Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.103599
Revised: December 23, 2024
Accepted: February 5, 2025
Published online: February 26, 2025
Processing time: 87 Days and 18.9 Hours
The global incidence of asthma, a leading respiratory disorder affecting more than 235 million people, has dramatically increased in recent years. Characterized by chronic airway inflammation and an imbalanced response to airborne irritants, this chronic condition is associated with elevated levels of inflammatory factors and symptoms such as dyspnea, cough, wheezing, and chest tightness. Conventional asthma therapies, such as corticosteroids, long-acting β-agonists, and anti-inflammatory agents, often evoke diverse adverse reactions and fail to reduce symptoms and hospitalization rates over the long term effectively. These limi
Core Tip: In this review, we provide an overview of the characteristics of stem cells, including embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells and adult stem cells, along with a summary of stem cell therapies for asthma and associated challenges. This review aims to guide future research endeavors on developing innovative stem cell therapies for asthma and other disorders.
- Citation: Chen QH, Zheng JY, Wang DC. Asthma and stem cell therapy. World J Stem Cells 2025; 17(2): 103599
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/103599.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.103599
Asthma, a chronic inflammatory airway disease affecting both children and adults, has seen a notable increase in incidence in recent years. The World Health Organization 2020 report indicates that approximately 235 million individuals suffer from asthma. Despite accounting for less than 1% of the total mortality rate from all causes, asthma significantly impacts human health and imposes a considerable economic burden. The cost of treating severe asthma cases can be 10 times higher than that of conventional therapy, consuming over 50% of global medical resources allotted for asthma[1].
Patients with asthma present with variable clinical and pathological manifestations including restricted airflow, lung tissue remodeling, and typical symptoms such as coughing, wheezing, and chest tightness. Infants frequently present with wheezing, and a significant proportion progress to chronic asthma by 6 years of age. However, three-quarters of school-aged children with asthma outgrow the disease by adulthood[2], while adults with asthma often experience incomplete remission[3,4]. Asthma is driven by an exaggerated T helper type 2 (Th2) immune response characterized by excess numbers of CD4+ T cells that produce interleukin 4 (IL-4) and IL-5. This response leads to the production of allergen-specific immunoglobulin E (IgE) and eosinophil accumulation that trigger chronic airway inflammation, culminating in airway remodeling marked by basement membrane thickening, goblet cell hyperplasia, smooth muscle cell proliferation, inflammatory cell infiltration, and mucus plug formation[5,6].
Traditional asthma therapies, such as corticosteroids, long-acting β-agonists, and anti-inflammatory agents, are often associated with a broad spectrum of adverse effects such as immunosuppression induced by long-term glucocorticoids use that can increase susceptibility to infection, as well as adrenocortical insufficiency, bone damage, electrolyte disorders, high blood pressure, and hyperglycemia[7-9]. Another drawback of conventional treatments lies in their inability to effectively reverse the asthma pathogenic process, thereby contributing to the high prevalence of severe and refractory cases that underscores the urgent need for innovative prevention and treatment strategies.
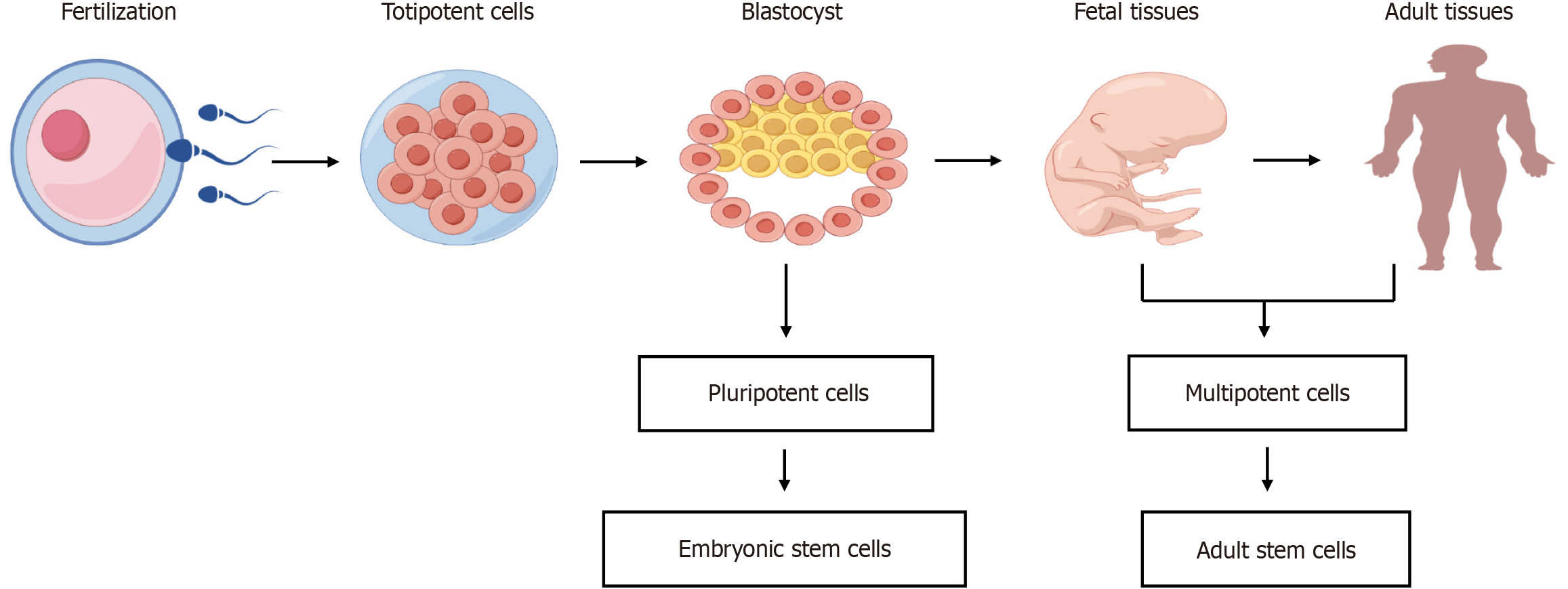
Stem cells (SCs), which have self-renewal and differentiation capabilities, were first identified in the hematopoietic system in the mid-20th century. A landmark 1963 study conducted by Becker et al[10] showed that simple infusion of bone marrow-derived cells into the blood of lethally irradiated animals can reconstitute all blood cell populations, rescuing the animals from death, while recent studies have highlighted their potential as therapies for lung diseases such as asthma[11]. Notably, SCs exhibit a spectrum of potencies, ranging from totipotent to pluripotent, multipotent, and unipotent, each with a progressively narrower range of differentiation potential. Totipotent SCs are capable of differentiating into all cell types found within the embryonic and extraembryonic tissues (e.g., placenta) of a developing organism; pluripotent cells can only differentiate into cell types found within the embryo proper, including germ cells and cells within any germ layer that can differentiate into any cell type found within a mature organism; multipotent cells are capable of differentiating into any cell type found within a specific germ layer; and unipotent cells can only differentiate into a limited number of cell types. Importantly, SCs can be derived using diverse methods from human embryos, adult somatic cells, or by enhancing the SC potential of differentiated somatic cells (Figure 1).
An overview of the characteristics of SCs is presented in this review, including embryonic SCs (ESCs), induced pluripotent SCs (iPSCs), mesenchymal SCs (MSCs), and adult SCs, along with a summary of SC therapies for asthma and associated challenges. This review aims to guide future research endeavors towards developing innovative SC therapies for asthma and other disorders.
ESCs, typically harvested from preimplantation blastocysts around 7 to 10 days post-fertilization, offer unparalleled regenerative capabilities. Their capacity for indefinite propagation and differentiation into essentially any specialized cell type within the body sets them apart as a powerful tool for disease treatment and tissue repair[12].
Lin et al[13] described a murine asthma model employed to uncover the association between the therapeutic benefits of human ESC (hESC)-derived MSCs (hESC-MSCs) and the expression of crucial mRNAs in lung tissue. These mRNAs are transcribed from genes encoding chemokine C-C motif ligand 11 (CCL11), CCL24, IL-13, IL-33, and the eosinophil-associated, ribonuclease A family, member 11, as assessed using a polymerase chain reaction array[13]. Ultimately, MSCs were successfully obtained from hESCs through a simple two-step protocol that eliminated the need for fluorescence-activated cell sorting, a process that could potentially damage the cells. Following intravenous injection into the tail veins of allergic mice, the transplanted hESC-MSCs suppressed allergic inflammation in the lung tissues of animals by reducing the expression of CCL11, CCL24, IL13, IL33 (expressed in Th2 cells) and the eosinophil-associated, ribonuclease A family, member 11 gene (expressed in eosinophils). Additionally, the transplanted cells restored previously diminished regulatory T cells (Tregs) levels in lung tissues to normal levels. These results suggest that allergic reactions induce elevated expression of these mRNAs, which MSC-mediated immunomodulation inhibits. It is noteworthy that hESC-MSCs share biological characteristics with bone marrow-derived MSCs (BMSCs), including expression of surface markers related to multilineage differentiation and immunomodulatory states, robust proliferation and regenerative capacity and low levels of heterogeneity.
Nevertheless, challenges persist, particularly in ensuring hESC-derived cells’ purity, safety, and efficacy before they are widely applied as asthma treatments in clinical settings. For example, MSC therapy has sometimes resulted in poor clinical outcomes due to inherent differences in characteristics among MSCs obtained from different donors[14]. It is, therefore, crucial to evaluate the efficiency of hESC-MSC differentiation by testing hESC-MSC cultures generated using the abovementioned protocol for potential contamination with other cell types, which could trigger unexpected side effects when transplanted. However, it is worth noting that in another study the vast majority of hESC-MSCs strongly expressed classical MSC markers cluster of differentiation 73 (CD73) (> 96.3%), CD90 (> 75.4%), and CD105 (> 99.7%) and were positive for other known MSC markers CD166 (> 45.0%), CD44 (> 98.2%), and CD146 (> 89.0%), while testing negative for the hematopoietic SC marker CD45 (< 8.0%)[13].
Although significant strides have been made in the field of ESC-related regenerative medicine, even highly pure populations of transplantable hESC-derived tissue-specific cells may be unsuitable for tissue regeneration due to low-level expression of human leukocyte antigen I that can trigger immunorejection despite their otherwise low immunogenicity. Accordingly, our laboratory developed a reliable culture and genetic selection procedure yielding the first pure population of transplantable hESCs derived from lung alveolar type II epithelial cells (hESC-ATIICs). After these cells were transplanted into the lungs of severe-combined immunodeficiency mice with bleomycin-induced acute lung injury[15], in vivo differentiation of hESC-ATIICs into ATICs was observed associated with repair of damaged lung tissue and long-term restoration of pulmonary function without teratoma formation. At study completion (10 days post-injury), the engrafted cells expressed ATIC phenotypic markers, strongly indicating ongoing or complete differentiation of transplanted ATIICs into ATICs, a noteworthy step towards overcoming the challenge of immunorejection within the context of ESC-derived cell transplantation-induced tissue repair. These results highlight an approach for overcoming immune rejection, paving the way for expanded clinical applications of hESCs and positioning them as a potential “universal donor” SC line with enhanced therapeutic potential.
In 2006, Takahashi and Yamanaka[16] introduced four transcription factors (TFs), organic cation transporter 3/4, sex determining region (SRY) box 2, c-Myc, and Kruppel-like factor 4, into mature cells lacking their expression. Intriguingly, a subset of these modified mature cells reverted to a significantly less-developed ESC-like state, highlighting the successful artificial expansion of cell pluripotency as a transformative achievement ushering in a new era in SC biology. Subsequently, iPSCs collected from an individual could differentiate into any other cell type found within that individual’s body, underscoring their potential value as a tool for evaluating the efficacy and safety of ‘personalized’ drug therapies. However, after extended culture, these cells exhibit noticeable changes in mRNA copy number during reprogramming and epigenetic memory following differentiation and increased in vivo tumorigenicity.
Interestingly, treatment of asthmatic mice with iPSC-MSCs or BMSCs prior to the antigenic challenge has been shown to reduce levels of Th2-induced immunoglobulins (e.g., IgE) and cytokines (e.g., IL-4, IL-5, IL-13) in bronchoalveolar and/or nasal lavage fluid[17,18]. Royce et al[19] demonstrated the superior protective effect of intranasally administered iPSCs and mesenchymoangioblast-derived MSCs against ovalbumin (OVA)-induced chronic allergic airway disease and asthma compared to corticosteroids.
In a study by Gao et al[20], two distinct sets of human iPSCs were employed to generate MSCs with robust proliferative capacity; heightened expression of recognized adult BMSC markers; and enhanced abilities to engage in adipogenesis, osteogenesis, and chondrogenesis. Specifically, urine cell-derived iPSCs were generated from cells isolated from human urine through reprogramming induced by electroporation of the plasmid pEP4EO2SET2K into the cells. Meanwhile, amniocyte-derived iPSCs were produced through retrovirus-mediated transduction of genes encoding organic cation transporter 4, SRY box 2, Kruppel-like factor 4, and c-Myc TFs into cells isolated from amniotic fluid. Notably, both types of iPSC-MSCs exhibited superior proliferative ability, longer life spans (over 50 passages), and lower rates of cell senescence than MSCs, highlighting their promise as a source of easily generated and well-tolerated MSCs for use in clinical applications. Additionally, these iPSC-MSCs inhibited dendritic cell differentiation, an effect attributed to both cell-cell interactions and iPSC-MSC secretion of IL-10. Unfortunately, safety assessments, including testing of iPSC-MSCs in immunodeficient mice, were lacking in these studies[20]. However, regarding the safety issue, previous studies demonstrated that two other iPSC-MSC clones suppressing allergic airway inflammation[17] were devoid of carcinogenic drifts during four months following their subcutaneous transplantation into severe-combined immunodeficiency mice[21]. In practice, incomplete and random reprogramming of iPSCs by TFs has been observed, associated with abnormal gene expression profiles and necessitating the rigorous screening of iPSCs using various techniques, such as whole genome sequencing, comparative genomic hybridization, single nucleotide polymorphism analysis, before deeming these iPSCs suitable for clinical applications. Within this context, our laboratory has successfully established a novel site-specific insertion-driven targeting strategy for efficiently generating mutation-free, reprogramming factor-free human iPSCs[22]. A refinement of the iPSC methodology is currently in progress, and it is poised to substantially increase the future utilization of iPSCs in therapeutic applications.
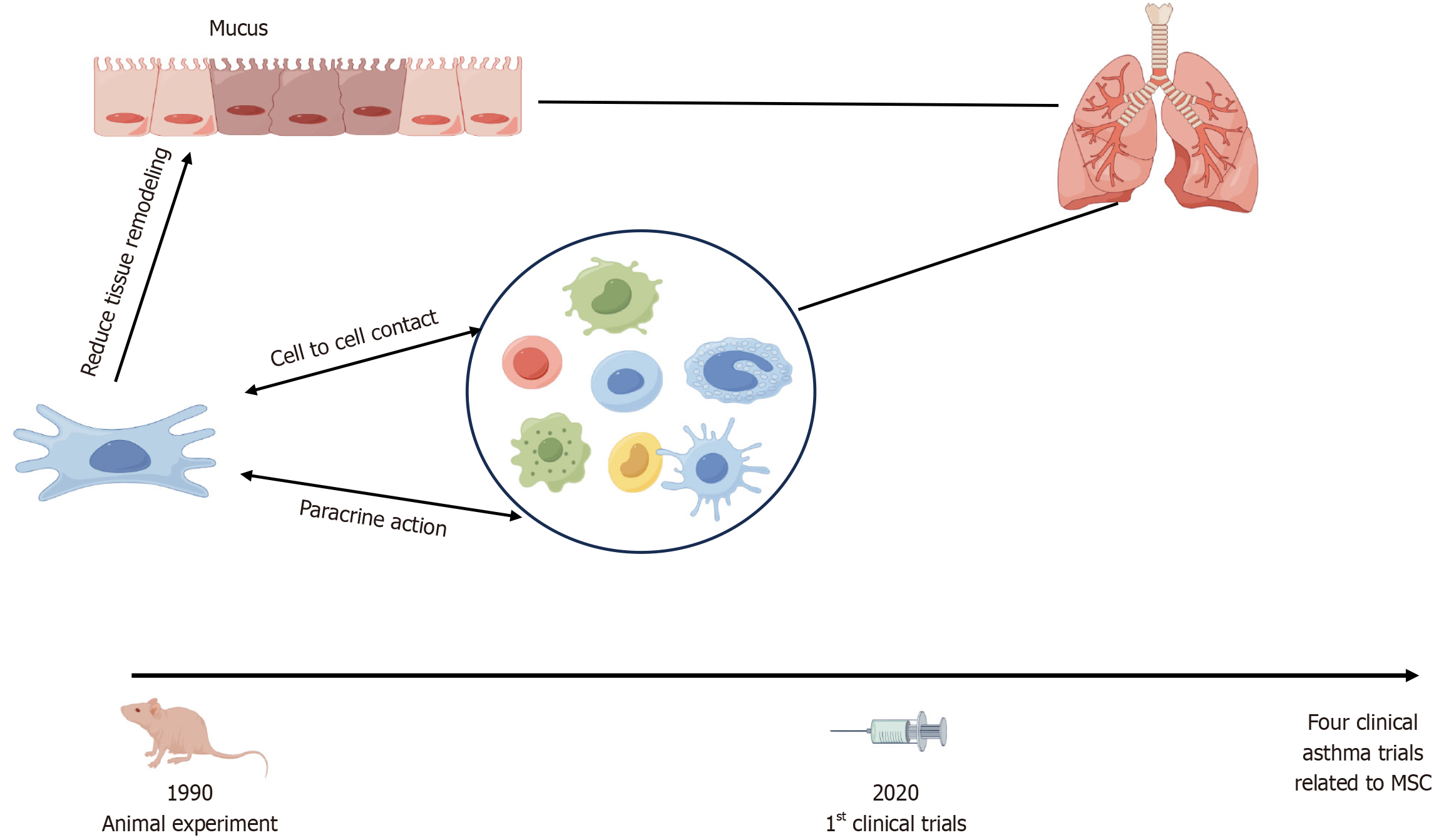
Over the past half-century, understanding the basic and clinical aspects of asthma MSC-mediated mechanisms has advanced significantly, resulting in the emergence of MSCs as the most extensively studied cell type in experimental cell therapy (Figure 2). Beyond their potential cell replacement applications, certain MSC cell types may be capable of altering the course of a disease without undergoing engraftment. This realization prompted researchers to explore the potential of MSCs to modulate cellular responses to injury or aberrant immune cell activity. In turn, these efforts led to the early identification of a population of BMSCs capable of generating various MSC-derived populations ex vivo, giving rise to the concept of MSCs as a customizable tool for regenerating specific types of tissues[23,24]. Currently, MSC-derived colonies arising in culture are recognized for their ability to generate cells that could be induced to differentiate into osteoblasts, adipocytes, or chondrocytes in vitro. MSCs may be administered in vivo via intravenous, intranasal, or intratracheal routes. However, an ongoing debate persists regarding the criteria for identifying these cells and their specific functions after in vivo administration.
MSCs effectively modulate immune responses by suppressing activated T and B cells, inhibiting M1 macrophage differentiation and reducing antigen-presenting cell costimulation[25]. They regulate the Th1/Th2 balance, suppress pathological T cell proliferation, and offer anti-inflammatory[26], antifibrotic[27], anti-apoptotic[28], antimicrobial[29], antioxidative[30], and pro-angiogenic benefits[31]. Additionally, MSCs enhance alveolar fluid clearance[32] and repair pulmonary endothelial and epithelial cell damage[33].
MSCs, specialized cells with critical roles in regulating immune system functions and managing immune responses triggering inflammatory diseases, do not home efficiently to target tissues when infused intravenously[34], resulting in limited MSC colonization and differentiation within target tissues[35]. Despite this limitation, MSCs exert immunoregulatory effects through cell-cell contact involving two key intercellular interaction molecules: Programmed death ligand 1, a costimulatory molecule, and tumor necrosis factor (TNF) ligand superfamily member 6 (TNFSF6)[36]. In one study, during T cell recruitment, BMSCs were found to regulate monocyte chemoattractant protein-1 secretion via a Fas-dependent mechanism, leading to T cell apoptosis through a TNFSF6-based mechanism. Subsequently, macrophages were observed to ingest apoptotic T cell debris and release elevated quantities of transforming growth factor beta (TGF-β), triggering enhanced Treg activity and immunotolerance.
MSCs have been evaluated as potential treatments for asthma. In a study conducted by Shin et al[37], the therapeutic effects of human umbilical cord-MSCs were evaluated in two murine models of severe asthma, alternaria (alternata-induced) and house dust mite/diesel exhaust particle-induced asthma. Their results revealed significant post-treatment reductions in airway hyperresponsiveness, lung eosinophil levels, and direct inhibition of Th2 cell and type 2 innate lymphoid cell activities. However, Volarevic et al[38] revealed that MSC populations exhibited notable diversity in secreted immunoregulatory factor profiles, encompassing TGF-β, hepatocyte growth factor, nitric oxide, indoleamine 2,3-dioxygenase, IL-10, IL-6, leukemia inhibitory factor, IL-1 receptor antagonist, galectins, TNF-stimulated gene 6 protein, human leukocyte antigen-G5, heme oxygenase-1, and prostaglandin E2[38].
Previously, researchers had speculated that the multidirectional differentiation potential of MSCs might lead to their differentiation into fibroblasts and myofibroblasts during asthma progression, triggering the pathological process of airway remodeling[39]. However, recent animal studies demonstrated that MSCs can improve airway remodeling in asthmatic mice[40,41], although the specific mechanism underlying this effect remains unclear. Meanwhile, the paracrine action of MSCs has been linked to the exosome release of biologically active substances that mirrors the observed MSC-induced immunomodulatory effect. MSC exosomes (MSC-Exo) maintained in a conditioned serum-free medium and isolated via serial centrifugations were non-immunogenic, well-tolerated by the human body, and equipped with membrane penetration and intrinsic homing capabilities.
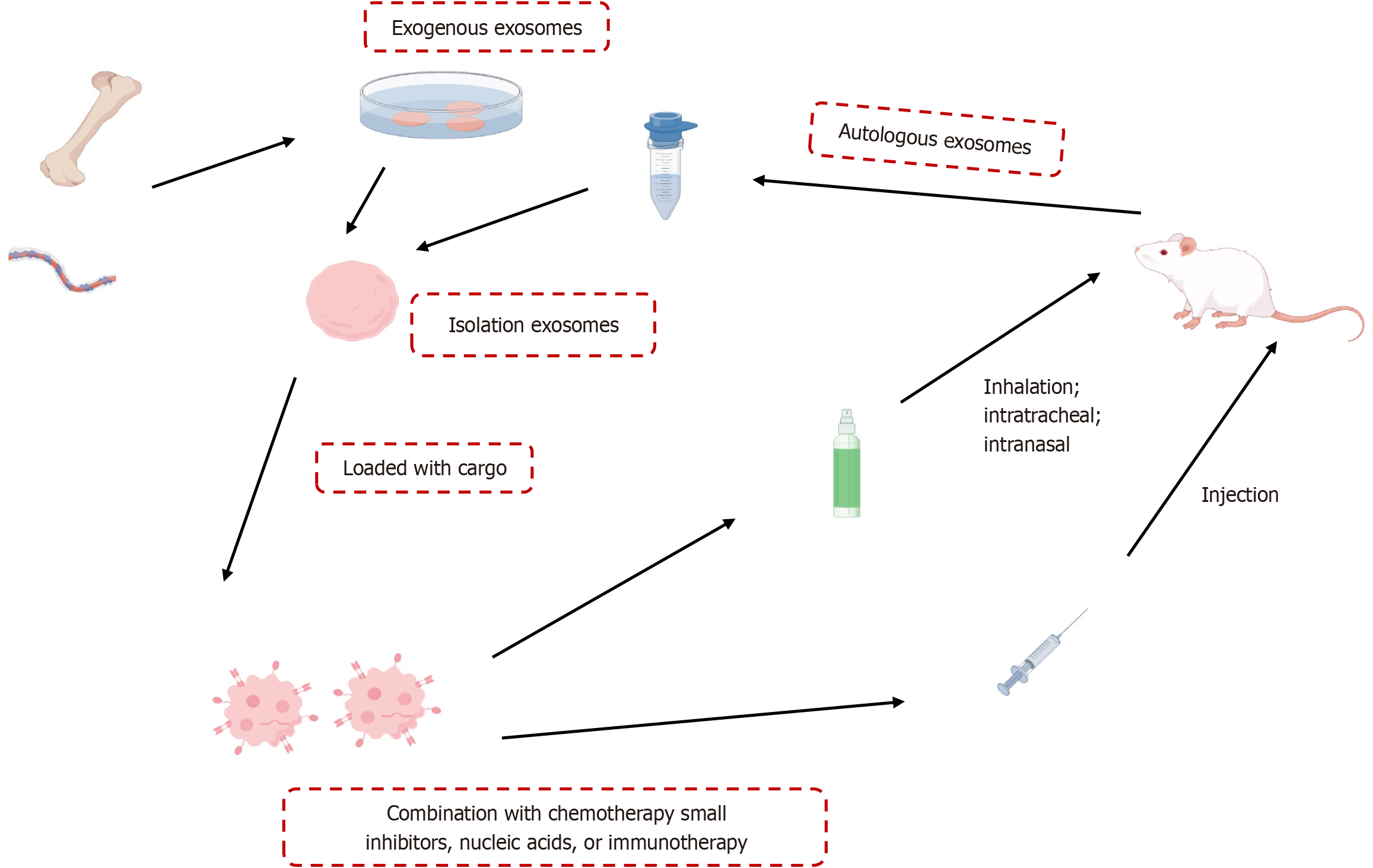
MSC-Exo modulate Treg activity by upregulating levels of immunosuppressive cytokines IL-10 and TGF-β1 produced by peripheral blood mononuclear cells of asthmatic patients, a process potentially influenced by antigen-presenting cells[42]. Furthermore, MSC-Exo reversed airway hyperresponsiveness, histopathological changes, and inflammation when administered intratracheally to a severe, steroid-resistant asthma mouse model by reshaping the macrophage polarization profile[43]. MSC-Exo may be an effective therapeutic strategy for Th17-dominant neutrophilic airway inflammation by inhibiting Th17 polarization through the Janus kinase 2/signal transducer and activator of transcription 3 pathway[44]. Kun et al[45] found that inhibiting the Notch1/Jagged1 pathway could facilitate the migration of allogeneic BMSCs to injured lung tissues, which promotes immune regulation, corrects the Th1/Th2 imbalance, and enhances the treatment of asthmatic airway inflammation. These effects suggest that MSCs can sense their environment and restore the T cell balance in individuals with disorders primarily associated with aberrant Th1 or Th2 responses[46]. Here, we highlight strategies of MSC-derived extracellular vesicle treatment in asthma models (Figure 3) and summarize findings from the past 3 years[44,47-57], showing that MSC-Exo hold promise as a cell-free therapeutic approach (Table 1). Exosomes are administered via intravenous, inhalation, intratracheal, and intranasal. Combined with chemotherapy, small inhibitors, nucleic acids, or immunotherapy could enhance the outcomes in treatment.
| Ref. | MSC-dosage and frequency | MSC-EV source | Asthma animal model | Asthma replication model | Animal numbers | Delivery | Cargo | EV markers |
| Firouzabadi et al[47], 2024 | 15 μg, 1 time | HBMSC | Male BALB/c mice | Sensitized and challenged with OVA | Total = 43, C = 11, A = 11, T = 21 | IV + IT | N/A | CD105, CD63 |
| Shan et al[48], 2022 | 20 μg, 9 times | HBMSC | BALB/c mice | Sensitized and challenged with OVA | Total = 30, C = 10, A = 10, T = 10 | IV | MiR-188, miR-124, miR-410, miR-223, miR-130a | CD81, TSG101 |
| Liu et al[49], 2022 | 100 μg, 3 times | MBMSC | BALB/c mice | Sensitized and challenged with OVA | Total = 24, C = 8, A = 8, T = 8 | IT | N/A | CD9, CD63, CD81, TSG101 |
| Feng et al[50], 2022 | NP | NP | Male BALB/c mice | Sensitized and challenged with OVA | Total = 15, C = 5, A = 5, T = 5 | NP | Some transfected with miR-301a-3 | CD63, CD9 |
| Li et al[51], 2023 | NP | MBMSC | Male SD rats | Sensitized and challenged with OVA | Total = 32, C = 8, A = 8, T = 16 | IV | MiR-223-3p | CD9, CD63, CD81 |
| Xu et al[52], 2023 | 40 μg, 4 times | Hypoxic, HUCMSC | Female BALB/c mice | Sensitized and challenged with OVA | Total = 21, C = 4, A = 5, IV T = 6, INH T = 6 | INH and IV | Some vesicles were transfected with miR-146a-5p | TSG101, HSP70 |
| Bandeira et al[53], 2023 | 2 × 109 particles, 1 time | HBMSC | C57BL/6 male mice | Sensitized and challenged with OVA | Total = 15, C = 5, A = 5, T = 5 | IN | N/A | Flotillin-1, CD81, and β-actin |
| Dehnavi et al[54], 2023 | NP | MAMSC | Female BALB/c mice | Sensitized and challenged with OVA | Total = 20, C = 5, A = 5, OVA-EV T = 5, normal EV T = 5 | SL | OVA | CD9, CD63 |
| Asadirad et al[55], 2023 | NP, 6 times | MAMSC | Female BALB/c mice | Sensitized and challenged with OVA | Total = 20, C = 5, A = 5, T = 10 | SL | OVA | CD9, CD63 |
| Luo et al[56], 2024 | 40 μg, 4 times | Hypoxic, HUCMSC | Female BALB/c mice | Sensitized and challenged with OVA | Total = 16, C = 4, A = 6 T = 6 | INH | N/A | TSG101, HSP70 |
| Liu et al[57], 2024 | NP, 3 times | HUCMSC | Female C57BL/6 mice | Sensitized and challenged with DFE | Total = 24, C = 6, A = 6, DFE + EVs T = 6, DFE + 146a-EVs T = 6 | INH | MiR-146a-5p | CD63, HSP70, TSG101 |
| He et al[44], 2024 | 2 × 1010 particles, 3 times | Human iPSC-MSCs | Female C57BL/6 mice | Sensitized with OVA and LPS, challenged with OVA | Total = 15, C = 5, A = 5, T = 5 | IV | N/A | CD9, CD63, Alix, TSG101, calnexin |
Moreover, the differential expression of numerous microRNAs (miRNAs), including miR-146a-5p[57], miR-223-3p[51], miR-138-5p[58], miRNA-let-7, miRNA-155, and miRNA-126, is associated with allergic airway inflammation[59], making these miRNAs potential therapeutic targets in human MSC-based therapy (part of these references are discussed in Table 1). At the same time, the miR-21/activin A receptor type 2A axis has emerged as a crucial mechanism implicated in asthmatic inflammation in both a mouse model of asthma and in human subjects with asthma[60]. Furthermore, human MSCs genetically modified to afford inhibition of miR-138-5p, showed enhanced ability to reduce inflammation and allergic reactions by activating sirtuin 1 and inhibiting the high-mobility group box 1/Toll-like receptor 4 pathway in an asthma mouse model[58]. Shan et al[48] found that human bone marrow-MSC-derived exosomes reduced BMSC proliferation and lung injury in asthmatic mice via the miR-188/Jumonji, AT-rich interactive domain 2/Wnt/β-catenin pathway. In addition, externally originating MSCs have been observed to migrate towards lung tissue, accumulating at damage sites and then differentiating into type I and type II alveolar epithelial cells that participate in tissue restoration and repair in vivo[61,62]. Although their roles in this process remain unclear, the perceived weak differentiation ability of MSCs has prompted researchers to focus increasingly on studying their immunomodulatory effects on the repair of damaged and diseased tissues instead of their transplantation and differentiation behaviors.
Placenta-derived MSCs exerted an anti-IL-5 effect in vitro and reduced IL-5 level in culture with peripheral blood mononuclear cells that had been isolated from different subgroups of children with asthma[63]. Concurrently, another study conducted around the same time demonstrated that therapeutic administration of human MSCs to a mouse model of allergic asthma positively impacted oxidative stress by decreasing nitrotyrosine levels in lung tissues[64]. Similarly, Hu et al[65] reported a reduction in inflammation and oxidative stress to improve therapeutic outcomes in lung injury of acute respiratory distress syndrome by a new technique of integrating biomaterials and therapeutic agents through the fusion of mitochondria with liposomes. Interactions with probiotics also exerted antioxidant effects through the action of antioxidant enzymes[66]. Surprisingly, even nonviable MSCs, such as apoptotic MSCs, have been shown to exert immunosuppressive effects in vivo[67].
Shortly after the abovementioned studies were completed, a 12-month study was conducted to evaluate the significance of repeated intravenous MSC infusions. The study’s findings revealed that 12-month administration of MSCs initiated after the onset of chronic allergic feline asthma did not lead to reduced airway inflammation and hyperresponsiveness, as assessed using computed tomographic measurements of airway remodeling. However, reduced airway remodeling was observed earlier, after 8 months of treatment[68]. More recently, researchers discovered that a single injection of human MSCs was significantly more effective than double injections in reducing OVA-induced airway inflammation in a mouse model. Although systemic delivery of cell therapy through infusion is thought to achieve optimal therapeutic efficiency and targeting of lung tissues after optimization of MSC size and treatment frequency[69], both local and systemic administration of a single dose of MSCs were shown to reduce inflammation and lung tissue remodeling in OVA- and aspergillus hyphal extract-induced allergic asthma models[70,71]. Conversely, a single dose of MSCs did not improve lung function or remodeling in house dust mite extract-induced allergic asthma. In contrast, multiple MSC doses reduce lung inflammation, stimulate tissue remodeling, improve lung mechanics, and promote T cell-mediated immunosuppression[70]. Consequently, these outcomes should be carefully considered in future clinical trials when evaluating potential MSC-based asthma therapies.
Most MSC-based trials are currently in their early stages (primarily Phase 1 or 2), with only a limited number progressing to Phase 3[72]. As of May 2020, 68 clinical trials related to MSCs and respiratory diseases were underway, as documented in the clinical registration research database[73]. Among these, coronavirus disease 2019 emerged as the most frequent target condition (31 ongoing trials), followed closely by acute respiratory distress syndrome and chronic obstructive pulmonary disease (10 trials each), idiopathic pulmonary fibrosis (six trials) and asthma (two trials). The remaining nine trials focused on a broad range of diseases, including cystic fibrosis, lung transplantation, pneumoconiosis, radiation-induced injury, and unspecified lung injury. In terms of trial phases, thirty trials were classified as Phase 1, 17 as combined Phase 1/2, 14 as Phase 2, two as combined Phase 2/3, and one as Phase 3.
In 2020, a report revealed treatment outcomes for three individuals who have severe asthma refractory to conventional therapies, including steroids, bronchodilators, and anti-IgE medications are not effective for severe asthma, but SC therapy is effective. These patients received a single intravenous treatment with autologous BMSCs (2 × 107 cells/patient) and then were monitored for 1 year for therapeutic and adverse effects. The study’s results demonstrated that administering autologous BMSCs via intravenous infusion was safe and effective in improving self-perceived quality of life, as assessed during the early post-procedure phase. Lung function and the 6-minute walk test measurements remained stable throughout[74]. These findings paved the way for future clinical investigations of treatments based on BMSCs or alternative cell types for patients diagnosed with severe asthma.
In 2023, Sharan et al[75] reported preliminary findings related to the first participant with asthma enrolled in a Phase 1 clinical trial (Safety of cultured allogeneic adult umbilical cord derived mesenchymal SC intravenous infusion for the treatment of pulmonary diseases, NCT05147688). This trial involved intravenous infusion of cultured MSCs derived from umbilical cord tissue, administered at a dosage of 100 million cells over a 40-minute period. Encouragingly, no adverse events or complications were noted at 2 months and 6 months post-treatment, while improvement in the participant’s condition persisted throughout the 6-month follow-up period. These results underscore the potential safety and efficacy of MSC-based therapies for pulmonary diseases, warranting further validation in more extensive clinical trials.
To date, four early-stage clinical studies employing SC therapies for asthma are registered in the clinical trials database, of which three are Phase 1 trials (http://clinicaltrials.gov) (Table 2). Notably, umbilical cord MSCs are the prevailing MSC type utilized in two of these trials, while allogeneic MSCs under evaluation in three of the four trials have exhibited robust immunomodulatory properties. As anticipated, MSCs were predominantly administered via the intravenous route (two trials), while intranasal delivery was employed in one trial. It is important to note that these studies are not designed to rule out potential adverse effects of therapy, including uncontrolled MSC proliferation, vascular blockage occurring after intravascular administration, and abnormal differentiation of injected MSCs. Nonetheless, before MSCs can be used for asthma treatment and other clinical applications, MSC dose/dosage, formulation, route of administration, frequency, and indications[76] must be optimized in animal models.
| NCT No | Title | Disease | Source MSCs | Auto/Allo? | Delivery | Phases | Enrollment | Ages eligible for study | Locations |
| 02192736 | Safety and feasibility study of intranasal MTF for treatment of asthma | Asthma | UCMSC-CM | Allogeneic | Intra-nasal | 1/2 | 20 | 18-65 years old | Panama |
| 03137199 | Allogeneic human MSCs via intravenous delivery in patients with mild asthma | Asthma | BMSCs | Allogeneic | IV | 1 | 6 | 18-65 years old | United States |
| 04883320 | Stem cell strategies for the treatment of chronic asthma | Asthma | MSC | Unspecified | Not provided | Not applicable | 15 | 18-70 years old | United Kingdom |
| 05147688 | Safety of cultured allogeneic adult umbilical cord-derived mesenchymal stem cells for pulmonary diseases | Pulmonary diseases, asthma | UCMSC | Allogeneic | IV | 1 | 20 | Child, adult, older adult | Antigua and Barbuda |
Importantly, MSCs derived from adult or newborn tissues of different donors exhibit limited proliferative capacities, significant variability in quality, rapid loss of differentiation potential, and lower therapeutic efficacy compared to corresponding features of iPSCs and ESCs. Nevertheless, MSCs are still considered an ideal therapeutic option due to their lower immunogenicity and greater ease of preparation. At the same time, iPSCs and ESCs, due to their high proliferation rates, could potentially serve as progenitor cells for generating artificially induced MCSs with therapeutic value. Meanwhile, explorations of the effects of MSC-Exo on the expression of genes related to asthma progression through gene editing could enhance MSC regenerative capacities and effectiveness. Notably, MSC-Exo hold great promise as an alternative to MSCs that may alleviate asthma by regulating the expression of novel miRNAs and other disease-related targets awaiting identification through more comprehensive approaches, such as transcriptomics and proteomics. Such research will enhance understanding of asthma-related pathways and pave the way for developing more targeted and effective therapeutic strategies.
Adult SCs, also known as tissue-specific SCs, are believed to reside in most tissues and persist throughout an individual’s lifetime. These cells are considered crucial for tissue maintenance and repair, particularly in tissues with high cell turnover, such as blood, skin, and intestines, where adult SCs have been clearly identified and studied experimentally[77-79]. Meanwhile, potential adult SC populations have also been reported in tissues with low cell turnover, such as muscle, brain, and kidney[80-83], although one report of adult SCs in lung tissues was retracted[84,85].
In animal models, substantial numbers of bone marrow-derived adult SCs that produce collagen type I and α-smooth muscle actin have been detected in specific tissues, such as lung tissues of mice with OVA-induced chronic asthma[86]. However, adult SC isolation from different tissues can be challenging and an obstacle hindering exploring its potential in regenerative medicine. Within this context, the application of iPSC technology has emerged as a significantly important tool that can stimulate local tissue adult SCs to engage in tissue repair through paracrine signaling and other mechanisms, offering a promising avenue for advancing regenerative medicine.
Although MSCs demonstrate promising therapeutic potential for asthma, MSC-based treatment strategies remain challenging. For example, restricted in vitro growth, stemness decline, and harsh microenvironments hinder transplanted MSCs’ therapeutic potential and clinical application prospects[87]. Allogeneic MSC infusion triggers immune memory and boosts innate responses. If their immunosuppressive function is not activated, MSCs can act like antigen-presenting cells and promote inflammation[88]. Adverse effects post-transplantation include fever, chills, headache, back pain, and numbness[89]. Several issues must be addressed before SCs can be harnessed for patient treatment in clinical settings.
The first concern revolves around tissue integration, whereby transplanted cells must seamlessly integrate into surrounding tissues to ensure physiologically beneficial outcomes. Intriguingly, certain types of SCs, such as hESC-derived endothelial cells, exhibit an inherent ability to assemble into tubular structures that can integrate within the vasculature of tissues when inoculated into animals as dispersed cells[90].
A second challenge is the high likelihood that transplanted cells will develop into tumours, with a particularly high risk noted for transplanted pluripotent cells, given their ability to generate teratomas in animal models[91]. Therefore, it is crucial to precisely determine the differentiation states of transplanted cells to avoid the delivery of residual pluripotent cells that might undergo abnormal differentiation in vivo. Additionally, culture procedures must be designed to minimize the proliferation of genetically aberrant and potentially hazardous cell types[92]. To address these concerns, assessing pluripotent or other cell types for genetic integrity before transplantation in vivo is essential.
A third challenge relates to the ability to control the differentiation of cells into specific cell types, as it can sometimes be challenging to obtain a desired cell type from pluripotent cells. Furthermore, achieving uniformity and consistency of differentiated cells may be difficult, especially when derived from certain progenitor cell types. While these challenges are daunting, they are not insurmountable, although overcoming each obstacle will require substantial effort and focus. Nevertheless, we remain confident that these hurdles will be overcome to pave the way for the continued incorporation of SCs in asthma treatment strategies[12].
This review covers fundamental SC traits, including ESCs, iPSCs, MSCs, and adult SCs. It sheds light on SC therapies for alleviating asthma. Although the mechanisms by which SCs alleviate asthma are unclear, results of animal experiments suggest that SC-based treatments may relieve asthma symptoms by effectively reducing both airway inflammation and tissue remodeling, improving oxidative stress responses and paracrine functions. Based on these findings, human clinical trials are underway to evaluate SC safety and effectiveness as treatments for asthma and other diseases.
Professor Da-Chun Wang, the important author of this review, died March 29, 2024. We would like to thank Professor Wang for the support, encouragement, and care. We will always miss him.
| 1. | Kerkhof M, Tran TN, Soriano JB, Golam S, Gibson D, Hillyer EV, Price DB. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73:116-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Guilbert T, Krawiec M. Natural history of asthma. Pediatr Clin North Am. 2003;50:523-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Ernst P, Cai B, Blais L, Suissa S. The early course of newly diagnosed asthma. Am J Med. 2002;112:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Bronnimann S, Burrows B. A prospective study of the natural history of asthma. Remission and relapse rates. Chest. 1986;90:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Akkoc T. Mesenchymal Stem Cells in Asthma. Adv Exp Med Biol. 2020;1247:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451-62; quiz 463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Asamoah F, Kakourou A, Dhami S, Lau S, Agache I, Muraro A, Roberts G, Akdis C, Bonini M, Cavkaytar O, Flood B, Izuhara K, Jutel M, Kalayci Ö, Pfaar O, Sheikh A. Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Papadopoulos NG, Agache I, Bavbek S, Bilo BM, Braido F, Cardona V, Custovic A, Demonchy J, Demoly P, Eigenmann P, Gayraud J, Grattan C, Heffler E, Hellings PW, Jutel M, Knol E, Lötvall J, Muraro A, Poulsen LK, Roberts G, Schmid-Grendelmeier P, Skevaki C, Triggiani M, Vanree R, Werfel T, Flood B, Palkonen S, Savli R, Allegri P, Annesi-Maesano I, Annunziato F, Antolin-Amerigo D, Apfelbacher C, Blanca M, Bogacka E, Bonadonna P, Bonini M, Boyman O, Brockow K, Burney P, Buters J, Butiene I, Calderon M, Cardell LO, Caubet JC, Celenk S, Cichocka-Jarosz E, Cingi C, Couto M, Dejong N, Del Giacco S, Douladiris N, Fassio F, Fauquert JL, Fernandez J, Rivas MF, Ferrer M, Flohr C, Gardner J, Genuneit J, Gevaert P, Groblewska A, Hamelmann E, Hoffmann HJ, Hoffmann-Sommergruber K, Hovhannisyan L, Hox V, Jahnsen FL, Kalayci O, Kalpaklioglu AF, Kleine-Tebbe J, Konstantinou G, Kurowski M, Lau S, Lauener R, Lauerma A, Logan K, Magnan A, Makowska J, Makrinioti H, Mangina P, Manole F, Mari A, Mazon A, Mills C, Mingomataj E, Niggemann B, Nilsson G, Ollert M, O'Mahony L, O'Neil S, Pala G, Papi A, Passalacqua G, Perkin M, Pfaar O, Pitsios C, Quirce S, Raap U, Raulf-Heimsoth M, Rhyner C, Robson-Ansley P, Alves RR, Roje Z, Rondon C, Rudzeviciene O, Ruëff F, Rukhadze M, Rumi G, Sackesen C, Santos AF, Santucci A, Scharf C, Schmidt-Weber C, Schnyder B, Schwarze J, Senna G, Sergejeva S, Seys S, Siracusa A, Skypala I, Sokolowska M, Spertini F, Spiewak R, Sprikkelman A, Sturm G, Swoboda I, Terreehorst I, Toskala E, Traidl-Hoffmann C, Venter C, Vlieg-Boerstra B, Whitacker P, Worm M, Xepapadaki P, Akdis CA. Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy. 2012;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 1194] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 10. | Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 799] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 11. | Quan Y, Wang D. Clinical potentials of human pluripotent stem cells in lung diseases. Clin Transl Med. 2014;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Blau HM, Daley GQ. Stem Cells in the Treatment of Disease. N Engl J Med. 2019;380:1748-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Lin YD, Fan XL, Zhang H, Fang SB, Li CL, Deng MX, Qin ZL, Peng YQ, Zhang HY, Fu QL. The genes involved in asthma with the treatment of human embryonic stem cell-derived mesenchymal stem cells. Mol Immunol. 2018;95:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18204] [Article Influence: 958.1] [Reference Citation Analysis (0)] |
| 17. | Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30:2692-2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Zhang R, Yin Z, Pan J, Zhai C, Athari SS, Dong L. Effect of transfected induced pluripotent stem cells with Decorin gene on control of lung remodeling in allergic asthma. J Investig Med. 2023;71:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Royce SG, Mao W, Lim R, Kelly K, Samuel CS. iPSC- and mesenchymoangioblast-derived mesenchymal stem cells provide greater protection against experimental chronic allergic airways disease compared with a clinically used corticosteroid. FASEB J. 2019;33:6402-6411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Gao WX, Sun YQ, Shi J, Li CL, Fang SB, Wang D, Deng XQ, Wen W, Fu QL. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 22. | Yan Q, Quan Y, Sun H, Peng X, Zou Z, Alcorn JL, Wetsel RA, Wang D. A site-specific genetic modification for induction of pluripotency and subsequent isolation of derived lung alveolar epithelial type II cells. Stem Cells. 2014;32:402-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1278] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 24. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 25. | Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018:3057624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 26. | Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, Bellusci S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell. 2017;21:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 319] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 28. | Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Silva-Carvalho AÉ, Cardoso MH, Alencar-Silva T, Bogéa GMR, Carvalho JL, Franco OL, Saldanha-Araujo F. Dissecting the relationship between antimicrobial peptides and mesenchymal stem cells. Pharmacol Ther. 2022;233:108021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Wang X, Zhao S, Lai J, Guan W, Gao Y. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. Int J Mol Sci. 2021;23:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012;302:L891-L908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Li JW, Wu X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur Rev Med Pharmacol Sci. 2015;19:2368-2378. [PubMed] |
| 33. | Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11:707-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 34. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 35. | Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 697] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 36. | Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 37. | Shin JW, Ryu S, Ham J, Jung K, Lee S, Chung DH, Kang HR, Kim HY. Mesenchymal Stem Cells Suppress Severe Asthma by Directly Regulating Th2 Cells and Type 2 Innate Lymphoid Cells. Mol Cells. 2021;44:580-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43:633-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 39. | Saunders R, Siddiqui S, Kaur D, Doe C, Sutcliffe A, Hollins F, Bradding P, Wardlaw A, Brightling CE. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123:376-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Royce SG, Rele S, Broughton BRS, Kelly K, Samuel CS. Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J. 2017;31:4168-4178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Mariñas-Pardo L, Mirones I, Amor-Carro O, Fraga-Iriso R, Lema-Costa B, Cubillo I, Rodríguez Milla MÁ, García-Castro J, Ramos-Barbón D. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy. 2014;69:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 43. | Dong B, Wang C, Zhang J, Zhang J, Gu Y, Guo X, Zuo X, Pan H, Hsu AC, Wang G, Wang F. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | He BX, Fang SB, Xie YC, Lou DX, Wu ZC, Li CG, Liu XQ, Zhou ZR, Huang LX, Tian T, Chen DH, Fu QL. Small extracellular vesicles derived from human mesenchymal stem cells prevent Th17-dominant neutrophilic airway inflammation via immunoregulation on Th17 cells. Int Immunopharmacol. 2024;133:112126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Kun W, Xiaomei C, Lei Y, Huizhi Z. Modulating Th1/Th2 drift in asthma-related immune inflammation by enhancing bone mesenchymal stem cell homing through targeted inhibition of the Notch1/Jagged1 signaling pathway. Int Immunopharmacol. 2024;130:111713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 47. | Firouzabadi SR, Mohammadi I, Ghafourian K, Kiani A, Hashemi SM. Correction: Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Asthma in Murine Models: A Systematic Review and Meta-analysis. Stem Cell Rev Rep. 2024;20:1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Shan L, Liu S, Zhang Q, Zhou Q, Shang Y. Human bone marrow-mesenchymal stem cell-derived exosomal microRNA-188 reduces bronchial smooth muscle cell proliferation in asthma through suppressing the JARID2/Wnt/β-catenin axis. Cell Cycle. 2022;21:352-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Liu W, Lin H, Nie W, Wan J, Jiang Q, Zhang A. Exosomal miR-221-3p Derived from Bone Marrow Mesenchymal Stem Cells Alleviates Asthma Progression by Targeting FGF2 and Inhibiting the ERK1/2 Signaling Pathway. Evid Based Complement Alternat Med. 2022;2022:5910874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Feng CY, Bai SY, Li ML, Zhao JY, Sun JM, Bao HJ, Ren Y, Su XM. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomal miR-301a-3p Regulates Airway Smooth Muscle Cells During Asthma by Targeting STAT3. J Asthma Allergy. 2022;15:99-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Li X, Yang N. Exosome miR-223-3p in the bone marrow-derived mesenchymal stem cells alleviates the inflammation and airway remodeling through NLRP3-induced ASC/Caspase-1/GSDMD signaling pathway. Int Immunopharmacol. 2023;123:110746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 52. | Xu X, Wang Y, Luo X, Gao X, Gu W, Ma Y, Xu L, Yu M, Liu X, Liu J, Wang X, Zheng T, Mao C, Dong L. A non-invasive strategy for suppressing asthmatic airway inflammation and remodeling: Inhalation of nebulized hypoxic hUCMSC-derived extracellular vesicles. Front Immunol. 2023;14:1150971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 53. | Bandeira E, Jang SC, Lässer C, Johansson K, Rådinger M, Park KS. Effects of mesenchymal stem cell-derived nanovesicles in experimental allergic airway inflammation. Respir Res. 2023;24:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 54. | Dehnavi S, Khodadadi A, Asadirad A, Ghadiri AA. Immune response modulation by allergen loaded into mesenchymal stem cell-derived exosomes as an effective carrier through sublingual immunotherapy. Immunobiology. 2023;228:152361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Asadirad A, Ghadiri AA, Amari A, Ghasemi Dehcheshmeh M, Sadeghi M, Dehnavi S. Sublingual prophylactic administration of OVA-loaded MSC-derived exosomes to prevent allergic sensitization. Int Immunopharmacol. 2023;120:110405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Luo X, Wang Y, Mao Y, Xu X, Gu W, Li W, Mao C, Zheng T, Dong L. Nebulization of Hypoxic hUCMSC-EVs Attenuates Airway Epithelial Barrier Defects in Chronic Asthma Mice by Transferring CAV-1. Int J Nanomedicine. 2024;19:10941-10959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Liu J, Xu Z, Yu J, Zang X, Jiang S, Xu S, Wang W, Hong S. MiR-146a-5p engineered hucMSC-derived extracellular vesicles attenuate Dermatophagoides farinae-induced allergic airway epithelial cell inflammation. Front Immunol. 2024;15:1443166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Tang H, Han X, Li T, Feng Y, Sun J. Protective effect of miR-138-5p inhibition modified human mesenchymal stem cell on ovalbumin-induced allergic rhinitis and asthma syndrome. J Cell Mol Med. 2021;25:5038-5049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Tang GN, Li CL, Yao Y, Xu ZB, Deng MX, Wang SY, Sun YQ, Shi JB, Fu QL. MicroRNAs Involved in Asthma After Mesenchymal Stem Cells Treatment. Stem Cells Dev. 2016;25:883-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Wu XB, Wang MY, Zhu HY, Tang SQ, You YD, Xie YQ. Overexpression of microRNA-21 and microRNA-126 in the patients of bronchial asthma. Int J Clin Exp Med. 2014;7:1307-1312. [PubMed] |
| 61. | Ai WJ, Li J, Lin SM, Li W, Liu CZ, Lv WM. R-Smad signaling-mediated VEGF expression coordinately regulates endothelial cell differentiation of rat mesenchymal stem cells. Stem Cells Dev. 2015;24:1320-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Lin SC, Liou YM, Ling TY, Chuang YH, Chiang BL. Placenta-Derived Mesenchymal Stem Cells Reduce the Interleukin-5 Level Experimentally in Children with Asthma. Int J Med Sci. 2019;16:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Malaquias MAS, Oyama LA, Jericó PC, Costa I, Padilha G, Nagashima S, Lopes-Pacheco M, Rebelatto CLK, Michelotto PV, Xisto DG, Brofman PRS, Rocco PRM, de Noronha L. Effects of mesenchymal stromal cells play a role the oxidant/antioxidant balance in a murine model of asthma. Allergol Immunopathol (Madr). 2018;46:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Hu H, Zhang W, Zhou Y, Zhao K, Kuang J, Liu X, Li G, Xi Y. Engineered mitochondrial ROS scavenger nanocomplex to enhance lung biodistribution and reduce inflammation for the treatment of ARDS. Adv Compos Hybrid Mater. 2024;7:194. [DOI] [Full Text] |
| 66. | Wen X, Qi LM, Zhao K. Influence of gut bacteria on type 2 diabetes: Mechanisms and therapeutic strategy. World J Diabetes. 2025;16:100376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 67. | Mathias LJ, Khong SM, Spyroglou L, Payne NL, Siatskas C, Thorburn AN, Boyd RL, Heng TS. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol. 2013;191:5914-5924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Trzil JE, Masseau I, Webb TL, Chang CH, Dodam JR, Cohn LA, Liu H, Quimby JM, Dow SW, Reinero CR. Long-term evaluation of mesenchymal stem cell therapy in a feline model of chronic allergic asthma. Clin Exp Allergy. 2014;44:1546-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Hur J, Kang JY, Kim YK, Lee SY, Jeon S, Kim Y, Jung CK, Rhee CK. Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma. J Korean Med Sci. 2020;35:e188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Castro LL, Kitoko JZ, Xisto DG, Olsen PC, Guedes HLM, Morales MM, Lopes-Pacheco M, Cruz FF, Rocco PRM. Multiple doses of adipose tissue-derived mesenchymal stromal cells induce immunosuppression in experimental asthma. Stem Cells Transl Med. 2020;9:250-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, Loi R, Cruz F, Dunaway CW, Steele C, Weiss DJ. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med. 2014;3:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1010] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 73. | Yen BL, Yen ML, Wang LT, Liu KJ, Sytwu HK. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID-19. Stem Cells Transl Med. 2020;9:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 74. | Aguiar FS, Melo AS, Araújo AMS, Cardoso AP, de Souza SAL, Lopes-Pacheco M, Cruz FF, Xisto DG, Asensi KD, Faccioli L, Salgado ABS, Landesmann MCPP, Goldenberg RCS, Gutfilen B, Morales MM, Rocco PRM, Lapa E Silva JR. Autologous bone marrow-derived mononuclear cell therapy in three patients with severe asthma. Stem Cell Res Ther. 2020;11:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Sharan J, Barmada A, Band N, Liebman E, Prodromos C. First Report in a Human of Successful Treatment of Asthma with Mesenchymal Stem Cells: A Case Report with Review of Literature. Curr Stem Cell Res Ther. 2023;18:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 76. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 810] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 77. | Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 179] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1556] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 79. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4336] [Article Influence: 240.9] [Reference Citation Analysis (0)] |
| 80. | Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 788] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 81. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3870] [Cited by in RCA: 3844] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 82. | Alexander MS, Rozkalne A, Colletta A, Spinazzola JM, Johnson S, Rahimov F, Meng H, Lawlor MW, Estrella E, Kunkel LM, Gussoni E. CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell. 2016;19:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Garcia SM, Tamaki S, Lee S, Wong A, Jose A, Dreux J, Kouklis G, Sbitany H, Seth R, Knott PD, Heaton C, Ryan WR, Kim EA, Hansen SL, Hoffman WY, Pomerantz JH. High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Reports. 2018;10:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogórek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 85. | Drazen JM. Retraction: Kajstura J et al. Evidence for Human Lung Stem Cells. N Engl J Med 2011;364:1795-806. N Engl J Med. 2018;379:1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Ou-Yang HF, Han XP, Zhao F, Ti XY, Wu CG. The role of bone marrow-derived adult stem cells in a transgenic mouse model of allergic asthma. Respiration. 2012;83:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Huang S, Li Y, Zeng J, Chang N, Cheng Y, Zhen X, Zhong D, Chen R, Ma G, Wang Y. Mesenchymal Stem/Stromal Cells in Asthma Therapy: Mechanisms and Strategies for Enhancement. Cell Transplant. 2023;32:9636897231180128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 88. | François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Averyanov A, Koroleva I, Konoplyannikov M, Revkova V, Lesnyak V, Kalsin V, Danilevskaya O, Nikitin A, Sotnikova A, Kotova S, Baklaushev V. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 2020;9:6-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 90. | Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 91. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10432] [Article Influence: 386.4] [Reference Citation Analysis (0)] |
| 92. | Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, Kashin S, Mekhoubad S, Ilic D, Charlton M, Saphier G, Handsaker RE, Genovese G, Bar S, Benvenisty N, McCarroll SA, Eggan K. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545:229-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |