INTRODUCTION
Fracture is one of the most pervasive injuries in the musculoskeletal system. The complex physiological process of fracture healing has been extensively studied[1]. Following bone injury, a variety of inflammatory cells participate in the response, and the inflammatory response at the injury site affects the process of osteogenic differentiation[2]. Bone tissue engineering is a critical and innovative field in the treatment of bone injuries. Multiple relevant studies have been conducted, offering a valuable clinical foundation for the treatment of bone defects[3,4]. Owing to their unique ability to differentiate into osteogenic lineages, several stem cell types, including adipose tissue-derived stem cells (ADSCs), bone marrow mesenchymal stem cells (MSCs), and embryonic stem cells, have garnered significant attention in bone tissue engineering in recent years[5]. Among these, ADSCs, which are tissue stem cells that possess multidirectional differentiation potential and are present in the stromal vascular fraction (SVF) of adipose tissue, offer several benefits, such as easy availability of materials, plentiful sources, and the ability to interact with other inflammatory cells[6]. Therefore, ADSCs are widely used as stem cell sources for bone tissue engineering research. Following bone injury, macrophages are essential for regulating inflammatory responses[7]. Under the chemotaxis of mediators released by macrophages, MSCs and pericytes derived from bone marrow and other tissues migrate toward the trauma site and differentiate into osteoblasts to contribute to tissue repair[8].
Complex interactions exist between macrophages and ADSCs, consequently affecting the activity, function, and potential clinical applications of ADSCs[9]. Our previous studies established a two-dimensional (2D) coculture model of macrophages with ADSCs[10], and we discovered that macrophages promoted the osteogenic differentiation of ADSCs. Although 2D culture is the standard technique for in vitro cell culture, its ability to accurately mimic the complex microenvironment of cells in vivo, including the intricate cellular matrix, vascular and nutrient transport, and growth patterns, is limited[11]. In 3D culture, each cell can be surrounded by surrounding cells or an extracellular matrix, which corresponds to the normal in vivo ecological niche[12]. It is a good simulation of cell-cell junctions and cell-substrate interactions. In addition, the 3D coculture models can have more cell connections and morphogenetic movements than can the 2D coculture models in terms of cell adhesion, cell migration, and inflammatory activation processes, which can better mimic the in vivo environment[13-15]. Using 3D culture technology, we further established a 3D coculture model of macrophages and ADSCs by inoculating the cells with biocompatible scaffolds[16] or allowing them to aggregate into small spheres[17] to further elucidate the relationship between macrophages and ADSCs[18,19].
Interestingly, the results of the 3D culture model showed that macrophages inhibited the osteogenic differentiation of ADSCs, which was completely opposite to the results of the 2D culture model. These results confirmed that both the 2D and 3D culture models exerted different influences on the macrophage-ADSC interaction. N-cadherin-mediated intercellular junctions also play an important role in interfering with the osteogenesis of ADSCs[20]. This suggests that under 3D culture conditions, intercellular junctions form complex interactions that may have a significant impact on cellular functions[21,22].
N-cadherin is a single-stranded transmembrane glycoprotein that promotes adhesion between homologous and heterotypic cells in a calcium-dependent manner. As a member of the calcineurin family, N-cadherin plays crucial roles in regulating the development and function of the brain, nervous system, hematopoietic system, vasculature, skeletal muscle, heart, and microenvironment[23]. N-cadherin promotes osteogenic differentiation in the early stages but is challenging to identify in fully differentiated osteoblasts, indicating varying roles in different stages of osteogenic differentiation[24]. Our previous study revealed that N-cadherin plays a different role in osteogenic differentiation at different time points and that N-cadherin is expressed in macrophages, whereas it is not expressed in ADSCs, which is consistent with previously reported findings. In addition, Wnt/β-catenin signaling plays a vital role in regulating the stem cell resting phase and drug resistance[25]. E-cadherin is also crucial for macrophage function through the Wnt/β-catenin signaling pathway. However, the effect of N-cadherin on macrophages has not been reported[26,27]. In our previous study, by investigating the effect of macrophages on the osteogenic differentiation of ADSCs, we found that intercellular connectivity exerted an important influence on cellular function in a 3D environment and that the differential expression of N-cadherin was an important factor affecting stem cell osteogenesis.
In previous studies, osteogenic 2D and 3D coculture models of ADSCs and macrophages were established to investigate the role of macrophages in the osteogenic differentiation of ADSCs. The degree of osteogenic differentiation was assessed through Alizarin red staining and quantitative analysis of osteogenic markers using polymerase chain reaction (PCR), while western blotting was employed to analyze proteins involved in relevant signaling pathways. By exploring the pathophysiological processes underlying ADSC osteogenesis, this study provides a refined protocol for studying osteogenic differentiation in ADSCs and offers novel insights into 3D osteogenesis, fracture repair, and regeneration of bone tissue after trauma.
MATERIALS AND METHODS
Acquisition and characterization of ADSCs
Young, healthy patients aged between 20 and 30 underwent liposuction to extract adipose tissue from their waist, abdomen, or thighs. All operations were performed through the ethical committee of Tongji Hospital. The tissue was digested with collagenase, filtered through a screen, and centrifuged in a density gradient to obtain the SVF. The SVFs were then inoculated into culture flasks at a density of 1 × 105/cm2, and the wall cells obtained after 3-5 passes were considered ADSCs[28]. The cells were either preserved through freezing or cultured for further progeny. To evaluate the differentiation potential of ADSCs, the cells were cultured in osteogenic, adipogenic, and chondrogenic induction media. Differentiation outcomes were assessed using Alcian blue staining for chondrogenesis, Oil Red O staining for adipogenesis, and Alizarin red staining for osteogenesis. Additionally, the expression of surface markers, including CD29, CD44, CD31, and CD34, was analyzed via flow cytometry to characterize the ADSCs.
Induced differentiation of the THP-1 cell line
The THP-1 monocyte cell line was purchased, cultured, and passaged in RPMI-1640 medium supplemented with 10% foetal bovine serum, which could be used for macrophage-induced differentiation after 3 generations. The induction protocol was as follows: THP-1 cells were induced to become macrophages after 48 hours by the addition of 50 ng/mL phorbol-12-myristate-13-acetate[29].
2D coculture of ADSCs-macrophages
To investigate the differences in ADSC and macrophage coculture under different conditions, 2D cocultures were divided into two groups. In the 2D-ADSC group, 5 × 104 ADSCs were cultured alone, and in the 2D-ADSC-THP-1 group, 5 × 104 ADSCs and 5 × 104 macrophages were cultured together. In 2D culture, the direct coculture method was used to obtain ADSCs and THP-1-differentiated macrophages, which were mixed according to the cell types and numbers of the above groups in 24-well plates and then cultured for 28 days by the addition of osteogenic medium (containing 10 nM dexamethasone, 100 μM ascorbic acid, and 10 mmol/L sodium β-glycerophosphate).
3D coculture of ADSCs-macrophages
To investigate the differences in ADSC and macrophage coculture under different conditions, 3D cocultures were divided into two groups. In the 3D-ADSC group, 5 × 104 ADSCs were cultured alone, and in the 3D-ADSC-THP-1 group, 5 × 104 ADSCs and 5 × 104 macrophages were cultured together. 3D culture was performed by the suspension drop method; 10 cm diameter Petri dishes were prepared with 15 mL of phosphate buffered saline (PBS), and the lid of each Petri dish was turned over and placed on an ultraclean table. For coculture, the concentration of macrophages was adjusted to 2.86 × 106/mL, and then the macrophages were mixed with an equal volume and concentration of ADSCs. For individual cultures, the concentration of ADSCs was adjusted to 2.86 × 106/mL, and then the ADSCs were mixed with an equal volume of medium. The cell suspension was mixed well, and the cell suspension was pushed onto the inside of the Petri dish lid with a micropipette at 35 μL per spot so that the cell suspension had a hemispherical protrusion. The lid was carefully flipped over the dish, and the mixture was incubated in the incubator for 3 days until the cells aggregated to form spheres. Finally, the pellets were transferred to 48-well nonadherent culture plates and incubated with osteogenic medium for 28 days.
For live-cell staining of cultured spheroplasts, the viability of the spheroplasts was assessed after spheroplast formation using the Calcein-AM/PI Toxicity Assay Kit (Yesen, China). The spheroids were washed twice with PBS and incubated in PBS containing 2 μM calcein-AM and 1.5 μM PI for 15 minutes at room temperature. The samples were observed via a SOPTOP CX40 microscope (Shanghai, China).
Alizarin red staining and Semiquantification
The cells were fixed in 4% paraformaldehyde for 10 minutes after 14 and 28 days of osteogenic differentiation and then washed with distilled water. After the cells were stained for 2 minutes with Alizarin red staining, they were cleaned once more with distilled water and photographed using a microscope. Alizarin red was analyzed by quantitative detection using the colorimetric method. After the distilled water was removed, the plates remained at room temperature until they were completely dry. After 280 μL of 10% acetic acid was added, the plate was agitated for 30 minutes. The mixture was then transferred to an EP tube and heated for 10 minutes to 85 °C before being chilled on ice for 5 minutes. Following 20 minutes of centrifugation at 16000 × g, 100 μL of the supernatant was transferred into a tube along with 40 μL of 10% ammonium hydroxide. After the finished solution was transferred to a 96-well plate, a microplate reader was used to measure the absorbance at 405 nm.
Quantitative real-time PCR
To extract total RNA from the cell precipitates, we used RNAiso Plus (Takara Biomedical Technology, China). To obtain stable cDNA, Hieff first-strand cDNA Synthesis Super Mix (Yeasen, China) was then used following the manufacturer’s instructions. To assess mRNA expression precisely, SYBR green real-time PCR Master Mix (Yeasen, China) was utilized. The primer sequences are shown in Supplementary Table 1.
Western blotting
Western blotting was used to identify protein expression. The cell samples were homogenized using cell lysis buffer (Cell Signaling Technology, United States), and the supernatant was obtained via centrifugation. The extracted proteins were separated on 10% or 8% SDS-polyacrylamide gels and subsequently transferred onto polyvinylidene fluoride membranes (Bio-Rad, United States) through electrotransfer. After the membranes were blocked for 1 hour, they were incubated overnight at 4 °C with primary antibodies. Following thorough washing, the membranes were incubated with HRP-conjugated secondary antibodies (Proteintech, Chicago, IL, United States) at a 1:5000 dilution for 2 hours at room temperature. Using the ECL western blotting kit (Proteintech, Chicago, IL, United States), the protein bands were detected and quantified using ImageLab software. The antibodies used are shown in Supplementary Table 2.
Statistical analysis
The data in the article are presented as the means ± SDs. Quantitative outcomes were evaluated using one-way ANOVA, with statistical significance defined as P < 0.05. All the statistical analyses were conducted using SPSS Statistics 25 software (IBM, New York, NY, United States).
RESULTS
Successful isolation and characterization of ADSCs
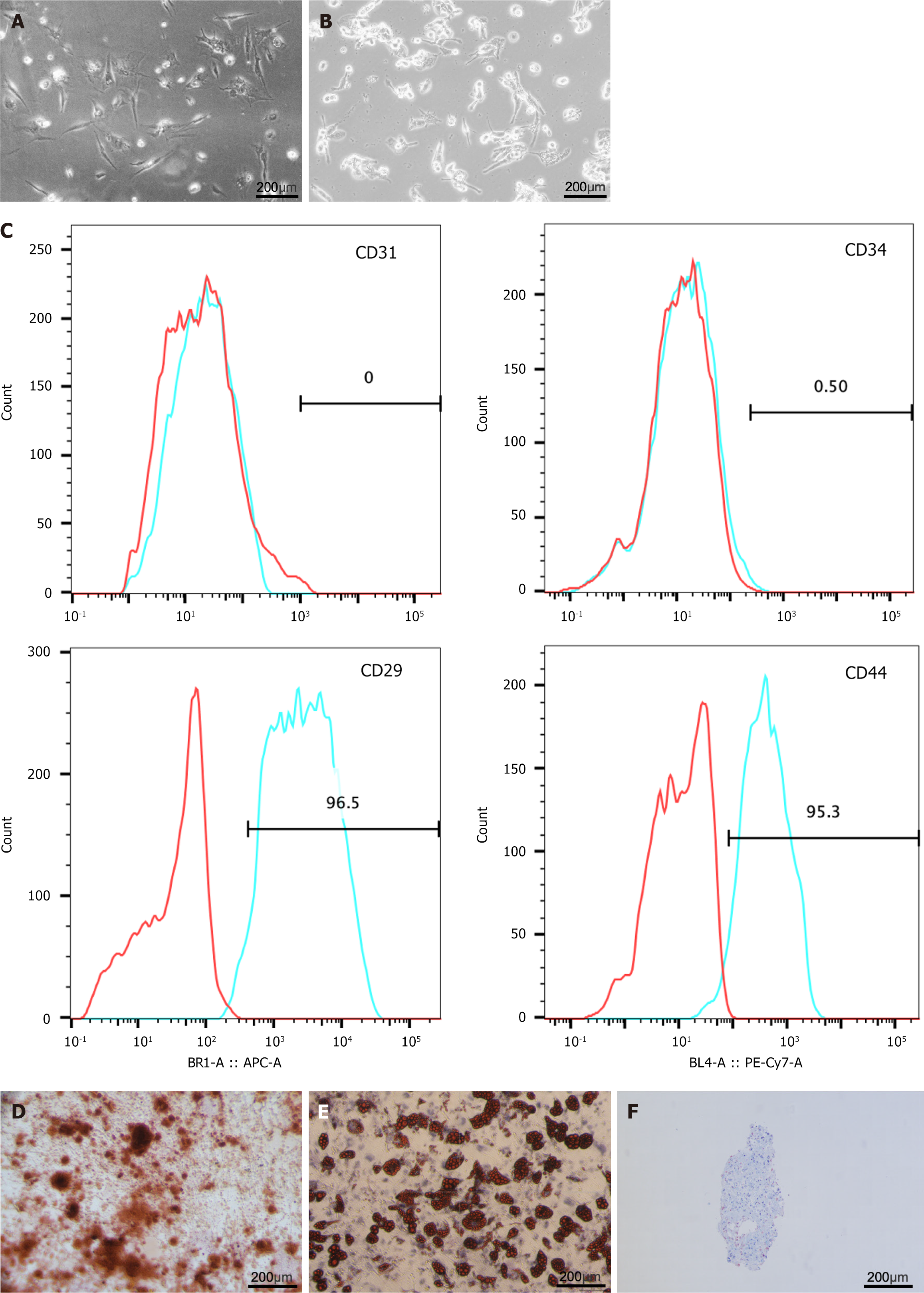
To obtain ADSCs, adipose tissue obtained from microtia surgery was digested with collagenase, screened, and subjected to density gradient centrifugation to obtain the SVF. After the SVFs were inoculated and passaged for 3-5 passages, we isolated and extracted human ADSCs. In passages 1-2, only a small number of cells adhered to the wall and were in a low-density state, forming a large, flattened monolayer. In passages 3-5, the stem cell growth morphology favored a long spindle shape and displayed a fibroblast-like morphology with abundant cytoplasm and nucleoli in parallel or swirling growth arrangements in a favorable culture state (Figure 1A). In the context of macrophage culture, normal THP-1 cells exhibited a complete morphology, good refractive qualities, and existed as single cells in suspension. After 48 hours of phorbol-12-myristate-13-acetate induction, the cells were wall-adherent round or oval shaped and larger in size (Figure 1B). ADSCs were incubated with fluorescence-conjugated antibodies and analyzed using flow cytometry. The results demonstrated that ADSCs were positive for the MSC surface markers CD29 and CD44 but negative for the endothelial marker CD31 and the hematopoietic marker CD34 (Figure 1C). Additionally, Alizarin red, Oil Red O, and Alcian blue staining confirmed the osteogenic, adipogenic, and chondrogenic differentiation capabilities of the ADSCs (Figure 1D-F). Therefore, ADSCs were successfully isolated and characterized.
Figure 1 The isolation, extraction and characterisation of adipose tissue-derived stem cell and THP1.
A: Cell culture micrographs of adipose tissue-derived stem cells; B: Cell culture micrographs of THP1; C: Flow cytometry showed adipose tissue-derived stem cells positive for CD29 and CD44, negative for CD31 and CD34; D-F: Osteogenic, adipogenic, and chondrogenic differentiations measured by Alizarin Red staining, Oil Red O staining, and Alcian Blue staining.
Osteogenic differentiation effects of macrophages on ADSCs in 2D or 3D culture
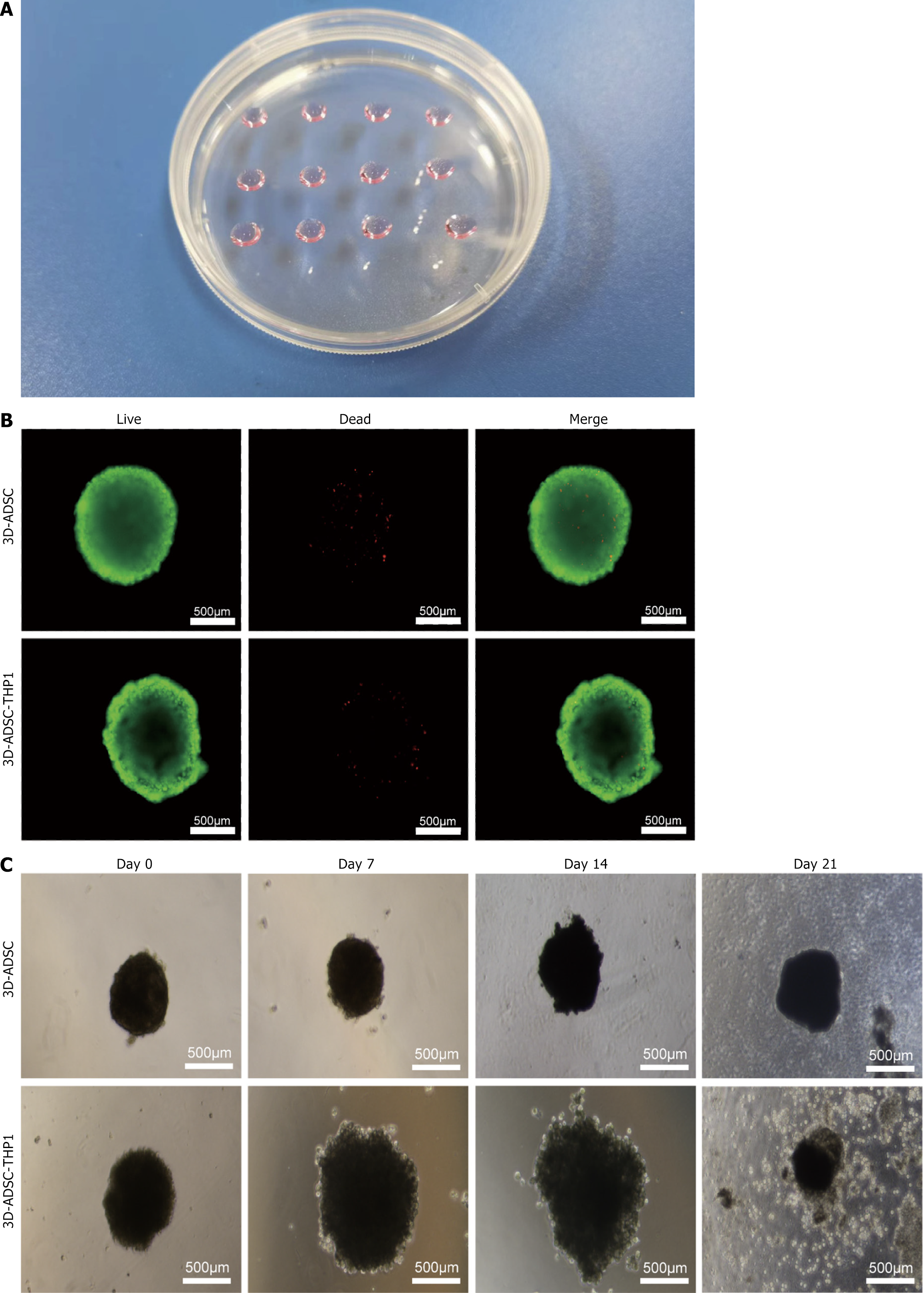
Figure 2A presents a physical image of the 3D cell culture model that was prepared using the hanging drop method. Live cell staining revealed that the cells inside the spheres had good viability with negligible cell death during sphere formation, and most of the cells demonstrated strong proliferative activity capacity (Figure 2B). Images captured by the microscope revealed variations in the spheres between the two groups of cells. The ADSC spheres had a smoother and more uniform structure, whereas the ADSC-THP-1 spheres were irregular and had a larger diameter. These cell spheres were cultured in nonadherent plates and adhered to the bottom of the plates, maintaining a roughly spherical shape in the first 14 days. After 21 days of culture, the cells were detached from the spheres and subsequently proliferated by adhering to the bottom of the culture plate (Figure 2C).
Figure 2 Three-dimensional culture of adipose tissue-derived stem cells.
A: Macro photographs of three-dimensional culture cell; B: Immunofluorescence staining of alive and dead stem cells; C: Three-dimensional cultured cell spheroids recorded on day 0, 7, 14, and 21, and the cells are gradually detached from the and migrated to the culture plate with the prolongation of culture time. 3D: Three-dimensional; ADSC: Adipose tissue-derived stem cell.
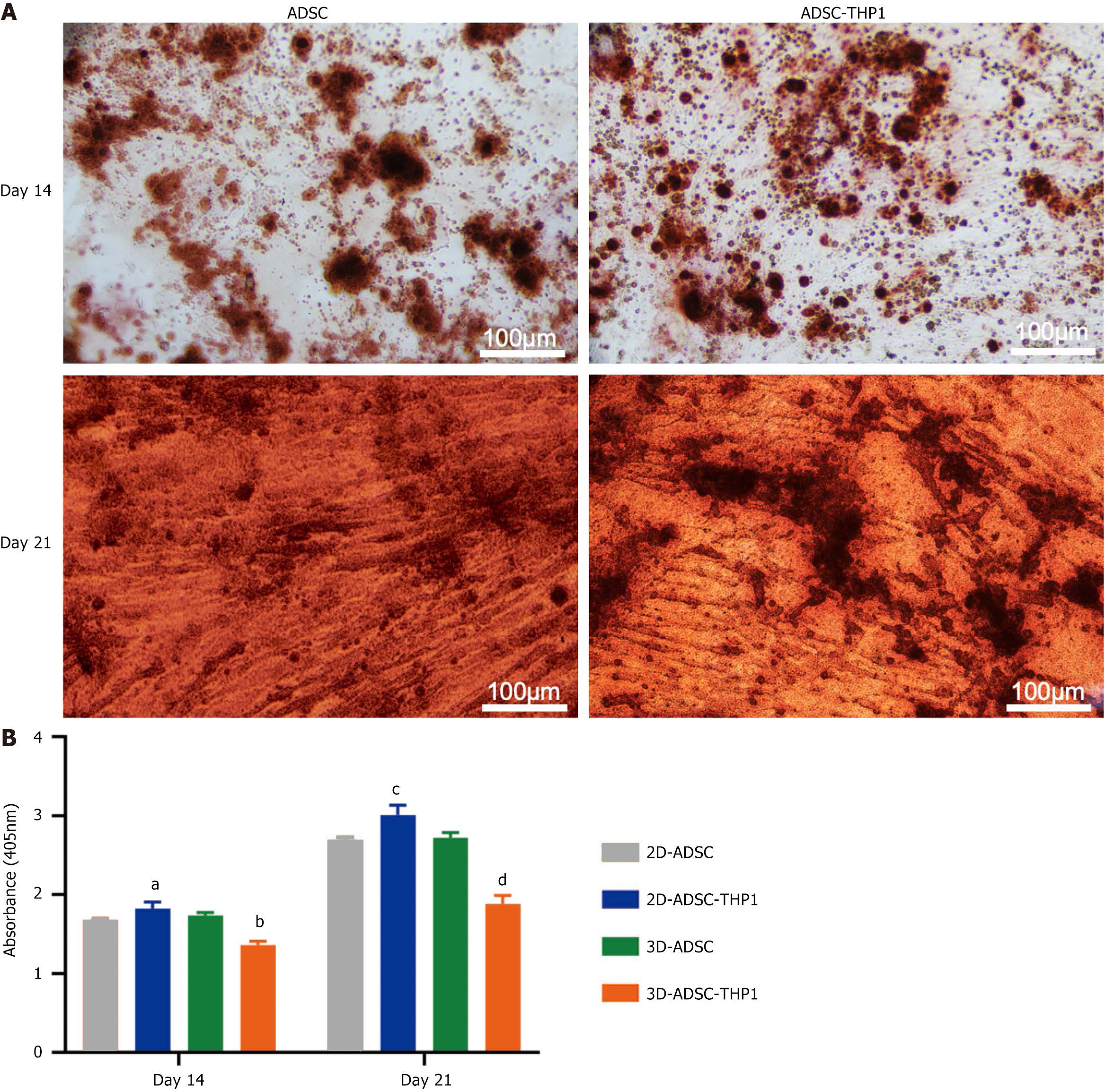
After 14 and 28 days of osteogenic culture, the 2D-ADSC-THP-1 group displayed greater calcium deposition than did the 2D-ADSC group, as shown by alizarin red staining (Figure 3A). As determined by colorimetry, macrophages effectively induced osteogenic differentiation of ADSCs in the 2D culture groups at 14 and 28 days. In contrast, macrophages hindered the osteogenic differentiation of ADSCs in the 3D culture groups. All the differences were statistically significant (Figure 3B).
Figure 3 Osteogenic differentiation of adipose tissue-derived stem cells.
A: Alizarin red staining of adipose tissue-derived stem cell (ADSC) and ADSC-THP1 in two-dimensional culture at 14 and 28 days; B: Semiquantitative measurement of calcium deposits formed during osteogenic differentiation of ADSCs in each group by alizarin red staining colorimetry. aP < 0.05 vs day14 two-dimensional-adipose tissue-derived stem cell, bP < 0.001 vs day14 three-dimensional-adipose tissue-derived stem cell, cP < 0.05 vs day28 two-dimensional-adipose tissue-derived stem cell, dP < 0.001 vs day28 three-dimensional-adipose tissue-derived stem cell. 3D: Three-dimensional; 2D: Two-dimensional; ADSC: Adipose tissue-derived stem cell.
Osteogenic marker gene expression was analyzed via quantitative real-time PCR. The results of alkaline phosphatase (ALP) gene expression on day 7 revealed no significant difference between the 2D-ADSC and 2D-ADSC-THP-1 groups, but the ALP expression in the 3D-ADSC-THP-1 group was lower than that in the 3D-ADSC group. On day 14, ALP gene expression was notably elevated in the 2D-ADSC-THP-1 group compared with the 2D-ADSC group. In contrast, the 3D-ADSC-THP-1 group presented significantly lower ALP gene expression than the 3D-ADSC group did (Figure 4A). On both day 7 and day 14, the expression levels of osteocalcin (OCN) and Runt-related transcription Factor 2 (RUNX2) were elevated in the 2D-ADSC-THP-1 group compared with those in the 2D-ADSC group, whereas they were reduced in the 3D-ADSC-THP-1 group compared with those in the 3D-ADSC group (Figure 4B and C).
Figure 4 Expression of osteogenic gene markers in adipose tissue-derived stem cells.
A: Relative expression of alkaline phosphatase in each group on day 7 and 14 (aP < 0.05 vs day 7 two-dimensional-adipose tissue-derived stem cell (2D-ADSC), bP < 0.001 vs day 7 three-dimensional (3D)-ADSC, cP < 0.001 vs day 14 2D-ADSC, dP < 0.01 vs day 14 3D-ADSC); B: Relative expression of Runt-related transcription factor 2 mRNA in each group on day 7 and 14 (aP < 0.05 vs day 7 2D-ADSC, bP < 0.01 vs day 7 3D-ADSC, cP < 0.01 vs day 14 2D-ADSC, dP < 0.01 vs day 14 3D-ADSC); C: Relative expression of osteocalcin in each group on day 7 and 14 (aP < 0.001 vs day 7 2D-ADSC, bP < 0.001 vs day 7 3D-ADSC, cP < 0.05 vs day 14 2D-ADSC, dP < 0.01 vs day 14 3D-ADSC). ALP: Alkaline phosphatase; RUNX2: Runt-related transcription factor 2; OCN: Osteocalcin; 3D: Three-dimensional; 2D: Two-dimensional; ADSC: Adipose tissue-derived stem cell.
The osteogenic differentiation function of ADSCs involves the Wnt signaling pathway
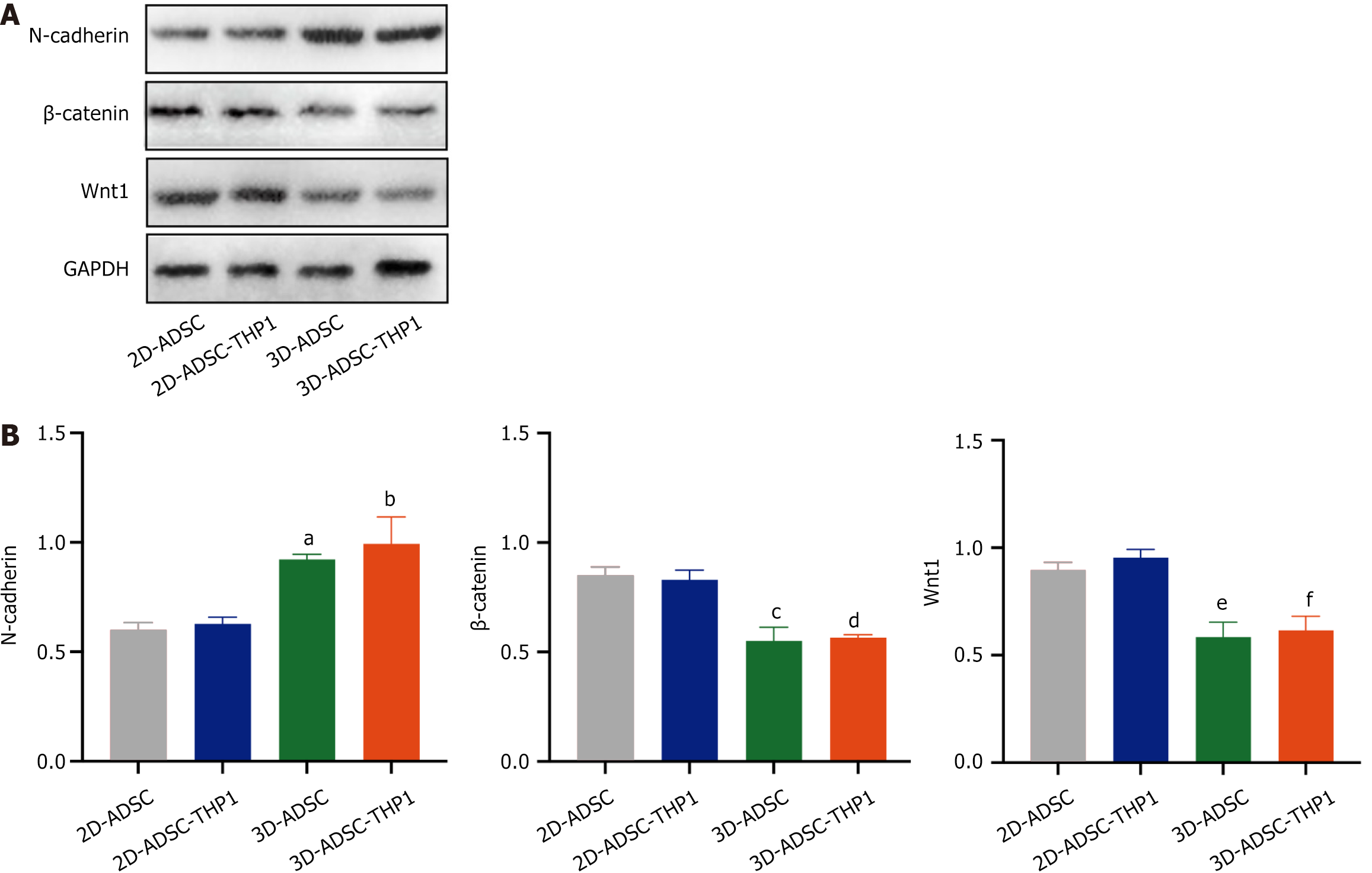
The levels of N-cadherin and proteins involved in the Wnt/β-catenin signaling pathway were quantified via western blotting analysis within each group (Figure 5A and B). The results revealed that the 3D-ADSC-THP-1 group presented a significantly greater level of N-cadherin expression than did the 2D-ADSC-THP-1 group (Figure 5B). In addition, the protein expression of β-catenin and Wnt1 was reduced in the 3D-ADSC-THP-1 group (Figure 5B). These findings indicate that 3D culture might inhibit the Wnt/β-catenin signaling pathway by increasing the expression of N-cadherin, thus inhibiting the osteogenic differentiation of ADSCs.
Figure 5 Quantification of pathway proteins.
A: Measurement of N-cadherin and Wnt1, β-catenin signaling pathway protein expression in each group using western blotting; B: Quantitative analysis of western blotting in each group. aP < 0.01 vs two-dimensional-adipose tissue-derived stem cell (N-cadherin), bP < 0.001 vs two-dimensional-adipose tissue-derived stem cell-THP1 (N-cadherin), cP < 0.001 vs two-dimensional-adipose tissue-derived stem cell (β-catenin), dP < 0.001 vs two-dimensional-adipose tissue-derived stem cell-THP1 (β-catenin), eP < 0.01 vs two-dimensional-adipose tissue-derived stem cell (Wnt1), fP < 0.001 vs two-dimensional-adipose tissue-derived stem cell-THP1 (Wnt1). 3D: Three-dimensional; 2D: Two-dimensional; ADSC: Adipose tissue-derived stem cell.
DISCUSSION
Fractures are prevalent injuries to the musculoskeletal system, with complex interactions occurring between macrophages and ADSCs during the healing process[30]. There have been no relevant studies exploring the changes in N-cadherin expression in macrophages and MSCs during osteogenesis in ADSCs, particularly in the context of 2D and 3D cultures. Therefore, this study established osteogenic 2D and 3D coculture models and investigated the relationship between macrophages and ADSCs through the induction of cell aggregation into small spheres.
The use of coculture systems has rapidly become a suitable tool for studying cell-cell interactions in tissue engineering and regenerative medicine[18,19]. Previous studies explored 2D coculture systems to determine the cell-cell interactions between macrophages and ADSCs[6]. However, traditional 2D culture inadequately mimics the real microenvironment of in vivo cell interactions, ignoring the effects of the complex cellular matrix, vascular transport, nutrient transport, and growth patterns compared with 3D culture when studying the interaction between macrophages and ADSCs[12,31,32]. For example, primary articular chondrocytes and hepatocytes rapidly lose their normal phenotype when removed from in vitro culture and placed in 2D cell cultures, but this loss can be mitigated or even reversed by 3D culture methods[33,34]. The aggregation of ADSCs into 3D multicellular spheroids enhances their resistance to hypoxia-induced cell injury and apoptosis, differentiation potential, and growth factor production and release[35,36]. Our study established a 3D coculture model of macrophages and ADSCs, which can better mimic the real microenvironment of cells in vivo and explore the relationship between macrophages and ADSCs more comprehensively.
Previous studies have explored the effects of macrophages on the osteogenic differentiation of MSCs but reported different effects. Fernandes et al[37] reported that macrophage-conditioned medium (CM) and oncostatin M in CM significantly promoted osteogenic differentiation[37]. However, Chen et al[38] reported that CM inhibited bone morphogenetic protein 2-induced osteogenic differentiation of human MSCs. The culture environment may be one of the factors influencing the results. In this study, we obtained different results in different culture models by establishing 2D and 3D osteogenic coculture models. Under 2D culture conditions, macrophages promoted ADSC osteogenic differentiation, whereas under 3D culture conditions, macrophages inhibited ADSC osteogenic differentiation. Compared with those in 2D culture, the intercellular adhesive junctions in 3D culture more precisely imitated the proliferation and osteogenic alterations of ADSCs in vivo. Moreover, the intricate intercellular bonds in the 3D culture setting could significantly impact the osteogenic differentiation of ADSCs.
N-cadherin is a classical cadherin that mediates intercellular adhesion between neural cells and many other types of nonneural cells[39]. Previous studies have demonstrated that N-calmodulin, which is expressed predominantly by osteoblasts, can control bone formation and bone mass[40]. N-calmodulin directly interacts with the Wnt coreceptors lipoprotein receptor-related protein 5 and lipoprotein receptor-related protein 6, thereby inhibiting Wnt/β-catenin signaling, osteoblast differentiation, and bone formation[41]. In this study, we further revealed that 3D culture conditions might inhibit the Wnt/β-catenin signaling pathway by increasing the expression of N-cadherin, thereby inhibiting the osteogenic differentiation of ADSCs.
This study provides a better understanding of the pathophysiological process of ADSC osteogenesis and a more extensive investigation of ADSC osteogenesis. On the contrary, exploration of the potential molecular mechanisms of ADSC osteogenesis provides a more theoretical basis for the clinical promotion of new targets for bone trauma repair. These results could provide a basis for the clinical application of ADSCs, enabling the rational modulation of macrophage activity or the use of macrophage-targeting drugs to increase the osteogenic differentiation capacity and efficiency of ADSCs. By modifying the culture environment, the osteogenic differentiation process can be precisely and directionally regulated. Moreover, employing a 3D coculture of macrophages and ADSCs allows simulation of the immune cell infiltration environment in bone defects, facilitating a deeper understanding of the functionality and potential osteogenic mechanisms of ADSCs as materials for bone tissue engineering.
However, there are still some issues to be considered in this study. First, the ADSCs used in this study were derived from a limited number of donors, which may not fully represent the characteristics of ADSCs across different ages, sexes, or pathological conditions. Although the 2D coculture model is more closely aligned with the in vivo environment than the 3D coculture model is, there are still some simplified treatments that may not fully replicate the mechanical microenvironment and biomechanical environment of bone tissue. Furthermore, the samples of ADSCs used in this study were derived from a limited number of donors, which may have resulted in an inability to fully represent the characteristics of ADSCs across different age groups, sexes, or pathological states, and different types of macrophages may have different degrees of influence on the osteogenic differentiation of ADSCs. Osteogenic differentiation is a highly dynamic, long-term process, and the signaling requirements and pathway activity may differ significantly at different stages of differentiation. A single observation of the results and the focus on only the N-cadherin-regulated Wnt signaling pathway may not fully capture the temporal dynamics of the signaling pathways. Future studies can further explore alternative molecular mechanisms to achieve a more comprehensive understanding of the osteogenic differentiation mechanism of ADSCs. In addition, this study was performed only at the cellular level, and future studies can further investigate the osteogenic differentiation effects of macrophages in animal experiments.
CONCLUSION
Macrophages promoted ADSC osteogenic differentiation in 2D culture conditions but inhibited it in 3D culture. Compared with 2D cultures, ADSCs in 3D cultures maintained high levels of activity. Additionally, the 3D culture environment might inhibit the Wnt/β-catenin signaling pathway by upregulating N-cadherin expression, ultimately hindering the osteogenic differentiation of ADSCs. Furthermore, the intercellular adhesive connections in the 3D-ADSC-THP-1 coculture were able to simulate the proliferation and osteogenic changes of ADSCs better than those in the other cocultures, thus influencing the osteogenic differentiation of ADSCs. By investigating the process of osteogenesis in ADSCs, this study provides novel ideas for exploring 3D osteogenesis in ADSCs, fracture repair, and other types of bone trauma repair.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade D
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade C
P-Reviewer: Gangadaran P; Kuhlmann C S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD