Published online Jan 26, 2025. doi: 10.4252/wjsc.v17.i1.97905
Revised: November 6, 2024
Accepted: January 2, 2025
Published online: January 26, 2025
Processing time: 221 Days and 17.9 Hours
Endometrial injury caused by repeated uterine procedures, infections, inflammation, or uterine artery dysfunction can deplete endometrial stem/progenitor cells and impair regeneration, thereby diminishing endometrial receptivity and evidently lowering the live birth, clinical pregnancy, and embryo implantation rates. Currently, safe and effective clinical treatment methods or gene-targeted therapies are unavailable, especially for severe endometrial injury. Umbilical cord mesenchymal stem cells and their extracellular vesicles are characterized by their simple collection, rapid proliferation, low immunogenicity, and tumorigenicity, along with their involvement in regulating angiogenesis, immune response, cell apoptosis and proliferation, inflammatory response, and fibrosis, Therefore, these cells and vesicles hold broad potential for application in endometrial repair. This article reviewed recent research on human umbilical cord mesenchymal stem cells as well as their extracellular vesicles in repairing endometrial injury.
Core Tip: This study explored the therapeutic potential of human umbilical cord mesenchymal stem cells (hucMSCs) as well as their extracellular vesicles in repairing endometrial injury. We demonstrated that hucMSCs promote endometrial regeneration through various mechanisms, such as cell proliferation, anti-fibrosis, angiogenesis, and immunomodulation. The findings highlighted the advantages of hucMSCs, including their low immunogenicity and ethical acceptability, making them a promising candidate for clinical applications. Our work addressed current challenges in hucMSC therapy, laying the groundwork for future standardized protocols and potentially offering new hope for females with endometrial dysfunction.
- Citation: Zhang WY, Wang HB, Deng CY. Advances in human umbilical cord mesenchymal stem cells-derived extracellular vesicles and biomaterial assemblies for endometrial injury treatment. World J Stem Cells 2025; 17(1): 97905
- URL: https://www.wjgnet.com/1948-0210/full/v17/i1/97905.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i1.97905
The synchronous development of high-quality embryos and normal endometrial receptivity is a crucial factor for successful pregnancies[1]. Endometrial injury severely affects endometrial receptivity, hindering embryo implantation and development, thereby leading to infertility and adverse perinatal outcomes. Currently, a gold-standard treatment is lacking, with most treatments failing to restore the regenerative characteristics of the endometrium.
As an early undifferentiated cell type, stem cells possess self-replication, high proliferation, and multidirectional differentiation capabilities[2] essential for endometrial regeneration[3]. Among them, human umbilical cord mesenchymal stem cells (hucMSCs), sourced from residual blood and discarded umbilical cord tissue after fetal cord ligation, participate in anti-inflammation, angiogenesis, immunomodulation, anti-apoptosis, and anti-fibrosis[4,5]. Compared to other stem cell sources, hucMSCs offer multiple advantages, including simple collection, rapid proliferation, low immunogenicity and tumorigenicity, and few ethical disputes.
Recently, hucMSCs have emerged as a research hotspot in reproductive medicine, especially for endometrial injury repair[6]. HucMSC-derived extracellular vesicles (hucMSC-EVs) are important communication carriers between stem cells[7]. Among the various molecules contained in EVs, microRNAs (miRNAs) are inseparably linked to tissue injury, repair, and regeneration[8] and have received more attention in endometrial repair and regeneration.
Despite numerous basic and clinical studies over the years investigating stem cells from various sources to promote endometrial regeneration and repair, little is known about the communication mechanism and regulatory role between hucMSCs and injured endometrial cells. A summary was conducted in this review on the regulatory mechanisms of endometrial regeneration, the pathophysiological characteristics of endometrial injury, and the existing data on hucMSC and hucMSC-EV treatment of endometrial injury repair, aiming to provide a scientific basis and reference significance for endometrial repair by hucMSCs.
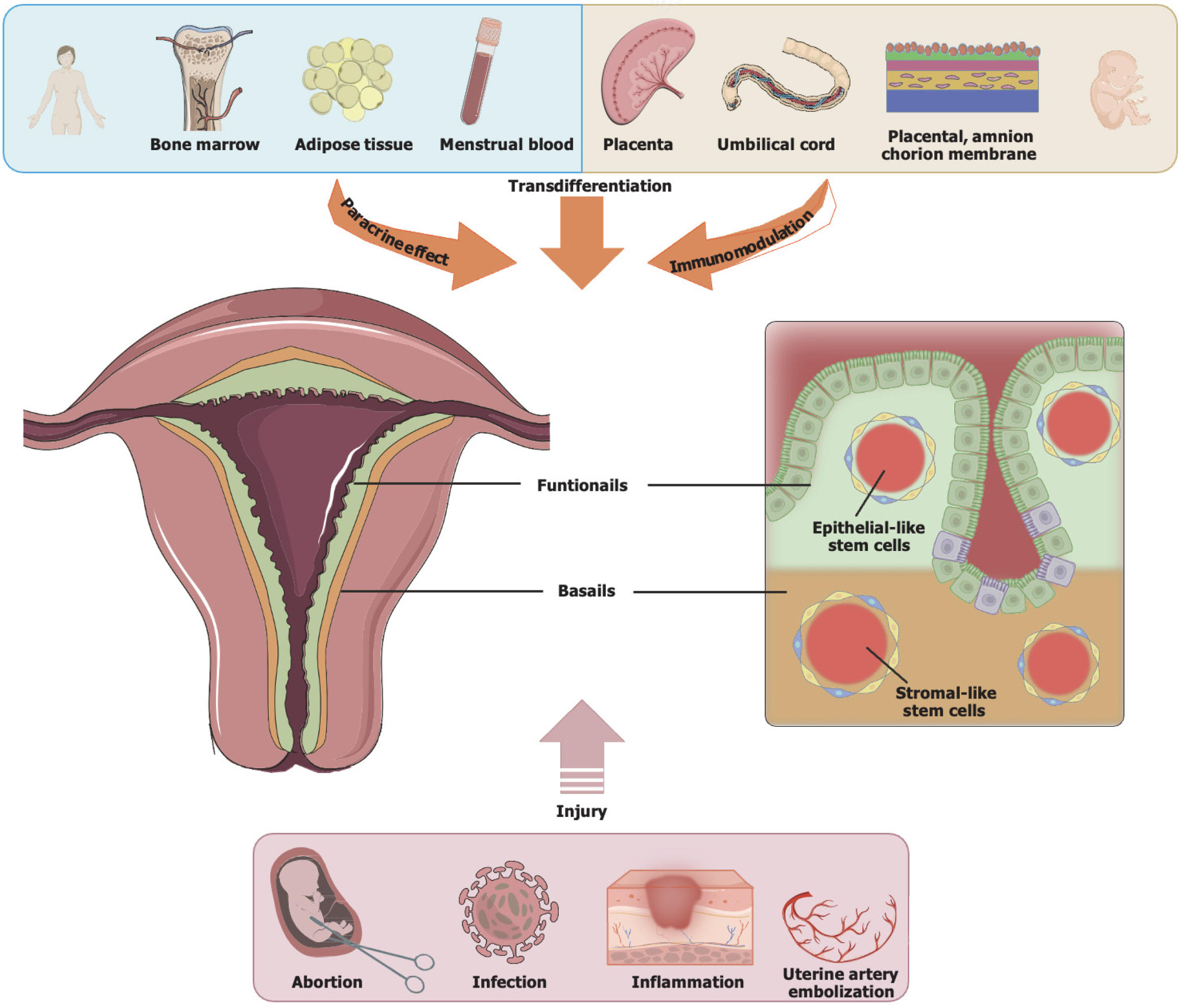
The endometrium undergoes changes regulated by estrogen and progesterone throughout the female reproductive cycle, controlling inflammatory, anti-inflammatory, and epigenetic signaling pathways. Over a female’s lifespan, 400-500 cyclical proliferation, differentiation, shedding, repair, and regeneration processes occur[9]. At the cellular level, the endometrium contains epithelial cells (glandular epithelia and luminal epithelia), stromal cells, immune cells, and blood vessels. At the tissue level, the endometrium comprises two layers: (1) The functional layer, containing epithelial cells, loose stroma, and spiral arteries, is susceptibility to sex hormones. The functional layer has a thickness of roughly 1 mm during the early proliferative phase, reaches 8-16 mm at ovulation, and is shed during the menstrual period. The luminal epithelia in the functional layer contain cilia that aid in clearing cell secretions, assist in sperm transport, and promote embryo implantation, thereby contributing significantly to determining endometrial receptivity[10]; and (2) The basal layer includes adenocarcinoma cells, dense stroma, and short straight arteries, with no sensitivity to sex hormones. Its thickness is approximately 1 mm, exhibiting strong proliferation and repair abilities. During the menstrual period, the basal layer is shed and subsequently forms a new functional layer[11] (Figure 1).
In the menstrual cycle, the endometrium undergoes repeated injury and scarless repair, demonstrating a high degree of dynamic regenerative capacity. Therefore, in 1978, Prianishnikov[12] first proposed the possible existence of endometrial stem/progenitor cells. In 2004, Chan et al[13] were the first to discover, identify, and purify a small number of stem cells with high cloning activity and multilineage differentiation potential in human total hysterectomy specimens. In 2006, the presence of epithelial stem cells and stromal stem cells within rodent endometrium was again traced by Chan and Gargett[14]. They suggested that this result was related to endometrial regeneration. Subsequently, numerous basic and clinical studies have demonstrated that endometrial stem/progenitor cells not only coexist in the endometrial functional and basal layers but also arise from bone marrow-derived stem cells in the circulatory system[15]. Such a regenerative process occurs not only in contexts, such as childbirth, endometrial resection, and menopause hormone therapy, but also in the menstrual cycle[16]. These findings highlighted the presence of endometrial stem/progenitor cells from varying sources and tissues, each contributing to the repair of various types of endometrial injuries, such as thinning, atrophy, and dysfunction.
The injured endometrial basal layer may result in endometrial fibrosis, a decrease in gland number, and invasion by inflammatory factors, thereby leading to excessive consumption of endometrial stem/progenitor cells and/or endometrial regeneration disorder. Among them, the injured endometrial basal layer can be caused by artificial abortion, diagnostic curettage, repeated manipulation of endometrial cavity, tuberculosis infection, and uterine artery embolization (Figure 1). The pathophysiological characteristics of endometrial injury include increased uterine artery blood flow resistance, poor vascular development, slow growth of glandular epithelium, endometrial fibrosis, and a decline in gland numbers, resulting in ischemia and hypoxia of endometrial cells and an obstacle to tissue regeneration[17].
Clinically, endometrial injury is manifested as thin endometrium or intrauterine adhesions (IUA), with its pathogenesis remaining unclear. Additionally, the related hypotheses are composed of neural reflex theory, abnormal regulation of signaling pathways, fibrosis hyperplasia theory, changes in the uterine microenvironment and fibrosis, abnormal differentiation of stem cells, and inflammatory responses caused by adhesive fibroblasts. Confirmed signaling pathways or factors mainly include transforming growth factor-β (TGF-β)/small mothers against decapentaplegic (Smad)[18], Wnt/β catenin[19], nuclear factor-κB signaling pathway[20], estrogen receptor-α, cellular communication network factor 2, matrix metalloproteinase-9[21], and ΔNp63[22].
Thin endometrium markedly declined embryo implantation rate, live birth rate, and clinical pregnancy rate, and its occurrence is linked to repeated implantation failure, recurrent miscarriage, and infertility[23], with an incidence rate of 2.4%-8.5%[24]. However, no consensus has been reached regarding thin endometrium. An endometrial thickness of < 8 mm on trigger day and < 7 mm in the frozen-thaw embryo transfer cycle were defined as thin endometrium, as proposed by the Canadian Fertility and Andrology Society guideline in 2019[25]. In 2019, the European Society of Human Repro
Endometrial repair includes endometrial epithelial regeneration, vascular repair, proteolytic enzyme action, and growth factor stimulation. The repair mechanisms are still unclear. Currently, three hypotheses are proposed: Surviving endometrial epithelial proliferation and differentiation; endometrial stem cell differentiation; and epithelial-mesenchymal transition (EMT). Clinical treatment methods consist of hysteroscopic adhesion resection, use of high-dose estrogen, oral medications to improve endometrial blood supply (such as low-dose aspirin, sildenafil citrate, and pentyl caffeate), intrauterine infusion of cytokines (such as platelet-rich plasma, growth hormone, and granulocyte colony-stimulating factor), and stem cell therapy[27]. However, hysteroscopic adhesion resection is ineffective for severe IUA, with a recurrence rate as high as 62.5% post-surgery[28]. Long-term high-dose estrogen use possibly enhances the risk of thrombosis and abnormal endometrial hyperplasia or carcinogenesis. The clinical efficacy of medications like low-dose aspirin remains uncertain. Intrauterine infusions of cytokines lack unified protocols and safety evaluations, and their efficacy in treating severe injuries is poor. Therefore, there is an urgent need for a safe, effective, and ethically acceptable treatment for endometrial injury. Recent studies have increasingly highlighted the critical role of stem cells, particularly MSCs, in the repair and regeneration of endometrial injury, and its role has garnered growing attention[29,30].
As a cell type that exists in an undifferentiated form, stem cells possess strong self-renewal, differentiation, and proliferation abilities. Based on their source, stem cells can be allocated into adult and embryonic stem cells. Stem cells were first isolated from the mouse blastocyst in 1981[31]. Under physiological conditions, stem cells participate in the growth, development, and homeostasis of tissues, organs, and whole systems. Under pathological conditions, stem cells regulate disease progression and tissue repair. Currently, stem cells have been applied in various fields, such as the production of stem cell-derived biomaterials for human tissues, the treatment of genetic diseases, regenerative medicine, and pharmacogenomics[32]. In 2007, the first report on stem cells targeting and promoting endometrial regeneration was published[33]. Additionally, stem cells have achieved significant outcomes in treating various infertility and reproductive system diseases, such as premature ovarian insufficiency, IUA, endometrial atrophy, pelvic organ prolapse, or endometriosis[34].
MSCs are adult stem cells originating from the embryonic early mesoderm layer, named for their ability to differentiate into interstitial cells. MSCs are isolatable and purifiable from several tissues, such as umbilical cord, bone marrow, neural tissue, fat, placenta, salivary gland, amniotic fluid, menstrual blood, and dental pulp[35,36]. Currently, MSCs used for endometrial regeneration mainly include adipose tissue-derived MSCs, menstrual blood-derived MSCs, bone marrow MSCs, and hucMSCs (Table 1). In 2011, Nagori et al[37] first reported injecting autologous bone marrow stem cells into the uterine cavity of patients with severe IUA, resulting in endometrial growth up to 8 mm and achieving clinical pregnancy through in vitro fertilization and embryo transfer.
| MSC type | Source | Mechanisms in endometrial repair | Key findings in endometrial injury studies | Ref. |
| Umbilical cord-derived MSCs | Endometrium | Proliferation, anti-fibrosis, angiogenesis | Support for endometrial regeneration by differentiating into functional endometrial cells | [85] |
| Bone marrow-derived MSCs | Bone marrow | Anti-inflammation, immunomodulation | Reduction of fibrosis and improvement in endometrial thickness and vascularization in preclinical models | [86] |
| Adipose tissue-derived MSCs | Adipose tissues | Paracrine signaling, anti-apoptosis | Significant potential in mitigating fibrosis and enhancing cellular regeneration | [87] |
| Amniotic MSCs | Amniotic membranes | Immunomodulation, anti-fibrosis | Success in reducing inflammation and supporting tissue repair of the endometrium in experimental settings | [88] |
| Menstrual blood-derived MSCs | Menstrual blood | Angiogenesis, epithelial-mesenchymal transition | Known for high proliferative potential and efficacy in improving endometrial receptivity and vascular repair | [89] |
HucMSCs are an MSC type derived from the residual blood and discarded umbilical cord tissue after umbilical cord ligation. They can be obtained from the entire umbilical cord (which may contain other cells) or separated and cultured from distinct compartments of the umbilical cord, including Wharton’s jelly MSCs, subamnion MSCs, amniotic membrane MSCs, and perivascular MSCs[38,39]. HucMSCs and their blood-derived progeny were previously regarded as medical waste. However, a study revealed that hucMSCs express low or no major histocompatibility complex I and II, along with low tumorigenicity and immunogenicity, positioning them as a crucial source of stem cells for immune rejection-free allogeneic transplantation[40]. Additionally, due to the non-invasive collection method and no ethical issues, hucMSCs are preferred for autologous and allogeneic transplantation compared to other sources of MSCs. They have been extensively studied and applied in tissue engineering and regenerative medicine, including reproductive medicine. However, the mechanisms of hucMSCs repairing injured endometrium are still unclear, and their long-term safety and application are still controversial. The majority of developed therapeutic methods remain in the experimental stage.
As research on MSCs advances, the therapeutic effects of MSCs are likely achieved through a large number of bioactive cytokines produced by their autocrine and paracrine secretion. These cytokines are secreted into the extracellular space, where they may be sorted into extracellular vesicles, including apoptotic bodies and microsomes, which can mediate intercellular communication.
Within this context, EVs have become a research hotspot resulting from their small size, low antigenicity and tumorigenicity, stable and easy-to-store content, non-invasive collection, and ease of large-scale production. EVs possess a lipid double-layer membrane structure, with a density of 1.09-1.18 g/mL and a diameter of 30-200 nm. They carry various nucleic acids, proteins, and lipids (including miRNA, long non-coding RNA, circular RNA, and messenger RNA), making them the main regulatory factors for cell-to-cell signaling. Current research has shown that in various injury repair models, hucMSC-EVs can promote angiogenesis, inhibit fibrosis, and facilitate collagen deposition through their autocrine and paracrine actions, even playing a more crucial role than MSCs themselves. To date, research on EV therapy has been reported, including reducing infarct size of myocardial infarction[41], alleviating liver fibrosis[42], promoting regenerative repair after liver injury[43], treating autoimmune demyelination[44], enhancing regenerative repair after skin injury[45], delaying the graft-vs-host disease onset[46], rescuing the bone marrow MSC function in lupus[47], protecting ischemic myocardium from ischemia/reperfusion injury[48], stimulating chondrocyte migration and proliferation in osteoarthritis[49], inhibiting tumor progression and angiogenesis[50], and promoting the dormancy of breast cancer metastatic lesions[51]. EVs have attracted considerable research interest as an alternative to traditional MSC-based cell therapy.
Increasing research indicates that hucMSCs and hucMSC-EVs can be directly loaded onto biomaterial scaffolds, which are then implanted into the uterine cavity or infused intravenously. These treatments can regulate various signaling pathways and participate in processes such as proliferation, apoptosis, migration, invasion, differentiation, fibrosis, immunomodulation, inflammatory responses, and EMT of stromal cells and endometrial epithelial cells, affecting immune cells and vascular endothelial cells, thereby promoting endometrial injury repair and improving reproductive outcomes (Table 2).
| No. | MSC origin | Vehicle type | Carrier | Stem cell transplant method | Model | Target (gene/pathway) | Biological functions | Reproductive outcome | Ref. |
| 1 | HucMSC | NA | HA/Gel hydrogel | Intrauterine injection | Endometrial mechanical injury rat | MEK/ERK1/2 | Fibrosis; inflammation; migration; proliferation | Endometrial thickness; number of glands; endometrial vessels; embryo implantation rate; live birth rate | [30] |
| 2 | HucMSC | NA | NA | NA | HESC | CCL2, HGF | Migration; invasion | NA | [90] |
| 3 | HucMSC | Exosome | NA | Femoral vein injection | Endometrial 95% ethanol injury rat | MiR-202-3p/MMP11 | ECM remodeling | NA | [65] |
| 4 | HucMSC | NA | GelMA/SerMA | Intrauterine injection | Endometrial 95% ethanol injury mouse | CD31, CK18, Ki67, vimentin, caspase 3 | Angiogenesis; proliferation; apoptosis; fibrosis | Endometrial thickness; embryo implantation rate | [58] |
| 5 | T10-HucMSC | Exosome | NA | Intrauterine injection | HESC treated with TGF-β endometrial mechanical injury rat | MiR-543N-cadherin α-SMA FN1 | Fibrosis; EMT; differentiation; apoptosis; migration | NA | [59] |
| 6 | HucMSC treated with TGF-β1 | Exosome | Cross-linked sodium hyaluronate | Intrauterine injection | Endometrial 95% ethanol injury rat | TGF-β1/Smad2/3 | Angiogenesis; fibrosis; proliferation; migration; collagen remodeling | Endometrial thickness; number of glands; embryo implantation rate | [60] |
| 7 | HucMSC | Exosome | NA | NA | EEC injury induced by hypoxia | MiR-663a/CDKN2A | Proliferation; apoptosis; migration; EMT | NA | [61] |
| 8 | T10-HucMSC | Exosome | NA | Intrauterine injection | Endometrial 95% ethanol injury mouse | HOXA10 | Fibrosis; angiogenesis; apoptosis | Endometrial thickness; number of glands; embryo implantation rate; Endometrial receptivity | [62] |
| 9 | UC-MSCbFGF | NA | Collagen scaffolds | Intrauterine injection | Uterine full-thickness defect rat | NA | Inflammation; angiogenesis; proliferation; regeneration | Endometrial thickness; embryo implantation rate | [91] |
| 10 | HucMSC | NA | PF-127 | Intrauterine injection | Endometrial 95% ethanol injury rat | IL-1β, bFGF, EGF, HGF | Angiogenesis; inflammation; fibrosis | Endometrial thickness; number of glands | [65] |
| 11 | HucMSC | NA | SF-SIS scaffolds | Transplanted in situ | Uterine full-thickness defect mouse | circPTP4A2/miR-330-5p/PDK2 | Fibrosis; mitochondrial metabolism | Number of glands; endometrial fibrosis area | [66] |
| 12 | HucMSC | NA | NA | Tail vein injection versus intrauterine injection | Endometrial 95% ethanol injury rat | IL-1β, TNF-α, bFGF | Angiogenesis; inflammation | Number of glands; endometrial receptivity | [92] |
| 13 | HucMSC | Exosome | NA | Tail vein injection | Mifepristone-induced HESC injury endometrial 95% ethanol injury rat | Integrin alpha, thrombospondin, laminin, collagen, VWF | ECM remodeling; protein degradation and absorption; inflammation; proliferation; apoptosis; migration; adhesion | Endometrial morphology and thickness; number of glands; endometrial fibrosis area; embryo implantation rate | [68] |
| 14 | HucMSC | NA | NA | NA | Endometrial mechanical injury mouse | MiR-455-5p/SOCS3/JAK/STAT455 | Fibrosis; proliferation; cell cycle progression | Number of glands; endometrial fibrosis area | [69] |
| 15 | HucMSC | NA | AAM | Transplanted in situ | Endometrial 95% ethanol injury rat | Keratin, vimentin, integrin β3, VEGF, MMP9 | ECM remodeling; proliferation; migration; inflammation | Endometrial morphology and thickness; number of glands; endometrial receptivity; pregnancy rate; birth weight; number of neonatal rats | [70] |
| 16 | HucMSC | NA | Collagen scaffolds | Hysteroscopic transplantation | 16 females experiencing infertility with thin endometrium (embryo transfers ≤ 5.5 mm) | ERα, PR | Angiogenesis; proliferation; hormone response; differentiation; angiogenesis | Endometrial thickness; uterine receptivity (endometrial volume and subendometrial blood flow); number of glands; miscarriage rate; pregnancy rate; live birth rate; birth defects | [55] |
| 17 | HucMSC | NA | NA | Intraperitoneal injection | Endometrial mechanical injury rat | TGF-β1/Smad3 | Fibrosis; angiogenesis; differentiation; migration | Endometrial thickness; endometrial fibrosis area; number of glands; pregnancy rate; number of fetuses | [93] |
| 18 | HucMSC | Exosome | Collagen scaffolds | Transplanted in situ | Endometrial mechanical injury rat | MiR-223-3p | Immunomodulation (infiltration and polarization of macrophages); collagen remodeling; hormone response; inflammation; endometrial regeneration; fibrosis; angiogenesis | Pregnancy rate | [71] |
| 19 | HucMSC | NA | HA-GEL | Transplanted in situ | Endometrial mechanical injury rhesus monkey | IL-4, IGF-1, EGF, IFN, VEGF | Fibrosis; angiogenesis; inflammation | Endometrial morphology and thickness; number of glands; endometrial receptivity | [84] |
| 20 | HucMSC | NA | Collagen scaffolds | Intrauterine injection | Endometrial mechanical injury rat | ER, CTGF, TGFβ1, FGF2 | Proliferation; hormone response; fibrosis | Number of glands; endometrial fibrosis area | [94] |
| 21 | HucMSC | Exosome | NA | NA | OGD/R-induced injured EEC | TLR4/RelA | Cell viability; cell death; inflammation | NA | [95] |
| 22 | HucMSC | NA | Collagen scaffolds | Transplanted in situ | HESC endometrial mechanical injury rat | TGF-b1, PDGF-BB, VEGF-A, Ki67 | Proliferation; apoptosis; hormone response; regeneration; collagen remodeling; fibrosis; angiogenesis | Endometrial morphology and thickness; number of glands; pregnancy rate | [96] |
| 23 | HucMSC | NA | Collagen scaffolds | Transplanted in situ (spread onto an 18F Foley catheter and placed into the uterine cavity) | 26 females experiencing infertility with recurrent IUA | ERα, vimentin, Ki67, VWF, ΔNP63 | Proliferation; differentiation; angiogenesis | IUA score; endometrial thickness; ongoing pregnancy rate (> 12 weeks active fetus); live birth rate; menstrual volume; miscarriage rate; histological changes in endometrium | [97] |
| 24 | HucMSC | NA | NA | Tail vein injection | Endometrial 95% ethanol injury rat | VEGF-A, MMP9, IFN-γ, TNF-α, IL-2, α-SMA, TGF-β | Fibrosis; proliferation; angiogenesis; inflammation; differentiation; regeneration; fibrosis | Endometrial morphology and thickness; number of glands; endometrial fibrosis area; pregnancy rate | [98] |
| 25 | HucMSC | NA | Collagen scaffolds | Intrauterine injection | Uterine full-thickness defect rat | MMP-9, α-SMA, VWF | Adhesion; migration; differentiation; angiogenesis; differentiation | Endometrial morphology and thickness; number of glands; pregnancy rate; number, size, and weight of fetuses | [56] |
| 26 | HucMSC | Exosome | NA | NA | Mifepristone-induced HESC injury | PTEN/AKT | Proliferation; apoptosis | NA | [57] |
| 27 | HucMSC | Exosome | NA | NA | Mifepristone-induced HESC injury | MiR-7162-3p/APOL6 | Apoptosis; regeneration | NA | [58] |
| 28 | HucMSC | Exosome | NA | NA | HESC treated with TGF-β1 | MiR-145-5p/ZEB2 | Fibrosis | NA | [54] |
| 29 | HucMSC | Exosome | NA | NA | HESC | NA | Proliferation | NA | [99] |
| 30 | WJMSC | NA | NA | Intrauterine injection | 1 female experiencing infertility with IUA; 1 female experiencing infertility with endometrial atrophy | NA | NA | Endometrial thickness; pregnancy rate | [80] |
| 31 | WJMSC | NA | NA | NA | Mifepristone-induced HESC injury | Circ6401miR-29b-1-5p/RAP1B | Angiogenesis; apoptosis | NA | [100] |
| 32 | WJMSC | NA | NA | Intrauterine injection | Endometrial 95% ethanol injury rat | TGF-β1 Rho/ROCK | Angiogenesis; regeneration | Endometrial thickness; number of glands | [101] |
| 33 | hAMSC | NA | NA | NA | Mifepristone-induced HESC injury | VEGF, caspase 3, caspase 8 | Proliferation; apoptosis | NA | [102] |
| 34 | hAMSC | NA | NA | Intrauterine injection | Endometrial 95% ethanol injury rat | Notch pathway | Differentiation; regeneration and repair | NA | [103] |
| 35 | hAMSC | NA | NA | Intrauterine injection | Endometrial mechanical injury rat | TNFα, IL-1β, bFGF, IL-6, VEGF | Angiogenesis; regeneration | Endometrial thickness; number of glands; endometrial fibrosis area | [52] |
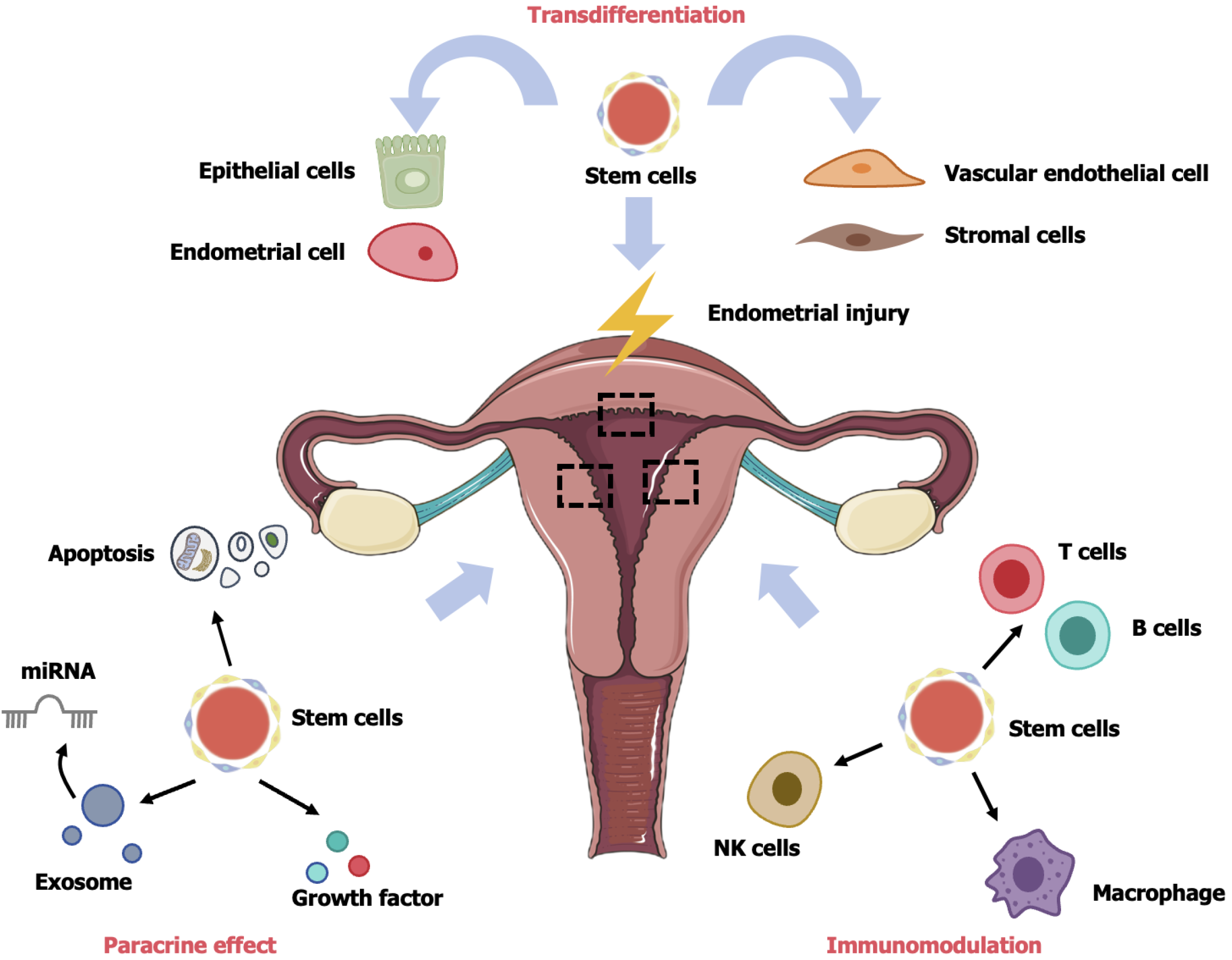
HucMSC therapy operates through three pathways: (1) Transdifferentiation; (2) Paracrine effect; and (3) Immunomodulation. Overall, hucMSCs migrate and localize to the injured site, differentiating into functional cells. Additionally, hucMSCs release growth factors and EVs through paracrine secretion, playing roles not only in cell proliferation, apoptosis, fibrosis, EMT, and angiogenesis but also in regulating the immune system to enhance tissue regeneration and control inflammation (Figure 2).
When the endometrial basal layer is injured (such as during artificial abortion, infection, inflammation, and uterine artery embolization), isolated and purified MSCs from several tissues, such as adipose tissues, umbilical cord, placenta, menstrual blood, and bone marrow, contribute to endometrial repair through transdifferentiating, paracrine, and immunomodulatory actions.
Regulation of cell proliferation and apoptosis: Human endometrium is a highly dynamic and regenerative tissue, and cell apoptosis and proliferation are crucial for endometrial regeneration and repair. A study conducted in 2014 found that treatment of IUA mice with bone marrow MSCs resulted in the detection of only a small amount of exogenous MSCs in the endometrium of the transplanted mice[52]. Furthermore, in rodent animal models, non-hematopoietic stem cell implantation did not lead to the formation of endometrial cell lines[53]. Therefore, it is suggested that, besides directly differentiating into endometrial cells, MSCs may also promote endometrial injury repair by regulating endometrial cell proliferation and apoptosis through paracrine actions.
Lv et al[54] demonstrated that hucMSC-EVs promote endometrial proliferation in a dose-dependent manner. Among 16 females experiencing infertility with thin endometrium, implantation of hucMSC-EVs loaded on collagen scaffolds led to increased endometrial cell proliferation, substantial endometrial thickening, and increased gland numbers[55]. HucMSC-EVs also inhibit the apoptosis of damaged endometrial stromal cells (human endometrial stromal cells) induced by mifepristone through miR-7162-3p/apolipoprotein L6 and phosphatase and tensin homolog/protein kinase B axis, thus promoting endometrial regeneration[56,57].
Anti-fibrosis activity: Normal endometrium undergoes natural repair and regeneration during the menstrual cycle, without scarring or functional loss. However, damaged endometrium cannot be normally repaired, becoming prone to fibrosis, scarring, and adhesions. This can lead to infertility caused by embryo implantation failure. Therefore, antifibrosis is essential for endometrial regeneration and repair. Various factors promoting and degrading organ fibrosis have been found to be inseparably linked to endometrial injury, such as basic fibroblast growth factor, TGF-β, Smad3, a disintegrin and metalloproteinase-15, platelet-derived growth factor, and a disintegrin and metalloproteinase-17. Among them, TGF-β mediates extracellular matrix (ECM) deposition, induces EMT, and facilitates epithelial cell differentiation into myofibroblasts, making it the strongest promoter of fibrosis.
By mediating the miR-145-5p/zinc finger E-box binding homeobox 2 axis, hucMSC-EVs inhibit endometrial fibrosis induced by TGF-β1[58]. Injecting methacrylate sericin and methacrylate gelatin hydrogels encapsulating hucMSCs into the uterine cavity leads to a remarkable decline in fibrotic endometrial area and collagen content, along with an elevation in endometrial thickness and embryo implantation rate in mice[59]. Endometrial fibrosis is prevented by hucMSC-EVs carrying miR-543 through N-cadherin downregulation, both in vivo and in vitro[60]. Additionally, the fibrotic endometrial area in IUA mice is notably decreased by hucMSCs encapsulated in silk fibroin small-intestinal submucosa[61]. The miR-455-5p upregulation in hucMSCs inhibits endometrial fibrosis in mice[62]. These findings indicate that hucMSCs or hucMSC-EVs can modulate endometrial fibrosis through multiple signaling pathways, thus improving endometrial receptivity.
Induction of EMT: EMT refers to a process in which epithelial cell cytoskeleton, under the influence of changes in the surrounding microenvironment (such as inflammation and injury), loses its native shape. Then, the cells become spindle-shaped, with a loss of polarity and dissolution of intercellular junctions. As a result, tightly connected epithelial cells transform into loose, low-differentiated, and highly proliferative stromal cells. EMT occurs in three stages: (1) Transformation of epithelial cells into interstitial direction during embryonic development; (2) Conversion of epithelial cells into fibroblasts during inflammatory responses, promoting the formation of fibrosis; and (3) Loss of polarity and surface adhesion molecules by epithelial cells during the invasion and metastasis of epithelial tumors, resulting in their transformation into the interstitial space. Under normal physiological conditions, the regeneration of uterine stroma and epithelial cells is promoted by the differentiation of endometrial stromal cells and their integration into the endometrial epithelium, highlighting the critical role of EMT in endometrial regeneration.
Emerging studies have suggested that hypoxia-induced damage in endometrial adenocarcinoma cells may involve modulation of key signaling pathways associated with EMT[63]. For example, Yuan et al[60] demonstrated that hucMSC-EVs carrying miR-543 regulate TGF-β1-triggered EMT in IUA mouse models and human endometrial epithelial cells. However, further investigations are required to clarify the exact mechanisms and therapeutic potential.
Reconstruction of ECM: As a highly dynamic structural network, the ECM undergoes continuous remodeling mediated by various matrix degradation enzymes, providing both mechanical and structural support for cells and tissues. It not only regulates signaling space transmission by binding soluble ligands and transmembrane receptors, but also modulates cell proliferation, migration, and differentiation in response to chemical and mechanical signals to maintain tissue function and homeostasis. However, when the endometrium is injured, the fibroblasts in the damaged area are activated through multiple signaling pathways, differentiate into myofibroblasts, and secrete ECM components to restore ECM structure. This repair process is disordered, resulting in the replacement of functional tissue with a stiff and disordered ECM[64], which is strongly correlated with fibrosis.
MiR-202-3p overexpression in hucMSC-EVs can promote endometrial repair by ECM remodeling through regulating target gene expression associated with cell differentiation, migration, and proliferation[65]. Moreover, ECM degradation is the main cause of endometrial injury, and ECM reconstruction occurs during the transplantation and repair process of hucMSCs, as revealed by Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analyses[66]. HucMSCs combined with human acellular amniotic matrix contribute to endometrial repair through ECM remodeling[67].
Promotion of angiogenesis: Endometrial injury results in an increase in uterine artery resistance, the suppression of new vessel formation, and the occurrence of ischemia and hypoxia, leading to loss of nutrition support for the endometrium and impairing endometrial regeneration. Therefore, angiogenesis is essential in endometrial regeneration and repair.
HucMSC-EVs can increase endometrial microvascular density in rats with thin endometrium through the TGF-β1/Smad2/3 signaling pathway, thereby improving pregnancy rates[68]. Overexpression of homeobox A10 in hucMSC-EVs reduces uterine blood flow resistance and vascular endothelial growth factor (VEGF) expression in mice with injured endometrium, promoting endometrial angiogenesis and improving reproductive outcomes[69]. In rats with thin endometrium treated with pluronic F-127s/hucMSCs, the abundance of vascular markers, such as nitric oxide synthase 3 and VEGFA, was raised, along with a marked elevation in the number of von Willebrand factor and VEGFA-positive vessels. Such results indicate that hucMSCs promote the angiogenic factor release, thereby enhancing the regeneration of thin endometrium[70]. In 2018, significant improvement in uterine blood flow was observed in 26 Chinese females experiencing infertility with thin endometrium who received intramuscular injections of hucMSC-EVs[22]. By integrating hucMSC-EVs into collagen scaffolds and implanting them into rats with endometrial injury, miRNA-223-3 significantly promoted angiogenesis and improved pregnancy rates[71].
Participation in immunomodulation: MSC-mediated immunomodulation depends on direct cell-cell interactions or cytokine secretion. Immunoregulation is achieved through interactions with monocytes/macrophages, T cells, dendritic cells, natural killer (NK) cells, and B cells. Notably, anti-inflammatory monocytes/macrophages and regulatory T cells (Treg) play crucial roles in immunomodulation[72]. MSCs and their EVs have been confirmed to participate in immu
The immune mechanism underlying endometrial injury remains unclear and may be related to vaginal microbial ecological imbalance (caused by frequent sexual intercourse, long-term antibiotic use, and decreased estrogen) and damage to the endometrial surface barrier (due to repeated manipulation of endometrial cavity). In 2022, Hua et al[77] found that in the IUA rabbit model, hucMSCs could differentiate into macrophages to participate in immunomodulation, secrete immunoregulatory factors, such as IL-6, IL-8, monocyte chemoattractant protein-1, CC-motif chemokine ligand 5, and toll-like receptor 4, to inhibit inflammation, and regulate the T helper 17/Treg balance through the nuclear factor-κB signaling pathway to suppress the immune response to injured endometrium. However, the specific mechanism involved requires further investigation with larger-scale basic and clinical studies.
Repair of damaged endometrium by miRNA: As a non-coding RNA that contains roughly 22 nucleotides, miRNA modulates gene expression through direct binding to the 3’ untranslated region of its target genes. As demonstrated by Azizi et al[78], miRNA is strongly related to scar repair, participating in regulating angiogenesis, inflammatory responses, collagen deposition, cell apoptosis and proliferation, and the expression and function of ECM components. Currently, more than 1000 miRNAs are encoded in the human genome, among which 56 exhibit significant differences in expression between normal endometrium and IUA tissues[79]. In recent years, EV-derived miRNAs have received considerable attention due to their resistance to RNAse degradation and stability in circulation. As effective carriers, these miRNAs can deliver miRNA to target cells to exert specific regulatory effects.
Multiple miRNAs have been confirmed to be involved in hucMSC-mediated repair of damaged endometrium. HucMSC-EVs have been reported to mediate miR-663a/cyclin dependent kinase inhibitor 2A to regulate the apoptosis, EMT, and proliferation of hypoxia-damaged endometrial adenocarcinoma cells and repair endometrial injury[63]. Wang et al[65] discovered that hucMSC-EVs promoted the repair of damaged endometrium through miR-202-3p upregulating collagen type I alpha 1 chain, collagen type III alpha 1 chain, collagen VI, and fibronectin. MiR-543 overexpression in hucMSC-EVs downregulates N-cadherin, preventing endometrial fibrosis[60]. HucMSCs regulate circular RNA protein tyrosine phosphatase 4A2/miR-330-5p/pyruvate dehydrogenase kinase 2 to improve IUA injury in mice, reducing endometrial fibrosis and fibrosis area and increase the endometrial gland numbers[61]. Additionally, miR-455-5p[62], miR-7162-3p[57], miR-223-3p[71], miR-145-5p[58], and miR-29b-1-5[80] have been reported to be associated with hucMSCs or hucMSC-EVs in promoting endometrial repair.
The transplantation methods for hucMSCs and hucMSC-EVs in treating endometrial injury include intrauterine infusion, intravenous injection, intraperitoneal injection, and stem cell-loaded scaffolds/hydrogels. However, during intrauterine infusion, there is an increased risk of stem cell suspension loss, resulting in reduced retention and survival rates of stem cells. During intravenous and intraperitoneal injections, stem cell migration and homing to the endometrium are limited, lowering their utilization rate. In contrast, scaffolds/hydrogels are capable of providing mechanical and structural support for stem cell survival, migration, and development, extending stem cell preservation time and facilitating endometrial regeneration, such as the promotion of angiogenesis the reduction in inflammatory responses, anti-fibrosis, and immunomodulation[81]. Therefore, some scholars have proposed that stem cells and scaffolds are two core components of endometrial repair, both of which have become hot topics in regenerative medicine[82,83].
The application of collagen scaffolds is the most widespread. Numerous studies have demonstrated that loading hucMSCs and hucMSC-EVs onto collagen scaffolds can significantly improve the fertility outcomes in rats with endometrial injury and in females experiencing infertility with thin endometrium, even achieving live births. In 2023, Zhang et al[30] loaded hucMSCs onto a hydrazide-grafted gelatin and oxidized hyaluronic acid (HA)-based HA/Gel hydrogels, which were then injected into the uterine cavity of rats with endometrial injury. Their findings revealed thickened endometrium, an increase in gland and vessel numbers, along with a marked improvement in live birth and embryo implantation rates.
Chen et al[59] encapsulated hucMSCs in methacrylate gelatin/methacrylate sericin hydrogels and facilitated fertility recovery and endometrial regeneration in mice through in situ injection. Zheng et al[61] constructed a scaffold seeded with hucMSCs and loaded it onto silk fibroin small-intestinal submucosa scaffolds, which repaired damaged endo
While hucMSCs and hucMSC-EVs show considerable promise for endometrial repair, several challenges and uncertainties must be addressed before clinical application. The key issues include: (1) No international unified standard exists for the isolation, culture, identification, preparation, and administration of hucMSCs and hucMSC-EVs, which hinders reproducibility and scalability; (2) The parameters, such as the appropriate number of transplanted cells, timing, frequency, treatment cycles, and administration routes, have not been established, which limits the development of effective protocols; (3) MSC-based treatment may have potential risks, such as alterations in immune function and possible tumorigenicity. Although no cases of MSC-related malignancy have been reported, large-scale, long-term prospective studies are required to confirm their safety; and (4) The exact mechanism by which damaged endometrium signals MSCs to initiate the repair mechanism remains unclear. A deeper understanding of these mechanisms is essential for optimizing MSC therapy. Addressing these gaps will be crucial to optimizing MSC therapy for endometrial injury repair.
In summary, hucMSCs and hucMSC-EVs offer a promising regenerative approach for treating endometrial injury by modulating key processes, such as cell apoptosis, proliferation, fibrosis, EMT, ECM remodeling, angiogenesis, and immunomodulation. These mechanisms collectively enhance endometrial receptivity and improve reproductive out
| 1. | Wu J, Huang J, Dong J, Xiao X, Li M, Wang X. The thicker the endometrium, the better the neonatal outcomes? Hum Reprod Open. 2023;2023:hoad028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | De Luca M, Aiuti A, Cossu G, Parmar M, Pellegrini G, Robey PG. Advances in stem cell research and therapeutic development. Nat Cell Biol. 2019;21:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Cousins FL, Filby CE, Gargett CE. Endometrial Stem/Progenitor Cells-Their Role in Endometrial Repair and Regeneration. Front Reprod Health. 2021;3:811537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Amissah OB, Huangfang X, Wang L, Dieu Habimana J, Lv L, Ding X, Li J, Chen M, Zhu J, Mukama O, Sun Y, Li Z, Huang R. Large-scale expansion of human umbilical cord-derived mesenchymal stem cells using PLGA@PLL scaffold. Bioresour Bioprocess. 2023;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Hu L, Zhou J, He Z, Zhang L, Du F, Nie M, Zhou Y, Hao H, Zhang L, Yu S, Zhang J, Chen Y. In Situ-Formed Fibrin Hydrogel Scaffold Loaded With Human Umbilical Cord Mesenchymal Stem Cells Promotes Skin Wound Healing. Cell Transplant. 2023;32:9636897231156215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Rodríguez-Eguren A, Gómez-Álvarez M, Francés-Herrero E, Romeu M, Ferrero H, Seli E, Cervelló I. Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine. Int J Mol Sci. 2022;23:15942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Mei Q, Mou H, Liu X, Xiang W. Therapeutic Potential of HUMSCs in Female Reproductive Aging. Front Cell Dev Biol. 2021;9:650003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Beavers KR, Nelson CE, Duvall CL. MiRNA inhibition in tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2015;88:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Hong IS. Endometrial stem/progenitor cells: Properties, origins, and functions. Genes Dis. 2023;10:931-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 10. | Bajpai K, Acharya N, Prasad R, Wanjari MB. Endometrial Receptivity During the Preimplantation Period: A Narrative Review. Cureus. 2023;15:e37753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 11. | Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol. 2005;68:85-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 446] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Maruyama T, Masuda H, Ono M, Kajitani T, Yoshimura Y. Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction. 2010;140:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Lv H, Zhao G, Jiang P, Wang H, Wang Z, Yao S, Zhou Z, Wang L, Liu D, Deng W, Dai J, Hu Y. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc Natl Acad Sci U S A. 2022;119:e2115912119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 18. | Zhao X, Sun D, Zhang A, Huang H, Li Y, Xu D. Candida albicans-induced activation of the TGF-β/Smad pathway and upregulation of IL-6 may contribute to intrauterine adhesion. Sci Rep. 2023;13:579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Wei X, Liu F, Zhang S, Xu X, Li J, Wang Q, Cai J, Wang S. Human Umbilical Cord Mesenchymal Stem Cell-Derived Conditioned Medium Promotes Human Endometrial Cell Proliferation through Wnt/β-Catenin Signaling. Biomed Res Int. 2022;2022:8796093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Lv H, Sun H, Wang L, Yao S, Liu D, Zhang X, Pei Z, Zhou J, Wang H, Dai J, Yan G, Ding L, Wang Z, Cao C, Zhao G, Hu Y. Targeting CD301(+) macrophages inhibits endometrial fibrosis and improves pregnancy outcome. EMBO Mol Med. 2023;15:e17601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Li C, Wang W, Sun S, Xu Y, Fang Z, Cong L. Expression and Potential Role of MMP-9 in Intrauterine Adhesion. Mediators Inflamm. 2021;2021:6676510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L, Zhou Q, Wang H, Dai C, Ding L, Xu B, Zhou Y, Hao J, Dai J, Hu Y. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 23. | Zhao D, Xie R, Li X. Comparison of pregnancy outcome after fresh embryo transfer between GnRH antagonist and GnRH agonist regimens in patients with thin endometrium. Front Med (Lausanne). 2023;10:1071014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Liao Z, Liu C, Cai L, Shen L, Sui C, Zhang H, Qian K. The Effect of Endometrial Thickness on Pregnancy, Maternal, and Perinatal Outcomes of Women in Fresh Cycles After IVF/ICSI: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:814648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online. 2019;39:49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G, Le Clef N, Massin N, Mastenbroek S, Polyzos N, Sunkara SK, Timeva T, Töyli M, Urbancsek J, Vermeulen N, Broekmans F. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;2020:hoaa009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 27. | Maged AM, El-Mazny A, Kamal N, Mahmoud SI, Fouad M, El-Nassery N, Kotb A, Ragab WS, Ogila AI, Metwally AA, Fahmy RM, Saad H, Shaeer EK, Salah N, Lasheen Y. The value of platelet-rich plasma in women with previous implantation failure: a systematic review and meta-analysis. J Assist Reprod Genet. 2023;40:969-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Zhu X, Péault B, Yan G, Sun H, Hu Y, Ding L. Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr Stem Cell Res Ther. 2019;14:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Zhang D, Du Q, Li C, Ding C, Chen J, He Y, Duan T, Feng Q, Yu Y, Zhou Q. Functionalized human umbilical cord mesenchymal stem cells and injectable HA/Gel hydrogel synergy in endometrial repair and fertility recovery. Acta Biomater. 2023;167:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 31. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5431] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 32. | Marofi F, Vahedi G, Biglari A, Esmaeilzadeh A, Athari SS. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front Immunol. 2017;8:1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 33. | Wolff EF, Wolff AB, Hongling Du, Taylor HS. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Kowalczyk A, Wrzecińska M, Czerniawska-Piątkowska E, Kupczyński R. Exosomes - Spectacular role in reproduction. Biomed Pharmacother. 2022;148:112752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 36. | Spitzhorn LS, Megges M, Wruck W, Rahman MS, Otte J, Degistirici Ö, Meisel R, Sorg RV, Oreffo ROC, Adjaye J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res Ther. 2019;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Zhang F, Wang XS, Tang B, Li PA, Wen Y, Yu PW. Long non-coding RNA FTX promotes gastric cancer progression by targeting miR-215. Eur Rev Med Pharmacol Sci. 2020;24:3037-3048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 39. | Xue G, Zhang J, Wu L, Sun S, Wu H, Hou Y, Wang J. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes with CYP450 metabolic enzyme activity induced by a liver injury microenvironment. Biochem Biophys Res Commun. 2023;647:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (4)] |
| 41. | Yuan J, Yang H, Liu C, Shao L, Zhang H, Lu K, Wang J, Wang Y, Yu Q, Zhang Y, Yu Y, Shen Z. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv Healthc Mater. 2023;12:e2202959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 42. | Cheng F, Yang F, Wang Y, Zhou J, Qian H, Yan Y. Mesenchymal stem cell-derived exosomal miR-27b-3p alleviates liver fibrosis via downregulating YAP/LOXL2 pathway. J Nanobiotechnology. 2023;21:195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 43. | Jiao Z, Ma Y, Wang Y, Liu T, Zhang Q, Liu X, Piao C, Liu B, Wang H. Protective Effect of Adipose-Derived Mesenchymal Stem Cell Secretome against Hepatocyte Apoptosis Induced by Liver Ischemia-Reperfusion with Partial Hepatectomy Injury. Stem Cells Int. 2021;2021:9969372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Fan J, Han Y, Sun H, Sun S, Wang Y, Guo R, Guo J, Tian X, Wang J, Wang J. Mesenchymal stem cell-derived exosomal microRNA-367-3p alleviates experimental autoimmune encephalomyelitis via inhibition of microglial ferroptosis by targeting EZH2. Biomed Pharmacother. 2023;162:114593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 45. | Tienda-Vázquez MA, Hanel JM, Márquez-Arteaga EM, Salgado-Álvarez AP, Scheckhuber CQ, Alanis-Gómez JR, Espinoza-Silva JI, Ramos-Kuri M, Hernández-Rosas F, Melchor-Martínez EM, Parra-Saldívar R. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells. 2023;12:1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Jaing TH, Chang TY, Chiu CC. Harnessing and honing mesenchymal stem/stromal cells for the amelioration of graft-versus-host disease. World J Stem Cells. 2023;15:221-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Zhao Y, Song W, Yuan Z, Li M, Wang G, Wang L, Liu Y, Diao B. Exosome Derived from Human Umbilical Cord Mesenchymal Cell Exerts Immunomodulatory Effects on B Cells from SLE Patients. J Immunol Res. 2023;2023:3177584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 48. | Cheng XF, He ST, Zhong GQ, Meng JJ, Wang M, Bi Q, Tu RH. Exosomal HSP90 induced by remote ischemic preconditioning alleviates myocardial ischemia/reperfusion injury by inhibiting complement activation and inflammation. BMC Cardiovasc Disord. 2023;23:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 49. | Zhou X, Liang H, Hu X, An J, Ding S, Yu S, Liu C, Li F, Xu Y. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Discov. 2020;6:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int J Mol Sci. 2020;21:5840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 51. | Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 536] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 52. | Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9:e96662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 53. | Ong YR, Cousins FL, Yang X, Mushafi AAAA, Breault DT, Gargett CE, Deane JA. Bone Marrow Stem Cells Do Not Contribute to Endometrial Cell Lineages in Chimeric Mouse Models. Stem Cells. 2018;36:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Lv CX, Duan H, Wang S, Gan L, Xu Q. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Promote Proliferation of Allogeneic Endometrial Stromal Cells. Reprod Sci. 2020;27:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Zhang Y, Shi L, Lin X, Zhou F, Xin L, Xu W, Yu H, Li J, Pan M, Pan Y, Dai Y, Zhang Y, Shen J, Zhao L, Lu M, Zhang S. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 56. | Wang J, Hu R, Xing Q, Feng X, Jiang X, Xu Y, Wei Z. Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Alleviate Mifepristone-Induced Human Endometrial Stromal Cell Injury. Stem Cells Int. 2020;2020:6091269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Shi Q, Wang D, Ding X, Yang X, Zhang Y. Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch Biochem Biophys. 2021;707:108887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Li X, Duan H, Wang S, Lv CX. Umbilical cord mesenchymal stem cell-derived exosomes reverse endometrial fibrosis by the miR-145-5p/ZEB2 axis in intrauterine adhesions. Reprod Biomed Online. 2023;46:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 59. | Chen L, Li L, Mo Q, Zhang X, Chen C, Wu Y, Zeng X, Deng K, Liu N, Zhu P, Liu M, Xiao Y. An injectable gelatin/sericin hydrogel loaded with human umbilical cord mesenchymal stem cells for the treatment of uterine injury. Bioeng Transl Med. 2023;8:e10328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 60. | Yuan D, Guo T, Qian H, Jin C, Ge H, Zhao Y, Zhu D, Lin M, Wang H, Yu H. Exosomal miR-543 derived from umbilical cord mesenchymal stem cells ameliorates endometrial fibrosis in intrauterine adhesion via downregulating N-cadherin. Placenta. 2023;131:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 61. | Zheng Y, Li L, Bi X, Xue R. circPTP4A2-miR-330-5p-PDK2 Signaling Facilitates In Vivo Survival of HuMSCs on SF-SIS Scaffolds and Improves the Repair of Damaged Endometrium. Oxid Med Cell Longev. 2022;2022:2818433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 62. | Sun D, Jiang Z, Chen Y, Shang D, Miao P, Gao J. MiR-455-5p upregulation in umbilical cord mesenchymal stem cells attenuates endometrial injury and promotes repair of damaged endometrium via Janus kinase/signal transducer and activator of transcription 3 signaling. Bioengineered. 2021;12:12891-12904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Salinas-Vera YM, Gallardo-Rincón D, Ruíz-García E, Silva-Cázares MB, de la Peña-Cruz CS, López-Camarillo C. The Role of Hypoxia in Endometrial Cancer. Curr Pharm Biotechnol. 2022;23:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Walraven M, Hinz B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol. 2018;71-72:205-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 65. | Wang S, Liu T, Nan N, Lu C, Liang M, Wang S, Wang H, He B, Chen X, Xu X, Zheng Y. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Facilitates Injured Endometrial Restoring in Early Repair Period through miR-202-3p Mediating Formation of ECM. Stem Cell Rev Rep. 2023;19:1954-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 66. | Zhang L, Li Y, Dong YC, Guan CY, Tian S, Lv XD, Li JH, Su X, Xia HF, Ma X. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep. 2022;12:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Wang S, Shi C, Cai X, Wang Y, Chen X, Han H, Shen H. Human Acellular Amniotic Matrix with Previously Seeded Umbilical Cord Mesenchymal Stem Cells Restores Endometrial Function in a Rat Model of Injury. Mediators Inflamm. 2021;2021:5573594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Zhang S, Wang D, Yang F, Shen Y, Li D, Deng X. Intrauterine Injection of Umbilical Cord Mesenchymal Stem Cell Exosome Gel Significantly Improves the Pregnancy Rate in Thin Endometrium Rats. Cell Transplant. 2022;31:9636897221133345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 69. | Wu M, Li Y, Wang Y, Li Y, Li J, Xie J, Zhao S, Sun L. HOXA10 Expressing UCMSCs Transplantation Improved Endometrial Receptivity on Endometrial Injury. Curr Stem Cell Res Ther. 2023;18:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 70. | Zhou S, Lei Y, Wang P, Chen J, Zeng L, Qu T, Maldonado M, Huang J, Han T, Wen Z, Tian E, Meng X, Zhong Y, Gu J. Human Umbilical Cord Mesenchymal Stem Cells Encapsulated with Pluronic F-127 Enhance the Regeneration and Angiogenesis of Thin Endometrium in Rat via Local IL-1β Stimulation. Stem Cells Int. 2022;2022:7819234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Xin L, Lin X, Zhou F, Li C, Wang X, Yu H, Pan Y, Fei H, Ma L, Zhang S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 72. | Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 481] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 73. | Gholami M, Ghorban K, Sadeghi M, Dadmanesh M, Rouzbahani NH, Dehnavi S. Mesenchymal stem cells and allergic airway inflammation; a therapeutic approach to induce immunoregulatory responses. Int Immunopharmacol. 2023;120:110367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 74. | Lu D, Jiao X, Jiang W, Yang L, Gong Q, Wang X, Wei M, Gong S. Mesenchymal stem cells influence monocyte/macrophage phenotype: Regulatory mode and potential clinical applications. Biomed Pharmacother. 2023;165:115042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 75. | Liu J, Liu Q, Chen X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front Immunol. 2020;11:1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Yan L, Li J, Zhang C. The role of MSCs and CAR-MSCs in cellular immunotherapy. Cell Commun Signal. 2023;21:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 77. | Hua Q, Zhang Y, Li H, Li H, Jin R, Li L, Xiang Y, Tian M, Wang J, Sun L, Wang Y. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium. Stem Cell Res Ther. 2022;13:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 78. | Azizi E, Mofarahe ZS, Naji M. MicroRNAs, small regulatory elements with significant effects on human implantation: a review. J Assist Reprod Genet. 2023;40:697-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Vishnoi A, Rani S. miRNA Biogenesis and Regulation of Diseases: An Updated Overview. Methods Mol Biol. 2023;2595:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 80. | Shi Q, Sun B, Wang D, Zhu Y, Zhao X, Yang X, Zhang Y. Circ6401, a novel circular RNA, is implicated in repair of the damaged endometrium by Wharton's jelly-derived mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B axis. Stem Cell Res Ther. 2020;11:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Li X, Lv HF, Zhao R, Ying MF, Samuriwo AT, Zhao YZ. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater Today Bio. 2021;11:100101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 82. | Liu Y, Jia D, Li L, Wang M. Advances in Nanomedicine and Biomaterials for Endometrial Regeneration: A Comprehensive Review. Int J Nanomedicine. 2024;19:8285-8308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Gargus ES, Rogers HB, McKinnon KE, Edmonds ME, Woodruff TK. Engineered reproductive tissues. Nat Biomed Eng. 2020;4:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 84. | Wang L, Yu C, Chang T, Zhang M, Song S, Xiong C, Su P, Xiang W. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6:eaba6357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 85. | Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li JH, Ma X, Xia HF. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 86. | Yao Y, Chen R, Wang G, Zhang Y, Liu F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther. 2019;10:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 87. | Shao X, Ai G, Wang L, Qin J, Li Y, Jiang H, Zhang T, Zhou L, Gao Z, Cheng J, Cheng Z. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 88. | Mao Y, Yang Y, Sun C, Zou Y, Zhang Y, Wu B, Li C, Huang J, Zhang W, Wang J. Human amniotic mesenchymal stem cells promote endometrium regeneration in a rat model of intrauterine adhesion. Cell Biol Int. 2023;47:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 89. | Zhang S, Zhang R, Yin X, Lu Y, Cheng H, Pan Y, Liu Y, Lin J. MenSCs Transplantation Improve the Viability of Injured Endometrial Cells Through Activating PI3K/Akt Pathway. Reprod Sci. 2023;30:3325-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 90. | Zhao Q, Larios K, Naaldijk Y, Sherman LS, Chemerinski A, Okereke K, Rameshwar P, Lemenze A, Douglas NC, Morelli SS. Mesenchymal stem cell secretome alters gene expression and upregulates motility of human endometrial stromal cells. Reproduction. 2023;166:161-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Wang L, Yao S, Huang F, Lv H, Liu D, Gao T, Wang B, Zhou Z, Cao C, Zhu Q, Weng Q, Zhao G, Hu Y. The UCMSC-bFGF/Scaffold System Accelerates the Healing of the Uterine Full-Thickness Injury. Tissue Eng Part A. 2023;29:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 92. | Zhuang M, Zhang W, Cheng N, Zhou L, Liu D, Yan H, Fang G, Heng BC, Sun Y, Tong G. Human umbilical cord mesenchymal stromal cells promote the regeneration of severe endometrial damage in a rat model. Acta Biochim Biophys Sin (Shanghai). 2022;54:148-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Zheng JH, Zhang JK, Kong DS, Song YB, Zhao SD, Qi WB, Li YN, Zhang ML, Huang XH. Quantification of the CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated to the dual injured uterus in SD rat. Stem Cell Res Ther. 2020;11:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 94. | Liu Y, Cai J, Luo X, Wen H, Luo Y. Collagen Scaffold with Human Umbilical Cord Mesenchymal Stem Cells Remarkably Improves Intrauterine Adhesions in a Rat Model. Gynecol Obstet Invest. 2020;85:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Liang L, Wang L, Zhou S, Li J, Meng L, Zhang H, Cui C, Zhang C. Exosomes derived from human umbilical cord mesenchymal stem cells repair injured endometrial epithelial cells. J Assist Reprod Genet. 2020;37:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, Ma L, Gao C, Zhang S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 97. | Zhang L, Zhang Y, Yu X, Xu H, Sui D, Zhao X. Alprostadil attenuates myocardial ischemia/reperfusion injury by promoting antioxidant activity and eNOS activation in rats. Acta Cir Bras. 2018;33:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Xu L, Ding L, Wang L, Cao Y, Zhu H, Lu J, Li X, Song T, Hu Y, Dai J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther. 2017;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 99. | Kaczynski JB, Rzepka JK. Endometrial regeneration in Asherman's syndrome and endometrial atrophy using Wharton's jelly-derived mesenchymal stem cells. Ginekol Pol. 2022;93:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 100. | Li J, Huang B, Dong L, Zhong Y, Huang Z. WJMSCs intervention may relieve intrauterine adhesions in female rats via TGFβ1mediated Rho/ROCK signaling inhibition. Mol Med Rep. 2021;23:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Yang X, Zhang M, Zhang Y, Li W, Yang B. Mesenchymal stem cells derived from Wharton jelly of the human umbilical cord ameliorate damage to human endometrial stromal cells. Fertil Steril. 2011;96:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Yu J, Zhang W, Huang J, Gou Y, Sun C, Zhang Y, Mao Y, Wu B, Li C, Liu N, Wang T, Huang J, Wang J. Management of intrauterine adhesions using human amniotic mesenchymal stromal cells to promote endometrial regeneration and repair through Notch signalling. J Cell Mol Med. 2021;25:11002-11015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Gan L, Duan H, Xu Q, Tang YQ, Li JJ, Sun FQ, Wang S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |