Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.102067
Revised: January 17, 2025
Accepted: February 24, 2025
Published online: March 26, 2025
Processing time: 164 Days and 14.8 Hours
As living biodrugs, mesenchymal stem cells (MSCs) have progressed to phase 3 clinical trials for cardiovascular applications. However, their limited immediate availability hampers their routine clinical use.
To validate our hypothesis that cryopreserved MSCs (CryoMSCs) are as safe and effective as freshly cultured MSC counterparts but carry logistical advantages.
Four databases were systematically reviewed for relevant randomized controlled trials (RCTs) evaluating the safety and efficacy of CryoMSCs from various tissue sources in treating patients with heart disease. A subgroup analysis was per
Seven RCTs (285 patients) met the eligibility criteria for inclusion in the meta-analysis. During short-term follow-up, CryoMSCs demonstrated a significant 2.11% improvement in left ventricular ejection fraction (LVEF) [WMD (95%CI) = 2.11 (0.66-3.56), P = 0.004, I2 = 1%], with umbilical cord-derived MSCs being the most effective cell type. However, the significant effect on LVEF was not sustained over the 12 months of follow-up. Subgroup analysis demonstrated a substantial 3.44% improvement in LVEF [WMD (95%CI) = 3.44 (1.46-5.43), P = 0.0007, I2 = 0%] when using MSCs with post-thaw viability exceeding 80%. There was no statistically significant difference in the frequency of major cardiac adverse events observed in rehospitalization or mortality in patients treated with CryoMSCs vs the control group.
CryoMSCs are a promising option for heart failure patients, particularly considering the current treatment options for cardiovascular diseases. Our data suggest that CryoMSCs could be a viable alternative or complementary treatment to the current options, potentially improving patient outcomes.
Core Tip: Our study yields significant findings that are crucial for regenerative medicine and cardiology. Our findings revealed that cryopreserved mesenchymal stem cells (CryoMSCs) treatment, compared to the control group, resulted in a 2.11% improvement in left ventricular ejection fraction during six months of follow-up, offering hope for potential future therapies. Left ventricular ejection fraction improvement was higher when using umbilical cord-derived mesenchymal stem cells or CryoMSCs with more than 80% post-thaw viability. The CryoMSCs treatment was safe, as there was no significant difference in the incidence of major cardiac adverse events compared to the control group. In addition, no significant effects on mortality and readmission were observed in the CryoMSCs group compared to the control group.
- Citation: Safwan M, Bourgleh MS, Haider KH. Clinical experience with cryopreserved mesenchymal stem cells for cardiovascular applications: A systematic review. World J Stem Cells 2025; 17(3): 102067
- URL: https://www.wjgnet.com/1948-0210/full/v17/i3/102067.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i3.102067
Cardiovascular diseases (CVDs), such as myocardial infarction and heart failure, are a pressing global health issue, contributing to 32% of all global deaths[1,2]. The current treatment options provide only symptomatic relief, failing to repair or regenerate the damaged myocardium or preserve declining cardiac function. In this context, cell-based therapy using mesenchymal stem cells (MSCs) has emerged as a promising solution for heart failure patients[3].
MSCs possess unique cell biology and characteristics, i.e., multilineage differentiation potential, soluble and insoluble factor release as part of their paracrine activity, low immunogenicity upon transplantation, anti-inflammatory and immunomodulatory properties, etc[4]. They have a robust nature that withstands the rigors of genetic modulation and can carry transgenes during cell-based gene therapy. Combined with their ease of availability from diverse tissue sources, notably bone marrow, adipose tissue, and umbilical cord, non-invasive isolation without moral and ethical strings places them near the ideal cell type for use in the cell-based therapy approach[5]. These characteristic features of MSCs are critical for their diverse clinical applications and have been extensively studied during experimental animal studies and clinical trials[6]. Currently, nearly 1500 registrations with clinicaltrials.gov to assess MSCs from different tissue sources in clinical settings for diverse pathological conditions[7]. Despite these advantages, logistic issues regarding their off-the-shelf availability in large numbers remain a significant limitation that is a critical impediment in routine clinical use[8]. Moreover, repeated tissue sampling for isolation, purification, and in vitro expansion adds to the cost of each procedure, besides being time-intensive, which limits their routine use in clinical practice in general and especially in the emergency rooms[9].
The low-temperature storage or cryopreservation of MSCs offers a cost-effective solution to the logistical issues and ensures their ready-made availability. This significantly reduces the time needed to isolate, purify, and expand the cells in vitro before use for every patient. The currently available cryopreservation protocols are believed to preserve the cells’ unique stemness characteristics, such as their ability to differentiate into multiple cell types and immunomodulatory properties. These characteristics are crucial for the therapeutic potential of MSCs. Despite the beneficial effects of cryopreservation’s known impact on MSC biology and viability, standardized preservation methods do not lead to significant variability across preclinical data. Various techniques have been explored to mitigate cellular damage post-cryopreservation, with dimethyl sulfoxide (DMSO) being the most common cryoprotectant despite its associated adverse effects[10,11].
The potential of cryopreserved MSCs (CryoMSCs) in clinical trials treating CVDs has been reported as a significant advancement. A recent meta-analysis of six randomized controlled trials (RCTs) involving 263 heart failure patients found that bone marrow-derived MSCs (BM-MSCs) significantly increased left ventricular ejection fraction (LVEF) by 6.37% at the end of the follow-up period compared to the control group[3]. This promising potential not only instills hope and optimism for the future of cardiovascular medicine but also inspires further research and development in this area. The use of CryoMSCs in clinical trials opens new doors for research and treatment, and their potential could significantly impact the field. Currently, several MSC-based products are available on the market, including Prochymal (Osiris Therapeutics, Canada), Cartistem (Medipost Co Ltd, Korea), and Stempeucel (Stempeutics Research), Cellgram-AMI (FCB Pharmicell, South Korea) (Alliance for Regenerative Medicine; https://alliancerm.org/available-products/), but their functionality and clinical efficacy are still under scrutiny. Despite the commercial availability and recent use of MSC-based products, there is a scarcity of published data comparing cryoMSCs with freshly cultured MSCs as living biodrugs to assess their safety and efficacy for patients with CVD.
The present systematic review and meta-analysis of cryoMSCs in patients with myocardial infarction and heart failure compared to freshly cultured MSC-based therapies aim to significantly contribute to cardiovascular medicine and stem cell therapy. The evidence-based insights into the efficacy and safety of cryoMSCs could pave the way for improved treatment strategies using off-the-shelf MSCs. These findings can potentially revolutionize the field, bringing an exciting new approach to cardiovascular medicine and inspiring future research and innovation in stem cell therapy, potentially dramatically improving patient outcomes.
Before any formal literature search or data analysis, a complete protocol for this study was prospectively developed and registered on the International Prospective Register of Systematic Reviews database dated June 6, 2024. The protocol is available online under the registration ID: CRD42024555501.
A comprehensive and meticulous search strategy was conducted across four databases, including PubMed, Cochrane CENTRAL, ClinicalTrials.gov, and Embase, from their inception until June 2024 to identify relevant RCTs. The search strategy incorporated common text words and medical subject headings (MeSH) such as “Mesenchymal Stem Cells”, “MSCs”, “Cryopreserved”, “Bone marrow Mesenchymal stem cells”, “umbilical cord Mesenchymal stem cells”, “myocardial infarction,” “ischemic heart disease”, “acute coronary syndrome”, “heart failure”, and “cardiomyopathy”. These terms were combined using specific algorithms, such as “Mesenchymal stem cells” and “Myocardial infarction”. Additionally, the reference lists of included studies were manually searched to identify any relevant RCTs not captured in the initial search. Our search was not restricted by language, and the strategy adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 statement[12], ensuring the thoroughness and validity of the research and instilling confidence in the validity of the findings.
For inclusion in the current systematic review and meta-analysis, a study was required to meet the following eligibility criteria: (1) It must be an RCT; (2) It assessed the efficacy of CryoMSCs; (3) It involved patients with myocardial infarction or heart failure; (4) It must include a control group; (5) The follow-up period was at least six months; and (6) It reported one of the following outcomes: Change in LVEF, six-minute walking distance test (6-MWD), major adverse cardiac events (MACE), or readmission for exacerbation of heart failure or myocardial infarction. Any study that did not fulfill these criteria or was not available in full text was considered ineligible for inclusion.
The primary outcome evaluated the efficacy of CryoMSCs, measured by the change in LVEF and 6-MWD compared to the change observed in the control arm. The secondary outcomes focused on the safety of CryoMSCs, assessed by the frequency of MACE across both arms during treatment. MACE encompassed various events, including mortality, arrhythmias, heart failure, recurrence of myocardial infarction, and readmission for cardiac reasons.
Two co-authors (Safwan M and Bourgleh MS) independently evaluated the eligibility of studies for meta-analysis based on the inclusion/exclusion criteria and utilized a standardized data extraction sheet. Each included study was examined, with the extraction of the following variables: (1) First author and publication year; (2) Trial location (country); (3) Type of stem cells; (4) Sample size; (5) Gender distribution; (6) Mean sample age; (7) Presence of co-morbidities; (8) Duration of follow-up for key endpoint measurements; (9) Dosage (number of cells transferred in millions); (10) Method of cell delivery (e.g., intravenous, intramyocardial, or intracoronary infusion); (11) New York Heart Association classification of study participants at baseline; (12) Assessment method/tools for study endpoints (e.g., electrocardiogram, echocardiogram, magnetic resonance imaging, cardiac computed tomography, and single-photon emission computed tomography); (13) LVEF (mean ± SD); and (14) Occurrence of MACE.
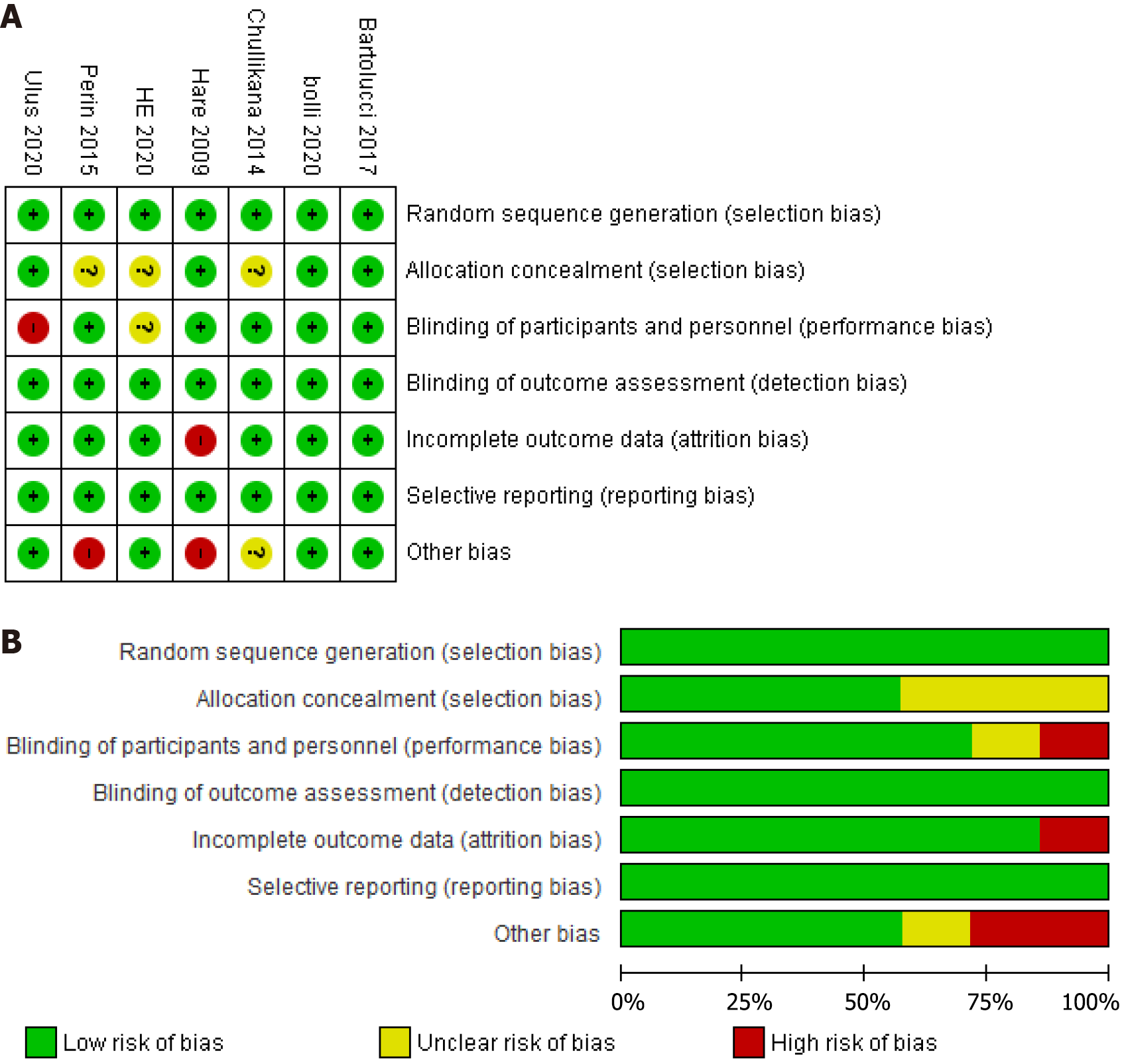
The same co-authors (Safwan M and Bourgleh MS) independently followed the Cochrane collaboration tool for bias assessment to evaluate the methodological quality of the included RCTs. The overall risk of bias was visually presented in a bias risk graph. In instances of disagreement between the authors, a third independent author (Haider KH) was consulted for resolution, ensuring the objectivity and independence of the quality assessment process and reassuring the authors about the results’ reliability. This rigorous quality assessment process adds further credibility to the findings, providing the audience with confidence in the reliability of the results.
Statistical data analysis was performed using Review Manager (RevMan) 5.4.1 software. The odds ratio (OR) was calculated and presented with confidence intervals (CI) for dichotomous outcomes, mortality, MACE, and readmission. For continuous outcomes, LVEF and 6MWD, any data extracted in mean ± SE or mean and CI were converted into mean ± SD using equations of the Cochrane Handbook[13]. Weighted mean difference (WMD) analysis was performed due to the consistent measurement units across all the studies for LVEF and 6MWD. Considering the expected heterogeneity between studies due to variations in sample sizes, countries, and doses, a random effect model was employed. To explore the potential variations in efficacy and safety based on the cell source, subgroup analysis was performed using two different cell sources: Bone marrow and umbilical cord tissue. Additionally, a subgroup analysis was conducted based on the release criteria for post-thaw viability, comparing groups with viability rates above 80% to those below 80%. Between-study heterogeneity was assessed using the I² statistic and interpreted as follows: 25% < I² < 75%: Low and unimportant heterogeneity; I² 25%-75%: Moderate heterogeneity; 75% < I² < 100%: High heterogeneity. The significance cutoff for statistical significance was set at a P value of less than 0.05. A sensitivity analysis was conducted in case high heterogeneity was observed between the studies.
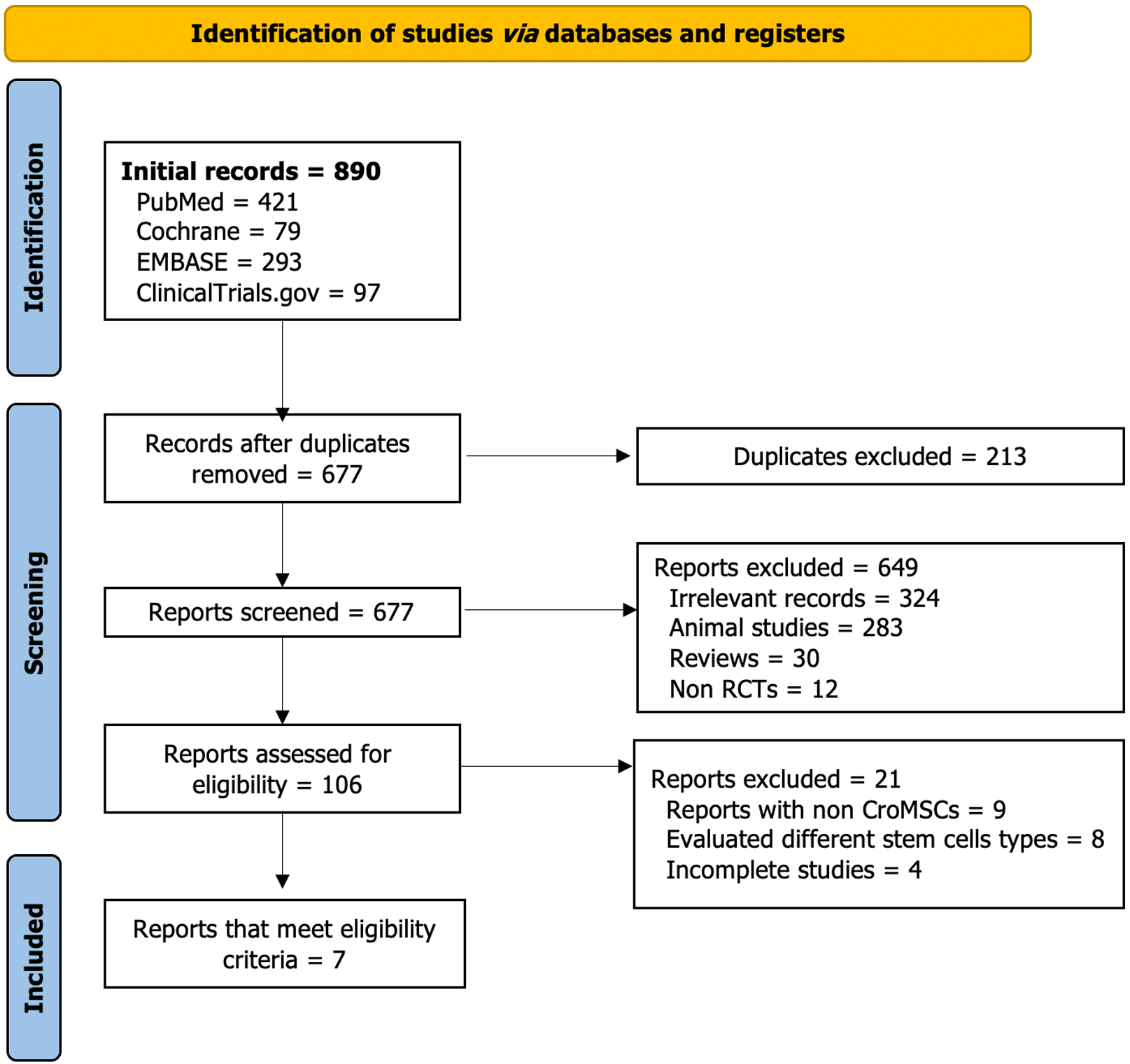
Figure 1 summarizes the process of systematically searching for eligible RCTs. Initially, a search was conducted across various databases, resulting in 890 records. After removing duplicates and performing title and abstract screening, 28 RCTs remained for full-text screening. Seven RCTs were included, and the remaining 21 were excluded based on the reasons outlined in Figure 1. The risk of bias for the included studies was assessed using the Cochrane collaboration tool[14]. The assessment was based on selection, performance, detection, attrition, and reporting biases. Figure 2 presents a graphical summary of the bias assessment.
The baseline characteristics of the included RCTs are detailed in Tables 1 and 2. The 7 RCTs included 285 heart disease patients, with 178 patients in the intervention and 107 in the control arms[15-21]. Four of the included RCTs used CryoBM-MSCs for the intervention[15-18], involving 164 patients, 103 in the intervention arm and 61 in the control arm. The percentage of male participants in the BM-MSCs studies ranged from 43% to 100% in the intervention group and 24% to 80% in the control group. The remaining three RCTs used cryopreserved umbilical cord-derived MSCs (CryoUC-MSCs)[19-21] with 121 patients, 75 in the intervention group and 46 in the control group. The percentage of male participants in the UC-MSCs studies ranged from 71% to 100% in the intervention group and 46% to 100% in the control groups. The included studies were published between 2009 and 2020 and were conducted across several countries: One each in India[16], Turkey[19], China[20], and Chile[21], while three RCTs were conducted in the United States[15,17,18]. The route of cell delivery varied among the included studies. Four studies employed the intramyocardial route[15,18-20], while the remaining three used the intravenous route[16,17,21].
| Ref. | He et al[20], 2020, China | Bartolucci et al[21], 2017, Chile | Ulus et al[19], 2020, Turkey | |
| Study type | RCT | RCT | Open-label RCT | |
| Phase | I | I/II | I/II | |
| Condition | MI | HF | MI | |
| Sample size | Total | 50 | 30 | 41 |
| Intervention (% male) | 35 (71.42) | 15 (80.0) | 25 (100) | |
| Control (% male) | 15 (46.67) | 15 (93.3) | 16 (100) | |
| Age (mean ± SD) | Intervention | 61 ± 8.2 | 57.33 ± 10.05 | 61.8 ± 10 |
| Control | 65.2 ± 7.9 | 57.20 ± 11.64 | 65.3 ± 6.8 | |
| BMI (mean ± SD) | Intervention | 25 ± 3.35 | 29.12 ± 2.88 | 26.5 ± 4.5 |
| Control | 23.59 ± 2.28 | 29.52 ± 4.00 | 26.6 ± 4.8 | |
| Number of smokers | Intervention | 11 (31.43) | 7 (46.7) | 21 (84) |
| Control | 3 (25.0) | 4 (26.7) | 15 (88.2) | |
| HTN | Intervention | 24 (68.57) | 7 (46.7) | 15 (60) |
| Control | 9 (75.0) | 8 (53.3) | 11 (64.7) | |
| DM | Intervention | 12 (34.29) | 5 (33.3) | 16 (66.7) |
| Control | 8 (66.7) | 7 (46.7) | 9 (52.9) | |
| NYHA; I (n), II (n), III (n), IV (n) | Intervention | III (4/8), IV (12/8) | N/S: 2.03 ± 0.61 | N/S: 1.9 ± 0.44 |
| Control | III (7) IV (5) | N/S: 1.67 ± 0.49 | N/S: 2.1 ± 0.37 | |
| Comparison | CABG only | Placebo | CABG only | |
| Follow-up | 3, 6, and 12 months | 3, 6, and 12 months | 1, 3, 6, and 12 months | |
| Assessment modality (yes/no) | ECG | No | Yes | Yes |
| Echo | No | Yes | Yes | |
| MRI | Yes (CMR) | Yes (CMR) | Yes | |
| Cardiac CT | No | No | No | |
| SPECT | No | No | Yes | |
| Measured outcomes | Serious adverse events at 12 months (primary), the efficacy of hUC-MSCs and collagen scaffold assessed according to the CV-CMR–based LVEF and infarct size at 3, 6, and 12 months after treatment, and NYHA (secondary) | Safety: Adverse events after IV infusion -/-. Efficacy: Primary, changes in LVEF, LVESV & LVEDV by Echo; LVEF, LVESV, and LVEDV by CMR; NYHA score (secondary) | LVEF, LV remodeling, myocardial mass, 6MWD, NYHA score change |
| Ref. | Chullikana et al[16], 2015, India | Hare et al[17], 2009, United States | Bolli et al[15], 2020, United States | Perin et al[18], 2015, United States | |
| Study type | RCT | RCT | RCT | RCT | |
| Phase | I/II | I | I | II | |
| Condition | MI | MI | HF | HF | |
| Sample size | Total | 20 | 53 | 31 | 60 |
| Intervention (% male) | 10 (100) | 34 (82.4) | 14 (43) | 45 (97.8) | |
| Control (% male) | 10 (80) | 19 (78.9) | 17 (24) | 15 (73.3) | |
| Age (mean ± SD) | Intervention | 47.31 ± 12.10 | 59 ± 12.3 | 54.7 ± 12.8 | 62.2 ± 10.3 |
| Control | 47.79 ± 6.48 | 55 ± 10.2 | 58.2 ± 11.2 | 62.7 ± 11.2 | |
| BMI (mean ± SD) | Intervention | 23.32 ± 3.74 | 29.8 ± 6.7 | 30.2 ± 9.0 | 29.8 ± 4.1 |
| Control | 24.86 ± 1.88 | 30.3 ± 4.3 | 30.4 ± 6.5 | 31.3 ± 9.2 | |
| Number of smokers | Intervention | N/A | 3 (8.8) | 5 (36) | 7 (15.6) |
| Control | N/A | 2 (10.5) | 3 (18) | 2 (13.3) | |
| HTN | Intervention | N/A | 16 (17.6) | 6 (43) | 29 (64.4) |
| Control | N/A | 9 (47.4) | 10 (59) | 9 (60) | |
| DM | Intervention | N/A | 6 (17.6) | 3 (21) | 13 (28.9) |
| Control | N/A | 1 (5.3) | 5 (29) | 2 (13.3) | |
| NYHA; I (n), II (n), III (n), IV (n) | Intervention | N/A | N/A | II (13), III (1) | II (31), III (14) |
| Control | N/A | N/A | II (13), III (4) | II (6), III (9) | |
| Comparison | Placebo (multiple electrolytes injection) | Placebo | Placebo | Placebo | |
| Follow-up, months | Six months till two years | Six months | 6 and 12 months | 3, 6, 12 months | |
| Assessment modality (yes/no) | ECG | No | Yes | Yes | No |
| Echo | Yes | Yes | No | Yes | |
| MRI | Yes | Yes | Yes (CMR) | No | |
| Cardiac CT | No | Yes | No | No | |
| SPECT | Yes | No | No | Yes | |
| Measured outcomes | Adverse events, LVEF (Echo & SPECT), total perfusion score, and total volume of infarct | Safety, adverse events, LVEF (Echo), and 6MWD | Safety and feasibility of allogenic MSC administration in this population (primary). Effects of allogenic MSC administration on LV function (LVEF, LVEDV, LVESV, scar morphology) and functional status (6MWD, MLHFQ) (secondary) | Safety (primary), LV volume, LVEF, 6MWD (secondary) |
In the included studies, the control group received a placebo treatment besides standard pharmacological or adjunct surgical intervention. For example, in three studies[15,18,21], the control group also received standard heart failure therapy, including beta blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or aldosterone antagonists. In two studies[19,20], the control group underwent coronary artery bypass graft surgery, while in one study[16], the control group received percutaneous coronary intervention. The remaining study[17] did not provide specific information on the medications or procedures administered to the control group.
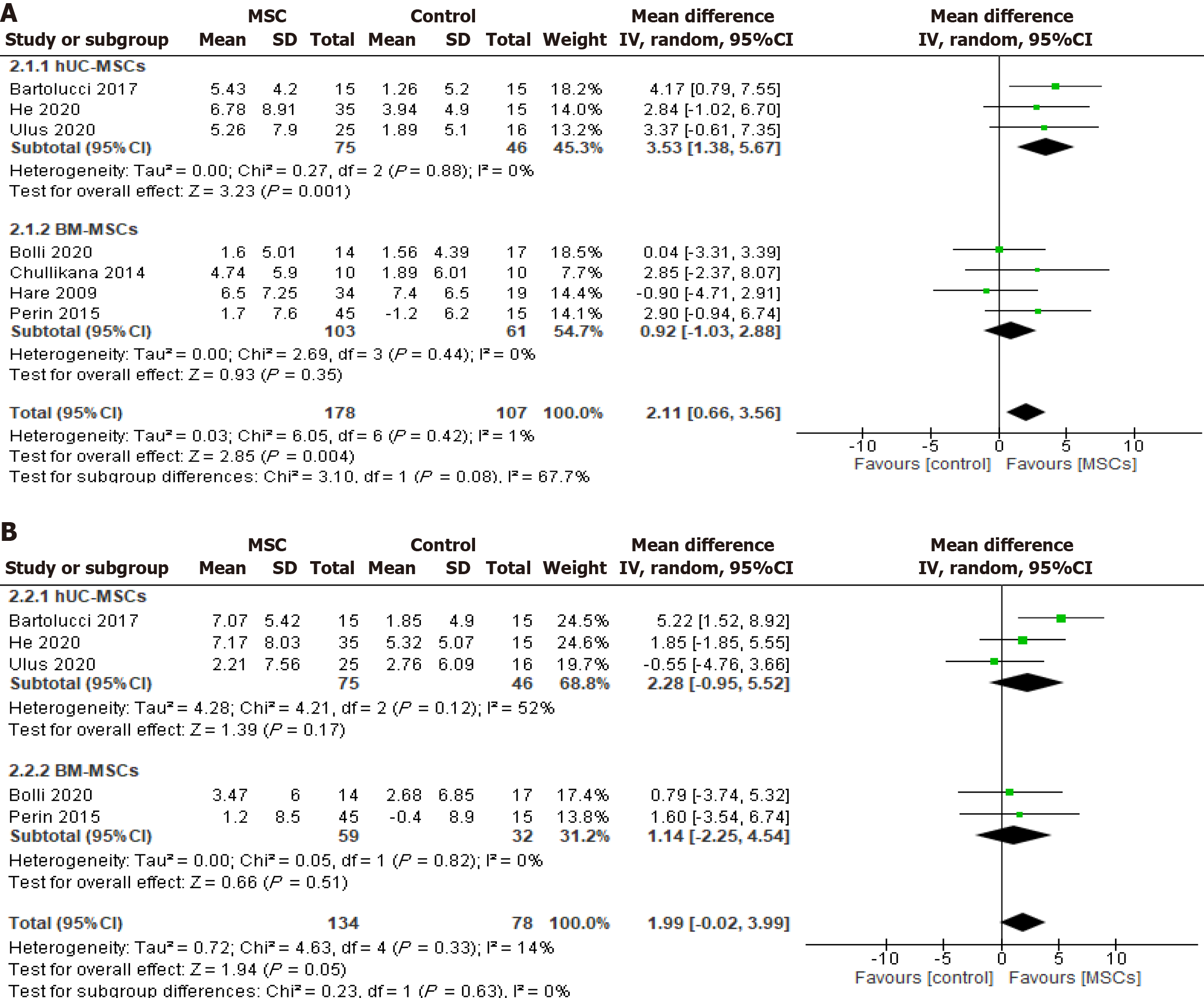
LVEF: Seven studies[15-21] reported changes in LVEF after six months of CryoMSCs-based treatment, including three studies[19-21] using UC-MSCs and four studies[15-18] using BM-MSCs. The pooled analysis showed a significant 2.11% improvement in LVEF in the MSC treatment groups compared to the control [WMD (95%CI) = 2.11 (0.66-3.56), P = 0.004, I2 = 1%]. In the subgroup analysis by cell type, the pooled 6-month LVEF changes recorded a significant 3.53% increase in the UC-MSCs studies [WMD (95%CI) = 3.53 (1.38-5.67), P = 0.001, I2 = 0%]. At the same time, the BM-MSCs treatment did not demonstrate any significant improvement compared to the control [WMD (95%CI) = 0.92 (-1.03 to 2.88), P = 0.35, I2 = 0%] (Figure 3A). Seven studies measured the LVEF change before and after 12 months of CryoMSCs treatment, including 3 UC-MSCs and 4 BM-MSCs studies. However, the pooled change in the treatment group was a 1.99% improvement compared to the control, which was not statistically significant [WMD (95%CI) = 1.99 (-0.02 to 3.99), P = 0.05, I2 = 14%) (Figure 3B).
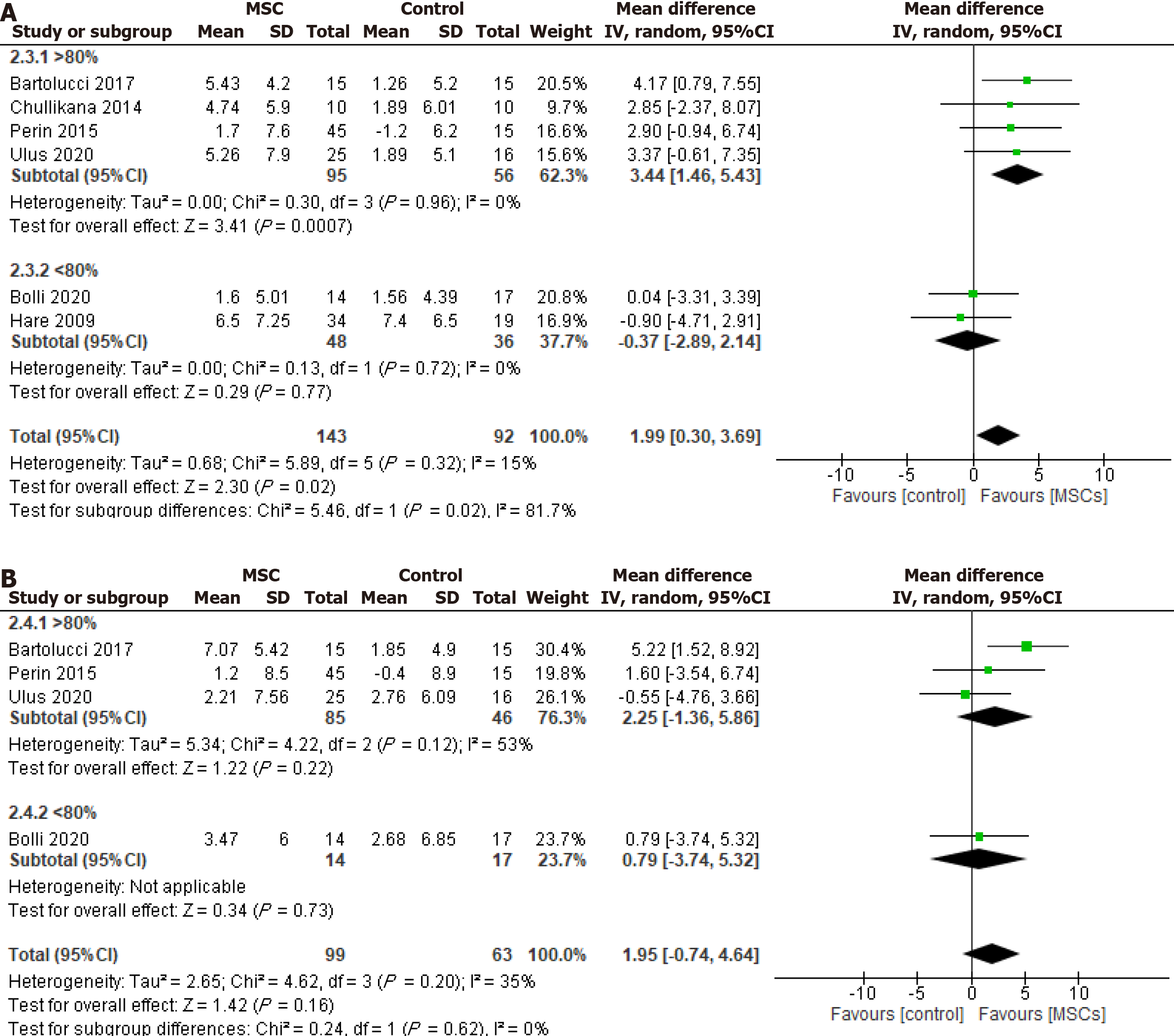
In the subgroup analysis based on post-thaw viability, the group with more than 80% post-thaw viable cells demon
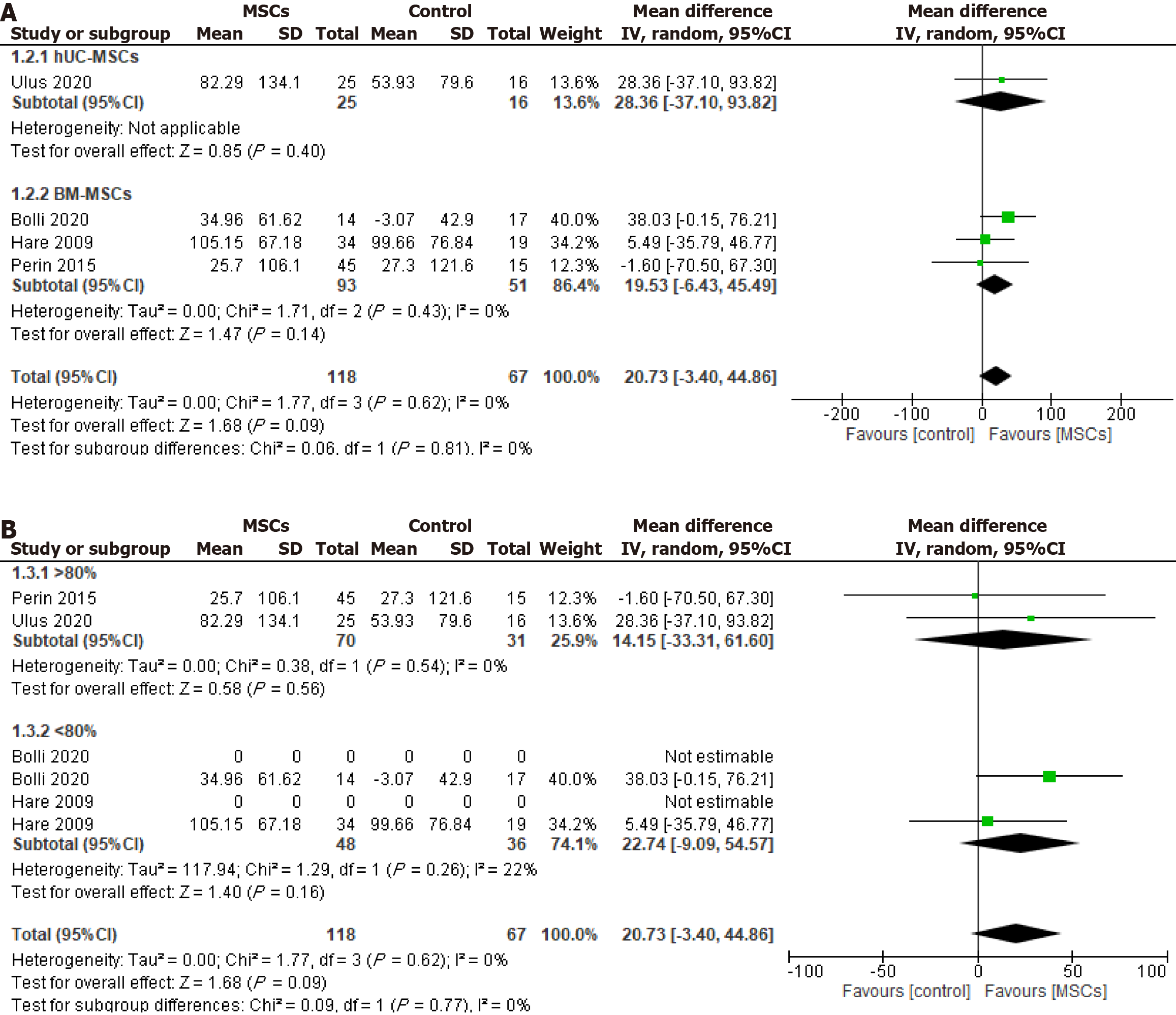
6MWD test: The 6MWD test results were reported in four included studies, one involving UC-MSCs and three involving BM-MSCs. The pooled analysis demonstrated no significant difference in 6MWD between the MSCs group and control group [WMD (95%CI) = 20.73 (-3.40.10 to 44.86), P = 0.09, I2 = 0%] for either the UC-MSCs studies [WMD (95%CI) = 28.36 (-37.10 to 93.82), P = 0.40] or the BM-MSCs studies [WMD (95%CI) = 19.53 (-6.43 to 45.49), P = 0.14, I2 = 0%] (Figure 5A). Furthermore, the subgroup analysis according to cellular post-thaw viability demonstrated no significant difference in 6MWD between the intervention and control groups, regardless of whether the viable cells were > 80% [WMD (95%CI) = 14.15 (-33.31 to 61.60), P = 0.56, I2 = 0%] or < 80% [WMD (95%CI) = 22.74 (-9.09 to 54.57), P = 0.16, I2 = 22%] (Figure 5B).
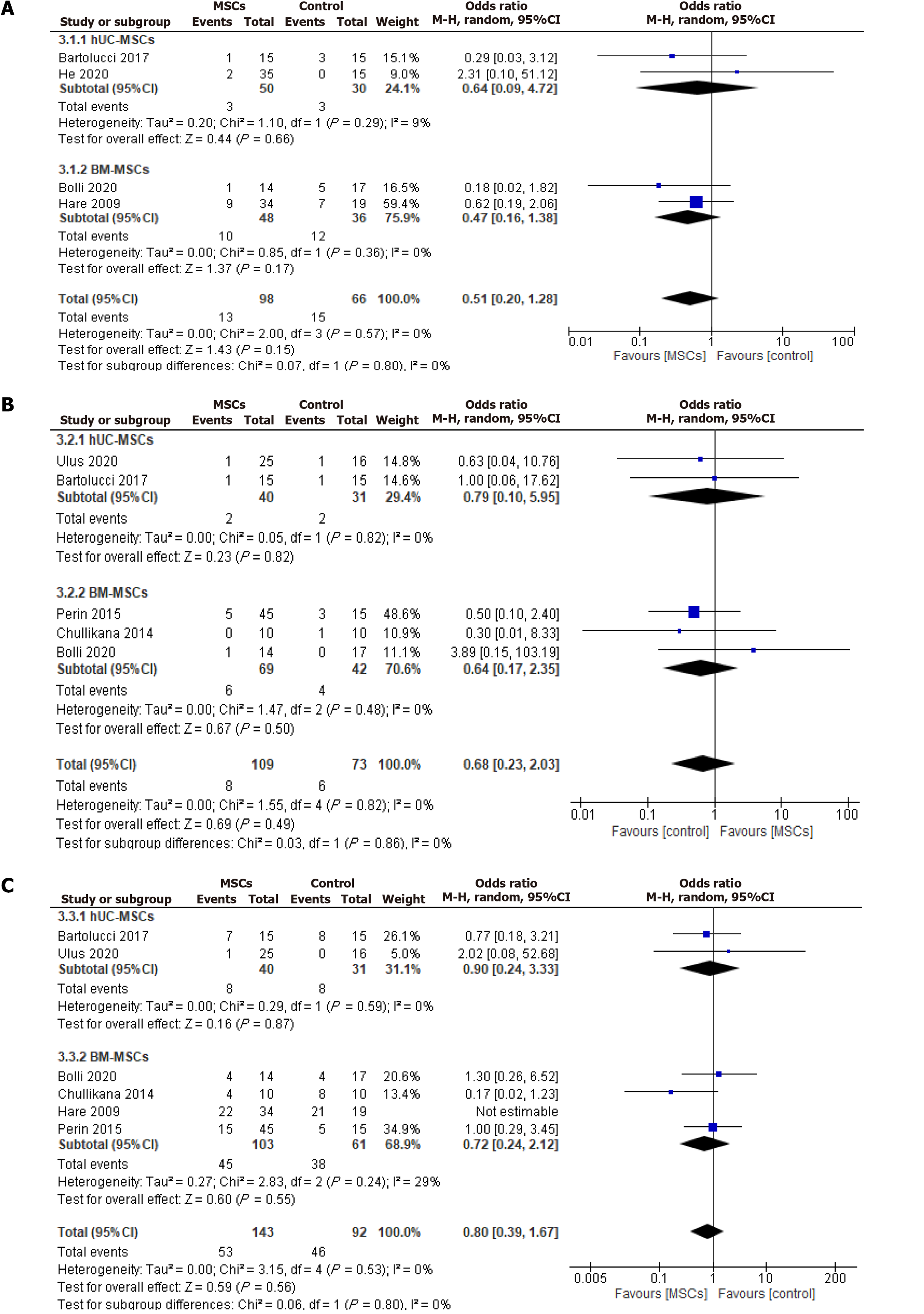
Rehospitalization: The rehospitalization incidence was reported during the follow-up period in two UC-MSCs and two BM-MSCs studies. The analysis showed no significant reduction in the overall OR of rehospitalization [OR (95%CI) = 0.51 (0.20-1.28), P = 0.15, I2 = 0%] for both UC-MSCs and BM-MSCs treated groups compared to the respective control groups [OR (95%CI) = 0.64 (0.09-4.72), P = 0.66, I2 = 9%] and [OR (95%CI) = 0.47 (0.16-1.38), P = 0.17, I2 = 0%] (Figure 6A).
Mortality: Two UC-MSCs RCTs and three BM-MSCs RCTs reported mortality from the included RCTs (Figure 6B). There were no significant differences in the OR of mortality between the intervention group and control group with either UC-MSCs studies [OR (95%CI) = 0.79 (0.10-5.95), P = 0.82, I2 = 0%] and BM-MSCs studies [OR (95%CI) = 0.64 (0.17-2.35), P = 0.50, I2 = 0%].
Six included studies reported the incidence of MACE, 2 UC-MSCs studies, and 4 BM-MSCs studies, such as ventricular tachycardia, supraventricular tachycardia, and angina and revascularization of myocardial infarction. The pooled analysis did not show a statistically significant difference in the overall MACE [OR (95%CI) = 0.80 (0.39-1.67), P = 0.56, I2 = 0%] between the CryoMSCs and the control group. Similarly, no statistically significant effect was seen when sub
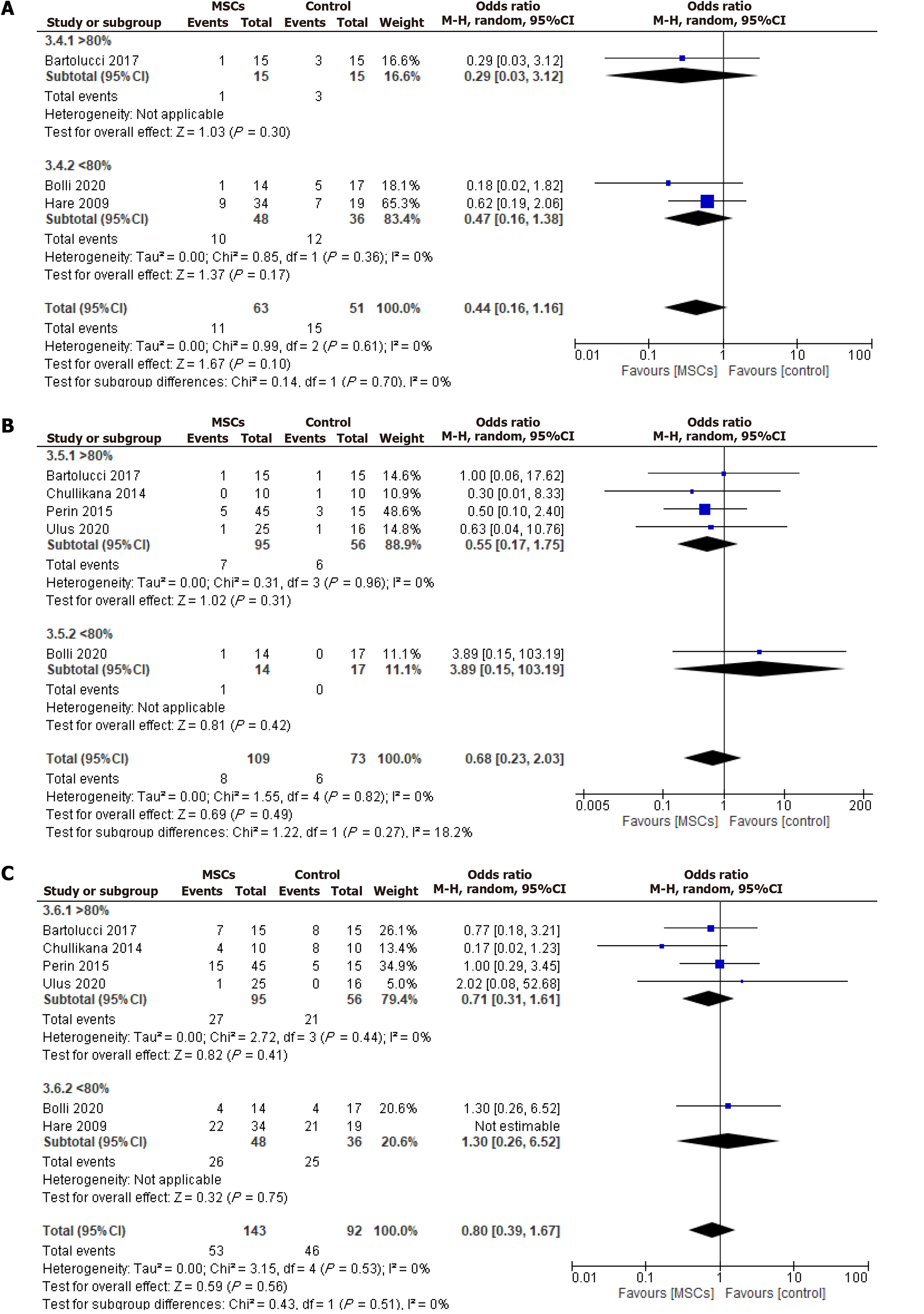
Based on cellular post-thaw viability, a subgroup analysis was conducted on adverse events, including rehospitalization, mortality, and MACE. The analysis found no significant differences between the MSCs and the control groups for either the > 80% viable cells or < 80% viable cells groups regarding rehospitalization, mortality, or MACE (Figure 7).
Our systematic review and meta-analysis are aimed at evaluating the safety and efficacy of the CryoMSCs for treating patients with myocardial infarction and heart failure. The significant findings of our study are: (1) CryoMSC treatment resulted in an overall 2.11% improvement in LVEF during 6 mo of follow-up compared to the control group; (2) The LVEF improvement was higher when using UC-MSCs or CryoMSCs with more than 80% post-thaw viability; (3) The functional benefits of treatment with CryoMSCs were not sustained during the 12-mo follow-up; (4) Treatment with CryoMSCs did not result in a statistically significant improvement of the 6MWD test compared to control; (5) Treatment with CryoMSCs was safe, as there was no significant difference in the incidence of MACE compared to the control group; and (6) No significant effects on mortality and readmission were observed in the CryoMSC group compared to the control group.
Unlike conventional drugs, which are mostly natural or synthetic, and modern-day biologics, which are substances of biological origin, pharmacology has advanced to the next generation of drugs: The living biodrugs, i.e., a novel fast-emerging group of medications for which product viability is a primary requirement. Depending upon their subsequent therapeutic application, living biodrugs can also be genetically modified to enhance their efficacy, such as chimeric antigen receptors-T cells nick-named TRUCKs and hematopoietic progenitor cells-based Food and Drug Administration-approved products (Allocord, Hemacord, Ducord, etc)[22]. MSCs are novel living biodrugs that may be used naïve or modified to deliver transgenes and drugs as payloads[23]. Logistic considerations for living biodrugs, especially off-the-shelf ready-to-use availability, differ from conventional pharmaceuticals and impede their clinical progress. Firstly, such an arrangement will mainly necessitate an allogenic source of cells. Although the published clinical data supports the use of allogenic MSCs on par with their autologous counterparts[24,25], the donor-related factors affecting their biology and functional heterogeneity, especially for long-term benefits, add to the potential uncertainty about their clinical outcome[26]. Their cryopreservation increases the uncertainty regarding their post-transplantation performance.
The factors affecting the safety and efficacy of cryopreserved cells encompass almost everything from tissue source[27] and cryo-banking to thawing and delivery. For example, whether the cryopreserved cells post-thaw be cultured or used directly after washing to remove the cryoprotectant remains an important consideration. Similarly, their viability post-thaw is critical to their safety and efficacy. The current study represents the first evaluation of CryoMSCs’ efficacy in patients with CVD and reveals a notable connection between MSCs viability post-thawing and their impact on LVEF. A subgroup analysis based on MSC type and post-thaw viability showed that treatment with UC-MSCs resulted in a significant 3.53% improvement in LVEF during 6 mo of follow-up compared to the control group. On the contrary, no significant LVEF change was observed after treatment with BM-MSCs. It is essential to mention that neither cell type significantly impacted the 12-mo follow-up, although UC-MSCs demonstrated better efficacy. The considerable variations in LVEF improvement observed between MSCs from two tissue sources may be attributed to the relatively primitive nature of UC-MSCs compared to the BM-MSCs[5,27]. These are significant findings as UC-MSCs, with their better efficacy, primitive nature, and non-invasive availability, are being widely cryopreserved to ensure ready-to-use obtainability. The preclinical studies support these data to indicate that MSCs isolated from healthy donors and cryopreserved in liquid nitrogen for extended periods can sustain their biology and stemness characteristics, i.e., paracrine signaling, differentiation potential, and proliferation capabilities[10,28]. This underscores the potential of UC-MSCs for future cryopreservation efforts, instilling a sense of optimism and hope for further research and development.
Changes in LVEF remain a significant predictor of prognosis in heart failure patients. Previous studies have shown that the hazard ratio for all-cause mortality increases by 39% for every 10% reduction in LVEF below 45%[29]. Despite this, relatively few studies have assessed the effectiveness of heart failure medications in improving LVEF. One study demonstrated that beta-blocker treatment led to an LVEF improvement of 4% to 4.9% in patients with a baseline LVEF below 40% and 1.9% in those with a baseline LVEF between 40% and 50%[30]. Another study on the impact of renin-angiotensin system inhibition in heart failure with preserved LVEF found a significant LVEF improvement (2.18%) after treatment[31]. Based on this evidence, the improvements observed in our analysis with CryoMSCs appear promising. However, it is important to note that in three of the included studies, both the intervention and control groups received standard therapy (e.g., beta blockers and/or renin-angiotensin system inhibitors). Consequently, we found that treatment with CryoMSCs resulted in a more sustained and pronounced improvement in LVEF compared to those receiving standard therapy alone. Moreover, it is difficult to delineate the effects of cell therapy from those of standard therapy during concomitant treatment.
The lack of sustained treatment effects with CryoMSCs after 12 months suggests that a single dose is insufficient for long-term cardiac improvement, and administering a second dose may sustain long-term outcomes. Possible explanations for the lack of sustained long-term effects include the progressive nature of heart failure, which often worsens insidiously due to the underlying neurohormonal imbalance and endothelial dysfunction, even in the absence of overt clinical symptoms[32]. Secondly, the permanence of the long-term therapeutic benefits may require the sustenance of the cell graft. Loss of cell graft may contribute to the loss of therapeutic benefits, which can be benefited by repeated cell administration until the recovery from heart failure is reached. Moreover, some studies have employed intravenous administration of cells, which offers advantages such as safety, ease of administration, and lower cost than intracoronary or intramyocardial routes. Although these alternative routes may be more effective, there is a need for specialized centers and a 1%-2% risk of complications like perforation and tamponade that hamper their routine application. Intravenous administration is associated with lower engraftment, which can limit the therapeutic benefits, especially when a single dose is used[33]. Lastly, due to the clinical trial design of the included studies, labeling the injected cells and tracking their migration to the myocardium proved challenging[17]. These factors underscore the potential advantages of administering multiple doses over time. A recent study by Attar et al[34] showed that administering repeated MSC doses led to a 4% improvement in LVEF at 6 months compared to a single dose. Although there is no data on a second dose administered after 6 mo for myocardial infarction or heart failure patients, studies have shown that a second dose given 6 months after the first can lead to significant functional improvements in knee osteoarthritis patients compared to a single dose[35,36]. These findings emphasize the potential of repeated MSC doses at various intervals to optimize treatment in cardiac patients, sparking further interest and research. We did not find a significant difference in the adverse cardiac events between the CryoMSCs and the control groups, suggesting the clinical safety of the cryopreserved cells. However, the included RCTs lack details regarding the cryopreservation methods, underscoring the need for further research.
Our study results were limited by the relatively small number of RCTs and the small sample size in the included RCTs. Another significant limitation of our study is the absence of RCTs that directly assess the clinical effectiveness of CryoMSCs. Given the topic’s significance, it is strongly recommended that future clinical studies explore this area further. Hence, there is a pressing need for more extensive RCTs to validate these findings and establish standardized cell preservation protocols. This urgent need for further research underscores the importance of our findings and the potential impact on cardiology and regenerative medicine.
In conclusion, our systematic review of the literature’s meta-analysis reveals that CryoMSCs significantly improved LVEF by an average of 2.11%, with superior outcomes observed when employing CryoMSCs with post-thaw viability exceeding 80%. Furthermore, the safety profile of CryoMSCs did not show a significant incidence of adverse events compared to the control. This suggests that CryoMSCs hold promise as a viable cell product for off-the-shelf use in patients with CVD, offering a positive outlook for the future of this treatment[37]. This promising outlook should encourage further research and development in this area.
| 1. | World Health Organization. Cardiovascular diseases (CVDs). [cited 15 August 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). |
| 2. | Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 954] [Reference Citation Analysis (0)] |
| 3. | Kalou Y, Al-Khani AM, Haider KH. Bone Marrow Mesenchymal Stem Cells for Heart Failure Treatment: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023;32:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Reference Citation Analysis (0)] |
| 4. | Alvarez-Viejo M, Haider KH (2022). Mesenchymal Stem Cells. In: Haider, K.H. (eds) Handbook of Stem Cell Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-16-6016-0_6-12. |
| 5. | Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 7. | National Library of Medicine. Search results for “mesenchymal stem cells”. [cited 18 February 2025]. Available from: https://clinicaltrials.gov/search?intr=mesenchymal%20stem%20cells. |
| 8. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 428] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 9. | Linkova DD, Rubtsova YP, Egorikhina MN. Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products. Cells. 2022;11:2691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 10. | Bahsoun S, Coopman K, Akam EC. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review. J Transl Med. 2019;17:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, Lakey JR. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021;30:963689721999617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 4619] [Article Influence: 1154.8] [Reference Citation Analysis (0)] |
| 13. | Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf). 2022;44:e588-e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 226] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 14. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24860] [Article Influence: 1775.7] [Reference Citation Analysis (3)] |
| 15. | Bolli R, Perin EC, Willerson JT, Yang PC, Traverse JH, Henry TD, Pepine CJ, Mitrani RD, Hare JM, Murphy MP, March KL, Ikram S, Lee DP, O'Brien C, Durand JB, Miller K, Lima JA, Ostovaneh MR, Ambale-Venkatesh B, Gee AP, Richman S, Taylor DA, Sayre SL, Bettencourt J, Vojvodic RW, Cohen ML, Simpson LM, Lai D, Aguilar D, Loghin C, Moyé L, Ebert RF, Davis BR, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Allogeneic Mesenchymal Cell Therapy in Anthracycline-Induced Cardiomyopathy Heart Failure Patients: The CCTRN SENECA Trial. JACC CardioOncol. 2020;2:581-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Chullikana A, Majumdar AS, Gottipamula S, Krishnamurthy S, Kumar AS, Prakash VS, Gupta PK. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 18. | Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res. 2015;117:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Ulus AT, Mungan C, Kurtoglu M, Celikkan FT, Akyol M, Sucu M, Toru M, Gul SS, Cinar O, Can A. Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial). Int J Stem Cells. 2020;13:364-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | He X, Wang Q, Zhao Y, Zhang H, Wang B, Pan J, Li J, Yu H, Wang L, Dai J, Wang D. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2016236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 22. | Holzinger A, Abken H. Treatment with Living Drugs: Pharmaceutical Aspects of CAR T Cells. Pharmacology. 2022;107:446-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 23. | Litvinova LS, Shupletsova VV, Khaziakhmatova OG, Daminova AG, Kudryavtseva VL, Yurova KA, Malashchenko VV, Todosenko NM, Popova V, Litvinov RI, Korotkova EI, Sukhorukov GB, Gow AJ, Weissman D, Atochina-Vasserman EN, Khlusov IA. Human Mesenchymal Stem Cells as a Carrier for a Cell-Mediated Drug Delivery. Front Bioeng Biotechnol. 2022;10:796111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Omar T. Ahmed OT, Ahmed ZT, Dairi AW, Zain Al-Abeden MS, Alkahlot MH, Alkahlot RH, Al Jowf GI, Eijssen LMT, Haider KH. The Inconclusive Superiority Debate of Allogeneic Vs. Autologous MSCs in Treating Patients with HFrEF: A Systematic Review and Meta-Analysis of RCTs. Stem Cell Res Ther. 2025;. |
| 25. | Li C, Zhao H, Cheng L, Wang B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021;11:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 26. | Safwan M, Bourgleh MS, Alshakaki H, Molhem A, Haider KH (2024a). Morbid Cell Status and Donor Age Significantly Alter Mesenchymal Stem Cell Functionality and Reparability. In: K. H. Haider (ed.), Handbook of Stem Cell Applications, Springer Nature Singapore Pte Ltd., pp.1359-1387, 2024. https://doi.org/10.1007/978-981-99-7119-0_6227. |
| 27. | Safwan M, Bourgleh MS, Aldoush M, Haider KH. Tissue-source effect on mesenchymal stem cells as living biodrugs for heart failure: Systematic review and meta-analysis. World J Cardiol. 2024;16:469-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 28. | Bárcia RN, Santos JM, Teixeira M, Filipe M, Pereira ARS, Ministro A, Água-Doce A, Carvalheiro M, Gaspar MM, Miranda JP, Graça L, Simões S, Santos SCR, Cruz P, Cruz H. Umbilical cord tissue-derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy. 2017;19:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA; Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738-3744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 618] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 30. | Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D; Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 467] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 31. | Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effect of renin-angiotensin system inhibition on cardiac structure and function and exercise capacity in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2021;26:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Anand I. Stable but Progressive Nature of Heart Failure: Considerations for Primary Care Physicians. Am J Cardiovasc Drugs. 2018;18:333-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Traverse JH. Is There a Role for Intravenous Stem Cell Delivery in Nonischemic Cardiomyopathy? Circ Res. 2017;120:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Attar A, Farjoud Kouhanjani M, Hessami K, Vosough M, Kojuri J, Ramzi M, Hosseini SA, Faghih M, Monabati A. Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial. Stem Cell Res Ther. 2023;14:264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 36. | Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, Paterson K, Boyd R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14:213-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (2)] |
| 37. | Yong KW, Wan Safwani WK, Xu F, Wan Abas WA, Choi JR, Pingguan-Murphy B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv Biobank. 2015;13:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |