Published online Jul 26, 2024. doi: 10.4252/wjsc.v16.i7.742
Revised: May 28, 2024
Accepted: June 24, 2024
Published online: July 26, 2024
Processing time: 128 Days and 22.3 Hours
Wharton’s jelly mesenchymal stem cells (WJ-MSCs) are gaining significant attention in regenerative medicine for their potential to treat degenerative diseases and mitigate radiation injuries. WJ-MSCs are more naïve and have a better safety profile, making them suitable for both autologous and allogeneic transplantations. This review highlights the regenerative potential of WJ-MSCs and their clinical applications in mitigating various types of radiation injuries. In this review, we will also describe why WJ-MSCs will become one of the most probable stem cells for future regenerative medicine along with a balanced view on their strengths and weaknesses. Finally, the most updated literature related to both preclinical and clinical usage of WJ-MSCs for their potential application in the regeneration of tissues and organs will also be compiled.
Core Tip: Stem cells, particularly Wharton’s jelly mesenchymal stem cells (WJ-MSCs), are pivotal in cell-based therapy due to their robust tissue repair abilities. While radiotherapy is a common cancer treatment, it often causes collateral damage to healthy tissues, reducing its efficacy. WJ-MSCs, resembling embryonic stem cells, exhibit superior differentiation and safety, making them ideal for both autologous and allogeneic transplants. This review emphasizes WJ-MSCs’ regenerative potential and clinical utility in alleviating radiation-induced injuries resulting from radiotherapy across various cancer types.
- Citation: Sharma P, Maurya DK. Wharton’s jelly mesenchymal stem cells: Future regenerative medicine for clinical applications in mitigation of radiation injury. World J Stem Cells 2024; 16(7): 742-759
- URL: https://www.wjgnet.com/1948-0210/full/v16/i7/742.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i7.742
Cancer cells are very aggressive in nature and hold their place in the top five most common causes of death worldwide[1]. Some of the common strategies being used in cancer treatment include radiotherapy (RT), chemotherapy, surgery, and their combinations. However, nowadays, RT is one of the main treatment modalities for treating cancer patients, either alone or in combination with chemotherapy and surgery[2]. RT is used as a definitive treatment or employed either to reduce tumor size before surgery or after surgery to eradicate small masses of tumor cells that remain after surgery, depending on the type of cancer[3,4]. There are diverse types of radiation therapies, such as external beam RT, brachytherapy, systemic radioisotope therapy, stereotactic body RT, stereotactic radiosurgery, proton, heavy particles RT, as well as fractionation regimens (e.g., hypofractionation, hyperfractionation, and accelerated fractionation)[5]. RT is one of the preferred treatment options for patients with solid tumors. While RT invariably exposes healthy cells to radiation along with the cancer cells and leads to different types of radiation injuries, several advancements, such as intensity-modulated RT (IMRT) or image-guided RT, have significantly reduced the normal tissue damage associated with conventional RT. However, healthy tissues lying in the path of radiation still get exposed. Therefore, there is still a need for a treatment modality that can regenerate the tissue damage caused by radiation exposure during treatment.
Currently, researchers have identified several strategies to address normal tissue damage caused by RT, including the development of radiation protectors and mitigators. Radioprotective agents, such as amifostine, protect normal tissues by scavenging free radicals and enhancing DNA repair, although further exploration is needed to fully understand their mechanisms and minimize side effects[6]. Fractionation, which involves dividing the total radiation dose into smaller sessions, allows normal cells time to repair, yet optimal schedules and individualized responses are areas for deeper study[7]. Advanced radiation techniques like IMRT and image-guided RT provide precise targeting to spare healthy tissues, though their long-term impacts and best practices require further investigation. Proton therapy, which precisely deposits radiation at the tumor site, minimizes collateral damage, but its cost-effectiveness and comparative efficacy need more comprehensive evaluation[8]. Present interventions for radiation-induced normal tissue damage include physical modalities, such as modified collimators and fractionation schedules, and pharmacological agents like essential fatty acids, vasoactive drugs, and antioxidants[9]. However, these procedures need more standardization. Notably, stem cell therapy, especially with mesenchymal stem cells (MSCs) has emerged as a highly promising approach for promoting tissue repair and regeneration post-radiation exposure[10]. Radiation-driven injuries cause significant damage at the cellular and molecular levels, leading to severe inflammation, tissue destruction, and impaired healing processes[11]. The body’s response to such injuries involves the release of cytokines and chemokines, which play crucial roles in signaling and attracting stem cells to the damaged sites[12]. These molecular mechanisms create an environment conducive to stem cell therapies, as the recruited stem cells can differentiate into various cell types, promote tissue regeneration, and favorably modulate inflammatory responses. This makes stem cell therapy particularly suitable for treating radiation-induced damage, offering potential for effective repair and recovery of affected tissues[10]. The potential of MSCs to enhance healing while minimizing adverse effects marks a significant advancement in RT support, though further research is needed to optimize cell types, dosages, and delivery methods[10].
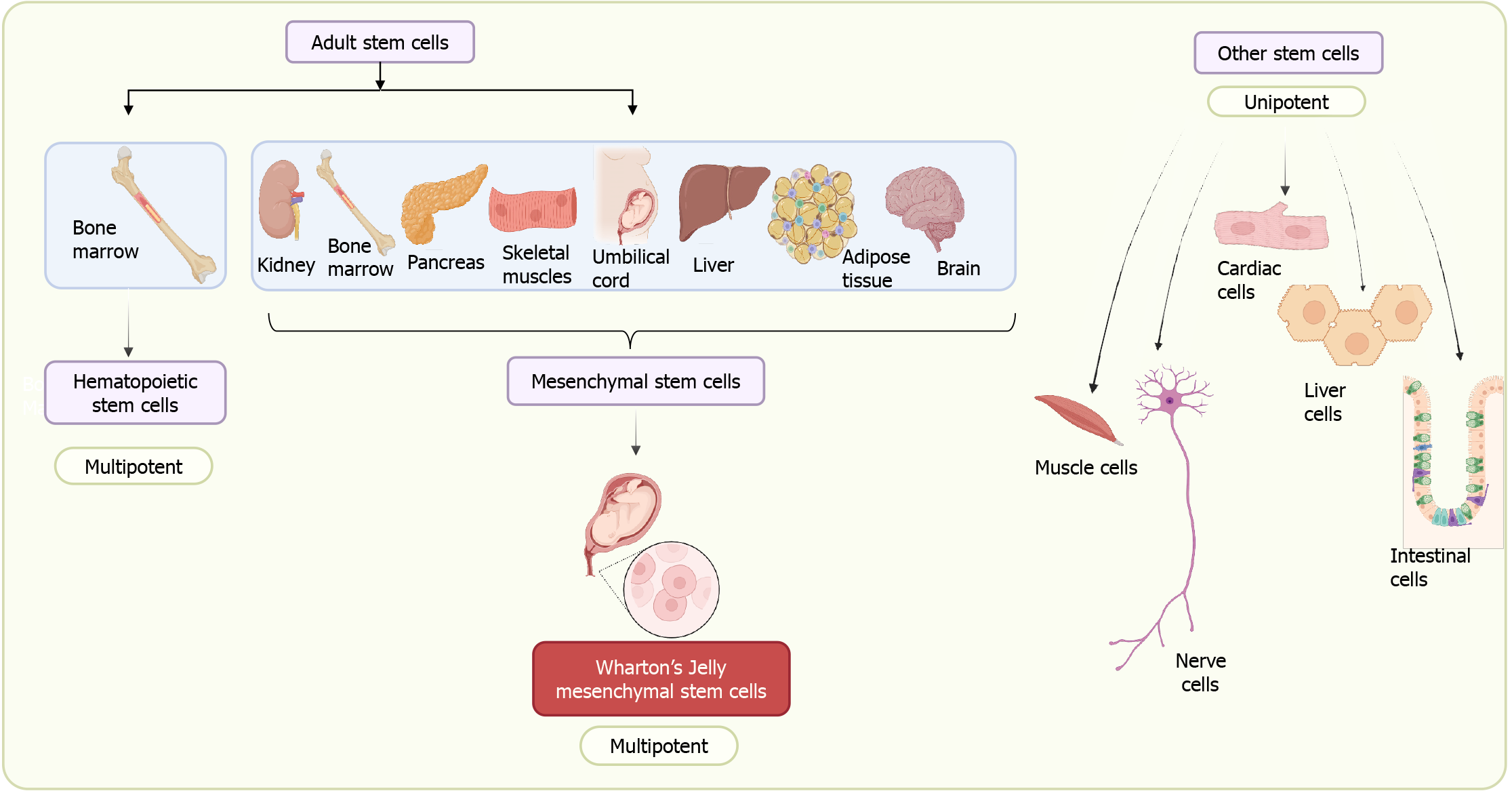
In the last two decades, the use of stem cells in the field of regenerative medicine has significantly increased because of their tremendous regenerative potential. Stem cells became integral to modern regenerative medicine in the 1950s, notably with the first successful bone marrow transplantation in 1956. This breakthrough hinted at future treatment possibilities, encouraging the refinement of clinical techniques[13]. While today stem cells are at the forefront of regenerative medicine with their unlimited division potential and ability to trans-differentiate, they hold promise as a leading source for repairing tissues and organs[14]. Several clinical trials are currently underway in the field of stem cell therapeutics[15]. Until now, stem cells have been isolated from various sources ranging from blastocysts to adult tissues. We can now induce the dedifferentiation of adult cells into pluripotent stem cells by expressing the pluripotency transcription factors Sox2, Oct3/4, cMyc, and Klf4[16]. Stem cells are categorized into embryonic stem cells (ESCs) and adult stem cells (ASCs) including fetal stem cells according to their respective origins[17]. Induced pluripotent stem cells (iPSCs) represent a class of pluripotent stem cells that can be generated from adult somatic cells through a process of “reprogramming”, accomplished by the transduction of pluripotency genes[16]. The isolation of ESCs poses many ethical issues compared to ASCs. At the same time, ESCs/iPSCs have limitations due to associated risks of immune rejection, teratoma formation, and tumorigenesis[18]. Different sources of stem cells have inherent advantages and disadvantages in terms of their derivation, potency, and biological efficacy[16-21] (Table 1). ASCs also referred to as somatic or tissue stem cells, are uncommon cell populations residing in the body throughout a significant portion of postnatal life. These cells play a crucial role in generating a limited range of mature cell types specific to the tissue they inhabit. These are again majorly classified into hematopoietic stem cells (HSCs) and MSCs on the basis of origin (Figure 1). HSCs isolated from the bone marrow have limited plasticity and can only differentiate into blood and blood-related lineages. On the other hand, MSCs are adaptable stromal cells with multipotent characteristics, possessing the ability to differentiate into various cell types such as adipocytes, myocytes, osteocytes, and chondrocytes[22]. The isolation of bone marrow MSCs (BM-MSCs) in the 1960s-1970s opened up new possibilities for their application[18] and has become one of the most studied MSCs since[19,23]. Subsequently, alternative sources of MSCs were explored, such as adipose tissue, dental pulps, and extra-embryonic tissues like the placenta, umbilical cord, and amnion. Table 2 shows a comparison of the three most commonly used MSCs. MSCs isolated from extra-embryonic tissues are more naïve and share features with ESCs compared to other MSCs. They have immuno-privileged characteristics, possess broader multipotent plasticity, and proliferate faster compared to adult MSCs[20,24]. The isolation of stem cells from the umbilical cord opens up several opportunities in the field of regenerative medicine. In this review, we mainly focus on MSCs isolated from Wharton’s jelly (WJ) of umbilical cord, which have tremendous therapeutic potential due to their inherent repair and regenerative abilities. Our emphasis will be on the possible applications of umbilical cord MSCs (UC-MSCs) in mitigating radiation injuries. We will first discuss their origin and unique features and then explore their possible applications in the treatment of different types of radiation injuries and their underlying mechanisms. Finally, we will discuss the challenges and future perspectives of MSCs found in the umbilical cord.
| Characteristics | Embryonic stem cells | Induced pluripotent stem cells | Adult stem cells | Ref. |
| Origin | Inner cell mass of blastocyst | Somatic cells | Postnatal adult tissue | [16] |
| Potency | Pluripotent | Pluripotent | Multipotent | [17] |
| Self-renewal | Yes | Yes | Limited | [17] |
| Teratoma formation | Yes | Yes | No | [18] |
| Tumorigenesis | Yes | Yes | No | [18] |
| Immune response | Immuno-privileged MHC-I and II present in low level | Not immuno-privileged MHC-I and II present in normal level | MSCs are immuno-privileged and immunosuppressive in nature | [18,19,20,21,22] |
| Ethical issue | Serious ethical issue | No ethical issue | No ethical issue | [21] |
| Characteristics | BM-MSCs | AD-MSCs | UC-MSCs | Ref. |
| Harvesting procedure | Invasive | Invasive | Non-invasive | [18] |
| Potency to differentiate | Low | Low | High | [18,20] |
| Proliferative potential | Low | Low | High | [18,20,21] |
| Immune modulatory properties | Good | Good | Good | [18] |
| Allogenic cell rejection | No | No | No | [18,22] |
| Ethical issue | No | No | No | [23] |
| Risk of tumorigenicity | No | No | No | [18] |
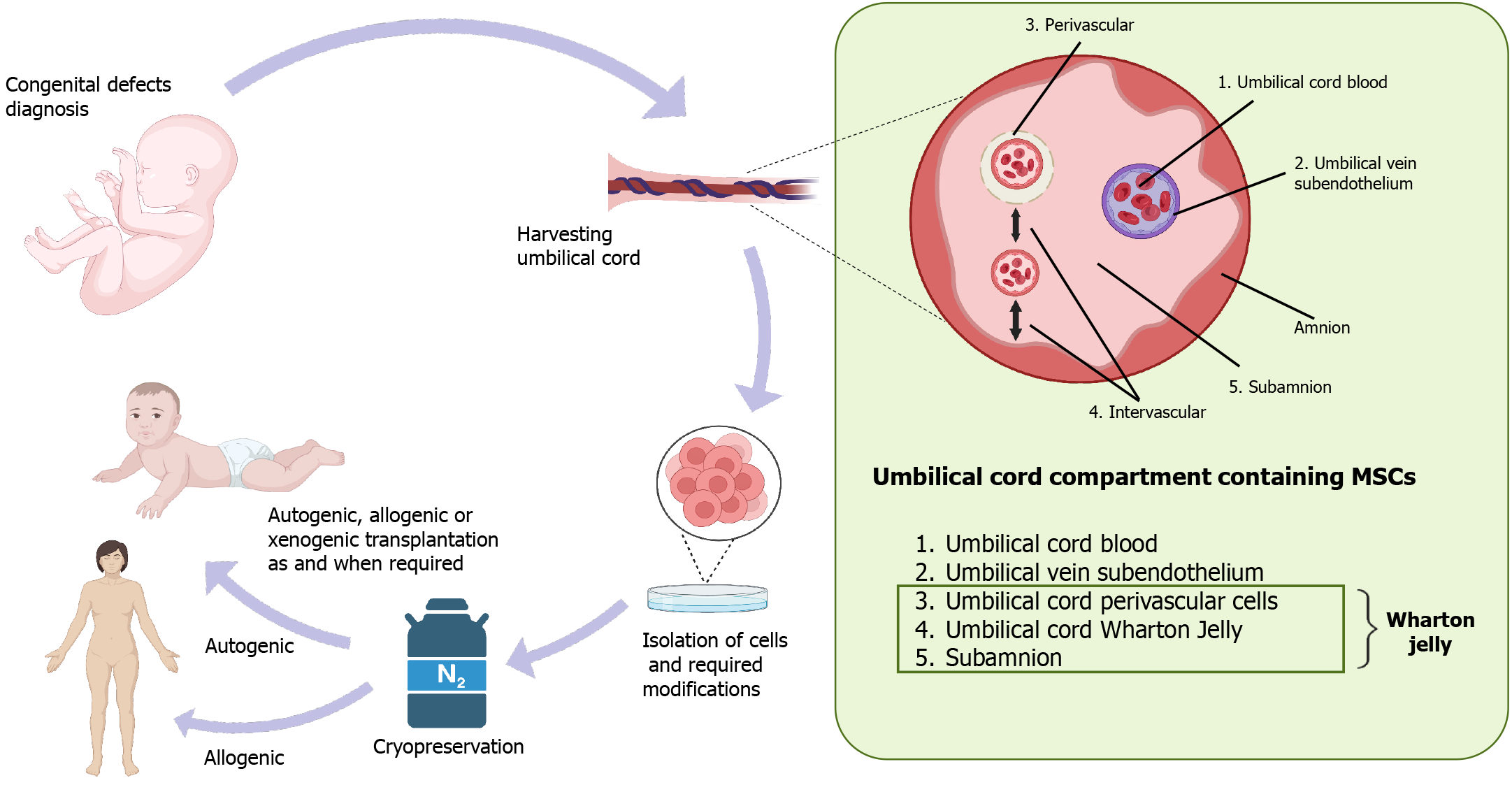
For decades, WJ-MSCs have seemed to be of particular interest because they can be harvested after delivery without any ethical issues. They have the capacity to expand at a faster rate than adult MSCs, in which expansion declines with aging, and they start showing immunological issues. Anatomically, the umbilical cord contains a specific mucous proteoglycan-rich matrix known as WJ. Within this matrix, there are two umbilical arteries and one umbilical vein, and the whole structure is covered by amniotic epithelium (Figure 2). The umbilical cord connects the developing baby with the placenta in the womb and supplies oxygen and nutrient-rich blood to sustain its growth. WJ, confined in the umbilical cord, prevents umbilical vessels from twisting, compression, or torsion during fetal movement, safeguarding proper blood supply to the fetus[25]. This unique anatomic architecture of the umbilical cord allows communication between the mother and the fetus through the fetoplacental membrane, hormone and cytokine interaction[26]. During fetal development, hematopoiesis takes place in the yolk sac and later in the aorta-gonad-mesonephros region, and these processes are linked to the presence of stem cells in the cord. There are two possible theories on the presence of stem cells in the umbilical cord: (1) Migration of fetal HSCs and MSCs toward the placenta, and during a second round of migration from the placenta to the liver and bone marrow, some cells get trapped and reside in the WJ of the umbilical cord[27]; or (2) These MSCs originate from mesenchyme already present in the umbilical cord matrix. Thus, these MSCs trapped in the WJ remain there for the duration of the gestational period[27]. Different researchers have named these MSCs with different names such as UC-MSCs, umbilical cord stem cells, WJ stem cells, or WJ-MSCs. Among all these names, WJ-MSCs is the most common. WJ-MSCs can be isolated from three regions: The perivascular zone, the intervascular zone, and the sub-amnion[28]. Studies show significant differences in the number and nature of stem cells among these three regions[29,30].
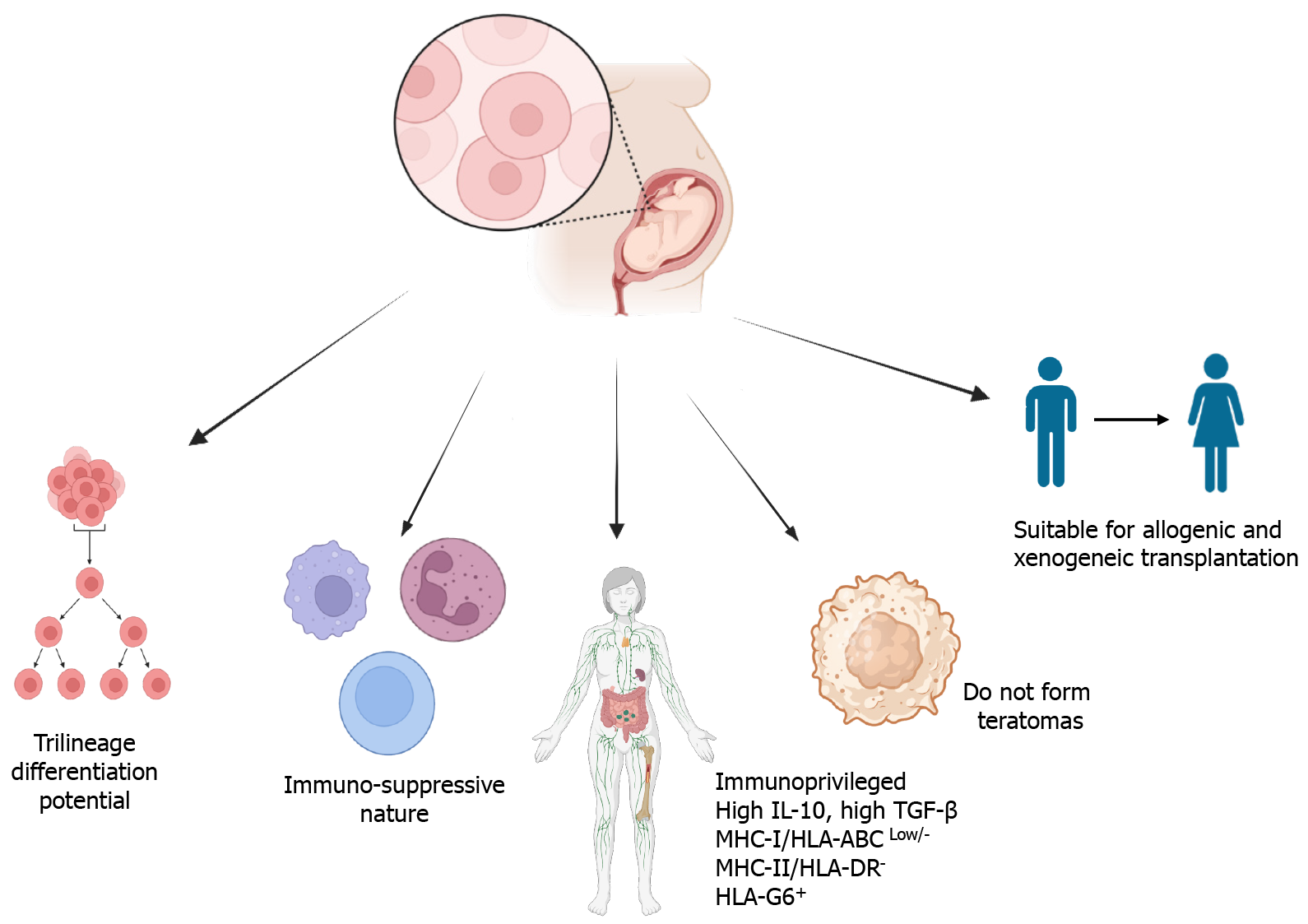
WJ-MSCs are a kind of multipotent stem cells that have several common features of ESCs. Their potency lies between pluripotent and multipotent stem cells (Figure 3). They significantly express ESC stemness markers Oct-4, Sox-2, and Nanog[31]. WJ-MSCs comply with all the measures of the International Society for Cellular Therapy for MSCs[32,33]. These criteria are as follows: Adherence to treated plastic for cell culture (polystyrene), morphologically spindle-shaped[34], high expression of MSCs markers such as CD29, CD44, CD73, CD90, CD105, and no expression of hematopoietic and endothelial markers such as HLA-DR, CD11b, CD14, CD31, CD34, and CD45, and in vitro tri-lineage differentiation potential (such as osteocytes, chondrocytes, and adipocytes)[35,36].
The amount of MSCs that can be obtained from bone marrow is very limited. Only 0.001% to 0.01% of mononuclear cells have been reported[23], while 1 g of adipose tissue yields approximately 5 × 103 stem cells, which is 500-fold greater than in the bone marrow[37]. The isolation efficiency from WJ is high and ranges from (1-5) × 104 cells/cm of umbilical cord[38]. WJ-MSCs have several advantages, such as cost-effectiveness, unlimited availability of tissue sources, easy collection, convenient transportation, no donor site morbidity, and highly proliferative potential without losing potency and functions, which make them superior to other sources of MSCs[39].
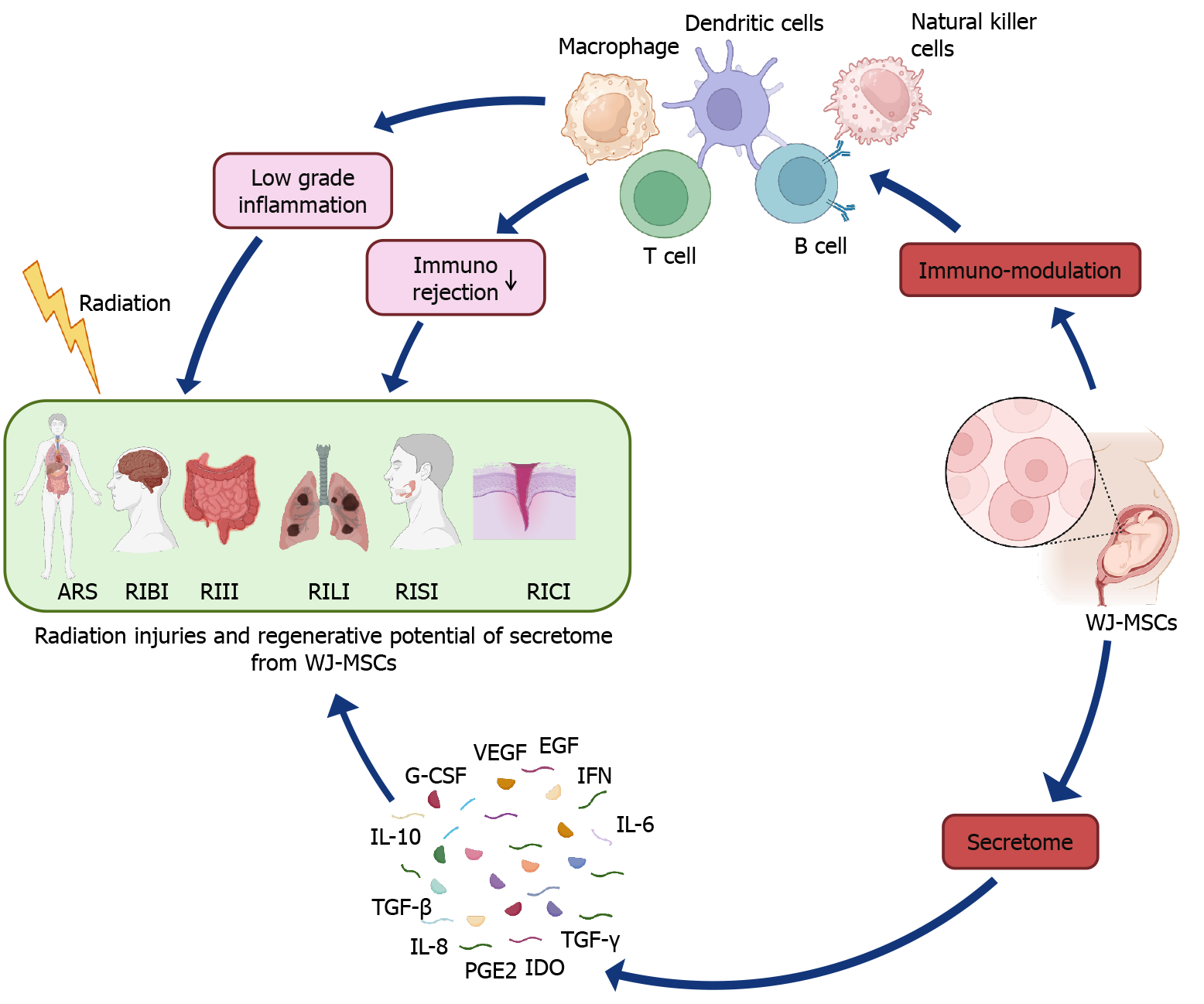
WJ-MSCs are multipotent, immunosuppressive, non-tumorigenic, and highly suitable for allogeneic and xenogeneic transplantation compared to other sources of MSCs[18,28,31,40]. WJ-MSCs are also capable of immune suppression and immune avoidance, similar to other types of MSCs. They are non-immunogenic because they express low levels of major histocompatibility class (MHC) I (HLA-ABC) and do not express MHC-II (HLA-DR) and co-stimulatory antigens (CD80, CD86) associated with the stimulation of both T and B cell reactions[40-43]. The low levels of expression of MHC class I protect WJ-MSCs from natural killer cell-mediated lysis[41]. Although BM-MSCs and WJ-MSCs are both MSCs, HLA-DR is considerably induced in BM-MSCs with interferon (IFN)-γ treatment, whereas this induction is very negligible in WJ-MSCs[42,44-46]. In addition, WJ-MSCs produce large amounts of tolerogenic factors such as interleukin (IL)-10, higher levels of transforming growth factor (TGF)-β, and express HLA-G, which is not true for BM-MSCs[40,42-44]. They also express high levels of leukocyte antigen G6 (HLA-G6), which is produced by trophoblasts and protects the embryo from immune-based destruction[43]. WJ-MSCs release secretory soluble mediators such as IL-6, IL-8, TGF-β, indoleamine-2,3-dioxygenase (IDO), vascular endothelial growth factor (VEGF), cyclooxygenase-2, prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), galectin-1, and HLA-G5, which are effective factors for immunosuppression[47-49]. The secretion of inhibitory cytokines such as IL-10, IL-6, IL-8, TGF-β2, and HGF inhibits T helper type 17 (Th17) cells and stimulates regulatory T (Tregs) cells. It inhibits the proliferation of activated T cells by secreting IDO and PGE2 and upregulates the expression of programmed death ligand 1[50-52]. WJ-MSCs can suppress allogenic-stimulated immune cells to a greater extent than either BM-MSCs or adipose-derived MSCs (AD-MSCs)[25]. WJ-MSCs infusion more effectively decreased the incidence and severity of graft-vs-host disease (GvHD) compared to human decidua mesenchymal stromal cells, hBM-MSCs, and human adipose-derived stem cells, which was mediated by the enrichment of myeloid-derived suppressor cells in GvHD target tissues[53]. hUC-MSC- extracellular vesicles are reported to prevent life-threatening acute GvHD by modulating immune responses[54]. In addition to immunomodulation, they have applications in regenerative medicine and tissue engineering.
Because of their primitive nature, immuno-privileged status, and inexhaustible source of stem cells, WJ-MSCs have greater potential in clinics for regenerative medicine. WJ-MSCs produce abundant amounts of tissue growth-promoting factors such as VEGF, granulocyte-colony stimulating factor (G-CSF), platelet-derived growth factor, TGF-β, IL-6, IL-8, and insulin-like growth factor-1 (IGF1)[35,39,45]. These unique features of WJ-MSCs make them an excellent alternative source of MSCs for allogeneic transplantation to repair and regenerate different organs and tissues, including skin, heart, fat, cartilage, bone, pancreas, neural and vascular/endothelial constituents[31,46,55-57], as well as xenogeneic transplan
In diseases like Parkinson’s disease, motor activities, the number of dopaminergic neurons, and levels of dopamine and tyrosine hydroxylase activities are reduced. WJ-MSCs have shown beneficial effects in improving the dopaminergic cells in Parkinson’s disease. Jalali et al[65] have shown that infusion of WJ-MSCs along with L-dopa/carbidopa improved their levels. Chronic treatment with WJ-MSCs, alone and in combination with L-Dopa, improved nociception and cognitive deficit in Parkinson’s disease rats, which may be the result of increasing IGF-1 and protecting the viability of dopaminergic neurons[66,67]. WJ-MSCs were readily differentiated into WJ Schwann cell-like cells, which effectively promoted the regeneration of peripheral nerves. Transplantation of WJ Schwann cell-like cells with acellular nerve grafts might be useful for assisting peripheral nerve regeneration[68].
Preclinical studies were conducted in a trinitrobenzene sulfonic acid-induced colitis animal model for hUC-MSCs, and it was observed that systemic infusion of hUC-MSCs could home to the inflamed colon and effectively ameliorate colitis[69]. In addition to the known suppressive effects on Th1-type immune responses, hUC-MSC-mediated modulation of IL-23/IL-17 regulated inflammatory reactions also plays an important role in the amelioration of colitis[69]. In another study, Chao et al[70] have shown that hUC-MSCs protected against experimental colitis by boosting the numbers of CD5 + B cells and IL-10-producing CD5 + Bregs and correcting Treg/Th17/Th1 imbalances. In a randomized controlled clinical trial, after UC-MSC infusion, steroid dosage significantly decreased, and the Crohn’s disease (CD) patients’ conditions also improved significantly. This indicates that UC-MSCs can attenuate immune malfunction in patients with CD. UC-MSCs therapy can significantly and safely improve the disease condition in patients with CD receiving a stable steroid dose[71]. A clinical trial for the use of WJ-MSCs in inflammatory bowel disease was started but it resulted in not being available (https://clinicaltrials.gov/ct2/show/NCT03299413).
WJ-MSC-derived extracellular vesicles have the potential to reduce cytokine storm reactions in patients with both chronic inflammatory diseases and viral infections[72]. Cytokine storm is recognized as one of the factors contributing to organ failure and mortality in patients with coronavirus disease 2019 (COVID-19). Therefore, a study was conducted on five patients with severe COVID-19 who were treated with WJ-MSCs (150 × 106 cells per injection). It was found that the levels of IL-10 and stromal cell-derived factor-1 increased after cell therapy, while the levels of VEGF, TGF-β, IFN-γ, IL-6, and tumor necrosis factor (TNF)-α decreased[73].
WJ-MSCs exhibit significant regenerative potential, making them a promising option for various therapeutic applica
Despite their potential therapeutic benefits, MSCs are hindered by concerns over their potential to promote cancer. Studies have shown that MSCs can support the stem cell phenotype of acute myeloid leukemia and protect acute promyelocytic leukemia cells from apoptosis[76]. Additionally, MSCs have been implicated in promoting cancer progression by inducing epithelial to mesenchymal transition, enhancing cancer cell migration, and increasing tumor growth and metastasis[76]. The specific mechanisms behind these cancer-promoting characteristics of MSCs remain unclear, highlighting significant obstacles to their adoption in cancer therapies. As for WJ-MSCs, they are an ideal candidate for regenerative medicine, not only because they have huge regenerative potential but also because they have been reported to be non-tumorigenic and even anti-tumorigenic, suggesting their safety for cancer therapy as well[77,78]. They have low immunogenicity and are not rejected by the host immune system[79,80]. The dosage or count of WJ-MSCs for infusion varies from 0.2 × 106/kg to 8.7 × 106/kg in various disease conditions. The cell counts to be administered are mostly calculated relative to body weight, although some clinical studies have also applied arbitrary counts[80]. Regarding WJ-MSC transplantation for diabetes mellitus, a dose of 1 × 106/kg has been reported several times.
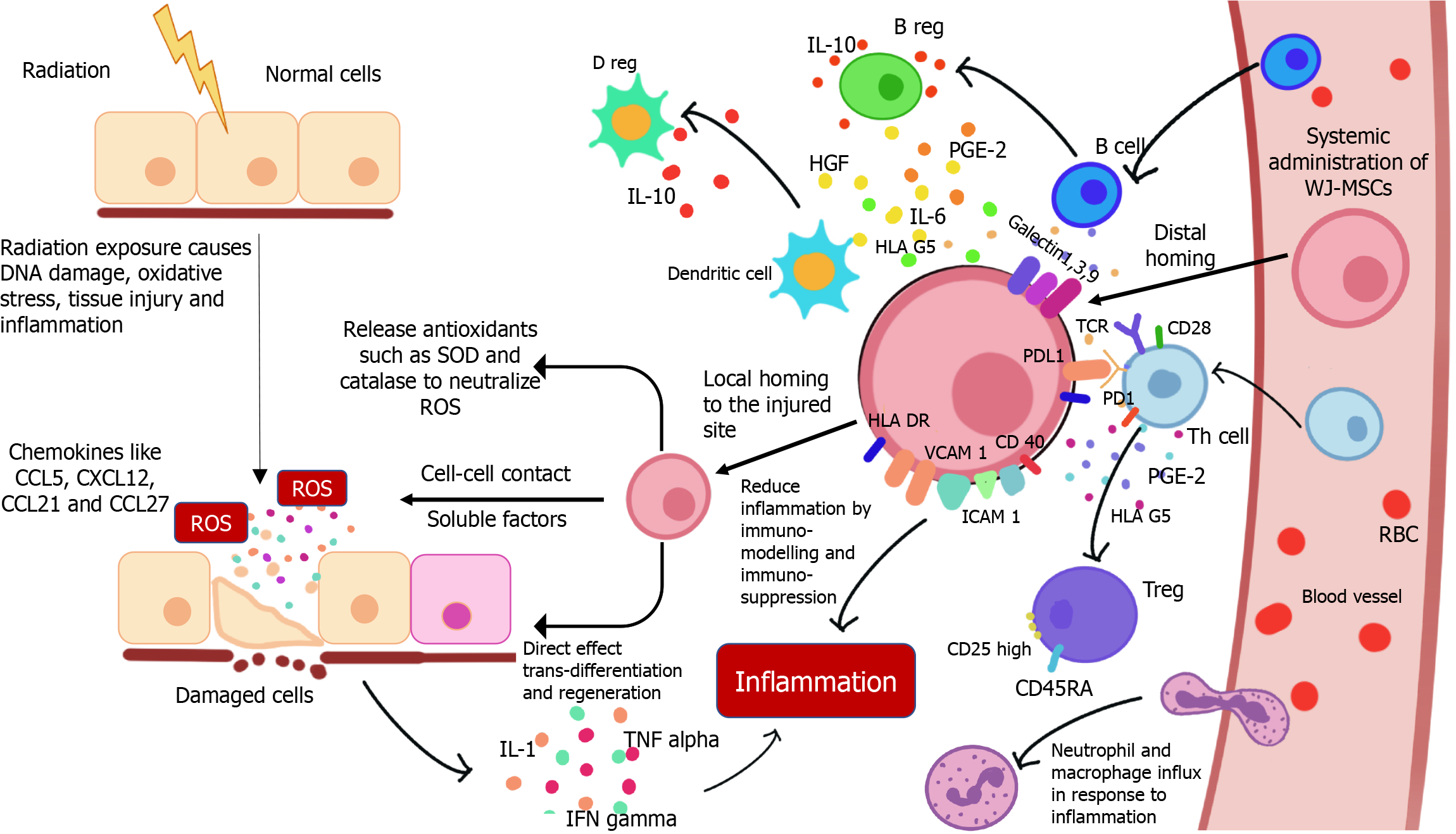
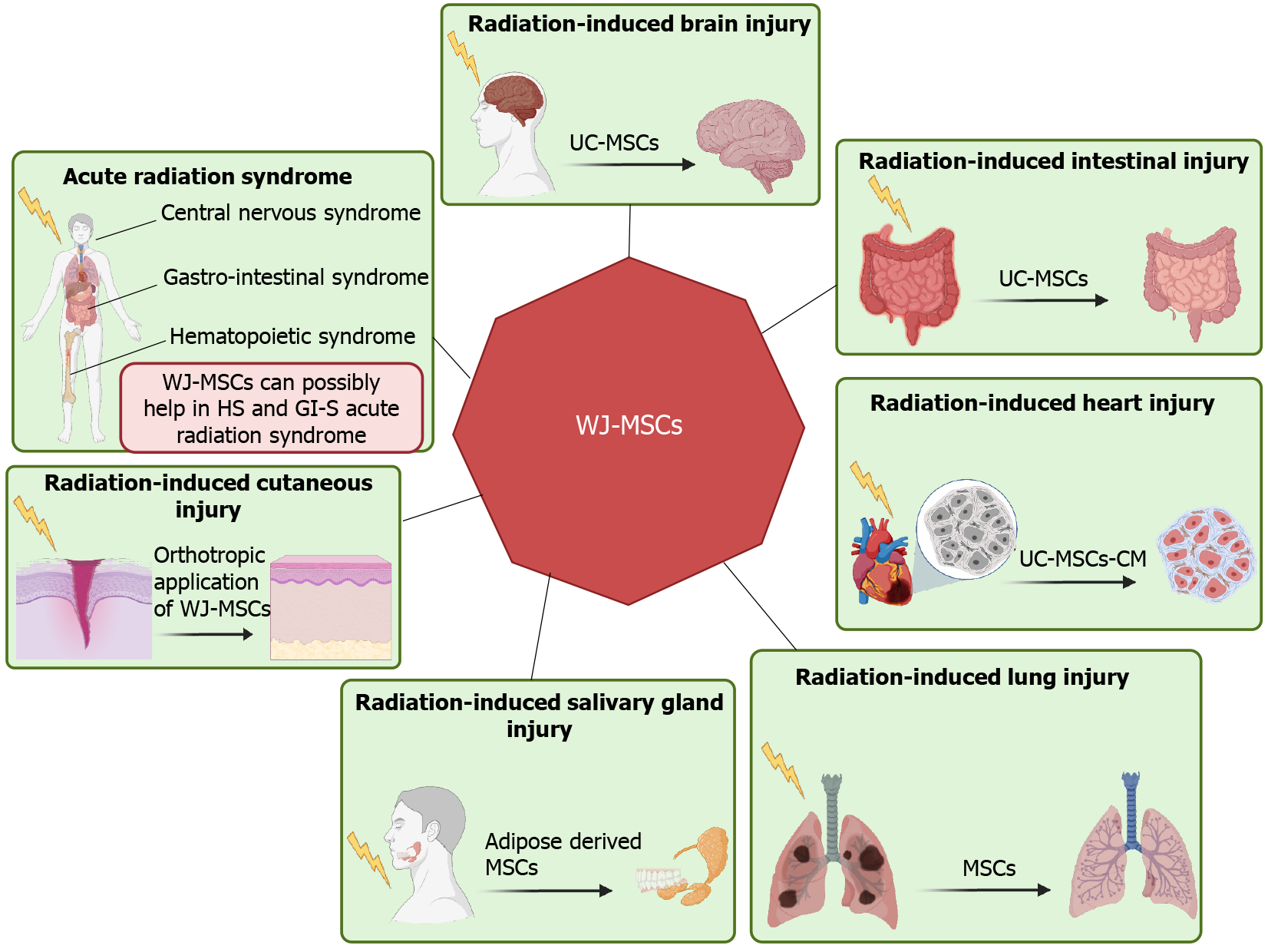
The molecular mechanisms underlying radiation injuries and their repair are complex, involving DNA damage, oxidative stress, and inflammatory responses (Figure 5). Better understanding of these mechanisms is crucial for leveraging the therapeutic potential of WJ-MSCs. These cells help mitigate radiation injury through paracrine signaling, immunomodulation, differentiation, and direct cell-to-cell interactions[81]. Ionizing radiation causes DNA damage, including single-strand and double-strand breaks, potentially leading to mutations and cell death[11]. WJ-MSCs exhibit strong DNA repair capabilities and secrete growth factors like HGF and IGF-1[82]. However, their ability to enhance DNA repair in neighboring cells requires further investigation. Radiation also generates reactive oxygen species (ROS), causing oxidative damage and impairing mitochondrial function. WJ-MSCs release antioxidant enzymes such as superoxide dismutase and catalase, which neutralize ROS[83]. Studies have shown that WJ-MSCs’ protective effects against oxidative stress involve paracrine signaling and extracellular vesicle release[83]. They express crucial ROS-managing enzymes[84] and can transfer healthy mitochondria to damaged cells via tunneling nanotubes, restoring mitochondrial function[85]. Additionally, radiation induces pro-inflammatory cytokines, leading to sustained inflammation and tissue damage[86]. WJ-MSCs migrate to injury sites using chemokine receptors and adhesion molecules, secreting radioprotective and tissue-regenerative factors to modulate the immune response[12]. They regulate immunity through cell-cell contact with T cells and produce soluble factors (PGE2, HGF, IL-6, IL-10, TGFβ1) that reduce T-cell proliferation, induce T-cell apoptosis, and promote regulatory T cells, thereby mitigating radiation-induced inflammation and promoting tissue regeneration[83]. During RT for cancer, damage to normal tissues near the tumor can occur, leading to the development of xerostomia in head and neck cancer, lung fibrosis in lung cancer, or enteritis/colitis in colon and pelvic cancer. These limitations compromise the therapeutic outcome of RT. Emerging studies using stem cells suggest that the infusion of WJ-MSCs could be beneficial in managing several of these RT complications (Figure 6). WJ-MSCs have shown significant potential in mitigating acute and late radiation side effects due to their anti-inflammatory, antioxidant, and regenerative properties. These cells can be administered early after RT or to treat established late effects by inhibiting fibrosis, enhancing vascular regeneration, and reducing chronic inflammation[87]. The potential of WJ-MSCs in managing radiation enteropathy, a common side effect of abdominal and pelvic RT, is also being explored[88].
Acute radiation syndromes (ARS), also known as triple syndrome (comprising hematopoietic, gastrointestinal, and central nervous syndromes), can develop after whole-body radiation exposure, either knowingly (such as in RT, reactor maintenance, or clean-up) or unknowingly (due to radiation accidents). Zhang et al[89] have demonstrated the beneficial role of umbilical cord blood stem cell transplantation in the recovery of hematopoietic syndrome in experimental mice. Kovalenko et al[90] have shown that the administration of 2 × 108 human umbilical cord blood mononucleated cells within 24-52 hours following irradiation, along with the antibiotic levaquin, significantly enhances the probability of survival compared to irradiated and untreated animals. Very recently, Bandekar et al[35] showed that therapeutic infusion of WJ-MSCs after lethal exposure to radiation (8.5 Gy) reduces the symptoms of ARS. WJ-MSCs have the capability to preferentially home into radiosensitive tissues like the spleen, bone marrow, and small intestine of irradiated mice, and secrete various soluble mediators while minimizing radiation toxicity[35]. However, the infusion of WJ-MSCs in normal mice results in their random distribution[91]. The transplanted xenogeneic WJ-MSCs produce human cytokines and enhance the production of mouse cytokines in irradiated mice. Among these, WJ-MSC-derived human IL-6 and G-CSF were found to play a causal role in radioprotection[35,92].
Radiation-induced cutaneous injury, also known as radiation dermatitis, manifests due to repeated exposure to radiation on the skin during RT. The skin is the first organ to come into contact with external RT, making it the most common type of radiation injury. It limits the duration and dose of radiation that can be delivered to the patient. Sun et al[93] showed that the orthotropic application of WJ-MSCs-derived conditioned media significantly increases the wound-healing rate by effectively promoting tissue repair and regeneration in radiation-damaged skin in rats, signifying the role of WJ-MSCs in acute radiation skin injury. The regenerative mechanism mediated by WJ-MSC-conditioned medium (CM) involves tissue regeneration due to the presence of secreted soluble factors that reduce inflammation, enhance alpha-smooth muscle actin expression, and promote angiogenesis, thereby increasing the total number of vessels in the healed wound skin[94]. Additionally, the CM from WJ-MSCs also accelerates scar-free wound healing. These findings point towards an urgent need for optimization and benchmarking of the method of isolation and preservation of conditioned media from WJ-MSCs for possible use as a therapeutic agent for the treatment of radiation-induced dermatitis[93].
During RT for head and neck cancer, the salivary glands are inevitably exposed to radiation, resulting in severe damage and the development of radiation-induced salivary gland injury (RISI). After undergoing RT for head and neck cancer, most patients experience xerostomia, dysphagia, dental caries, and other issues that negatively impact their social and professional lives. This is due to the irreversible loss of acinar cells, sterilization of primitive glandular stem cells, and decreased saliva secretion (known as hyposalivation) caused by radiation exposure, respectively[95]. In the early stages of exposure, there are no morphological changes, but saliva secretion diminishes, possibly due to cell membrane damage caused by radiation-induced ROS or alterations in signaling pathways and ion channels such as Aqp5[96]. However, in the later stages, significant morphological changes occur, including atrophy and loss of acinar cells, duct dilation, and infiltration of chronic inflammatory cells[97]. Until now, neither the prophylactic use of amifostine (to prevent radiation-induced xerostomia)[98] nor symptomatic treatment strategies (such as pilocarpine, which stimulates saliva secretion) have provided satisfactory relief from symptoms. However, IMRT has been shown to be effective in reducing the dose delivered to the parotid glands, thus potentially reducing the risk of parotid gland injury[99]. Because radiation-induced xerostomia results from the loss of stem cells, the infusion of stem cells may aid in the regeneration of salivary glands. There have been reports on the use of adult tissue stem cells, such as HSCs, MSCs, and salivary stem/progenitor cells, for rescuing radiation-induced xerostomia.
Although there is currently no report on the effect of WJ-MSCs on RISI, other sources of stem cells have shown beneficial effects, and the same is expected for WJ-MSCs. Schwarz et al[100] demonstrated that intra-glandular infusion of BM-MSCs was retained in inflamed glands, while intravenous infusion reached both normal and damaged submandibular glands. Xiong et al[101] showed that transplantation of AD-MSCs was beneficial in alleviating xerostomia, possibly by aiding in the regeneration of salivary glands after intra-glandular transplantation. They observed that the transplanted AD-MSCs survived and differentiated into salivary epithelial cells.
RT is one of the key treatment modalities for thoracic cancers such as lymphoma, lung, breast, and esophageal cancer. Radiation-induced lung injury (RILI) develops post-RT due to severe cell damage after repeated radiation exposure. Manifestations of RILI include early-stage radiation pneumonitis (1-6 months after RT) and late-stage pulmonary fibrosis (1-2 years after RT). Although high-dose steroids can effectively treat acute radiation pneumonitis, there is currently no approved causative treatment for late-onset pulmonary radiation damage such as pulmonary fibrosis[87]. Radiation exposure results in an increase in reactive oxygen and nitrogen levels in epithelial and endothelial cells, causing damage. These damaged cells start producing pro-inflammatory cytokines, which alter vasodilation and vascular permeability and recruit cells of the immune system, resulting in chronic inflammation. These damages lead to the loss of epithelial and endothelial cells, causing blood-air dysfunction and increased vascular permeability. In the late stage, they ultimately develop fibrosis. The development of fibrosis causes damage to tissue architecture, which interferes with gaseous exchange, resulting in dyspnea, accumulation of fluid in the interstitial space, and ultimately respiratory failure and death. Despite significant advancements in the safety of RT, on average, 10%-30% of patients develop symptoms of RILI after thoracic RT[102]. RILI not only affects the quality of life of the patients but also increases the chances of death. Until now, apart from amifostine, we do not have any other treatment regimens for it. In the last few decades, cell-based therapy employing MSCs has played a significant role in reducing lung fibrosis by promoting the repair of damaged tissue as well as secreting anti-inflammatory mediators and anti-fibrotic factors. They are also known to suppress T-cell activity and reduce B-cell activation and proliferation. Under inflammatory conditions, MSCs secrete IDO, PGE2, and IL-10, which have a regulatory function.
Hao et al[103] showed that intratracheal transplantation of UC-MSCs in a canine model reduced oxidative stress, inflammatory reactions, and TGF-β-Smad2/3 pathways, thereby reducing RILI. Zhang et al[104] reported that CXCR4-overexpressing WJ-MSCs preferentially home to damaged lung tissues and show improved therapeutic potential for the treatment of RILI. The protection offered by WJ-MSCs was associated with a reduction of radiation-induced increase in stromal cell-derived factor-1, TGF-β1, alpha-smooth muscle actin, and collagen I levels, as well as a protection from radiation-induced decrease in the expression of E-cadherin, leading to the moderation of RILI.
During thoracic exposure to radiation, heart injury is also associated with lung injury. Radiation causes fibrosis in all components of the heart and significantly increases the risk of coronary artery disease, cardiomyopathy, valvulopathy, arrhythmias, and pericardial disease[105]. Heart injury comprises myocardial, coronary artery, pericardial, valvular, and conduction system diseases, which have been observed in breast cancer and Hodgkin’s lymphoma patients[106-108]. Chen et al[109] assessed the therapeutic effect of human UC-MSCs-CM on radiation-induced myocardial fibrosis. They found that irradiated human cardiac fibroblasts cultured with UC-MSCs-CM showed greater viability. Inhibited nuclear factor-kappa B activity decreased the expression of several pro-fibrotic cytokines, including TGF-β1, IL-6, and IL-8, followed by mitigated collagen deposition and fibrosis. Meanwhile, changes in oxidation markers (malondialdehyde) and antioxidant enzyme levels reflected reduced oxidative stress[109]. However, specific nutritional factors released by MSCs that are involved in myocardial protection from ionizing radiation were not clarified[109].
During RT for abdominal and pelvic cancers, exposure of radiation to the intestine is unavoidable. This radiation exposure causes serious damage to intestinal villi, leading to mucosal erosion, intestinal vascular permeabilization, chronic inflammation, and eventually developing into radiation enteritis/proctitis/colitis, intestinal ischemia, mucositis, ulcers, necrosis, or even perforation. The development of these conditions after RT worsens the quality of life of these patients. Depending on the total dose of RT, size of the radiation field, course time, and division method, radiation enteritis can be divided into acute phase and chronic phase[20,23]. Acute radiation enteritis occurs within 1-2 weeks and is characterized by main manifestations such as nausea, emesis, stomachache, acute diarrhea, and tenesmus. On the other hand, chronic radiation enteritis generally occurs after several months or years and is characterized mucous bloody stool, intestinal stenosis, anal pendant expansion, and even intestinal obstruction. Bandekar et al[35] have shown that infusion of WJ-MSCs helps in the recovery of Lgr5 + stem cells in mice.
This is one of the most common types of injury that takes place during RT for head and neck cancer. It involves damage to the cerebral-vascular system, inflammatory response, and oxidative stress in the brain, which causes progressive cognitive dysfunction. Radiation exposure to the brain depletes the neuronal stem/precursor cell pools primarily residing in the neurogenic region of the hippocampus, leading to cognitive deficits. Therefore, transplantation of stem cells may be a promising option for restoring cognitive function in the brain. Very recently, Wang et al[110] have shown that infusion of UC-MSCs to 15 Gy whole body irradiated mice inhibits brain injury and imparts a neuroprotective effect. It inhibits neuro-inflammation by decreasing the levels of the inflammatory cytokines (TNF-α and IL-6) and increasing the level of IL-10, significantly improving the learning and memory of the mice. These studies demonstrated profound beneficial effects of either ESCs or neuronal stem cells (derived from iPSCs) in ameliorating the adverse effects of radiation on the brain in a preclinical rat model[111,112].
The first clinical trial to test the feasibility and efficacy of WJ-MSC therapy was registered in 2008. By May 2024, the public clinical trials database, https://www.clinicaltrials.gov/, had shown 48 clinical trials using WJ-MSCs for a wide range of therapeutic applications (Table 3, keywords used: “Wharton’s jelly mesenchymal stem cells” or “umbilical cord mesenchymal stem cells”). Most of these trials are safety studies (phase I) and proof of concept (phase II), with very few in phase III (comparison of a new treatment to the standard treatment).
| No. | Study title | Clinical trial code numbers | Conditions | Status/conclusions |
| 1 | Randomized study of coronary revascularization surgery with injection of WJ-MSCs and placement of an epicardial extracellular matrix | NCT04011059 | Cardiovascular diseases: Heart failure, coronary artery disease | Not yet recruiting |
| Mesenchymal stem cell transplantation, regenerative medicine | ||||
| 2 | MSCs for prevention of MI-induced HF | NCT05043610 | Myocardial infarction: Acute, anterior wall | Recruiting |
| Cardiac remodeling, ventricular | ||||
| STEMI, regenerative medicine | ||||
| Heart failure | ||||
| 3 | The application of the umbilical cord mesenchymal stem cells in the complex treatment of non-ischemic heart failure | NCT04325594 | Chronic heart failure | Completed. Result not available |
| Non-ischemic cardiomyopathy, dilated cardiomyopathy | ||||
| 4 | Treatment of degenerative disc disease with allogenic mesenchymal stem cells (MSV) | NCT01860417 | Degenerative disc disease | Completed. Result available. But not concluded yet |
| Intervertebral disc disease | ||||
| Low back pain | ||||
| 5 | Treatment of knee osteoarthritis with allogenic mesenchymal stem cells | NCT01586312 | Arthritis of knee | Completed. Result available. But not concluded yet |
| 6 | Ultrasound-guided treatments for shoulder pain in wheelchair users with spinal cord injury | NCT04136743 | Spinal cord injuries | Recruiting |
| Tendinopathy | ||||
| Rotator cuff tears | ||||
| Shoulder pain | ||||
| 7 | Ultrasound-guided injections for meniscal injuries in active-duty military | NCT04274543 | Tibial meniscus injuries | Recruiting |
| Knee injuries and disorders | ||||
| 8 | 3D tissue engineered bone equivalent for treatment of traumatic bone defects | NCT03103295 | Bone defects | Unknown status |
| 9 | Safety and feasibility study of the CELLSPAN esophageal implant (CEI) in patients requiring short segment esophageal replacement | NCT05877300 | Esophageal diseases | Not yet recruiting |
| 10 | Micro-fragmented adipose tissue and complex Crohns’ anal fistulas | NCT03555773 | Crohn disease, perianal fistula | Completed. Result not available |
| 11 | Micro-fragmented adipose Tissue (Lipogems®) injection for chronic shoulder pain in persons with spinal cord injury | NCT03167138 | Shoulder pain | Unknown status |
| Shoulder impingement syndrome | ||||
| Rotator cuff impingement syndrome, rotator cuff tendinitis, rotator cuff syndrome of shoulder and allied disorders | ||||
| Spinal cord injuries | ||||
| 12 | Encapsulated mesenchymal stem cells for dental pulp regeneration | NCT03102879 | Periapical periodontitis | Completed. Result available. But no conclusion |
| 13 | Allogeneic cord blood cells for adults with severe acute contusion spinal cord injury | NCT04331405 | Spinal cord contusion | Completed. Result not available |
| 14 | Wharton’s jelly-derived mesenchymal stem cells in osteoarthritis | NCT03866330 | Osteoarthritis: Hip, knee, glenohumeral | Unknown status |
| 15 | Cardiovascular clinical project to evaluate the regenerative capacity of cardiocell in patients with acute myocardial infarction (AMI) | NCT03404063 | Myocardial infarction | Completed. Result not available |
| 16 | Randomized clinical trial to evaluate the regenerative capacity of cardiocell in patients with chronic ischaemic heart failure (CIHF) | NCT03418233 | Heart failure | Completed. Result not available |
| 17 | Cardiovascular clinical project to evaluate the regenerative capacity of cardiocell in patients with no-option critical limb ischemia (N-O CLI) | NCT03423732 | Critical limb ischemia | Unknown status |
| 18 | Transplantation of allogeneic MSC in patients with pulp necrosis and chronic apical periodontitis | NCT04545307 | Pulp Necroses|Apical Periodontitis | Completed. Result not available |
| 19 | Efficacy of intradiscal injection of autologous bm-MSC in subjects with chronic LBP due to multilevel lumbar IDD | NCT05066334 | Intervertebral Disc Degeneration|Chronic Low-back pain | Recruiting |
| 20 | Allogeneic ADSCs and platelet-poor plasma fibrin hydrogel to treat the patients with burn wounds (ADSCs-BWs) | NCT03113747 | Second- or third-degree burns | Unknown status |
| 21 | Allogeneic mesenchymal stromal cells in elderly patients with hip fracture | NCT02630836 | Femoral neck fracture | Withdrawn |
| 22 | Umbilical cord blood-derived mesenchymal stem cells in regeneration of sweat glands and body repair | NCT02304562 | Sweat gland diseases | Unknown status |
| 23 | Residual dental pulp tissue and cord blood stem cells | NCT04040127 | Irreversible pulpitis | Withdrawn |
| 24 | Treatment of osteoarthritic knee with high tibial osteotomy and implantation of allogenic human umbilical cord blood-derived stem cells | NCT04234412 | Osteoarthritis, knee | Unknown status |
| 25 | Umbilical cord blood collection and processing for hypoplastic left heart syndrome patients | NCT01856049 | Hypoplastic left heart syndrome | Recruiting |
| 26 | Stem cell educator therapy in type 1 diabetes | NCT03390231 | Type 1 diabetes | Unknown status |
| 27 | Use of Wharton Jelly in diabetic nephropathy | NCT03288571 | Diabetic nephropathies | Not yet recruiting |
| 28 | Efficacy of Wharton jelly in erectile dysfunction | NCT03751735 | Erectile dysfunction associated with type 2 diabetes mellitus | Completed. Result not available |
| 29 | Safety of Wharton Jelly in erectile dysfunction | NCT02945449 | Erectile dysfunction associated with type 2 diabetes mellitus | Completed. Result not available |
| 30 | Treatment of COVID-19 patients using Wharton’s jelly-mesenchymal stem cells | NCT04313322 | Use of stem cells for COVID-19 treatment | Recruiting |
| 31 | Use of mesenchymal stem cells in inflammatory bowel disease | NCT03299413 | Inflammatory bowel diseases | Active, not recruiting |
| 32 | Intrathecal administration of expanded Wharton’s jelly mesenchymal stem cells in chronic traumatic spinal cord injury | NCT03003364 | Spinal cord injury, chronic | Completed. Result not available |
| 33 | Evaluation of umbilical cord-derived Wharton’s jelly stem cells for the treatment of acute graft versus host disease | NCT03158896 | Acute graft versus host disease | Active, not recruiting |
| 34 | Use of Wharton Jelly-derived mesenchymal stem cells for knee osteoarthrosis | NCT02963727 | Knee osteoarthrosis | Recruiting |
| 35 | Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells | SHGM56733164 | Retinitis pigmentosa | Completed. Result not available |
| Inherited retinal dystrophy | ||||
| 36 | Safety and efficacy of intravenous Wharton’s jelly-derived mesenchymal stem cells in acute respiratory distress syndrome due to COVID-19 | NCT04625738 | Acute respiratory distress syndrome | Not yet recruiting |
| 37 | Wharton’s jelly-derived mesenchymal stem cells in osteoarthritis | NCT03866330 | Osteoarthritis: Hip, knee, glenohumeral | Recruiting |
| 38 | Intracoronary human Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) transfer in patients with acute myocardial infarction (AMI) | NCT01291329 | ST-elevation myocardial infarction | Completed. Result not available |
| 39 | Therapeutic potential of stem cell conditioned medium on chronic ulcer wounds | NCT04134676 | Chronic ulcer | Not yet recruiting |
| 40 | Effect of implanting allogenic cytokines derived from human amniotic membrane (HAM) and mesenchymal stem cells derived from human umbilical cord Wharton’s jelly (HUMCWJ) on pain and functioning of knee osteoarthritis | NCT03337243 | Knee osteoarthritis | Completed. Result not available |
| Knee pain chronic | ||||
| Joint disease | ||||
| Musculoskeletal disease | ||||
| 41 | Intracoronary or intravenous infusion human Wharton’s jelly-derived mesenchymal stem cells in patients with ischemic cardiomyopathy | NCT02368587 | Ischemic cardiomyopathy | Not yet recruiting |
| 42 | Therapeutic treatment of amyotrophic lateral sclerosis | NCT02881476 | Amyotrophic lateral sclerosis | Unknown |
| 43 | Pericardial matrix with mesenchymal stem cells for the treatment of patients with infarcted myocardial tissue | NCT03798353 | Myocardial infarction | Recruiting |
| 44 | A research study looking at specific tissue of the umbilical cord | NCT01166776 | Varices of umbilical cord | Completed. Result not available |
| 45 | Efficacy and safety evaluation of mesenchymal stem cells for the treatment of patients with respiratory distress due to COVID-19 | NCT04390139 | COVID-19, SARS-CoV-2 | Recruiting |
| Adult respiratory distress syndrome | ||||
| 46 | Treatment of spinal cord injuries with (AutoBM-MSCs) versus (WJ-MSCs) | NCT04288934 | Spinal cord injuries | Recruiting |
| 47 | Cell therapy using umbilical cord-derived mesenchymal stromal cells in SARS-CoV-2-related ARDS | NCT04333368 | SARS-CoV-2 | Recruiting |
| Severe acute respiratory distress syndrome | ||||
| 48 | Role of stem cells in improving implantation rates in ICSI patients | NCT01649752 | Assess the efficacy of differentiated and undifferentiated stem cell therapy in improving endometrial receptivity | Unknown |
| 49 | Wharton’s jelly-derived mesenchymal stromal cell repeated treatment of adult patients diagnosed with type I diabetes | NCT03973827 | Type 1 diabetes | Recruiting |
The pre-clinical observations of WJ-MSCs for mitigating radiation injuries show its significant potential in managing the side effects of RT. The future application of WJ-MSCs in treating radiation injuries is promising, with several novel techniques emerging. Enhanced delivery technologies like nanotechnology, hydrogels, and microencapsulation are key areas of focus. Nanoparticles and nanocarriers can protect cells during transit and deliver them precisely to damaged sites, while hydrogels offer a supportive matrix for improved cell retention and function. Microencapsulation enhances the therapeutic effectiveness of WJ-MSCs by protecting them. Personalized medicine aims to tailor WJ-MSCs treatments based on a patient’s genetic profile and specific injury characteristics, potentially improving outcomes. Genetic manipulation of WJ-MSCs to express higher levels of therapeutic factors such as VEGF can enhance their regenerative potential. Increasing their resistance to apoptosis can also improve their survival and efficacy in the hostile post-radiation environment. Further research into WJ-MSCs’ ability to repair DNA in damaged cells can reveal mechanisms to minimize radiation harm and enable genetic modifications for more effective regeneration. Combining WJ-MSC’s therapy with other treatments, such as antioxidants or anti-inflammatory agents, can amplify healing effects. Pharmaceutical agents that enhance MSCs homing and engraftment, like CXCR4 agonists and heparin, are also promising. Thus, future strategies to enhance the therapeutic potential of WJ-MSCs involve genetic modification, preconditioning of MSCs, rigorous screening, advancing research to fully understand their mechanisms of action, and standardized production and enhanced delivery techniques. Personalized treatment protocols, harmonizing regulatory guidelines, and developing innovative delivery systems are essential steps. Several challenges still need to be addressed before transferring WJ-MSCs from the bench to the bedside. These challenges include: Do WJ-MSCs remain immuno-privileged and maintain their hypo-immunogenicity and paracrine properties after differentiation? What will be their post-transplantation status? How much cell count/dose and post-RT time should be selected for desired benefits? Robust clinical trials with long-term follow-up are crucial to fully realize the therapeutic potential of WJ-MSCs in mitigating radiation injuries.
In conclusion, there are different sources of MSCs, and each has its own merits and demerits in terms of derivation, ethical issues, and safety for applications. Various pieces of evidence show that WJ-MSCs do not impose any ethical concerns, risk of forming teratomas, or immunorejection, which exist with BM-MSCs, ESCs, or iPSCs. Thus, WJ-MSCs-based therapy may offer an alternative to allogeneic bone marrow transplantation in accidental radiation exposure scenarios. Like other stem cells, WJ-MSCs also have limitations, such as different researchers following different isolation protocols. Therefore, clear guidelines, standardization and regulatory improvements are essential for widespread clinical adoption of WJ-MSCs. Owing to their unique properties, WJ-MSCs will be at the forefront of stem cell therapy for ameliorating radiation injuries developed after RT. We believe that in the future, more preclinical and clinical studies will be initiated to improve the quality of life for cancer patients who have undergone RT.
The authors thanks to Dr. Santosh Kumar Sandur, Head RB & HSD for critical review of the manuscript.
| 1. | Rana JS, Khan SS, Lloyd-Jones DM, Sidney S. Changes in Mortality in Top 10 Causes of Death from 2011 to 2018. J Gen Intern Med. 2021;36:2517-2518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 2. | Ringborg U, Bergqvist D, Brorsson B, Cavallin-Ståhl E, Ceberg J, Einhorn N, Frödin JE, Järhult J, Lamnevik G, Lindholm C, Littbrand B, Norlund A, Nylén U, Rosén M, Svensson H, Möller TR. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001--summary and conclusions. Acta Oncol. 2003;42:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 402] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 510] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Marcu LG. Altered fractionation in radiotherapy: from radiobiological rationale to therapeutic gain. Cancer Treat Rev. 2010;36:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Wasserman TH, Brizel DM. The role of amifostine as a radioprotector. Oncology (Williston Park). 2001;15:1349-54; discussion 1357. [PubMed] |
| 7. | Asur R, Butterworth KT, Penagaricano JA, Prise KM, Griffin RJ. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015;356:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Wu YY, Fan KH. Proton therapy for prostate cancer: current state and future perspectives. Br J Radiol. 2022;95:20210670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Rezvani M. Therapeutic Potential of Mesenchymal Stromal Cells and Extracellular Vesicles in the Treatment of Radiation Lesions-A Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, Malard O, Stewart F, Tamarat R, van Luijk P, Limoli CL. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid Redox Signal. 2014;21:338-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Talapko J, Talapko D, Katalinić D, Kotris I, Erić I, Belić D, Vasilj Mihaljević M, Vasilj A, Erić S, Flam J, Bekić S, Matić S, Škrlec I. Health Effects of Ionizing Radiation on the Human Body. Medicina (Kaunas). 2024;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Rustad KC, Gurtner GC. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv Wound Care (New Rochelle). 2012;1:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 621] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 15. | Petrosyan A, Martins PN, Solez K, Uygun BE, Gorantla VS, Orlando G. Regenerative medicine applications: An overview of clinical trials. Front Bioeng Biotechnol. 2022;10:942750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18157] [Article Influence: 955.6] [Reference Citation Analysis (0)] |
| 17. | Batista CE, Mariano ED, Marie SK, Teixeira MJ, Morgalla M, Tatagiba M, Li J, Lepski G. Stem cells in neurology--current perspectives. Arq Neuropsiquiatr. 2014;72:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Liau LL, Ruszymah BHI, Ng MH, Law JX. Characteristics and clinical applications of Wharton's jelly-derived mesenchymal stromal cells. Curr Res Transl Med. 2020;68:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9:226-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 525] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 22. | Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122:3824-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15191] [Article Influence: 584.3] [Reference Citation Analysis (0)] |
| 24. | Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med. 2008;12:730-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-11712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Fahmy M. Anatomy of the Umbilical Cord. Umbilicus and Umbilical Cord. Cham: Springer, 2018. |
| 27. | Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, Yang X, Liu B, Mao N. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood. 2008;111:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 29. | Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 30. | Nanaev AK, Kohnen G, Milovanov AP, Domogatsky SP, Kaufmann P. Stromal differentiation and architecture of the human umbilical cord. Placenta. 1997;18:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A. Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 32. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12664] [Article Influence: 703.6] [Reference Citation Analysis (2)] |
| 33. | Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Ranjbaran H, Abediankenari S, Mohammadi M, Jafari N, Khalilian A, Rahmani Z, Momeninezhad Amiri M, Ebrahimi P. Wharton's Jelly Derived-Mesenchymal Stem Cells: Isolation and Characterization. Acta Med Iran. 2018;56:28-33. [PubMed] |
| 35. | Bandekar M, Maurya DK, Sharma D, Checker R, Gota V, Mishra N, Sandur SK. Xenogeneic transplantation of human WJ-MSCs rescues mice from acute radiation syndrome via Nrf-2-dependent regeneration of damaged tissues. Am J Transplant. 2020;20:2044-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Amati E, Perbellini O, Rotta G, Bernardi M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M, Astori G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Ther. 2018;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 741] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 38. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 39. | Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Tien CH, Jeschke MG. Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014;5:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 41. | Jyothi Prasanna S, Sowmya Jahnavi V. Wharton's Jelly Mesenchymal Stem Cells as Off-The-Shelf Cellular Therapeutics: A Closer Look into their Regenerative and Immunomodulatory Properties. Open Tissue Eng Regen Med J. 2011;4:28-38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 43. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 44. | Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 45. | Choi M, Lee HS, Naidansaren P, Kim HK, O E, Cha JH, Ahn HY, Yang PI, Shin JC, Joe YA. Proangiogenic features of Wharton's jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int J Biochem Cell Biol. 2013;45:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Hou T, Xu J, Wu X, Xie Z, Luo F, Zhang Z, Zeng L. Umbilical cord Wharton's Jelly: a new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Eng Part A. 2009;15:2325-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. 2014;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 48. | Ding DC, Chou HL, Chang YH, Hung WT, Liu HW, Chu TY. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016;25:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, Cong Y, Chen H, Lu L, Shi S, Wang D, Chen W, Sun L. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine. 2019;45:341-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, Zhang Q, Yang Y. Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Sci Rep. 2016;6:37566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 51. | Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018;2018:8429042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 52. | Song Y, Lim JY, Lim T, Im KI, Kim N, Nam YS, Jeon YW, Shin JC, Ko HS, Park IY, Cho SG. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J Stem Cells. 2020;12:1032-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Wang R, Wang X, Yang S, Xiao Y, Jia Y, Zhong J, Gao Q, Zhang X. Umbilical cord-derived mesenchymal stem cells promote myeloid-derived suppressor cell enrichment by secreting CXCL1 to prevent graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy. 2021;23:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, Zhao S, Luo L, Wei H, Guan L, Gao Z, Li Y, Wang L, Liu D, Gao C. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016;25:1874-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 55. | Nekanti U, Mohanty L, Venugopal P, Balasubramanian S, Totey S, Ta M. Optimization and scale-up of Wharton's jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton's Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 57. | Fu X, Qu Z, Sheng Z. Potentiality of mesenchymal stem cells in regeneration of sweat glands. J Surg Res. 2006;136:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Fong CY, Subramanian A, Biswas A, Gauthaman K, Srikanth P, Hande MP, Bongso A. Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton's jelly stem cells. Reprod Biomed Online. 2010;21:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 60. | Du T, Cheng J, Zhong L, Zhao XF, Zhu J, Zhu YJ, Liu GH. The alleviation of acute and chronic kidney injury by human Wharton's jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy. 2012;14:1215-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Tsai MT, Li WJ, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, Chen W, Sun L, Wang S. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 63. | Nilforoushzadeh MA, Raoofi A, Afzali H, Gholami O, Zare S, Nasiry D, Khodaverdi Darian E, Rustamzadeh A, Alavi S, Ahmadi R, Alimohammadi A, Razzaghi Z, Safaie Naraghi Z, Mahmoudbeyk M, Amirkhani MA, Mousavi Khaneghah A. Promotion of cutaneous diabetic wound healing by subcutaneous administration of Wharton's jelly mesenchymal stem cells derived from umbilical cord. Arch Dermatol Res. 2023;315:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Mathen C, Ghag Sawant M, Gupta R, Dsouza W, Krishna SG. Evaluation of Potential Application of Wharton's Jelly-Derived Human Mesenchymal Stromal Cells and its Conditioned Media for Dermal Regeneration using Rat Wound Healing Model. Cells Tissues Organs. 2021;210:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Jalali MS, Sarkaki A, Farbood Y, Azandeh SS, Mansouri E, Ghasemi Dehcheshmeh M, Saki G. Neuroprotective effects of Wharton's jelly-derived mesenchymal stem cells on motor deficits due to Parkinson's disease. Iran J Basic Med Sci. 2021;24:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Jalali MS, Sarkaki A, Farbood Y, Azandeh SS, Mansouri E, Ghasemi Dehcheshmeh M, Saki G. Transplanted Wharton's jelly mesenchymal stem cells improve memory and brain hippocampal electrophysiology in rat model of Parkinson's disease. J Chem Neuroanat. 2020;110:101865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Jalali MS, Saki G, Farbood Y, Azandeh SS, Mansouri E, Ghasemi Dehcheshmeh M, Sarkaki A. Therapeutic effects of Wharton's jelly-derived Mesenchymal Stromal Cells on behaviors, EEG changes and NGF-1 in rat model of the Parkinson's disease. J Chem Neuroanat. 2021;113:101921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Choi SJ, Park SY, Shin YH, Heo SH, Kim KH, Lee HI, Kim JK. Mesenchymal Stem Cells Derived from Wharton's Jelly Can Differentiate into Schwann Cell-Like Cells and Promote Peripheral Nerve Regeneration in Acellular Nerve Grafts. Tissue Eng Regen Med. 2021;18:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, Gu DS, Wang D, Du W, Zhu D, Han ZC. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Chao K, Zhang S, Qiu Y, Chen X, Zhang X, Cai C, Peng Y, Mao R, Pevsner-Fischer M, Ben-Horin S, Elinav E, Zeng Z, Chen B, He Y, Xiang AP, Chen M. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5(+) B regulatory cells. Stem Cell Res Ther. 2016;7:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018;12:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Khanh VC, Fukushige M, Chang YH, Hoang NN, Yamashita T, Obata-Yasuoka M, Hamada H, Osaka M, Hiramatsu Y, Ohneda O. Wharton's Jelly Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduce SARS-CoV2-Induced Inflammatory Cytokines Under High Glucose and Uremic Toxin Conditions. Stem Cells Dev. 2021;30:758-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Saleh M, Vaezi AA, Aliannejad R, Sohrabpour AA, Kiaei SZF, Shadnoush M, Siavashi V, Aghaghazvini L, Khoundabi B, Abdoli S, Chahardouli B, Seyhoun I, Alijani N, Verdi J. Cell therapy in patients with COVID-19 using Wharton's jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 74. | Ouzin M, Kogler G. Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 75. | El-Kadiry AE, Rafei M, Shammaa R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front Med (Lausanne). 2021;8:756029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 76. | Stucky A, Gao L, Li SC, Tu L, Luo J, Huang X, Chen X, Li X, Park TH, Cai J, Kabeer MH, Plant AS, Sun L, Zhang X, Zhong JF. Molecular Characterization of Differentiated-Resistance MSC Subclones by Single-Cell Transcriptomes. Front Cell Dev Biol. 2022;10:699144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 77. | Doi C, Maurya DK, Pyle MM, Troyer D, Tamura M. Cytotherapy with naive rat umbilical cord matrix stem cells significantly attenuates growth of murine pancreatic cancer cells and increases survival in syngeneic mice. Cytotherapy. 2010;12:408-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Maurya DK, Doi C, Kawabata A, Pyle MM, King C, Wu Z, Troyer D, Tamura M. Therapy with un-engineered naïve rat umbilical cord matrix stem cells markedly inhibits growth of murine lung adenocarcinoma. BMC Cancer. 2010;10:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 80. | Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy. 2017;19:1351-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 81. | Pochon C, Notarantonio AB, Laroye C, Reppel L, Bensoussan D, Bertrand A, Rubio MT, D'Aveni M. Wharton's jelly-derived stromal cells and their cell therapy applications in allogeneic haematopoietic stem cell transplantation. J Cell Mol Med. 2022;26:1339-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Nie WB, Zhang D, Wang LS. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des Devel Ther. 2020;14:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 83. | Puig-Pijuan T, de Godoy MA, Pinheiro Carvalho LR, Bodart-Santos V, Lindoso RS, Pimentel-Coelho PM, Mendez-Otero R. Human Wharton's jelly mesenchymal stem cells protect neural cells from oxidative stress through paracrine mechanisms. Future Sci OA. 2020;6:FSO627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 85. | Sanchez V, Villalba N, Fiore L, Luzzani C, Miriuka S, Boveris A, Gelpi RJ, Brusco A, Poderoso JJ. Characterization of Tunneling Nanotubes in Wharton's jelly Mesenchymal Stem Cells. An Intercellular Exchange of Components between Neighboring Cells. Stem Cell Rev Rep. 2017;13:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 87. | Nicolay NH, Lopez Perez R, Debus J, Huber PE. Mesenchymal stem cells – A new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Chang PY, Qu YQ, Wang J, Dong LH. The potential of mesenchymal stem cells in the management of radiation enteropathy. Cell Death Dis. 2015;6:e1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Zhang X, Xia Y, Ji C. Human umbilical cord blood stem cells applied in therapy for ionizing radiation injury. Integr Mol Med. 2015;2:284-287. [DOI] [Full Text] |
| 90. | Kovalenko OA, Azzam EI, Ende N. Human umbilical-cord-blood mononucleated cells enhance the survival of lethally irradiated mice: dosage and the window of time. J Radiat Res. 2013;54:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Maurya DK, Doi C, Pyle M, Rachakatla RS, Davis D, Tamura M, Troyer D. Non-random tissue distribution of human naïve umbilical cord matrix stem cells. World J Stem Cells. 2011;3:34-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 92. | Maurya DK, Bandekar M, Sandur SK. Soluble factors secreted by human Wharton's jelly mesenchymal stromal/stem cells exhibit therapeutic radioprotection: A mechanistic study with integrating network biology. World J Stem Cells. 2022;14:347-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 93. | Sun J, Zhang Y, Song X, Zhu J, Zhu Q. The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats. Cell Transplant. 2019;28:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 94. | Sun W, Luo L, Feng Y, Cai Y, Zhuang Y, Xie RJ, Chen X, Chen H. Aggregation-Induced Emission Gold Clustoluminogens for Enhanced Low-Dose X-ray-Induced Photodynamic Therapy. Angew Chem Int Ed Engl. 2020;59:9914-9921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 95. | Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 659] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 96. | Coppes RP, Meter A, Latumalea SP, Roffel AF, Kampinga HH. Defects in muscarinic receptor-coupled signal transduction in isolated parotid gland cells after in vivo irradiation: evidence for a non-DNA target of radiation. Br J Cancer. 2005;92:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Ma C, Xie J, Chen Q, Wang G, Zuo S. Amifostine for salivary glands in high-dose radioactive iodine treated differentiated thyroid cancer. Cochrane Database Syst Rev. 2009;2009:CD007956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Ballivy O, Galiana Santamaría R, Lozano Borbalas A, Guedea Edo F. Clinical application of intensity-modulated radiotherapy for head and neck cancer. Clin Transl Oncol. 2008;10:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Schwarz S, Huss R, Schulz-Siegmund M, Vogel B, Brandau S, Lang S, Rotter N. Bone marrow-derived mesenchymal stem cells migrate to healthy and damaged salivary glands following stem cell infusion. Int J Oral Sci. 2014;6:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Xiong X, Shi X, Chen F. Human adipose tissuederived stem cells alleviate radiationinduced xerostomia. Int J Mol Med. 2014;34:749-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Käsmann L, Dietrich A, Staab-Weijnitz CA, Manapov F, Behr J, Rimner A, Jeremic B, Senan S, De Ruysscher D, Lauber K, Belka C. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 103. | Hao Y, Ran Y, Lu B, Li J, Zhang J, Feng C, Fang J, Ma R, Qiao Z, Dai X, Xiong W, Liu J, Zhou Q, Hao J, Li R, Dai J. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Canine Radiation-Induced Lung Injury. Int J Radiat Oncol Biol Phys. 2018;102:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Zhang C, Zhu Y, Wang J, Hou L, Li W, An H. CXCR4-Overexpressing Umbilical Cord Mesenchymal Stem Cells Enhance Protection against Radiation-Induced Lung Injury. Stem Cells Int. 2019;2019:2457082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Belzile-Dugas E, Eisenberg MJ. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J Am Heart Assoc. 2021;10:e021686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 106. | Banfill K, Giuliani M, Aznar M, Franks K, McWilliam A, Schmitt M, Sun F, Vozenin MC, Faivre Finn C; IASLC Advanced Radiation Technology committee. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol. 2021;16:216-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 107. | van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 108. | Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, Yao H, Wu SH. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 109. | Chen ZY, Hu YY, Hu XF, Cheng LX. The conditioned medium of human mesenchymal stromal cells reduces irradiation-induced damage in cardiac fibroblast cells. J Radiat Res. 2018;59:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Wang G, Ren X, Yan H, Gui Y, Guo Z, Song J, Zhang P. Neuroprotective Effects of Umbilical Cord-Derived Mesenchymal Stem Cells on Radiation-Induced Brain Injury in Mice. Ann Clin Lab Sci. 2020;50:57-64. [PubMed] |
| 111. | Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, Limoli CL. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:19150-19155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 112. | Acharya MM, Martirosian V, Christie LA, Riparip L, Strnadel J, Parihar VK, Limoli CL. Defining the optimal window for cranial transplantation of human induced pluripotent stem cell-derived cells to ameliorate radiation-induced cognitive impairment. Stem Cells Transl Med. 2015;4:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |