Published online Jan 26, 2025. doi: 10.4252/wjsc.v17.i1.101485
Revised: November 11, 2024
Accepted: January 8, 2025
Published online: January 26, 2025
Processing time: 125 Days and 22 Hours
Human mesenchymal stromal cells (MSCs) possess regenerative potential due to pluripotency and paracrine functions. However, their stemness and immunomodulatory capabilities are sub-optimal in conventional two-dimensional (2D) culture.
To enhance the efficiency and therapeutic efficacy of MSCs, an in vivo-like 3D culture condition was applied.
MSCs were cultured on polystyrene (2D) or in a gellan gum-based 3D system. In vitro, prostaglandin-endoperoxide synthase 2, indoleamine-2,3-dioxygenase, heme oxygenase 1, and prostaglandin E synthase gene expression was quantified by quantitative real-time polymerase chain reaction. MSCs were incubated with lipopolysaccharide (LPS)-treated mouse splenocytes, and prostaglandin E2 and tumor necrosis factor-alpha levels were measured by enzyme linked immuno
Gellan gum polymer-based 3D culture significantly increased expression of octamer-binding transcription factor 4 and Nanog homeobox stemness markers in human MSCs compared to 2D culture. This 3D environment also heightened expression of cyclooxygenase-2 and heme-oxygenase 1, enzymes known for immunomodulatory functions, including production of prostaglandins and heme degradation, respectively. MSCs in 3D culture secreted more prostaglandin E2 and effectively suppressed tumor necrosis factor-alpha release from LPS-stimulated splenocytes and surpassed the efficiency of MSCs cultured in 2D. In a murine neuroinflammation model, intravenous injection of 3D-cultured MSCs significantly reduced ionized calcium-binding adaptor molecule 1 and glial fibrillary acidic protein expression, mitigating chronic inflammation more effectively than 2D-cultured MSCs.
The microenvironment established in 3D culture serves as an in vivo mimetic, enhancing the immunomodulatory function of MSCs. This suggests that engineered MSCs hold significant promise a potent tool for cell therapy.
Core Tip: Clinical trials utilizing mesenchymal stromal cells (MSCs) have been on the rise, but several barriers to broader adoption of MSCs as therapeutics remain to be addressed. Our study presents compelling evidence demonstrating the remarkable enhancement of immunomodulatory capabilities in functional polymer-based MSCs through three-dimensional culture. We specifically applied these optimized MSCs to a brain inflammation model and found a prolonged and more potent anti-inflammatory effect in vivo compared to conventionally cultured MSCs. The polymer-based three-dimensional cultures we propose could be an easy tool for mass culture for clinical applications.
- Citation: Kim OH, Kang H, Chang ES, Lim Y, Seo YJ, Lee HJ. Extended protective effects of three dimensional cultured human mesenchymal stromal cells in a neuroinflammation model. World J Stem Cells 2025; 17(1): 101485
- URL: https://www.wjgnet.com/1948-0210/full/v17/i1/101485.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i1.101485
Mesenchymal stromal cells (MSCs) are widely utilized in cell-based therapies due to their multipotency, anti-inflammatory properties, immunomodulatory capabilities, and regenerative potential, mediated through paracrine signaling and cell-to-cell interactions[1-3]. MSCs have been extensively investigated as therapeutic candidates for various conditions including bone and cartilage diseases, immune-related disorders, and neurological disorders, with clinical applications approved by the Food and Drug Administration for treating acute graft-versus-host disease[1,4]. However, clinical trial outcomes have been suboptimal in terms of MSC maintenance, therapeutic efficacy, and persistence after infusion[5]. Studies suggest that manipulation of the biochemical and biophysical microenvironment such that it more closely resembles the stem cell niche can influence the fate of MSCs and enhance their therapeutic potential[2,6,7].
As MSCs naturally reside within a three-dimensional (3D) stem cell niche in vivo, there is emerging evidence that 3D culture platforms inspired by nature can bolster the immunomodulatory function of MSCs. Commonly utilized 3D culture platforms for MSCs include MSC spheroids and biomaterial-based scaffolds. For example, MSCs in spheroid form have shown a potentiated suppressive effect on tumor necrosis factor-alpha (TNF-α) secretion by stimulated macrophages compared to 2D culture on tissue culture plates, both when stimulated with lipopolysaccharide (LPS) or LPS in combination with interferon-gamma[8,9]. However, the generation of MSC spheroids is often labor-intensive (e.g., hanging drop method) or leads to high variation in size and morphology (e.g., low-attachment surface)[10]. Additionally, the use of biomaterials to create stem cell niche-mimicking architectures has shown promise. For instance, MSCs pre-conditioned with interferon-gamma exhibited anti-inflammatory properties when cultured on silk fibroin nanofiber scaffolds and improved survival in a sepsis murine model[11,12]. Similarly, chitosan membrane enhanced the expression of leukaemia inhibitory factor, interleukin (IL)24, TP53, transforming growth factor-beta 3, platelet-derived growth receptor alpha, and prostaglandin-endoperoxide synthase 2 (PTGS2) in MSCs, likely attributable to increased cell-substrate interaction on chitosan films and upregulation of calcium-associated genes[13]. However, these methods lack standardization and may not be suitable for large-scale cultivation.
Recently, a novel 3D suspension culture system using the polysaccharide polymer derived from gellan gum, was developed. This system allows human embryonic stem cells, induced pluripotent stem cells, and hepatocytes derived from these cells to float in the culture medium without cytotoxicity[14]. Here, we investigated the effects of 3D culture using gellan gum derived polymer on MSC characteristics. The cross-linked structure formed in the medium enabled even dispersion and suspension of cells, transforming the spindle-shaped MSCs into round-shaped cells resembling their in vivo counterparts in 3D floating culture compared to 2D plastic culture. Furthermore, expression of the PTGS2, indoleamine-2,3-dioxygenase (IDO), and heme oxygenase 1 genes and their protein products, which are involved in immunomodulation, were increased in MSCs cultured in 3D. Prostaglandin E2 (PGE2) secretion was also elevated in 3D culture, which in turn effectively suppressed TNF-α, a pro-inflammatory cytokine. Overall, this nature-inspired culture condition enhances effectively promotes the immunomodulatory function of MSCs, which holds promising potential for cell therapy applications.
Human bone marrow MSCs were derived from whole bone marrow from independent human donors (commercially available by AllCells, Alameda, CA, United States). For 2D culture, cells were cultured in Modified Eagle Medium-a (MEM-a, Thermo Fisher Scientific, Waltham, MA, United States) supplemented with 20% fetal bovine serum (GW Vitek, Geumcheon-gu, Seoul, Korea), and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, United States) in a humidified atmosphere of 5% CO2 at 37°C. For 3D culture of MSCs, the bottom of the culture well was coated with 4% pluronic F-127 (Sigma-Aldrich, St. Louis, MO, United States) for at least 16 hours to prevent adherence of the cells to the well plate. 3D medium was prepared by supplementing MEM-a with 0.05% (v/v) FCeM Advance preparation kit (Nissan Chemical Corporation, Tokyo, Japan) according to the manufacturer’s instruction. Cells were seeded at a density of 50000 cells/well using 2D or 3D medium in 24-well plates and then cultured for 24 hours.
For intracellular staining, cells were treated with FOXP3 Fix/Perm solution (FOXP3 Fix/Perm Buffer Set, BioLegend, San Diego, CA, United States) for 30 minutes at room temperature (RT). Then the cell suspensions were stained with anti-octamer-binding transcription factor 4 (Oct4) (1:100, 653703, BioLegend, San Diego, CA, United States), and anti-Nanog homeobox (Nanog) (1:100, 674001, BioLegend, San Diego, CA, United States) antibodies for 1 hour at RT, followed by staining with secondary antibody (Alexa fluor 647, A28181, Thermo Fisher Scientific, Waltham, MA, United States) for 30 minutes at RT. Cells were resuspended in fluorescence-activated cell sorting buffer (1% bovine serum albumin, 10% sodium azide, and 2 mmol/L ethylenediaminetetraacetic acid) and analyzed on an Attune NxT Acoustic Focusing Cytometer (Invitrogen, Carlsbad, CA, United States).
Cells were harvested in RIPA buffer (Thermo Fisher Scientific, Waltham, MA, United States) containing 1% protease inhibitor cocktail (GenDEPOT, Katy, TX, United States) and 1% phosphatase inhibitor cocktail (GenDEPOT, Katy, TX, United States). Equal amounts of protein were separated by 10% sodium-dodecyl sulfate gel electrophoresis and then transferred to a nitrocellulose membrane. The membranes were blocked with 5% fat-free milk and incubated with primary antibodies overnight at 4°C. Antibodies used in immunoblotting were as follows; anti-cyclooxygenase-2 (COX-2, 1:1000, ab102005, Abcam, Cambridge, United Kingdom), anti-heme-oxygenase1 (HO1, 1:1000, ab13243, Abcam), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000, sc-365062, Santa Cruz Biotechnology). The membranes were washed and then probed with the appropriate horseradish peroxide-conjugated secondary antibodies at RT. The immuno-reactive proteins were detected using an enhanced chemiluminescence detection system (Thermo Fisher Scientific, Waltham, MA, United States). The intensities of protein bands were quantified using Image J. Quantification of relative protein levels was performed by comparing each protein to GAPDH.
Total RNA was extracted from 2D- and 3D-cultured MSCs using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction and quantified with a UV/VIS Nano spectrophotometer (Nabi, MicroDigital Co.,Ltd., Bundang-gu, Seongnam-si, Gyeonggi-do, Korea). Reverse transcription of RNA was performed using a Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, United States). Quantitative real-time polymerase chain reaction (PCR) was performed using the Power SYBR Green PCR Master Mix reagent (Applied Biosystems, Foster city, CA, United States) and QuantStudio3 Real-Time PCR (Applied Biosystems, Foster city, CA, United States). The relative quantification of target genes was normalized to that of GAPDH, and the 2-∆∆Ct method was used to calculate gene expression. The primer sequences used here are listed in Supplementary material.
In 3D cell culture conditions, cell morphology and dispersibility were evaluated using a fluorescent live/dead staining protocol. Live cells and dead cells were stained using a LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR, United States) following the manufacturer’s instructions. Briefly, cells were washed in Dulbecco’s phosphate buffered saline 3 times. Live cells (green) and dead cells (red) were stained with 2 mmol/L calcein AM and 4 mmol/L EthD-1 at 37°C for 30 minutes. Z-stack images of these cells were then acquired using a confocal laser-scanning microscope (LSM700, Carl Zeiss, Jena, Germany). Bright-field images were examined with a phase contrast microscope (IX-81, Olympus, Tokyo, Japan).
Murine splenocytes were isolated from 5-week old ICR μice for co-culture with MSCs. Briefly, the spleen was mechanically dissociated through a 70-μm strainer in order to exclude all connective tissue, lysed in Red Blood Cell Lysing Buffer Hybri-Max (Sigma-Aldrich, St. Louis, MO, United States), and resuspended in medium (MEM-a, 20% fetal bovine serum, 1% P/S) after washing with phosphate buffered saline (PBS) for culture with MSCs. Splenocyte-MSC co-cultures were plated at a ratio of 30 to 1. LPS (1 mg/mL, Sigma Aldrich, St. Louis, MO, United States) was added 30 minutes after plating. Following 4 hours incubation at 37 °C, the supernatant was collected and TNFα was quantified using the CymaxTM Mouse TNFα enzyme linked immunosorbent assay (ELISA) kit (Ab Frontiers, Geumcheon-gu, Seoul, Korea). Similarly, IL-10 was quantified using the Mouse IL-10 Quantikine ELISA kit (R&D systems, Minneapolis, MN, United States) following the manufacturer’s instructions.
Human bone marrow-derived MSCs were seeded in 2D and 3D culture systems. After 24 hours of incubation at 37°C, the supernatant was collected and PGE2 was quantified using a PGE2 ELISA kit (R&D Systems Inc., Minneapolis, MN, United States). To block PGE2 production, the cells were treated with 10 mmol/L indomethacin (Sigma-Aldrich, St. Louis, MO, United States), a COX2 inhibitor, and cultured for 24 hours. The concentrations of PGE2 were measured by ELISA according to the instructions provided by the manufacturer.
Blood was collected for analysis via a cardiac puncture at the time of sacrifice. The samples were centrifuged (3000 rpm, 15 minutes, 4°C), and the serum was stored at -80°C. The concentrations of IL-6 (Ab Frontiers) and IL-10 (R&D Systems Inc., Minneapolis, MN, United States) were measured using ELISA kits according to the instructions provided by the manufacturer.
The animal protocol was designed to minimize pain or discomfort to the animals. Male ICR mice (20-25 g) were purchased from DBL (Eumseong-gun, Chungcheongbuk-do, Korea), caged at a density of 5 mice per cage, and maintained on a normal laboratory diet and tap water ad libitum in an air-conditioned room (21 ± 2°C) with a 12 hours light cycle. All experimental procedures were approved by the Institutional Animal Care Use Committee of Chung-Ang University (Approval ID: A2021023). For the neuroinflammation model, mice were anesthetized with 0.2 mg/kg avertin (Sigma-Aldrich, St. Louis, MO, United States) and then placed in a stereotaxic frame (RWD Life Science, CA, United States). Vehicle (sterile saline) or LPS (Escherichia coli serotype 055:B5, Sigma Aldrich, St. Louis, MO, United States; 5 mg in 2 mL of sterile saline) was administered into the cerebral lateral ventricle (-0.22 mm anterior/posterior, -1.1 mm medial/lateral, and -3.3 mm dorsal/ventral from bregma) using a 10 mL Hamilton syringe (Hamilton) at a rate 0.5 mL/minute. The needle was left in place for an additional 5 minutes after injection and then retracted slowly from the brain. Twenty-four hours after the LPS injection procedure, MSCs were isolated from the 2D or 3D culture, and mice received a tail vein injection of either a mixture of one million MSCs in 0.2 mL PBS, or 0.2 mL PBS alone. The mice were divided into four groups: (1) Sham control group (n = 4); (2) LPS + vehicle group (n = 8); (3) LPS + 2D MSC group (n = 8); and (4) LPS + 3D MSC group (n = 8).
At either 1 or 5 days post-MSC injection, animals were deeply anesthetized and transcardially perfused with 10% neutral buffered formalin (Biosesang, Seongnam-si, Gyeonggi-do, Korea). Harvested brains were stored in 10% neutral buffered formalin for 24 hours and then sequentially transferred to and sunk in 15%, 20%, 25%, and 30% sucrose solutions for cryoprotection. After overnight storage in 30% sucrose, the brains were removed from the solution and stored at -80°C until needed. Brains were sectioned at 20-μm and then processed for immunohistochemistry. Sections were incubated with 0.3% H2O2 for 30 minutes, placed in a blocking buffer for 1 hour at 37°C, and then incubated with primary antibodies against glial fibrillary acidic protein (Rabbit aGFAP, 1:200, Z0334, Agilent Technologies, Santa Clara, CA, United States) and ionized calcium binding adapter molecular 1 (Rabbit aIba1, 1:200, GTX100042, GeneTex, Irvine, CA, United States) overnight at RT. After washing, the sections were incubated with appropriate biotinylated secondary antibodies (1:200, Vector Laboratories Inc., San Francisco, CA, United States), followed by avidin-biotin-peroxide (1:200, Vector Labo
All data were analyzed with the GraphPad Prism 9 software (GraphPad, Inc., La Jolla, CA, United States) for statistical significance and are expressed as the mean ± SEM. Differences in gene expression were analyzed using the unpaired t-test. One-way analysis of variance (ANOVA) and the Holm-Sidak method for multiple comparisons were used evaluate differences in histological measurements. P values of < 0.05, < 0.01, < 0.001, and < 0.0001 were considered to be signi
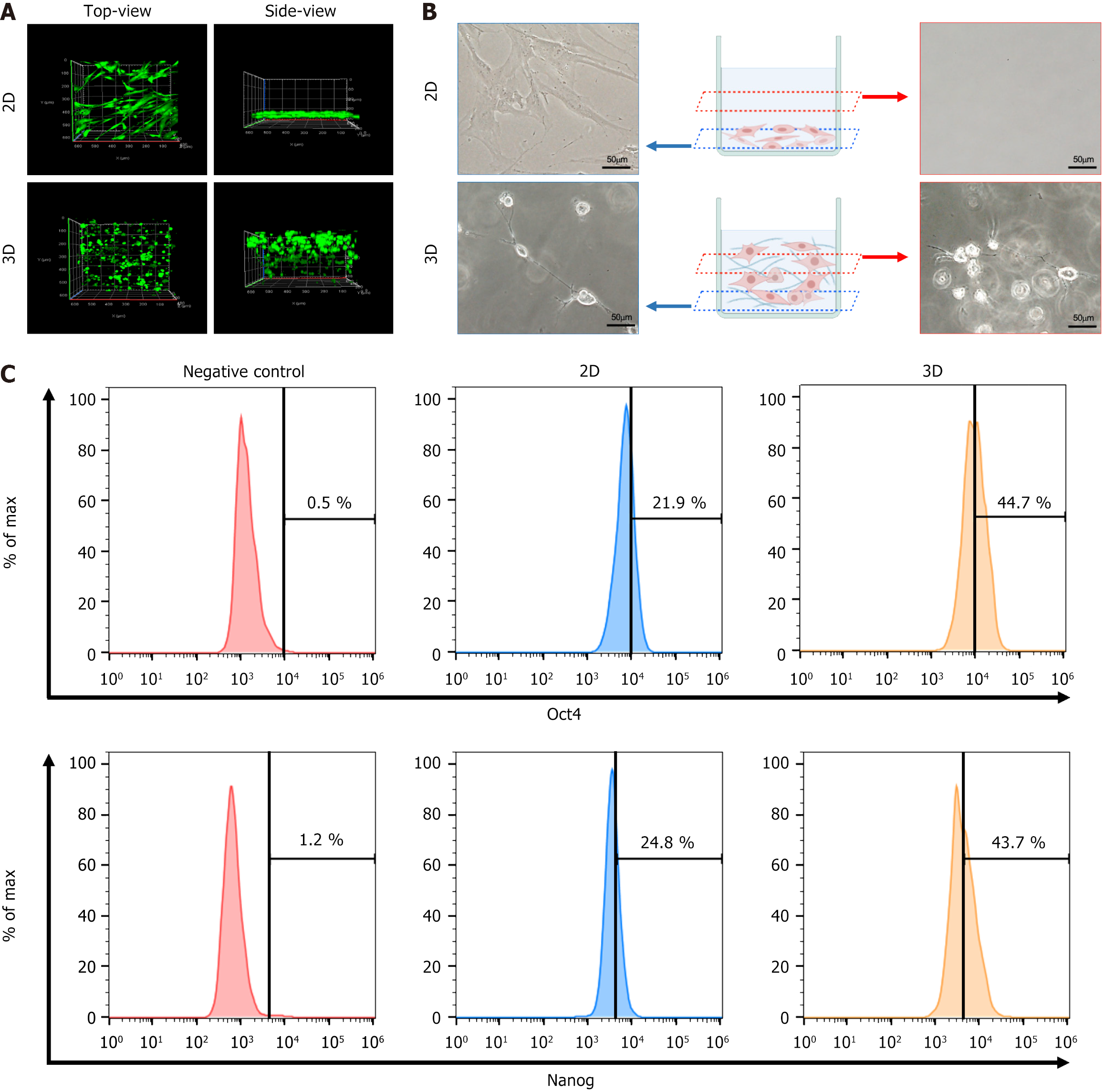
In order to investigate the characteristics of MSCs cultured in a 3D setting, the distribution of 3D-cultured MSCs in a medium containing the polymer (FP003) was compared to 2D-cultured MSCs in a medium without the polymer. A total of 50000 cells were seeded on adherent or non-adherent culture plates, and after 24 hours, the live cells were stained with calcein AM. Confocal imaging analysis revealed distinct morphological differences between the two groups. From a top-down view, 2D cells appeared elongated, while 3D cells were round. When observed from the side, 2D cells were primarily located at the bottom of the culture plates, whereas 3D cells were distributed throughout the medium from top to bottom (Figure 1A). Further, under brightfield imaging conditions (Figure 1B), 2D-cultured cells displayed an elongated shape, while 3D-cultured cells exhibited a rounded morphology with long branches and interconnected neighboring cells. It is noteworthy that the 3D culture system provided significantly different and unique microenvironments for MSCs compared to the traditional 2D cell monolayers.
To confirm the potential priming effects of 3D culture on MSCs, the expression levels of stemness markers, such as Oct4 and Nanog, were analyzed. Fluorescence-activated cell sorting revealed a significant upregulation of Oct4 expression in 3D-cultured cells compared to 2D-cultured cells (21.9% in 2D MSCs vs 44.7% in 3D MSCs). Similarly, Nanog positive cells made up 24.8% of 2D MSCs and 43.7% of 3D MSCs (Figure 1C). These findings indicate that MSCs cultured in a 3D environment exhibited enhanced stemness.
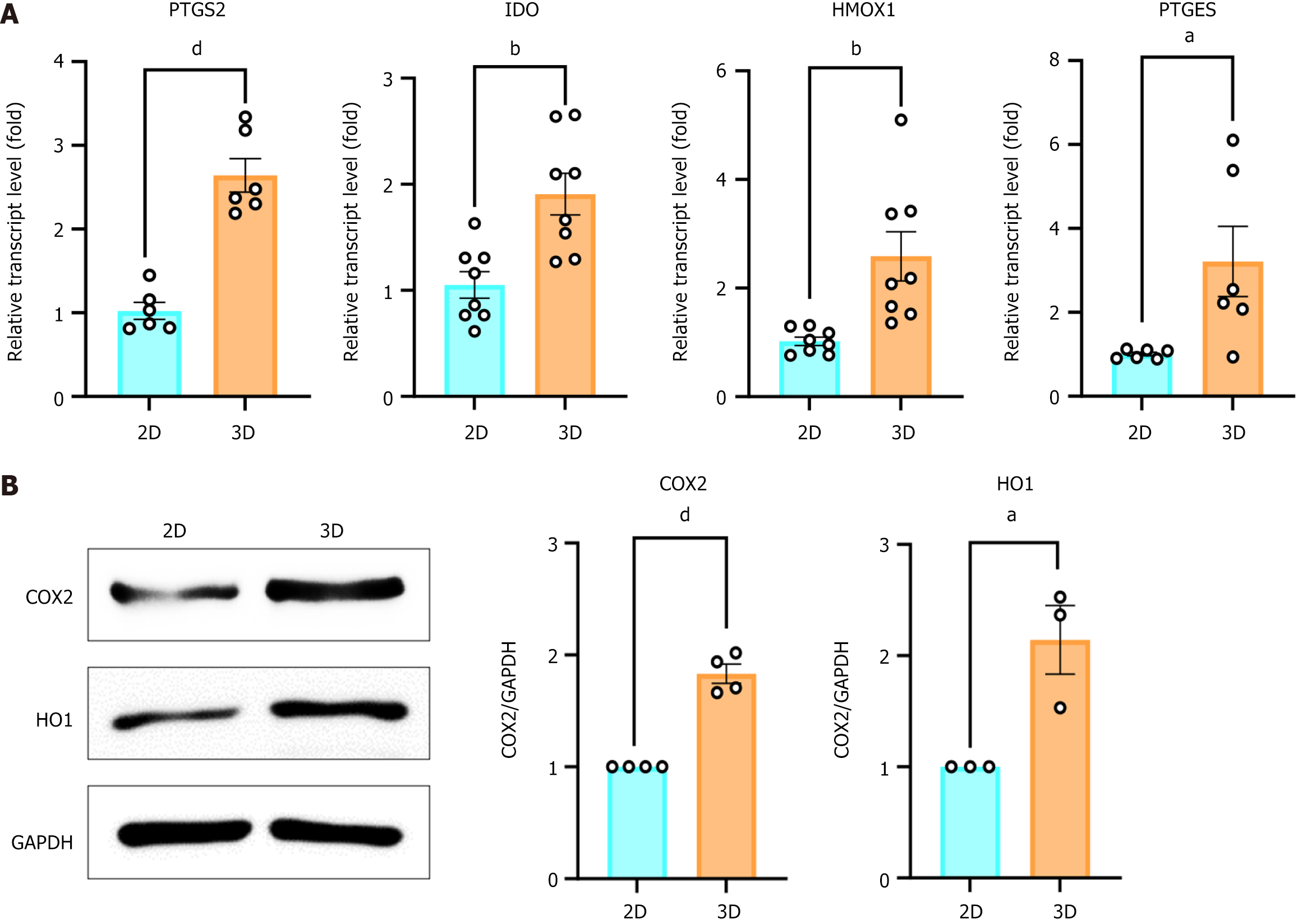
MSCs possess anti-inflammatory and immunomodulatory effects mediated by the secretion of paracrine factors. To investigate whether the 3D floating conditions stimulate the expression of immunomodulatory cytokines in MSCs, the mRNA expression levels of PTGS2, IDO, heme oxygenase 1, and PGE synthase were analyzed. After 24 hours, the mRNA expression levels of these cytokines were significantly increased in the 3D floating conditions (Figure 2A). Furthermore, the protein expression levels of COX2 and heme-oxygenase 1 were also significantly elevated in the 3D floating conditions (Figure 2B). These results indicate that MSCs cultured in a 3D environment exhibit an increased release of immunomodulatory cytokines.
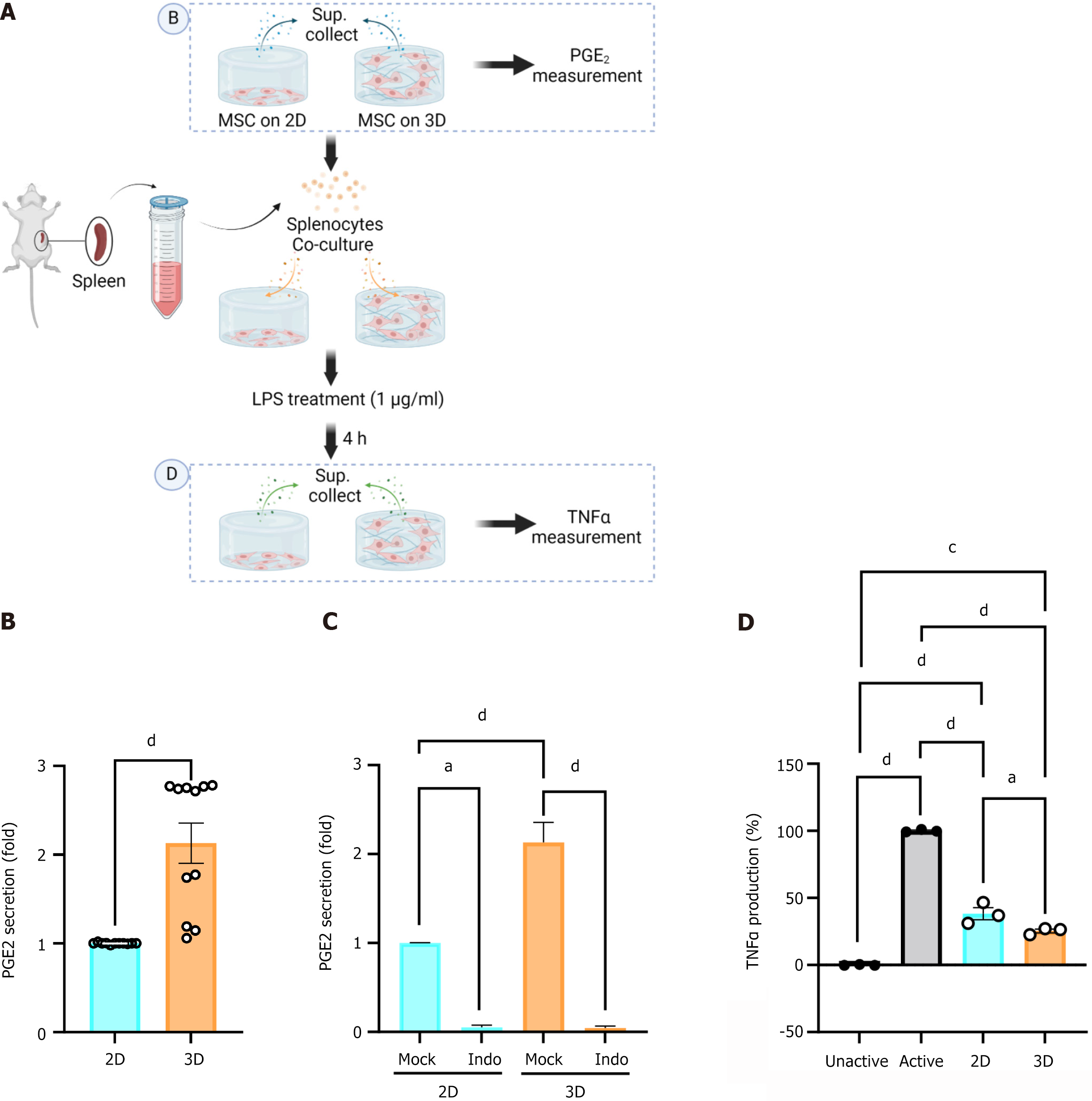
Figure 2 demonstrated that 3D-cultured MSCs exhibit a substantial increase in both protein and mRNA expression of PTGS2 (its corresponding protein is COX2), a critical enzyme involved in the production of PGE2, a well-known immunomodulator. The supernatant from both 2D and 3D cultures of MSCs was collected as depicted in Figure 3A for PGE2 measurement (Figure 3B). PGE2 secretion was significantly upregulated in supernatant collected from the 3D culture condition, exhibiting a 2.5-fold increase in PGE2 over supernatant collected from the 2D condition (Figure 3B). Furthermore, when indomethacin, a selective inhibitor of COX2, was administered at a concentration of 10 μM in both the 2D and 3D cultures, PGE2 secretion was completely blocked in both conditions (Figure 3C). These findings indicate that immunomodulatory function is dependent on COX2 in both 2D and 3D cultures.
To assess the anti-inflammatory function of MSCs, we established a co-culture system of MSCs with murine immune cells, as depicted in Figure 3A. Isolated mouse splenocytes were co-cultivated with MSCs on both 2D and 3D platforms in the presence of 1 mg/mL of LPS for 4 hours. Following the experiment, the supernatant from each condition was used for TNFα measurement (Figure 3D). Co-cultivation with MSCs suppressed TNFα production relative to splenocytes cultured without MSCs. Notably, when MSCs were cultured in a 3D environment, TNFα suppression was more pronounced, indicating an enhanced immunomodulatory effect (Figure 3D). Thus, MSCs grown in a 3D floating culture system effectively enhance immunomodulation.
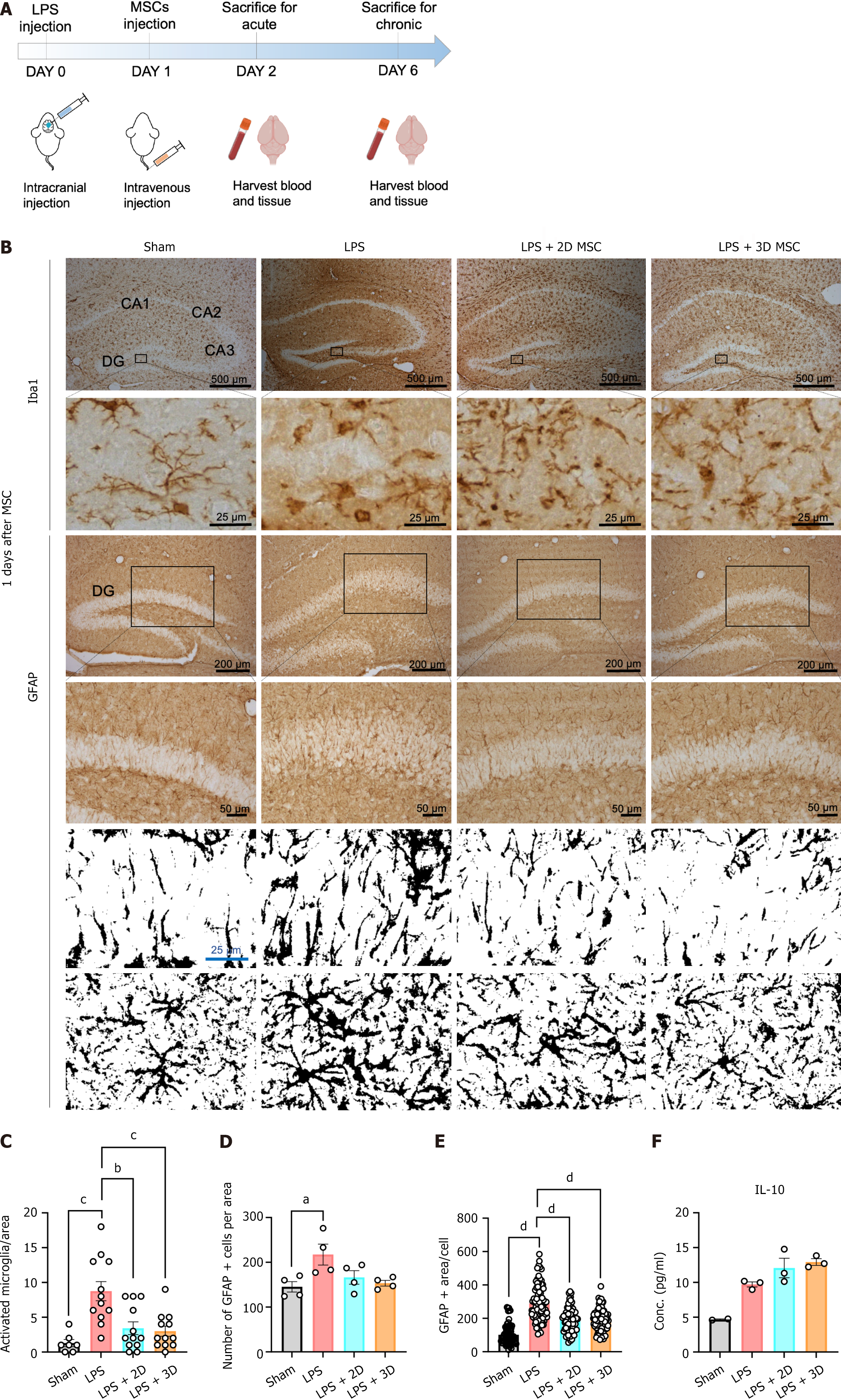
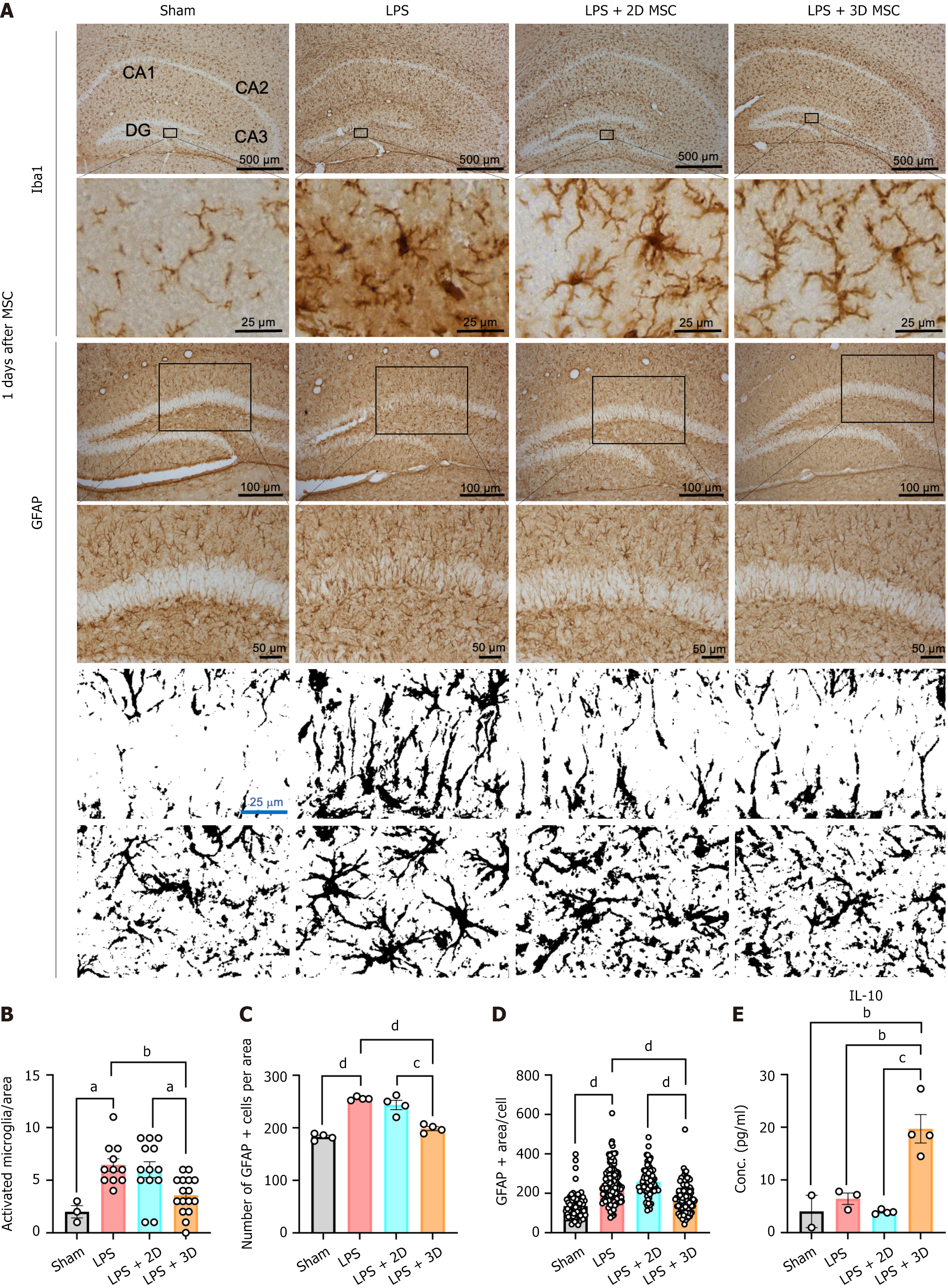
Our in vitro findings confirmed that 3D culture of MSCs enhances the secretion of anti-inflammatory and immune modulatory factors. To validate these anti-inflammatory effects in vivo, we applied 3D-cultured MSCs to a LPS-induced mouse model of neuroinflammation. The anti-inflammatory effects of MSCs from each culture condition were investigated by examining the staining patterns of the microglia marker Iba1, and the astrocyte marker GFAP, in brain tissue at day 1 and day 5 after cell infusion (Figure 4A). LPS administration resulted in strong staining of brain tissue, particularly in the dentate gyrus and CA3 regions. However, LPS-induced expression of Iba1 and GFAP was significantly attenuated by MSC injection, irrespective of the culture condition (Figures 4B and 5A). Upon stimulation, microglia undergo morphological changes from a ramified shape to an amoeboid-shaped “activated” state. Therefore, the microglial phenotype in each state was analyzed. Counting the number of amoeboid cells in the ipsilateral lesion revealed a significant decrease in both the 2D and 3D MSC groups compared to the vehicle group at day 1 after cell infusion (Figure 4C). Another indicator of the inflammatory response, GFAP staining, was also assessed. In the dentate gyrus, a large number of GFAP-positive cells were observed in the vehicle group. Consistent with the Iba1 results, the injection of both 2D and 3D MSCs led to a significant reduction in the GFAP-positive area compared to the vehicle group at day 1 after cell infusion (Figure 4D and E). These findings demonstrate that MSCs suppress the inflammatory response in the mouse brain induced by LPS, with no differential effect between the 2D and 3D culture conditions at day 1 after cell injection. However, the protective effect of 2D MSCs diminished over time, and the number of Iba1- or GFAP-positive cells became comparable to the vehicle group at day 5 after cell infusion. Surprisingly, 3D MSCs continued to reduce the activation of microglia and astrocytes in response to LPS (Figure 5B-D). In line with the immunohistochemistry results, the serum level of the anti-inflammatory cytokine IL-10 was significantly enhanced in the 3D MSC group, while there were no differences between the vehicle group and the 2D MSC group at day 5 after cell infusion (Figure 5E). At 1 day after cell infusion, the serum IL-10 levels were generally similar among the groups (Figure 4F). Based on these results, we have confirmed that 2D MSCs do not exert a lasting inhibitory effect on LPS-induced neuroinflammation, while MSCs cultured in a 3D environment continue to effectively alleviate neuroinflammation over an extended period.
Cell-based therapies have made significant strides in utilizing various cell products derived from autologous peripheral blood lymphocytes, allogeneic or autologous MSCs, hematopoietic cells, fibroblasts, and chondrocytes[15,16]. Although MSCs are recognized as easily obtainable and the most suitable choice among various cell sources for application to a wide range of pathologies[12,17-19], their clinical application still faces challenges in terms of efficiency and effectiveness. These challenges include cellular senescence, malignant transformation, and chromosomal abnormalities that arise from prolonged culture, which are inevitably induced during the process of producing MSCs at scale[20,21]. Moreover, the transient nature of the effects observed in cell therapy necessitates repeated treatments[22,23]. Therefore, further research is imperative to optimize the function and persistence of MSCs post-transplantation and mitigate adverse effects associated with cell therapy.
In this study, we have demonstrated that the creation of an in vivo-like 3D culture environment enhances the immunomodulatory function of MSCs and prolongs the persistence of their protective effects after administration in a mouse model of neuroinflammation. MSCs cultured in the 3D environment induced significant transformations in cellular morphology and microarchitecture, leading to elevated expression of stemness markers Oct4 and Nanog, surpassing the transformation efficiency of 2D MSCs (Figure 1). Furthermore, the 3D cultured MSCs exhibited significantly enhanced immunomodulatory gene expression and TNFα suppression (Figures 2 and 3).
To investigate the protective effects of MSCs in a therapeutic context, we employed a murine neuroinflammation model. Neuroinflammation is a complex response to brain injury characterized by the activation of glial cells, release of inflammatory mediators such as cytokines and chemokines, and the generation of reactive oxygen and nitrogen species[24]. When overactivated, this cascade of events can lead to further cellular damage and loss of neuronal functions[25]. Zhao et al[26] also reported that LPS-induced neuroinflammation in the mouse brain can cause severe cognitive impairment through microglia activation. Our study revealed significant brain-wide activation of glial cells, including astrocytes and microglia, following ventricular injection of LPS. However, intravenous administration of MSCs effectively attenuated glial activation at day 1 post-injection in both 2D and 3D conditions. Interestingly, under chronic conditions at day 5, only 3D MSCs demonstrated sustained anti-inflammatory effects on glial cells in the brain, accompanied by an enhanced serum IL-10 level in the periphery. In contrast, the protective effect of 2D MSCs had already diminished by day 5 post-injection. These findings are likely attributable to the significantly elevated expression levels of immunoregulation-related molecules and the increased secretion of PGE2 by 3D MSCs compared to their 2D counterparts (Figures 2 and 3). An additional hypothesis posits that the 3D culture conditions augment the expression of adhesion-related molecules on the MSC surface[27]. Enhanced adhesion molecules, such as integrins, may consequently improve the survival and persistence of MSCs following transplantation. The enrichment of adhesion molecules, such as integrin alpha 2, provide MSCs with a beneficial microenvironment for adhesion and adaptation in vivo after transplantation[28,29]. Additionally, integrin alpha 2 has been shown to improve the survival rate of transplanted MSCs[30]. The molecular mechanisms involved remain to be elucidated in future studies.
Notably, peripheral infusion of MSCs has been demonstrated to repair neuronal damage induced by neuroinflammation. Despite often exhibiting low engraftment rates in damaged tissues, numerous animal studies have unequivocally shown that injected MSCs can facilitate tissue repair and functional recovery without differentiating into specialized cells of the target tissue[31]. Consequently, MSCs appear to exert their regenerative effects primarily through paracrine mechanisms rather than tissue-specific differentiation. In this study, peripherally administered MSCs effectively ameliorated brain inflammation, underscoring their therapeutic potential. The enhanced expression of adhesion molecules on MSCs may significantly prolong their in vivo survival post-transplantation, thereby extending the duration of their immunomodulatory effects. Eggenhofer et al[23] reported that intravenously infused MSCs are predominantly short-lived, with most being sequestered in the lungs within an hour post-infusion and only a few detected in other organs. Moreover, within 24 hours of infusion, the majority of MSCs were found to be dead and localized in the liver. However, increased expression of adhesion molecules on MSCs may improve their attachment to endothelial cells, including those in the pulmonary arteries, cardiac microvessels, and umbilical veins[32]. This enhanced adhesion capacity could effectively and sustainably mitigate inflammation in damaged tissues. Taken together, our study indicates that an in vivo-like 3D culture system represents an effective approach to maximize the efficacy of MSCs as cellular therapeutics. This system enhances therapeutic efficiency and prolongs the durability of MSCs after transplantation by creating a favorable microenvironment that supports adhesion, adaptation, and sustained immunomodulatory effects.
Here, MSCs derived from a 3D culture system demonstrated enhanced expression of immunomodulatory and anti-inflammatory molecules compared to those derived from traditional 2D culture in vitro. While both 2D and 3D MSCs exhibited acute protective effects on neuroinflammation in the mouse brain, only 3D MSCs showed sustained and more potent anti-inflammatory activity in a mouse model of neuroinflammation following intravenous injection, suggesting our simple and standardized 3D culture method can significantly augment the therapeutic benefit of MSCs.
| 1. | Lee S, Kim HS, Min BH, Kim BG, Kim SA, Nam H, Lee M, Kim M, Hwang HY, Leesong AI, Leesong MM, Kim JH, Shin JS. Enhancement of anti-inflammatory and immunomodulatory effects of adipose-derived human mesenchymal stem cells by making uniform spheroid on the new nano-patterned plates. Biochem Biophys Res Commun. 2021;552:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | Wang J, Donohoe E, Canning A, Moosavizadeh S, Buckley F, Brennan MÁ, Ryan AE, Ritter T. Immunomodulatory function of licensed human bone marrow mesenchymal stromal cell-derived apoptotic bodies. Int Immunopharmacol. 2023;125:111096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20:613-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 5. | Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, Moll G, Cox CS Jr. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front Immunol. 2019;10:1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 6. | Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017;26:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 7. | Wu YN, Law JB, He AY, Low HY, Hui JH, Lim CT, Yang Z, Lee EH. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine. 2014;10:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724-13729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 761] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 9. | Zimmermann JA, McDevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16:331-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Petrenko Y, Syková E, Kubinová Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Kim OH, Yoon OJ, Lee HJ. Silk fibroin scaffolds potentiate immunomodulatory function of human mesenchymal stromal cells. Biochem Biophys Res Commun. 2019;519:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kim OH, Park JH, Son JI, Yoon OJ, Lee HJ. Bone Marrow Mesenchymal Stromal Cells on Silk Fibroin Scaffolds to Attenuate Polymicrobial Sepsis Induced by Cecal Ligation and Puncture. Polymers (Basel). 2021;13:1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Yeh HY, Liu BH, Hsu SH. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials. 2012;33:8943-8954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Chen YC, Chang WC, Lin SP, Minami M, Jean C, Hayashi H, Rival-Gervier S, Kanaki T, Wu SC, Pain B. Three-dimensional culture of chicken primordial germ cells (cPGCs) in defined media containing the functional polymer FP003. PLoS One. 2018;13:e0200515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1972] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 16. | van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, Tan HL, Harper JV, Hassan NJ, Jakobsen BK, Jorritsma A, Blank CU, Schumacher TN, Haanen JB. Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor. Mol Ther. 2015;23:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Krasilnikova OA, Baranovskii DS, Lyundup AV, Shegay PV, Kaprin AD, Klabukov ID. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev Rep. 2022;18:1974-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Ringdén O, Moll G, Gustafsson B, Sadeghi B. Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome. Front Immunol. 2022;13:839844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 20. | Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 22. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 23. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 24. | Wang W, Chang R, Wang Y, Hou L, Wang Q. Mitophagy-dependent mitochondrial ROS mediates 2,5-hexanedione-induced NLRP3 inflammasome activation in BV2 microglia. Neurotoxicology. 2023;99:50-58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Milatovic D, Zaja-milatovic S, Breyer RM, Aschner M, Montine TJ. Neuroinflammation and oxidative injury in developmental neurotoxicity. In: Reproductive and Developmental Toxicology (Second Edition). United States: Academic Press, 2017: 1051-1061. |
| 26. | Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9:5790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 544] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 27. | Bou-Ghannam S, Kim K, Grainger DW, Okano T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci Rep. 2021;11:8170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Cui LL, Nitzsche F, Pryazhnikov E, Tibeykina M, Tolppanen L, Rytkönen J, Huhtala T, Mu JW, Khiroug L, Boltze J, Jolkkonen J. Integrin α4 Overexpression on Rat Mesenchymal Stem Cells Enhances Transmigration and Reduces Cerebral Embolism After Intracarotid Injection. Stroke. 2017;48:2895-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, Docheva D. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Di Matteo B, El Araby MM, D'Angelo A, Iacono F, Nannini A, Vitale ND, Marcacci M, Respizzi S, Kon E. Adipose-Derived Stem Cell Treatments and Formulations. Clin Sports Med. 2019;38:61-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Semon JA, Nagy LH, Llamas CB, Tucker HA, Lee RH, Prockop DJ. Integrin expression and integrin-mediated adhesion in vitro of human multipotent stromal cells (MSCs) to endothelial cells from various blood vessels. Cell Tissue Res. 2010;341:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |