Published online Jan 26, 2025. doi: 10.4252/wjsc.v17.i1.98777
Revised: October 12, 2024
Accepted: December 3, 2024
Published online: January 26, 2025
Processing time: 198 Days and 19.2 Hours
Mesenchymal stem cells (MSCs) are promising candidates for regenerative therapy due to their self-renewal capability, multilineage differentiation potential, and immunomodulatory effects. The molecular characteristics of MSCs are influenced by their location. Recently, epidural fat (EF) and EF-derived MSCs (EF-MSCs) have garnered attention due to their potential benefits to the spinal microenvironment and their high expression of neural SC markers. However, their clinical applications are limited due to cell senescence and limited accessibility of EF. Although many studies have attempted to establish an immortalized, stable SC line, the characteristics of immortalized EF-MSCs remain to be clarified.
To establish and analyze stable immortalized EF-MSCs.
The phenotypes of EF-MSCs were analyzed using optical microscopy. Cell immortalization was performed using lentiviral vectors. The biomolecular characteristics of the cells were analyzed by immunoblotting, quantitative PCR, and proteomics.
The immortalized EF-MSCs demonstrated a significantly extended lifespan compared to the control group, with well-preserved adipogenic potential and SC surface marker expression. Introduction of human telomerase reverse transcriptase genes markedly increased the lifespan of EF-MSCs. Proteomics analysis revealed substantial increase in the expression of DNA replication pathway components in immortalized EF-MSCs.
Immortalized EF-MSCs exhibited significantly enhanced proliferative capacity, retained adipogenic potential, and upregulated the expression of DNA replication pathway components.
Core Tip: Epidural fat-derived mesenchymal stem cells (EF-MSCs) are potential candidates to treat neurodegenerative diseases. However, the clinical implication of EF-MSCs has encountered many limitations including difficulty in isolating the fat from the epidural region and limited proliferation ability of the SCs. Therefore, precise biomolecular characteristics of the EF-MSC remain unclear. To overcome these problems, we immortalized EF-MSCs through lentiviral transduction, and these stable EF-MSCs were analyzed by phenotypical interpretation and proteomics. In this study, immortalized EF-MSCs demonstrated notably increased lifespan and adipogenic potential. These findings suggest that stable EF-MSCs could be potential tools for developing effective regenerative medicine strategies.
- Citation: Lee SW, Lim YJ, Kim HY, Kim W, Park WT, Ma MJ, Lee J, Seo MS, Kim YI, Park S, Choi SK, Lee GW. Immortalization of epidural fat-derived mesenchymal stem cells: In vitro characterization and adipocyte differentiation potential. World J Stem Cells 2025; 17(1): 98777
- URL: https://www.wjgnet.com/1948-0210/full/v17/i1/98777.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i1.98777
Mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into mesodermal-derived tissues, including muscle, adipose tissue, fibrous tissue, chondrocytes, bones, and nerves[1]. Research has demonstrated that MSCs hold significant promise for regenerative cell therapy due to their multipotency, self-renewal ability, and immunomodulatory effects[2]. Consequently, there have been extensive efforts to isolate MSCs from sources such as bone marrow, umbilical cord, peripheral blood, and adipose tissue[3].
Adipose tissue-derived MSCs have been identified as promising candidates for cellular and regeneration therapy. Typically isolated from subcutaneous fat tissue, these MSCs offer significant advantages over those derived from other sources, including greater accessibility, abundance, and minimally invasive collection methods[4]. Research indicates that adipocytes undergo distinct metabolic processes based on their anatomical location. For example, Ibrahim[5] demon
Epidural fat (EF) is a type of adipose tissue with a metameric distribution surrounding the spinal canal and dura mater[8]. Traditionally, it is considered a clinically insignificant tissue, primarily serving as a space-filling material and reducing mechanical stress on the spinal cord, often leading to its removal during surgery. However, recent studies have challenged this view, suggesting that EF is more than just a filler tissue, with its volume positively correlating with the prognosis of various spinal disorders[9,10]. Consequently, there is a growing interest in EF-derived MSCs (EF-MSCs). Shah et al[11] highlighted the potential role of EF-MSCs in preserving the spinal region’s microenvironment. Similarly, Solmaz et al[12] reported that EF-MSCs exhibit significantly higher expression of neural SC markers (e.g., nestin, glial fibrillary acidic protein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase) compared to subcutaneous fat-derived MSCs. These findings led to the hypothesis that EF-MSCs could serve as promising candidates for regenerative or cellular therapy targeting degenerative spine diseases. Despite their potential, the clinical application of EF-MSCs is hindered by several challenges, including the complex anatomical location of EF and the limited lifespan of the SCs. EF is situated in the epidural space, making MSC isolation more difficult compared to sources such as subcutaneous fat[13]. Furthermore, MSCs isolated from EF typically exhibit limited passage ability because of cell senescence[14]. These limitations necessitate further research to overcome these obstacles. One promising approach is immortalization, a technique that involves the transduction of specific genes, such as human telomerase reverse transcriptase (hTERT) and human papilloma virus E6 and E7[15]. Immortalization enables SCs to bypass senescence and achieve unlimited proliferation, allowing the establishment of stable SC lines[16]. Building on these findings, we hypothesized that immortalizing EF-MSCs represents a viable strategy for advancing cell therapy research.
All procedures involving cell cultures derived from human samples were conducted with approval from the institutional review board and Ethical Committee of Yeungnam University Medical Center (IRB No. 2017-07-032-001). The isolated fat tissues were briefly disinfected with 70% ethanol and minced into smaller pieces, after which debris was removed using sterile surgical scissors. The remaining adipose tissue was digested with 2 mg/mL collagenase (17018-029; Gibco, Waltham, MA, United States) at 37 °C in a shaking incubator for 30 minutes. Following digestion, the collagenase was neutralized by adding an equal volume of Dulbecco’s Modified Eagle Medium (DMEM, 17018-029; Gibco) supplemented with 20% fetal bovine serum (FBS) (26140-079; Gibco) and 2% penicillin-streptomycin (15150-122; Gibco). The resulting homogenate was filtered through a 70 μm sterile nylon mesh cell strainer (93070; SPL Life Sciences, Pocheon-si, South Korea) and centrifuged at 3000 rpm for 5 minutes to isolate the stromal vascular fraction (SVF). Then the SVF was resuspended in DMEM and centrifuged again at 3000 RPM for 5 minutes to eliminate cell debris and other precipitates. The resulting cell pellet was carefully resuspended in 1 mL cell culture medium (DMEM supplemented with 10% FBS and 1% penicillin-streptomycin) and seeded onto sterile cell culture dishes (200100; SPL). After 2 hours, non-adherent materials, including cellular debris and red blood cells, were washed away with phosphate-buffered saline (PBS) (SH30256.01; Cytiva, Marlborough, MA, United States) and fresh cell culture medium. When the cells reached 70%-80% confluence, they were detached using 0.05% trypsin-ethylenediamine tetraacetic acid (25300-062; Gibco) and replated for further expansion.
Lentiviral vectors encoding hTERT and E6/E7 were obtained from Applied Biological Materials, Inc. (Richmond, British Columbia, Canada). Passage 5 EF-MSCs at 50%-60% confluence were transfected using a mixture of lentiviral vector suspension and fresh cell culture medium at a 1:1 ratio, supplemented with 8 μg/mL polybrene. The transfected EF-MSCs were incubated overnight at 37 °C in a humidified atmosphere with 5% CO2, and the culture medium was replaced 3 days post-infection. Following transfection, the immortalized EF-MSCs were naturally selected based on their ability to sustain continuous growth.
To confirm immortalization, EF-MSCs are evaluated using quantitative reverse transcriptase polymerase chain reaction. RNA was extracted from the EF-MSCs using a commercial cell RNA Extraction Kit (9767A; Takara Bio Inc., Shiga, Japan) and quantified with the NanoDrop One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). cDNA synthesis was performed using a cDNA Synthesis Kit (6110A; Takara) following the manufacturer’s protocol. For quantitative polymerase chain reaction (qPCR), the cDNA samples were combined with TB Green Premix (RR071A; Takara) and 5 pmol primers. The amplification process was conducted on the CFX96TM Real-Time System (Bio-Rad, Hercules, CA, United States). β-Actin served as the internal control to normalize mRNA expression. The primer sequences used in this study are provided in Supplementary Table 1.
To assess the phenotypic characteristics of SCs, positive and negative SC surface markers were analyzed using fluorescence-activated single cell sorting (FACS). Briefly, EF-MSCs were seeded in 6-well plates and harvested using 0.05% trypsin-ethylenediamine tetraacetic acid once they reached 60%-70% confluence. Then the cells were washed with ice-cold FACS buffer (PBS containing 10% FBS and 1% sodium azide) and incubated with primary antibodies diluted in PBS containing 3% bovine serum albumin (BSA) (A0100-010; GenDEPOT, Katy, TX, United States) for 30 minutes at room temperature. After incubation, the cells were washed three times with ice-cold PBS and subsequently incubated with fluorescently labeled secondary antibodies, also diluted in 3% BSA/PBS, in the dark for 30 minutes at room temperature. Following this, the cells were washed three times with ice-cold PBS, resuspended in 3% BSA/PBS, and analyzed using the BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, United States). Detailed information about the antibodies used for FACS analysis is provided in Table 1.
| Name | Sources | Dilution | Catalog number | Company |
| CD105 | Mouse | 1:200 | NB110-58718 | Novus |
| CD90 | Mouse | 1:200 | 14-0909-82 | Thermo Fisher Scientific |
| CD73 | Mouse | 1:200 | NBP2-48480 | Novus |
| CD45 | Rabbit | 1:200 | GTX116018 | GeneTex |
| CD34 | Rabbit | 1:200 | 60180-1-Ig | Proteintech |
| HLA-DR | Rabbit | 1:200 | bsm-52535R | Bioss Antibodies |
| Alexa Fluor 488-donkey anti-mouse IgG | Donkey | 1:500 | ab150105 | Abcam |
| Alexa Fluor 555-donkey anti-rabbit IgG | Donkey | 1:500 | ab150066 | Abcam |
We conducted a SC adipocyte differentiation assay to assess the stemness of EF-MSCs. The cells were differentiated into adipocytes using the commercial StemPro Adipogenesis Differentiation Kit (A1007001; Gibco). Briefly, EF-MSCs were seeded EF-MSCs in a 12-well cell culture plate at 50%-60% confluency and incubated with the adipocyte differentiation medium, as recommended by the manufacturer, for 1 week. To visualize lipid droplets, the EF-MSCs were fixed in 4% paraformaldehyde at room temperature for 20 minutes and then stained using an Oil Red O kit (LL-0052; Lifeline, Oceanside, CA, United States). Images were captured with the Leica DM 5000 optical microscope (Leica, Wetzlar, Germany) and Oil Red O quantification by measuring five random fields using ImageJ software (version 1.54k).
Normal and adipogenic EF-MSCs were seeded as previously described. The cells were harvested using protein lysis buffer containing 1 × Laemmli’s sodium dodecyl sulfate (SDS) sample buffer (GenDepot; L1100-001). Equal volumes of the samples were then heated at 95 °C for 10 minutes and subjected to 10% SDS polyacrylamide gel electrophoresis. After protein separation, the proteins were electrotransferred to nitrocellulose membranes using a semi-dry blotting system (Bio-Rad). The membranes were blocked in EveryBlot blocking buffer (12010020; Bio-Rad) for 10 minutes at room temperature, followed by overnight incubation with primary antibodies, including anti-β-actin (1:2000, #MA5-15739; Thermo Fisher Scientific) and anti-CCAAT/enhancer-binding protein alpha (C/EBPα) (1:2000, 2295; Cell Signaling Technology, Danvers, MA, United States) at 4 °C. Then the membranes were washed three times with Tris-buffered saline with Tween buffer and incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit and goat anti-mouse) in antibody dilution buffer for 1 hour at room temperature. Finally, the protein bands were visualized using Clarity Western Enhanced Chemiluminescence substrate (#1705061; Bio-Rad) and captured with the ChemiDoc MP Imaging System (Bio-Rad). Protein expression intensity was measured using ImageJ software.
To evaluate the senescence of EF-MSCs, β-galactosidase staining was performed following the manufacturer’s instructions (#9860; Cell Signaling Technology). Briefly, EF-MSCs were washed three times with Dulbecco’s PBS, fixed at room temperature for 20 minutes, and then incubated overnight in β-galactosidase staining solution in a 37 °C dry incubator. The cells were subsequently mounted in 70% glycerol. Images were captured using the Leica DM 5000 optical microscope, and β-galactosidase activity was quantified from five random fields using ImageJ software (version 1.54k).
Data from each experiment are expressed as the mean ± standard deviation, and P values among the samples were determined using the unpaired t-test (MS Excel).
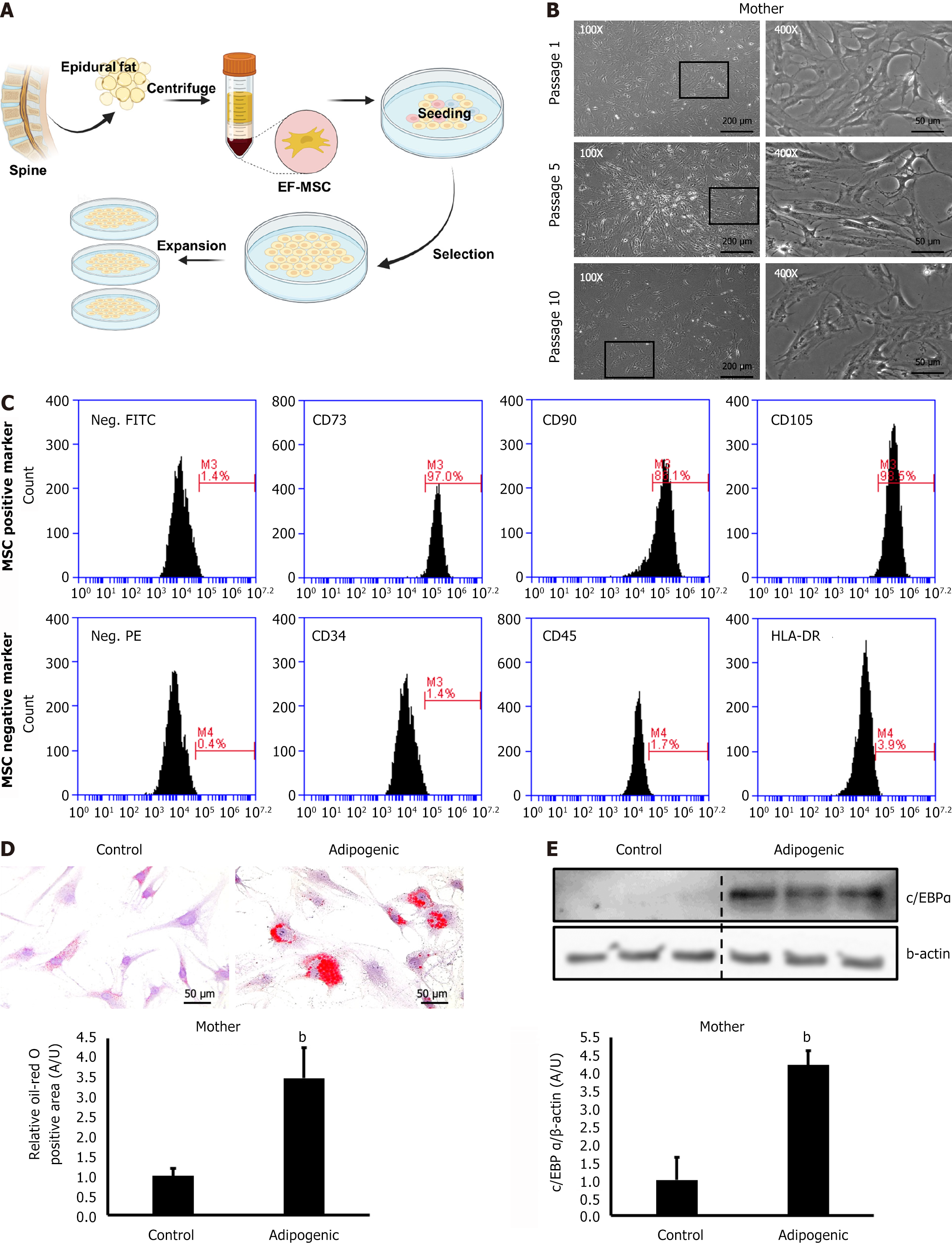
Human adipose tissue-derived MSCs were successfully isolated from EF using the methods outlined in this study. The cells from the SVF were seeded into cell culture plates (Figure 1A). The isolated EF-MSCs exhibited a spindle-shaped morphology and demonstrated continuous growth up to passage 10 (Figure 1B). The cells were characterized for SC surface markers using FACS analysis. Approximately 90% of the cells expressed cluster of differentiation 73 (CD73), CD90, and CD105, which are common markers for SCs, whereas fewer than 4% exhibited hematopoietic cell markers, including CD34, CD45, and human leukocyte antigen-DR (Figure 1C). Differentiation assays and Oil Red O staining revealed a significant increase in lipid droplets in EF-MSCs incubated in differentiation medium compared to control cells (Figure 1D). Consistently, immunoblot analysis showed increased C/EBPα expression in the EF-MSCs incubated in adipogenic medium compared to those in the control group (Figure 1E). These findings demonstrate that EF-MSCs isolated from human patients possess both proliferative and adipogenic potential.
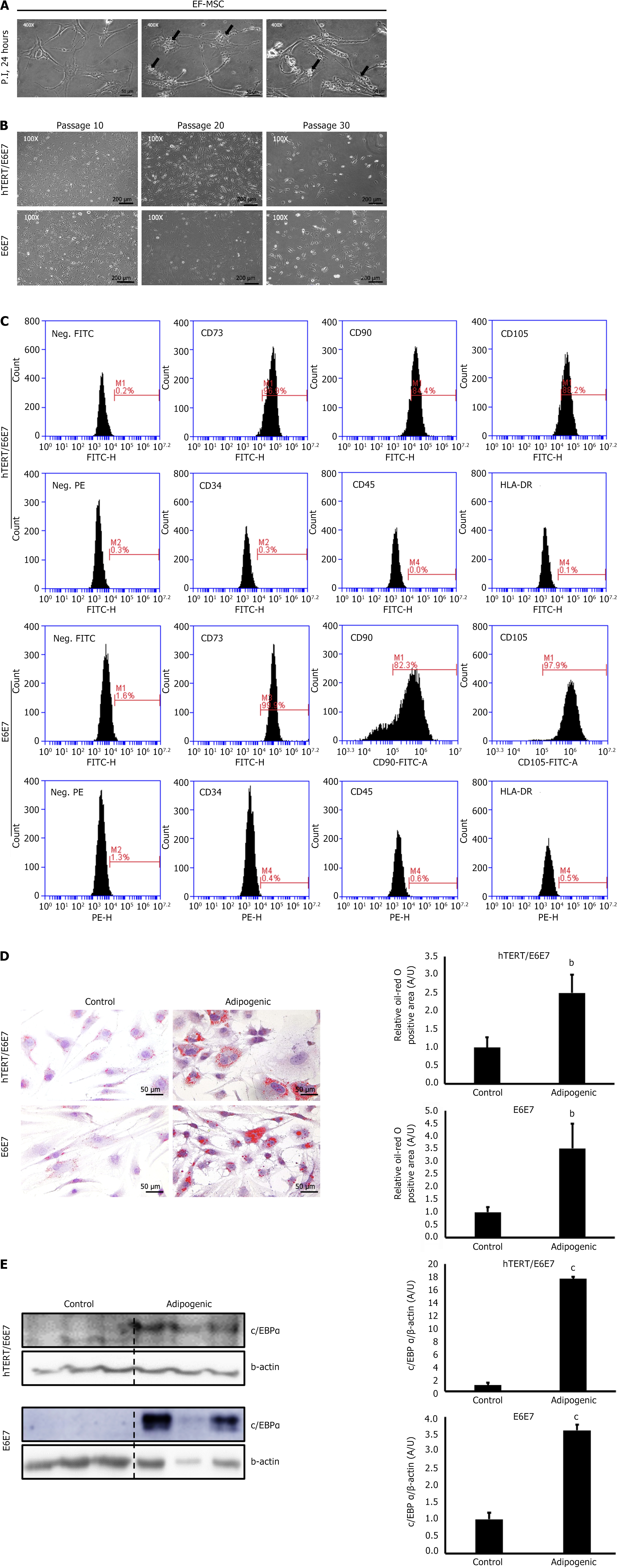
For SC immortalization, immortalization genes were transfected into passage 5 EF-MSCs using lentiviral vectors with polybrene. Following transfection, the cells exhibited vacuolar degeneration, indicating mild cell injury due to the lentiviral vectors (Figure 2A). To confirm gene expression in the transfected EF-MSCs, qPCR analysis was performed (Supplementary Figure 1). These results confirmed successful transfection with the viral vectors. As expected, the proliferative ability of EF-MSCs was well preserved until the cells reached a senescent state, around passage 30 (Figure 2B). FACS analysis further showed that immortalized passage 20 EF-MSCs retained the expression of SC surface markers (Figure 2C). Moreover, differentiation assays and western blot analysis demonstrated that the adipogenic potential of the EF-MSCs remained intact, despite the transfection of immortalization genes (Figure 2D and E). These findings indicate that the immortalization did not alter the SC characteristics of the EF-MSCs.
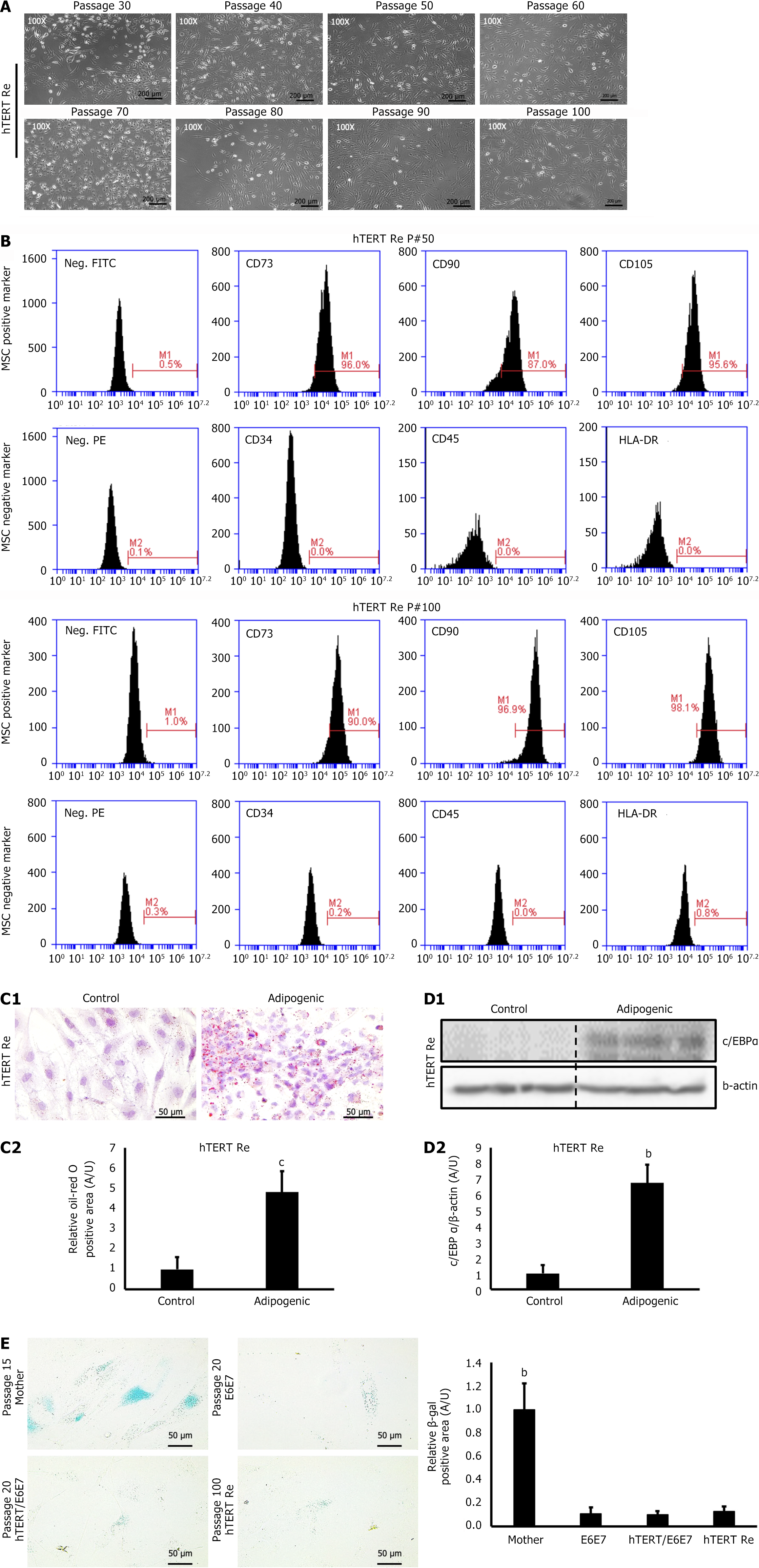
In the present study, the proliferative ability of the immortalized EF-MSCs gradually declined around passage 30. To restore the cell growth capacity, we transfected the cells with hTERT. Following transfection, the retransfected EF-MSCs (hTERT Re) maintained their proliferative ability for more than 100 passages without exhibiting signs of senescence or morphological changes (Figure 3A). These cells also retained the expression of SC surface marker proteins (Figure 3B). Consistent with this finding, the adipogenic differentiation assay performed on retransfected passage 100 EF-MSCs showed that their differentiation ability was preserved, even after prolonged culture (Figure 3C and D). Given that the parent EF-MSCs stopped proliferating around passage 15, we hypothesized that immortalization successfully extended the lifespan of the EF-MSCs. In line with this, the β-galactosidase assay showed a significantly reduced positive area in the immortalized EF-MSCs (Figure 3E). These data suggest that immortalization, combined with retransfection of hTERT, can significantly increase the lifespan of EF-MSCs without inducing tumorigenesis or compromising their stemness.
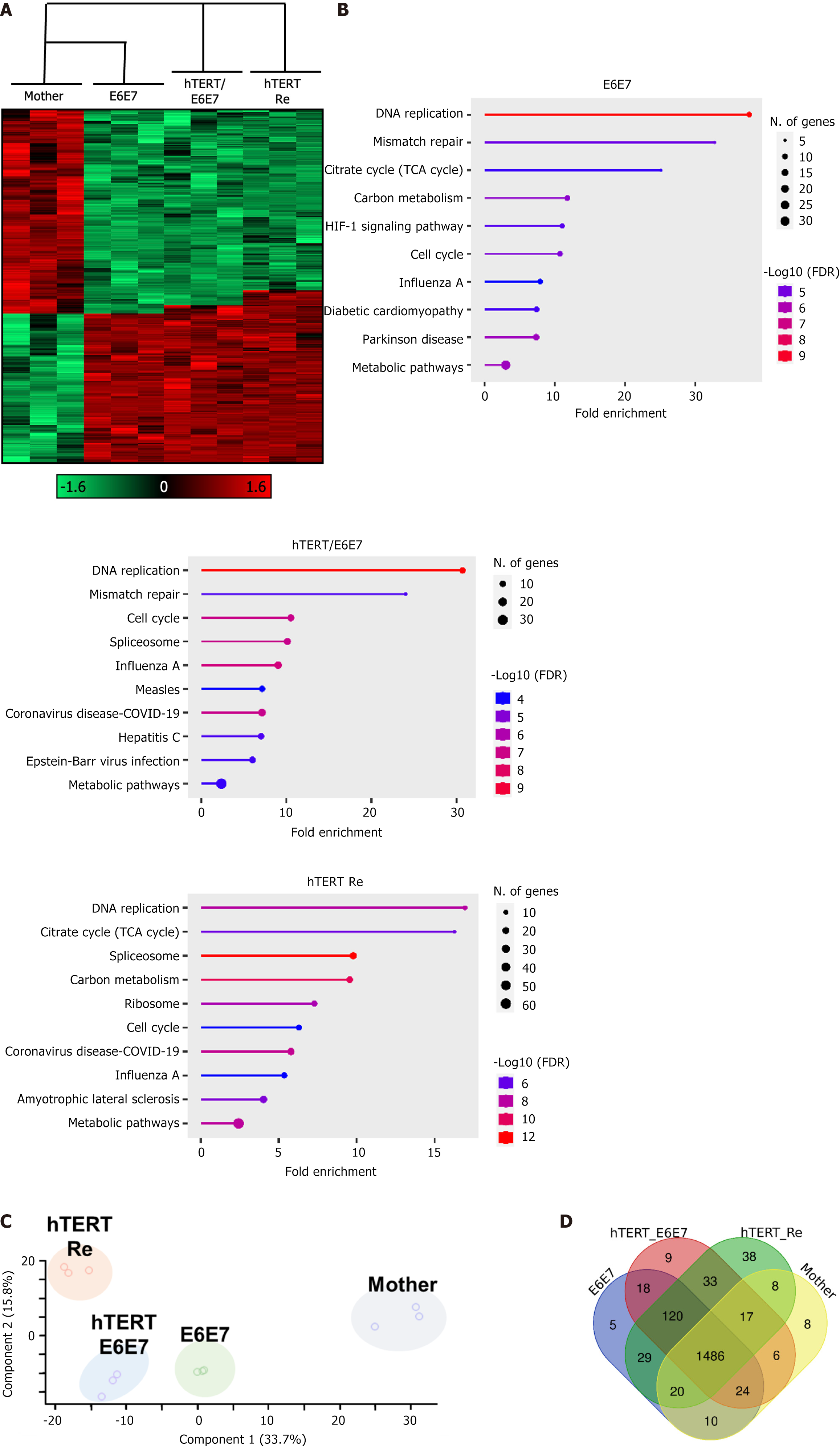
We analyzed the protein expression patterns of each EF-MSCs subtype (mother, E6E7, hTERT/E6E7, and hTERT-retransfected) using proteomics analysis. The heatmap revealed distinct protein expression profiles in the immortalized EF-MSCs (e.g., E6E7, hTERT/E6E7, and hTERT-Re) compared to the mother cell group (Figure 4A). Consistently, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed a significant increase in the expression of genes associated with DNA replication, the cell cycle, and tricarboxylic acid (Figure 4B). Next, we performed principal component analysis to visualize the differences between the mother and immortalized EF-MSCs. Principal component analysis demonstrated that the immortalized EF-MSCs formed a distinct cluster, with increasing separation from the mother group, and the distances between the mother and immortalized cells generally expanded with the number of transfected genes (Figure 4C). Furthermore, Venn diagram analysis revealed that the number of differentially expressed proteins increased in response to the transfection of immortalizing genes (Figure 4D). These findings indicate that immortalized EF-MSCs exhibit significantly higher expression of proteins involved in DNA replication and metabolic pathways, and that gene transfection substantially altered the gene expression patterns compared to the mother cells.
Recently, there has been growing interest in adipose tissue-derived MSCs due to their easy accessibility and multilineage differentiation potential. However, several challenges hinder their clinical application, including cell senescence and limited self-renewal capacity[17]. Moreover, many researchers have suggested that the limited lifespan of SCs is a significant barrier to their practical use[18,19]. As a result, various studies have focused on establishing immortalized cell lines to enhance the clinical applicability of stable cell lines[20]. Immortalized human fetal neural SCs (CTX0E03) are already being used in clinical applications, showing promising therapeutic effects on preclinical and clinical trials for various neurodegenerative syndromes[21-25]. Additionally, immortalized MSCs are promising candidates for regenerative medicine due to their therapeutic potential[26]. Chiu et al[27] demonstrated that immortalized bone marrow MSCs significantly enhance muscle regeneration in mice, while Kim et al[28] demonstrated that immortalized adipose tissue-derived MSCs reduce systemic inflammation and improve cardiac function in patients with infarcted myocardium. These findings highlight that immortalization is a valuable strategy for improving the clinical applicability of SCs. However, to the best of our knowledge, no comprehensive proteomics analysis of signaling pathway in immortalized MSCs has been conducted.
In this study, we successfully isolated EF-MSCs that demonstrated in vitro proliferation and adipogenic potential (Figure 1). However, by passage 15, most EF-MSCs showed signs of senescence, included inhibited proliferation and morphological changes. These findings clearly highlighted the limited expansion capacity of EF-MSCs. To address this limitation, we immortalized EF-MSCs using lentiviral transduction. The resulting immortalized EF-MSCs (e.g., E6/E7 and hTERT/E6E7) not only maintained key SC characteristics, such as stemness marker expression and adipogenic potential, but also exhibited an extended lifespan (Figure 2). Proteomics analysis revealed significant changes in protein expression patterns in immortalized EF-MSCs compared to the parental cells. Notably, KEGG pathway enrichment analysis showed that genes associated with DNA replication and mismatch repair were significantly upregulated in the immortalized EF-MSCs. This upregulation may explain how these immortalized cells preserved both stemness and adipogenic potential despite the gene transduction (Figure 4). Overall, our findings demonstrate that stable, immortalized EF-MSC cell lines retain key SC features. Given the challenges associated with isolating EF-MSCs due to the anatomical location of EF, it is anticipated that immortalized EF-MSC could serve as valuable in vitro models for regeneration therapy research. We hope that this study will contribute to enhancing the clinical application of EF-MSCs.
Although the immortalized cells in this study exhibited enhanced proliferation properties compared to the parental cells, they also exhibited cell senescence-related features, such as limited proliferation, growth arrest, and phenotypic changes between passages 30 and 35. Since cell division is primarily influenced by telomere length and hTERT activity, we hypothesized that supplementing with hTERT transfection could restore the proliferative capacity of aged SCs[29]. To test this, we retransfected passage 30 hTERT/E6E7 EF-MSCs with hTERT-containing lentiviral vectors. As expected, the additional transduction of hTERT significantly promoted cell growth, and the proliferative ability of the re-immortalized EF-MSCs was sustained for more than 100 passages (Figure 3). These results suggest that supplementary hTERT trans
Although the retransfected EF-MSCs exhibited SC surface markers and adipogenic potential, indicating multi-lineage potency, some minor issues remain. KEGG analysis revealed that the transfected EF-MSCs (e.g., E6E7 and hTERT/E6E7) showed a significant increase in the expression of components involved in DNA replication and mismatch repair pathways. In contrast, the hTERT-Re EF-MSCs exhibited an increase in the expression of only the DNA replication pathway components, not those involved in DNA mismatch repair (Figure 4B). As a result, the hTERT-Re EF-MSCs displayed distinct protein expression patterns and exhibited faster cell proliferation compared to the other control groups. These findings raise concerns regarding the use of immortalized EF-MSCs at excessively high passage numbers.
In this study, we successfully established immortalized EF-MSC and analyzed them using proteomics techniques. To the best of our knowledge, this is the first study focused on the immortalization of EF-MSCs. We believe that creating immortalized cell lines and understanding their characteristics are essential steps for the practical application of MSCs. Therefore, the findings from this study provide a valuable reference for both the research and clinical use of EF-MSCs.
| 1. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 2. | Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front Bioeng Biotechnol. 2020;8:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 3. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 4. | Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, Hui Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019;114:108765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 5. | Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1493] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 6. | Nahmgoong H, Jeon YG, Park ES, Choi YH, Han SM, Park J, Ji Y, Sohn JH, Han JS, Kim YY, Hwang I, Lee YK, Huh JY, Choe SS, Oh TJ, Choi SH, Kim JK, Kim JB. Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics. Cell Metab. 2022;34:458-472.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 7. | Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233:901-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Reina MA, Franco CD, López A, Dé Andrés JA, van Zundert A. Clinical implications of epidural fat in the spinal canal. A scanning electron microscopic study. Acta Anaesthesiol Belg. 2009;60:7-17. [PubMed] |
| 9. | Megan Sions J, Angelica Rodriguez C, Todd Pohlig R, Evan Hicks G, Charles Coyle P. Epidural Fat and Its Association with Pain, Physical Function, and Disability Among Older Adults with Low Back Pain and Controls. Pain Med. 2018;19:1944-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lee GW, Mun JU, Ahn MW. The impact of posterior epidural adipose tissue on postoperative outcomes after posterior decompression surgery for lumbar spinal stenosis: A prospectively randomized non-inferiority trial. J Orthop Surg (Hong Kong). 2020;28:2309499019896871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Shah S, Mudigonda S, Mitha AP, Salo P, Krawetz RJ. Epidural fat mesenchymal stem cells: Important microenvironmental regulators in health, disease, and regeneration: Do EF-MSCs play a role in dural homeostasis/maintenance? Bioessays. 2021;43:e2000215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Solmaz B, Şahin A, Keleştemur T, Kiliç E, Kaptanoğlu E. Evidence that osteogenic and neurogenic differentiation capability of epidural adipose tissue-derived stem cells was more pronounced than in subcutaneous cells. Turk J Med Sci. 2020;50:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Wang Y, Ma X, Zhang L, Wang C. Role of epidural fat in the local milieu: what we know and what we don't. Connect Tissue Res. 2024;65:102-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 15. | Takeda Y, Mori T, Imabayashi H, Kiyono T, Gojo S, Miyoshi S, Hida N, Ita M, Segawa K, Ogawa S, Sakamoto M, Nakamura S, Umezawa A. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J Gene Med. 2004;6:833-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Villa A, Navarro-Galve B, Bueno C, Franco S, Blasco MA, Martinez-Serrano A. Long-term molecular and cellular stability of human neural stem cell lines. Exp Cell Res. 2004;294:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Miana VV, González EAP. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018;12:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Binato R, de Souza Fernandez T, Lazzarotto-Silva C, Du Rocher B, Mencalha A, Pizzatti L, Bouzas LF, Abdelhay E. Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif. 2013;46:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Liu J, Ding Y, Liu Z, Liang X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front Cell Dev Biol. 2020;8:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 20. | Voloshin N, Tyurin-Kuzmin P, Karagyaur M, Akopyan Z, Kulebyakin K. Practical Use of Immortalized Cells in Medicine: Current Advances and Future Perspectives. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 21. | Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Puangmalai N, Somani A, Thangnipon W, Ballard C, Broadstock M. A genetically immortalized human stem cell line: a promising new tool for Alzheimer's disease therapy. EXCLI J. 2015;14:1135-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 24. | Muir KW, Bulters D, Willmot M, Sprigg N, Dixit A, Ward N, Tyrrell P, Majid A, Dunn L, Bath P, Howell J, Stroemer P, Pollock K, Sinden J. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91:396-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Yoon Y, Kim HS, Jeon I, Noh JE, Park HJ, Lee S, Park IH, Stevanato L, Hicks C, Corteling R, Barker RA, Sinden JD, Song J. Implantation of the clinical-grade human neural stem cell line, CTX0E03, rescues the behavioral and pathological deficits in the quinolinic acid-lesioned rodent model of Huntington's disease. Stem Cells. 2020;38:936-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 428] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 27. | Chiu CH, Chang TH, Chang SS, Chang GJ, Chen AC, Cheng CY, Chen SC, Fu JF, Wen CJ, Chan YS. Application of Bone Marrow-Derived Mesenchymal Stem Cells for Muscle Healing After Contusion Injury in Mice. Am J Sports Med. 2020;48:1226-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Kim JH, Choi SC, Park CY, Park JH, Choi JH, Joo HJ, Hong SJ, Lim DS. Transplantation of Immortalized CD34+ and CD34- Adipose-Derived Stem Cells Improve Cardiac Function and Mitigate Systemic Pro-Inflammatory Responses. PLoS One. 2016;11:e0147853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Liu JP. Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J. 1999;13:2091-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |