Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.102421
Revised: January 23, 2025
Accepted: March 3, 2025
Published online: April 26, 2025
Processing time: 188 Days and 1.7 Hours
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) can traverse the blood-brain barrier due to their small size. This characteristic makes them a research hotspot for the treatment of Parkinson’s disease (PD) and is expected to be a potentially revolutionary strategy for treating PD. Despite this, no summary of clinical trial results has been reported.
To assess the efficacy and durability of MSC-EVs in treating PD.
Systematic searches were conducted in four electronic databases until June 2024 to collect studies on the use of MSC-EVs for this purpose. Thirteen relevant randomized controlled trials, encompassing 16 experiments, were selected for inclusion.
Behavioral assessments, including the rotarod and apomorphine turning behavior tests, indicated improvements in motor coordination (P < 0.00001); the Pole test and the Wire-hang test showed enhanced limb motor agility and synchronization (P = 0.003 and P < 0.00001, respectively). Histopathologically, there was a reduction in inflammatory markers such as tumor necrosis factor-α and interleukin-6 (P = 0.03 and P = 0.01, respectively) and an increase in tyrosine hydroxylase-positive cells in the lesion areas (P < 0.00001).
MSC-EV therapy for PD is a gradual process, with significant improvements observable more than 2 weeks after administration and lasting at least 8 weeks. This study is the first to demonstrate the efficacy and durability of MSC-EV treatment in PD.
Core Tip: Mesenchymal stem cell extracellular vesicles (MSC-EVs) have become a popular direction for tissue engineering research in recent years and have been shown to have many advantages, especially in neurological diseases. Unfortunately, the treatment of Parkinson’s disease (PD) with MSC-EVs is still in the clinical void stage due to insufficient guideline protocols. Our study was the first to validate the efficacy and durability of MSC-EVs in the treatment of PD and to draw a number of valuable guiding conclusions. The results of our analyses are of great reference value for future clinical translation of stem cell exosomes for PD.
- Citation: Wang XS, Wang Y, Xu Y, Zhang SR, Zhang Y, Peng LL, Wu N, Ye JS. Effectiveness of mesenchymal stem cell-derived extracellular vesicles therapy for Parkinson’s disease: A systematic review of preclinical studies. World J Stem Cells 2025; 17(4): 102421
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/102421.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.102421
Parkinson’s disease (PD), the second most prevalent neurodegenerative disorder globally, poses a significant health threat to individuals over the age of 65[1]. It is characterized by mental disorders such as depression and anxiety, cognitive decline, and motor difficulties including resting tremors and impaired initiation of movement. Current interventions aimed at slowing the progression of PD encompass pharmacological and nonpharmacological treatments. Pharmacological approaches, primarily involving levodopa as dopamine replacement therapy[2], are associated with numerous side effects during long-term use[3]. Conversely, nonpharmacological treatments are tailored to the patient’s specific dysfunctions, focusing on rehabilitation and physical therapy for movement[4], speech[5], and swallowing[6,7]. However, existing treatments neither halt nor reverse the progression of the disease[8], highlighting the critical need for novel and effective therapies. Stem cells are currently showing amazing potential as a drug replacement therapy for PD[9]. Mesenchymal stem cells (MSCs), as a type of adult stem cells, seem to be more advantageous in the treatment of neurodegenerative diseases due to their reduced risk of immune rejection and fewer ethical concerns[10].
The transfer of toxic α-synuclein (α-syn) oligomers and protofibrils, similar to prions, between cells are implicated in the pathogenesis of the disease[11-13]. Recent studies indicate that MSCs not only enhance α-syn clearance[14] but also ameliorate PD through neurotropism, autophagy modulation, and immunomodulation[15]. However, like most therapeutic agents, MSCs cannot cross the blood-brain barrier[16]. In recent years, research has revealed that the beneficial effects of MSCs in treating PD are largely due to MSC extracellular vesicles (MSC-EVs)[17-19].
EVs with diameters of 40-100 nm contain biologically active substances such as microRNA (miRNA), lipids, and proteins[20]. MSC-EVs not only promote cell proliferation and multidirectional differentiation but also modulate the inflammatory microenvironment and mitigate inflammatory diseases through the delivery of nucleic acids, cytokines, chemokines, and immunomodulatory factors[21]. Compared to treatments using the cells themselves, using MSC-EVs for PD reduces the risk of side effects such as uncontrolled differentiation of MSCs and potential activation of allogeneic immune responses[22]. Additionally, MSC-EVs can cross the blood-brain barrier and capillaries, migrate, and persist at brain lesion sites, thereby reducing inflammation and potentially repairing damaged neurons. This is a result difficult to achieve with cell therapy alone[23,24]. Animal studies suggest that MSC-EVs may be more effective than levodopa in treating PD[25]. Currently, MSC-EVs as an innovative treatment for PD is undergoing preclinical studies worldwide. This systematic review and meta-analysis was conducted to assess the effectiveness of MSC-EVs in treating PD, along with potential precautions and therapeutic mechanisms.
The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis[26]. The registration number for this study is CRD42024534456.
Two researchers (Wang XS and Wang Y) searched PubMed, Embase, Cochrane Library, and Web of Science databases using the medical subject headings terms “extracellular vesicles”, “exosomes”, and “Parkinson’s disease” from the inception of these databases to April 10, 2024. Detailed search terms and arithmetic symbols were integral to the literature search strategy (Supplementary Table 1). Additionally, references from the literature were manually searched for potential studies.
Studies were selected based on the PICOS criteria: Participant (P): Animals modeled to exhibit PD, irrespective of species, age, or sex. Intervention (I): The intervention consisted of MSC-EVs. Comparison (C): Controls included injections of phosphate buffered saline, saline, or no treatment. Outcome (O): Assessments included behavioral tests such as the rotarod, apomorphine turning behavior, Pole, and Wire-hang tests, histopathological evaluation of tyrosine hydroxylase (TH)-positive cell percentages in the substantia nigra and striatum, and measurements of immune markers interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-β. Study design (S): Only randomized controlled trials were included.
The exclusion criteria included: (1) Non-English articles; (2) Studies that were not randomized controlled trials, such as case reports, reviews, conference presentations, letters, surveys, or satisfaction studies; (3) Studies not published as full-text articles or where the full text was not downloadable; (4) Treatments using MSCs that were preconditioned, such as those involving genetically transfected cell EVs or EVs following cell differentiation, which could affect the results; and (5) Studies where animals were not sacrificed immediately post-treatment, potentially affecting outcome metrics.
Data were extracted by two researchers (Xu Y and Zhang SR) from the final included studies. Subsequently, two other researchers (Wang XS and Wang Y) organized and verified the data. Discrepancies were discussed and resolved by the entire research team. Persistent disagreements were referred to a third party (Zhang Y and Ye JS) to reach a consensus.
Studies with the following outcomes were selected for this meta-analysis. Behavioral research: (1) Rotarod test: The duration of an animal’s run on a rotating bar is recorded, providing an objective measure of motor function; (2) Apomorphine turning behavior test: Apomorphine induces abnormal turning in rats, enabling assessment of dopamine depletion in PD models by recording the number of rotations over a specified period; (3) Pole test: Used to assess motor slowing and agility in animal models of PD; and (4) Wire-hang test: Used to assess synchronization of limb movements. Histopathology research: (1) Immunohistochemical detection of the percentage of TH-positive cells in the substantia nigra and striatum; and (2) Measurement of tissue concentrations of inflammatory factors IL-6, TNF-α, and IL-β.
Basic information, such as study details and designs, was also collected. This included the first author, year, country, method of PD induction, and species. Study design details collected included the number of models in each group, route of injection, EV source, injection dose, and outcome metrics. When essential data were missing in the paper, details were requested from the corresponding author by email.
Note that when there was more than one parallel experiment in an article, we split it into two separate experiments. As in one study, multiple experimental groups had different EV injection doses or different routes of EV administration from the beginning of treatment for the model. However, when data were extracted from the same experimental group at different periods, we did not consider it as two individual studies but presented them differently in the outcome statistics and performed subgroup analysis to ensure the rigor of the analysis. Therefore, 13 studies containing 16 experiments were finally included.
The risk of bias in the included studies was assessed using the Center for Systematic Evaluation of Laboratory Animal Experiments bias risk tools. These tools evaluate several aspects, including sequence generation, baseline characteristics, allocation concealment, random housing, blinding of investigators, random outcome assessment, blinding of outcome assessor, incomplete outcome data, selective outcome reporting, and other potential sources of bias[27].
Statistical analysis was conducted using Review Manager 5.4 and Stata14 software. Results were treated as continuous variables and expressed as standardized mean differences (SMD) with 95% confidence intervals (CI). The heterogeneity of the experiments was assessed using the Cochrane Q statistic and I2 test, with P < 0.05 considered statistically significant. An I2 value greater than 50% indicated significant heterogeneity among experiments[28]. A fixed-effects model was applied in cases of homogeneity, whereas a random-effects model was employed in the presence of heterogeneity. Sensitivity analyses were conducted for the primary outcome indicator when its heterogeneity exceeded 50% to identify the source of heterogeneity. Subgroup analyses were undertaken when the data set was adequate and variables were controllable. For outcomes involving more than ten experiments, publication bias was evaluated using funnel plots and Egger’s test. The stability of the conclusions was verified using the trim-and-fill method if publication bias was detected. When data were presented as statistical plots, means and standard deviations or standard errors were extracted using Engauge Digitizer. Mean standard error values were converted to standard deviations for the final analysis.
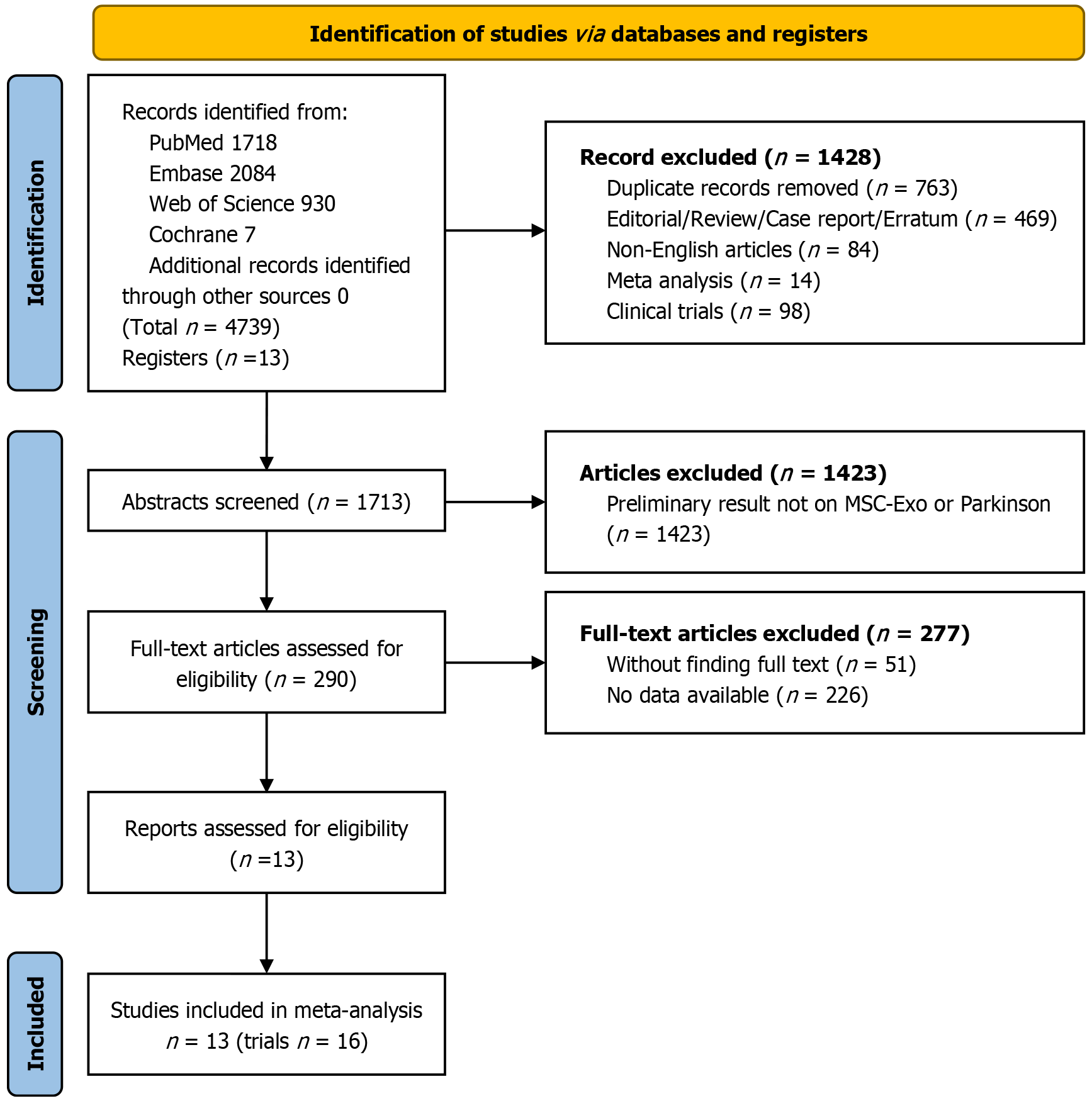
A search of PubMed, Embase, Web of Science, and Cochrane Library databases using medical subject headings terms and free words yielded 1718, 2084, 930, and 7 studies, respectively. No additional studies were identified through manual searching. Thirteen studies, comprising 16 independent trials, were included in the final analysis, as illustrated in Figure 1.
Given that EV therapy is an emerging approach for PD, all included studies were conducted between 2019 and 2023. These studies originated from four countries: China[29-36], Latvia[37,38], Egypt[25,39], and Portugal[18]. All published articles utilized rodents as research models. The total sample size was 265 animals, with 143 treated using MSC-EVs and the remainder receiving placebo or no treatment. Seven of the thirteen studies employed the 6-hydroxydopamine (6-OHDA) model for inducing PD, three utilized the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model, two used the rotenone model, and one study involved A53T transgenic mice. Two studies employed varying routes of injection and MSC-EV doses[35,36] and were treated as independent trials. Four studies reported assessment results at different timepoints post-MSC-EV treatment[18,29,32,35]. Since the data were extracted from the same set of models, these were not divided into separate trials but were instead presented in the results and analyzed in subgroup analyses. Detailed information is available in Supplementary Table 2.
Of the 13 studies included, only two specifically reported the method of randomization, while the others merely noted “random” and were thus deemed unclear. Six studies provided detailed information that experimental and control groups were comparable at baseline; the others did not specify. Only one study reported concealed grouping. Nine studies documented the random housing of animals, maintaining identical conditions and environments across all experiments. No studies detailed the blinding of animal keepers or the randomization of outcome assessment. Two studies reported blinding of outcome evaluators, and three confirmed that all animals were included in the final analysis; the rest were indistinguishable. Twelve studies reported all expected outcomes, but the results of one study partially deviated from the anticipated outcomes. No studies disclosed other potential sources of bias. Overall, the methodological quality of these experiments was deemed reliable and acceptable (Supplementary Table 3).
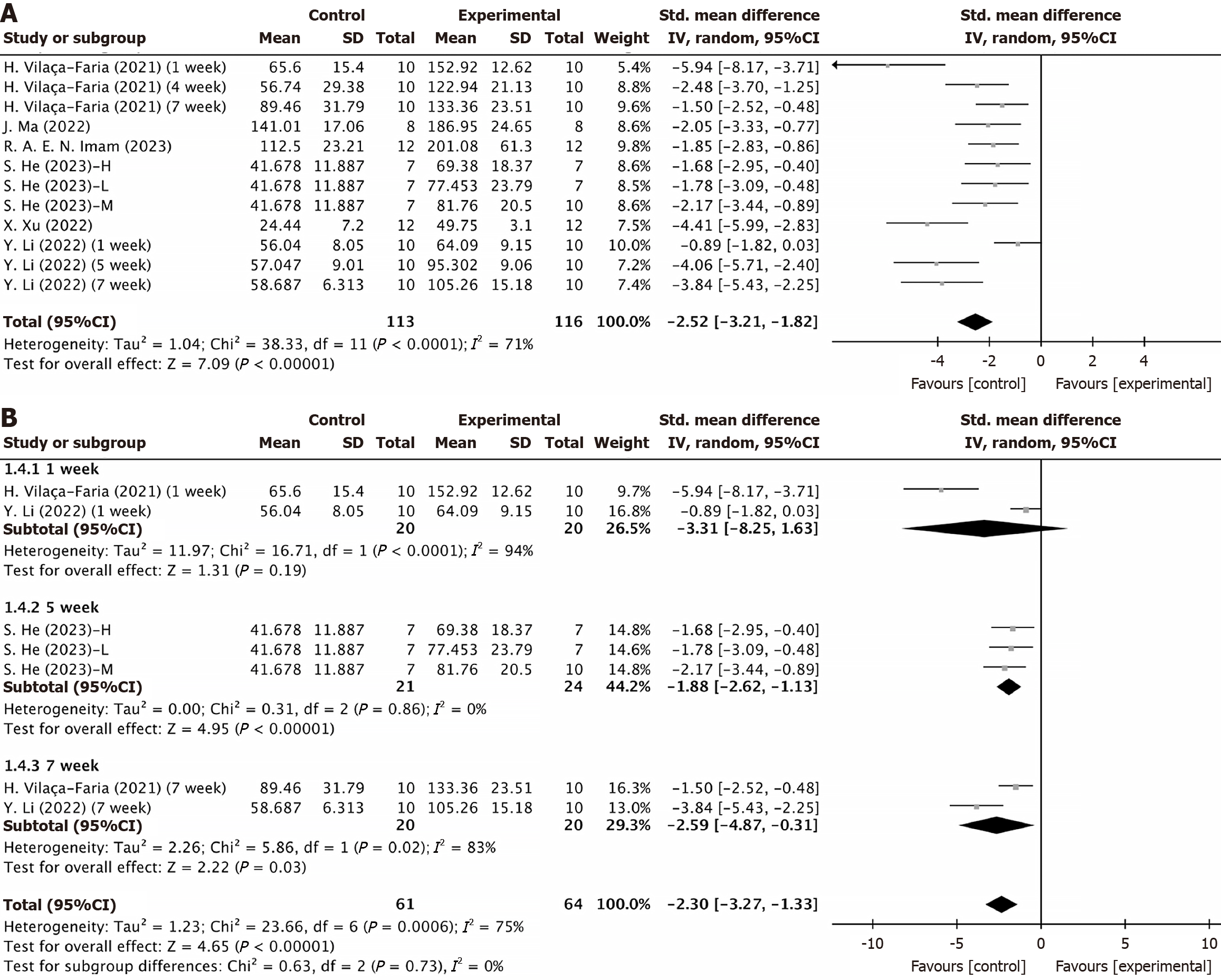
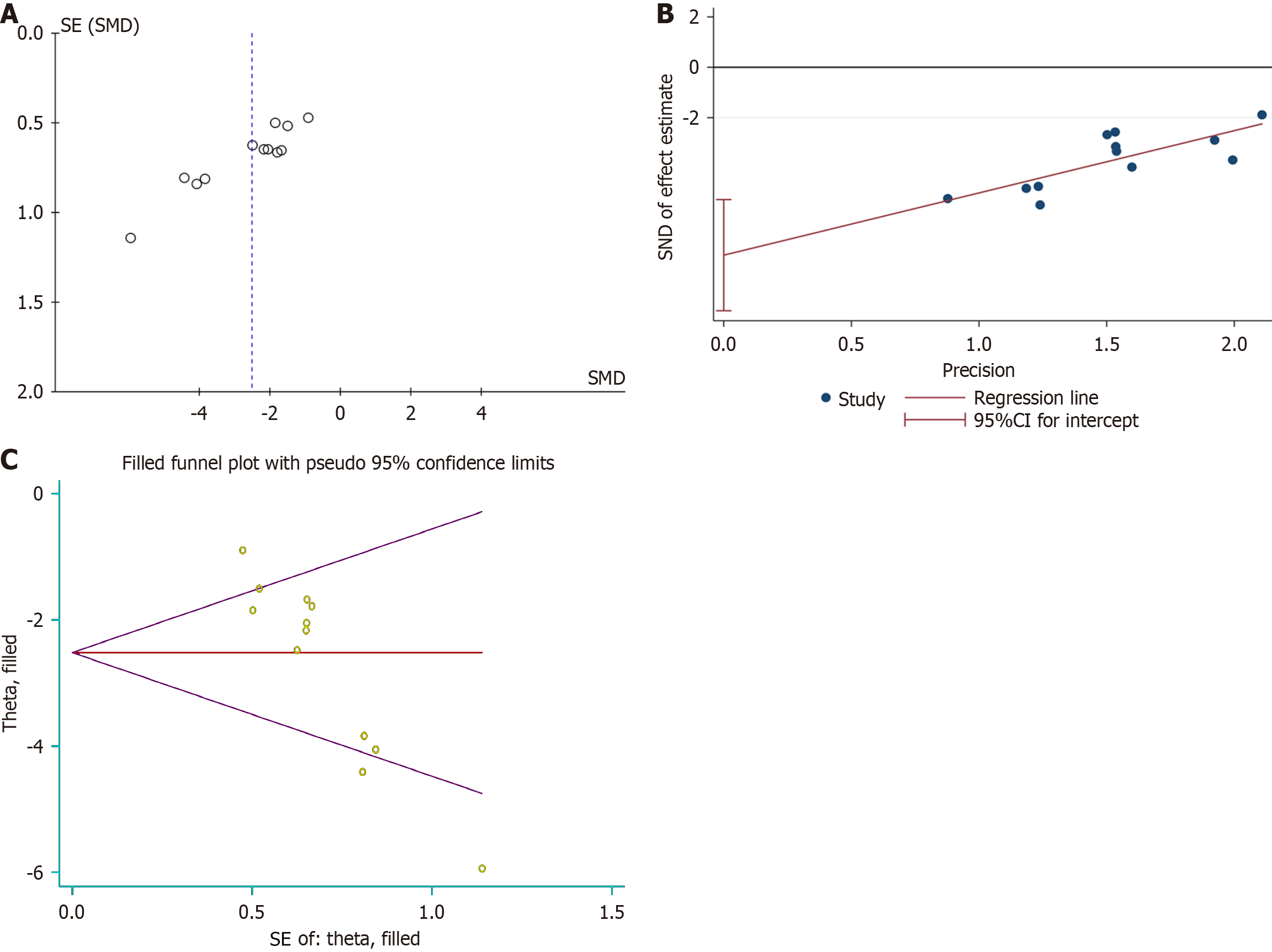
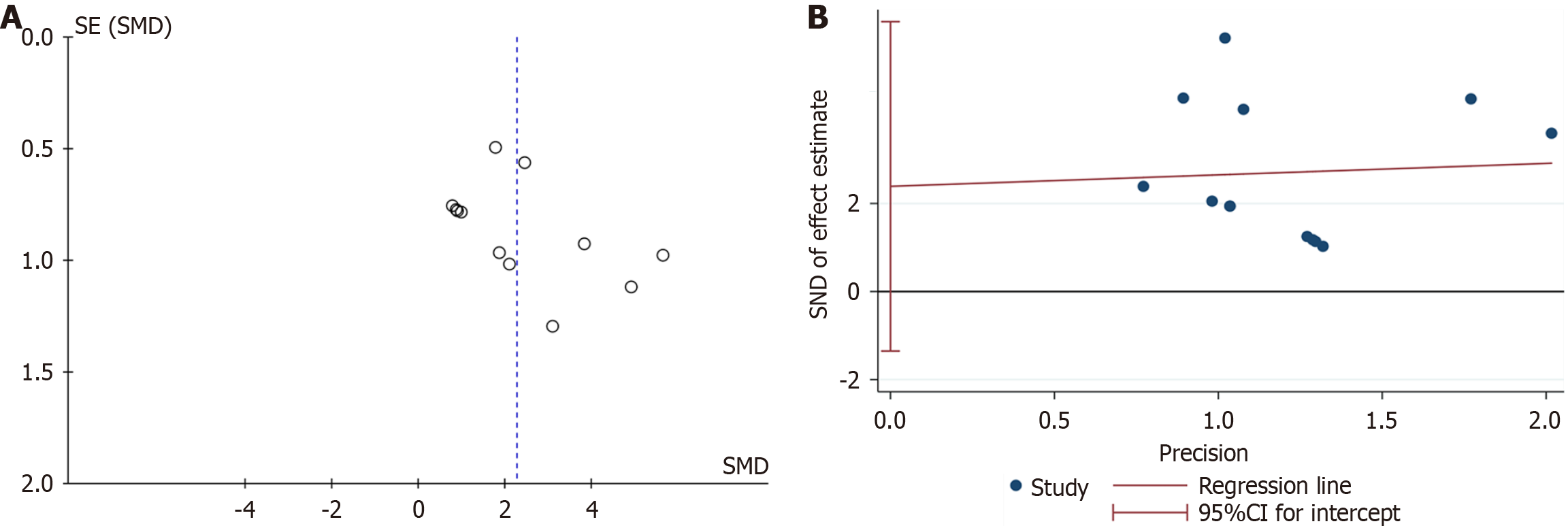
Rotarod test: Six studies, encompassing eight experiments, reported rotarod test results for both the experimental and control groups. Two of these studies provided results at different timepoints for the same models[18,32], resulting in a total of 12 experimental records. The Q test and I2 test indicated significant heterogeneity among studies (P < 0.0001, I2 = 71% > 50%). The random-effects model showed: SMD = -2.51, 95%CI: -3.21 to -1.82, P < 0.00001 (Figure 2A). Subgroup analyses based on various injection routes, EV sources, and follow-up times identified no sources of heterogeneity (Supplementary Figure 1A and B, Figure 2B). At the 1-week follow-up, no significant differences were observed between the groups (P = 0.19 > 0.05). However, significant differences emerged at the 5-week and 7-week follow-ups (P < 0.00001 and P = 0.03 < 0.05) (Figure 2B). Despite heterogeneity, sensitivity analysis confirmed a stable statistical difference between the groups (Supplementary Figure 2A). Funnel plots indicated asymmetry (Figure 3A), and Egger’s test (t =
| Method | Pooled Est | 95%CI | Asymptotic | No. of studies | ||
| Lower | Upper | z value | P value | |||
| Fixed | 0.112 | 0.078 | 0.161 | -11.835 | 0.000 | 12 |
| Random | 0.081 | 0.040 | 0.162 | -7.094 | 0.000 | |
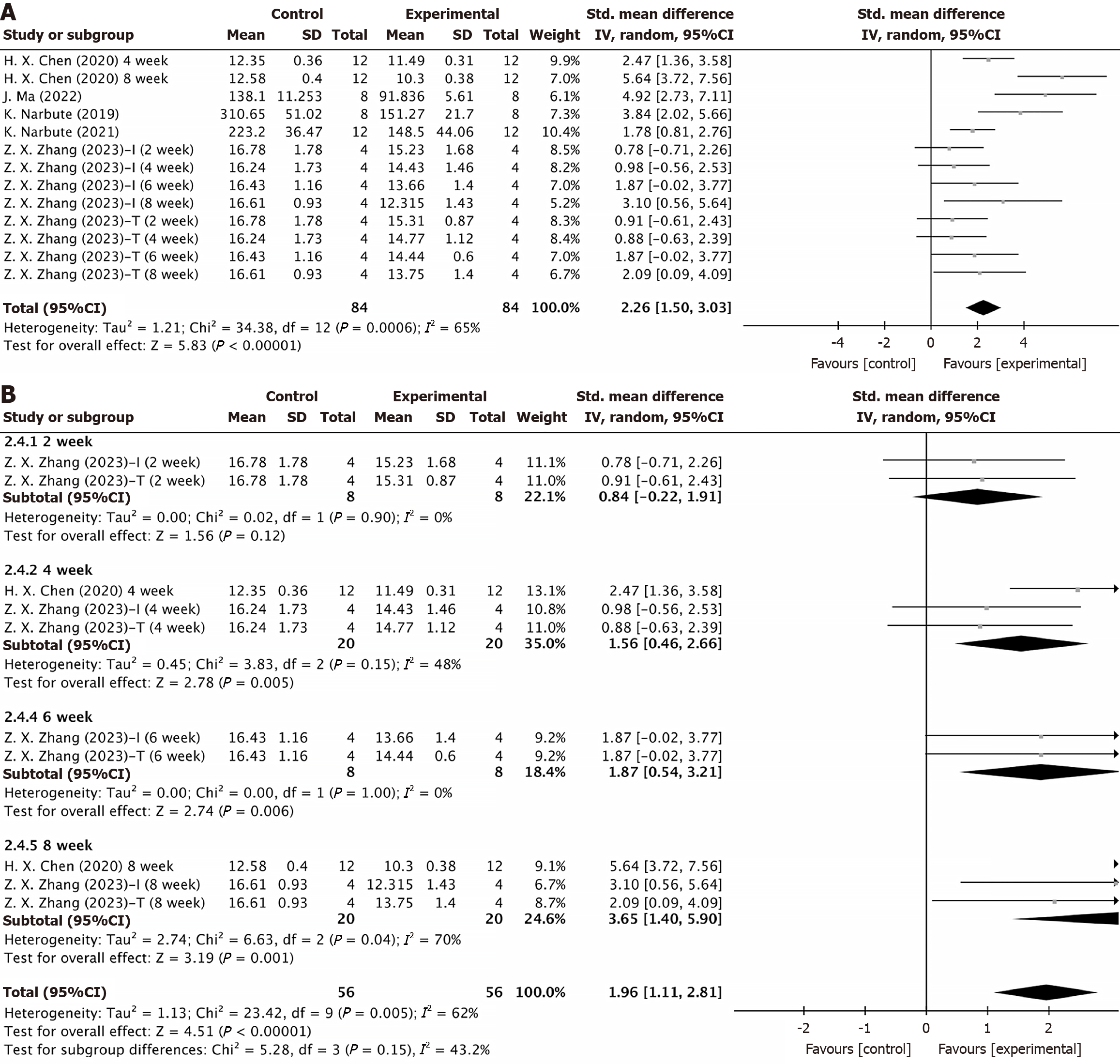
Apomorphine turning behavior test: Five studies, encompassing six experiments, conducted the apomorphine turning behavior test on two groups of animals. Data from two studies were collected at different timepoints[29,35], resulting in 13 experimental records included in the statistical analysis. The results showed that the duration of turning behavior was significantly shorter in the EV group than in the control group (SMD = 2.26, 95%CI: 1.50-3.03, P < 0.00001). However, the results displayed considerable heterogeneity (P < 0.0001, I2 = 65%) (Figure 4A). Subgroup analyses by injection route, EV source, and post-treatment follow-up time did not reveal sources of heterogeneity (Supplementary Figure 3A and B, Figure 4B). The subgroup analysis indicated no significant difference at week 2 post-treatment (P = 0.12 > 0.05), but significant differences were observed at weeks 4, 6, and 8 (P = 0.005, P = 0.006, and P = 0.001) (Figure 4B). Sensitivity analysis confirmed the stability of these results (Supplementary Figure 2B). The funnel plot indicated no significant publication bias (Figure 5A), and Egger’s test (t = 1.41, P = 0.187 > 0.05) confirmed the absence of publication bias (Figure 5B). These results suggested that MSC-EV intervention was effective, with stable and reliable outcomes.
Pole test: A meta-analysis of seven experiments demonstrated a statistically significant difference in the Pole test between the treatment and control groups (SMD = 1.09, 95%CI: 0.38-1.80, P = 0.003 < 0.05) (Supplementary Figure 4A). High heterogeneity was observed between the groups (I2 = 64% > 50%). However, excluding the study by Bai et al[31] reduced the heterogeneity significantly (I2 = 43% < 50%) (Supplementary Figure 4B), identifying it as the source of heterogeneity. Overall, the analysis confirmed that the MSC-EV intervention produced reliable statistical differences between the two groups.
Wire-hang test: Two studies, including four experiments, reported results for the Wire-hang test. The meta-analysis revealed a statistically significant difference between the two groups (SMD = -1.53, 95%CI: -2.08 to -0.97, P < 0.00001) (Supplementary Figure 5). The absence of increased heterogeneity due to differences in the injected dose (I2 = 0% < 50%) supports the reliability of the behavioral test results, indicating that EVs significantly improved the behavior of the animals.
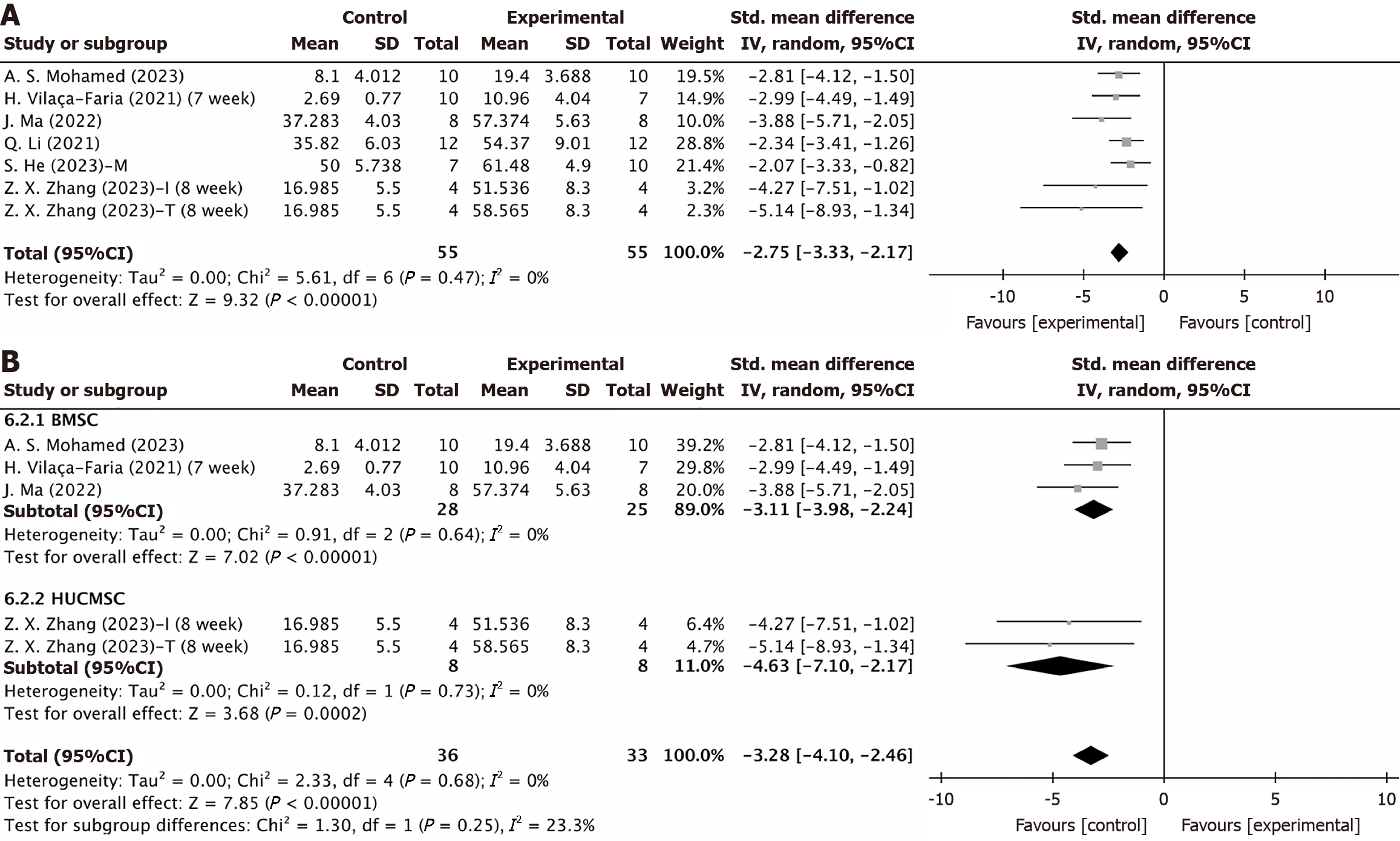
Number of TH-positive cells in the substantia nigra: TH, the rate-limiting enzyme for dopamine synthesis in neurons, serves as the gold standard for identifying dopamine neurons. Seven experiments involving 119 animals assessed the number of TH-positive cells in the substantia nigra, revealing a significant therapeutic effect following MSC-EV injection (SMD = -2.75, 95%CI: -3.33 to -2.17, P < 0.0001) with low heterogeneity (P = 0.47, I2 = 0% < 50%) (Figure 6A). EVs from various MSC sources did not significantly alter the efficacy of MSC-EVs in restoring TH levels (Figure 6B), indicating that EVs can regulate dopamine levels by increasing the number of TH-positive cells in the substantia nigra, irrespective of the source of the EVs.
Number of TH-positive cells in the striatum: Two studies reported on the percentage of TH-positive cells in the striatum, showing that EVs significantly enhanced TH levels in the striatum of Parkinsonian animals (SMD = -3.69, 95%CI: -4.49 to -2.89, P < 0.00001), with stable and reliable results (I2 = 0% < 50) (Supplementary Figure 6).
Inflammatory factor: Two studies measured plasma concentrations of IL-6, IL-β, and TNF-α. One study, conducted by Li et al[32], evaluated these markers at weeks 1, 5, and 7. The results demonstrated that MSC-EVs effectively reduced plasma concentrations of IL-6 and TNF-α in Parkinsonian animals (IL-6: SMD = 1.57, 95%CI: 0.35-2.79, P = 0.01 < 0.05; TNF-α: SMD = 1.49, 95%CI: 0.17-2.82, P = 0.03 < 0.05) (Supplementary Figure 7A and B). MSC-EVs did not improve IL-β concentrations (P = 0.08 > 0.05). However, a statistical difference emerged between the two groups when the record from Li et al[32] at week 1 was excluded (P = 0.03 < 0.05) (Supplementary Figure 8A and B).
PD, a prevalent neurodegenerative disorder, is marked by movement impairments such as dyskinesia and rigidity. Pathologically, it is characterized by the loss of dopamine neurons in the substantia nigra and the accumulation of α-syn. Initially, α-syn exists as non-toxic soluble monomers, but upon oxidative stress it aggregates into oligomers and eventually forms toxic Lewy bodies[40].
In recent years, stem cell-derived EVs have emerged as significant in treating neurodegenerative diseases, potentially revolutionizing therapy for conditions like PD[41,42]. Behavioral tests (rotarod test, apomorphine turning behavior test, Pole test, Wire-hang test) demonstrated that MSC-EV intervention significantly improved motor function, agility, limb synchronization, and balance in Parkinsonian animals[43-45]. Studies on its therapeutic mechanisms utilized two main animal models: Neurotoxin-induced and transgenic. We included both models in our analysis, involving neurotoxins like 6-OHDA, MPTP, and rotenone as well as A53T transgenic mice. MPTP is capable of rapidly inducing motor symptoms in PD and is suitable for evaluating the degree of improvement in motor function during the treatment of PD with MSC-EVs. However, it may not be suitable for assessing the neuroprotective effects of MSC-EVs due to its insignificant pathological changes[46]. 6-OHDA is able to induce more pronounced neuronal damage in animals and is suitable for testing the neuroprotective effects of MSC-EVs. In addition, its unilateral damage feature facilitates the assessment of MSC-EV efficacy through behavioral tests (e.g., the apomorphine turning behavior test)[47]. Rotenone is able to induce pathological features in animals that more closely resemble those of PD in humans, making it suitable for long-term studies of the intervention effects of MSC-EVs on neurodegenerative processes[48]. The A53T gene-editing model, on the other hand, is more suitable for studying the effects of MSC-EVs on α-syn pathology[49]. Overall, however, all of the studies we included validated the effectiveness of MSC-EVs in treating PD.
MSC-EVs can inhibit the developmental process of PD by expressing biologically active substances that act as messengers transmitting biological signals between cells under both normal and pathophysiological conditions. In addition, MSC-EVs not only have similar therapeutic characteristics to MSCs but also exhibit low immunogenicity, long-term safety, and no cytotoxicity. These features confer significant advantages, as MSC-EVs cannot transform into malignant precursor cells. Most importantly, as a cellular secretagogue, MSC-EVs raise fewer ethical concerns and therefore have potential advantages for the treatment of PD. EVs from various MSC sources, including dental pulp, bone marrow, umbilical cord, adipose tissue, and trophoblasts, were analyzed. Subgroup analysis showed that regardless of the source, treatment with MSC-EVs consistently resulted in significant improvements compared to control groups. Further analysis confirmed that the source of MSCs did not significantly impact the positive treatment outcomes for PD.
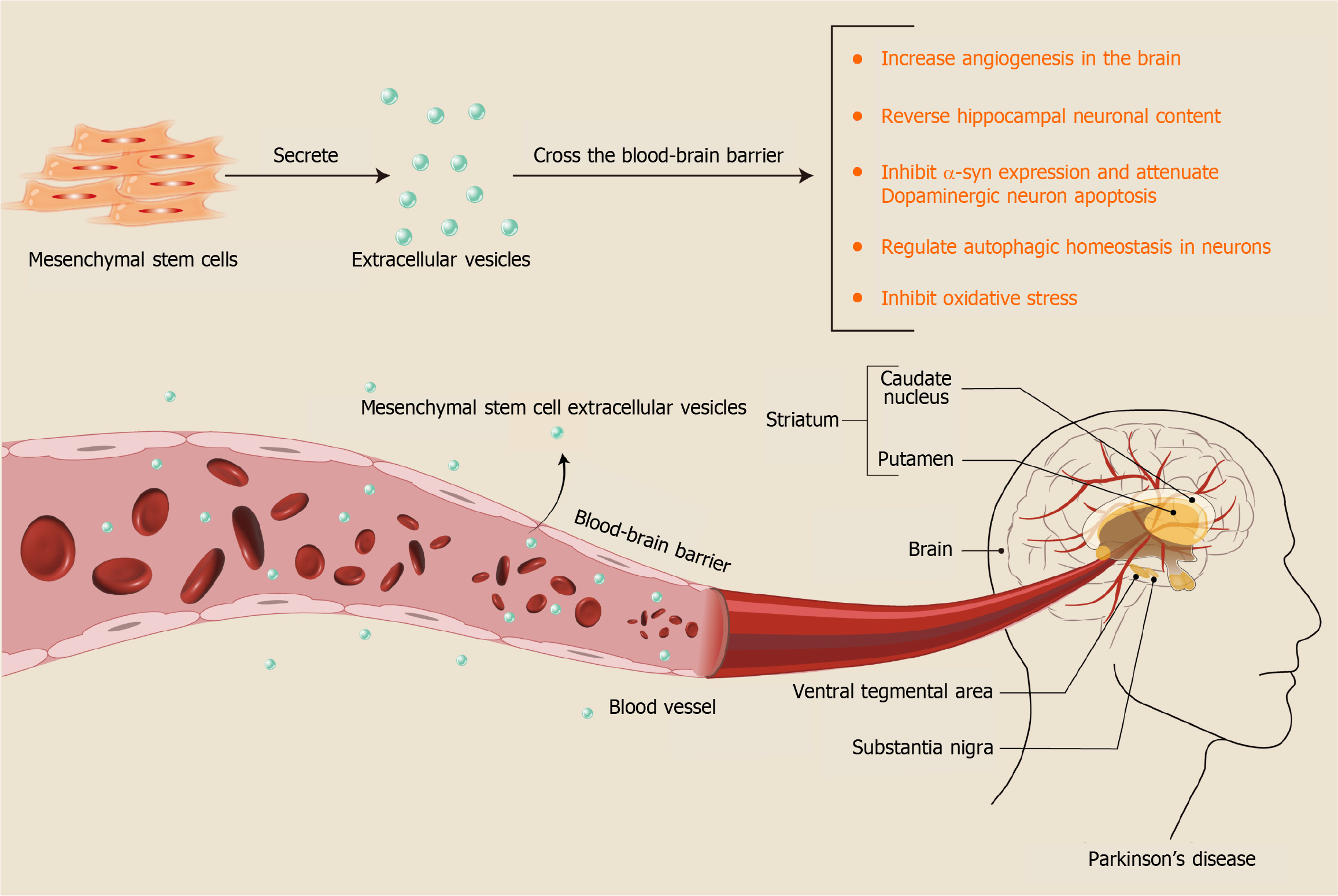
The route of injection is also critical. Results from various administration routes including the tail vein, nasal, and intracranial confirmed the effectiveness of MSC-EVs. However, research suggests that nasal delivery might bypass the blood-brain barrier more effectively than intravenous administration, leading to better brain accumulation[50]. Studies indicate that MSC-EVs can be rapidly transported to brain regions via olfactory neurons, predominantly through neuronal uptake, peaking after 1 h. This intervention reduces amyloid beta protein deposition and inhibits microglial cell activation[51], thereby remodeling the inflammatory microenvironment around dopamine neurons. MSC-EVs are currently under clinical trials for Alzheimer’s disease, demonstrating safety and tolerability when administered intranasally[51]. This provides insights into potential MSC-EV therapies for PD in future clinical applications.
According to previous research, the dosage of MSCs for treating diseases generally depends on the weight of the patient. Unfortunately, no unified standard exists for the dose size of EVs in PD treatment, whether in preclinical or clinical trials. The injection doses used in the studies analyzed varied, preventing a determination of whether different doses affect the effectiveness of the treatment. However, behavioral tests were conducted using EV concentrations of 2.44 × 1010, 7.32 × 1010, and 1.22 × 1011 particles/mL, and it was found that the therapeutic efficacy of MSC-EVs for PD did not depend on the injected dose[36].
Subgroup analyses based on follow-up times revealed that in the behavioral studies, no significant improvement in motor and coordination functions was observed 1 to 2 weeks after EV injection (1 week: Rotarod test: P = 0.19 > 0.05; 2 weeks: Apomorphine turning behavior test P = 0.12 > 0.05). The statistical difference between the two groups peaked at 4-5 weeks, then slightly decreased, but differences were consistently observed from week 4 to week 8. These results suggested a long-term effect of MSC-EV intervention, although extended follow-up beyond 8 weeks is necessary to assess the therapeutic durability.
TH catalyzes the conversion of tyrosine to levodopa, the first rate-limiting step in catecholamine biosynthesis, which includes dopamine. TH expression and activity directly regulate dopamine production. TH immunohistochemistry was utilized to evaluate the survival of dopaminergic neurons. Analysis of the results revealed that MSC-EV treatment increased TH-positive cells in the substantia nigra and striatum, indicating a reversal in dopamine production levels and suggesting gradual neuronal function improvement.
Inflammatory cytokine levels are elevated in the serum and cerebrospinal fluid of patients with PD. Overexpression of α-syn in these patients can induce microglial cell polarization toward a proinflammatory phenotype, resulting in the production of inflammatory cytokines such as TNF-α, IL-6, and IL-2, along with enzymes like cyclooxygenase-2 and inducible nitric oxide synthase, and the generation of free radicals[52,53]. These inflammatory factors further enhance intraneuronal α-syn transmission, perpetuating a vicious cycle[54]. Reducing α-syn aggregation has been identified as a potential effective treatment for PD[55]. The results of our meta-analysis indicated that IL-6 and TNF-α levels in PD animal tissues improved following EV intervention. However, IL-β expression did not show a significant difference between the two groups (P = 0.08). This discrepancy could be related to the initial weeks following EV treatment. When data from an early trial[32] was excluded, a significant reduction in IL-β levels was observed post-EV treatment (P = 0.03). Overall, EVs reduced inflammatory cytokine production, potentially through the downregulation of α-syn expression levels.
MSC-EV therapy for PD demonstrated significant results, primarily due to its distinct advantages. The efficacy of neurological drugs is often hindered by the blood-brain barrier. However, MSC-EVs can effectively traverse this barrier, establishing a safe and efficient system for drug delivery in neurological diseases[56]. MSC-EVs have been employed as carriers for targeted drug delivery in tissue injuries. Unlike traditional lipid carriers, EVs are abundant in functionally active components, underscoring their substantial potential for clinical application. For instance, MSC-EVs contain numerous functional miRNAs that can be released and absorbed to facilitate transcellular gene regulation[57].
N6-methyladenosine (m6A) modification, prevalent in the brain, plays a critical role in neurodevelopment and is implicated in neurodegenerative diseases due to its dysregulation[58]. MSC-EVs can modulate in vivo somatic m6A levels by inhibiting the m6A demethylase enzyme (FTO), which consequently suppresses α-syn expression and dopaminergic neuron apoptosis. This indicates that the therapeutic benefits of MSC-EVs in treating PD may partly arise through m6A modification. Researchers have administered FTO-targeted small interfering RNA via MSC-EVs in an animal model of PD. Laser scanning confocal microscopy revealed that MSC-EVs effectively penetrated the blood-brain barrier and effectively delivered si-FTO to the site of nerve injury, enhancing their capability to inhibit FTO expression and prevent dopaminergic neuronal death[19]. It has been demonstrated that mir-627-5p, a component of MSC-EVs, alleviated nonalcoholic fatty liver disease by reducing FTO expression[59]. Therefore, the inhibitory effect of MSC-EVs on FTO expression may also be mediated through the exosomal miR-627-5p.
Dysregulation of autophagy plays a crucial role in the development of PD[60]. The expression of miR-106b-3p in EVs was found to be downregulated in the blood of patients with PD, and this miRNA is closely associated with autophagy[61]. MSC-EVs, containing miR-106b-3p, could potentially treat PD by enhancing autophagy. Further, overexpressing miR-106b-3p in EVs has been shown to strengthen their ability to promote autophagy and rescue neurons[29,31]. Another study demonstrated that MSC-EV treatment significantly modulated autophagy-related biomarkers, including micro
It has also been shown that MSC-EVs reduced the expression of the oxidative stress marker 4-hydroxynonenal due to their miR-181a-2-3p content[64]. The underlying mechanism involves inhibiting oxidative stress in PD by downregulating the NADPH oxidase 4 (NOX4)/p38 mitogen-activated protein kinase axis, which is influenced by early growth response transcription factor 1[33]. Treatment using trophoblast MSC EVs demonstrated that these EVs could reduce oxidative stress by inhibiting NOX4 expression[36]. In summary, MSC-EVs intervention could treat PD by reducing NOX4 expression and thus inhibiting oxidative stress. In addition, NOX4 overexpression can inhibit intercellular adhesion molecule-1[65]. MSC-EVs may further promote cerebral angiogenesis and ultimately inhibit the development of PD by inhibiting NOX4.
An imbalance in intracellular cholesterol in neurons can induce PD by leading to tau protein phosphorylation[66]. The Wnt signaling pathway, crucial for coordinating reverse cholesterol transport and inflammatory responses, plays a role in maintaining intracellular cholesterol homeostasis. MSC-EVs also regulate phospholipid metabolism and neuronal cholesterol levels through the Wnt5a-LRP1 cascade, which reverses hippocampal neuronal degradation and thus treats PD[34].
Dopamine replacement therapy is the current gold-standard treatment for PD. Long-term usage, however, leads to reduced motor function, diminished effectiveness, and adverse effects[3]. As dopamine neurons diminish in the brain, the efficacy of dopamine medications decreases. Research suggests that MSC-EVs exerts a superior therapeutic effect compared to levodopa[25]. MSC-EVs function by reducing α-syn, enhancing miRNA-34b expression, inhibiting Lewy bodies’ aggregation, and increasing TH activity, which stimulates dopamine production. Furthermore, miRNA-34b boosts Parkin and DJ-1 production, mitigating oxidative stress in PD through enhanced glutathione synthesis[25,67]. In turn, glutathione synthesis can further inhibit the development of PD by reducing α-syn aggregation caused by lipid peroxide accumulation[68].
MSC-EVs not only enhance motor function in Parkinsonian animals but also ameliorate sleep disorders associated with the disease by increasing peroxisome proliferator-activated receptor activity in the striatum and restoring mitochondrial membrane potential balance[69]. MSC-EVs address PD through multiple pathways and mechanisms (Figure 7). Cur
Despite the noteworthy conclusions derived from this study, it is imperative to acknowledge its inherent limitations. Primarily, while the findings indicate the maintenance of the efficacy of MSC-EVs in treating PD for a period of 8 weeks, the available studies did not provide sufficient data beyond this timeframe. This limitation precludes a definitive assessment of the long-term effectiveness of the therapy. In addition, we are also concerned about the effect of the injection dose of MSC-EVs on effectiveness. While some studies have indicated that MSC-EVs do not exhibit dose-dependency in the treatment of PD, this conclusion could not be drawn from the studies included in this analysis. Finally, although MSC-EVs have a lower likelihood of triggering immune rejection compared to MSCs, the quality control and standardized production of MSC-EVs still face challenges. MSC-EVs from different sources vary in composition and function, which may affect therapeutic outcomes. Additionally, individual differences among patients should be considered in clinical applications. Future research should focus on the quality control of MSC-EVs and the development of personalized treatment protocols to ensure their safety and efficacy in clinical use.
More and more studies have concluded that avoiding progressive loss of neurons in the midbrain substantia nigra is an important aspect of treating PD. Drugs such as levodopa can only slow down the progression of the disease to a certain extent, and prolonged use may lead to side effects. Meanwhile, even cell replacement therapies currently being resea
Our findings indicated that MSC-EVs improved motor and coordination functions in animals and reduced cellular inflammatory factor levels of TNF-α, IL-β, and IL-6 in tissues. Moreover, MSC-EVs reversed TH-positive cell levels in the substantia nigra and striatum of Parkinsonian animals. Subgroup analysis revealed that neither the MSC source of EVs nor the injection route significantly impacted treatment effectiveness. Furthermore, the therapeutic response appeared not to be dose-dependent. Improvements in dyskinesia were gradual. Significant changes were not evident within the first 2 weeks post-injection, but notable improvement occurred after more than 2 weeks, with effects lasting at least 8 weeks. Ultimately, our analysis confirmed the efficacy and durability of MSC-EVs in treating PD at the animal level, yet larger preclinical studies and clinical trials are essential to corroborate these findings and establish a standard protocol for MSC-EVs in PD treatment.
| 1. | Yi Z, Mao Y, He C, Zhang Y, Zhou J, Feng XL. Medication adherence and costs of medical care among patients with Parkinson's disease: an observational study using electronic medical records. BMC Public Health. 2024;24:1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1649] [Article Influence: 329.8] [Reference Citation Analysis (0)] |
| 3. | Huang YT, Chen YW, Lin TY, Chen JC. Suppression of presynaptic corticostriatal glutamate activity attenuates L-dopa-induced dyskinesia in 6-OHDA-lesioned Parkinson's disease mice. Neurobiol Dis. 2024;193:106452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Corcos DM. Importance of upper and lower body resistance exercise for preventing and reversing sarcopenia in Parkinson's disease. Parkinsonism Relat Disord. 2024;123:106104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Lee SJ, Dvorak AL, Manternach JN. Therapeutic Singing and Semi-Occluded Vocal Tract Exercises for Individuals with Parkinson's Disease: A Randomized Controlled Trial of a Single Session Intervention. J Music Ther. 2024;61:132-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Yeo MS, Hwang J, Lee HK, Kim SJ, Cho SR. Therapeutic singing-induced swallowing exercise for dysphagia in advanced-stage Parkinson's disease. Front Neurol. 2024;15:1323703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Chung CL, Mak MK, Hallett M. Transcranial Magnetic Stimulation Promotes Gait Training in Parkinson Disease. Ann Neurol. 2020;88:933-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi GP, Corti S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int J Mol Sci. 2020;21:3103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 9. | Shariati A, Nemati R, Sadeghipour Y, Yaghoubi Y, Baghbani R, Javidi K, Zamani M, Hassanzadeh A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: A promising frontier. Eur J Cell Biol. 2020;99:151097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Vissers C, Ming GL, Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv Drug Deliv Rev. 2019;148:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1232] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 12. | Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112:E5308-E5317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 554] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 13. | Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6729] [Cited by in RCA: 7282] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 14. | Shin JY, Kim DY, Lee J, Shin YJ, Kim YS, Lee PH. Priming mesenchymal stem cells with α-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res Ther. 2022;13:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 15. | Venkatesh K, Sen D. Mesenchymal Stem Cells as a Source of Dopaminergic Neurons: A Potential Cell Based Therapy for Parkinson's Disease. Curr Stem Cell Res Ther. 2017;12:326-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Soni N, Tripathi A, Mukherjee S, Gupta S, Mohanty S, Basu A, Banerjee A. Bone marrow-derived extracellular vesicles modulate the abundance of infiltrating immune cells in the brain and exert an antiviral effect against the Japanese encephalitis virus. FASEB Bioadv. 2022;4:798-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, Teixeira FG, Salgado AJ. Bone Marrow Mesenchymal Stem Cells' Secretome Exerts Neuroprotective Effects in a Parkinson's Disease Rat Model. Front Bioeng Biotechnol. 2019;7:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Vilaça-Faria H, Marote A, Lages I, Ribeiro C, Mendes-Pinheiro B, Domingues AV, Campos J, Lanceros-Mendez S, Salgado AJ, Teixeira FG. Fractionating stem cells secretome for Parkinson's disease modeling: Is it the whole better than the sum of its parts? Biochimie. 2021;189:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Geng Y, Long X, Zhang Y, Wang Y, You G, Guo W, Zhuang G, Zhang Y, Cheng X, Yuan Z, Zan J. FTO-targeted siRNA delivery by MSC-derived exosomes synergistically alleviates dopaminergic neuronal death in Parkinson's disease via m6A-dependent regulation of ATM mRNA. J Transl Med. 2023;21:652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020;15:6917-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 21. | Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int J Mol Sci. 2021;22:1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | d'Angelo M, Cimini A, Castelli V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson's Disease. Int J Mol Sci. 2020;21:5241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Baharlooi H, Azimi M, Salehi Z, Izad M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int J Stem Cells. 2020;13:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Peng H, Li Y, Ji W, Zhao R, Lu Z, Shen J, Wu Y, Wang J, Hao Q, Wang J, Wang W, Yang J, Zhang X. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson's Disease. ACS Nano. 2022;16:869-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 25. | Mohamed AS, Abdel-Fattah DS, Abdel-Aleem GA, El-Sheikh TF, Elbatch MM. Biochemical study of the effect of mesenchymal stem cells-derived exosome versus L-Dopa in experimentally induced Parkinson's disease in rats. Mol Cell Biochem. 2023;478:2795-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 27. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2467] [Article Influence: 224.3] [Reference Citation Analysis (2)] |
| 28. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25789] [Article Influence: 1121.3] [Reference Citation Analysis (0)] |
| 29. | Chen HX, Liang FC, Gu P, Xu BL, Xu HJ, Wang WT, Hou JY, Xie DX, Chai XQ, An SJ. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. 2020;11:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 30. | Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson's disease. Mol Ther Nucleic Acids. 2021;23:1334-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 31. | Bai X, Dong Q, Zhao L, Yao Y, Wang B. microRNA-106b-containing extracellular vesicles affect autophagy of neurons by regulating CDKN2B in Parkinson's disease. Neurosci Lett. 2021;760:136094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Li Y, Li Z, Gu J, Xu X, Chen H, Gui Y. Exosomes isolated during dopaminergic neuron differentiation suppressed neuronal inflammation in a rodent model of Parkinson's disease. Neurosci Lett. 2022;771:136414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Ma J, Shi X, Li M, Chen S, Gu Q, Zheng J, Li D, Wu S, Yang H, Li X. MicroRNA-181a-2-3p shuttled by mesenchymal stem cell-secreted extracellular vesicles inhibits oxidative stress in Parkinson's disease by inhibiting EGR1 and NOX4. Cell Death Discov. 2022;8:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Xu X, Li Z, Zuo H, Chen H, Gui Y. Mesenchymal stem cell-derived exosomes altered neuron cholesterol metabolism via Wnt5a-LRP1 axis and alleviated cognitive impairment in a progressive Parkinson's disease model. Neurosci Lett. 2022;787:136810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 35. | Zhang ZX, Zhou YJ, Gu P, Zhao W, Chen HX, Wu RY, Zhou LY, Cui QZ, Sun SK, Zhang LQ, Zhang K, Xu HJ, Chai XQ, An SJ. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson's disease and neuronal damage through inhibition of microglia. Neural Regen Res. 2023;18:2291-2300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | He S, Wang Q, Chen L, He YJ, Wang X, Qu S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson's disease model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med. 2023;21:747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 37. | Narbute K, Piļipenko V, Pupure J, Dzirkale Z, Jonavičė U, Tunaitis V, Kriaučiūnaitė K, Jarmalavičiūtė A, Jansone B, Kluša V, Pivoriūnas A. Intranasal Administration of Extracellular Vesicles Derived from Human Teeth Stem Cells Improves Motor Symptoms and Normalizes Tyrosine Hydroxylase Expression in the Substantia Nigra and Striatum of the 6-Hydroxydopamine-Treated Rats. Stem Cells Transl Med. 2019;8:490-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 38. | Narbute K, Pilipenko V, Pupure J, Klinovičs T, Auders J, Jonavičė U, Kriaučiūnaitė K, Pivoriūnas A, Kluša V. Time-Dependent Memory and Gait Improvement by Intranasally-Administered Extracellular Vesicles in Parkinson's Disease Model Rats. Cell Mol Neurobiol. 2021;41:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | El Nasser Imam RA, Aboulhoda BE, Abdallah NM, Aboulkhair AG, Aboelkomsan EAF, Badr AM, Alghamdi MA, Al Badawi EA, Morsy SA, Hassan FE, Abd El-Galil TI. Exosomes Extracted from Adipose Tissue Stem Cells Alleviate Rotenone-Induced Nigrostriatal Neuro-Degeneration, Glial Cell Activation, Synucleinopathy and Motor Incoordination via Modulation of Autophagy/MiRNA7/MiRNA21. J Biol Reg Homeos Ag. 2023;37:6891-6910. [DOI] [Full Text] |
| 40. | Vaz M, Soares Martins T, Henriques AG. Extracellular vesicles in the study of Alzheimer's and Parkinson's diseases: Methodologies applied from cells to biofluids. J Neurochem. 2022;163:266-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 41. | Jarmalavičiūtė A, Pivoriūnas A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol Res. 2016;113:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Ma Y, Wang K, Pan J, Fan Z, Tian C, Deng X, Ma K, Xia X, Huang Y, Zheng JC. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol Dis. 2019;124:322-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Chompoopong S, Jarungjitaree S, Punbanlaem T, Rungruang T, Chongthammakun S, Kettawan A, Taechowisan T. Neuroprotective Effects of Germinated Brown Rice in Rotenone-Induced Parkinson's-Like Disease Rats. Neuromolecular Med. 2016;18:334-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Campos FL, Carvalho MM, Cristovão AC, Je G, Baltazar G, Salgado AJ, Kim YS, Sousa N. Rodent models of Parkinson's disease: beyond the motor symptomatology. Front Behav Neurosci. 2013;7:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 45. | Wu X, Meng X, Tan F, Jiao Z, Zhang X, Tong H, He X, Luo X, Xu P, Qu S. Regulatory Mechanism of miR-543-3p on GLT-1 in a Mouse Model of Parkinson's Disease. ACS Chem Neurosci. 2019;10:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Chia SJ, Tan EK, Chao YX. Historical Perspective: Models of Parkinson's Disease. Int J Mol Sci. 2020;21:2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 47. | Lal R, Singh A, Watts S, Chopra K. Experimental models of Parkinson's disease: Challenges and Opportunities. Eur J Pharmacol. 2024;980:176819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 48. | Ibarra-Gutiérrez MT, Serrano-García N, Orozco-Ibarra M. Rotenone-Induced Model of Parkinson's Disease: Beyond Mitochondrial Complex I Inhibition. Mol Neurobiol. 2023;60:1929-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 49. | Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, Gehmeyr M, Volkmann J, Brotchie JM, Ahsan F, Haack B, Monoranu CM, Keber U, Yeghiazaryan R, Pagenstecher A, Heckel T, Bischler T, Wischhusen J, Koprich JB, Lutz MB, Ip CW. Neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson's disease mice. Brain Behav Immun. 2022;101:194-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 50. | Chen Y, Zhang C, Huang Y, Ma Y, Song Q, Chen H, Jiang G, Gao X. Intranasal drug delivery: The interaction between nanoparticles and the nose-to-brain pathway. Adv Drug Deliv Rev. 2024;207:115196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 51. | Xie X, Song Q, Dai C, Cui S, Tang R, Li S, Chang J, Li P, Wang J, Li J, Gao C, Chen H, Chen S, Ren R, Gao X, Wang G. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer's disease: a phase I/II clinical trial. Gen Psychiatr. 2023;36:e101143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 52. | Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 53. | Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci. 2018;12:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 55. | Matsui H, Takahashi R. Current trends in basic research on Parkinson's disease: from mitochondria, lysosome to α-synuclein. J Neural Transm (Vienna). 2024;131:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 56. | Ren Z, Qi Y, Sun S, Tao Y, Shi R. Mesenchymal Stem Cell-Derived Exosomes: Hope for Spinal Cord Injury Repair. Stem Cells Dev. 2020;29:1467-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 57. | Liu M, Zhang L, Zhao Q, Jiang X, Wu L, Hu Y. Lower-Molecular-Weight Chitosan-Treated Polyethyleneimine: a Practical Strategy For Gene Delivery to Mesenchymal Stem Cells. Cell Physiol Biochem. 2018;50:1255-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Jiang L, Li X, Wang S, Yuan Z, Cheng J. The role and regulatory mechanism of m(6)A methylation in the nervous system. Front Genet. 2022;13:962774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 59. | Cheng L, Yu P, Li F, Jiang X, Jiao X, Shen Y, Lai X. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 60. | Yang Q, Mao Z. Parkinson disease: a role for autophagy? Neuroscientist. 2010;16:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Wang S, Huang Y, Zhou C, Wu H, Zhao J, Wu L, Zhao M, Zhang F, Liu H. The Role of Autophagy and Related MicroRNAs in Inflammatory Bowel Disease. Gastroenterol Res Pract. 2018;2018:7565076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Wang J, Ran Y, Li Z, Zhao T, Zhang F, Wang J, Liu Z, Chen X. Salsolinol as an RNA m6A methylation inducer mediates dopaminergic neuronal death by regulating YAP1 and autophagy. Neural Regen Res. 2025;20:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 63. | Xue C, Li X, Ba L, Zhang M, Yang Y, Gao Y, Sun Z, Han Q, Zhao RC. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson's Disease. Aging Dis. 2021;12:1211-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 64. | Mack JM, de Menezes Moura T, Bobinski F, Martins DF, Cunha RA, Walz R, Fernandes PA, Markus RP, Dafre AL, Prediger RD. Neuroprotective effects of melatonin against neurotoxicity induced by intranasal sodium dimethyldithiocarbamate administration in mice. Neurotoxicology. 2020;80:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Zhao W, Feng H, Guo S, Han Y, Chen X. Danshenol A inhibits TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep. 2017;7:12953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Galvagnion C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson's Disease. J Parkinsons Dis. 2017;7:433-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 67. | Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1561] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 68. | Perfeito R, Ribeiro M, Rego AC. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch Toxicol. 2017;91:1245-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Li Z, Li Y, Xu X, Gu J, Chen H, Gui Y. Exosomes rich in Wnt5 improved circadian rhythm dysfunction via enhanced PPARγ activity in the 6-hydroxydopamine model of Parkinson's disease. Neurosci Lett. 2023;802:137139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |