TO THE EDITOR
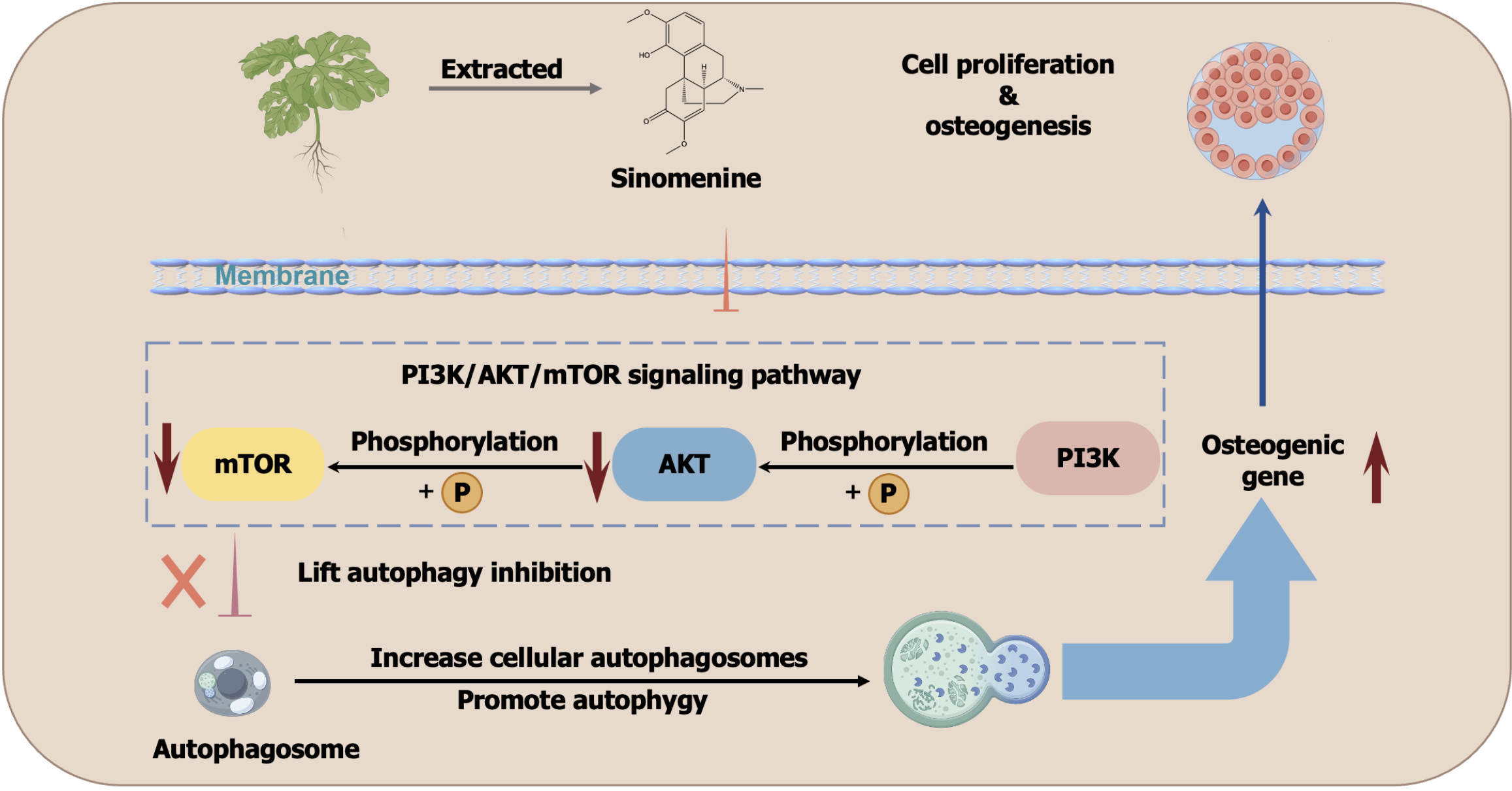
Xiao et al[1] reported on the anti-osteoporosis activity of the natural product sinomenine (SIN), which is a traditional Chinese medicine. The major underlying mechanism of SIN involves the inhibition of phosphorylation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin signalling pathway in bone marrow mesenchymal stromal cells, which increases the autophagic capacity and promotes osteogenic differentiation. Bone loss antagonism, osteogenic differentiation promotion, autophagy induction and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin inhibition were assessed. The overall outline of this work is shown in Figure 1 (modified from the original work).
Figure 1 Scheme of the study by Xiao et al[1].
This figure is modified from the original work[1]. mTOR: Mammalian target of the rapamycin; AKT: Protein kinase B; PI3K: Phosphatidylinositol 3-kinase.
It is important to consider the delivery of SIN to bone marrow mesenchymal stromal cells or ovariectomised mice in the original study. Based on the methodology, SIN was dissolved in dimethyl sulfoxide (DMSO) and subsequently diluted to the required concentration for injection. In animal studies, the resulting SIN solution was administrated via intraperitoneal injection. For clinical application, intraperitoneal injection must be modified to intravenous or intramuscular injection. A more challenging problem is that DMSO-containing solvents are not favourable for developing an injection formulation because it has several safety concerns. First, DMSO is highly permeable in the mucous membranes[2]. For injectable formulations, DMSO may be utilised for other ingredients, which include potentially harmful impurities, into the body along with it, thus increasing the risk of side effects or unpredictable systemic reactions. Second, DMSO irritates the skin and mucous membranes and may cause discomfort or inflammation at the injection site[3]. Such irritation renders the use of the injection unsuitable or unsafe. Third, DMSO may react with other components from the injectables to affect the stability and potency of the drug. For instance, it may cause chemical degradation of the drug[4]. The reason for using DMSO by Xiao et al[1] was that SIN showed a low water solubility[5] and dissolving it in DMSO could achieve higher solubility. We have developed alternative techniques to solubilise SIN in water, which can avoid the use of DMSO and reduce safety concerns.
From the perspective of pharmaceutics, several techniques for solubilization, including amorphisation, emulsification, micellisation, nano-crystallisation and host-guest inclusion exist. Notably, amorphisation does not significantly improve the solubility of SIN (with only an approximate 7% increment), as shown previously[6]. Although emulsification optimises the solubility of SIN, emulsifiers have stability issues and may interact with the drug to affect its bioavailability[7]. Micellisation involves the encapsulation of SIN in micelles to improve solubility; however, the material utilised to prepare micelles may affect the rate of drug release, whereas the formulation and concentration of the micelles must be optimised to avoid potential interactions[8]. Nano-crystallisation can significantly enhance the solubility and dissolution rate of SIN; however, the preparation process for nano-crystallisation is complex and costly[9]. Additionally, the stability of the nanoparticles and potential particle aggregation must be addressed. Host-guest inclusion improves the solubility of SIN[10], which effectively prevents recrystallisation of the drug in an aqueous phase by encapsulating SIN in the hydrophobic inner cavity of the host molecule, thus optimising its solubility and bioavailability. Compared with the solubilization methods listed above, host-guest inclusion has the advantages of improved stability, lower cost and an increased release rate and bioavailability of the drug. Therefore, we conclude that host-guest inclusion holds promise for SIN solubilization.
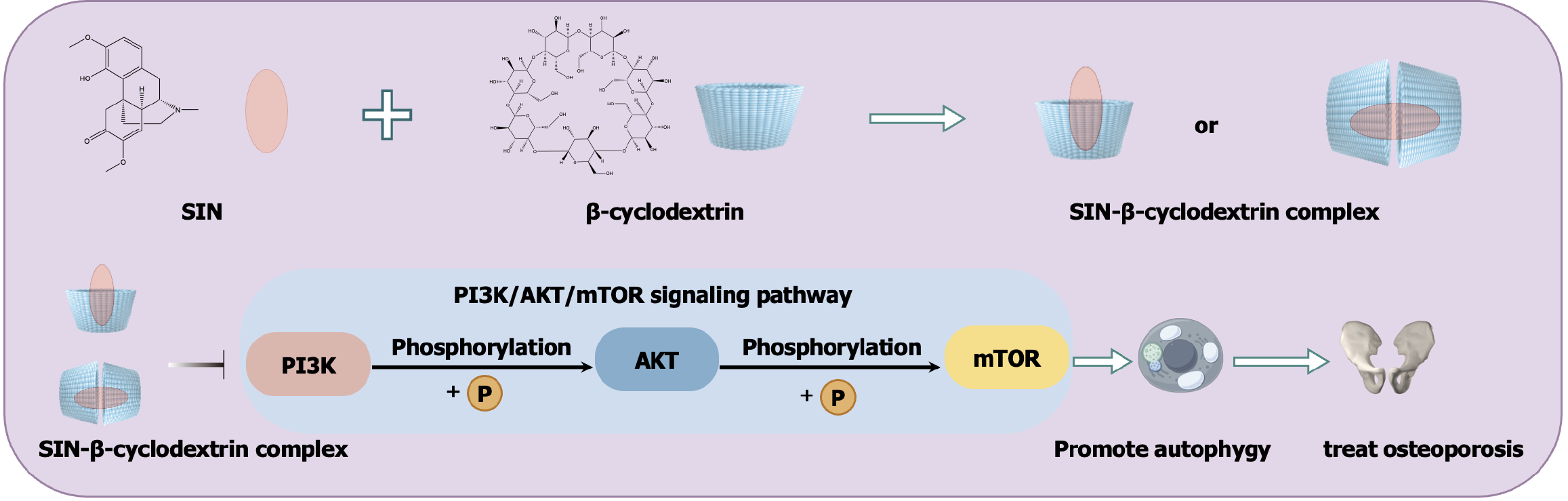
Host-guest inclusion is defined as a drug molecule wholly or partially encapsulated into the molecular cavity of the encapsulation agent[11]. Herein, the ‘host’ and ‘guest’ refer to the encapsulation agent and the drug, respectively. Materials that are commonly utilised as encapsulation agents include cyclodextrins, calixarene and cucurbits. Of these, cyclodextrins are widely considered to be the best materials for host-guest inclusion because of their excellent biocompatibility, efficient encapsulation ability, mature preparation process and good drug stability[12]. There are three major categories of cyclodextrins, namely α-, β-, and γ-cyclodextrin. β-cyclodextrin has a larger cavity inner diameter compared to α-cyclodextrin and a lower production cost compared to γ-cyclodextrin, and therefore it has rapidly become a research hotspot in recent years. According to the literature[13], β-cyclodextrin is widely employed because of its strong interaction with various guest molecules. Several derivatives of β-cyclodextrin have been developed, such as 2-hydroxypropyl-β-cyclodextrin, sulfobutyl aether-β-cyclodextrin and amino-β-cyclodextrin. They exhibit superior solubilization effects, better drug stability, lower toxicity and side effects and optimised drug release properties compared with common β-cyclodextrin[14]. Some commercially available injections use cyclodextrin as the solubiliser, such as Cylert® (containing 2-hydroxypropyl-β-cyclodextrin), DepoCyte® (containing sulfobutyl aether-β-cyclodextrin) and Kytril® (containing a mixture of α, β, and γ-cyclodextrins)[15]. Noteworthily, a previous study synthesised a β-cyclodextrin-grafted polymer to encapsulate SIN, which showed a high interaction intensity with the guest molecule. Overall, we believe that the β-cyclodextrin inclusion complex can effectively solubilise SIN, thus circumventing the need for DMSO and facilitating its clinical application (Figure 2).
Figure 2 Illustration of the scheme to solubilise sinomenine with β-cyclodextrin and its application.
SIN: Sinomenine; mTOR: Mammalian target of the rapamycin; AKT: Protein kinase B; PI3K: Phosphatidylinositol 3-kinase.
To facilitate the clinical translation of the SIN-β-cyclodextrin complex, several prerequisites must be fulfilled. First, a safety assessment, which includes toxicity and allergic reactions, must be carried out on the SIN-β-cyclodextrin complex. Second, pharmacodynamic evaluation is required to ensure that the complex has the desired therapeutic effect. Stability testing studies are also required to ensure that it is stable during storage and use. Finally, compliance and regulatory approvals are required and SIN-β-cyclodextrin complexes must comply with relevant drug regulations and clinical trial requirements to obtain official approval. Once all the above are achieved, the clinical application of the relevant products will be possible.
In conclusion, in the study of Xiao et al[1] evaluating SIN to modulate autophagy for the treatment of osteoporosis, considering the safety issue of DMSO solubilization, we proposed a safer method, which involves the preparation of SIN-β-cyclodextrin complexes instead of DMSO co-solubilization. After comparing different methods of solubilising SIN, we contend that the use of the host-guest inclusion method with β-cyclodextrin and its derivatives is superior for the solubilization of SIN. However, host-guest inclusion technology has several disadvantages such as limited release control, stability issues and toxicity risks. These disadvantages can affect the clinical transformation of SIN-β-cyclodextrin complexes by complicating dose optimization, controlling release profiles, and ensuring long-term safety. Considering the future clinical application of SIN-β-cyclodextrin complexes, issues including safety, efficacy, stability and the relevant laws and regulations must be addressed. Once these issues are resolved, the clinical application of SIN-β-cyclodextrin complexes to treat osteoporosis may be achieved.