Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.101929
Revised: March 7, 2025
Accepted: June 25, 2025
Published online: July 26, 2025
Processing time: 162 Days and 1.3 Hours
Spinal cord injury (SCI) often results in irreversible neurological deficits; there
To explore the role of XIST in enhancing NSC function and its therapeutic po
We used in vitro and in vivo models to examine the effects of XIST on NSCs. XIST was overexpressed in NSCs, and its impact on mitochondrial function, neuronal differentiation, and the insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2)/carnitine palmitoyl transferase 1A (CPT1A) pathway was assessed using a series of biochemical assays, quantitative PCR, and Seahorse XF24 ana
Overexpression of XIST in NSCs significantly increased mitochondrial membrane potential, ATP production, and oxygen consumption rate. XIST also promoted NSC proliferation and neuronal differentiation while inhibiting astrocytic differentiation. Mechanistically, XIST regulated CPT1A expression post-transcriptionally by interacting with IGF2BP2. In vivo XIST-treated mice exhibited im
These findings suggested that XIST modulated mitochondrial function and neural differentiation in NSCs through the IGF2BP2/CPT1A pathway. While preliminary in vivo results are encouraging, further studies are needed to determine the long-term therapeutic relevance and underlying mechanisms of XIST in SCI recovery.
Core Tip: This study highlighted the critical role of long non-coding RNA X inactive-specific transcript (XIST) in enhancing neural stem cell function for spinal cord injury (SCI) treatment. XIST significantly improved motor recovery and reduced inflammation in mouse models of SCI by promoting mitochondrial function and neuronal differentiation. These findings suggested that XIST regulated carnitine palmitoyl transferase 1A expression via the insulin-like growth factor 2 mRNA binding protein 2 pathway, providing a promising therapeutic target for the development of effective interventions against irreversible neurological deficits in SCI. Further exploration of the long-term effects of XIST may advance its clinical applications.
- Citation: Zeng SX, Ye JT, Huang SH, Liu RX. X inactive-specific transcript regulates mitochondrial function and neuronal differentiation of stem cells via IGF2BP2/CPT1A axis in models of spinal cord injury. World J Stem Cells 2025; 17(7): 101929
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/101929.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.101929
Spinal cord injury (SCI) is a severe condition that causes irreversible neurological deficits[1]. SCI represents a profound medical challenge characterized by severe and often irreversible neurological deficits that significantly compromise the patient’s quality of life and functional capabilities[2,3]. SCI affects not only motor and sensory functions but also bowel, bladder, and respiratory functions[3]. The restoration of function following SCI relies heavily on the regeneration of damaged neural tissues and re-establishment of neural circuits. Among various therapeutic approaches, neural stem cells (NSCs) have shown great potential because of their inherent potential to differentiate into various neuronal lineages, thereby contributing to neural repair and functional recovery[4].
The role of long noncoding RNAs (lncRNAs) in regulating stem cell function and differentiation is widely studied, with that of the X inactive-specific transcript (XIST) being one of the most studied[5-7]. XIST has been implicated in various cellular processes including gene expression regulation[8], chromatin modification[9], and mitochondrial function[10]. Although XIST is known to influence gene expression and chromatin modification, its specific role in NSCs, especially in SCI, remains largely unexplored. Elucidating how XIST modulates NSC behavior under injury conditions may provide novel insights into the molecular mechanisms governing neuronal differentiation and recovery.
Mitochondrial dysfunction is a hallmark of SCI and contributes significantly to the neuronal death cascade following injury. This dysfunction disrupts energy metabolism, leading to inadequate ATP production necessary for cellular functions and is particularly detrimental to neural tissues that rely on a high energy supply[11,12]. Carnitine palmitoyl transferase 1A (CPT1A) is pivotal for mitochondrial function, particularly in patients with SCI. Mitochondrial dysfu
This study investigated the role of XIST in regulating mitochondrial oxidative phosphorylation (OXPHOS) and neuronal differentiation in NSCs, focusing on its interaction with the insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) and CPT1A pathways. We hypothesized that XIST enhances mitochondrial function and promotes neuronal differentiation in NSCs, thereby alleviating SCI. We conducted a series of in vitro and in vivo experiments to elucidate key molecular mechanisms and evaluate the therapeutic potential of targeting XIST for SCI recovery.
NSCs were isolated and cultured as previously described[19]. Briefly, NSCs were obtained from neonatal C57BL/6N mice (strain 213; Charles River Laboratories, MA, United States). The isolated cells were collected and centrifuged at 500 g for 5 min. To maintain optimal conditions the culture medium was refreshed every 3 days. On day 10, the neurospheres formed were dissociated into individual NSCs and produced in an adherent medium with 10% fetal bovine serum.
The NSCs were transfected with a human XIST-encoding plasmid using Lipofectamine 3000 (Thermo Fisher Scientific, MA, United States). NSCs were grown in 6-well plates until they reached approximately 70% confluency. The XIST-encoding plasmid was mixed with Lipofectamine 3000 and P3000 reagent in Opti-MEM, and the transfection mixture was applied to the cells. Forty-eight hours after transfection, XIST overexpression was confirmed using quantitative PCR (qPCR) and western blot analysis. For the knockdown of CPT1A, NSCs were transfected with a CPT1A-specific small interfering RNA using Lipofectamine RNAiMAX. CPT1A small interfering RNA and Lipofectamine RNAiMAX were diluted in Opti-MEM, combined, and incubated at room temperature for 5 min before adding to the cells. After a 48-h incubation period, the efficiency of CPT1A knockdown was assessed by qPCR.
The oxygen consumption rate (OCR) was measured using a Seahorse XF24 Analyzer (Agilent Technologies, CA, United States) to assess mitochondrial function. Twenty thousand NSCs were seeded per well, and the cells were left to grow overnight. OCR was recorded under basal conditions and following the sequential addition of oligomycin (1 μM), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (1 μM), and antimycin A/rotenone (0.5 μM each) to assess ATP-linked, maximal, and non-mitochondrial respiration.
qPCR was used to measure target gene expression. Total RNA was extracted from cells or tissues using TRIzol reagent (Invitrogen, CA, United States), and a NanoDrop spectrophotometer (Thermo Scientific, MA, United States) was used to measure the concentration and purity of the extracted RNA. The procedure called for 2 min of initial denaturation at
CCK-8 (Dojindo, Kumamoto, Japan) was used to measure cell proliferation according to the manufacturer’s instructions. In 96-well plates 2000 NSCs were planted per well, and the cells were grown for predetermined periods under normal conditions (37 °C, 5% CO2). The absorbance was measured at 450 nm.
To explore the potential regulatory relationship between XIST and CPT1A expression, we used the bioinformatics tool StarBase (https://rnasysu.com/encori/index.php). This platform enables prediction of RNA interactions and regulatory pathways.
As previously reported real-time qPCR (RT-PCR) was used to quantify the mitochondrial DNA (mtDNA) copy number[20]. Total genomic DNA was extracted using a DNeasy kit (QIAGEN, Shanghai, China), and RT-PCR analysis was performed to determine the amount of mtDNA. The ratio of nuclear DNA (18s rRNA) to mtDNA (cytochrome oxidase I) was used to calculate relative mtDNA concentration. Primer sequences are as follows: Cytochrome oxidase I forward: 5’-GATGAGTGGGAAAGGGGTAA-3’, reverse: 5’-GGGAGGATGAGTGGAGGAGC-3’; and 18s rRNA forward: 5’-CTTAGAGGGACAAGTGGCGTTC-3’, reverse: 5’-GCTGAGCCAGTCAGTGTAG-3’ (Table 1).
| Gene name | Forward 5’-3’ | Reverse 5’-3’ |
| XIST (human) | GTAGGTGTGCTGATAACCAAGGC | GGGAAAGGAAGATTGAGGGTGG |
| XIST (mouse) | CAAGAAGAAGGATTGCCTGGATTT | GCGAGGACTTGAAGAGAAGTTCTG |
| MAP2 (human) | AGGCTGTAGCAGTCCTGAAAGG | CTTCCTCCACTGTGACAGTCTG |
| GFAP (human) | CACCTACAGGAAATTGCTGGAGG | CCACGATGTTCCTCTTGAGGTG |
| CPT1A (human) | GATCCTGGACAATACCTCGGAG | CTCCACAGCATCAAGAGACTGC |
| CPT1A (mouse) | GGCATAAACGCAGAGCATTCCTG | CAGTGTCCATCCTCTGAGTAGC |
| GAPDH (human) | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| GAPDH (mouse) | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
The JC-1 Mitochondrial Membrane Potential Detection kit (ab113850; Abcam, Cambridge, United Kingdom) was used to measure the mitochondrial membrane potential (ΔΨm). After incubation the cells were examined using a fluorescence microplate reader and washed with PBS. In cells with high ΔΨm, JC-1 fluoresces red (about 590 nm) instead of green (about 530 nm), indicating healthy mitochondria. The red/green fluorescence ratio was calculated with a lower ratio reflecting a reduction in ΔΨm. Each experiment was performed thrice to guarantee data accuracy and repeatability.
Inflammatory cytokine levels in the spinal cord tissues were quantified using ELISA kits. The supernatants were collected. Supernatants were transferred to a 96-well plate pre-coated with specific antibodies and incubated with the detection antibody and streptavidin-HRP.
NSCs were collected, and cytoplasmic and nuclear RNA fractions were isolated using the PARIS™ Kit (Thermo Fisher Scientific, MA, United States). Before reverse transcription into cDNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, CA, United States), RNA purity and integrity were assessed using a NanoDrop spectrophotometer and agarose gel electrophoresis, respectively. qPCR was performed to evaluate XIST expression levels in both the cytoplasmic and nuclear fractions using U6 snRNA and GAPDH as nuclear and cytoplasmic controls, respectively. The primer sequences for U6 snRNA were as follows: Forward: 5’-CTCGCTTCGGCAGCACA-3’ and reverse primer: 5’-AACGCTTCACGAATTTGCGT-3’.
To investigate the relationship between XIST and CPT1A, we used the Magna RIPTM RNA-Binding Protein Immunoprecipitation kit (Millipore, MA, United States). NSCs were lysed and incubated overnight at 4 °C with magnetic beads attached to either an anti-CPT1A antibody (ab220789; Abcam, Cambridge, United Kingdom) or control IgG antibody (ab200699; Abcam, Cambridge, United Kingdom). After extensive washing the RNA-protein complexes were eluted, purified, and reverse transcribed into cDNA. The association between XIST and CPT1A was confirmed by qPCR using XIST-specific primers, which showed that XIST RNA was considerably enriched in samples immunoprecipitated with the CPT1A antibody compared to the IgG control.
An ATP assay kit (ab83355; Abcam, Cambridge, United Kingdom) was used to measure the amount of ATP in the spinal cord samples. After homogenizing the tissues in assay buffer, the supernatants were employed for analysis after the tissues were centrifuged for 5 min at 4 °C at 12000 × g. In a 96-well plate the supernatant was mixed with ATP reaction mix. All experiments were performed in triplicate to guarantee accuracy, and ATP quantities were determined by comparing the luminescence findings to a reference curve.
NSCs were treated with actinomycin D (5 μg/mL) to inhibit RNA synthesis and harvested at 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, and 16 h post-treatment. The TRIzol reagent was used to extract total RNA and reverse transcribe it into cDNA. The expression of the target mRNAs were quantified by qPCR, using GAPDH as a normalization control.
Forty-eight 8-week-old C57BL/6N mice, weighing 20-25 g, were individually housed in a specific pathogen-free-grade facility under controlled conditions of 22-25 °C and 60%-65% relative humidity. The mice were kept under a 12-h light/dark cycle and had unlimited access to food and drinks. After a 1-week acclimation period, the experimental procedures began following a health assessment of the mice. The mice were randomly split into four groups of 12 animals each: (1) Sham: Mice that did not undergo spinal cord transection surgery and did not receive saline injections; (2) SCI: Mice underwent spinal cord transection surgery and received saline injections at four sites around the injured area using a microinjection pump; (3) Negative control (NC): Mice underwent SCI surgery followed by intrathecal injection of 20 nmol/mL NC (empty lentiviral vector) (SCI + NC) at four sites around the injured area using a microinjection pump[21,22]; and (4) XIST (SCI + XIST): Mice underwent SCI surgery and received intrathecal injections of 20 nmol/mL XIST lentiviral vector at four sites around the injured area using a microinjection pump. The animals were treated for 3 days.
Mice scheduled for SCI surgery were administered intraperitoneal injections of xylazine (5 mg/kg) or ketamine (70 mg/kg) to induce anesthesia. The fur was shaved and the skin was sanitized with iodine tincture and 70% ethanol when the corneal response was no longer present. An incision was made dorsal to the midline to expose the paravertebral muscles. Laminectomy was performed at the T9-T10 vertebral level. Following surgery, the mice were housed in warm cages with free access to food and drinks to aid recovery. Body weight was recorded before surgery and 6 weeks post-surgery. Six weeks later, the mice were perfused with 4% paraformaldehyde for 20 min and approximately 2 mm of spinal cord tissue was harvested from the injury site for analysis.
GraphPad Prism (version 9.0) and IBM SPSS (version 23.0) were used for statistical analysis. Data are shown as the
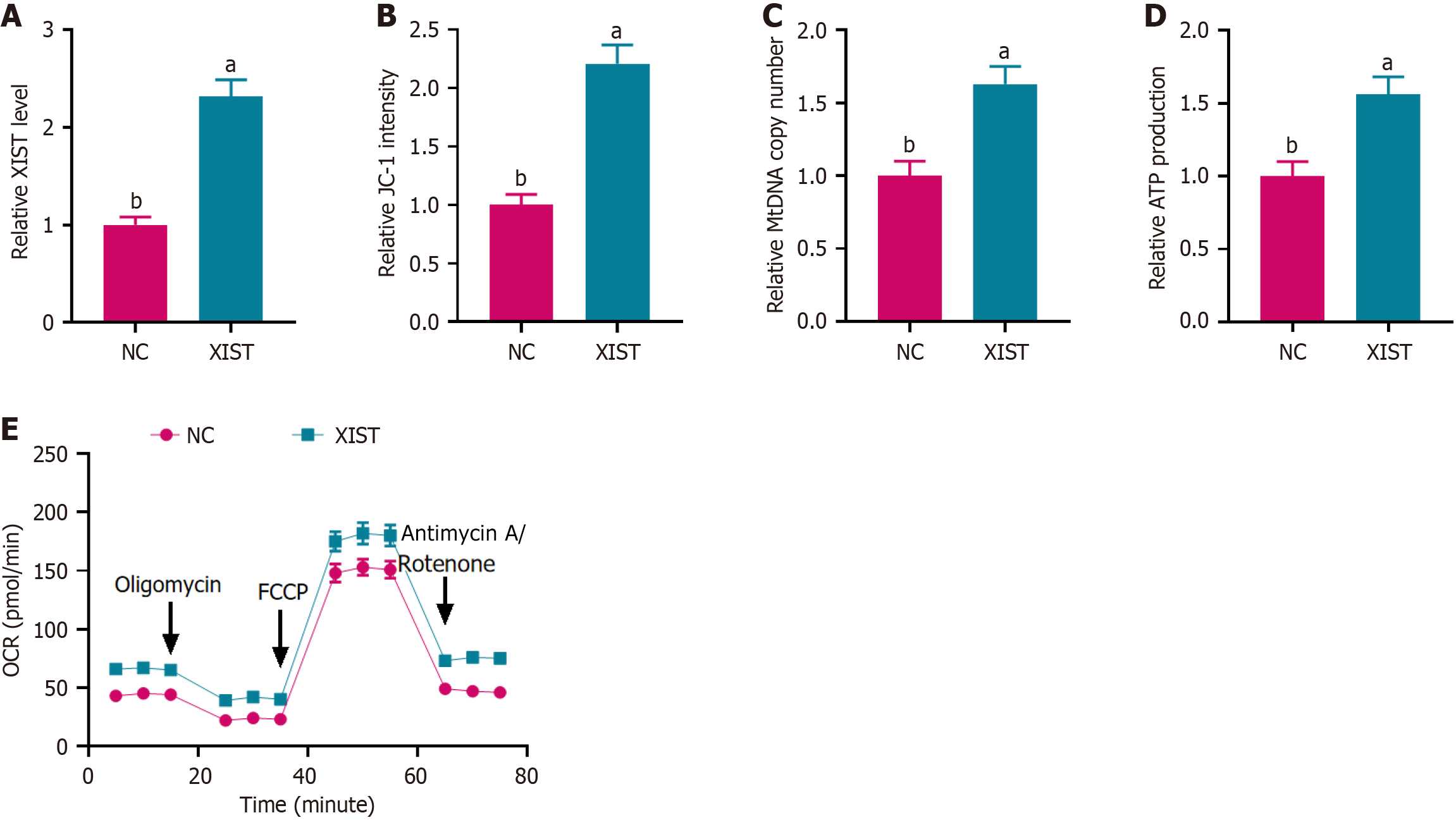
To explore the role of XIST in NSCs, we first overexpressed XIST and assessed its transfection efficiency by PCR. XIST expression was significantly upregulated in the XIST overexpression group compared with the NC group (Figure 1A).
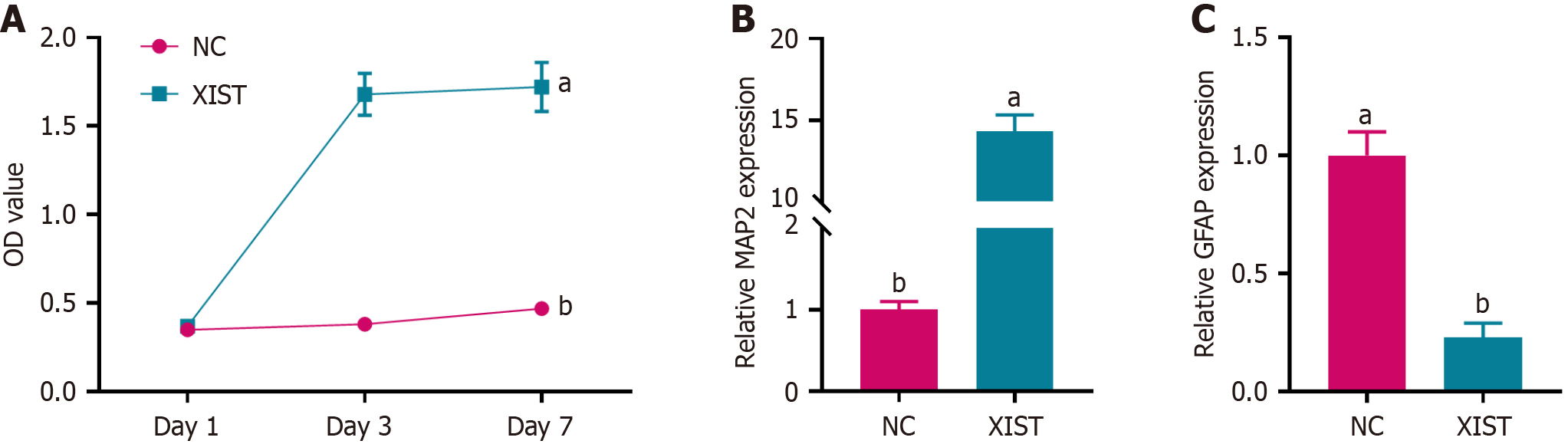
To assess the effect of XIST on NSC proliferation, we performed a CCK-8 assay. As shown in Figure 2A, NSCs transfected with XIST exhibited a significant increase in cell proliferation compared with the NC group, particularly on days 3 and 7. Furthermore, we examined the expression of neuronal and astrocytic markers using RT-PCR on day 7. Figure 2B shows that the expression of microtubule-associated protein 2 (MAP2), a neuronal marker, was a 3.2-fold increase in the XIST group compared with the NC group. Conversely, the expression of glial fibrillary acidic protein (GFAP), an astrocytic marker, was significantly downregulated in the XIST group (Figure 2C). XIST enhanced NSC proliferation and promoted neuronal differentiation while inhibiting astrocytic differentiation.
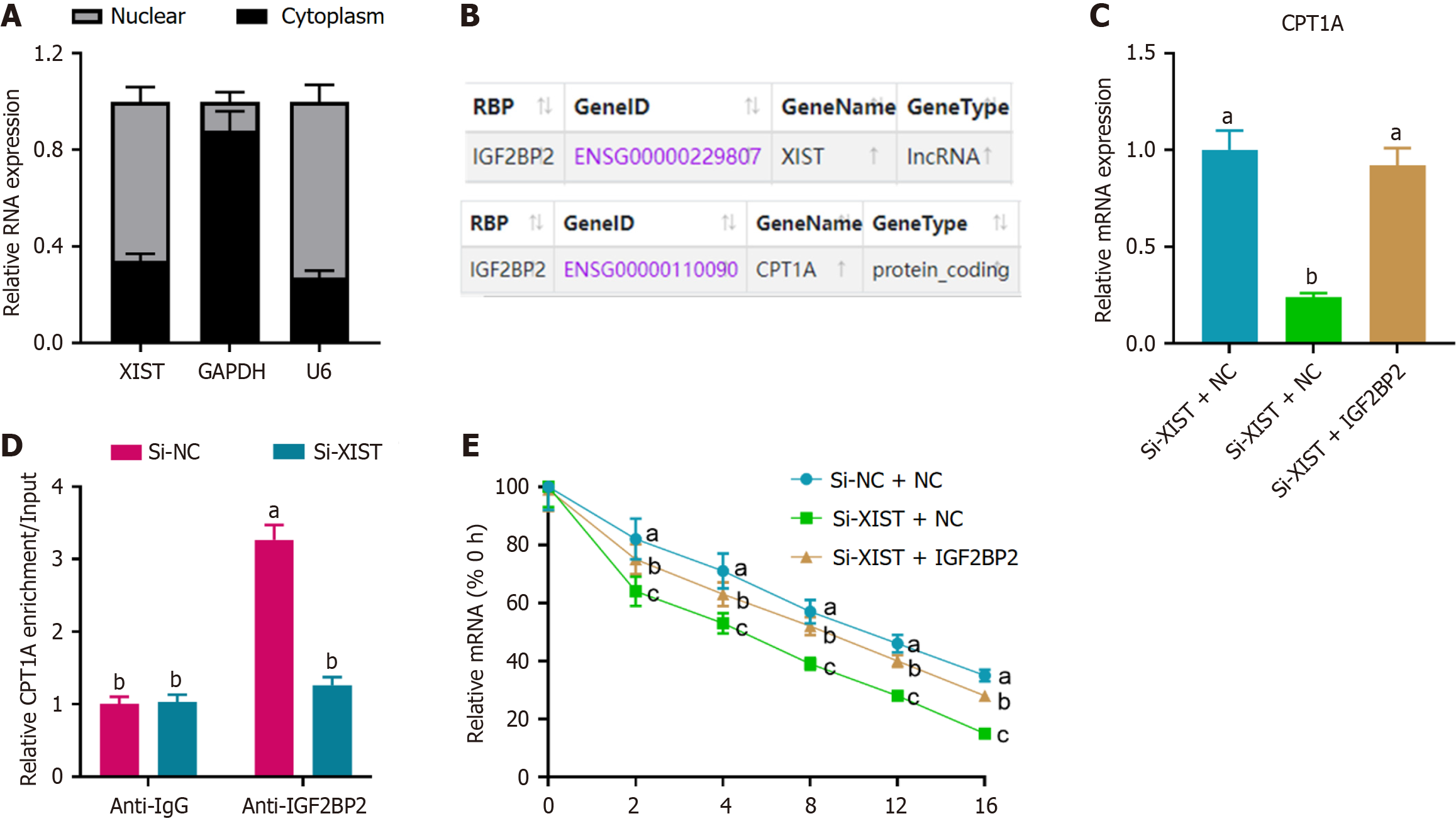
To elucidate the mechanism by which XIST modulated OXPHOS, we determined the subcellular localization of XIST in NSCs. Cytoplasmic and nuclear RNA fractionation experiments revealed that XIST was primarily localized in the nucleus of the NSCs with a smaller portion present in the cytoplasm (Figure 3A). Using StarBase, we predicted that XIST regulates CPT1A expression via the IGF2BP2 pathway (Figure 3B). To investigate the effect of XIST on CPT1A expression, we performed RT-PCR, which showed that XIST knockdown significantly reduced CPT1A expression. This effect was reversed by IGF2BP2 knockdown (Figure 3C). Additionally, RNA immunoprecipitation assays demonstrated an interaction between XIST and CPT1A, revealing that CPT1A is enriched in IGF2BP2. This interaction was diminished by XIST knockdown (Figure 3D). Finally, we assessed the effects of XIST on CPT1A mRNA stability. Knockdown of XIST reduced CPT1A mRNA stability, an effect that was reversed by IGF2BP2 knockdown (Figure 3E). XIST regulated CPT1A post-transcriptional expression by interacting with IGF2BP2.
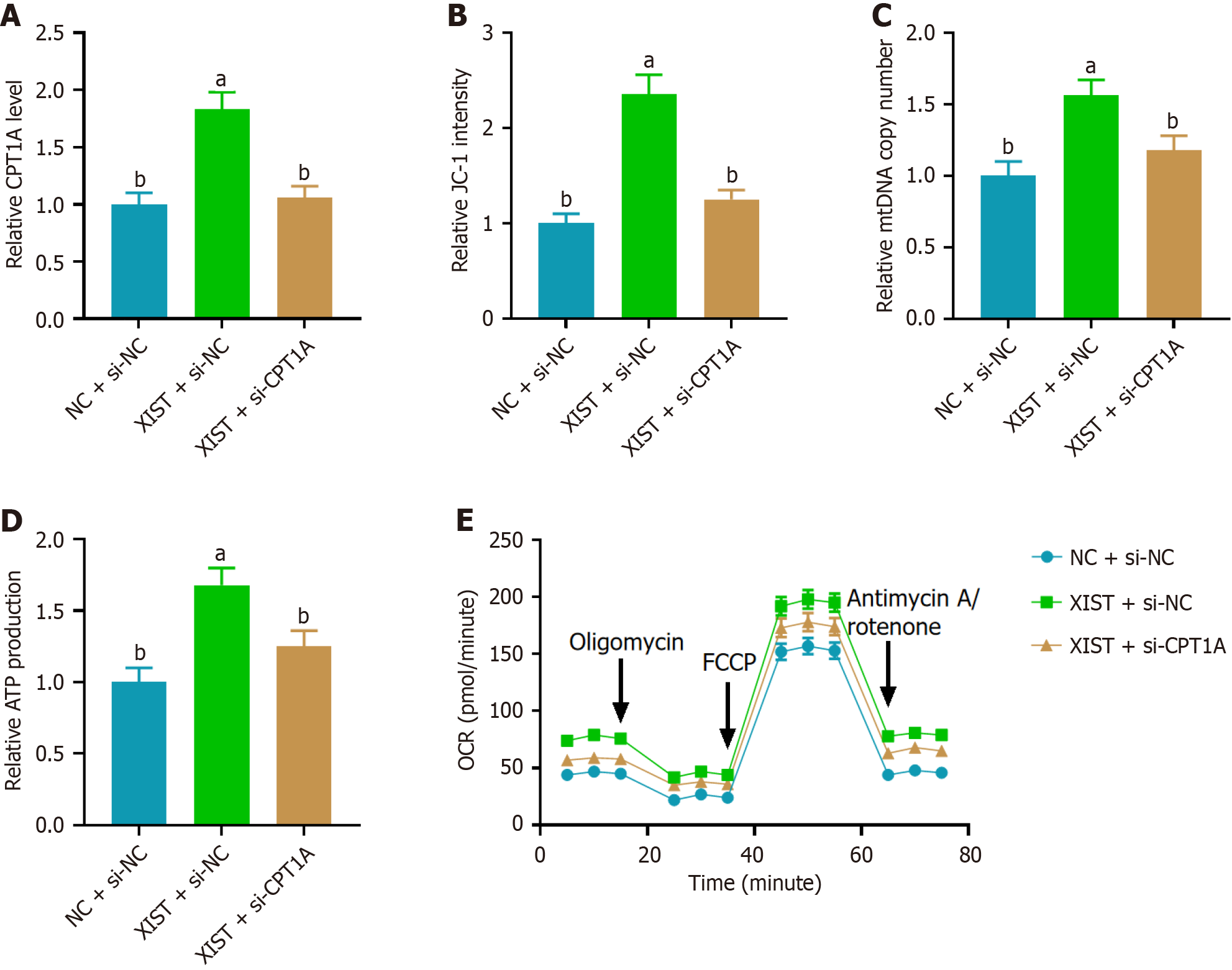
To investigate the role of CPT1A in mediating the effects of XIST on mitochondrial function, we first assessed CPT1A expression by RT-PCR. As shown in Figure 4A, XIST overexpression resulted in a 2.5-fold increase in CPT1A expression, while CPT1A knockdown (si-CPT1A) reversed this effect. Next, we evaluated ΔΨm using the JC-1 assay, which measures the red/green fluorescence ratio. XIST overexpression increased approximately 1.9-fold JC-1 fluorescence, indicating enhanced ΔΨm, whereas si-CPT1A reversed this effect (Figure 4B). We further assessed the mitochondrial function by measuring the relative mtDNA copy number. XIST overexpression led to a 2.2-fold increase in relative mtDNA copy number, which was reversed by CPT1A knockdown (Figure 4C). ATP production was measured to evaluate mito
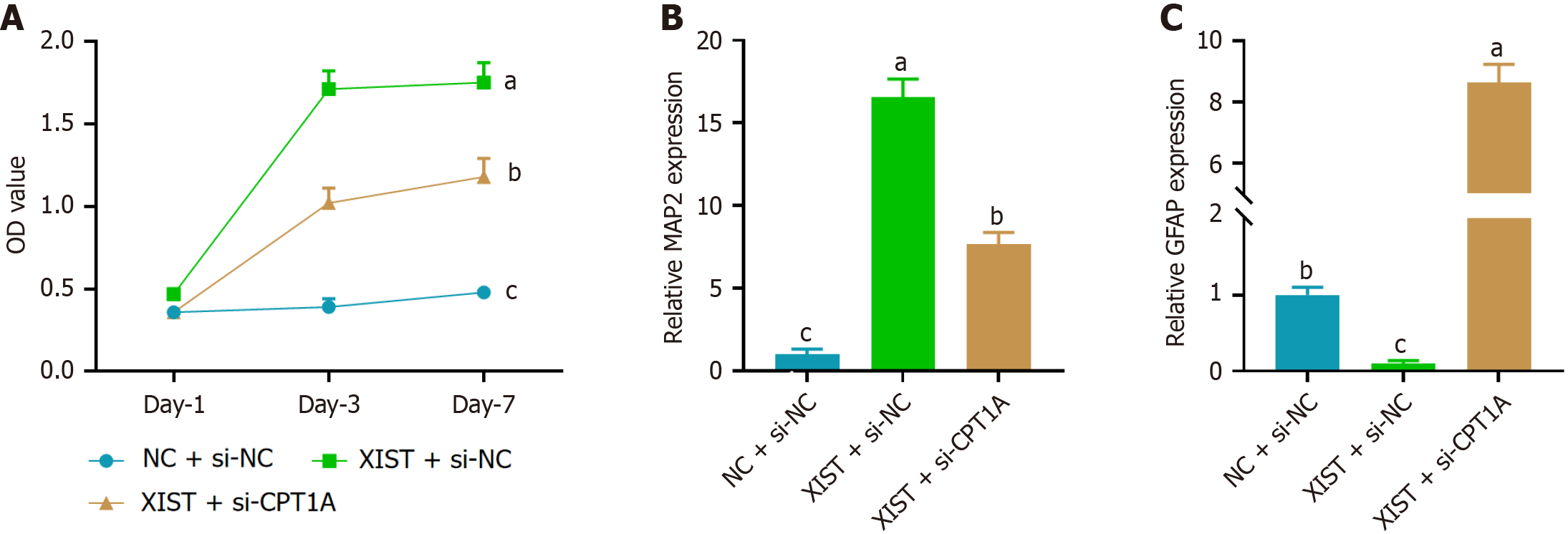
To assess the effect of CPT1A on XIST-mediated NSC proliferation and differentiation, we first performed a CCK-8 assay. As shown in Figure 5A, XIST overexpression significantly enhanced NSC proliferation, which was reversed by si-CPT1A. Next, we examined the expression of neuronal and astrocytic markers on day 7 by RT-PCR. XIST overexpression led to a marked increase in the expression of the neuronal marker MAP2 (Figure 5B) while simultaneously reducing the expression of the astrocytic marker GFAP (Figure 5C). CPT1A knockdown reversed these effects, demonstrating that CPT1A is essential for the function of XIST in enhancing NSC proliferation and neuronal differentiation while supp
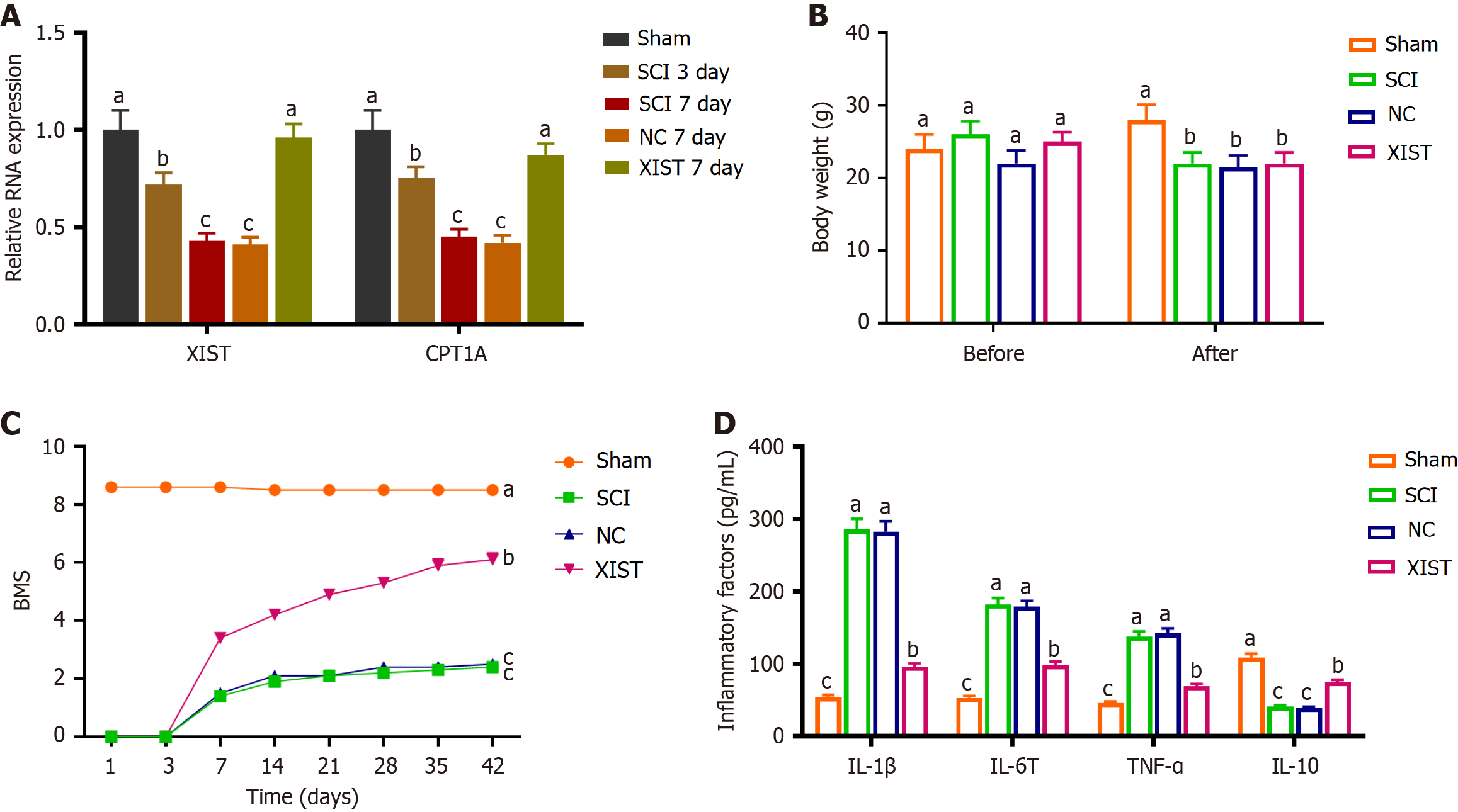
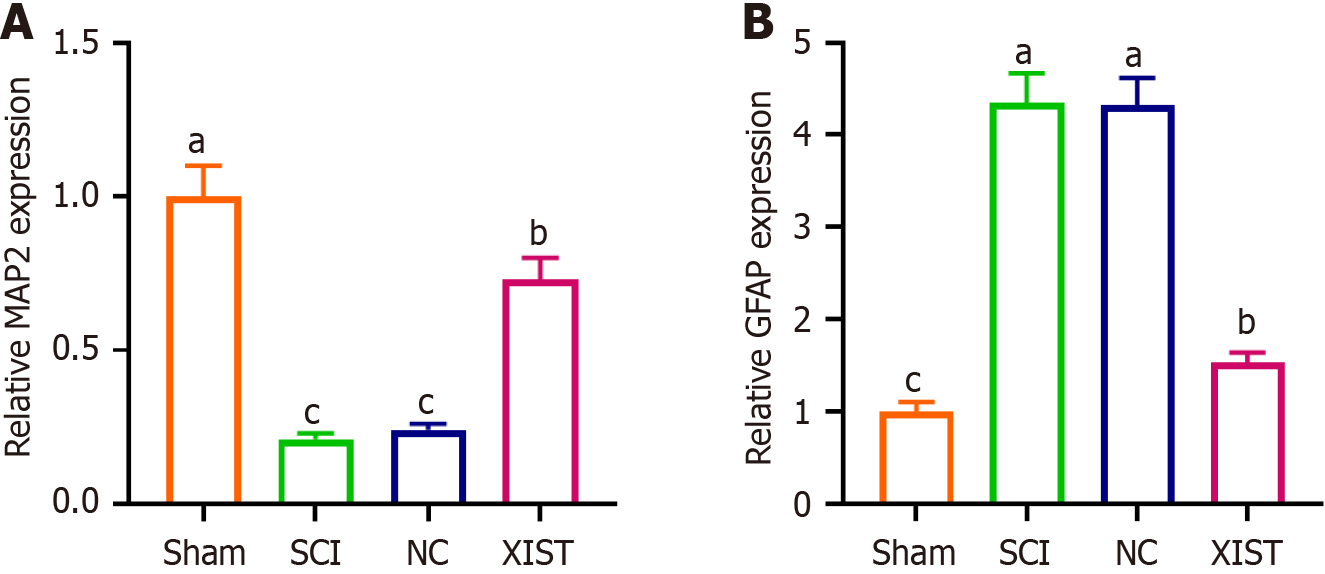
The expression of XIST and CPT1A markedly decreased on days 3 and 7 after SCI. Conversely, no significant differences in XIST and CPT1A expression were observed between the SCI day 7 group and NC day 7 group. These findings indicated a temporal reduction in the expression of these genes post-injury with the expression levels stabilizing by day 7. Notably, compared with the NC day 7 group, XIST and CPT1A expression was elevated in the XIST day 7 group (Figure 6A). The body weights of the mice were recorded preoperatively and 6 weeks postoperatively. Prior to the surgical procedure, no notable variations in body weight were observed between the groups. No notable variations in body weight were detected between the SCI, NC, and XIST groups. This suggests that the treatments administered to the NC and XIST groups did not cause weight alterations in SCI mice (Figure 6B). The Basso Mouse Scale (BMS) scores decreased significantly in the SCI group compared with those in the sham group. No notable variations in the BMS scores were observed between the SCI and NC groups. However, BMS scores were considerably higher in the XIST group than in the NC group (Figure 6C). The results indicated an increase in inflammatory markers in the SCI model, which was reversed by XIST treatment (Figure 6D). Furthermore, RT-PCR analysis of MAP2 and GFAP expression indicated that MAP2 expression decreased considerably, whereas GFAP expression increased considerably in the SCI group. Treatment with XIST reversed these effects (Figure 7).
This study provided significant insights into the role of XIST in regulating mitochondrial OXPHOS and neuronal differentiation in NSCs and its potential therapeutic implications in SCI. These findings demonstrated that XIST via the IGF2BP2/CPT1A pathway enhanced mitochondrial function, promoted NSC proliferation, and facilitated neuronal differentiation, alleviating SCI. These results expanded our understanding of the molecular pathways involved in NSC-mediated neural repair. XIST may serve as a candidate regulatory molecule for further exploration in SCI models.
Research has shown that XIST overexpression in NSCs can improve mitochondrial function, including enhanced mitochondrial membrane potential and increased ATP production[23]. This suggests that XIST is integral to maintaining mitochondrial homeostasis and is crucial for the proliferation and differentiation of NSCs[24]. Mitochondrial dysfunction is a well-established contributor to the pathophysiology of SCI as it exacerbates neuronal death and impedes the regeneration of neural tissues[25]. In the present study XIST overexpression mitigated these detrimental effects by enhancing mitochondrial function and promoting neural repair and functional recovery after SCI. This aligns with findings suggesting that interventions targeting mitochondrial health could potentially enhance recovery after SCI[26]. In addition to its effects on mitochondrial function, XIST overexpression influences the fate of NSCs by promoting neuronal differentiation and inhibiting astrocytic differentiation. This dual role of XIST in enhancing mitochondrial function and directing NSC differentiation towards a neuronal lineage is of potential therapeutic value as it can improve the efficacy of NSC-based therapies for SCI by increasing the generation of neurons and reducing the formation of glial scar tissue, which often impedes neural regeneration. Moreover, recent research has shown that the inhibition of lncRNA XIST can promote M2 polarization of microglia, thereby aggravating SCI via the miR-124-3p/interferon regulatory factor 1 axis[27]. This highlights the complex role of XIST in SCI where its inhibition may exacerbate injury through inflammatory pathways in contrast to its protective role in mitochondrial function and NSC differentiation. Integrating these findings with those of our study suggests that the role of XIST in SCI is multifaceted, affecting both inflammatory responses and cellular metabolism.
XIST modulates the expression of CPT1A, a key enzyme involved in mitochondrial FAO, by interacting with IGF2BP2. CPT1A knockdown reversed the beneficial effects of XIST overexpression on mitochondrial function and NSC differentiation, highlighting the importance of the IGF2BP2/CPT1A pathway in mediating the effects of XIST. This finding is particularly significant as it provides a novel mechanistic link between XIST and mitochondrial regulation in NSCs that could be exploited for therapeutic interventions in SCI. By interacting with IGF2BP2 XIST can influence metabolic pathways crucial for effective mitochondrial function and stem cell differentiation[28]. CPT1A knockdown reverses the beneficial effects of XIST overexpression on mitochondrial function[29]. This finding suggests that the role of CPT1A is not merely supplementary but is fundamental to the mechanisms through which XIST exerts its effects. The implications of this are profound as they demonstrate that disruptions in CPT1A expression can negate the positive effects of XIST, highlighting its crucial role in CC-induced mitochondrial regulation[16,29].
The involvement of IGF2BP2 in this pathway indicates its critical role in mediating the effects of XIST on CPT1A and mitochondrial function. IGF2BP2 stabilizes mRNAs that encode mitochondrial components and regulate energy metabolism, thereby steering NSC fate decisions toward differentiation and functionality[28]. This illustrates how IGF2BP2 not only serves as a bridge between XIST and CPT1A but also reinforces the hypothesis that these interactions are vital for maintaining metabolic homeostasis within stem cells[30-32].
While our study provided novel insights into the role of XIST in regulating mitochondrial function and directing NSC differentiation, several limitations must be acknowledged. First, the functional assessment of SCI recovery relied on BMS scores and inflammatory cytokine measurements, which although widely accepted do not provide direct cellular or histological evidence of neuronal regeneration. Second, while our in vitro results demonstrated a clear increase in MAP2 and decrease in GFAP following XIST overexpression, we did not quantify the exact proportions of differentiated cell types or perform in vivo histological analyses such as immunohistochemistry or lineage tracing to confirm these outcomes within the spinal cord microenvironment. Third, we did not employ conditional XIST knockout models or additional functional assays to further validate the observed effects. These limitations do not detract from the mechanistic value of our findings, but we acknowledge that additional in vivo and translational studies are necessary to fully define the therapeutic potential of XIST in SCI[33]. Moreover, although this study identified the IGF2BP2/CPT1A pathway as a key mediator of the effects of XIST, other potential pathways and interacting partners may also contribute to its role in NSCs. To gain a more thorough understanding of how XIST controls NSC function and aids SCI recovery, future studies should attempt to clarify these processes.
This study provided compelling evidence that lncRNA XIST via the IGF2BP2/CPT1A pathway enhances mitochondrial function and promotes neuronal differentiation in NSCs, thereby offering a potential therapeutic strategy for SCI. However, further studies are required to validate these findings in more complex models and explore additional pathways involved in XIST-mediated neural repair.
| 1. | Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80:S9-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 585] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 2. | Lee S, Nam H, Joo KM, Lee SH. Advances in Neural Stem Cell Therapy for Spinal Cord Injury: Safety, Efficacy, and Future Perspectives. Neurospine. 2022;19:946-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Gao L, Peng Y, Xu W, He P, Li T, Lu X, Chen G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020;2020:2853650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Guo W, Zhang X, Zhai J, Xue J. The roles and applications of neural stem cells in spinal cord injury repair. Front Bioeng Biotechnol. 2022;10:966866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 5. | Richart L, Picod-Chedotel ML, Wassef M, Macario M, Aflaki S, Salvador MA, Héry T, Dauphin A, Wicinski J, Chevrier V, Pastor S, Guittard G, Le Cam S, Kamhawi H, Castellano R, Guasch G, Charafe-Jauffret E, Heard E, Margueron R, Ginestier C. XIST loss impairs mammary stem cell differentiation and increases tumorigenicity through Mediator hyperactivation. Cell. 2022;185:2164-2183.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Wutz A. Xist function: bridging chromatin and stem cells. Trends Genet. 2007;23:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Xu K, Li YD, Ren LY, Song HL, Yang QY, Xu DL. Long non-coding RNA X-Inactive Specific Transcript (XIST) interacting with USF2 promotes osteogenic differentiation of periodontal ligament stem cells through regulation of WDR72 transcription. J Periodontal Res. 2023;58:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Yang X, Zhang S, He C, Xue P, Zhang L, He Z, Zang L, Feng B, Sun J, Zheng M. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 9. | Sado T, Hoki Y, Sasaki H. Tsix silences Xist through modification of chromatin structure. Dev Cell. 2005;9:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Wu XJ, Xie Y, Gu XX, Zhu HY, Huang LX. LncRNA XIST promotes mitochondrial dysfunction of hepatocytes to aggravate hepatic fibrogenesis via miR-539-3p/ADAMTS5 axis. Mol Cell Biochem. 2023;478:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Liu C, Liu Y, Ma B, Zhou M, Zhao X, Fu X, Kan S, Hu W, Zhu R. Mitochondrial regulatory mechanisms in spinal cord injury: A narrative review. Medicine (Baltimore). 2022;101:e31930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Fico A, Fiorenzano A, Pascale E, Patriarca EJ, Minchiotti G. Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cell Mol Life Sci. 2019;76:1459-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Slater PG, Domínguez-Romero ME, Villarreal M, Eisner V, Larraín J. Mitochondrial function in spinal cord injury and regeneration. Cell Mol Life Sci. 2022;79:239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Rabchevsky AG, Michael FM, Patel SP. Mitochondria focused neurotherapeutics for spinal cord injury. Exp Neurol. 2020;330:113332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Chew S, Kolosowska N, Saveleva L, Malm T, Kanninen KM. Impairment of mitochondrial function by particulate matter: Implications for the brain. Neurochem Int. 2020;135:104694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Mørkholt AS, Oklinski MK, Larsen A, Bockermann R, Issazadeh-Navikas S, Nieland JGK, Kwon TH, Corthals A, Nielsen S, Nieland JDV. Pharmacological inhibition of carnitine palmitoyl transferase 1 inhibits and reverses experimental autoimmune encephalitis in rodents. PLoS One. 2020;15:e0234493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Audano M, Pedretti S, Crestani M, Caruso D, De Fabiani E, Mitro N. Mitochondrial dysfunction increases fatty acid β-oxidation and translates into impaired neuroblast maturation. FEBS Lett. 2019;593:3173-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Morant-Ferrando B, Jimenez-Blasco D, Alonso-Batan P, Agulla J, Lapresa R, Garcia-Rodriguez D, Yunta-Sanchez S, Lopez-Fabuel I, Fernandez E, Carmeliet P, Almeida A, Garcia-Macia M, Bolaños JP. Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat Metab. 2023;5:1290-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 19. | Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 691] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 20. | Wang GY, Xu X, Xiong DY, Deng L, Liu W, Huang XT. CPT1A as a potential therapeutic target for lipopolysaccharide-induced acute lung injury in mice. Sci Rep. 2024;14:1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Miguel V, Tituaña J, Herrero JI, Herrero L, Serra D, Cuevas P, Barbas C, Puyol DR, Márquez-Expósito L, Ruiz-Ortega M, Castillo C, Sheng X, Susztak K, Ruiz-Canela M, Salas-Salvadó J, González MAM, Ortega S, Ramos R, Lamas S. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J Clin Invest. 2021;131:e140695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 22. | Zhu D, Peng T, Zhang Z, Guo S, Su Y, Zhang K, Wang J, Liu C. Mesenchymal stem cells overexpressing XIST induce macrophage M2 polarization and improve neural stem cell homeostatic microenvironment, alleviating spinal cord injury. J Tissue Eng. 2024;15:20417314231219280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Li X, Gao Y, Tian F, Du R, Yuan Y, Li P, Liu F, Wang C. miR-31 promotes neural stem cell proliferation and restores motor function after spinal cord injury. Exp Biol Med (Maywood). 2021;246:1274-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Bai G, Jiang L, Meng P, Li J, Han C, Wang Y, Wang Q. LncRNA Neat1 Promotes Regeneration after Spinal Cord Injury by Targeting miR-29b. J Mol Neurosci. 2021;71:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Park JY, Wang PY, Matsumoto T, Sung HJ, Ma W, Choi JW, Anderson SA, Leary SC, Balaban RS, Kang JG, Hwang PM. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ Res. 2009;105:705-712, 11 p following 712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Czermiński JT, Lawrence JB. Silencing Trisomy 21 with XIST in Neural Stem Cells Promotes Neuronal Differentiation. Dev Cell. 2020;52:294-308.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Yang T, Ou J, Yildirim E. Xist exerts gene-specific silencing during XCI maintenance and impacts lineage-specific cell differentiation and proliferation during hematopoiesis. Nat Commun. 2022;13:4464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 28. | Schmidt J, Quintá HR. Mitochondrial dysfunction as a target in spinal cord injury: intimate correlation between pathological processes and therapeutic approaches. Neural Regen Res. 2023;18:2161-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Scholpa NE, Schnellmann RG. Mitochondrial-Based Therapeutics for the Treatment of Spinal Cord Injury: Mitochondrial Biogenesis as a Potential Pharmacological Target. J Pharmacol Exp Ther. 2017;363:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Yang J, Gong Z, Dong J, Bi H, Wang B, Du K, Zhang C, Chen L. lncRNA XIST inhibition promotes M2 polarization of microglial and aggravates the spinal cord injury via regulating miR-124-3p / IRF1 axis. Heliyon. 2023;9:e17852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Cao J, Mu Q, Huang H. The Roles of Insulin-Like Growth Factor 2 mRNA-Binding Protein 2 in Cancer and Cancer Stem Cells. Stem Cells Int. 2018;2018:4217259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 32. | Tang M, Dong X, Xiao L, Tan Z, Luo X, Yang L, Li W, Shi F, Li Y, Zhao L, Liu N, Du Q, Xie L, Hu J, Weng X, Fan J, Zhou J, Gao Q, Wu W, Zhang X, Liao W, Bode AM, Cao Y. CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma. Cell Death Dis. 2022;13:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 33. | Hosseini SM, Borys B, Karimi-Abdolrezaee S. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Brain. 2024;147:766-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |