wish to acknowledge Xing-Fang Pan, Professor of China, University of Tianjin University of Traditional Chinese Medicine, for her help in revising the review.
| 1. | Fabián O, Kamaradová K. Morphology of inflammatory bowel diseases (IBD). Cesk Patol. 2022;58:27-37. [PubMed] |
| 2. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 646] [Article Influence: 107.7] [Reference Citation Analysis (2)] |
| 3. | Bitton A, Vutcovici M, Sewitch M, Suissa S, Brassard P. Mortality Trends in Crohn's Disease and Ulcerative Colitis: A Population-based Study in Québec, Canada. Inflamm Bowel Dis. 2016;22:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Lin WC, Weng MT, Tung CC, Chang YT, Leong YL, Wang YT, Wang HY, Wong JM, Wei SC. Trends and risk factors of mortality analysis in patients with inflammatory bowel disease: a Taiwanese nationwide population-based study. J Transl Med. 2019;17:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Nguyen GC, Targownik LE, Singh H, Benchimol EI, Bitton A, Murthy SK, Bernstein CN, Lee K, Cooke-Lauder J, Kaplan GG. The Impact of Inflammatory Bowel Disease in Canada 2018: IBD in Seniors. J Can Assoc Gastroenterol. 2019;2:S68-S72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Windsor JW, Kaplan GG. Evolving Epidemiology of IBD. Curr Gastroenterol Rep. 2019;21:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 7. | Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 766] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 8. | Troncone E, Monteleone G. The safety of non-biological treatments in Ulcerative Colitis. Expert Opin Drug Saf. 2017;16:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Xu J, Wang X, Chen J, Chen S, Li Z, Liu H, Bai Y, Zhi F. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10:12204-12222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Ricart E. Current status of mesenchymal stem cell therapy and bone marrow transplantation in IBD. Dig Dis. 2012;30:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 12. | Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98:151041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 14. | Wang M, Liu W, Ge J, Liu S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. 2023;14:1147718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 15. | Liao HY, Satyanarayanan SK, Lin YW, Su KP. Clinical efficacy and immune effects of acupuncture in patients with comorbid chronic pain and major depression disorder: A double-blinded, randomized controlled crossover study. Brain Behav Immun. 2023;110:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 16. | Ding SS, Hong SH, Wang C, Guo Y, Wang ZK, Xu Y. Acupuncture modulates the neuro-endocrine-immune network. QJM. 2014;107:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Patel M, Urits I, Kaye AD, Viswanath O. The role of acupuncture in the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 2020;34:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Jiang LH, Li PJ, Wang YQ, Jiang ML, Han XY, Bao YD, Deng XL, Wu WB, Liu XD. Anti-inflammatory effects of acupuncture in the treatment of chronic obstructive pulmonary disease. J Integr Med. 2023;21:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Bao C, Wu L, Wang D, Chen L, Jin X, Shi Y, Li G, Zhang J, Zeng X, Chen J, Liu H, Wu H. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn's disease: A randomized controlled trial. EClinicalMedicine. 2022;45:101300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Zhou YF, Sun N, Cheng SR, Deng XD, Ye XY, Li ZJ, Zhou J, Xu GX, Qu YZ, Huang LY, Sun RR, Liang FR. Effectiveness and safety of acupuncture therapy for inflammatory bowel disease: a protocol of systematic review and meta-analysis. BMJ Open. 2021;11:e045090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Du Y, Zhang L, Liu W, Rao C, Li B, Nan X, Li Z, Jiang H. Effect of acupuncture treatment on post-stroke cognitive impairment: A randomized controlled trial. Medicine (Baltimore). 2020;99:e23803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Han KH, Cho KH, Han C, Cui S, Lin L, Baek HY, Kim J. The effectiveness and safety of acupuncture treatment on sciatica: A systematic review and meta-analysis. Complement Ther Med. 2022;71:102872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Gao H, Ding W. Effect and mechanism of acupuncture on endogenous and exogenous stem cells in disease treatment: A therapeutic review. Life Sci. 2023;331:122031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Ahn SM, Kim YR, Shin YI, Ha KT, Lee SY, Shin HK, Choi BT. Therapeutic Potential of a Combination of Electroacupuncture and TrkB-Expressing Mesenchymal Stem Cells for Ischemic Stroke. Mol Neurobiol. 2019;56:157-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Jin H, Zhang YT, Yang Y, Wen LY, Wang JH, Xu HY, Lai BQ, Feng B, Che MT, Qiu XC, Li ZL, Wang LJ, Ruan JW, Jiang B, Zeng X, Deng QW, Li G, Ding Y, Zeng YS. Electroacupuncture Facilitates the Integration of Neural Stem Cell-Derived Neural Network with Transected Rat Spinal Cord. Stem Cell Reports. 2019;12:274-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784-5788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Raza A, Yousaf W, Giannella R, Shata MT. Th17 cells: interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol. 2012;8:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Zhao J, Lu Q, Liu Y, Shi Z, Hu L, Zeng Z, Tu Y, Xiao Z, Xu Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J Immunol Res. 2021;2021:8816041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Mao L, Kitani A, Strober W, Fuss IJ. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front Immunol. 2018;9:2566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 31. | Bank S, Julsgaard M, Abed OK, Burisch J, Broder Brodersen J, Pedersen NK, Gouliaev A, Ajan R, Nytoft Rasmussen D, Honore Grauslund C, Roug S, Galsgaard J, Sprogøe Høyer Finsen D, Lindby K; Danish IBD Genetics Working Group, Sørensen J, Larsen L, Rohr Andersen M, Brandslund I, Thomassen M, Green A, Bo Bojesen A, Bek Sørensen S, Vogel U, Andersen V. Polymorphisms in the NFkB, TNF-alpha, IL-1beta, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:890-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Maerten P, Shen C, Colpaert S, Liu Z, Bullens DA, van Assche G, Penninckx F, Geboes K, Vanham G, Rutgeerts P, Ceuppens JL. Involvement of interleukin 18 in Crohn's disease: evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2893] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 35. | Boshagh MA, Foroutan P, Moloudi MR, Fakhari S, Malakouti P, Nikkhoo B, Jalili A. ELR positive CXCL chemokines are highly expressed in an animal model of ulcerative colitis. J Inflamm Res. 2019;12:167-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lee DS, Lee KL, Jeong JB, Shin S, Kim SH, Kim JW. Expression of Chemokine CCL28 in Ulcerative Colitis Patients. Gut Liver. 2021;15:70-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 38. | Martinez-Fierro ML, Garza-Veloz I, Rocha-Pizaña MR, Cardenas-Vargas E, Cid-Baez MA, Trejo-Vazquez F, Flores-Morales V, Villela-Ramirez GA, Delgado-Enciso I, Rodriguez-Sanchez IP, Ortiz-Castro Y. Serum cytokine, chemokine, and growth factor profiles and their modulation in inflammatory bowel disease. Medicine (Baltimore). 2019;98:e17208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807-4812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 196] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 370] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | Keshavarzian A, Sedghi S, Kanofsky J, List T, Robinson C, Ibrahim C, Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992;103:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 186] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2831] [Cited by in RCA: 2671] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 44. | Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 584] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 46. | Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Ding DC, Shyu WC, Lin SZ, Li H. The role of endothelial progenitor cells in ischemic cerebral and heart diseases. Cell Transplant. 2007;16:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1039] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 49. | Hwang NS, Zhang C, Hwang YS, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 51. | Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9:985-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 52. | Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med. 1995;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 880] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 54. | Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 1309] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 55. | Che J, Wang H, Dong J, Wu Y, Zhang H, Fu L, Zhang J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci Ther. 2024;30:e14454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 56. | Cao H, Ji X, Wang Q, Guan X, Wei W, Li Y, Zou W, Liu J. PTBP-1 and TNF-α/NF-κB are involved in repair mechanisms of human umbilical cord mesenchymal stem cell transplantation in mice with spinal cord injury. Am J Transl Res. 2022;14:4443-4456. [PubMed] |
| 57. | Wang T, Jian Z, Baskys A, Yang J, Li J, Guo H, Hei Y, Xian P, He Z, Li Z, Li N, Long Q. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257:120264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 58. | Stavely R, Robinson AM, Fraser S, Filippone RT, Stojanovska V, Eri R, Apostolopoulos V, Sakkal S, Nurgali K. Bone marrow-derived mesenchymal stem cells mitigate chronic colitis and enteric neuropathy via anti-inflammatory and anti-oxidative mechanisms. Sci Rep. 2024;14:6649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 59. | Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702-4717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Jo H, Eom YW, Kim HS, Park HJ, Kim HM, Cho MY. Regulatory Dendritic Cells Induced by Mesenchymal Stem Cells Ameliorate Dextran Sodium Sulfate-Induced Chronic Colitis in Mice. Gut Liver. 2018;12:664-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 62. | Wang ZX, Wang CQ, Li XY, Feng GK, Zhu HL, Ding Y, Jiang XJ. Mesenchymal stem cells alleviate atherosclerosis by elevating number and function of CD4(+)CD25 (+)FOXP3 (+) regulatory T-cells and inhibiting macrophage foam cell formation. Mol Cell Biochem. 2015;400:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 64. | Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 683] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 65. | Yang FY, Chen R, Zhang X, Huang B, Tsang LL, Li X, Jiang X. Preconditioning Enhances the Therapeutic Effects of Mesenchymal Stem Cells on Colitis Through PGE2-Mediated T-Cell Modulation. Cell Transplant. 2018;27:1352-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 66. | Li F, Guo X, Chen SY. Function and Therapeutic Potential of Mesenchymal Stem Cells in Atherosclerosis. Front Cardiovasc Med. 2017;4:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 68. | Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1019] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 69. | Casado JG, Tarazona R, Sanchez-Margallo FM. NK and MSCs crosstalk: the sense of immunomodulation and their sensitivity. Stem Cell Rev Rep. 2013;9:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Meisel R, Brockers S, Heseler K, Degistirici O, Bülle H, Woite C, Stuhlsatz S, Schwippert W, Jäger M, Sorg R, Henschler R, Seissler J, Dilloo D, Däubener W. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, Zamani M, Javidi K, Naimi A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol. 2020;235:9185-9210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Park HJ, Kim J, Saima FT, Rhee KJ, Hwang S, Kim MY, Baik SK, Eom YW, Kim HS. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem Biophys Res Commun. 2018;498:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | An JH, Song WJ, Li Q, Kim SM, Yang JI, Ryu MO, Nam AR, Bhang DH, Jung YC, Youn HY. Prostaglandin E(2) secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, Kim YL, Seo KW, Kang KS. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13:1792-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1673] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 76. | Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 77. | Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Lee YS, Jun YH, Lee J. Oral administration of bone marrow-derived mesenchymal stem cells attenuates intestinal injury in necrotizing enterocolitis. Clin Exp Pediatr. 2024;67:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 79. | Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Gu L, Ren F, Fang X, Yuan L, Liu G, Wang S. Exosomal MicroRNA-181a Derived From Mesenchymal Stem Cells Improves Gut Microbiota Composition, Barrier Function, and Inflammatory Status in an Experimental Colitis Model. Front Med (Lausanne). 2021;8:660614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Tak LJ, Kim HY, Ham WK, Agrahari G, Seo Y, Yang JW, An EJ, Bang CH, Lee MJ, Kim HS, Kim TY. Superoxide Dismutase 3-Transduced Mesenchymal Stem Cells Preserve Epithelial Tight Junction Barrier in Murine Colitis and Attenuate Inflammatory Damage in Epithelial Organoids. Int J Mol Sci. 2021;22:6431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C, Turner JR. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology. 2021;161:1924-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 285] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 83. | Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 84. | Jiang Y, Song J, Xu Y, Liu C, Qian W, Bai T, Hou X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021;275:119254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 85. | Yang X, He M, Tang Q, Wang Z, Jin D, Wu X, Yang Y, Ma D, Sun M, Li T. Assessment of anti-inflammatory efficacy of acupuncture in patients with inflammatory bowel disease: A systematic review and meta-analysis. Complement Ther Med. 2023;74:102946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | Zhang H, He W, Hu XF, Li YZ, Liu YM, Ge WQ, Zhanmu OY, Chen C, Lan YY, Su YS, Jing XH, Zhu B, Pan HL, Yu LL, Li M. Electroacupuncture Reduces Visceral Pain Via Cannabinoid CB2 Receptors in a Mouse Model of Inflammatory Bowel Disease. Front Pharmacol. 2022;13:861799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Wang CY, Zeng LL, Geng Y, Wang X, Zhang HJ, Yang H, Wu Q, Yu SG. [Effect of Electroacupuncture Stimulation of "Guanyuan" (CV 4) and "Zusanli" (ST 36) on Spleen Lymphocytes Treg/Th 17 Immune Balance in Ulcerative Colitis Mice]. Zhen Ci Yan Jiu. 2016;41:55-59. [PubMed] |
| 88. | Liu F, Wang Y, Lyu K, Du X, Zhou M, Shi J, Na R, Guo Y, Wang G, Xu W, Zheng T. Acupuncture and its ability to restore and maintain immune homeostasis. QJM. 2024;117:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 89. | Hong G. Research Progress on the Mechanism of Acupuncture in the Prevention and Treatment of Allergic Rhinitis. Altern Ther Health Med. 2023;29:228-232. [PubMed] |
| 90. | Song S, An J, Li Y, Liu S. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1β and promoting Nrf2/HO-1. Mol Immunol. 2019;106:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 91. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2269] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 92. | Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2367] [Cited by in RCA: 3555] [Article Influence: 323.2] [Reference Citation Analysis (0)] |
| 93. | Zhao Y, Zhou B, Zhang G, Xu S, Yang J, Deng S, Yao Z, Geng Q, Ouyang B, Xia T. The effect of acupuncture on oxidative stress: A systematic review and meta-analysis of animal models. PLoS One. 2022;17:e0271098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 94. | Chen H, Su H, Yuan L, Miao YF, Zhang YM, Li J, Tang WF. [Administration of Electroacupuncture and Da-Cheng-Qi Decoction Has a Synergetic Effect in Relieving Injury of Pancreas, Lung and Large Intestine and Inflammatory Reactions in Rats with Acute Pancreatitis]. Zhen Ci Yan Jiu. 2018;43:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Zhou CH, Xue F, Xue SS, Sang HF, Liu L, Wang Y, Cai M, Zhang ZJ, Tan QR, Wang HN, Peng ZW. Electroacupuncture Pretreatment Ameliorates PTSD-Like Behaviors in Rats by Enhancing Hippocampal Neurogenesis via the Keap1/Nrf2 Antioxidant Signaling Pathway. Front Cell Neurosci. 2019;13:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Yu JB, Shi J, Gong LR, Dong SA, Xu Y, Zhang Y, Cao XS, Wu LL. Role of Nrf2/ARE pathway in protective effect of electroacupuncture against endotoxic shock-induced acute lung injury in rabbits. PLoS One. 2014;9:e104924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Fang JQ, Zhu SX, Zhang Y, Wang F, Zhu QY. [Effect of electroacupuncture on expression of phosphorylated P 38 MAPK and IL-1beta in frontal lobe and hippocampus in rats with Alzheimer's disease]. Zhen Ci Yan Jiu. 2013;38:35-39. [PubMed] |
| 98. | Tang Y, Shao S, Guo Y, Zhou Y, Cao J, Xu A, Wu J, Li Z, Xiang D. Electroacupuncture Mitigates Hippocampal Cognitive Impairments by Reducing BACE1 Deposition and Activating PKA in APP/PS1 Double Transgenic Mice. Neural Plast. 2019;2019:2823679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Huang TI, Hsieh CL. Effects of Acupuncture on Oxidative Stress Amelioration via Nrf2/ARE-Related Pathways in Alzheimer and Parkinson Diseases. Evid Based Complement Alternat Med. 2021;2021:6624976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Xue X, You Y, Tao J, Ye X, Huang J, Yang S, Lin Z, Hong Z, Peng J, Chen L. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Hu T, Lu MN, Chen B, Tong J, Mao R, Li SS, Dai P, Tan YX, Xiyang YB. Electro-acupuncture-induced neuroprotection is associated with activation of the IGF-1/PI3K/Akt pathway following adjacent dorsal root ganglionectomies in rats. Int J Mol Med. 2019;43:807-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 102. | Shang HX, Wang AQ, Bao CH, Wu HG, Chen WF, Wu LY, Ji R, Zhao JM, Shi Y. Moxibustion combined with acupuncture increases tight junction protein expression in Crohn's disease patients. World J Gastroenterol. 2015;21:4986-4996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 103. | Liu GH, Zhuo XC, Huang YH, Liu HM, Wu RC, Kuo CJ, Chen NH, Chuang LP, Lin SW, Chen YL, Yang HY, Lee TY. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology (Basel). 2022;11:962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Mengzhu S, Yujie Z, Yafang S, Jing G, Yuhang W, Chen X, Dongmei GU, Jianhua S, Lixia P. Electroacupuncture alleviates water avoidance stress-induced irritable bowel syndrome in mice by improving intestinal barrier functions and suppressing the expression of inflammatory cytokines. J Tradit Chin Med. 2023;43:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 105. | Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells. 2017;35:1446-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 106. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 107. | Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 108. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 109. | Yun WS, Choi JS, Ju HM, Kim MH, Choi SJ, Oh ES, Seo YJ, Key J. Enhanced Homing Technique of Mesenchymal Stem Cells Using Iron Oxide Nanoparticles by Magnetic Attraction in Olfactory-Injured Mouse Models. Int J Mol Sci. 2018;19:1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Shao Y, Zhou F, He D, Zhang L, Shen J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed Pharmacother. 2019;109:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 111. | Chou KJ, Lee PT, Chen CL, Hsu CY, Huang WC, Huang CW, Fang HC. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp Cell Res. 2017;350:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 113. | Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, Silver KH, Shin J, Ewald G, Farr MJ, Anwaruddin S, Plat F, Fisher SJ, AuWerter AT, Pastore JM, Aras R, Penn MS. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur Heart J. 2015;36:2228-2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 114. | Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 115. | Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 116. | Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 513] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 117. | Bobis-Wozowicz S, Miekus K, Wybieralska E, Jarocha D, Zawisz A, Madeja Z, Majka M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp Hematol. 2011;39:686-696.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D, Xu T. CXCR-7 receptor promotes SDF-1α-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res. 2014;1575:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Zhao TT, Liu JJ, Zhu J, Li H, Wang YC, Zhao Y, Shao SJ, Guo HD, Mou FF. SDF-1/CXCR4-Mediated Stem Cell Mobilization Involved in Cardioprotective Effects of Electroacupuncture on Mouse with Myocardial Infarction. Oxid Med Cell Longev. 2022;2022:4455183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 120. | Xuan SS, Zhao Y, Zheng Y, Zhu J, Li H, Lu PP, Shao SJ, Guo HD, Mou FF. Electroacupuncture improves cardiac function after myocardial infarction by regulating the mobilization and migration of endogenous stem cells. Acupunct Med. 2023;41:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 121. | Wang Z, Xia L, Cheng J, Liu J, Zhu Q, Cui C, Li J, Huang Y, Shen J, Xia Y. Combination Therapy of Bone Marrow Mesenchymal Stem Cell Transplantation and Electroacupuncture for the Repair of Intrauterine Adhesions in Rats: Mechanisms and Functional Recovery. Reprod Sci. 2024;31:2318-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 122. | Geng Y, Chen D, Zhou J, Lu J, Chen M, Zhang H, Wang X. Synergistic Effects of Electroacupuncture and Mesenchymal Stem Cells on Intestinal Ischemia/Reperfusion Injury in Rats. Inflammation. 2016;39:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Yang Y, Xu HY, Deng QW, Wu GH, Zeng X, Jin H, Wang LJ, Lai BQ, Li G, Ma YH, Jiang B, Ruan JW, Wang YQ, Ding Y, Zeng YS. Electroacupuncture facilitates the integration of a grafted TrkC-modified mesenchymal stem cell-derived neural network into transected spinal cord in rats via increasing neurotrophin-3. CNS Neurosci Ther. 2021;27:776-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Deng L, Zhou L, Zhu Y, Fan G, Tang H, Zheng Y, Gao X, Guo K, Zhou P, Yang C. Electroacupuncture Enhance Therapeutic Efficacy of Mesenchymal Stem Cells Transplantation in Rats With Intracerebral Hemorrhage. Stem Cell Rev Rep. 2022;18:570-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Liu Z, Ding Y, Zeng YS. A new combined therapeutic strategy of governor vessel electro-acupuncture and adult stem cell transplantation promotes the recovery of injured spinal cord. Curr Med Chem. 2011;18:5165-5171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 126. | Yan Q, Ruan JW, Ding Y, Li WJ, Li Y, Zeng YS. Electro-acupuncture promotes differentiation of mesenchymal stem cells, regeneration of nerve fibers and partial functional recovery after spinal cord injury. Exp Toxicol Pathol. 2011;63:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 127. | Zhang K, Liu Z, Li G, Lai BQ, Qin LN, Ding Y, Ruan JW, Zhang SX, Zeng YS. Electro-acupuncture promotes the survival and differentiation of transplanted bone marrow mesenchymal stem cells pre-induced with neurotrophin-3 and retinoic acid in gelatin sponge scaffold after rat spinal cord transection. Stem Cell Rev Rep. 2014;10:612-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Ding Y, Yan Q, Ruan JW, Zhang YQ, Li WJ, Zhang YJ, Li Y, Dong H, Zeng YS. Electro-acupuncture promotes survival, differentiation of the bone marrow mesenchymal stem cells as well as functional recovery in the spinal cord-transected rats. BMC Neurosci. 2009;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Salazar TE, Richardson MR, Beli E, Ripsch MS, George J, Kim Y, Duan Y, Moldovan L, Yan Y, Bhatwadekar A, Jadhav V, Smith JA, McGorray S, Bertone AL, Traktuev DO, March KL, Colon-Perez LM, Avin KG, Sims E, Mund JA, Case J, Deng X, Kim MS, McDavitt B, Boulton ME, Thinschmidt J, Li Calzi S, Fitz SD, Fuchs RK, Warden SJ, McKinley T, Shekhar A, Febo M, Johnson PL, Chang LJ, Gao Z, Kolonin MG, Lai S, Ma J, Dong X, White FA, Xie H, Yoder MC, Grant MB. Electroacupuncture Promotes Central Nervous System-Dependent Release of Mesenchymal Stem Cells. Stem Cells. 2017;35:1303-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Liu L, Yu Q, Hu K, Wang B, Zhang Y, Xu Y, Fu S, Yu X, Huang H. Electro-Acupuncture Promotes Endogenous Multipotential Mesenchymal Stem Cell Mobilization into the Peripheral Blood. Cell Physiol Biochem. 2016;38:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Li H, Ye XF, Su YS, He W, Zhang JB, Zhang Q, Zhan LB, Jing XH. Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis. Chin J Integr Med. 2023;29:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 132. | Saadh MJ, Mikhailova MV, Rasoolzadegan S, Falaki M, Akhavanfar R, Gonzáles JLA, Rigi A, Kiasari BA. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur J Med Res. 2023;28:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 133. | Zhuang Y, Xing JJ, Li J, Zeng BY, Liang FR. History of acupuncture research. Int Rev Neurobiol. 2013;111:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 134. | Faria M, Teixeira M, Pinto MJ, Sargento P. Efficacy of acupuncture on cancer pain: A systematic review and meta-analysis. J Integr Med. 2024;22:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Shen J, Hao C, Yuan S, Chen W, Tong T, Chen Y, Shahzad Aslam M, Yan S, Li J, Zeng J, Liu S, Chen Y, Jiang Y, Li P, Meng X. Acupuncture alleviates CUMS-induced depression-like behaviors of rats by regulating oxidative stress, neuroinflammation and ferroptosis. Brain Res. 2024;1826:148715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 136. | Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3:CD004046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 137. | Guo X, Ma T. Effects of Acupuncture on Neurological Disease in Clinical- and Animal-Based Research. Front Integr Neurosci. 2019;13:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Albarenque SM, Zwacka RM, Mohr A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011;7:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 139. | Li W, Zhou Y, Yang J, Zhang X, Zhang H, Zhang T, Zhao S, Zheng P, Huo J, Wu H. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J Exp Clin Cancer Res. 2015;34:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 140. | Pais I, Correia N, Pimentel I, Teles MJ, Neves E, Vasconcelos J, Guimarães J, Azevedo N, Moreira Pinto A, Machado J, Efferth T, Greten HJ. Effects of acupuncture on leucopenia, neutropenia, NK, and B cells in cancer patients: a randomized pilot study. Evid Based Complement Alternat Med. 2014;2014:217397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 141. | Yu Y, Kasahara T, Sato T, Guo SY, Liu Ya, Asano K, Hisamitsu T. Enhancement of splenic interferon-gamma, interleukin-2, and NK cytotoxicity by S36 acupoint acupuncture in F344 rats. Jpn J Physiol. 1997;47:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Jin H, Li M, Jeong E, Castro-Martinez F, Zuker CS. A body-brain circuit that regulates body inflammatory responses. Nature. 2024;630:695-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 124] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 143. | Lee CH, Jung HS, Lee TY, Lee SR, Yuk SW, Lee KG, Lee BH. Studies of the central neural pathways to the stomach and Zusanli (ST36). Am J Chin Med. 2001;29:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | Li YX, Xiao XL, Zhong DL, Luo LJ, Yang H, Zhou J, He MX, Shi LH, Li J, Zheng H, Jin RJ. Effectiveness and Safety of Acupuncture for Migraine: An Overview of Systematic Reviews. Pain Res Manag. 2020;2020:3825617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 145. | Li J, Li YX, Luo LJ, Ye J, Zhong DL, Xiao QW, Zheng H, Geng CM, Jin RJ, Liang FR. The effectiveness and safety of acupuncture for knee osteoarthritis: An overview of systematic reviews. Medicine (Baltimore). 2019;98:e16301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 146. | Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, Long Q. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9:5956-5975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 147. | Xiao K, He W, Guan W, Hou F, Yan P, Xu J, Zhou T, Liu Y, Xie L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 148. | Zhang Q, Lei X, Wang F, He X, Liu L, Hou Y, Liu Y, Jin F, Chen C, Li B. ERK1-mediated immunomodulation of mesenchymal stem cells ameliorates inflammatory disorders. iScience. 2023;26:107868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 149. | Li J, Deng X, Ji X, Shi X, Ying Z, Shen K, Xu D, Cheng Z. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf-2/ARE and NF-κB signaling pathways. PeerJ. 2020;8:e9928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 150. | Song IH, Jung KJ, Lee TJ, Kim JY, Sung EG, Bae YC, Park YH. Mesenchymal stem cells attenuate adriamycin-induced nephropathy by diminishing oxidative stress and inflammation via downregulation of the NF-kB. Nephrology (Carlton). 2018;23:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 151. | Han M, Cao Y, Guo X, Chu X, Li T, Xue H, Xin D, Yuan L, Ke H, Li G, Wang Z. Mesenchymal stem cell-derived extracellular vesicles promote microglial M2 polarization after subarachnoid hemorrhage in rats and involve the AMPK/NF-κB signaling pathway. Biomed Pharmacother. 2021;133:111048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 152. | Wang X, Yang Y, Li W, Hao M, Xu Y. Umbilical mesenchymal stem cell-derived exosomes promote spinal cord functional recovery through the miR-146b/TLR4 -mediated NF-κB p65 signaling pathway in rats. Biochem Biophys Rep. 2023;35:101497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 153. | Dai N, Tang C, Liu H, Huang S. Effect of electroacupuncture on inhibition of inflammatory response and oxidative stress through activating ApoE and Nrf2 in a mouse model of spinal cord injury. Brain Behav. 2021;11:e2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Li J, Hao M, Liu M, Wang J, Gao D, Han S, Yu D. Transcutaneous Electrical Acupoint Stimulation Pretreatment Alleviates Cerebral Ischemia-Reperfusion Injury in Rats by Modulating Microglia Polarization and Neuroinflammation Through Nrf2/HO-1 Signaling Pathway. Neurochem Res. 2023;48:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 155. | Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, Cao Y, Fisher M, Liu CZ. Acupuncture Attenuates Inflammation in Microglia of Vascular Dementia Rats by Inhibiting miR-93-Mediated TLR4/MyD88/NF-κB Signaling Pathway. Oxid Med Cell Longev. 2020;2020:8253904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 156. | Lou Y, Yu Q, Xu K, Tu Y, Balelang MF, Lu G, Zhu C, Dai Q, Geng W, Mo Y, Wang J. Electroacupuncture preconditioning protects from lung injury induced by limb ischemia/reperfusion through TLR4 and NFκB in rats. Mol Med Rep. 2020;22:3225-3232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 157. | Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Liu GH, Liu HM, Chen YS, Lee TY. Effect of Electroacupuncture in Mice with Dextran Sulfate Sodium-Induced Colitis and the Influence of Gut Microbiota. Evid Based Complement Alternat Med. 2020;2020:2087903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |