INTRODUCTION
Acute respiratory distress syndrome (ARDS) is an important cause of respiratory failure in critically ill patients, with a mortality rate of 30%-40%[1,2]. The course of pulmonary fibrosis is also associated with the occurrence of ARDS, exhibiting lung injury, inflammation, and fibrosis. Pulmonary fibrosis is one of the main reasons for the high mortality rate among ARDS patients; however, there are no effective treatment options for pulmonary fibrosis[3-5]. There is an urgent need to identify safer and more effective pulmonary fibrosis treatment options to improve the outcomes of patients with ARDS.
Bone marrow mesenchymal stromal cells (MSCs) have antifibrotic effects; however, their clinical applications are limited by tumorigenicity and ethical issues[6-8]. MSC-derived microvesicles (MSC-MVs) are heterogeneous subcellular structures secreted by MSCs that play important roles in tissue and organ damage repair. Research has shown that MSC-MVs partially inhibit the apoptosis of renal tubular epithelial cells and alleviate renal ischemia-reperfusion injury by transferring miR-21[9]. Du et al[10] reported that MSC-MVs alleviate renal fibrosis after partial nephrectomy through M2 macrophage polarization. MSC-MVs not only have strong tissue repair capabilities but also exert antitumor effects[11]. MSC-MVs derived from adipose tissue inhibit ovarian cancer cell proliferation, apoptosis, and necrosis through different signaling pathways[12]. MSC-MVs have been shown to exert antifibrotic effects in idiopathic pulmonary fibrosis mouse models[13]. Moreover, MSC-MVs have been shown to inhibit ARDS-related pulmonary fibrosis[14]; however, the specific effects and mechanisms have yet to be fully established.
Hepatocyte growth factor (HGF) is an important antifibrotic factor[15-17]. In an ARDS animal model, HGF significantly inhibited lung fibrous tissue proliferation in a dose-dependent manner, reduced hydroxyproline (PcNA) levels in lung tissues, and reversed pulmonary fibrosis[18]. Previously, we reported that MSC-MVSs inhibited pulmonary vascular endothelial fibrous tissue proliferation through HGF, thereby maintaining pulmonary vascular endothelial barrier functions[19]. Therefore, HGF may be an important factor in the inhibition of pulmonary fibrosis by MSC-MVs.
In this study, we investigated the effects of MSC-MVs on pulmonary fibrosis and lung functions in an ARDS model mice. Moreover, we investigated the significance of HGF in the MSC-MV-mediated inhibition of ARDS-related pulmonary fibrosis. Our findings elucidate the role and mechanisms of MSC-MVs in ARDS-induced pulmonary fibrosis and provide insight into new treatment strategies.
MATERIALS AND METHODS
Culture of MSCs and gene interference
Mouse bone marrow-derived MSCs were purchased from Guangzhou Saiye Company (Guangzhou, China), cultured in a specialized medium (Guangzhou Saiye Company) and incubated at 37 °C in a 5% CO2 atmosphere. The cell culture medium was changed every 3 days, and the cells were used in experiments after they had passed through 3-7 generations. On the basis of our previous method[19], we used lentivirus-mediated gene transfection technology to obtain MSCs with low expression of the HGF gene (siHGF-MSCs).
Isolation and identification of MSC-MVs
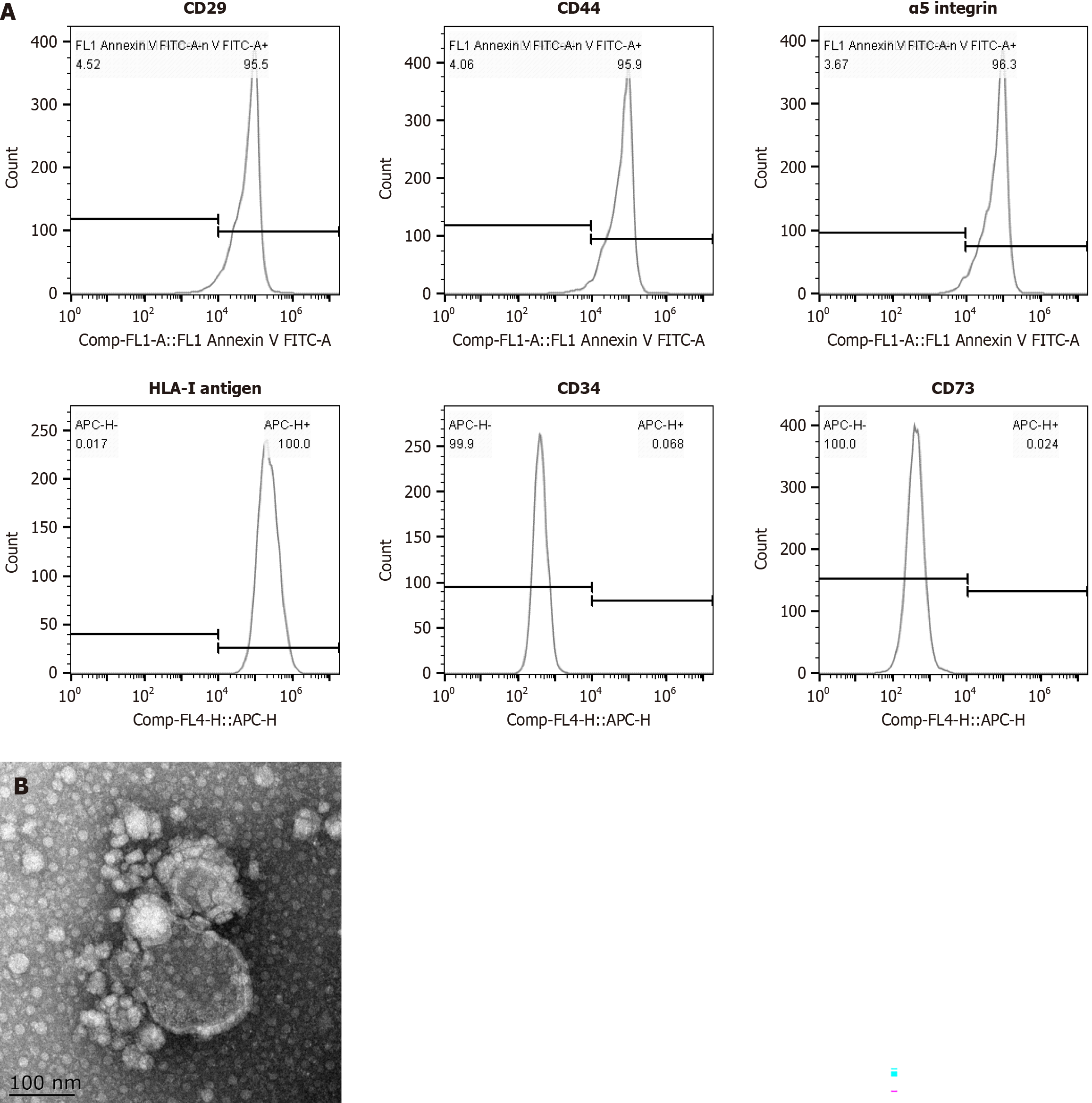
In accordance with our previous method[19], the MSC-MVs were separated from the MSCs by ultracentrifugation. The culture bottles containing 1 × 106 MSCs were incubated overnight with serum-free DMEM, followed by 0.5% fetal bovine serum. MSC-MVs were obtained by centrifugation at 2000 × g for 20 minutes, followed by centrifugation at 100000 × g and 4 °C for 1 hour. The pellets were washed with serum-free DMEM and ultracentrifuged to obtain the MSC-MVs, which were stored at -80 °C for further assays. In accordance with Teresa’s method[20], flow cytometry was performed to detect the MSC-MVs markers [positive expression of surface molecules (CD29, CD44, α5 integrins, HLA-I antigen) and negative expression of CD34 and CD73 (Figure 1)]. The morphologies of the MSC-MVs were analyzed with electron microscopy and imaging.
Figure 1 Detection of mesenchymal stromal cell-derived microvesicle markers by flow cytometry and transmission and scanning electron microscopy.
A: The expression of surface molecules (CD29, CD44, α5 integrins and the HLA-I antigen) was positive, whereas the expression of CD34 and CD73 was negative; B: Transmission and scanning electron microscopy were performed on purified mesenchymal stromal cell-derived microvesicles to reveal their spheroid morphologies and confirm their sizes.
Study ethics declarations
Animal care and interventions were performed in accordance with the National Institute of Health Guide for the Care and Use of Experimental Animals, and the animal protocol (YXYLL-2022-103) was approved by the Institutional Animal Care and Use Committees of the Yangzhou University School of Medicine. This study was carried out in compliance with the ARRIVE guidelines.
Animals and groups
Male C57BL/6 mice (aged 6-8 weeks and weighing 18-20 g) were purchased from Changzhou Cavens Experimental Animal Co., Ltd (Changzhou, China). The C57BL/6 mice were subjected to adaptive care for 2 days under controlled relative humidity (55% ± 10%) and temperature (25 ± 2 °C) conditions. Great care was taken to minimize their suffering. This protocol was prepared before the study. Briefly, 36 mice were randomly divided into six groups (6 mice in each group), as follows: Normal saline control group; ARDS group; ARDS pulmonary fibrosis group; MSC group; MSC-MVs group; and siHGF-MSC-MVs group. Lipopolysaccharide (LPS) (2 mg/kg) was instilled into the airways of all groups except the control group. The blank control group was injected with the same volume of normal saline. The ARDS mouse fibrosis model was generated via three LPS injections. Briefly, after weighing the 6-8-week-old C57BL/6 mice, they were intraperitoneally injected with 2% pentobarbital sodium (50 mg/kg) for anesthesia and fixed in the supine position; their airways were opened and LPS (1.5 mg/kg) was dropped into the airway. Twenty-four hours later, they were intraperitoneally injected with LPS (3 mg/kg). After 48 hours, the ARDS pulmonary fibrosis models were finally established by injecting 3 mg/kg LPS into the airways of the mice in the ARDS pulmonary fibrosis, MSC, MSC-MVs and siHGF-MSC groups. After 24 hours, MSCs (5 × 105/100 μL), MSC-MVs (100 μg/100 μL), or siHGF-MSC-MVs (100 μg/100 μL), respectively, were injected into the tail vein in the MSC group, MSC-MVs group and siHGF-MSC group.
MSC-MVs homing detection
The MSCs and MSC-MVs were dyed with a near-infrared dye (NIR815; Thermo Fisher Scientific, Waltham, MA, United States), as instructed by the manufacturer. After the ARDS pulmonary fibrosis models were established, they were injected with labeled MSCs or MSC-MVs via the tail vein. In vivo imaging was performed at 24 hours and 72 hours using the CRi Maestro Fluorescence Imaging System (Cambridge Research & Instrumentation, Inc, Woburn, MA, United States). The distributions of MSC-MVs and MSCs in the mouse bodies were determined at wavelengths ranging from 640-660 nm.
Assessment of pulmonary fibrosis and lung injury
Lung tissues were paraffin-embedded, sliced, stained with hematoxylin and eosin (referred to as HE), and evaluated for lung injury using the Smith scoring method to assess the pathological damage and repair status. The degree of pulmonary fibrosis was analyzed via Masson-stained sections. After the animals were euthanized, their left lungs were resected, weighed (wet weight), and placed in a 70 °C constant temperature oven for 72 hours to achieve a constant weight. After reweighing (dry weight), net weight of the two lungs was calculated as the wet/dry (W/D) ratio. Each group of mice was administered 20 g/L Evans blue through the tail vein. After the mice were sacrificed, their left atria were sliced open and uniformly injected with 10 mL of normal saline from the right ventricle to flush the lung tissues thoroughly. The lungs were resected, and the surface water of the lungs was removed. The wet weights were measured. The lung tissues were homogenized with 4 mL formamide and the Evans blue was extracted and centrifuged. The absorbance values of the supernatants were read at 620 nm, after which the amounts of extracted dye were determined using a standard curve. The permeability of the alveolar vascular barriers was determined by the amount of extracted dye per unit of tissue.
Respiratory mechanics and functional detection
After the intervention in each group, the mice were intubated via the trachea, and respiratory mechanics-related indicators of the mice in each group were measured via an experimental small animal lung function tester. The total expiratory resistance, lung compliance, forced vital capacity (FVC) and forced expiratory volume at 0.1 seconds (Fev0.1) were measured in each group. After treatment, arterial blood samples were obtained via left ventricular puncture, and the arterial partial pressure of oxygen (PO2) was measured using an automated blood gas analyzer. The oxygenation indices [PO2/oxygen inhalation (FiO2)] were calculated on the basis of the concentration of FiO2.
Immunohistochemical detection of pulmonary fibrosis-related indicators
After the experiments, the mice were euthanized and their right lower lung tissues were excised and fixed in 10% neutral formaldehyde. The tissues were paraffin-embedded and subjected to immunohistochemistry to assess type I collagen, type III collagen, transforming growth factor-β (TGF-β) and α-smooth muscle actin (α-SMA) protein expression. Under low magnification, the cells were classified as strongly positive (brown), moderately positive (brown yellow), or weakly positive (light yellow) on the basis of the degree of positive staining. The protein expression levels of type I and III collagens and TGF-β and α-SMA were quantified via the use of an immunohistochemical average absorbance tester.
Western blot analysis of pulmonary fibrosis-related indicators
The mice were euthanized, and their right lower lungs were excised for protein extraction. The proteins were incubated with antibodies (against HGF, α-SMA, type I collagen, type III collagen, and GADPH) and secondary antibodies. Finally, the target protein bands were visualized with a chemiluminescence imager for quantitative image analysis.
Determination of hydroxyproline and fibrosis-related inflammatory factor levels
Lung tissues were obtained from the middle lobe of the right lung to measure hydroxyproline levels using an alkaline hydrolysis kit. Briefly, the lungs were mixed with 2 mL of buffered saline (PBS) supplemented with 1 mL of 6 mol/L HCl for hydrolysis. Then, 25 μL of normal saline was added to 1 mL of chloramine (1.4%). After 20 minutes at room temperature, 1 mL of Erlich solution was added to the mixture and incubated at 65 °C for 15 minutes. The concentrations of hydroxyproline in the lung tissues were measured via a spectrophotometer. Mouse serum samples were removed from the -80 °C freezer for analysis of serum TGF-β, HGF, type III procollagen N-terminal aminopeptide (PIIIP), interleukin (IL)-1β and IL-10 expression via ELISA.
Statistical analysis
The data are presented as mean ± standard deviation. Statistical analyses were performed using the SPSS 21.0 software package (IBM Corp., Armonk, NY, United States). For group comparisons, one-way analysis of variance (i.e. ANOVA) followed by Bonferroni’s post hoc correction was used. Statistical significance was considered at P < 0.05.
RESULTS
MSC-MVs homed to the lungs of ARDS pulmonary fibrosis model mice
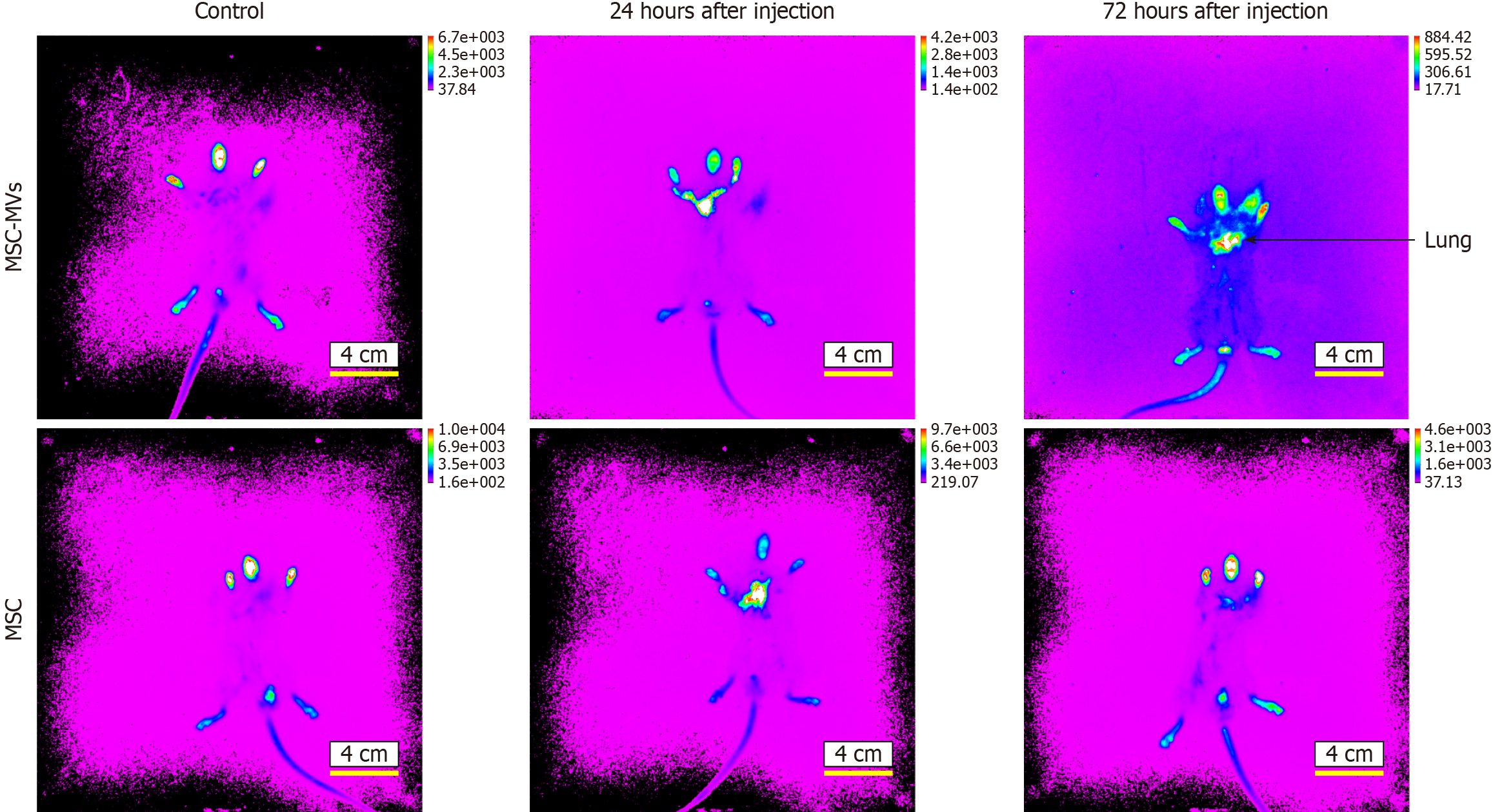
Near-infrared live imaging was performed to detect homing in the ARDS model mice after injection of MSCs or MSC-MVs. MSCs and MSC-MVs gradually migrated and homed to damaged lung tissues after tail vein injection. After 24 hours, the MSCs and MSC-MVs initially migrated into the damaged lung tissues. After 72 hours, the MSC-MVs distinctly homed to the lung tissues, with significantly increased fluorescence intensity (Figure 2). In summary, the MSC-MVs that were injected into the tail veins of ARDS pulmonary fibrosis model mice were mainly associated with injured lung tissues.
Figure 2 Microvesicles derived from mesenchymal stromal cells homing detection by the near-infrared dye NIR815.
At 24 hours after injection with mesenchymal stromal cell-derived microvesicles (MSC-MVs), the fluorescence appears mainly yellow in lung, and at 72 hours after injection, the fluorescence appears mainly yellow and red with significantly increased fluorescence intensity.
Effects of MSC-MVs on lung injury and pulmonary fibrosis in ARDS pulmonary fibrosis model mice
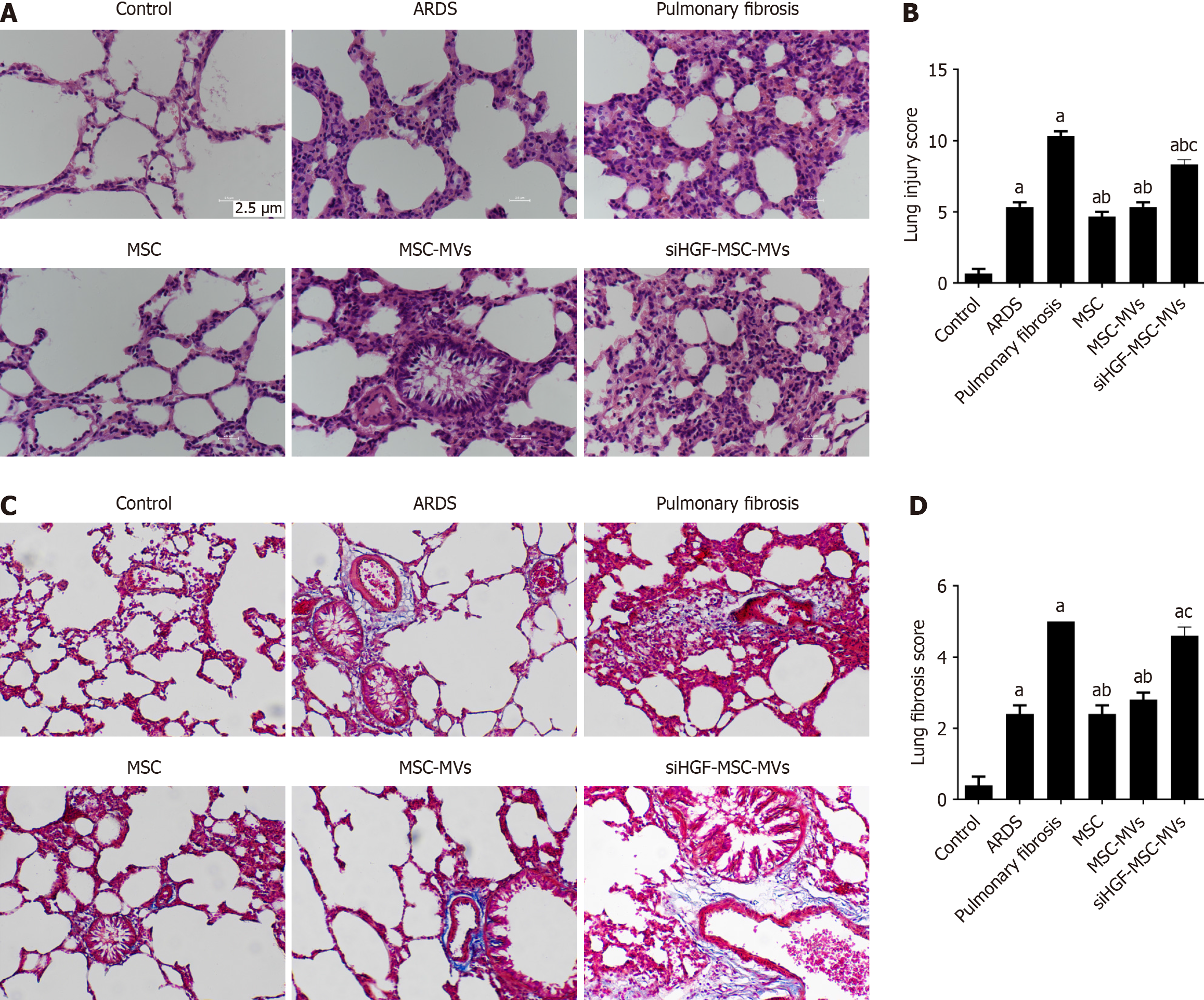
We compared the effects of MSC-MVs on lung injury and fibrosis in ARDS pulmonary fibrosis model mice via HE and Masson staining, respectively. Compared with those in the control group, the ARDS group showed significantly greater lung injury and pulmonary fibrosis scores. After the injection of the MSC-MVs, there were significant reductions in lung injury and pulmonary fibrosis scores. However, suppression of HGF expression markedly inhibited the above effects of MSC-MVs (Figure 3). These findings suggest that MSC-MVs inhibit ARDS-related pulmonary fibrosis partly through HGFs.
Figure 3 Immunohistochemical detection of the effects of mesenchymal stromal cell-derived microvesicles on pulmonary and fibrosis-related indicators in acute respiratory distress syndrome pulmonary fibrosis mouse models.
A: Hematoxylin and eosin staining of lung tissues from each group; B: Lung injury scores of pathological sections from each group; C: Masson staining of lung tissues from each group; D: Lung fibrosis scores of pathological sections from each group. aP < 0.05, vs the control group; bP < 0.05, vs the pulmonary fibrosis group; cP < 0.05, vs the mesenchymal stromal cell-derived microvesicles (MSC-MVs) group (n = 6). Control, acute respiratory distress syndrome (ARDS), pulmonary fibrosis, MSC, MSC-MVs and low hepatocyte growth factor (HGF)-MSC-MVs represent the control group, ARDS group, pulmonary fibrosis group, MSC group, MSC-MVs group and low HGF-MSC-MVs group, respectively.
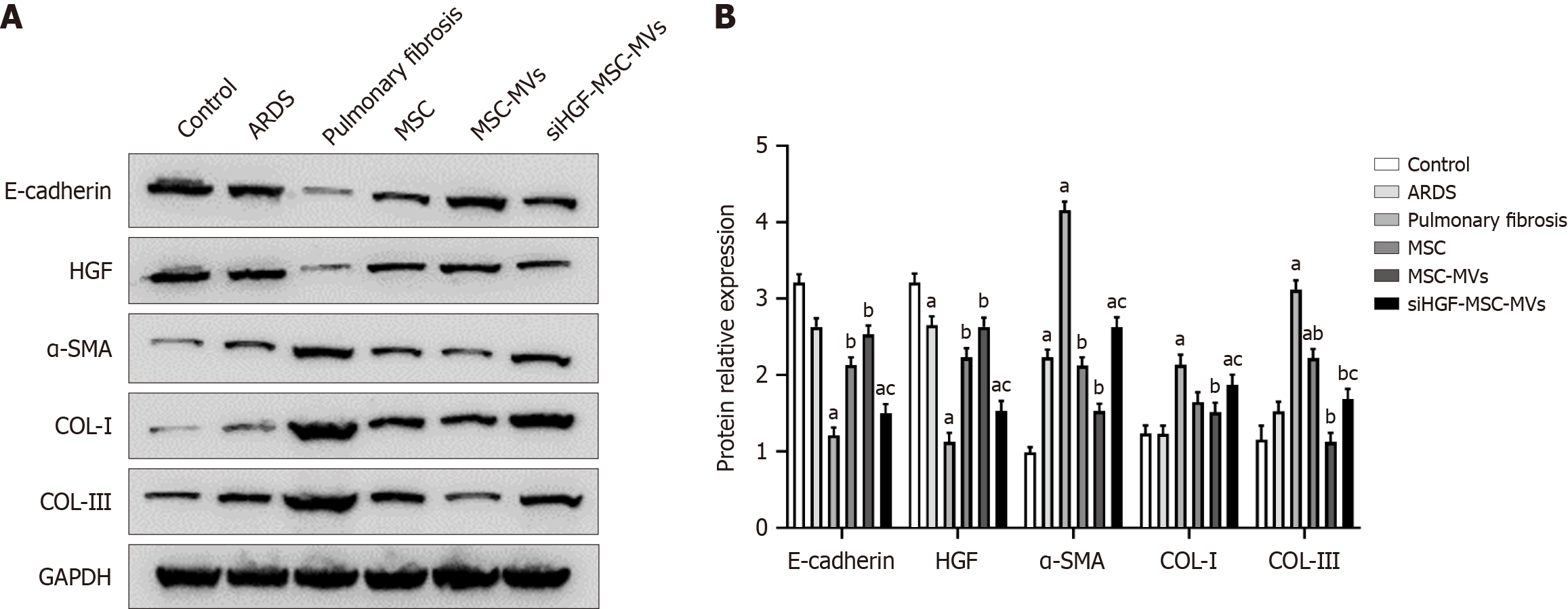
Effects of MSC-MVs on pulmonary fibrosis-related indicators in ARDS pulmonary fibrosis model mice
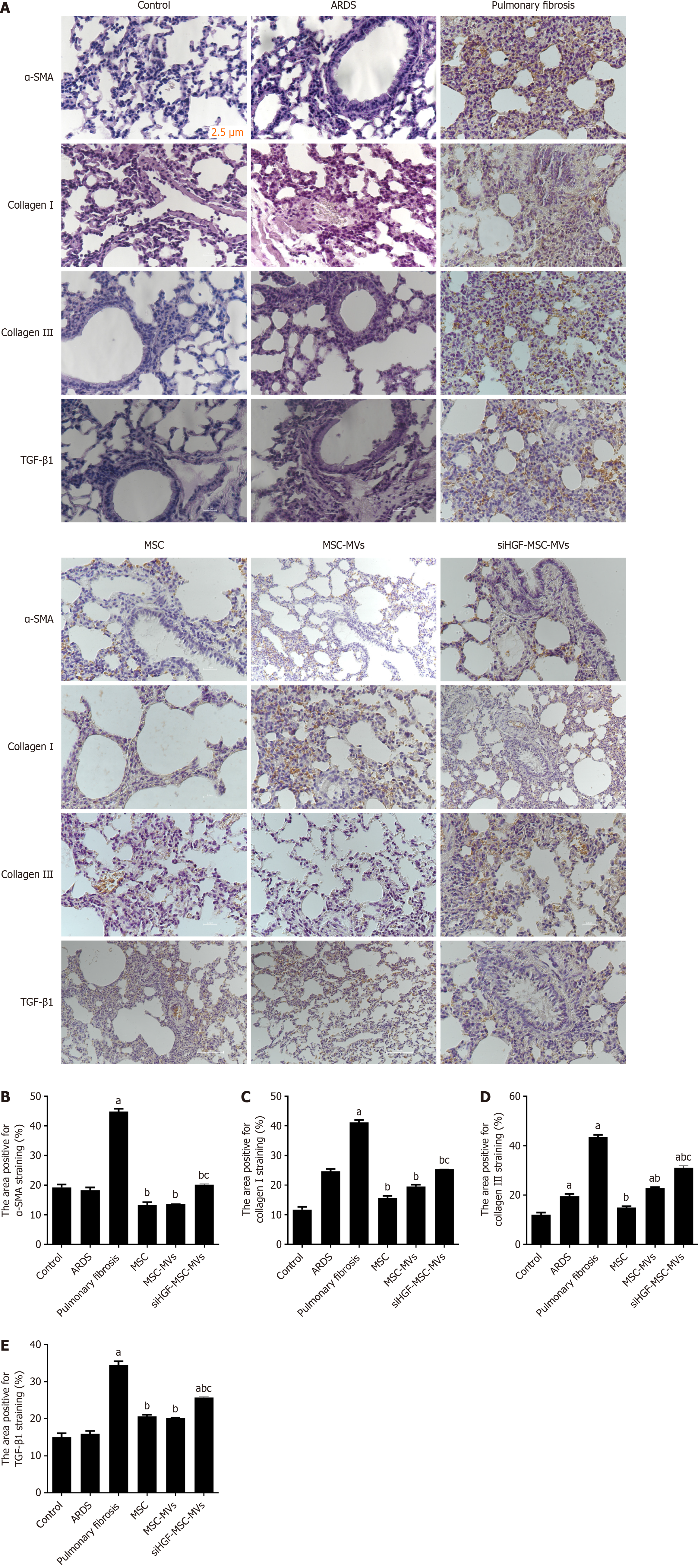
We further performed immunohistochemistry and western blot assays to investigate the effects of MSC-MVs on expression of fibrosis-related protein markers in the ARDS pulmonary fibrosis model mice. Compared with the ARDS pulmonary fibrosis group, the MSC-MVs group exhibited suppressed expression of type I collagen antigen, type III collagen antigen, and the proteins TGF-β and α-SMA, whereas the siHGF-MVs group exhibited significantly elevated expression of those proteins (Figures 4 and 5). Thus, MSC-MVs inhibit the expression of pulmonary fibrosis-related proteins in ARDS pulmonary fibrosis model mice partly through HGFs.
Figure 4 Effects of mesenchymal stromal cell-derived microvesicles on acute respiratory distress syndrome-related pulmonary fibrosis and lung injury.
A: Immunohistochemical images of pathological sections of lung tissues from each group; B: α-smooth muscle actin (α-SMA) protein expression; C: Type I collagen protein expression; D: Type III collagen protein expression; E: Transforming growth factor-β1 (TGF-β1) protein expression. aP < 0.05, vs the control group; bP < 0.05 vs the pulmonary fibrosis group; cP < 0.05 vs the mesenchymal stromal cell-derived microvesicles (MSC-MVs) group (n = 6). Control, acute respiratory distress syndrome (ARDS), pulmonary fibrosis, MSC, MSC-MVs and low hepatocyte growth factor (HGF)-MSC-MVs represent the control group, ARDS group, pulmonary fibrosis group, MSC group, MSC-MVs group and low HGF-MSC-MVs group, respectively.
Figure 5 Western blot analysis of the effects of mesenchymal stromal cell-derived microvesicles on pulmonary fibrosis-related proteins in acute respiratory distress syndrome pulmonary fibrosis model mice.
A: Western blot bands of fibrosis-related proteins in the lung tissues of each group; B: Relative expression of fibrosis-related proteins. aP < 0.05, vs the control group; bP < 0.05, vs the pulmonary fibrosis group; cP < 0.05, vs the mesenchymal stromal cell-derived microvesicles (MSC-MVs) group (n = 6). Control, acute respiratory distress syndrome (ARDS), pulmonary fibrosis, MSC, MSC-MVs and low hepatocyte growth factor (HGF)-MSC-MVs represent the control group, ARDS group, pulmonary fibrosis group, MSC group, MSC-MVs group and low HGF-MSC-MVs group, respectively. α-SMA: α-smooth muscle actin; COL: Collagen.
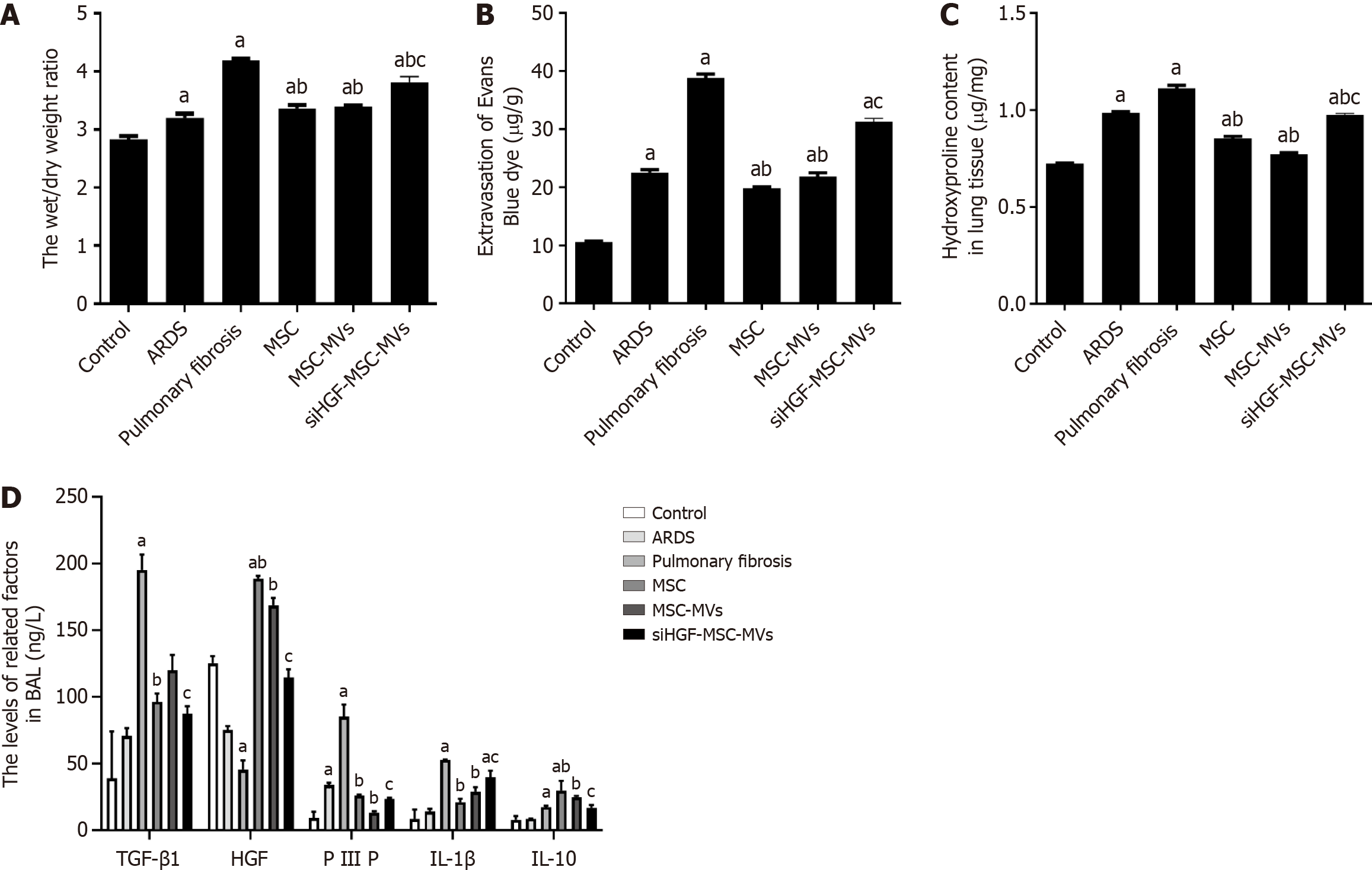
Effects of MSC-MVs on hydroxyproline in ARDS pulmonary fibrosis model mice
Hydroxyproline levels in lung tissues are correlated with pulmonary fibrosis severity. In this study, we found that the hydroxyproline levels in the pulmonary fibrosis group were significantly greater than those in the control group; those in the MSC-MVs treatment group were significantly lower than those in the pulmonary fibrosis group; whereas those in the siHGF-MSC-MVs group were significantly greater than those in the MSC-MVs group. Therefore, MSC-MVs suppressed hydroxyproline levels in the lung tissues of ARDS pulmonary fibrosis model mice partly through HGFs (Figure 6A).
Figure 6 Effects of mesenchymal stromal cell-derived microvesicles on pulmonary vascular endothelial permeability and related proteins in acute respiratory distress syndrome pulmonary fibrosis mouse models.
A: Lung wet/dry weight ratios for each group; B: Evans blue detection of vascular endothelial permeability in each group; C: Hydroxyproline levels in each group; D: Expression of inflammatory factors in each group detected by ELISA. aP < 0.05, vs the control group; bP < 0.05, vs the pulmonary fibrosis group; cP < 0.05, vs the mesenchymal stromal cell-derived microvesicles (MSC-MVs) group (n = 6). Control, acute respiratory distress syndrome (ARDS), pulmonary fibrosis, MSC, MSC-MVs and low hepatocyte growth factor (HGF)-MSC-MVs represent the control group, ARDS group, pulmonary fibrosis group, MSC group, MSC-MVs group and low HGF-MSC-MVs group, respectively. IL: Interleukin; PIIIP: Type III procollagen N-terminal aminopeptide; TGF-β1: Transforming growth factor-β1.
Effects of MSC-MVs on pulmonary vascular permeability and inflammatory indicators in ARDS pulmonary fibrosis model mice
Lung vascular permeability was assessed by measuring W/D ratios and Evans blue leakage. The lung W/D ratio and Evans blue leakage in the ARDS pulmonary fibrosis group were greater than those in the control group and were significantly lower after MSC-MVs injection. However, the above effects of MSC-MVs were significantly inhibited by HGF suppression (Figure 6B and C). These findings indicate that MSC-MVs inhibited pulmonary vascular permeability in ARDS pulmonary fibrosis partly through HGFs. ELISA was subsequently performed to detect the expression of inflammatory factors related to pulmonary fibrosis. Compared with those in the control group, the expression levels of TGF-β, PIIIP, IL-1β, and IL-10 in the pulmonary fibrosis group were significantly greater, whereas the HGF levels were lower. However, TGF-β, PIIIP, IL-1β, and IL-10 expression in the MSC-MVs group were significantly lower while HGF levels were higher than those of the pulmonary fibrosis group. The above effects of MSC-MVs were significantly inhibited by the suppression of HGF expression (Figure 6D). Therefore, MSC-MVs inhibited the expression of fibrosis-related inflammatory factors through HGFs.
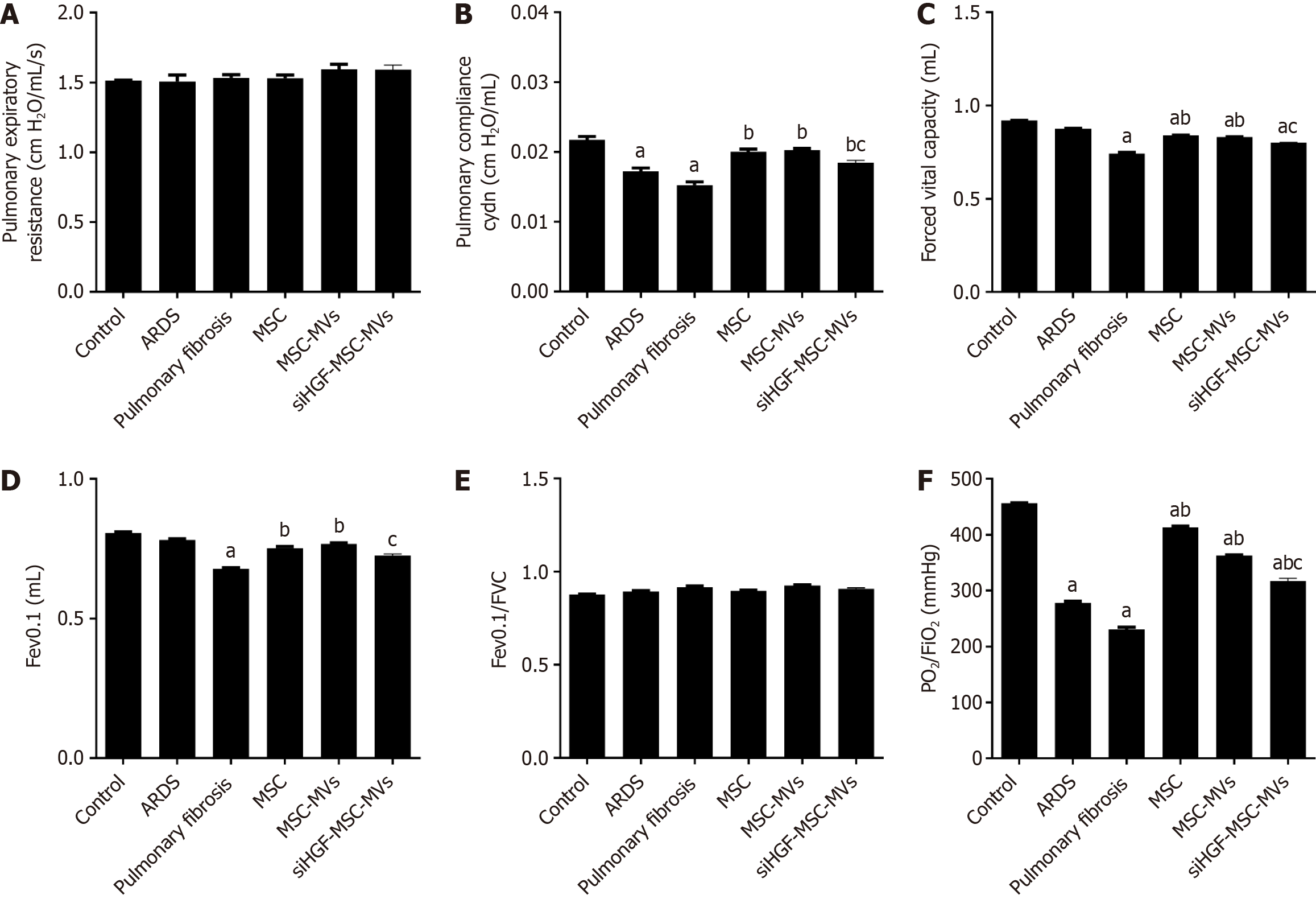
Effects of MSC-MVs on respiratory mechanics and lung functions in ARDS pulmonary fibrosis model mice
Pulmonary compliance, Fev0.1, FVC and PO2/FiO2 in the ARDS pulmonary fibrosis model group were significantly lower than those in the control group, and MSC-MVs significantly improved these indicators after tail vein injection. However, the above effects of MSC-MVs were significantly inhibited by HGF suppression. There were no significant differences in respiratory resistance or the Fev0.1/FVC among the groups (Figure 7). Therefore, MSC-MVs improved lung ventilation functions and oxygenation indices in ARDS pulmonary fibrosis mouse models partly through HGFs.
Figure 7 Effects of mesenchymal stromal cell-derived microvesicles on respiratory mechanics and lung functions in acute respiratory distress syndrome pulmonary fibrosis mouse models.
A: Pulmonary expiratory resistance; B: Pulmonary compliance; C: Forced expiratory volume in 0.1 seconds (Fev0.1); D: Forced vital capacity (FVC), E: Fev0.1/FVC; F: Pressure of oxygen (PO2)/oxygen inhalation (FiO2); G: Transmission and scanning electron microscopy were performed on purified mesenchymal stromal cell-derived microvesicles (MSC-MVs) to reveal their spheroid morphologies and confirm their sizes. aP < 0.05, vs the control group; bP < 0.05, vs the pulmonary fibrosis group; cP < 0.05, vs the MSC-MVs group (n = 6). Control, acute respiratory distress syndrome (ARDS), pulmonary fibrosis, MSC, MSC-MVs and low hepatocyte growth factor (HGF)-MSC-MVs represent the control group, ARDS group, pulmonary fibrosis group, MSC group, MSC-MVs group and low HGF-MSC-MVs group, respectively.
DISCUSSION
Pulmonary fibrosis is strongly associated with poor prognostic outcomes in ARDS patients[21-23]. Currently, there are no effective measures for treating pulmonary fibrosis[24,25]. MSC-MVs have anti-pulmonary fibrosis effects; however, their specific effects and mechanisms on ARDS-related pulmonary fibrosis have not been fully established[26]. We found that after MSC-MVs were injected into the tail vein of ARDS pulmonary fibrosis model mice, they first migrated and homed to damaged lung tissues, inhibited the expression of ARDS pulmonary fibrosis-related factors and fibrosis-related factors partly through HGFs, improved lung compliance and ventilation functions, and increased oxygenation indices. The findings of this study provide a new direction for the treatment of ARDS-related pulmonary fibrosis.
Pulmonary fibrosis develops in the early stages of ARDS and occurs throughout the entire process of ARDS[27]. We established ARDS model mice by instilling LPS into their airways. On the 7th day of ARDS model establishment, the lung fibrosis score and fibrosis-related protein expression in the mice, including type I collagen antigen, type III collagen antigen, TGF-β and α-SMA, were significantly increased; therefore, significant pulmonary fibrosis occurred on the 7th day of ARDS. Previous studies on pulmonary fibrosis have focused mostly on bleomycin-induced pulmonary fibrosis models[28,29]. On the basis of a previous study[30], we successfully established ARDS pulmonary fibrosis models via three LPS injections. After pulmonary fibrosis was induced, pulmonary fibrosis scores and the expression of type I collagen antigen, type III collagen antigen, TGF-β and α-SMA significantly increased, whereas lung compliance Fev0.1, FVC and ventilation functions significantly decreased, which was consistent with the pathological and pathophysiological changes in pulmonary fibrosis.
MSCs have good anti-pulmonary fibrosis effects[31], but their applications are limited by their tumorigenicity and ethical considerations[32]. MSC-MVs can avoid these challenges and are convenient for storage, which is conducive to large-scale production[33]. MSC-MVs exhibited good anti-pulmonary fibrosis effects in bleomycin-induced pulmonary fibrosis mouse models; however, their specific effects and the mechanisms involved in alleviating ARDS-related pulmonary fibrosis have yet to be established[34,35]. In this study, we found that most of the MSC-MVs and MSCs homed to the injured lung tissues and significantly inhibited the expression of type I collagen antigen, type III collagen antigen, TGF-β and α-SMA in the ARDS pulmonary fibrosis model mice, with reduced pulmonary fibrosis scores, improved lung compliance, Fev0.1 and FVC, and increased oxygenation indices. Like MSCs, MSC-MVs homed toward the site of lung injury, and there were no significant differences in the inhibition of pulmonary fibrosis between the two. Therefore, MSC-MVs can replace MSCs for anti-ARDS pulmonary fibrosis treatment.
HGF is a cell repair factor that promotes epithelial cell proliferation and collagen degradation and inhibits epithelial cell apoptosis. It is one of the few growth factors that can inhibit fibrosis[36-38]. MSC-MVs contain abundant mRNAs, such as HGF mRNA[39]; RNA transfer is an important way by which MSC-MVs exert damage-repair effects[40]. Previously, we reported that MSC-MVs alleviate pulmonary vascular endothelial injury and reduce pulmonary vascular endothelial permeability through HGF mRNA transfer[19]. In this study, lentivirus transfection was used to suppress HGF mRNA expression in MSC-MVs. The ability of MSC-MVs to inhibit ARDS-related pulmonary fibrosis score, affect the expression of type I collagen antigen, type III collagen antigen, TGF-β and α-SMA, and improve lung compliance and lung ventilation functions such as Fev0.1, FVC, and oxygenation indices, which were significantly inhibited after suppression of HGF mRNA in MSC-MVs. These findings imply that HGF mRNA transfer is one of the main mechanisms through which MSC-MVs exert their anti-ARDS pulmonary fibrosis effects. During ARDS-induced pulmonary fibrosis, the MSC-MVs administered through the tail vein first homed to the injured lung, after which the HGF transcribed from the mRNA in the MSC-MVs bound to the c-Met receptors in pulmonary vascular epithelial cells, thereby exerting anti-pulmonary fibrosis effects. Therefore, HGF mRNA transfer is one of the mechanisms by which MSC-MVs resist ARDS-induced pulmonary fibrosis.
MSC-MVs have advantages over MSCs, such as easy mass production, easy storage, no immunogenicity, and no ethical issues[41]. Therefore, MSC-MVs have broader therapeutic prospects than do MSCs in the treatment of ARDS-related pulmonary fibrosis. However, the treatment of pulmonary fibrosis caused by ARDS with MSC-MVs is still in the preliminary research stage, and the following problems remain: (1) There is still a lack of standard methods for the extraction, purification, and identification of MSC-MVs; (2) The timing, dosage, and administration methods of MSC-MV treatment need to be clarified; and (3) The mechanism by which MSC-MVs combat pulmonary fibrosis is not very clear. Future research should focus on how to obtain MSC-MVs in a standardized manner, clarify the timing, dosage and administration of MSC-MVs treatment, explore the mechanism of MSC-MVs treatment for pulmonary fibrosis, and carry out further clinical research[42,43].
This study has several limitations. First, researchers have not determined whether the same or better effects can be obtained by administering MSC-MVs intratracheally instead of intravenously. Second, MSC-MVs were administered 24 hours after the establishment of the pulmonary fibrosis model. However, the antifibrotic effects of MSC-MVs administered at different times after pulmonary fibrosis may vary. In addition, finally, only a single dose of MSC-MVs was administered, and two or more doses after the development of pulmonary fibrosis may have improved the anti-fibrosis effects. These limitations should be addressed in future studies.