Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.98693
Revised: December 9, 2024
Accepted: January 23, 2025
Published online: February 26, 2025
Processing time: 234 Days and 22.7 Hours
Skeletal muscle atrophy results from disruptions in the growth and metabolism of striated muscle, leading to a reduction or loss of muscle fibers. This condition not only significantly impacts patients’ quality of life but also imposes substantial socioeconomic burdens. The complex molecular mechanisms driving skeletal muscle atrophy contribute to the absence of effective treatment options. Recent advances in stem cell therapy have positioned it as a promising approach for addressing this condition. This article reviews the molecular mechanisms of muscle atrophy and outlines current therapeutic strategies, focusing on mesenchymal stem cells, induced pluripotent stem cells, and their derivatives. Additionally, the challenges these stem cells face in clinical applications are discussed. A deeper understanding of the regenerative potential of various stem cells could pave the way for breakthroughs in the prevention and treatment of muscle atrophy.
Core Tip: Muscle atrophy can exert a considerable influence on the quality of life of patients. The intricate molecular mechanisms of muscle atrophy give rise to a scarcity of effective treatment options. Recent advancements in stem cell therapy imply that it is a promising solution to this problem. This review summarizes the molecular mechanisms of muscle atrophy, presents current treatment strategies with a focus on mesenchymal stem cells, induced pluripotent stem cells, and their derivatives. Finally, it discusses the challenges encountered in the clinical application of these stem cells.
- Citation: Wang YJ, Chen ZH, Shen YT, Wang KX, Han YM, Zhang C, Yang XM, Chen BQ. Stem cell therapy: A promising therapeutic approach for skeletal muscle atrophy. World J Stem Cells 2025; 17(2): 98693
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/98693.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.98693
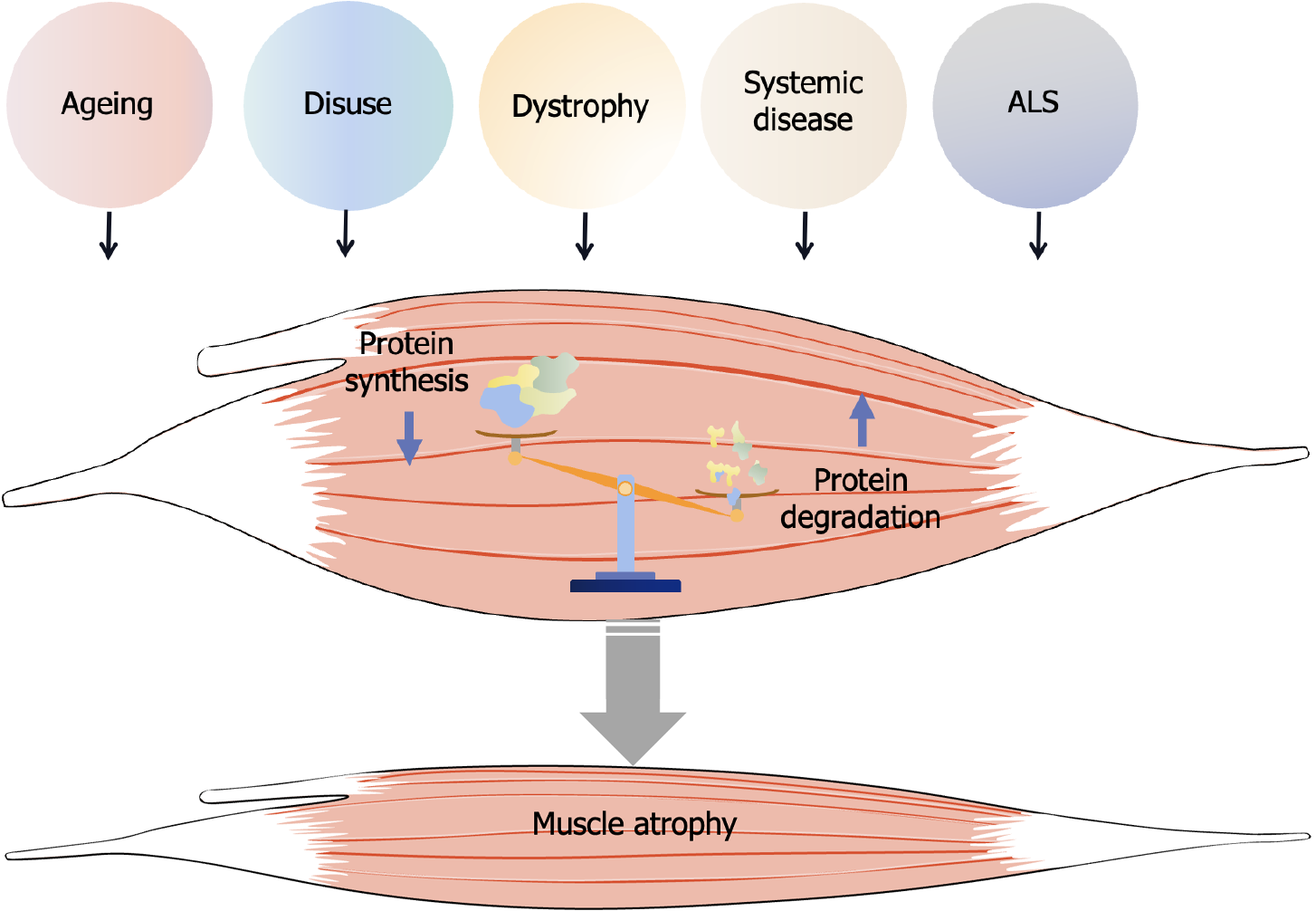
Muscle atrophy occurs in various physiological or pathological circumstances, including aging, disuse, dystrophy, amyotrophic lateral sclerosis (ALS), and systemic diseases such as cancer, sepsis, diabetes, chronic kidney disease and chronic obstructive pulmonary disease (Figure 1)[1-4]. Skeletal muscle atrophy causes a progressive decrease in the body’s response to various stresses and chronic diseases, leading to a reduced quality of life and imposing a heavy socioeconomic burden, thus adversely impacting the prognosis of related diseases[5]. As such, the prevention and management of skeletal muscle atrophy has garnered increasing attention from researchers. Despite ongoing efforts, the pathogenesis of skeletal muscle atrophy remains incompletely understood, and aside from rehabilitation, no effective treatment options fully restore muscle function. However, with advancing research into therapeutic strategies for muscle atrophy, stem cell-based approaches have shown promising potential for preventing and treating atrophy-related disorders. The transplantation or injection of various stem cells has demonstrated efficacy in mitigating muscle atrophy and has become an integral part of the treatment strategy[6,7]. Additionally, exosomes derived from stem cells have been shown to modulate the balance between protein synthesis and degradation, further promoting muscle repair. This review explores the potential of stem cell-based therapies and their derivatives in treating muscle atrophy, offering promising prospects for clinical application.
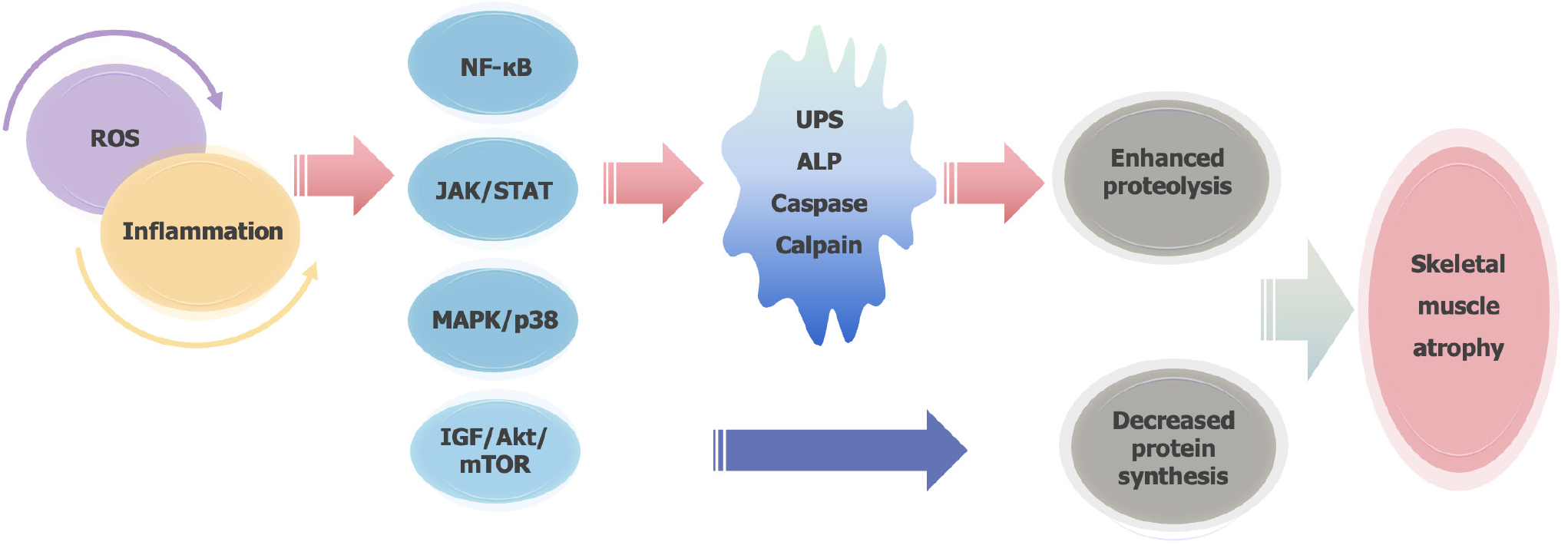
Skeletal muscle atrophy refers to the reduction or loss of muscle fibers due to nutritional disorders or diseases, leading to decreased muscle mass. Muscle mass is primarily regulated by the balance between protein synthesis and degradation under normal physiological conditions. Disruptions in the body’s microenvironment can result in an imbalance in skeletal muscle homeostasis, contributing to atrophy[8,9]. Recent findings suggest that skeletal muscle atrophy is linked to reduced protein synthesis and increased proteolysis, driven by factors such as inflammation, oxidative stress, autophagy, and apoptosis[10-12]. The key mechanisms of proteolysis include the ubiquitin-proteasome system, the autophagy-lysosome pathway, caspase-mediated proteolysis, and calpain-mediated proteolysis (Figure 2)[11,13,14]. Muscle RING-Finger protein 1 (MuRF-1) and Muscle Atrophy F-box protein are strongly associated with muscle protein degradation and serve as reliable biomarkers for this process. Moreover, the insulin-like growth factor/protein kinase B (Akt)/mechanistic target of rapamycin signaling pathway, which is critical for protein synthesis in skeletal muscle, is a major regulator of muscle atrophy[15].
Studies indicate that various triggering factors, including oxidative stress and inflammation either promote proteolytic processes or inhibit protein synthesis pathways, thereby leading to skeletal muscle atrophy[9,16-18]. Our previous research proposed a novel framework for understanding denervation-induced muscle atrophy, which involves four stages: Oxidative stress, inflammation, atrophy, and atrophic fibrosis[19]. Denervated skeletal muscles lose their contractile function, resulting in reduced blood flow to the affected muscles. This, in turn, induces a hypoxic environment that produces excess reactive oxygen species (ROS). The excessive ROS causes oxidative stress damage, triggering the release of inflammatory factors and activating inflammatory pathways, which cascade into a sustained inflammatory response. This response further accelerates muscle atrophy through reduced protein synthesis and enhanced proteolysis, ultimately leading to muscle atrophy and fibrosis[20]. Long-term oxidative stress injury and inflammation alter the microenvironment of skeletal muscle cells[21,22]. In particular, various inflammatory cytokines activate multiple signaling pathways in vivo, including nuclear factor-κB, Janus kinase/signal transducer and activator of transcription (JAK/STAT), and mitogen activated protein kinase (MAPK)/p38 pathway, leading to enhanced protein proteolysis and reduced protein synthesis in skeletal muscle[11,23,24]. In addition, denervation-induced muscular atrophy is likewise associated with the diminished availability of neurotrophic factors and lowered levels of activity[25]. In conclusion, the complex molecular mechanisms of muscle atrophy have not been well understood, and still needed to be further investigated to provide potential therapeutic strategies for muscle atrophy.
Skeletal muscle atrophy is characterized by a significant reduction in muscle mass, resulting from the disruption of the dynamic equilibrium between protein synthesis and degradation. Given this imbalance, a series of unfavorable changes occur in muscle function and structure. As muscle tissue plays a crucial role in movement, energy metabolism, storage, circulation, and protection[26,27]. Therefore, muscle atrophy can have a significant impact on quality of life. Consequently, the prevention and treatment of muscle atrophy are critical. At present, rehabilitation therapy is the major clinical treatment for muscle atrophy, and the muscle strength is partially restored with the assistance of various rehabilitation exercises. However, rehabilitation exercises require patients to meet certain basic conditions[28]. For example, patients who have been bedridden for long time or have mobility problems due to fractures or nerve deficits are unable to undergo rehabilitation[7]. In addition, rehabilitation exercises need to be conducted according to strict standards, otherwise secondary injuries are likely to occur. Therefore, massage or acupuncture is mostly used to prevent disuse muscular atrophy in the patients who are unable to undergo rehabilitation[29,30]. Although rehabilitation therapy has minimal side effects and no obvious sequelae, it is not very effective in treating muscle atrophy in certain populations[31-33]. So that pursuing more effective treatment measures may be beneficial in patients with several of muscular disorders.
Current therapies focus on how to promote protein synthesis, inhibit protein degradation and facilitate muscle regeneration to prevent or reduce muscle atrophy[7,34]. To date, numerous experiments have shown that anti-inflammatory and antioxidant drugs effectively alleviate the progression of muscle atrophy and significantly improve muscle mass and function[17,35,36]. While the exact molecular mechanisms remain to be fully elucidated, treatment with these drugs has been shown to induce significant changes in blood flow, mitochondrial activity, and apoptosis[17]. Scavenging accumulated ROS, improving autophagy and protecting mitochondria can alleviate skeletal muscle atrophy[37-39]. Transforming growth factor-β1 (TGF-β1) is an essential regulator of skeletal muscle several physio-pathologic proceedings, such as myogenesis, regeneration, and muscle atrophy. Injection of TGF-β1 into mice tibialis anterior induced muscle atrophy, leading to decreased fiber diameter, increased MuRF-1 levels and ROS contents. The antioxidant activity of N-acetyl-L-cysteine is used to inhibit the production of ROS in the target muscle, thereby reducing TGF-β-induced muscle atrophy[37]. Other drugs with potent antioxidant properties, such as astaxanthin, nobiletin, pyrroloquinoline quinone, isoquercitrin, and salidroside, have been shown to significantly reduce ROS levels in muscle[7,40,41]. Consequently, the mitochondrial function is stabilized and enhanced, which further reduces apoptosis caused by oxidative stress and abnormalities in mitochondria, and finally inhibits the progression of muscle atrophy[18,36,39,42]. Overall, inhibiting inflammation and reducing ROS accumulation may influence protein synthesis and degradation through multiple pathways, thereby suppressing skeletal muscle atrophy. However, most of these anti-inflammatory and antioxidant drugs have been primarily tested in pre-clinical studies, and their efficacy and safety in human applications remain to be thoroughly investigated. In recent years, stem cell therapies with superior biological properties have emerged as promising treatments for muscle atrophy. Furthermore, stem cell-based interventions have shown substantial benefits in animal models, indicating their potential for future clinical applications.
Stem cell transplantation offers a promising approach for treating various diseases due to the unique self-renewal capability of stem cells and their ability to differentiate into multiple lineages, including osteocytes, neurons, chondrocytes, hepatocytes, and adipocytes. This therapeutic strategy has also been applied to enhance the regenerative capacity of skeletal muscle following injury or degradation due to diseases such as muscular dystrophy[43,44]. In hereditary muscular dystrophies, such as facioscapulohumeral muscular dystrophy (FSHD), stem cell therapy has demonstrated significant improvements over non-transplanted controls, notably by reducing fibrosis and enhancing contractile-specific force[45]. Additionally, hematopoietic stem cells have also been discovered to alleviate muscle atrophy caused by Friedreich’s ataxia and Pompe disease[46,47]. Transplantation of bone marrow mesenchymal stem cells (BMSCs) or injection of their secretory vesicles significantly improves symptoms of muscle atrophy with effective control of inflammation[48]. Due to their excellent properties such as proliferation and directed differentiation, stem cells have considerable promise for therapy of muscle atrophy. Some clinical trials have indicated that stem cell therapy is capable of addressing certain intractable hereditary motor neuron (MN) diseases, such as ALS and spinal muscular atrophy muscular atrophy[49-53]. Herein, we summarized the potential role of mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and stem cell derivatives as the basis for the treatment of skeletal muscle atrophy.
MSCs is one of the most promising multipotent stem cell types widely used in regenerative medicine. MSCs can be isolated from a variety of tissues, such as adipose tissue, bone marrow, cranial neural crest, umbilical cords, and allogeneic placenta[16,54-56]. Despite cells from different tissues showing similar surface markers, they exhibited substantial differences in cell abundance, proliferation capacity, immune regulation, and differentiation capacity[57]. It has been reported that several MSCs including BMSCs, adipose-derived stem cells (ADSCs) and umbilical cord-derived MSCs (UC-MSCs) are effective in promoting skeletal muscle regeneration in acute and chronic diseases[58-61].
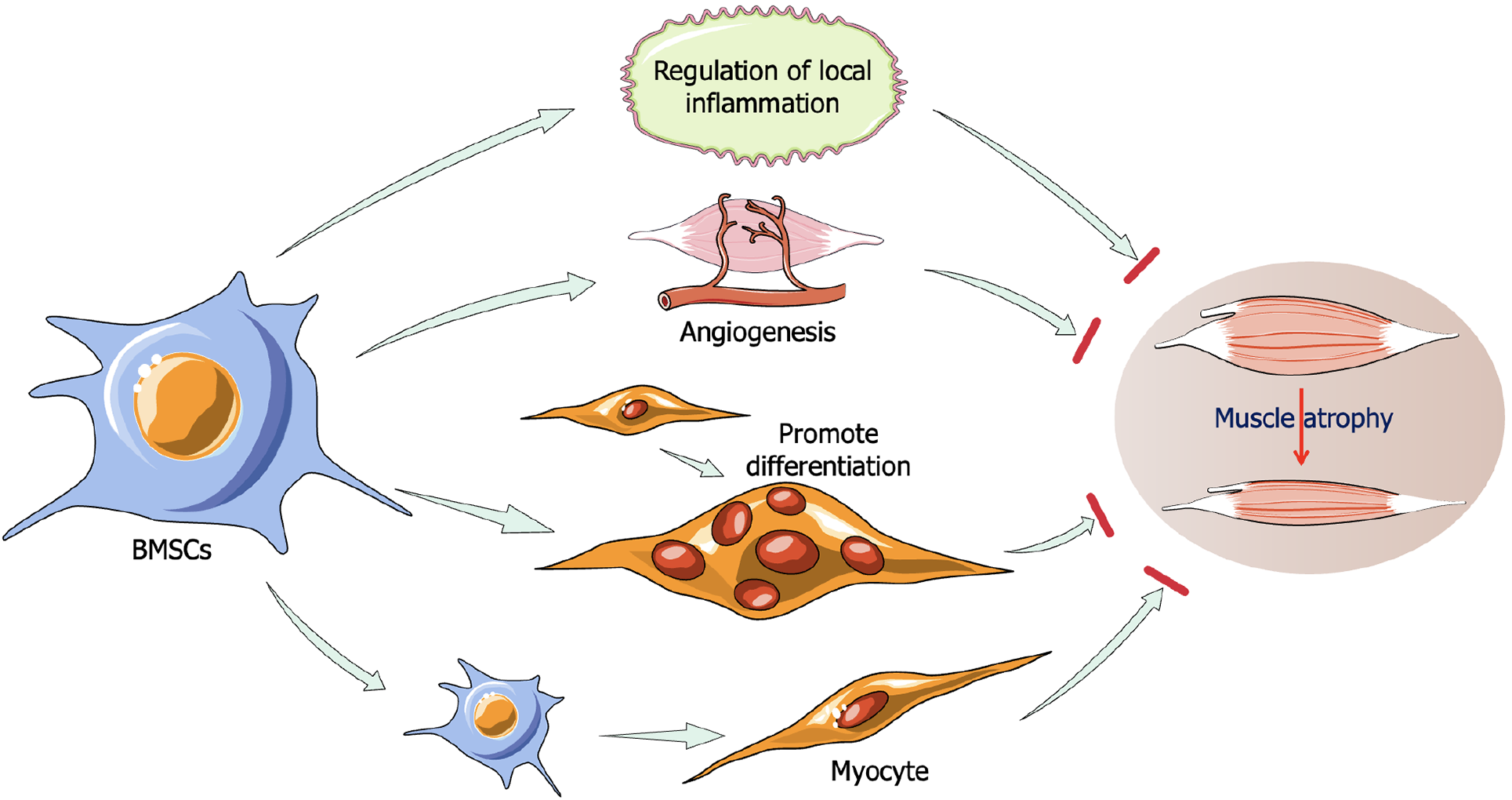
BMSCs in the treatment of muscle atrophy: The application of MSCs has become an established treatment for muscle diseases[62]. MSC injection has been shown to significantly improve muscle mass parameters, including mean fiber diameter, muscle mass, and myonuclear count in the extensor digitorum longus of rats following denervation[63]. ALS, a severe and progressive neurodegenerative disease characterized by the degeneration and loss of MNs, leads to muscle atrophy and loss of motor control[2]. Intrathecal transplantation of BMSCs in ALS mouse models significantly alleviated disease symptoms, delayed disease progression, and improved motor outcomes[64,65]. Studies have demonstrated that BMSCs possess regenerative potential for skeletal muscle[63,66]. Notably, BMSC transplantation increases the number of muscle stem cells and significantly reduces muscle fibrosis. In rats with acute muscle injury, intramuscular BMSC injection downregulated the protein levels of inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), thereby promoting muscle healing. Following BMSC transplantation, damaged muscle fibers were regenerated, and impaired blood vessels were largely restored. Furthermore, fibrosis progression in injured muscles was inhibited through the suppression of TGF-β1 downstream signaling[67]. Allogeneic BMSCs, when directly injected into acutely injured rat muscle, promoted myofibrogenesis and accelerated functional muscle recovery[68]. MSCs also enhance myotube differentiation, with co-cultures of muscle stem cells and BMSCs showing significant improvements in myotube formation[69]. BMSCs have shown therapeutic potential for dystrophic muscular atrophy as well[70]. In the mdx mouse model of Duchenne muscular dystrophy, peritoneal cavity transplantation of BMSCs significantly alleviated pathological malnutrition in the diaphragm, substantially extending lifespan[66]. Recent studies indicate that hypoxic preconditioning increases the production and secretion of stromal cell-derived factor-1 (SDF-1) and vascular endothelial growth factor in BMSCs. Transplanting hypoxic pretreated BMSCs into injured muscles improves the formation of new blood vessels and enhance muscle repair ability[71]. Thus, BMSCs have demonstrated considerable promise as a therapeutic approach for preventing muscle atrophy and promoting regeneration (Figure 3).
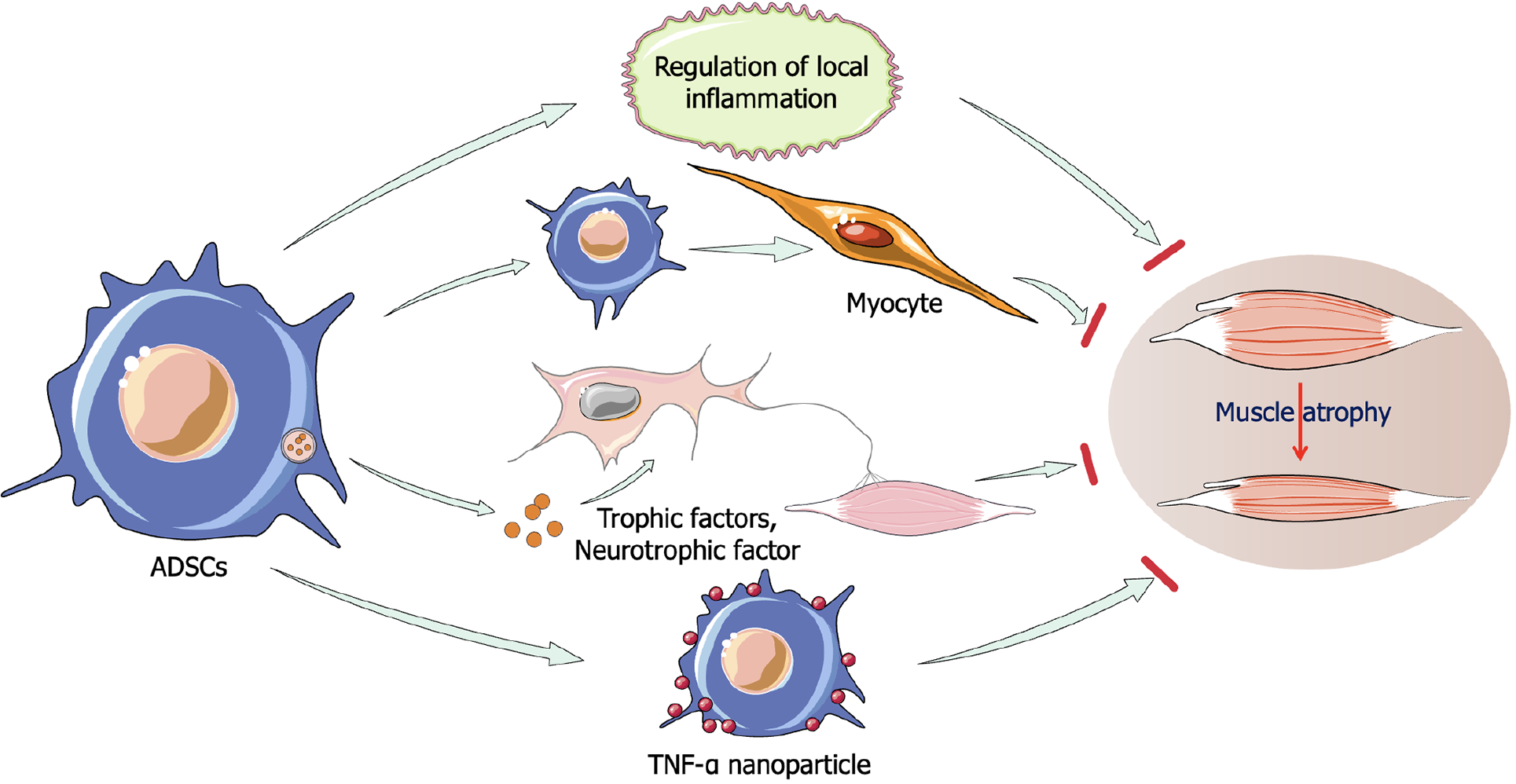
ADSCs in the treatment of muscle atrophy: A study has highlighted that both allogeneic BMSCs and ADSCs exhibit significant regenerative effects in treating skeletal muscle lacerations in rats, with ADSCs showing superior outcomes[72]. Local injection of ADSCs improves muscle strength and resistance to fatigue, primarily by regulating inflammation and gene products involved in regenerative processes. One study suggested that transplantation of a Matrigel 3D matrix containing TGFβ antibody-pretreated ADSCs into the injured gastrocnemius muscle of mice resulted in improved muscle mass and structure, reduced fibrosis, ameliorated the inflammatory microenvironment, and promoted skeletal muscle regeneration[73]. ADSCs can alleviate immune responses and support muscle reconstruction, with cytokine therapy further enhancing these effects. Preconditioning ADSCs with IL-4, SDF-1, or IL-11 overexpression further boosts their regenerative potential in damaged muscle tissue[74,75]. Recently, a novel approach involving the tethering of nanoparticles releasing TNF-α on the surface of ADSCs to stimulate their secretory activity has been shown to enhance perfusion, walking ability, and muscle mass recovery in ischemic hindlimb mice, providing a more efficient method for clinical stem cell therapy compared to previous pre-treatment strategies[76]. ADSCs have also demonstrated significant therapeutic potential in ALS, where they protect neurons and improve motor function by increasing neurotrophic factor expression[77,78]. These findings underscore the potential of ADSCs in treating muscle-wasting diseases (Figure 4).
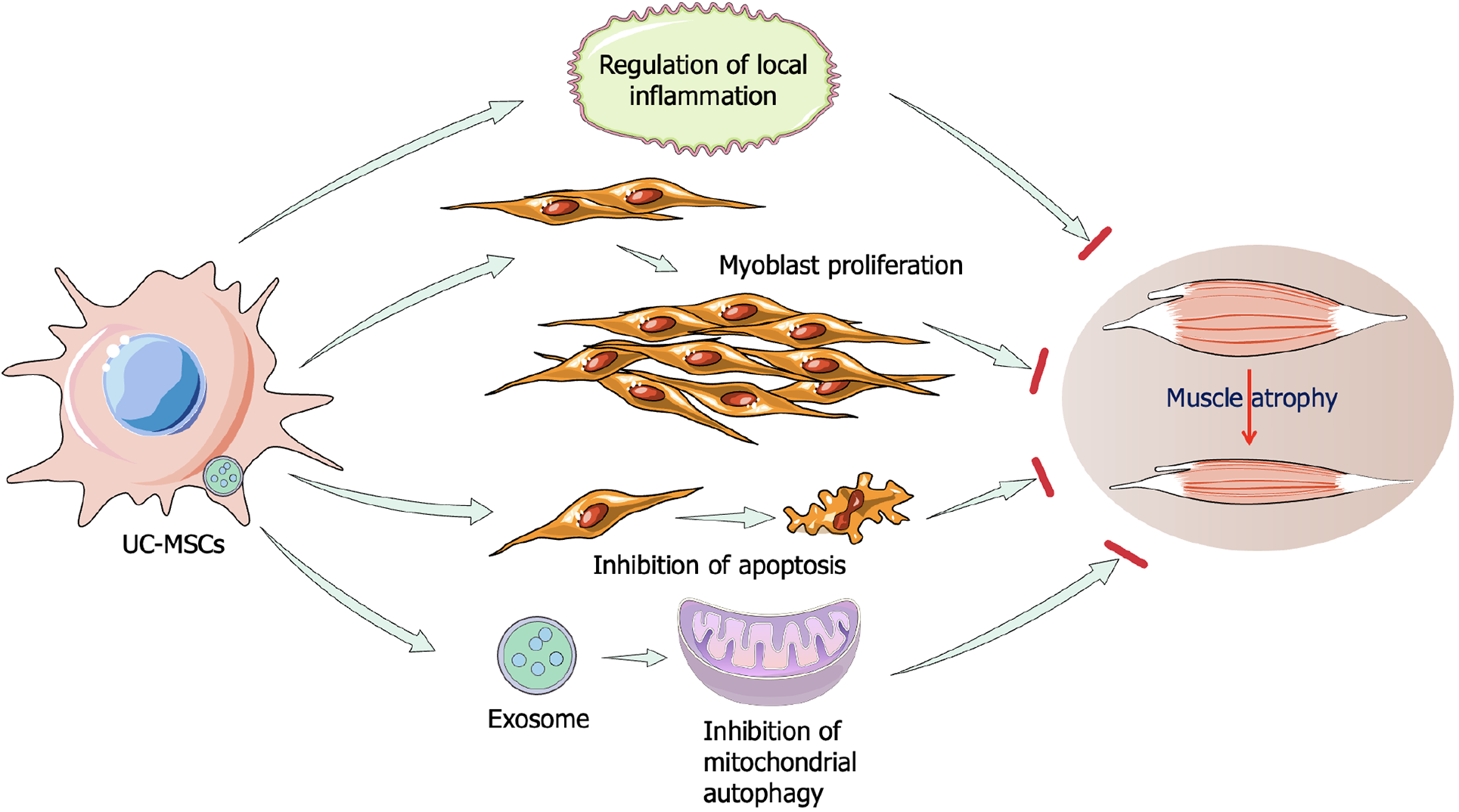
UC-MSCs in the treatment of muscle atrophy: UC-MSCs are gaining traction as a promising clinical tool due to their noninvasive collection and superior proliferation capacity, alongside lower immunogenicity compared to other MSCs like BMSCs[79]. As early as 2016, Kim et al[80] demonstrated that conditioned medium from UC-MSCs protects muscle cells from hindlimb suspension-induced atrophy. The injection of UC-MSCs activated the expression of muscle-specific markers such as desmin and skeletal muscle actin in satellite cells, stimulating myoblast proliferation and regulating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by downregulating MuRF-1 and Muscle Atrophy F-box protein[80]. In senescence-accelerated mouse prone 10 mice, UC-MSC injection inhibited apoptosis and inflammation while improving mitochondrial biogenesis via the AMP-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor gamma coactivator 1-alpha axis, thereby alleviating sarcopenia-related skeletal muscle atrophy[81]. Muscle atrophy is also associated with chronic metabolic and endocrine diseases such as obesity, hyperthyroidism, and type 2 diabetes mellitus[82,83]. A recent study evaluating the effects of human UC-MSCs (hUC-MSCs) on obesity- and diabetes-induced muscle atrophy found that exosomes derived from hUC-MSCs alleviated muscle atrophy symptoms by enhancing AMPK/UNC-51-like kinases 1 (ULK1)-mediated autophagy[84]. Therefore, UC-MSCs represent a promising therapeutic option for muscle atrophy prevention and regeneration (Figure 5).
In summary, MSCs from various sources offer effective strategies for mitigating muscle atrophy symptoms and maintaining muscle homeostasis by regulating local inflammation, promoting muscle regeneration, and protecting nerve innervation. These findings demonstrate the significant therapeutic potential of MSCs in treating muscle atrophy.
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and iPSCs, are actively explored for applications in regenerative medicine, drug screening, and disease modeling[85]. ESCs, derived from early embryos at the blastocyst stage, are pluripotent and capable of differentiating into all cell types within an organism[86]. However, due to ethical concerns, challenges in generating high-purity lineage-specific cell lines, and the potential risk of tumorigenesis, ESCs are seldom used in direct disease treatment and are primarily employed in the in vitro cultivation of tissues and organs[87]. In contrast, iPSCs offer distinct advantages, sharing key properties with ESCs, such as differentiation potential, electrophysiological characteristics, biochemistry, and telomerase activity[88]. Compared to muscle cells or MSCs directly transplanted into patients, iPSCs are more abundant, can be easily isolated and purified using specific markers, and demonstrate robust differentiation capabilities[89-91]. Furthermore, iPSCs mitigate the risk of immune rejection, as they avoid allogeneic transplantation issues[92,93].
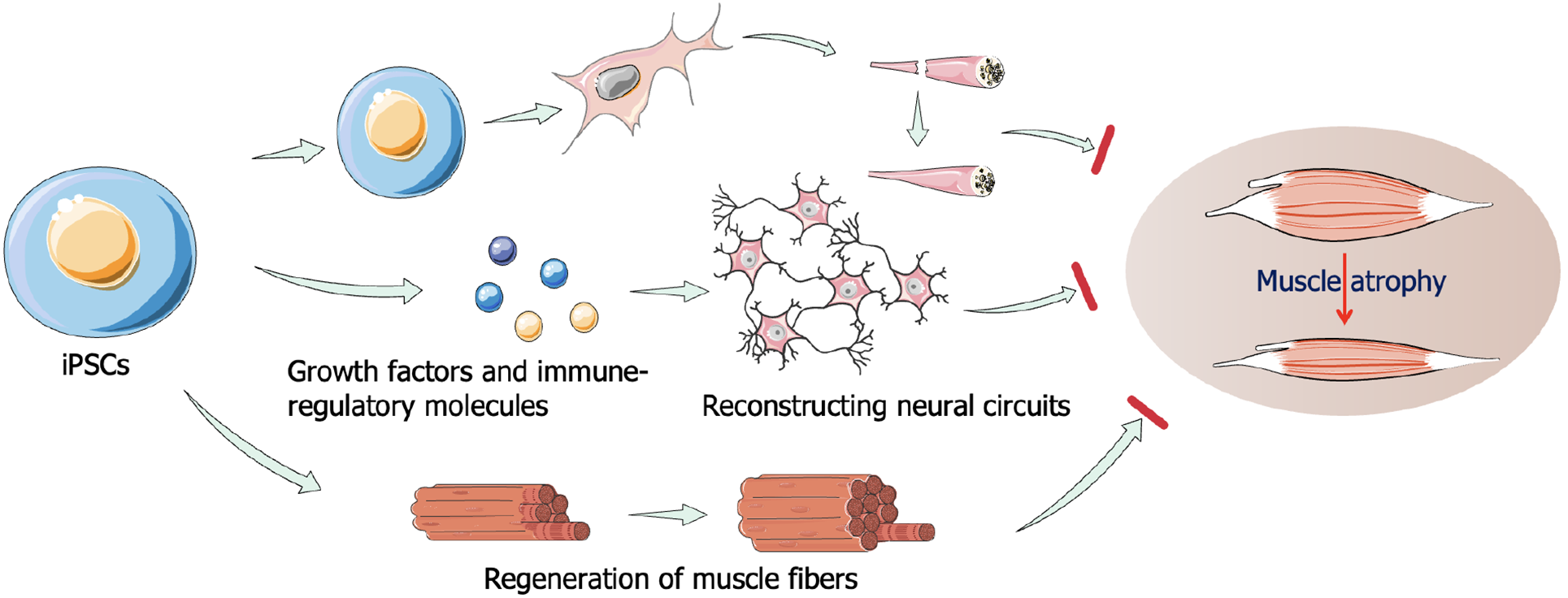
Differentiation of human iPSCs into myoblasts presents a promising approach for both autologous and allogeneic therapies in the treatment of muscle atrophy[91,94,95]. Human PSC-derived myogenic progenitor cells (MPCs), generated using both transgene-free and transgene-dependent protocols, have demonstrated potential in skeletal muscle engraftment[95]. Using a fluorescent reporter system, studies have shown that these MPCs promote myofiber regeneration in both local injury models and mdx mice[96]. PSC-derived PAX7+ MPCs represent a compelling strategy for muscle regeneration, enabling large-scale production of human myogenic progenitors[97]. Furthermore, under optimized 3D culture conditions, PSC-derived PAX7+ MPCs are capable of regenerating functional skeletal muscle[98]. In immunocompromised NSG mice, iSKM bundles derived from these cells were shown to survive, gradually vascularize, and maintain muscle function when implanted into the hind-limb muscles. Recent studies have directed human PSCs toward the differentiation of embryonic muscle progenitors, which subsequently developed into primary and secondary embryonic muscle, eventually forming three-dimensional muscle structures[99]. These tissue-engineered muscles exhibited high levels of maturation and function that closely resemble normal human muscle. After skeletal muscle atrophy, recovery is limited, even with sufficient blood supply and appropriate rest. In most cases, fibroblasts proliferate to repair damaged muscle tissue, leading to muscle mass reduction and impaired mobility. FSHD, a genetically dominant progressive myopathy characterized by muscle fibrosis, atrophy, and fat replacement, is one such condition. In an FSHD mouse model, the transplantation of MPCs derived from PSCs significantly improved muscle contractility compared to non-transplanted controls[45]. Moreover, the transplantation of human iPSC-derived MNs has shown potential in treating neuromuscular atrophy in mice with sciatic nerve injury[100]. Regeneration of neuromuscular junctions is vital for functional recovery in denervated muscles affected by trauma or MN diseases. P75+ neural stem cells derived from human iPSCs have been shown to enhance the secretion of soluble factors that promote neuromuscular regeneration. When injected into denervated gastrocnemius muscle, these NCSC spheres significantly improved neuromuscular function recovery[101]. iPSCs also show promise in treating ALS. In an ALS mouse model, transplantation of neural stem cells derived from iPSCs improved the microenvironment by secreting growth factors and immunomodulatory molecules, which likely contributed to the reconstruction of neural circuits and restoration of motor control in ALS-affected muscles[102,103]. Therefore, iPSCs have the potential to treat muscle atrophy and provide a new technical idea for personalized treatment of dystrophic muscular atrophy in the future (Figure 6). The available results have shown that this method is feasible and has the potential for application and development in clinical practice.
The regenerative potential of MSCs stems not only from their direct transplantation into target tissues and subsequent differentiation but also from their secretions, particularly exosomes, which activate the regenerative capacity of resident cells. Exosomes contain various bioactive components, including growth factors, cytokines, mRNAs, signaling lipids, and regulatory microRNAs[104]. Although MSCs from different tissues and donors secrete distinct protein profiles, their actions in vitro can vary; they are nonetheless capable of regulating apoptosis in dystrophic myogenic cells and promoting cell migration and proliferation[105]. Skeletal muscle regeneration is closely linked to the biological factors secreted by exosomes, which exert significant bioactive effects on damaged muscle tissue[106,107]. Increasing evidence suggests that exosomes derived from stem cells offer promising therapeutic potential for muscle regeneration (Table 1).
| Resouce | Mechanism | Function | Ref. |
| UC-MSCs | Releasing circHIPK3, while circHIPK3 down-regulates miR-421, leading to increased expression of FOXO3a, thereby inhibiting IL-1β and IL-18 | Accelerate repair of ischemic muscle injury in mice | [108] |
| hUC-MSCs or hUC-MSC-derived exosomes induce the activation of AMPK/ULK1 signal and enhancement of autophagy | Alleviate muscle atrophy induced by diabetes and obesity in mice | [84] | |
| Down-regulating IL-6 and IL-1β, up-regulating IL-10, and regulating inflammation of injured nerves | Promote the recovery of motor function and regeneration of axons, alleviated denervated gastrocnemius atrophy in rats | [109] | |
| Regulating the miR-132-3p/FoxO3 axis | Improve age-related muscle atrophy in a mouse model | [110] | |
| ADSCs | Promoting peripheral nerve regeneration via optimizing SC function | Alleviate denervated muscle atrophy in rats | [111] |
| Inhibiting inflammation, enhancing proliferation of muscle satellite cells, resisting cell senescence and promoting angiogenesis | Maintain muscle homeostasis, promote muscle regeneration in a mouse model of cardiotoxin-induced injury | [112,113] | |
| Preventing the atrophy, fatty infiltration, inflammation, and vascularization of muscles | Decrease atrophy and degeneration associated with torn rotator cuffs, and improve muscle regeneration in rats | [114] | |
| Improving motor performance; protecting lumbar motoneurons, neuromuscular junction, and muscle, decreasing glial cells activation | Protect neuromuscular junction and muscle in SOD1 (G93A) murine model | [115] | |
| BMSCs | Promoting M2 macrophages polarization, reducing the production of inflammatory cytokines, upregulating anti-inflammatory factors expression | Attenuate muscle injury and promote muscle healing in mice with hit injury | [116] |
| Foxo1 expression was inhibited by up-regulating the miR486-5p/Foxo1 axis | Inhibit dexamethasone-induced muscle atrophy in mice | [117] | |
| M2 macrophage polarization is regulated by NF-κB pathway to reduce inflammatory pain, and myeloid axon regeneration is enhanced by MEK/ERK pathway | Improve denervated muscle atrophy and further promote functional recovery in rats | [106,107] |
Exosomes secreted by hUC-MSCs (hUC-MSC-Exos) have been confirmed to be effective against muscle injury. In a mouse model of ischemic induced skeletal muscle injury, purified hUC-MSC-Exos accelerates skeletal muscle regeneration by releasing circHIPK3[108]. This led to the downregulation of miR-421, resulting in Forkhead box O3a (FOXO3a) expression, inhibition of pyroptosis, and decreased release of IL-1β and IL-18[108]. Similarly, Song et al[84] reported that hUC-MSC-Exos alleviated diabetes-associated muscle atrophy by enhancing autophagy through the AMPK/ULK1 axis. In a rat sciatic nerve defect model, hUC-MSC-Exos promoted nerve regeneration and attenuated gastrocnemius muscle atrophy by modulating inflammation - downregulating IL-1β and IL-6, upregulating IL-10[109]. A recent study suggest that hUC-MSC-Exos may improve age-related and dexamethasone-induced muscle atrophy in a mouse model by regulating the miR-132-3p/FoxO3 axis[110]. These studies suggest that the application of exosomes derived from UC-MSCs have great potential for muscle atrophy.
The protective effect of exosomes secreted by human ADSCs on denervated muscle atrophy has been confirmed in rat studies, where exosomes secreted by human ADSCs significantly promoted nerve regeneration and alleviated muscle atrophy following sciatic nerve dissection[111]. Exosomes secreted by ADSCs (ADSC-Exos) enhanced Schwann cell proliferation, migration, and myelination, while also stimulating the release of neurotrophic factors through the upregulation of key genes. Furthermore, ADSC-Exos contributed to muscle homeostasis by inhibiting inflammation, promoting muscle satellite cell proliferation, resisting cellular senescence, and stimulating angiogenesis[112-114]. ADSC-Exos play a critical role in skeletal muscle regeneration at various stages by modulating key signaling pathways, including MAPK, Wnt, PI3K/Akt, and JAK/STAT[113]. For example, catenin derived from ADSC-Exos activates the classical Wnt pathway, supporting myogenic maintenance of satellite cells, while also coordinating their self-renewal and migration via the non-classical Wnt pathway[115]. In the early stages of muscle regeneration, ADSC-Exos activate MAPK/extracellular signal-regulated kinase 1/2 and MAPK/JNK pathways, promoting myoblast proliferation and preventing premature differentiation[116]. At later stages, the p38/MK2 and MEF2 pathways drive myogenic differentiation by enhancing MyoD transcriptional activity[117], while the MAPK/extracellular signal-regulated kinase 5 pathway facilitates multinucleated myotube formation. Additionally, ADSC-Exos influence the PI3K/Akt pathway to upregulate protein synthesis and suppress protein degradation, counteracting muscle atrophy. However, the potential inhibitory effect of PI3K/Akt on autophagy via mechanistic target of rapamycin and FOXO suggests that its role in muscle regeneration may not be entirely beneficial[118]. Moreover, ADSC-Exos regulate the JAK1/STAT1/3 pathway to prevent premature differentiation of myoblasts, while JAK2/STAT2/3 and JAK3/STAT1/3 pathways promote myogenic differentiation. ADSC-Exos have also been shown to reduce muscle-related protein degeneration and muscle atrophy while enhancing regeneration and biomechanical properties in torn rotator cuff muscles in rats[119]. Additionally, repeated administration of ADSC-Exos reduced glial cell activation, protected muscles, MNs, and neuromuscular junctions, and improved motor performance in SOD1-G93A mice[120]. Proteomic analysis of ADSC-Exos revealed their influence on ALS-related pathways, including oxidative stress response, cell apoptosis, cell adhesion, and PI3K/Akt signaling[121]. In conclusion, extensive evidence highlights the significant potential of ADSC-Exos in the treatment of muscle diseases.
Exosomes secreted by BMSCs (BMSC-Exos) have shown therapeutic potential for muscle injury and atrophy. Intramuscular injection of BMSC-Exos reduces inflammation following muscle contusion in mice, mitigates muscle fibrosis, promotes regeneration, and enhances muscle biomechanical properties[122]. A study also demonstrated that BMSC-Exos counteract dexamethasone-induced muscle atrophy through the miR486-5p/Foxo1 pathway[123]. Recently, a BMSC-Exos-loaded electroconductive nerve dressing has been developed for treating diabetic peripheral nerve injury. Implantation of the BMSC-Exos-loaded electroconductive nerve dressing complex around damaged sciatic nerves enhances myelinated axon regeneration, ameliorates gastrocnemius muscle atrophy, and promotes functional recovery in diabetic rats[107]. Furthermore, supplementation with exosomes secreted by iPSCs in acellular nerve grafts through optimized chemical extraction processes has been shown to repair long-distance peripheral nerve defects, accelerating motor recovery and reversing denervated muscle atrophy[124]. Skin-derived precursors (SKPs), pluripotent cells derived from the dermis, are another promising stem cell source. Artificial nerve grafts incorporating SKP-derived Schwann cells can alleviate denervation-induced muscle atrophy[125]. In our recent study, direct intramuscular injection of SKP-SC-extracellular vesicles alleviated blood flow reduction, oxidative stress, and inflammation in target muscles, thus reducing skeletal muscle atrophy caused by denervation[126]. Overall, stem cell-derived exosomes mitigate inflammation, reduce fibrosis, accelerate muscle regeneration, and promote functional recovery[122,127]. In conclusion, stem cell-derived exosomes present substantial promise for clinical applications, with exosomes secreted by MSCs offering a potential alternative to whole-cell regenerative therapies.
Despite the considerable promise of stem cells in treating muscle atrophy and related diseases, challenges surrounding their source and biological characteristics, particularly ethical concerns related to the use of human ESCs, remain significant. Furthermore, other types of stem cells present notable limitations that impede the advancement of stem cell therapies. The potential risks of immune rejection and tumor formation after transplantation are significant issues worthy of attention. The approaches to mitigate these risks include strategies such as immunosuppression, immune matching, and stem cell genetic engineering. However, each strategy confronts its own unique challenges and may have varying degrees of impact on patient safety[128].
MSCs are widely utilized in medicine due to their pluripotency, broad tissue origins, and minimal ethical concerns. While MSC-based therapies offer advantages over conventional treatments in clinical trials, their therapeutic outcomes often fall short of expectations. Key challenges, including stem cell homing, immune modulation, and other factors, need to be addressed for successful MSC application. Under various conditions, such as ischemia or injury, MSCs - whether autologous or exogenous - migrate directionally from the vascular endothelium to the affected tissues, where they colonize and survive[129,130]. The activation of MSCs occurs in response to elevated inflammatory factors like IL-1 and TNF-α, which generate a concentration gradient of ligands around the target tissue, promoting MSC migration towards ischemic and hypoxic regions[131,132]. SDF-1 and its receptor C-X-C motif chemokine receptor 4 are the most extensively studied cytokines involved in cell homing[126]. SDF-1 is upregulated at injury sites, acting on MSC migration in a dose-dependent manner, thus enhancing BMSC homing for tissue repair[133,134]. However, maintaining MSCs at specific target sites, such as muscles, remains a significant challenge. MSCs secrete paracrine factors capable of tissue remodeling and repair, act as drug carriers, and modulate immune responses[135-138]. MSCs can detect injury sites and elicit stronger immune responses in low-immune states, while inhibiting immune cell function when inflammation is excessive in the damaged area[139]. They exhibit dual, opposing functions - pro-inflammatory or anti-inflammatory - depending on the inflammatory factors present in the local environment[66,140]. The key to optimizing their therapeutic effects lies in understanding how to modulate or exploit the immune-inducing properties of MSCs. Additionally, factors contributing to suboptimal therapeutic outcomes include the age of MSC donors, variations in cell isolation and culture protocols, the method of administration, and individual differences among recipients[141-143]. Long-term culture impairs the differentiation potential of MSCs, and the same cell batch may exhibit heterogeneous differentiation, which can skew experimental results. Moreover, various biological and experimental factors introduce safety risks in clinical and basic research applications of stem cells, such as tumor formation, infection, necrosis from tissue blockage due to stem cell homing[144], and other exogenous risks[145,146]. Although MSCs have good therapeutic effects in animal models of muscle diseases, biosafety evaluation standards for stem cell experiments and therapy are lacking. The heterogeneity of MSCs is one of the factors contributing to the variation in treatment outcomes, which presents challenges in determining the optimal stem cell therapy protocol, dosage, and timing[57]. These issues necessitate further research and clinical practice for resolution.
There was no unified and effective differentiation method for iPSCs to differentiate into muscle precursor cells, and their purification protocols are also inconsistent[95,147]. For transgene-free protocols, due to discrepancy in purification and transplantation times among the protocols, none of previously reported optimal purification strategies have been reproducibly validated. For transgene-dependent protocols, a risk factor that may not be ignored is the induced expression of proto-oncogenes introduced via viral vectors or existing in the host cells. It remains unclear whether other unknown genes are involved in the reprogramming process or if genetic and epigenetic changes occur during this process[148]. Consequently, several challenges remain before iPSCs can be reliably applied in clinical settings.
In the current social, scientific and ethical context, the use of stem cell derivatives as a cell-free therapy is rapidly emerging as an alternative with additional advantages. It can avoid safety issues related to the use of living cells, ethical issues related to the origin of the cells, and immunocompatibility issues. MSC-derived exosomes, which are characterized by low-immunogenicity, high biocompatibility, safety and low toxicity, provide a new approach to deliver therapeutic agents to target cells, which is the best alternative to stem cell therapy[149-151]. Although exosomes are generally considered to have great clinical therapeutic value, there are still many drawbacks such as extremely low concentration of active ingredients, poor specificity, and difficulty in traceability, which restrict the clinical use of exosomes. It is also worthy of note that cell-free therapeutic products might take a considerable amount of time for administration and could result in drug resistance as well as some adverse reactions[152].
It is crucial to prove the safety and efficacy of stem cells and their derived exosomes therapy through clinical trials. Evaluating long-term efficacy means tracking changes in cell survival, integration, and treatment response over time, and identifying any adverse events or delayed complications. This requires establishing standardized methods and guidelines[128]. Also, currently, there’s no clear consensus or standard about the administration route, dose, and treatment time[57]. These require the establishment of standardized methods and guidelines. Of course, in addition to the ethical controversy related to the use of ESCs mentioned earlier, issues such as informed consent from patients, the experimental nature of the treatment, and fair access to emerging therapies should also be considered in the consideration of stem cell clinical use. Solving these multifaceted challenges requires not only the efforts of researchers and clinicians, but also coordinated efforts with regulatory agencies and ethics committees, which will be key to realizing the potential of stem cell therapy for muscle atrophy.
The rapid advancements in stem cell research have opened new avenues for treating previously incurable diseases. Numerous clinical trials have demonstrated promising outcomes in addressing cardiovascular, neurological, and immune disorders[151,153-155]. This review systematically analyzes the therapeutic application of stem cells including BMSCs, ADSCs, UCMSCs, and iPSCs in skeletal muscle atrophy due to various causes. In addition, we also summarize the advantages of existing therapeutic strategies and further research development directions in order to mitigate or eliminate therapeutic deficiencies and establish a relatively sound therapeutic evaluation system. Cell-free therapies based on stem cell derivatives have better prospects compared to conventional cell transplantation therapy[156,157]. A full understanding of the capabilities of different types of stem cells, especially their derivatives, in muscle repair and regeneration would be a breakthrough in the treatment of muscle atrophy.
We would like to thank all the professionals who contributed to the discussion and elaboration of this review.
| 1. | Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 520] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 2. | Wang K, Liu Q, Tang M, Qi G, Qiu C, Huang Y, Yu W, Wang W, Sun H, Ni X, Shen Y, Fang X. Chronic kidney disease-induced muscle atrophy: Molecular mechanisms and promising therapies. Biochem Pharmacol. 2023;208:115407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 3. | Shen Y, Li M, Wang K, Qi G, Liu H, Wang W, Ji Y, Chang M, Deng C, Xu F, Shen M, Sun H. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front Endocrinol (Lausanne). 2022;13:917113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Chang M, Cai Y, Gao Z, Chen X, Liu B, Zhang C, Yu W, Cao Q, Shen Y, Yao X, Chen X, Sun H. Duchenne muscular dystrophy: pathogenesis and promising therapies. J Neurol. 2023;270:3733-3749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 5. | Yin L, Li N, Jia W, Wang N, Liang M, Yang X, Du G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol Res. 2021;172:105807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 6. | Qazi TH, Duda GN, Ort MJ, Perka C, Geissler S, Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019;10:501-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Huang L, Li M, Deng C, Qiu J, Wang K, Chang M, Zhou S, Gu Y, Shen Y, Wang W, Huang Z, Sun H. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants (Basel). 2022;12:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 8. | You Z, Huang X, Xiang Y, Dai J, Jiang J, Xu J. Molecular feature of neutrophils in immune microenvironment of muscle atrophy. J Cell Mol Med. 2022;26:4658-4665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Sun H, Sun J, Li M, Qian L, Zhang L, Huang Z, Shen Y, Law BY, Liu L, Gu X. Transcriptome Analysis of Immune Receptor Activation and Energy Metabolism Reduction as the Underlying Mechanisms in Interleukin-6-Induced Skeletal Muscle Atrophy. Front Immunol. 2021;12:730070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, Chen X, Yao X, Gu X, Qi L, Zhou C, Sun H. Oxidative stress: Roles in skeletal muscle atrophy. Biochem Pharmacol. 2023;214:115664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 11. | Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, Deng C, Shen Y, Zhu J, Wang W, Xu L, Sun H. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants (Basel). 2022;11:1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 12. | Liang W, Xu F, Li L, Peng C, Sun H, Qiu J, Sun J. Epigenetic control of skeletal muscle atrophy. Cell Mol Biol Lett. 2024;29:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 13. | Huang Z, Zhong L, Zhu J, Xu H, Ma W, Zhang L, Shen Y, Law BY, Ding F, Gu X, Sun H. Inhibition of IL-6/JAK/STAT3 pathway rescues denervation-induced skeletal muscle atrophy. Ann Transl Med. 2020;8:1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | He Q, Qiu J, Dai M, Fang Q, Sun X, Gong Y, Ding F, Sun H. MicroRNA-351 inhibits denervation-induced muscle atrophy by targeting TRAF6. Exp Ther Med. 2016;12:4029-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Ito N, Ruegg UT, Takeda S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int J Mol Sci. 2018;19:2804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Yan Y, Li M, Lin J, Ji Y, Wang K, Yan D, Shen Y, Wang W, Huang Z, Jiang H, Sun H, Qi L. Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function. Front Pharmacol. 2022;13:947387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 17. | Zhang L, Li M, Wang W, Yu W, Liu H, Wang K, Chang M, Deng C, Ji Y, Shen Y, Qi L, Sun H. Celecoxib alleviates denervation-induced muscle atrophy by suppressing inflammation and oxidative stress and improving microcirculation. Biochem Pharmacol. 2022;203:115186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 18. | Shen Y, Zhang Q, Huang Z, Zhu J, Qiu J, Ma W, Yang X, Ding F, Sun H. Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation. Front Physiol. 2020;11:988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Shen Y, Zhang R, Xu L, Wan Q, Zhu J, Gu J, Huang Z, Ma W, Shen M, Ding F, Sun H. Microarray Analysis of Gene Expression Provides New Insights Into Denervation-Induced Skeletal Muscle Atrophy. Front Physiol. 2019;10:1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Wan Q, Zhang L, Huang Z, Zhang H, Gu J, Xu H, Yang X, Shen Y, Law BY, Zhu J, Sun H. Aspirin alleviates denervation-induced muscle atrophy via regulating the Sirt1/PGC-1α axis and STAT3 signaling. Ann Transl Med. 2020;8:1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Sun J, Yang H, Yang X, Chen X, Xu H, Shen Y, Ding F, Gu X, Zhu J, Sun H. Global alternative splicing landscape of skeletal muscle atrophy induced by hindlimb unloading. Ann Transl Med. 2021;9:643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ma W, Cai Y, Shen Y, Chen X, Zhang L, Ji Y, Chen Z, Zhu J, Yang X, Sun H. HDAC4 Knockdown Alleviates Denervation-Induced Muscle Atrophy by Inhibiting Myogenin-Dependent Atrogene Activation. Front Cell Neurosci. 2021;15:663384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Liang Z, Zhang T, Liu H, Li Z, Peng L, Wang C, Wang T. Inflammaging: The ground for sarcopenia? Exp Gerontol. 2022;168:111931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 24. | Irazoki A, Martinez-Vicente M, Aparicio P, Aris C, Alibakhshi E, Rubio-Valera M, Castellanos J, Lores L, Palacín M, Gumà A, Zorzano A, Sebastián D. Coordination of mitochondrial and lysosomal homeostasis mitigates inflammation and muscle atrophy during aging. Aging Cell. 2022;21:e13583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Chu XL, Song XZ, Li Q, Li YR, He F, Gu XS, Ming D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen Res. 2022;17:2185-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 26. | Grgic J, Schoenfeld BJ, Mikulic P. Effects of plyometric vs. resistance training on skeletal muscle hypertrophy: A review. J Sport Health Sci. 2021;10:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Chen X, Ji Y, Liu R, Zhu X, Wang K, Yang X, Liu B, Gao Z, Huang Y, Shen Y, Liu H, Sun H. Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med. 2023;21:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 28. | Howard EE, Pasiakos SM, Fussell MA, Rodriguez NR. Skeletal Muscle Disuse Atrophy and the Rehabilitative Role of Protein in Recovery from Musculoskeletal Injury. Adv Nutr. 2020;11:989-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Lawrence MM, Van Pelt DW, Confides AL, Hunt ER, Hettinger ZR, Laurin JL, Reid JJ, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE, Miller BF. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol (Oxf). 2020;229:e13460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Su Z, Hu L, Cheng J, Klein JD, Hassounah F, Cai H, Li M, Wang H, Wang XH. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J Appl Physiol (1985). 2016;120:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Lv T, Mo Y, Yu T, Zhang Y, Shao S, Luo Y, Shen Y, Lu M, Wong SG. An Investigation into the Rehabilitative Mechanism of Tuina in the Treatment of Sciatic Nerve Injury. Evid Based Complement Alternat Med. 2020;2020:5859298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Peng S, Tian Y, Chang W, Yang Y, Li S, Ni J, Zhu W. Current state of research on acupuncture for the treatment of amyotrophic lateral sclerosis: A scoping review. Front Neurol. 2022;13:1019156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Rahiminezhad E, Sadeghi M, Ahmadinejad M, Mirzadi Gohari SI, Dehghan M. A randomized controlled clinical trial of the effects of range of motion exercises and massage on muscle strength in critically ill patients. BMC Sports Sci Med Rehabil. 2022;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Huang Z, Zhu J, Ma W, Sun H. Strategies and potential therapeutic agents to counter skeletal muscle atrophy. Biotarget. 2018;2:8. [DOI] [Full Text] |
| 35. | Qiu J, Fang Q, Xu T, Wu C, Xu L, Wang L, Yang X, Yu S, Zhang Q, Ding F, Sun H. Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy. Front Physiol. 2018;9:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Xu T, Yang X, Wu C, Qiu J, Fang Q, Wang L, Yu S, Sun H. Pyrroloquinoline quinone attenuates cachexia-induced muscle atrophy via suppression of reactive oxygen species. J Thorac Dis. 2018;10:2752-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Abrigo J, Rivera JC, Simon F, Cabrera D, Cabello-Verrugio C. Transforming growth factor type beta (TGF-β) requires reactive oxygen species to induce skeletal muscle atrophy. Cell Signal. 2016;28:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Scalabrin M, Engman V, Maccannell A, Critchlow A, Roberts LD, Yuldasheva N, Bowen TS. Temporal analysis of skeletal muscle remodeling post hindlimb ischemia reveals intricate autophagy regulation. Am J Physiol Cell Physiol. 2022;323:C1601-C1610. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Wang HH, Sun YN, Qu TQ, Sang XQ, Zhou LM, Li YX, Ren FZ. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int J Mol Sci. 2022;23:11963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 40. | Huang Z, Fang Q, Ma W, Zhang Q, Qiu J, Gu X, Yang H, Sun H. Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation. Front Pharmacol. 2019;10:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Wu C, Tang L, Ni X, Xu T, Fang Q, Xu L, Ma W, Yang X, Sun H. Salidroside Attenuates Denervation-Induced Skeletal Muscle Atrophy Through Negative Regulation of Pro-inflammatory Cytokine. Front Physiol. 2019;10:665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Yang X, Ji Y, Wang W, Zhang L, Chen Z, Yu M, Shen Y, Ding F, Gu X, Sun H. Amyotrophic Lateral Sclerosis: Molecular Mechanisms, Biomarkers, and Therapeutic Strategies. Antioxidants (Basel). 2021;10:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Salles J, Chanet A, Guillet C, Vaes AM, Brouwer-Brolsma EM, Rocher C, Giraudet C, Patrac V, Meugnier E, Montaurier C, Denis P, Le Bacquer O, Blot A, Jourdan M, Luiking Y, Furber M, Van Dijk M, Tardif N, Yves Boirie Y, Walrand S. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: role in sarcopenia. Commun Biol. 2022;5:1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 44. | Saeki S, Tokutake K, Takasu M, Kurimoto S, Asami Y, Onaka K, Saeki M, Hirata H. Functional Reconstruction of Denervated Muscle by Xenotransplantation of Neural Cells from Porcine to Rat. Int J Mol Sci. 2022;23:8773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 45. | Azzag K, Bosnakovski D, Tungtur S, Salama P, Kyba M, Perlingeiro RCR. Transplantation of PSC-derived myogenic progenitors counteracts disease phenotypes in FSHD mice. NPJ Regen Med. 2022;7:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Rocca CJ, Goodman SM, Dulin JN, Haquang JH, Gertsman I, Blondelle J, Smith JLM, Heyser CJ, Cherqui S. Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich's ataxia. Sci Transl Med. 2017;9:eaaj2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Yoon JK, Schindler JW, Loperfido M, Baricordi C, DeAndrade MP, Jacobs ME, Treleaven C, Plasschaert RN, Yan A, Barese CN, Dogan Y, Chen VP, Fiorini C, Hull F, Barbarossa L, Unnisa Z, Ivanov D, Kutner RH, Guda S, Oborski C, Maiwald T, Michaud V, Rothe M, Schambach A, Pfeifer R, Mason C, Biasco L, van Til NP. Preclinical lentiviral hematopoietic stem cell gene therapy corrects Pompe disease-related muscle and neurological manifestations. Mol Ther. 2024;32:3847-3864. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Kono Y, Kajita H, Okada T, Nakagawa R, Fujita T, Konishi S. Mesenchymal Stem Cells Promote IL-6 Secretion and Suppress the Gene Expression of Proinflammatory Cytokines in Contractile C2C12 Myotubes. Biol Pharm Bull. 2022;45:962-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Reis ALG, Maximino JR, Lage LAPC, Gomes HR, Pereira J, Brofman PRS, Senegaglia AC, Rebelatto CLK, Daga DR, Paiva WS, Chadi G. Proteomic analysis of cerebrospinal fluid of amyotrophic lateral sclerosis patients in the presence of autologous bone marrow derived mesenchymal stem cells. Stem Cell Res Ther. 2024;15:301. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Berry JD, Cudkowicz ME, Windebank AJ, Staff NP, Owegi M, Nicholson K, McKenna-Yasek D, Levy YS, Abramov N, Kaspi H, Mehra M, Aricha R, Gothelf Y, Brown RH. NurOwn, phase 2, randomized, clinical trial in patients with ALS: Safety, clinical, and biomarker results. Neurology. 2019;93:e2294-e2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Forotti G, Nizzardo M, Bucchia M, Ramirez A, Trombetta E, Gatti S, Bresolin N, Comi GP, Corti S. CSF transplantation of a specific iPSC-derived neural stem cell subpopulation ameliorates the disease phenotype in a mouse model of spinal muscular atrophy with respiratory distress type 1. Exp Neurol. 2019;321:113041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Mohseni R, Hamidieh AA, Shoae-Hassani A, Ghahvechi-Akbari M, Majma A, Mohammadi M, Nikougoftar M, Shervin-Badv R, Ai J, Montazerlotfelahi H, Ashrafi MR. An open-label phase 1 clinical trial of the allogeneic side population adipose-derived mesenchymal stem cells in SMA type 1 patients. Neurol Sci. 2022;43:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Yeo CJJ, Tizzano EF, Darras BT. Challenges and opportunities in spinal muscular atrophy therapeutics. Lancet Neurol. 2024;23:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Meesuk L, Suwanprateeb J, Thammarakcharoen F, Tantrawatpan C, Kheolamai P, Palang I, Tantikanlayaporn D, Manochantr S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci Rep. 2022;12:19509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Oguma Y, Kuroda Y, Wakao S, Kushida Y, Dezawa M. Single-cell RNA sequencing reveals different signatures of mesenchymal stromal cell pluripotent-like and multipotent populations. iScience. 2022;25:105395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 56. | Tsai YC, Cheng TS, Liao HJ, Chuang MH, Chen HT, Chen CH, Zhang KL, Chang CH, Lin PC, Huang CF. Mesenchymal Stem Cell Secreted-Extracellular Vesicles are Involved in Chondrocyte Production and Reduce Adipogenesis during Stem Cell Differentiation. Tissue Eng Regen Med. 2022;19:1295-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 57. | Li J, Wu Z, Zhao L, Liu Y, Su Y, Gong X, Liu F, Zhang L. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther. 2023;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 58. | Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, Ezquer M, Cabello-Verrugio C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxid Med Cell Longev. 2016;2016:9047821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 59. | Li TS, Shi H, Wang L, Yan C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med Sci Monit. 2016;22:4651-4660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Schilling BK, Schusterman MA 2nd, Kim DY, Repko AJ, Klett KC, Christ GJ, Marra KG. Adipose-derived stem cells delay muscle atrophy after peripheral nerve injury in the rodent model. Muscle Nerve. 2019;59:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Artioli GG, De Oliveira Silvestre JG, Guilherme JP, Baptista IL, Ramos GV, Da Silva WJ, Miyabara EH, Moriscot AS. Embryonic stem cells improve skeletal muscle recovery after extreme atrophy in mice. Muscle Nerve. 2015;51:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Li WY, Zhu GY, Yue WJ, Sun GD, Zhu XF, Wang Y. KLF7 overexpression in bone marrow stromal stem cells graft transplantation promotes sciatic nerve regeneration. J Neural Eng. 2019;16:056011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Mierzejewski B, Michalska Z, Jackowski D, Streminska W, Janczyk-Ilach K, Koblowska M, Iwanicka-Nowicka R, Gromadka A, Ciemerych MA, Brzoska E. The miR151 and miR5100 Transfected Bone Marrow Stromal Cells Increase Myoblast Fusion in IGFBP2 Dependent Manner. Stem Cell Rev Rep. 2022;18:2164-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | de Munter JPJM, Mey J, Strekalova T, Kramer BW, Wolters EC. Why do anti-inflammatory signals of bone marrow-derived stromal cells improve neurodegenerative conditions where anti-inflammatory drugs fail? J Neural Transm (Vienna). 2020;127:715-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Sykova E, Cizkova D, Kubinova S. Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis. Front Cell Dev Biol. 2021;9:695900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Świerczek-Lasek B, Tolak L, Bijoch L, Stefaniuk M, Szpak P, Kalaszczynska I, Streminska W, Ciemerych MA, Archacka K. Comparison of Muscle Regeneration after BMSC-Conditioned Medium, Syngeneic, or Allogeneic BMSC Injection. Cells. 2022;11:2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 67. | Helal MAM, Shaheen NEM, Abu Zahra FA. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol Immunotoxicol. 2016;38:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Andrade BM, Baldanza MR, Ribeiro KC, Porto A, Peçanha R, Fortes FS, Zapata-Sudo G, Campos-de-Carvalho AC, Goldenberg RC, Werneck-de-Castro JP. Bone marrow mesenchymal cells improve muscle function in a skeletal muscle re-injury model. PLoS One. 2015;10:e0127561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Maeda Y, Yonemochi Y, Nakajyo Y, Hidaka H, Ikeda T, Ando Y. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci Rep. 2017;7:3305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Klimczak A, Kozlowska U, Kurpisz M. Muscle Stem/Progenitor Cells and Mesenchymal Stem Cells of Bone Marrow Origin for Skeletal Muscle Regeneration in Muscular Dystrophies. Arch Immunol Ther Exp (Warsz). 2018;66:341-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Archacka K, Grabowska I, Mierzejewski B, Graffstein J, Górzyńska A, Krawczyk M, Różycka AM, Kalaszczyńska I, Muras G, Stremińska W, Jańczyk-Ilach K, Walczak P, Janowski M, Ciemerych MA, Brzoska E. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res Ther. 2021;12:448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Moussa MH, Hamam GG, Abd Elaziz AE, Rahoma MA, Abd El Samad AA, El-Waseef DAA, Hegazy MA. Comparative Study on Bone Marrow-Versus Adipose-Derived Stem Cells on Regeneration and Re-Innervation of Skeletal Muscle Injury in Wistar Rats. Tissue Eng Regen Med. 2020;17:887-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Grabowska I, Zimowska M, Maciejewska K, Jablonska Z, Bazga A, Ozieblo M, Streminska W, Bem J, Brzoska E, Ciemerych MA. Adipose Tissue-Derived Stromal Cells in Matrigel Impacts the Regeneration of Severely Damaged Skeletal Muscles. Int J Mol Sci. 2019;20:3313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Archacka K, Bem J, Brzoska E, Czerwinska AM, Grabowska I, Kasprzycka P, Hoinkis D, Siennicka K, Pojda Z, Bernas P, Binkowski R, Jastrzebska K, Kupiec A, Malesza M, Michalczewska E, Soszynska M, Ilach K, Streminska W, Ciemerych MA. Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells. Cells. 2020;9:1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Yang W, Zhang S, Ou T, Jiang H, Jia D, Qi Z, Zou Y, Qian J, Sun A, Ge J. Interleukin-11 regulates the fate of adipose-derived mesenchymal stem cells via STAT3 signalling pathways. Cell Prolif. 2020;53:e12771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Leong J, Hong YT, Wu YF, Ko E, Dvoretskiy S, Teo JY, Kim BS, Kim K, Jeon H, Boppart M, Yang YY, Kong H. Surface Tethering of Inflammation-Modulatory Nanostimulators to Stem Cells for Ischemic Muscle Repair. ACS Nano. 2020;14:5298-5313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Ciervo Y, Gatto N, Allen C, Grierson A, Ferraiuolo L, Mead RJ, Shaw PJ. Adipose-derived stem cells protect motor neurons and reduce glial activation in both in vitro and in vivo models of ALS. Mol Ther Methods Clin Dev. 2021;21:413-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Wang X, Zhang Y, Jin T, Botchway BOA, Fan R, Wang L, Liu X. Adipose-Derived Mesenchymal Stem Cells Combined With Extracellular Vesicles May Improve Amyotrophic Lateral Sclerosis. Front Aging Neurosci. 2022;14:830346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 79. | Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W, Wang S, Song J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11:519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 80. | Kim MJ, Kim ZH, Kim SM, Choi YS. Conditioned medium derived from umbilical cord mesenchymal stem cells regenerates atrophied muscles. Tissue Cell. 2016;48:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Piao L, Huang Z, Inoue A, Kuzuya M, Cheng XW. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem Cell Res Ther. 2022;13:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 82. | Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 784] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 83. | Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento-Villareal R, Sun Z, Brunetti L, Hyoung Park J, Kaipparettu BA, Putluri N, Auetumrongsawat V, Yarasheski K, Qualls C, Villareal DT. Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab. 2019;30:261-273.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 84. | Song J, Liu J, Cui C, Hu H, Zang N, Yang M, Yang J, Zou Y, Li J, Wang L, He Q, Guo X, Zhao R, Yan F, Liu F, Hou X, Sun Z, Chen L. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia Muscle. 2023;14:915-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 85. | Nishimura K, Fukuda A, Hisatake K. Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming. Int J Mol Sci. 2019;20:2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Genet M, Torres-Padilla ME. The molecular and cellular features of 2-cell-like cells: a reference guide. Development. 2020;147:dev189688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 87. | Liu B, Li M, Zhang L, Chen Z, Lu P. Motor neuron replacement therapy for amyotrophic lateral sclerosis. Neural Regen Res. 2022;17:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Mora C, Serzanti M, Consiglio A, Memo M, Dell'Era P. Clinical potentials of human pluripotent stem cells. Cell Biol Toxicol. 2017;33:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Novelli G, Spitalieri P, Murdocca M, Centanini E, Sangiuolo F. Organoid factory: The recent role of the human induced pluripotent stem cells (hiPSCs) in precision medicine. Front Cell Dev Biol. 2022;10:1059579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Zhang L, Ma XJ, Fei YY, Han HT, Xu J, Cheng L, Li X. Stem cell therapy in liver regeneration: Focus on mesenchymal stem cells and induced pluripotent stem cells. Pharmacol Ther. 2022;232:108004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 91. | Iberite F, Gruppioni E, Ricotti L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regen Med. 2022;7:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 92. | Ohuchi K, Funato M, Kato Z, Seki J, Kawase C, Tamai Y, Ono Y, Nagahara Y, Noda Y, Kameyama T, Ando S, Tsuruma K, Shimazawa M, Hara H, Kaneko H. Established Stem Cell Model of Spinal Muscular Atrophy Is Applicable in the Evaluation of the Efficacy of Thyrotropin-Releasing Hormone Analog. Stem Cells Transl Med. 2016;5:152-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 501] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 94. | Pinton L, Khedr M, Lionello VM, Sarcar S, Maffioletti SM, Dastidar S, Negroni E, Choi S, Khokhar N, Bigot A, Counsell JR, Bernardo AS, Zammit PS, Tedesco FS. 3D human induced pluripotent stem cell-derived bioengineered skeletal muscles for tissue, disease and therapy modeling. Nat Protoc. 2023;18:1337-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 95. | Kim H, Perlingeiro RCR. Generation of human myogenic progenitors from pluripotent stem cells for in vivo regeneration. Cell Mol Life Sci. 2022;79:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Sun C, Kannan S, Choi IY, Lim H, Zhang H, Chen GS, Zhang N, Park SH, Serra C, Iyer SR, Lloyd TE, Kwon C, Lovering RM, Lim SB, Andersen P, Wagner KR, Lee G. Human pluripotent stem cell-derived myogenic progenitors undergo maturation to quiescent satellite cells upon engraftment. Cell Stem Cell. 2022;29:610-619.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Kim H, Selvaraj S, Kiley J, Azzag K, Garay BI, Perlingeiro RCR. Genomic Safe Harbor Expression of PAX7 for the Generation of Engraftable Myogenic Progenitors. Stem Cell Reports. 2021;16:10-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun. 2018;9:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 99. | Shahriyari M, Islam MR, Sakib SM, Rinn M, Rika A, Krüger D, Kaurani L, Gisa V, Winterhoff M, Anandakumar H, Shomroni O, Schmidt M, Salinas G, Unger A, Linke WA, Zschüntzsch J, Schmidt J, Bassel-Duby R, Olson EN, Fischer A, Zimmermann WH, Tiburcy M. Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy. J Cachexia Sarcopenia Muscle. 2022;13:3106-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 100. | Pepper JP, Wang TV, Hennes V, Sun SY, Ichida JK. Human Induced Pluripotent Stem Cell-Derived Motor Neuron Transplant for Neuromuscular Atrophy in a Mouse Model of Sciatic Nerve Injury. JAMA Facial Plast Surg. 2017;19:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Adami R, Bottai D. NSC Physiological Features in Spinal Muscular Atrophy: SMN Deficiency Effects on Neurogenesis. Int J Mol Sci. 2022;23:15209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 102. | Abati E, Bresolin N, Comi GP, Corti S. Preconditioning and Cellular Engineering to Increase the Survival of Transplanted Neural Stem Cells for Motor Neuron Disease Therapy. Mol Neurobiol. 2019;56:3356-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Forostyak S, Forostyak O, Kwok JCF, Romanyuk N, Rehorova M, Kriska J, Dayanithi G, Raha-Chowdhury R, Jendelova P, Anderova M, Fawcett JW, Sykova E. Transplantation of Neural Precursors Derived from Induced Pluripotent Cells Preserve Perineuronal Nets and Stimulate Neural Plasticity in ALS Rats. Int J Mol Sci. 2020;21:9593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Wang W, Li M, Chen Z, Xu L, Chang M, Wang K, Deng C, Gu Y, Zhou S, Shen Y, Tao F, Sun H. Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem Pharmacol. 2022;198:114954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Assoni A, Coatti G, Valadares MC, Beccari M, Gomes J, Pelatti M, Mitne-Neto M, Carvalho VM, Zatz M. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017;26:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 106. | Youssef El Baradie KB, Hamrick MW. Therapeutic application of extracellular vesicles for musculoskeletal repair & regeneration. Connect Tissue Res. 2021;62:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Yang Q, Su S, Liu S, Yang S, Xu J, Zhong Y, Yang Y, Tian L, Tan Z, Wang J, Yu Z, Shi Z, Liang F. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact Mater. 2023;26:194-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 108. | Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, Zhu B, Zhao R, Yu XY, Li Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728-6742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 109. | Ma Y, Dong L, Zhou D, Li L, Zhang W, Zhen Y, Wang T, Su J, Chen D, Mao C, Wang X. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23:2822-2835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 110. | Ma H, Jing Y, Zeng J, Ge J, Sun S, Cui R, Qian C, Qu S, Sheng H. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate muscle atrophy via the miR-132-3p/FoxO3 axis. J Orthop Translat. 2024;49:23-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Chen J, Ren S, Duscher D, Kang Y, Liu Y, Wang C, Yuan M, Guo G, Xiong H, Zhan P, Wang Y, Machens HG, Chen Z. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 2019;234:23097-23110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 112. | Mellows B, Mitchell R, Antonioli M, Kretz O, Chambers D, Zeuner MT, Denecke B, Musante L, Ramachandra DL, Debacq-Chainiaux F, Holthofer H, Joch B, Ray S, Widera D, David AL, Huber TB, Dengjel J, De Coppi P, Patel K. Protein and Molecular Characterization of a Clinically Compliant Amniotic Fluid Stem Cell-Derived Extracellular Vesicle Fraction Capable of Accelerating Muscle Regeneration Through Enhancement of Angiogenesis. Stem Cells Dev. 2017;26:1316-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Zhu A, Liu N, Shang Y, Zhen Y, An Y. Signaling pathways of adipose stem cell-derived exosomes promoting muscle regeneration. Chin Med J (Engl). 2022;135:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017;6:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 115. | Figeac N, Zammit PS. Coordinated action of Axin1 and Axin2 suppresses β-catenin to regulate muscle stem cell function. Cell Signal. 2015;27:1652-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Gan L, Zheng L, Yao L, Lei L, Huang Y, Zeng Z, Fang N. Exosomes from adipose-derived mesenchymal stem cells improve liver fibrosis by regulating the miR-20a-5p/TGFBR2 axis to affect the p38 MAPK/NF-κB pathway. Cytokine. 2023;172:156386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 117. | Fan W, Gao XK, Rao XS, Shi YP, Liu XC, Wang FY, Liu YF, Cong XX, He MY, Xu SB, Shen WL, Shen Y, Yan SG, Luo Y, Low BC, Ouyang H, Bao Z, Zheng LL, Zhou YT. Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate p38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration. Mol Cell Biol. 2018;38:e00211-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Nowosad A, Jeannot P, Callot C, Creff J, Perchey RT, Joffre C, Codogno P, Manenti S, Besson A. p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy-lysosomal pathway and coordinate cell cycle and cell growth. Nat Cell Biol. 2020;22:1076-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 119. | Wang C, Song W, Chen B, Liu X, He Y. Exosomes Isolated From Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs. Am J Sports Med. 2019;47:3247-3255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 120. | Bonafede R, Turano E, Scambi I, Busato A, Bontempi P, Virla F, Schiaffino L, Marzola P, Bonetti B, Mariotti R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int J Mol Sci. 2020;21:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 121. | Bonafede R, Brandi J, Manfredi M, Scambi I, Schiaffino L, Merigo F, Turano E, Bonetti B, Marengo E, Cecconi D, Mariotti R. The Anti-Apoptotic Effect of ASC-Exosomes in an In Vitro ALS Model and Their Proteomic Analysis. Cells. 2019;8:1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 122. | Luo Z, Lin J, Sun Y, Wang C, Chen J. Bone Marrow Stromal Cell-Derived Exosomes Promote Muscle Healing Following Contusion Through Macrophage Polarization. Stem Cells Dev. 2021;30:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 123. | Li Z, Liu C, Li S, Li T, Li Y, Wang N, Bao X, Xue P, Liu S. BMSC-Derived Exosomes Inhibit Dexamethasone-Induced Muscle Atrophy via the miR-486-5p/FoxO1 Axis. Front Endocrinol (Lausanne). 2021;12:681267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 124. | Pan J, Zhao M, Yi X, Tao J, Li S, Jiang Z, Cheng B, Yuan H, Zhang F. Acellular nerve grafts supplemented with induced pluripotent stem cell-derived exosomes promote peripheral nerve reconstruction and motor function recovery. Bioact Mater. 2022;15:272-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 125. | Yu M, Gu G, Cong M, Du M, Wang W, Shen M, Zhang Q, Shi H, Gu X, Ding F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021;134:190-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 126. | Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, Shen Y, Gong L, Zhang Q, Shen M, Gu X, Sun H. SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants (Basel). 2021;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 127. | Iyer SR, Scheiber AL, Yarowsky P, Henn RF 3rd, Otsuru S, Lovering RM. Exosomes Isolated From Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function After Muscle Injury. Am J Sports Med. 2020;48:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 128. | Pan Y, Li L, Cao N, Liao J, Chen H, Zhang M. Advanced nano delivery system for stem cell therapy for Alzheimer's disease. Biomaterials. 2025;314:122852. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 129. | Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 130. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 131. | Goodman SB, Lin T. Modifying MSC Phenotype to Facilitate Bone Healing: Biological Approaches. Front Bioeng Biotechnol. 2020;8:641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 133. | Zhang F, Leong W, Su K, Fang Y, Wang DA. A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng Part A. 2013;19:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Chen L, Li Y, Chen W, Han N, Li K, Guo R, Liu Z, Xiao Y. Enhanced recruitment and hematopoietic reconstitution of bone marrow-derived mesenchymal stem cells in bone marrow failure by the SDF-1/CXCR4. J Tissue Eng Regen Med. 2020;14:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Al-Kharboosh R, ReFaey K, Lara-Velazquez M, Grewal SS, Imitola J, Quiñones-Hinojosa A. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin Proc Innov Qual Outcomes. 2020;4:443-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 136. | Ogay V, Sekenova A, Li Y, Issabekova A, Saparov A. The Therapeutic Potential of Mesenchymal Stem Cells in the Treatment of Atherosclerosis. Curr Stem Cell Res Ther. 2021;16:897-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Sarsenova M, Issabekova A, Abisheva S, Rutskaya-Moroshan K, Ogay V, Saparov A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int J Mol Sci. 2021;22:11592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 138. | Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13:619-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F, Liu C, Liu J, Luo Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev Rep. 2022;18:1525-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 140. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 141. | Pezzi A, Amorin B, Laureano Á, Valim V, Dahmer A, Zambonato B, Sehn F, Wilke I, Bruschi L, Silva MALD, Filippi-Chiela E, Silla L. Effects Of Hypoxia in Long-Term In Vitro Expansion of Human Bone Marrow Derived Mesenchymal Stem Cells. J Cell Biochem. 2017;118:3072-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 142. | Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 143. | Stubbendorff M, Deuse T, Hua X, Phan TT, Bieback K, Atkinson K, Eiermann TH, Velden J, Schröder C, Reichenspurner H, Robbins RC, Volk HD, Schrepfer S. Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue. Stem Cells Dev. 2013;22:2619-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 144. | Zhuang X, Hu X, Zhang S, Li X, Yuan X, Wu Y. Mesenchymal Stem Cell-Based Therapy as a New Approach for the Treatment of Systemic Sclerosis. Clin Rev Allergy Immunol. 2023;64:284-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 145. | Drela K, Stanaszek L, Nowakowski A, Kuczynska Z, Lukomska B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. Stem Cells Int. 2019;2019:7012692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 146. | Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 147. | Al Tanoury Z, Rao J, Tassy O, Gobert B, Gapon S, Garnier JM, Wagner E, Hick A, Hall A, Gussoni E, Pourquié O. Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro. Development. 2020;147:dev187344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 148. | Shamsian A, Sahebnasagh R, Norouzy A, Hussein SH, Ghahremani MH, Azizi Z. Cancer cells as a new source of induced pluripotent stem cells. Stem Cell Res Ther. 2022;13:459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 149. | Xiong J, Hu H, Guo R, Wang H, Jiang H. Mesenchymal Stem Cell Exosomes as a New Strategy for the Treatment of Diabetes Complications. Front Endocrinol (Lausanne). 2021;12:646233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 150. | Rezaie J, Nejati V, Mahmoodi M, Ahmadi M. Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochem Pharmacol. 2022;203:115167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 151. | Li Y, Kamei Y, Kambe M, Ebisawa K, Oishi M, Takanari K. Peripheral Nerve Regeneration Using Different Germ Layer-Derived Adult Stem Cells in the Past Decade. Behav Neurol. 2021;2021:5586523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Shan Y, Zhang M, Tao E, Wang J, Wei N, Lu Y, Liu Q, Hao K, Zhou F, Wang G. Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges. Signal Transduct Target Ther. 2024;9:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Reference Citation Analysis (0)] |
| 153. | Winkler T, Perka C, von Roth P, Agres AN, Plage H, Preininger B, Pumberger M, Geissler S, Hagai EL, Ofir R, Pinzur L, Eyal E, Stoltenburg-Didinger G, Meisel C, Consentius C, Streitz M, Reinke P, Duda GN, Volk HD. Immunomodulatory placental-expanded, mesenchymal stromal cells improve muscle function following hip arthroplasty. J Cachexia Sarcopenia Muscle. 2018;9:880-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 154. | Jiang L, Mee T, Zhou X, Jia X. Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells. Stem Cell Rev Rep. 2022;18:544-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 155. | Kolagar TA, Farzaneh M, Nikkar N, Khoshnam SE. Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances and Limitations. Curr Stem Cell Res Ther. 2020;15:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 156. | Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 244] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 157. | Luo W, Zhang H, Wan R, Cai Y, Liu Y, Wu Y, Yang Y, Chen J, Zhang D, Luo Z, Shang X. Biomaterials-Based Technologies in Skeletal Muscle Tissue Engineering. Adv Healthc Mater. 2024;13:e2304196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |