Published online Jul 26, 2024. doi: 10.4252/wjsc.v16.i7.760
Revised: May 18, 2024
Accepted: June 14, 2024
Published online: July 26, 2024
Processing time: 133 Days and 23 Hours
Non-alcoholic fatty liver disease (NAFLD) has emerged as a significant health challenge, characterized by its widespread prevalence, intricate natural pro
Core Tip: This review highlights the increasing incidence of non-alcoholic fatty liver disease and its complex progression, focusing on the underlying mechanisms of disease pathogenesis and contemporary treatment modalities. It delves into the therapeutic potential of mesenchymal stem cells, examining their classification, roles, and primary functions in the context of their use in various diseases. The review specifically aims to clarify how mesenchymal stem cells can mitigate non-alcoholic fatty liver disease progression by modulating molecular pathways involved in glycolipid metabolism, inflammation, oxidative stress, endoplasmic reticulum stress, and fibrosis.
- Citation: Jiang Y, Yusoff NM, Du J, Moses EJ, Lin JT. Current perspectives on mesenchymal stem cells as a potential therapeutic strategy for non-alcoholic fatty liver disease. World J Stem Cells 2024; 16(7): 760-772
- URL: https://www.wjgnet.com/1948-0210/full/v16/i7/760.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i7.760
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease occurring globally. Recent research has shown that the prevalence of NAFLD over the past four decades is estimated to be around 30%, with varying rates across different regions such as 44% in Latin America and 28% in the Asia Pacific[1]. This increase in prevalence is closely linked to the rising trends of obesity, type 2 diabetes mellitus (T2DM), insulin resistance (IR), cardiovascular disease (CVD), and hypertension[2]. Over the last two decades, numerous clinical trials have investigated targets in the development of drugs for non-alcoholic steatohepatitis (NASH). However, owing to the disease’s inherent heterogeneity and the intricate nature of its pathogenesis, few drugs have yet received approval for clinical intervention[3].
Mesenchymal stem cells (MSCs), commonly found in various tissues, exhibit intrinsic capabilities for high self-renewal, excellent biocompatibility, and low immunogenicity. These properties have positioned MSCs and their secretory factors as therapeutic innovations for various diseases[4]. Extensive research is currently being carried out to explore the potential of MSCs as a therapeutic approach to NAFLD. Preliminary studies have highlighted their potential efficacy in alleviating liver damage, improving liver function, and inducing liver regeneration[5]. This review aims to highlight and summarize the emerging therapeutic potential of MSCs in treating NAFLD, with a focus on elucidating the mechanisms that ameliorate the disease. For this review, articles published in English were searched on PubMed using the keywords “non-alcoholic fatty liver disease”, “metabolic-associated fatty liver disease”, “fatty liver”, “non-alcoholic steatohepatitis”, “mesenchymal stem cells”, and “stem cells”.
NAFLD is characterized by its heterogeneity, as it includes a spectrum of liver diseases. This diversity in disease manifestations has positioned NAFLD as a major contributor to liver transplantation worldwide, primarily due to its progression to cirrhosis and hepatocellular carcinoma (HCC)[6]. Previous studies indicate that patients with NAFLD have a greater risk of all-cause mortality than the general population, including increased risks for extrahepatic diseases such as CVD, chronic kidney disease, and certain cancers[6]. Notably, close relatives of individuals with NAFLD-induced cirrhosis face a 12-fold increase in the risk of developing advanced fibrosis, which can be attributed to genetic factors[7].
NAFLD typically progresses through stages that include steatosis, fibrosis, cirrhosis, and eventually HCC. However, the progression of the disease is unpredictable, with variable shifts in disease severity and the stages of fibrosis. Assessing liver tissue damage involves monitoring the fibrosis status and detecting the onset of cirrhosis, which are critical for understanding the development of this disease. A 4-year study involving 1773 adults with NAFLD demonstrated that increasing mortality rates are correlated with increased fibrosis severity, with stages F3 and F4 also associated with an increased chance of liver complications, T2DM, and reduced kidney function[8]. NAFLD progresses rapidly in patients with NASH, which further increases the risk of HCC and associated morbidities[6]. In contrast, NAFLD patients without cirrhosis exhibit a significantly lower incidence of HCC[9].
The intricate mechanisms underlying the onset and progression of NAFLD remain elusive. Historically, the pathogenesis of NAFLD can be explained by the two-hit hypothesis. The first hit, marking disease initiation, involves lipid accumulation and IR. These imbalances promote hepatic lipogenesis and reduce the degradation of free fatty acids, leading to hepatic steatosis[10]. The subsequent stage, or the second hit, increases the susceptibility of the liver to inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress. During this phase, the resulting increase in mitochondrial reactive oxygen species (ROS) can trigger inflammation (steatohepatitis), fibrosis, and lead to cirrhosis[10]. However, as research has progressed, this two-hit hypothesis seems insufficient to fully capture the complexity of NAFLD pathogenesis.
An improved understanding of the pathogenic mechanisms in NAFLD has led to the emergence of a multiple-hit hypothesis. This hypothesis posits that the disease results from various intertwined factors, encompassing genetic, environmental, and microbiological elements, and affects multiple organs, including the liver, pancreas, gut, and adipose tissue[11]. In the initial phase of the disease, IR and obesity are considered foundational triggers that lead to fat accumulation and liver lipotoxicity. Subsequent contributors to NAFLD progression include elevated levels of free fatty acids and triglycerides, which trigger the disease from mere steatosis to more complex stages[12]. Other contributing factors include oxidative stress, liver-derived inflammatory mediators (cytokines from Kupffer cells), and agents from external sources such as adipokines and lipopolysaccharides originating from the gut microbiota. Collectively, these factors lead to hepatocellular injury, apoptosis, compensatory hepatocyte regeneration, liver fibrosis, and NAFLD progression[11,12].
At present, no pharmacological treatments have been officially approved for NAFLD, despite an extensive understanding of its epidemiology, pathogenesis, and progression. Generally, interventions include lifestyle changes such as a hypocaloric diet and physical exercise, as well as weight loss induced by drugs or bariatric surgery.
Diet modification plays a critical role in NAFLD management. Diets highly recommended for NAFLD patients include hypocaloric diets, high-protein diets, and the Mediterranean diet. Particularly, the Mediterranean diet is frequently advocated as it contains a rich source of bioactive compounds and phytochemicals, such as omega-3 fatty acids and phytosterols, known for their antioxidant and anti-inflammatory benefits[13].
In addition to dietary changes, exercise has shown positive effects on NAFLD outcomes in animal models by altering liver metabolism. This alteration involves the regulation of SREBP-1 levels through AMPK activation, which potentially increases peroxisome proliferator-activated receptor (PPAR)-γ expression. Additional benefits observed from regular exercise include improved insulin sensitivity, reduced oxidative stress, and mitigated hepatocyte apoptosis through the upregulation of antioxidative enzymes and anti-inflammatory cytokines[14]. However, studies on NAFLD patients are relatively limited in terms of exercise type, duration, and intensity[15].
Weight loss achieved through these dietary and exercise interventions has been demonstrated to improve NAFLD outcomes, including enhanced glucose control, which subsequently reduces the risks of diabetes and CVD[15]. Specifically, in patients with NASH, weight reductions of up to 10% have been associated with decreased hepatic steatosis and fibrosis regression[16]. Additionally, biopsy-confirmed NASH patients have pharmacological treatment options, which include vitamin E and pioglitazone. Vitamin E, acting as an antioxidant, neutralizes free radicals and reduces oxidative stress. Treating NASH patients with 800 IU/day of vitamin E has been shown to improve liver histology compared to a placebo[17]. However, vitamin E can cause side effects such as bleeding, heart failure, and prostate cancer; therefore, its long-term high-dose usage should be evaluated against international guidelines. Pioglitazone is used to treat NASH in both diabetic and non-diabetic patients. Both groups have shown improvements in fibrosis scores, insulin sensitivity, and significant reductions in hepatic triglyceride content[18]. However, its use comes with potential side effects, including increased risks of certain cancers such as bladder cancer, weight gain, fluid retention, and cardiovascular events. Other alternatives to improve NAFLD/NASH outcomes include bariatric surgery and liver transplantation. However, extensive clinical trials are required for more conclusive results before these can be implemented as a standard course of treatment.
MSCs are multipotent stem cells that are derived from a diverse array of stromal regenerative cells obtained from various tissues. These sources include bone marrow, adipose tissues, umbilical cord, dental pulp, menstrual blood, synovium, amniotic fluid, and placenta. The International Mesenchymal and Tissue Stem Cell Committee has proposed specific criteria for the identification of human MSCs. Specifically, MSCs should adhere to plastic under standard conditions. Furthermore, flow cytometry analysis should reveal that these cells express the CD markers CD105, CD73, and CD90, and not express CD45, CD34, CD14 or CD11b, CD79a, CD19, or HLA-DR. Additionally, these cells should demonstrate the ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro[19].
MSCs have become a vital component of regenerative medicine due to their inherent self-renewal capability and ability to differentiate into various cell lineages. Additionally, MSCs secrete many factors that play pivotal roles in preserving tissue equilibrium, aiding in repair, and promoting regeneration[20]. The complex process of wound healing, involving cell migration, proliferation, matrix remodeling, angiogenesis, and re-epithelialization, is significantly improved by MSC intervention. MSCs accelerate healing by promoting angiogenesis, cell proliferation, collagen production, inflammation moderation, and overall tissue regeneration through the release of various chemotactic agents[21,22].
MSCs also exert significant immunomodulatory effects via both the innate and adaptive immune systems. They establish direct interactions with numerous immune cells, such as T cells, B cells, natural killer cells, macrophages, monocytes, dendritic cells, and neutrophils, through cell-to-cell contact and paracrine signaling[23]. MSCs promote the secretion of anti-inflammatory mediators such as interleukin (IL)-10 and transforming growth factor-β while attenuating the secretion of proinflammatory cytokines, including IL-1 and IL-17. Additionally, MSCs secrete chemokines that attract immune cells, thus enhancing their immunosuppressive functions[24]. Recent studies have clarified the role of MSCs and their secretory factors in anti-inflammatory processes. Their mode of action relies on interactions with various cellular targets, such as macrophages, microglia, chondrocytes, endothelial cells, fibroblasts, and neural stem cells[25].
Due to their intrinsic trophic, immunomodulatory, and homing attributes, MSCs possess great therapeutic potential. Specifically, they have a natural ability to seek and settle in sites of tissue injury, inflammation, or tumors, thus providing them with a distinct advantage in targeted interventions through immunomodulation, bioactive secretion, and cellular differentiation. In line with these characteristics, MSCs have also been shown to serve as delivery systems to transport therapeutic agents, genes, or proteins directly to specific tissues or tumor sites[26].
The therapeutic potential of MSCs for various medical conditions has been demonstrated in numerous animal models and clinical trials. These conditions include organ injuries to the heart, liver, kidneys, and lungs, often resulting from trauma or cellular changes[27], as well as neurological disorders such as Alzheimer’s disease, in which MSCs have replaced dying neurons and alleviated inflammation[28]. Additionally, MSCs are currently being studied for the treatment of stroke, Parkinson’s disease, and Huntington’s disease. MSCs have also shown capabilities in mitigating oxidative stress and providing protective effects against endocrine disorders such as diabetes[14]. Conditions such as thyroid irregularities, osteoporosis, adrenal insufficiency, and pituitary gland irregularities have also been ameliorated by MSC administration[29].
Numerous preclinical studies have highlighted the therapeutic potential of MSCs in alleviating lung injuries and fibrosis in respiratory conditions, including bronchopulmonary dysplasia, asthma, acute lung injuries, and chronic pulmonary diseases in adults[30]. MSCs have shown benefits for rheumatoid arthritis by reducing inflammation and decelerating its progression[31]. They also provide therapeutic benefits for conditions like systemic lupus erythematosus, multiple sclerosis, and Crohn’s disease, as evidenced in both preclinical and clinical studies[32]. Research suggests that MSCs can differentiate into cardiomyocytes both in vitro and in vivo. This capability could potentially benefit patients with dilated cardiomyopathy by rejuvenating endothelial function and enhancing coronary circulation[15]. Furthermore, MSCs are being investigated for the treatment of other conditions, including fractures, infertility, and obesity. Nevertheless, challenges remain concerning the safety, efficacy, and long-term impacts of MSC treatments.
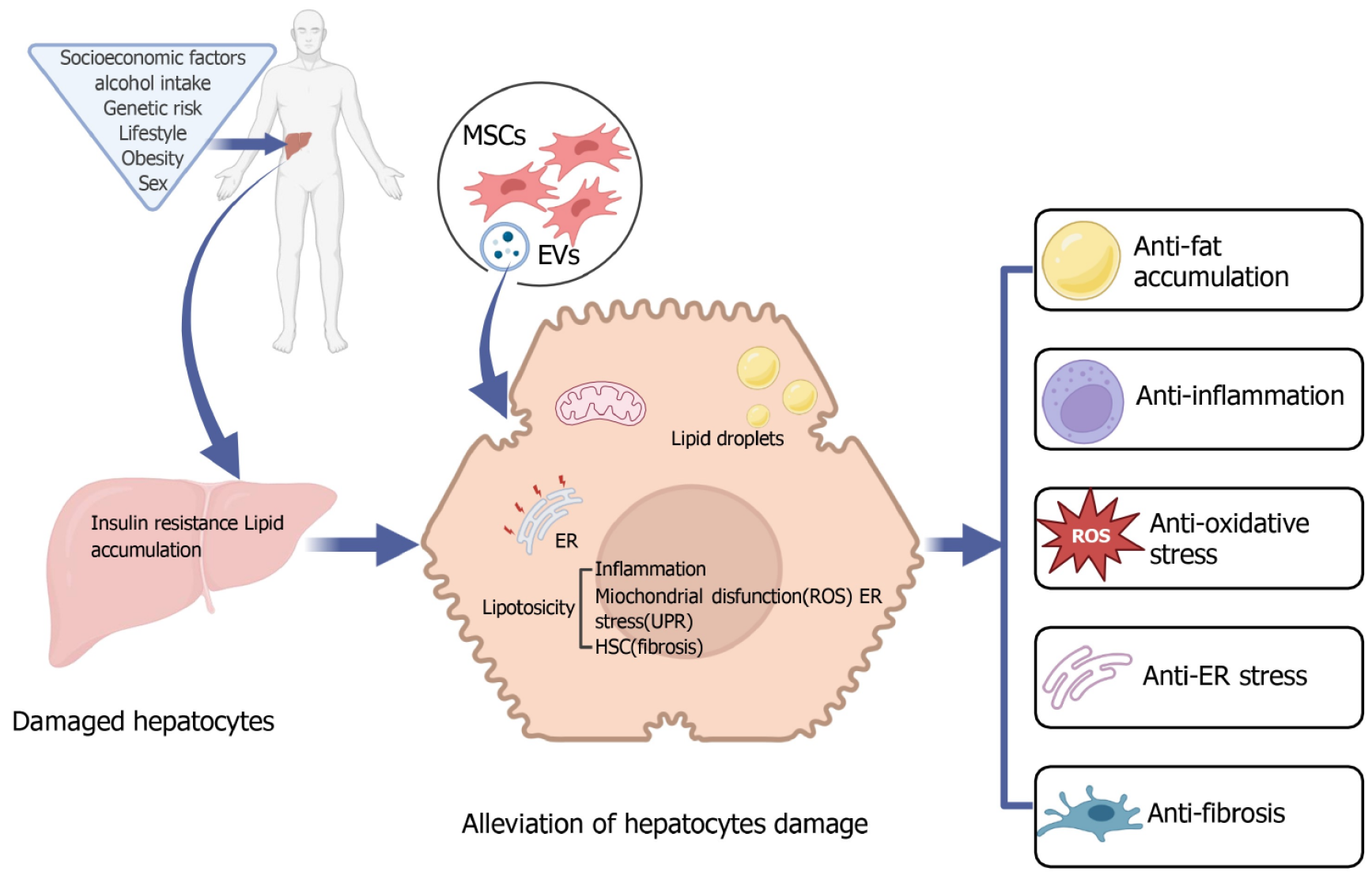
NAFLD/NASH is a leading cause of chronic liver disease, resulting in significant morbidity and mortality. Currently, NAFLD/NASH not only represents a mounting global concern but also signifies a considerable unmet medical need due to the lack of licensed drugs. MSCs are characterized by their rapid proliferation, minimal immunogenicity, and low tumorigenicity. Possessing self-renewal and multi-differentiation capabilities, they provide immunomodulatory and tissue repair functions, primarily through the secretion of trophic factors, cytokines, and chemokines[20]. Due to these beneficial biological characteristics, both MSCs and their derivatives emerge as promising cellular therapies for various diseases, including NAFLD/NASH (Figure 1).
Emerging evidence underscores the central role of liver IR and abnormalities in glucose and lipid metabolism in the pathophysiological features of NAFLD[10]. Various therapeutic measures have been explored to address these challenges. Among these, MSCs and their secretory factors are notable for their ability to mitigate the harmful effects of a high-fat diet on liver health, and address issues such as steatosis, liver dysfunction, and metabolic imbalances in NAFLD rat models and hepatocytes induced by palmitate[33-37]. Among specific types of MSCs, umbilical cord MSCs (UC-MSCs) have been recognized for their positive influence on lipid metabolism. This effect is achieved by their ability to upregulate the expression of genes associated with fatty acid oxidation and lipogenesis, consistent with the observed increased expression of protein in the HNF4α-CES2 pathway[38]. Additionally, exosomal miR-627-5p sourced from UC-MSCs promotes glucose and lipid metabolism, thereby alleviating liver damage in NAFLD. This protective mechanism operates by inhibiting the expression of obesity-associated genes[39]. On the other hand, transplantations involving adipose-derived stem cells (ADSCs) have shown promise in reversing pathological hepatic changes, reducing lipid accumulation, and restoring liver function in NAFLD models[40,41]. Concurrently, ADSC-derived extracellular vesicles (ADSC-EVs), which are rich in miR-223-3p, have been shown to suppress lipid accumulation by targeting the E2F1 gene, suggesting a potential strategy for slowing NAFLD progression[42]. Hepatocyte growth factor derived from menstrual blood-derived stem cells actively downregulates Rnf186 expression, which in turn promotes an increase in hepatic glycogen storage and reduces lipid buildup in NAFLD. This process is mediated through the AMPK-mechanistic target of rapamycin pathway[43].
With respect to NASH-focused research, investigations have shown notable decreases in hepatic lipid content following the administration of MSCs. An innovative method, in which mitochondria from human MSCs are transplanted into mouse hepatocytes with NASH, has been identified as a potential strategy to amplify lipid utilization by enhancing mitochondrial oxidative capacities, regardless of diverse pathogenic incidents[44,45]. Further exploration revealed that the Notch signaling pathway is instrumental in decreasing apoptosis in steatotic hepatocytes and promoting cellular proliferation in hepatocytes from NASH mice following ADSC treatment[46]. Concurrently, cotreatments involving bone marrow-derived MSCs (BMSCs) and BMSC exosomes (BMSC-Exos) led to marked reductions in hepatic steatosis and ballooning in NASH. Specifically, BMSC-Exos (120 μg/kg) displayed significant antisteatotic properties that are characterized by the downregulated expression of fatty acid synthesis genes (SREB1, 2 and ACC) and lipid uptake molecules (CD36), along with the upregulated expression of fatty acid oxidation genes (PPARα and CPT1)[47]. Another investigation highlighted that the fact that antioxidant-fortified MSCs diminished oxidative stress, subsequently reducing fat accumulation in the liver. This strategy has emerged as a potential remedy for conditions such as obesity and prediabetes and might alleviate complications such as NAFLD[48]. Conversely, a separate study presented evidence suggesting that treatments with either MSCs or MSCs-EVs did not significantly change fat accumulation in NASH, as assessed through histological analysis[49]. Despite the promise of MSCs and their secretions in the treatment of liver glucose and lipid imbalances in NAFLD/NASH, more research needs to be done to obtain consistent and reliable outcomes.
Fat accumulation in the liver, which is characteristic of NAFLD/NASH, induces endoplasmic reticulum (ER) stress and oxidative stress, which in turn leads to increased lipid peroxidation and increased levels of ROS. This biochemical disturbance activates inflammatory pathways, prompting an immune response that attracts inflammatory cells to the liver. Once present, these cells release inflammatory cytokines, thereby intensifying liver inflammation and causing subsequent damage. Recent studies in NAFLD mice have illustrated the effectiveness of MSCs and MSC-Exos in significantly reducing inflammatory cell infiltration in liver lobules[33,37]. Similarly, the transplantation of ADSCs has shown profound benefits in NAFLD, notably reducing the expression levels of critical proinflammatory markers such as tumor necrosis factor (TNF)-α and IL-6[41]. Furthermore, MSCs and MSC-conditioned medium (MSC-CM) have demonstrated efficacy in neutralizing inflammation and cellular apoptosis in T2DM/NAFLD, with the protective effect attributed to the Sirt1 protein[36,50]. Additionally, stem cell transplantation results in decreased plasma inflammatory cytokine and low-density lipoprotein levels, while increasing the expression of Sirt1 and HO-1, which contributes to the mitigation of NAFLD progression[51]. In the quest to optimize therapeutic strategies, combining antioxidant treatments with MSC delivery stands out as a promising approach to addressing liver inflammation and offers a comprehensive solution to the challenges posed by obesity, prediabetes, and related complications such as NAFLD[48].
As the therapeutic landscape evolves, the potential of MSCs to mitigate hepatic inflammation in NASH has gained prominence by targeting key mediators. Importantly, the levels of significant contributors to hepatic inflammation, such as TNF-α, tissue inhibitor of metalloproteinases-1, and matrix metalloproteinase-12, were decreased following MSC intervention in NASH mice, thereby effectively reducing inflammation[44]. Moreover, MSC treatment has shown promise in delaying the onset of NASH in obese mice. This protective effect appears to stem from limiting the growth of proinflammatory lymphocyte subgroups and maintaining anti-inflammatory profiles, rather than reversing metabolic syndrome, thus aiding in the mitigation of NASH[52,53]. Another discovery was that MSC transplantation inhibited NASH progression by reducing hepatic inflammation, as demonstrated by the decreased expression of the acute-phase protein serum amyloid A, inflammation-associated markers such as lipocalin 2, and the proinflammatory cytokine TNF-α[54,55]. The ability of ADSCs to hinder NASH progression by mitigating IL-17-mediated and other inflammatory effects underscores their therapeutic potential, showing outcomes comparable to those of both NASH-specific uncultured adipose tissue-derived stromal cells (u-ADSCs) and wild-type u-ADSCs[56,57]. An integrated approach, employing either a combination of MSCs and MSC-EVs or solely MSC-EVs led to a noticeable increase in the number of anti-inflammatory macrophages in the liver, showing their robust immunomodulatory potency in NASH[45,49,58]. From a mechanistic standpoint, MSC-centric treatments exert their anti-inflammatory effects through diverse mechanisms, including modulating the levels of inflammatory cytokines and altering macrophage phenotypes. MSC-Exos promoted anti-inflammatory macrophage phenotypes both in vitro and in vivo, thus emphasizing their therapeutic potential in NASH based on moderating inflammation and restoring cellular balance[59]. Hepatoprotective effects were observed with MSCs from various sources; for instance, both stem cell-derived conditioned medium and amnion-derived MSCs preserved hepatocyte health and inhibited proinflammatory macrophage activation[60,61]. Complementing these findings, antioxidant-fortified MSCs have shown promise in reducing inflammation and alleviating fatty liver conditions associated with diet-triggered obesity[48,62]. Emerging research underscores the transformative potential of MSC-based treatments in mitigating hepatic inflammation in NASH.
In NAFLD, the increased production of ROS causes damage to cellular proteins and DNA, with persistent oxidative stress subsequently leading to cell injury and apoptosis. Concurrently, the accumulation of fatty acids induces ER stress, which interferes with protein folding and, over time, impairs liver function. Importantly, MSC therapies offer significant benefits in these areas. In studies of NAFLD rats and palmitate-induced HepG2 cells, treatment with MSCs adjusted the intracellular calcium balance through SERC modulation, thereby reducing ER stress and enhancing metabolic function[34]. Similarly, transplantation of ADSCs provides dual benefits by alleviating abnormalities in lipid metabolism and oxidative stress in NAFLD[41]. Both in vivo and in vitro studies of diabetes-related NAFLD involving the implantation of BMSCs have revealed significant mitochondrial transfer. The recipient steatotic cells demonstrated increased OXPHOS activity, ATP production, and membrane potential, and reduced ROS levels[35]. Additionally, interventions using MSC-CMs have shown enhanced antioxidant capabilities and improved mitochondrial function via SIRT1-mediated effects in T2DM/NAFLD[50]. Moreover, treatment with MSC-Exos significantly reduced ROS and oxidative stress by targeting KEAP-1 via miR-24-3p in NAFLD[37].
In the pathogenesis of NASH, oxidative stress and ER stress play interconnected and complementary roles, collectively driving the progression of disease. Recent advancements in therapeutic research have highlighted the potential of MSC-centered treatments and emphasized their effectiveness in attenuating oxidative stress and its subsequent effects on NASH. Specifically, MSC therapies have shown their potential for enhancing oxidative capacity to restore metabolic and tissue balance[44,53]. Delving further into this therapeutic framework, hUC-MSC-Exos have been shown to significantly suppress NASH-induced oxidative stress by reducing malondialdehyde, CYP2E1, and ROS levels; enhancing superoxide dismutase and glutathione activities in hepatocytes; increasing the p-nuclear factor erythroid 2-related factor2/nuclear factor erythroid 2-related factor2 protein ratio; and increasing NQO-1 expression[45]. In a parallel therapeutic approach, combined treatment involving BMSCs and BMSC-Exos demonstrated a significant antiapoptotic effect, as indicated by a substantial decrease in the Bax/Bcl2 ratio and increased expression of mitochondrial mitophagy-related genes, including Parkin, PINK1, ULK1, BNIP3 L, ATG5, ATG7, and ATG12 in NASH[47]. Additionally, exosomes derived from curcumin-preconditioned MSCs significantly downregulated the expression of ASK-JNK-BAX genes involved in mitochondrial stress and apoptosis[63]. These findings underscore the complex interplay between oxidative stress, ER stress, and MSC-driven interventions, as well as the effectiveness of these interventions in addressing NASH.
Fibrosis plays a crucial role in the pathogenesis of NAFLD/NASH. Extended liver damage, particularly from excessive fat accumulation, triggers the activation of specialized stellate cells that produce collagen, leading to fibrosis. Emerging research has consistently highlighted the therapeutic potential of MSCs in ameliorating liver fibrosis in NAFLD/NASH. For instance, MSC therapies have led to significant improvements in NASH, with a nearly 50% reduction in collagen content[33,44]. This evidence is supported by other findings that emphasize the therapeutic benefits of MSC trans
A further promising therapeutic avenue involves modulating the gut-liver axis; a conditioned medium derived from stem cells from human exfoliated deciduous teeth (SHED-CM) successfully prevented fibrosis progression in a NASH mouse model[61]. Similarly, ADSC-EVs significantly reduced fiber accumulation and Kupffer cell and HSC activation in rats with NASH[49]. However, the therapeutic outcomes of treatments derived from MSCs are not universally consistent. For instance, there was a significant initial 12.4-fold increase in molecular fibrosis associated with NASH, which returned to normal after three months of treatment with MSC-Exos-curcumin. In contrast, treatments using only MSC-Exos resulted in a temporary 40%-50% reduction in fibrosis, which unfortunately rebounded after three months[63]. Another study suggested that treatment with MSC-EVs effectively reduced liver fibrosis in the NASH mouse model, despite an increase in profibrotic M2 macrophage polarization[58]. Taken together, these findings highlight the immense therapeutic potential of MSCs and their derivatives in treating liver fibrosis associated with NAFLD/NASH, although their effectiveness can vary in magnitude and duration.
MSCs and MSC-derived secretory factors play a crucial role in enhancing insulin sensitivity, reducing hepatic lipid accumulation, and moderating inflammatory responses. Additionally, they effectively counteract oxidative stress and ER stress, which are central contributors to NASH progression, while exhibiting anti-fibrotic capabilities that inhibit disease progression. Given the findings from both in vitro[34,35,37,39,42,43,45,46,50,59,63,64] (Table 1) and in vivo[34-47,49-61,63-66] (Table 2) studies, MSC-based therapies stand out as promising strategies, offering an integrative solution to address and possibly reverse the multifaceted challenges of NAFLD/NASH.
| Cell sources | Passage number | Cell models | Biological effects |
| Rat BM MSCs | P3-4 | HepG2 cells | rMSCs alleviated cellular lipotoxicity and metabolic disturbance, primarily by regulating ER stress and calcium homeostasis via SERCA[34] |
| Mouse BM MSCs | P5-10 | HepG2 cells | mBMSCs restored disordered glucose and lipid metabolism, as well as mitochondrial dysfunction in T2DM/NAFLD[35] |
| Human UC MSCs derived miR-24-3p | Not reported | Primary hepatocytes | hUC-MSC derived miR-24-3p suppressed lipid accumulation, ROS generation, and inflammatory response through targeting KEAP-1 signaling[37] |
| Human UC MSCs derived miR-627-5p | P3 | L-02 cells | hUC-MSC-derived miR-627-5p improved glucose and lipid metabolism by targeting FTO[39] |
| Mouse AD MSCs derived miR-223-3p | P2 or above | NCTC1469 cells | mADSC-EV-derived miR-223-3p exhibited suppressive effects on lipid accumulation and liver fibrosis by inhibiting the target gene E2F1[42] |
| Human MenSCs | P2-3 | L-02/AML12 cells | Hepatocyte growth factor secreted by MenSCs in NAFLD promoted hepatic glycogen storage and attenuated lipid accumulation through the downregulation Rnf186[43] |
| Human UC MSCs derived Exos | Not reported | HepG2/AML12 cells | hUC-MSC-Exos attenuated steatosis in hepatocytes and inhibited oxidative stress in NASH[45] |
| Mouse AD MSCs | P5-6 | Murine hepatocyte cell line H2.35 | mADSCs treatment alleviated lipotoxicity-induced apoptosis in steatotic hepatocytes by activating the Notch signaling pathway[46] |
| Human UC MSCs derived CM | Not reported | L-02 cells | hMSC-CM enhanced liver mitochondrial function while reducing inflammation and apoptosis by upregulating SIRT1[50] |
| Human UC MSCs derived Exos | P4-7 | HepRG cells | hUC-MSC-Exos reduced inflammatory cytokines by inducing macrophage anti-inflammatory phenotypes[59] |
| Human CB MSCs derived Exos with curcumin | Not reported | HepG2 cells | MSC-Exos with curcumin improved cell viability and inhibited lipogenesis, while the anti-apoptotic pathway involved the downregulation of ASK1, JNK, and BAX genes[63] |
| Human UC MSC derived Exos | P3-4 | L-02/AML12 cells | MSC-Exos inhibit lipid accumulation by promoting the β-oxidation of fatty acids and suppressing fatty acid synthesis[64] |
| MSC source | Passage number | Animal models | Other instructions | Biological effect |
| Mouse CB MSCs | P5-8 | Mouse | 1 × 106 cells/mouse injected (i.v.) at weeks 21 and 23 | mMSCs transplantation decreased high-fat-induced weight gain, expansion of subcutaneous adipose tissue, steatosis, lobular inflammation, and liver fibrogenesis[33] |
| Rat BM MSCs | P3-4 | Rat | 2 × 106 cells/rat injected (i.v.) at weeks 18 and 20 | rMSCs administration improved lipid metabolism and insulin sensitivity, and inhibited ER stress in the liver[34] |
| Mouse BM MSCs | P5-10 | Mouse | 1 × 107 cells/kg body weight injected (i.v.) | mBMSCs restored disordered glucose levels, reduced fat accumulation, and corrected mitochondrial dysfunction in mice with diabetes-associated NAFLD[35] |
| Human UC MSCs | P5 | Rat | 1 × 106 cells/rat injected (i.v.) at weeks 1 and 5 | hUC-MSCs in combination with liraglutide improved glycolipid metabolism, insulin resistance, and liver injury in T2DM/NAFLD rats by downregulating TLR4/NF-κB inflammatory pathway and ameliorating oxidative stress[36] |
| Human UC MSCs derived miR-24-3p | Not reported | Mouse | 120 μg/mouse injected (i.v.) weekly for 16 weeks | hUC-MSC-derived miR-24-3p alleviated lipid accumulation, inflammation, and oxidative stress in NAFLD[37] |
| Human UC MSCs | P3 | Mouse | 1 × 106 cells/mouse injected (i.v.) once a week for 6 weeks | hUC-MSCs improved glucose homeostasis and lipid metabolism, and alleviated hepatic steatosis and liver damage in obese T2DM/NAFLD mice[38] |
| Human UC MSCs derived miR-627-5p | P3 | Rat | 100 μg/rat injected (i.v.) once a week for 2 months | hUC-MSC-derived miR-627-5p improved glucose and lipid metabolism, and alleviated liver damage in NAFLD[39] |
| Rat AD MSCs | P3-15 | Rat | 2 × 106 cells/rat injected (p.v.) | rADSCs improved liver function and lipid metabolism, thereby exerting hepatoprotective effects[40] |
| Rat AD MSCs | P3 | Rat | 2 × 106 cells/rat injected (p.v.) | rADSCs improved liver function; reduced lipid accumulation, oxidative stress, and inflammation; and decelerated the progression of NAFLD in the rat model[41] |
| Mouse AD MSCs derived miR-223-3p | P2 or above | Mouse | 100 μg/mouse injected (i.v.) twice a week for last 6 weeks | mADSC-EV-derived miR-223-3p attenuated lipid accumulation and fibrosis by negatively regulating E2F1 expression[42] |
| Human MenSCs | P2-3 | Mouse | 5 × 105 cells/mouse injected (i.v.) at weeks 16, 19, and 22 | Hepatocyte growth factor secreted by MenSCs in fatty liver diseases promoted hepatic glycogen storage and attenuated lipid accumulation in NAFLD[43] |
| Human BM MSCs | P6-15 | Mouse | (0.9-1) × 106 cells/mouse via splenic injection | hBMSCs reduced hepatic lipid content, inflammation, and fibrosis, as well as restored metabolic and tissue homeostasis, by donating human mitochondria to mouse hepatocytes[44] |
| Human UC MSCs derived Exos | Not reported | Mouse | 100 μg/mouse injected (i.v.) twice a week for final 2 weeks | hUC-MSC-Exos attenuated steatosis, inflammatory responses, and oxidative stress in hepatocytes via the Nrf2/NQO-1 pathway[45] |
| Mouse AD MSCs | P5-6 | Mouse | 1 × 105 cells/mouse via splenic injection twice every 2 weeks for 12 weeks | mADSCs reduced apoptosis of steatotic hepatocytes and restored cellular proliferation by activating Notch signaling[46] |
| Rat BM MSCs/ Rat BM MSCs derived Exos | P4 | Rat | 1 × 106 cells/mouse injected (i.v.)/15/30/120 μg/kg body weight injected (i.v.) twice per week for 6 weeks | rBMSCs and rBMSC-Exos reduced lipid accumulation, hepatotoxicity, oxidation, and hepatocyte apoptosis, and activated mitochondrial mitophagy[47] |
| Human AD MSCs/ Human AD MSCs derived EVs | P4 | Mouse | 1 × 106 cells/mouse injected (i.v.)/ 1.0/2.5/5.0 μg injected (i.v.) at week 12 | hADSCs or hADSC-EVs exhibited anti-inflammatory and anti-fibrotic effects in the NASH model[49] |
| Human UC MSCs derived CM | Not reported | Mouse | 200 μL cells/mouse injected (i.v.) every 3 days for 2 months | hMSC-CM improved glucose tolerance, insulin sensitivity, and mitochondrial function, and alleviated liver dysfunction, lipid accumulation, inflammation, and apoptosis by upregulating SIRT1[50] |
| Human BM MSCs | Not reported | Mouse | 1 × 107 cells/mouse injected (p.v.) | hMSCs decreased the inflammatory cytokines, LDL levels, IR, and oxidative stress in NAFLD with T2DM[51] |
| Mouse BM MSCs | P3 | Mouse | 0.5 × 106 cells/mouse injected (i.v.) at weeks 33 and 37 | mMSCs administration prevented the onset of NASH in obese mice[52] |
| Murine CB MSCs | P5-8 | Mouse | 1 × 106 cells/mouse injected (i.v.) at weeks 6 and 7 | mMSCs reduced weight loss, hepatic lipid peroxidation, steatosis, ballooning, lobular inflammation, and fibrogenesis in NASH[53] |
| Human BM MSCs | Not reported | Mouse | 1.5 × 106 cells/mouse injected (i.v.) at day 42 | Hepatocyte-like cells derived from hBMSCs attenuated liver lipid accumulation and inflammation, and enhanced the regenerative capacity of the liver in NASH[54] |
| Human UC MSCs | Not reported | Mouse | 1 × 106 cells/mouse injected (i.v.) at week 10 | hMSCs alleviated hepatic steatosis, inflammation, and fibrosis, and reversed microbiome and metabolome disorders[55] |
| Uncultured mouse AD MSCs | Not reported | Mouse | 1 × 106/7.5 × 105 cells/mouse via splenic injection at weeks 24 and 26 | u-ADSCs derived from a NASH mouse model and wild-type mice had similar effects in reducing inflammation and fibrosis in NASH[56] |
| Mouse AD MSCs | Not reported | Mouse | 1 × 105 cells/mouse via splenic injected at weeks 4 and 8 | mADSCs administration prevented the progression of NASH fibrosis by suppressing IL-17-mediated inflammation[57] |
| Human ESC MSCs derived EVs | Not reported | Mouse | 1 and 10 μg/50 μL/mouse (i.p.) every other day for last 4 weeks | hMSC-EVs increased the number of anti-inflammatory M2 macrophages and suppressed fibrosis in NASH[58] |
| Human UC MSCs derived Exos | P4-7 | Mouse | 20 mg/kg body weight injected (i.v.) twice a week for 6 weeks | hUC-MSC-Exos regulated the anti-inflammatory phenotype of macrophages and reversed PPARα protein expression in liver cells[59] |
| Human AM MSCs derived EVs | Not reported | Rat | 15 μg/kg body weight injected (i.v.) at weeks 3 and 4 | AMSC-EVs alleviated inflammation and fibrosis in a NASH rat model[60] |
| Human SHEDs derived CM | P8-12 | Mouse | 0.5 mL cells/mouse injected (i.v.) once a week from week 10 to 12 | SHED-CM treatment inhibited liver fibrosis, inflammation, and parenchymal cell apoptosis in NASH[61] |
| Human CB MSCs derived Exos with curcumin | Not reported | Mouse | 15 μg/kg body weight injected (i.v.) | Exosomes derived from curcumin-preconditioned MSCs ameliorated NASH, protected against recurrence, and regulated inflammatory response, oxidative stress, and mitochondrial-dependent apoptosis[63] |
| Human UC MSC derived Exos | P3-4 | Mouse | 10 mg/kg body weight injected (i.v.) for last 4 weeks | hUC-MSC-Exos effectively reduced lipid deposition and improved liver function in an NAFLD mouse model via CAMKK1-mediated regulation of lipid homeostasis[64] |
| Rat AD MSCs stimulated with LPS | P3-5 | Rat | 1.5 × 106 cells/rat injected (i.v.) at week 8 for 6 weeks | ADSCs stimulated with LPS showed potential to alleviate NAFLD by reducing the expression of inflammatory genes and the levels of ROS[65] |
| Human UC MSCs | P5 | Mouse | 1.5 × 106 cells/mouse injected (i.v.) at week 32 | hUC-MSCs administration alleviated obesity, improved glucose metabolism, and reduced hepatic steatosis, inflammation, and fibrosis in NASH[66] |
In the context of NAFLD/NASH treatment, MSCs have emerged as a promising therapeutic avenue due to their unique cellular properties and demonstrated efficacy. They can be applied to address a range of pathological challenges, including glycemic-lipid metabolism, inflammation, oxidative stress, ER stress, and fibrosis. Emerging research has consistently highlighted the potential of MSCs in inhibiting the progression of NAFLD/NASH. Importantly, these mechanisms are not biologically isolated but exhibit intersecting and complementary mechanisms, which enhance the effects of one another. For instance, MSC intervention can directly alleviate fat accumulation in glycemic lipid metabolism, subsequently reducing hepatic inflammatory responses[50]. This reduction in inflammation further leads to the suppression of oxidative stress[45]. Furthermore, the intrinsic antioxidative capabilities of MSCs may provide additional reinforcement against oxidative challenges and aid in alleviating ER stress caused by excessive protein accumulation, thereby promoting the functional balance of hepatocytes[34]. Additionally, MSCs can indirectly inhibit the activation of HSCs by impacting inflammation and glycemic-lipid metabolism, thus preventing the onset of fibrosis[44]. Consequently, the multifaceted mechanisms of MSCs may synergize, creating a comprehensive, multilevel therapeutic approach for NAFLD/NASH patients.
Considering the complex and multifactorial nature of NAFLD/NASH, relying solely on MSC-based treatments may not adequately address the disease. Consequently, integrating MSCs with various strategies, such as pharmacotherapy, lifestyle modifications, gene therapy, cell therapy, and biomaterials, has emerged as a research priority, with the aim of developing a holistic approach to this disease. For instance, combining MSCs with specific anti-inflammatory and antioxidant drugs could enhance their therapeutic efficacy, potentially reducing drug dosages and associated side effects[63]. Adopting beneficial dietary habits, engaging in regular exercise, and implementing weight loss strategies are crucial for improving liver function in individuals with NAFLD/NASH[16]. Significantly, weight reduction directly contributes to decreased fat accumulation in the liver. Therefore, integrating MSC therapy with these lifestyle adjustments may synergistically improve the therapeutic outcomes of patients. Gene engineering provides renewed perspectives and innovative insights for NAFLD treatment[67], and its integration with MSC therapies opens up new therapeutic possibilities. Furthermore, combining MSCs with other cells with therapeutic potential or using biomaterials as delivery mechanisms may further refine and enhance the effectiveness of treatment[47,63]. These multifaceted strategies promise a more holistic, efficient, and tailored approach to treating NAFLD. However, the safety and efficacy of these integrated strategies require further clinical validation.
Although the use of MSCs for treating NAFLD/NASH is promising, practical applications still face a series of challenges and limitations. The variability in the therapeutic properties of MSCs associated with their origin is essential, yet comprehensive studies comparing their efficacy in treating NAFLD are lacking. This diversity necessitates careful research for optimal source selection[26]. Moreover, the survival and homing capabilities of MSCs are compromised under challenging conditions such as low oxygen tension, fluid pressure stress, and interactions with whole blood components postinjection. This process is further complicated by hypoxia, oxidative stress, and inflammation at targeted sites[68]. Alarmingly, significant concerns regarding potential risks, including tumorigenesis and adverse immune reactions must be considered. Certain MSCs, particularly BMSCs that have not been genetically modified, may exhibit chromosomal abnormalities even during early passages, potentially leading to the formation of malignant tumors[69]. Furthermore, considering the relationship between the injection frequency, dosage, and therapeutic effect of MSCs in maintaining long-term efficacy is crucial. Moreover, determining whether single or cotreatments with MSCs might have adverse effects is essential for their appropriate application in treating NAFLD[70].
MSC-EV or MSC-Exo therapy is superior to cell therapy in terms of safety, efficacy, and versatility, and reduces the potential risks of long-term mal-differentiation of engrafted cells and tumor formation[70]. However, several challenges must be addressed before this strategy can be used in clinical practice. For instance, the rapid clearance of MSC-Exos from the body might limit their long-term therapeutic effects[71]. In addition, the heterogeneity of MSC-Exos resulting from different culture conditions and cell passages poses another challenge. Therefore, standardized isolation protocols and defined culture conditions are required to produce more homogenous populations of MSC-Exos[72,73]. Another issue that requires resolution is the long-term storage, preservation, and transportation of exosomes, including whether lyophilization alters the characteristics of exosomes[70,73]. Moreover, current efforts focus on using encapsulation to increase longevity in the body[74]. For example, with advancements in bioengineering and cellular manipulation technologies, the upcoming trend regarding exosome utilization will involve engineering exosomes to be more specific and applicable in highly complicated areas of medicine[75].
MSCs and their derived secretions have shown significant potential benefits for the treatment of NAFLD/NASH. However, research on the treatment of NAFLD with MSCs is still in the exploratory stage prior to widespread clinical application. The number of studies on treating NAFLD with MSCs is small, and most of these studies are based on rodents. Thus, the scope of research needs to be expanded further using larger animals[70]. In addition, the specific therapeutic mechanisms and targets of MSCs and intrinsic liver cells are unclear; questions such as how MSCs aid in repairing damaged liver, regulate immune responses, and interact with other liver cells, such as HSCs and Kupffer cells, should be answered[23]. Importantly, the different progression stages of NAFLD and the specific conditions of patients may require different treatment strategies[46]. Further study is required to investigate whether MSCs retain the same therapeutic properties in clinical settings as they do in vivo and in vitro.
In summary, MSCs offer a promising therapeutic direction for NAFLD/NASH through the alteration of underlying molecular pathways, including glycolipid metabolism, inflammation, oxidative stress, ER stress, and fibrosis. By thoroughly understanding their mechanisms of action and integrating them with other treatment modalities, more effective and safer therapeutic strategies may be developed. As we advance in this field of research, it is imperative that we continuously monitor the outcomes of MSCs in clinical trials, ensuring positive results not only in laboratory settings but also in real-world clinical environments.
| 1. | Allen AM, Lazarus JV, Younossi ZM. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J Hepatol. 2023;79:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1502] [Article Influence: 250.3] [Reference Citation Analysis (0)] |
| 3. | Domingues I, Leclercq IA, Beloqui A. Nonalcoholic fatty liver disease: Current therapies and future perspectives in drug delivery. J Control Release. 2023;363:415-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Shi J, Zhao YC, Niu ZF, Fan HJ, Hou SK, Guo XQ, Sang L, Lv Q. Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: Progress and prospect. World J Stem Cells. 2021;13:49-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Han J, Lee C, Hur J, Jung Y. Current Therapeutic Options and Potential of Mesenchymal Stem Cell Therapy for Alcoholic Liver Disease. Cells. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 349] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 7. | Johnston MP, Patel J, Byrne CD. Causes of Mortality in Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcohol Related Fatty Liver Disease (AFLD). Curr Pharm Des. 2020;26:1079-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J; NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 708] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 9. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1217] [Article Influence: 304.3] [Reference Citation Analysis (0)] |
| 10. | Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From "two hit theory" to "multiple hit model". World J Gastroenterol. 2018;24:2974-2983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (5)] |
| 11. | Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 12. | Nassir F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 13. | Tsigalou C, Konstantinidis T, Paraschaki A, Stavropoulou E, Voidarou C, Bezirtzoglou E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 15. | Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. 2022;292:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 157] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 16. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 333] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 17. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1172] [Article Influence: 586.0] [Reference Citation Analysis (1)] |
| 18. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 574] [Article Influence: 191.3] [Reference Citation Analysis (1)] |
| 19. | Fernández Vallone VB, Romaniuk MA, Choi H, Labovsky V, Otaegui J, Chasseing NA. Mesenchymal stem cells and their use in therapy: what has been achieved? Differentiation. 2013;85:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 21. | Zhao H, Li Z, Wang Y, Zhou K, Li H, Bi S, Wang Y, Wu W, Huang Y, Peng B, Tang J, Pan B, Wang B, Chen Z, Zhang Z. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front Cell Dev Biol. 2023;11:1029671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 22. | Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, Chen L. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (38)] |
| 23. | Hu C, Wu Z, Li L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci. 2020;16:893-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Qian X, An N, Ren Y, Yang C, Zhang X, Li L. Immunosuppressive Effects of Mesenchymal Stem Cells-derived Exosomes. Stem Cell Rev Rep. 2021;17:411-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J, Guo L, Zhang X, Zhou X, Xu X, Yan X, Wang Y, Zhang J, Xu A, Tse HF, Lian Q. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13:580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 301] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 26. | Takayama Y, Kusamori K, Nishikawa M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin Drug Deliv. 2021;18:1627-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells. 2020;12:814-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Dong ZF, Zhang JY. Immunomodulatory role of mesenchymal stem cells in Alzheimer's disease. Life Sci. 2020;246:117405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 508] [Article Influence: 169.3] [Reference Citation Analysis (0)] |
| 30. | Behnke J, Kremer S, Shahzad T, Chao CM, Böttcher-Friebertshäuser E, Morty RE, Bellusci S, Ehrhardt H. MSC Based Therapies-New Perspectives for the Injured Lung. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 31. | Lv X, Wang L, Zou X, Huang S. Umbilical Cord Mesenchymal Stem Cell Therapy for Regenerative Treatment of Rheumatoid Arthritis: Opportunities and Challenges. Drug Des Devel Ther. 2021;15:3927-3936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Tyndall A, Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009;43:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Wang H, Zhang H, Huang B, Miao G, Yan X, Gao G, Luo Y, Chen H, Chen W, Yang L. Mesenchymal stem cells reverse high-fat diet-induced non-alcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol Med Rep. 2018;17:3769-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Li L, Zeng X, Liu Z, Chen X, Li L, Luo R, Liu X, Zhang J, Liu J, Lu Y, Cheng J, Chen Y. Mesenchymal stromal cells protect hepatocytes from lipotoxicity through alleviation of endoplasmic reticulum stress by restoring SERCA activity. J Cell Mol Med. 2021;25:2976-2993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Bi Y, Guo X, Zhang M, Zhu K, Shi C, Fan B, Wu Y, Yang Z, Ji G. Bone marrow derived-mesenchymal stem cell improves diabetes-associated fatty liver via mitochondria transformation in mice. Stem Cell Res Ther. 2021;12:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Xu X, Wang W, Lin L, Chen P. Liraglutide in combination with human umbilical cord mesenchymal stem cell could improve liver lesions by modulating TLR4/NF-kB inflammatory pathway and oxidative stress in T2DM/NAFLD rats. Tissue Cell. 2020;66:101382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Du X, Li H, Han X, Ma W. Mesenchymal stem cells-derived exosomal miR-24-3p ameliorates non-alcohol fatty liver disease by targeting Keap-1. Biochem Biophys Res Commun. 2022;637:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 38. | Li B, Cheng Y, Yu S, Zang L, Yin Y, Liu J, Zhang L, Mu Y. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Nonalcoholic Fatty Liver Disease in Obese Type 2 Diabetic Mice. Stem Cells Int. 2019;2019:8628027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Cheng L, Yu P, Li F, Jiang X, Jiao X, Shen Y, Lai X. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 40. | Liao N, Pan F, Wang Y, Zheng Y, Xu B, Chen W, Gao Y, Cai Z, Liu X, Liu J. Adipose tissue-derived stem cells promote the reversion of non-alcoholic fatty liver disease: An in vivo study. Int J Mol Med. 2016;37:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Pan F, Liao N, Zheng Y, Wang Y, Gao Y, Wang S, Jiang Y, Liu X. Intrahepatic transplantation of adipose-derived stem cells attenuates the progression of non-alcoholic fatty liver disease in rats. Mol Med Rep. 2015;12:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Niu Q, Wang T, Wang Z, Wang F, Huang D, Sun H, Liu H. Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte. 2022;11:572-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Du J, Jiang Y, Liu X, Ji X, Xu B, Zhang Y, Liu Y, Zhang T, Lin J. HGF Secreted by Menstrual Blood-Derived Endometrial Stem Cells Ameliorates Non-Alcoholic Fatty Liver Disease Through Downregulation of Hepatic Rnf186. Stem Cells. 2023;41:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 44. | Nickel S, Christ M, Schmidt S, Kosacka J, Kühne H, Roderfeld M, Longerich T, Tietze L, Bosse I, Hsu MJ, Stock P, Roeb E, Christ B. Human Mesenchymal Stromal Cells Resolve Lipid Load in High Fat Diet-Induced Non-Alcoholic Steatohepatitis in Mice by Mitochondria Donation. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 45. | Kang Y, Song Y, Luo Y, Song J, Li C, Yang S, Guo J, Yu J, Zhang X. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radic Biol Med. 2022;192:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 46. | Ishida K, Seki A, Kawaguchi K, Nasti A, Yamato M, Inui H, Komura T, Yamashita T, Arai K, Yamashita T, Mizukoshi E, Honda M, Wada T, Harada K, Kaneko S, Sakai Y. Restorative effect of adipose tissue-derived stem cells on impaired hepatocytes through Notch signaling in non-alcoholic steatohepatitis mice. Stem Cell Res. 2021;54:102425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | El-Derany MO, AbdelHamid SG. Upregulation of miR-96-5p by bone marrow mesenchymal stem cells and their exosomes alleviate non-alcoholic steatohepatitis: Emphasis on caspase-2 signaling inhibition. Biochem Pharmacol. 2021;190:114624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Domingues CC, Kundu N, Kropotova Y, Ahmadi N, Sen S. Antioxidant-upregulated mesenchymal stem cells reduce inflammation and improve fatty liver disease in diet-induced obesity. Stem Cell Res Ther. 2019;10:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Watanabe T, Tsuchiya A, Takeuchi S, Nojiri S, Yoshida T, Ogawa M, Itoh M, Takamura M, Suganami T, Ogawa Y, Terai S. Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regen Ther. 2020;14:252-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 50. | Yang M, Cui Y, Song J, Cui C, Wang L, Liang K, Wang C, Sha S, He Q, Hu H, Guo X, Zang N, Sun L, Chen L. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem Biophys Res Commun. 2021;546:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Li M, Guo K, Vanella L, Taketani S, Adachi Y, Ikehara S. Stem cell transplantation upregulates Sirt1 and antioxidant expression, ameliorating fatty liver in type 2 diabetic mice. Int J Biol Sci. 2015;11:472-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Ezquer M, Ezquer F, Ricca M, Allers C, Conget P. Intravenous administration of multipotent stromal cells prevents the onset of non-alcoholic steatohepatitis in obese mice with metabolic syndrome. J Hepatol. 2011;55:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Wang H, Wang D, Yang L, Wang Y, Jia J, Na D, Chen H, Luo Y, Liu C. Compact bone-derived mesenchymal stem cells attenuate nonalcoholic steatohepatitis in a mouse model by modulation of CD4 cells differentiation. Int Immunopharmacol. 2017;42:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Winkler S, Borkham-Kamphorst E, Stock P, Brückner S, Dollinger M, Weiskirchen R, Christ B. Human mesenchymal stem cells towards non-alcoholic steatohepatitis in an immunodeficient mouse model. Exp Cell Res. 2014;326:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Yang Z, Xia Q, Lu D, Yue H, Zhang J, Li Y, Zhang B, Li X, Cao M. Human mesenchymal stem cells treatment improved hepatic lesions and reversed gut microbiome disorder in non-alcoholic steatohepatitis. Aging (Albany NY). 2020;12:21660-21673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Yano M, Nasti A, Seki A, Ishida K, Yamato M, Inui H, Ogawa N, Inagaki S, Ho TTB, Kawaguchi K, Yamashita T, Arai K, Yamashita T, Mizukoshi E, Inoue O, Takashima S, Usui S, Takamura M, Honda M, Wada T, Kaneko S, Sakai Y. Characterization of adipose tissue-derived stromal cells of mice with nonalcoholic fatty liver disease and their use for liver repair. Regen Ther. 2021;18:497-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Yamato M, Sakai Y, Mochida H, Kawaguchi K, Takamura M, Usui S, Seki A, Mizukoshi E, Yamashita T, Yamashita T, Ishida K, Nasti A, Tuyen HTB, Komura T, Yoshida K, Wada T, Honda M, Kaneko S. Adipose tissue-derived stem cells prevent fibrosis in murine steatohepatitis by suppressing IL-17-mediated inflammation. J Gastroenterol Hepatol. 2019;34:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Zhang B, Zhang B, Lai RC, Sim WK, Lam KP, Lim SK. MSC-sEV Treatment Polarizes Pro-Fibrotic M2 Macrophages without Exacerbating Liver Fibrosis in NASH. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 59. | Shi Y, Yang X, Wang S, Wu Y, Zheng L, Tang Y, Gao Y, Niu J. Human umbilical cord mesenchymal stromal cell-derived exosomes protect against MCD-induced NASH in a mouse model. Stem Cell Res Ther. 2022;13:517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 60. | Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, Fu Q, Maehara O, Suda G, Sakamoto N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018;2018:3212643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 61. | Muto H, Ito T, Tanaka T, Yokoyama S, Yamamoto K, Imai N, Ishizu Y, Maeda K, Honda T, Ishikawa T, Kato A, Ohshiro T, Kano F, Yamamoto A, Sakai K, Hibi H, Ishigami M, Fujishiro M. Conditioned medium from stem cells derived from human exfoliated deciduous teeth ameliorates NASH via the Gut-Liver axis. Sci Rep. 2021;11:18778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Lopez-Yus M, García-Sobreviela MP, Del Moral-Bergos R, Arbones-Mainar JM. Gene Therapy Based on Mesenchymal Stem Cells Derived from Adipose Tissue for the Treatment of Obesity and Its Metabolic Complications. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Tawfeek GA, Kasem HA. Curcumin preconditioned mesenchymal stem cells derived exosomes transplantation ameliorate and protect against non- alcoholic steatohepatitis by regulation the expression of key genes of inflammation and oxidative stress. Transpl Immunol. 2023;78:101837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 64. | Yang F, Wu Y, Chen Y, Xi J, Chu Y, Jin J, Yan Y. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate liver steatosis by promoting fatty acid oxidation and reducing fatty acid synthesis. JHEP Rep. 2023;5:100746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 65. | Afarin R, Aslani F, Asadizade S, Jaberian Asl B, Mohammadi Gahrooie M, Shakerian E, Ahangarpour A. The Effect of Lipopolysaccharide-Stimulated Adipose-Derived Mesenchymal Stem Cells on NAFLD Treatment in High-Fat Diet-Fed Rats. Iran J Pharm Res. 2023;22:e134807. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Hu J, Li S, Zhong X, Wei Y, Sun Q, Zhong L. Human umbilical cord mesenchymal stem cells attenuate diet-induced obesity and NASH-related fibrosis in mice. Heliyon. 2024;10:e25460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 67. | Kim U, Kim N, Shin HY. Modeling Non-Alcoholic Fatty Liver Disease (NAFLD) Using "Good-Fit" Genome-Editing Tools. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Yu S, Yu S, Liu H, Liao N, Liu X. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. 2023;14:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 69. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 70. | Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 71. | Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 72. | Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, Wong SHD. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics. 2022;12:207-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 73. | Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 197] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 74. | Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 75. | Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |