Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.591
Revised: February 20, 2024
Accepted: April 2, 2024
Published online: May 26, 2024
Processing time: 115 Days and 4.5 Hours
Aplastic anemia (AA) presents a significant clinical challenge as a life-threatening condition due to failure to produce essential blood cells, with the current the
To assess the therapeutic potential of ginsenoside Rg1 on AA, specifically its protective effects, while elucidating the mechanism at play.
We employed a model of myelosuppression induced by cyclophosphamide (CTX) in C57 mice, followed by administration of ginsenoside Rg1 over 13 d. The investigation included examining the bone marrow, thymus and spleen for pathological changes via hematoxylin-eosin staining. Moreover, orbital blood of mice was collected for blood routine examinations. Flow cytometry was employed to identify the impact of ginsenoside Rg1 on cell apoptosis and cycle in the bone marrow of AA mice. Additionally, the study further evaluated cytokine levels with enzyme-linked immunosorbent assay and analyzed the expression of key proteins in the MAPK signaling pathway via western blot.
Administration of CTX led to significant damage to the bone marrow’s structural integrity and a reduction in hematopoietic cells, establishing a model of AA. Ginsenoside Rg1 successfully reversed hematopoietic dysfunction in AA mice. In comparison to the AA group, ginsenoside Rg1 provided relief by reducing the induction of cell apoptosis and inflammation factors caused by CTX. Furthermore, it helped alleviate the blockade in the cell cycle. Treatment with ginsenoside Rg1 significantly alleviated myelosuppression in mice by inhibiting the MAPK signaling pathway.
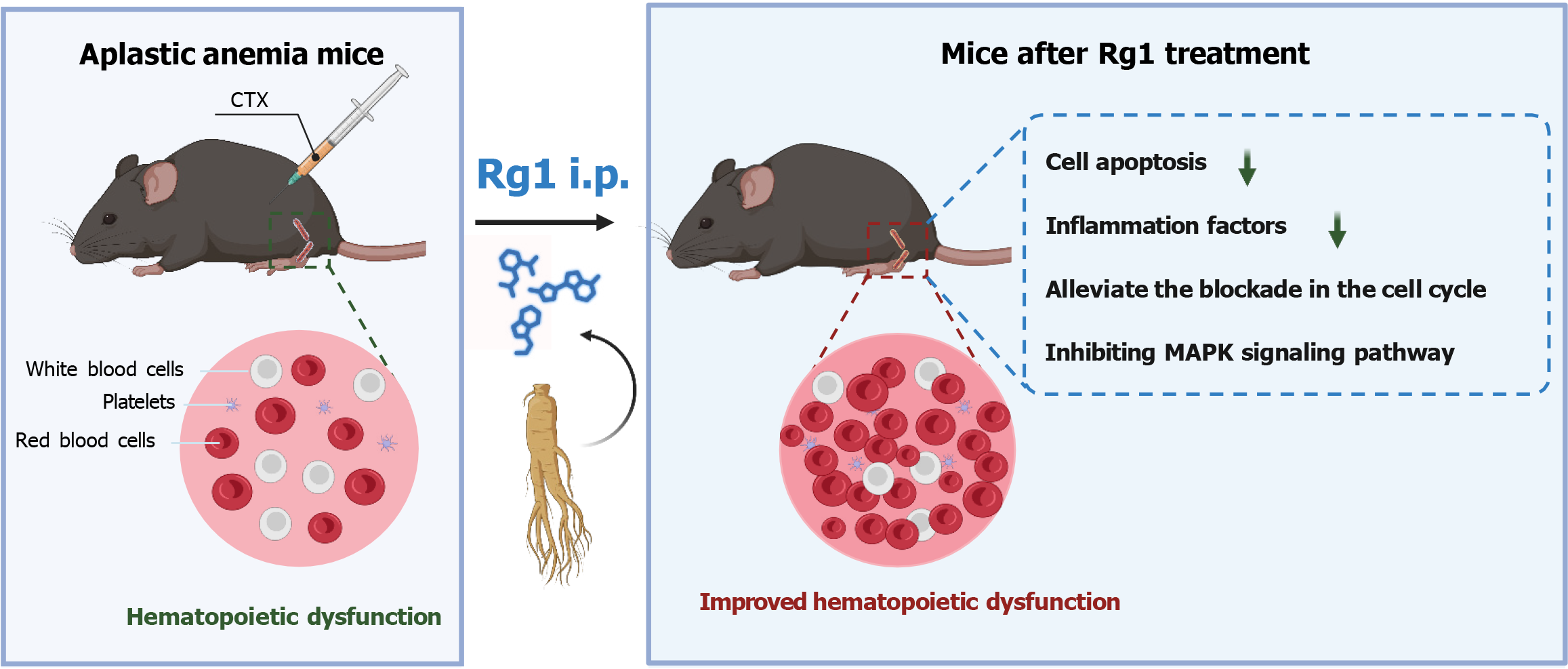
This study suggested that ginsenoside Rg1 addresses AA by alleviating myelosuppression, primarily through modulating the MAPK signaling pathway, which paves the way for a novel therapeutic strategy in treating AA, highlighting the potential of ginsenoside Rg1 as a beneficial intervention.
Core Tip: This study evaluated ginsenoside Rg1’s efficacy in aplastic anemia (AA) treatment, focusing on its impact on bone marrow pathology, hematopoiesis, and inflammation in a cyclophosphamide-induced myelosuppression C57 mice model. It demonstrated that ginsenoside Rg1 not only reduces cell apoptosis and inflammation but also promotes hematopoietic recovery by inhibiting the MAPK signaling pathway, offering a potential therapeutic strategy for AA.
- Citation: Wang JB, Du MW, Zheng Y. Effect of ginsenoside Rg1 on hematopoietic stem cells in treating aplastic anemia in mice via MAPK pathway. World J Stem Cells 2024; 16(5): 591-603
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/591.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.591
Aplastic anemia (AA) is an abnormality of primary or secondary bone marrow hematopoietic stem cells (HSCs) and/or hematopoietic microenvironment caused by physical, chemical and biological factors[1]. AA affects individuals across all age groups, primarily presenting as pancytopenia, a condition that significantly increases the risk of severe infections, anemia, and bleeding complications[2]. The primary cause of AA is the abnormal activation and overuse of T lym
Ginseng (Panax ginseng C. A. Meyer) is a perennial herb of the Araliaceae family and is a traditional Chinese medicine that has the functions of invigorating vitality, invigorating body fluid and solidifying hair, invigorating the spleen and benefiting the lungs, and soothing the nerves[8]. Ginsenosides, as the main active components of ginseng, are mainly classified into dammarane-type tetracyclic triterpene saponins, ocotillol-type tetracyclic triterpenoid saponins, and oleanolic acid-type saponins[9]. Recent research has revealed that ginsenosides possess a broad spectrum of pharmacological benefits, encompassing myocardial protection, modulation of glucose and lipid metabolism, antioxidative properties, anti-tumor activity, and therapeutic effects on hematological disorders[10-13]. Ginsenosides can promote the proliferation and differentiation of erythroid, granulosa monoline, and megakaryocyte cell lines, which may be related to its effect on hematopoietic growth factors[14]. Moreover, research has demonstrated that total saponins of Panax ginseng enhance the production of erythropoietin (EPO), granulocyte-macrophage colony-stimulating factor (CSF), interleukin-3 (IL-3), IL-6 and receptors EPOR and GM-CSFRα[15]. Furthermore, ginsenosides have been shown to promote the upregulation of GATA-2 mRNA expression and protein synthesis in hematopoietic progenitor cells. This increase in GATA-2 activity not only enhanced its DNA binding affinity but also regulated the expression of genes related to proliferation, thereby stimulating the proliferation of blood cells[16].
The growth, development, division, and death of cells, as well as the recognition, transmission, and amplification of various biochemical reaction signals within cells, are all related to the MAPK signaling pathway[17,18]. Studies have found that ginsenoside Rg1 inhibited the expression of inflammatory factors (IL-1β and IL-6) through the MAPK pathway, and reduced the nerve injury of ischemia-reperfusion[19]. Ginsenoside Rg1 can also promote the proliferation of bone marrow endothelial progenitor cells through the MAPK pathway[20].
In this study, a C57 mice model of bone marrow suppression was established by cyclophosphamide (CTX), and the pathological changes of the bone marrow were observed by hematoxylin-eosin (HE) staining, which proved that the model was successful. Orbital blood was taken from the mice to examine the composition of cells in the blood. The cellular components and protein expression changes of the MAPK signaling pathway in bone marrow were detected to verify whether ginsenoside Rg1 can alleviate myelosuppression in mice through the MAPK signaling pathway, and provide a new theoretical basis for clinical treatment of AA.
A total of 25 male C57BL/6 mice of specific pathogen free grade (6-8 wk old) were selected and freely ingested water and food. Room temperature was maintained at 20-24 °C and relative humidity at 40%-60%. The mice were purchased from Servicebio Technology Co., Ltd (Wuhan Optics Valley Biolake, Hubei, China). For the CTX group, 200 mg of powder was dissolved in 20 mL 0.9% saline water according to previous article[21]. The mice were randomly divided into five groups: Control group, Control + Rg1 (15 mg/kg) group, CTX + saline group, CTX + Rg1 (10 mg/kg) group, and CTX + Rg1 (15 mg/kg) group, with 5 mice in each group, in which the CTX and ginsenoside Rg1 were purchased from Sigma (St Louis, MO, United States).
Blood was extracted from the orbit when the mice in each group were under anesthetic sedation according to IACUC procedure. Blood routine examination, including white blood cells, red blood cells, hemoglobin, platelets, neutrophil, and lymphocytes, was performed using the whole blood by a routine blood test instrument[22] (RJ-0C107223; Mindray, Nanshan, Shenzhen, China).
According to the protocol of HE Staining Kit (Solarbio, Beijing, China), the sections (4-μm-thick) from bone marrow, thymus, and spleen slices of each group of mice were stained with HE for 1 min and differentiated in the acidic liquid alcohol for 30 s. Following the staining periods, sections were dehydrated by 95% ethanol for 50 s. Finally, sections were cleared in xylene and mounted[23]. Images were obtained by Ci-L microscope (Nikon, Tokyo, Japan), and analyzed by the Image J software (La Jolla, CA, United States).
Bone marrow cells were collected for flow cytometric analysis[24]. Bone marrow cells were then seeded into six-well plates at a density of 105 cells per well in 2 mL of medium supplemented with 10% fetal bovine serum (Gibco, ThermoFisher Scientific, Waltham, MA, United States). After incubation for different time intervals, cells were harvested and washed twice by phosphate-buffered saline (PBS) and trypsinized by 0.25% trypsin. Then, harvested cells were centrifuged at 1500 rpm for 5 min. After resuspension in 5 mL of PBS, the cells were analyzed by flow cytometry (Attune Cytometry; BD Biosciences, Franklin Lakes, NJ, United States).
We prepared the bone marrow or cell lysates for western blot[25]. For this, we utilized Radio Immunoprecipitation Assay cell lysis buffer and acquired the supernatant by centrifuging at 12000 rpm for 15 min at 4 °C. The Bicinchoninic Acid Assay Kit (Beyotime, Jiangsu, China) was employed to measure the total protein content. From the supernatant samples, 20 μg were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred onto a polyvinylidene fluoride membrane. Following this, the membrane was blocked at room temperature in TBS-T for 2 h and then washed three times with PBS. To conduct the immunoblot, the primary antibody was incubated overnight at 4 °C. Protein samples were incubated with Caspase3 (1:2000; Proteintech, Wuhan, China), Caspase9 (1:2000; Proteintech), Bcl2 (1:2000; Proteintech), Bax (1:2000; Proteintech), IL-6 (1:2000; Proteintech), CCL2 (1:2000; Proteintech), IL-1β (1:2000; Proteintech), P38 (1:2000; Abcam, Cambridge, United Kingdom), p-P38 (1:1000; Abcam), JNK (1:1500; Abcam), p-JNK (1:1000; Abcam), ERK (1:1000; Abcam), p-ERK (1:1000; Abcam). After incubation with the secondary antibody at room temperature for 2 h, the membrane was washed again and detected using electrochemiluminescence. The sizes of the bands were measured using Scion Image 4.0 software (Scion Corporation, Frederick, MD, United States). The sample loading amount was adjusted to a reference standard of GAPDH (1:8000; Proteintech) or β-actin (1:10000; Abclonal, Woburn, MA, United States).
To perform primary culture of bone marrow HSCs, bone marrow was obtained from control group mice (normal group) and mice injected with CTX (AA group)[26]. The bone marrow was then filtered using 100-mesh steel and aspirated and blown with a Pasteur pipette several times to create a suspension of single cells. This suspension was then placed in a 10 cm culture dish at a concentration of 1 × 106 cells/mL using the whole bone marrow culture method. Each dish was filled with 8 mL of culture medium, which includes 100 U/mL penicillin (Procell Life Science & Technology, China), 100 μg/mL streptomycin (Procell Life Science & Technology), 15% fetal bovine serum (Gibco), and regular low-sugar Dulbecco’s Modified Eagle’s Medium (Gibco). The culture dishes were then placed in a humidified incubator at 37 °C and 5% CO2 for primary culture. Then, to investigate the therapeutic effects of Rg1 on cellular sources of AA in vitro, add various concentrations of Rg1 (5 μM, 10 μM, and 15 μM) with different AA groups.
We quantified the levels of IL-10, IL-6, IL-1β, and tumor necrosis factor-alpha (TNF-α) levels in serum and cell culture supernatant were conducted using enzyme-linked immunosorbent assay (ELISA) kits (Beyotime). The assays were meticulously performed in strict accordance with the protocol specified by the manufacturer’s instructions, ensuring accurate and reliable measurement of these cytokines[27].
SPSS 24.0 software was used for statistical analysis of experimental data, measurement data were expressed as mean ± SD, and t-test (comparison between two groups) or LSD analysis of variance was used when normal distribution and homogeneity of variance were satisfied (comparison between multiple groups). Dunnett’s-T3 was used to compare uneven variance, and P < 0.05 was considered statistically significant.
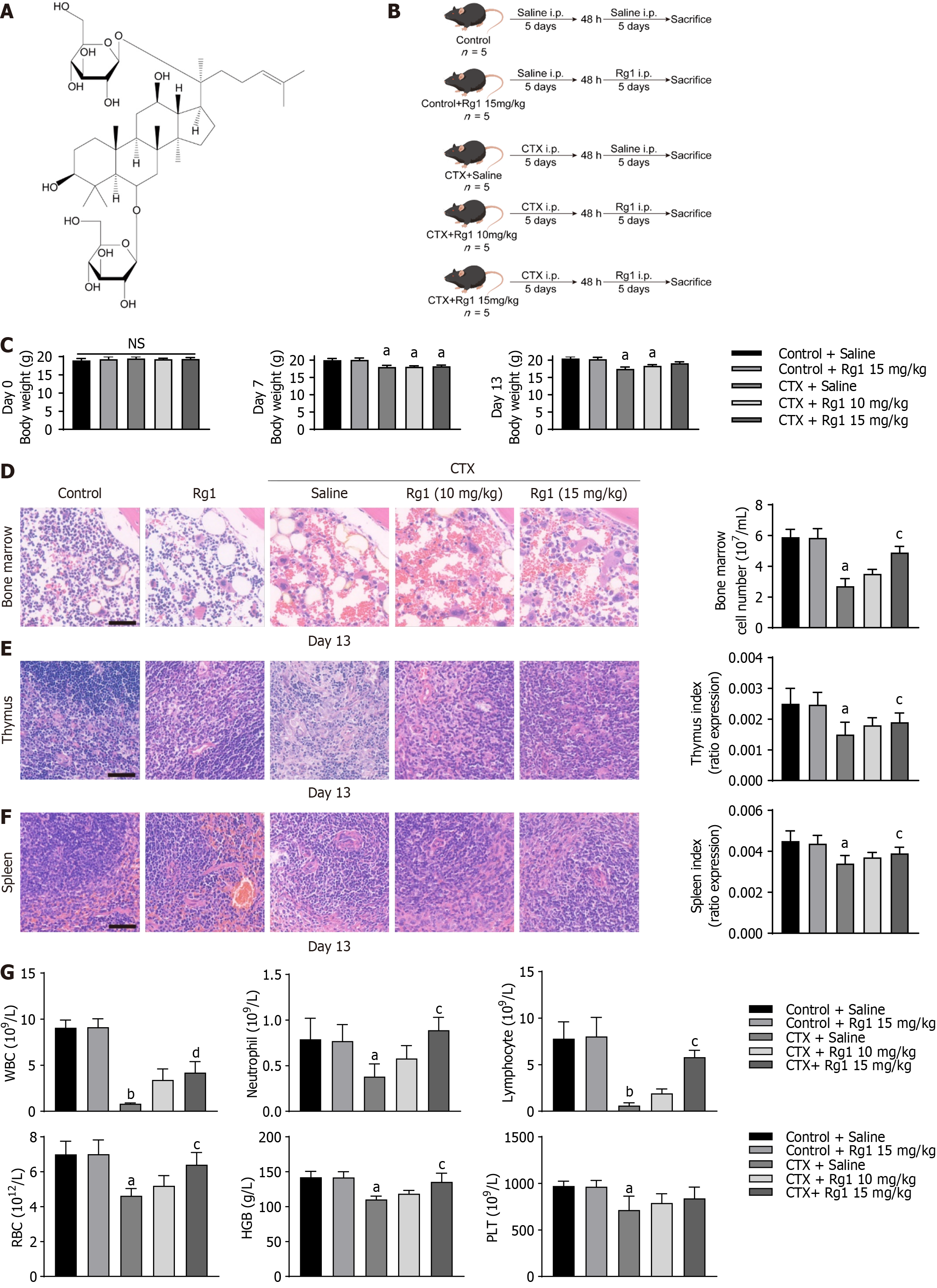
To investigate the inhibitory effect of ginsenoside Rg1 on CTX model mice, we injected different doses of ginsenoside Rg1 based on the CTX model (Figure 1A and B). Notably, mice in the CTX group exhibited a reduction in body weight compared to the control group after 7 and 13 d of treatment; however, there was no significant difference of body weight in the CTX + Rg1 (15 mg/kg) group (Figure 1C). Additionally, the number of bone marrow cells, along with the thymus and spleen indices, significantly decreased in mice following CTX injection. This decline was effectively mitigated by the administration injection of Rg1 (15 mg/kg), as depicted in Figure 1D-F, indicating that Rg1 may ameliorate CTX-induced myelosuppression. Furthermore, we investigated the effect of ginsenoside Rg1 on the bone marrow hematopoietic function of CTX mice. Without a doubt, the counts of white blood cells (WBC), neutrophil, lymphocytes, red blood cells (RBC), hemoglobin (HGB), and platelets (PLT) were significantly decreased in the CTX group mice compared to the control group. In contrast to the CTX group, the counts of WBC, neutrophil, lymphocytes, RBC, and HGB in mice of CTX + Rg1 (15 mg/kg) group were markedly increased, but not PLT count (Figure 1G). These outcomes suggest that ginsenoside Rg1 can partially reverse the hematopoietic dysfunction induced by CTX in mice.
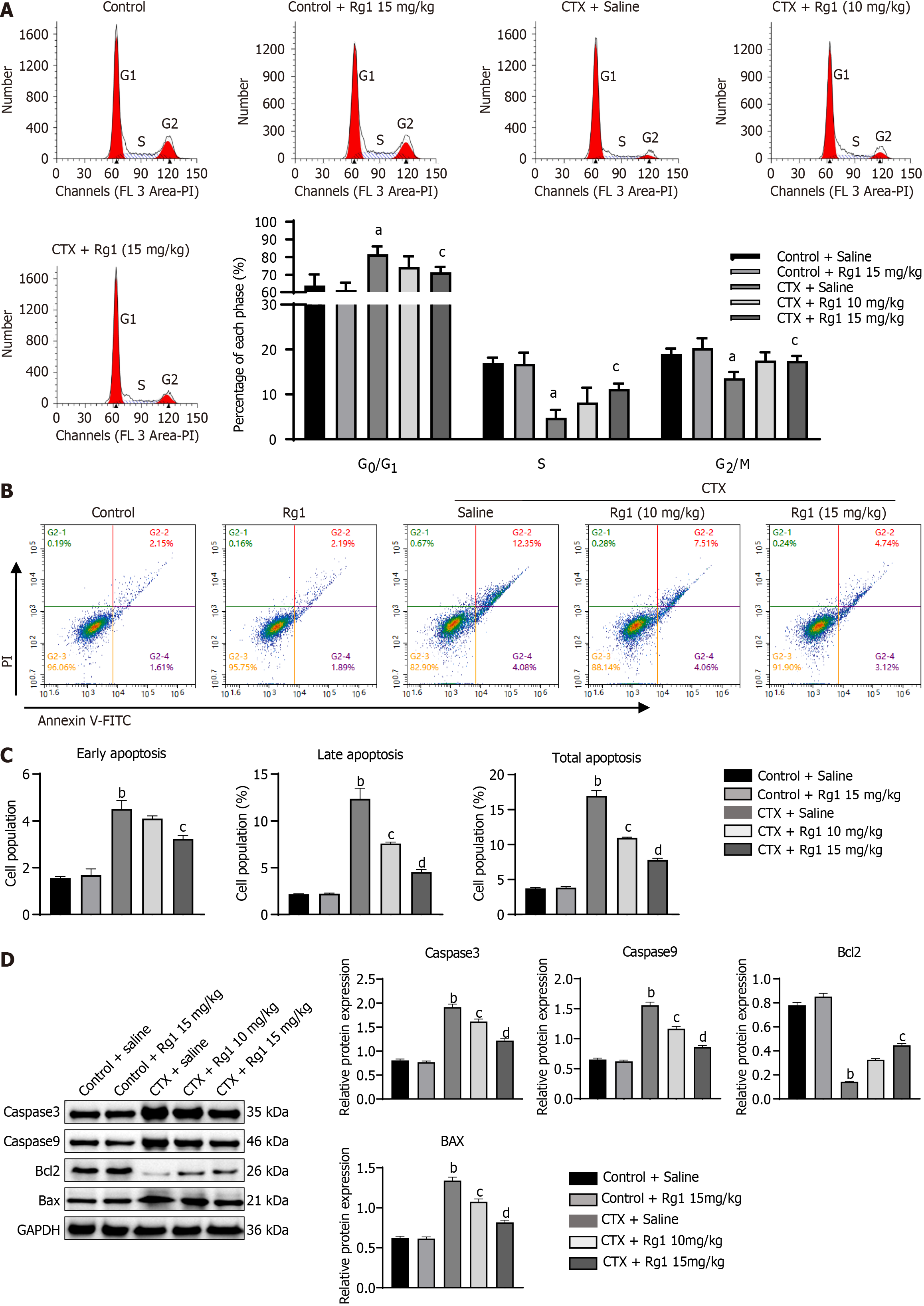
Next, we investigated the potential influence of ginsenoside Rg1 on the self-renewal of HSCs in AA mice. The alterations in the cell cycle and apoptosis of HSCs were evaluated individually among various treatment groups. The results showed that CTX significantly increased the number of G0/G1 and decreased the number of S and G2/M phase cells, which indicates that CTX significantly suppressed cell growth. However, these changes were remarkably reversed by ginsenoside Rg1 (15 mg/kg) treatment (Figure 2A). Furthermore, flow cytometry showed that CTX significantly increased apoptosis of HSCs compared with the control group. However, ginsenoside Rg1 (10 mg/kg and 15 mg/kg) partially reversed apoptosis of HSCs (Figure 2B and C). The expression of the classic apoptotic markers (Caspase3, Caspase9, and Bax) were significantly increased in CTX group when compared to the control group, while the expression of the anti-apoptotic molecule Bcl2 was inhibited. Interestingly, the administration of ginsenoside Rg1 had a significant reversing effect on this phenomenon (Figure 2D).
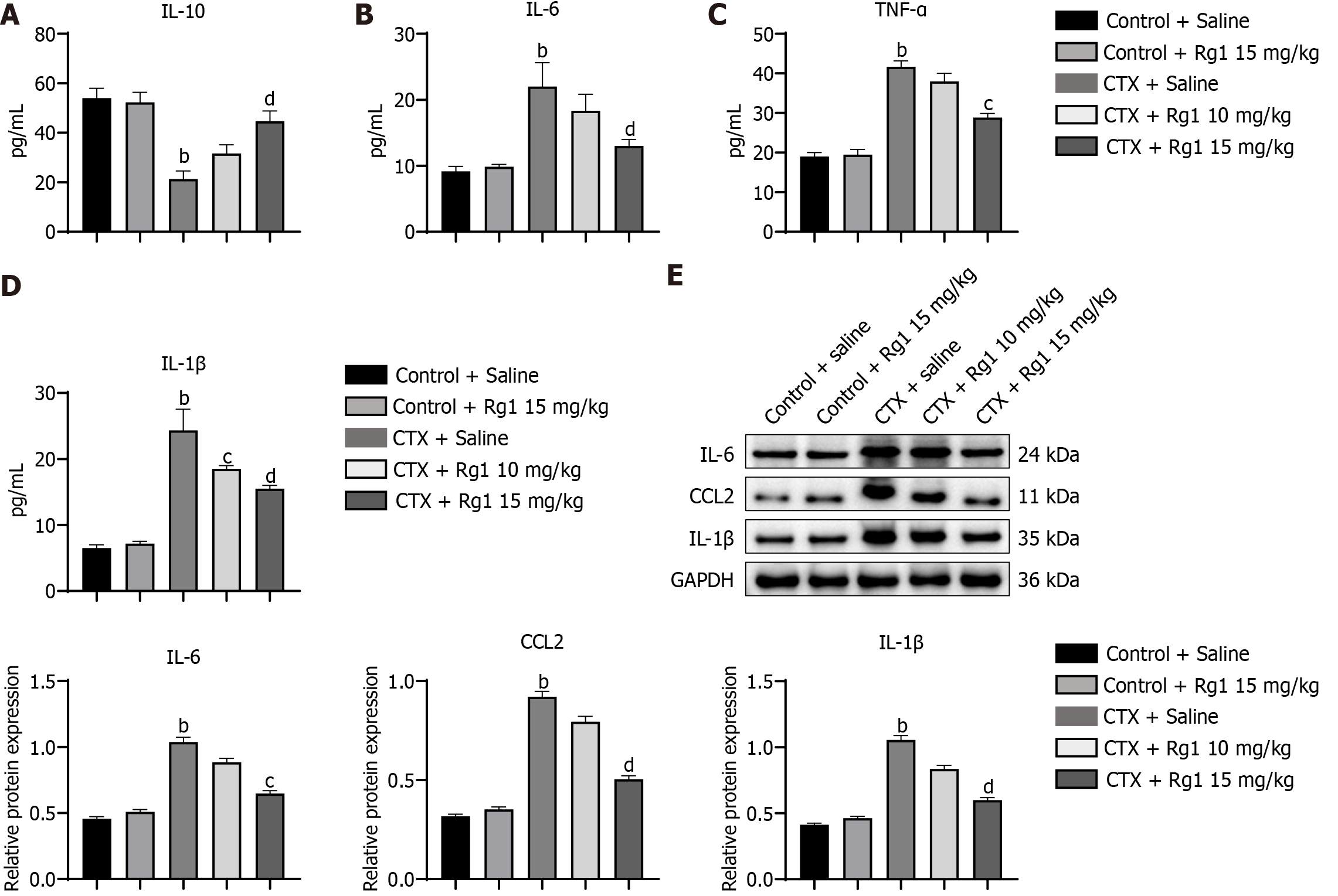
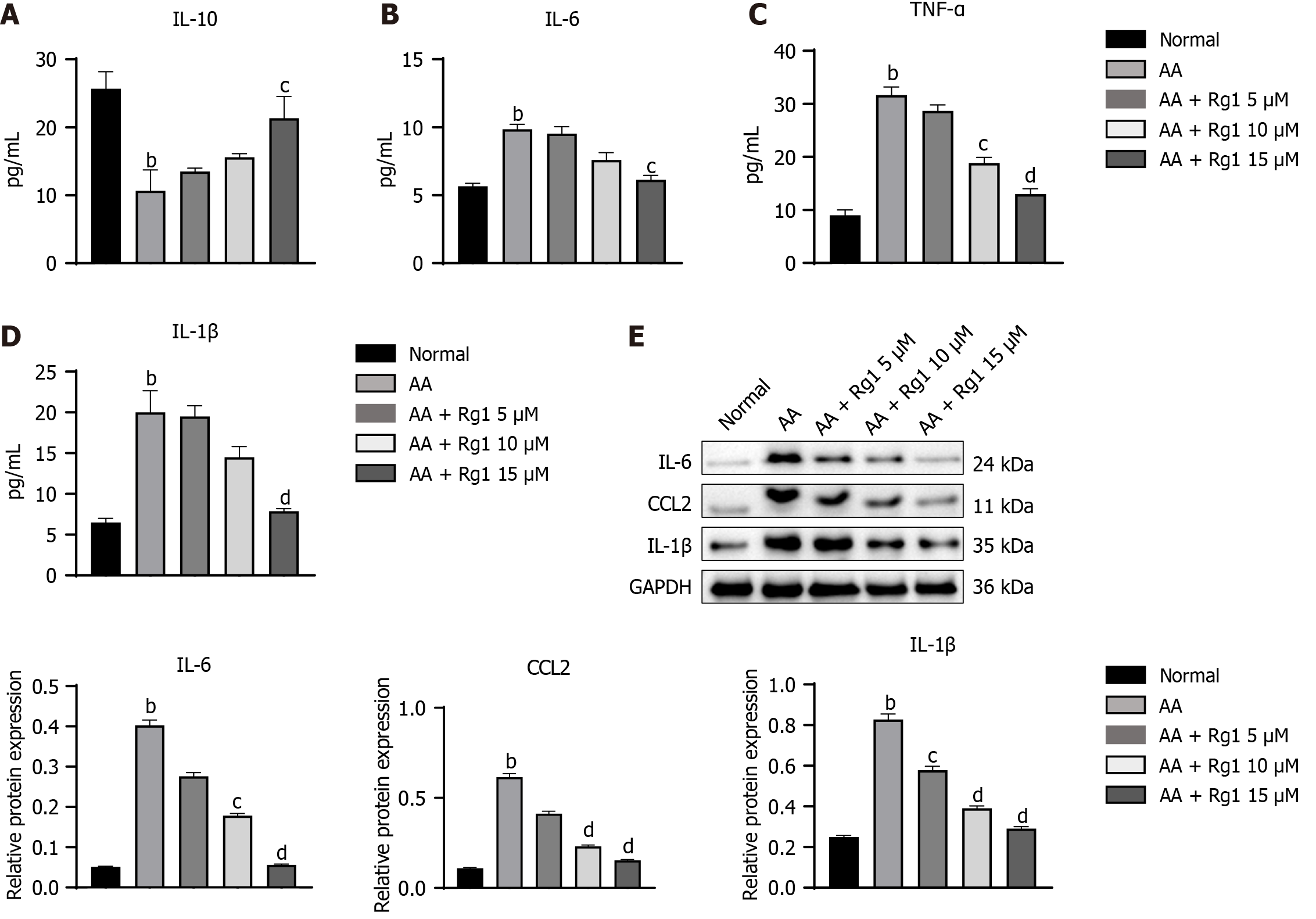
In order to evaluate the effect of ginsenoside Rg1 on the regulation of bone marrow microenvironment, we performed ELISA to measure the inflammatory cytokines (IL-10, IL-6, TNF-α, and IL-1β) in the bone marrow. As shown in Figure 3A-D, the expression of IL-6, TNF-α, and IL-1β induced by CTX was suppressed in the ginsenoside Rg1 group, while the expression of IL-10 was promoted by ginsenoside Rg1. Meanwhile, western blot analysis also showed the same trend of these cytokines in the bone marrow (Figure 3E).
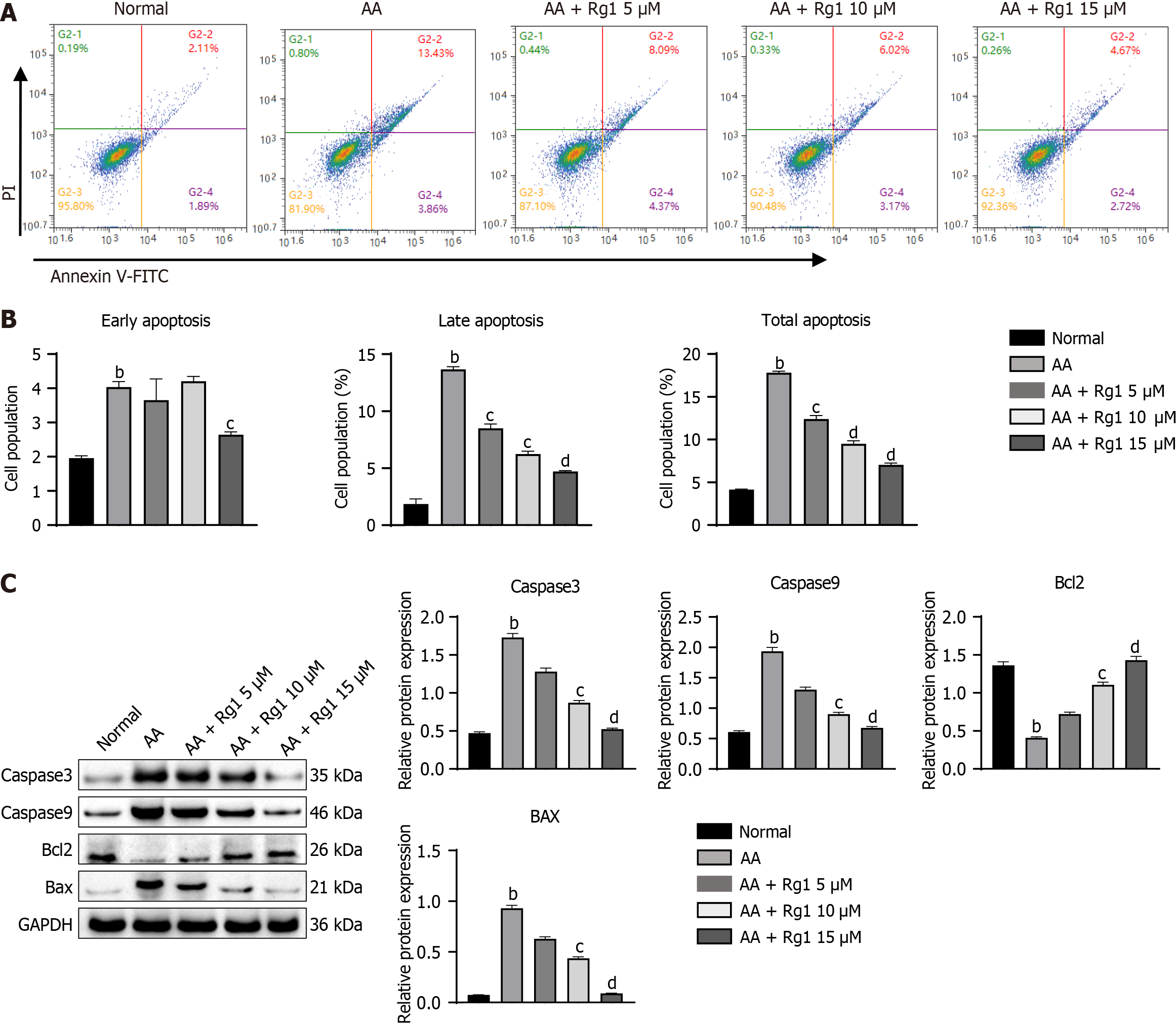
Next, we investigated the effects of ginsenoside Rg1 on the in vitro growth, proliferation, and apoptosis of HSCs from AA mice. Compared with HSCs cells from the control group mice, HSCs cells from AA mice showed a significant increase in apoptosis ability, including early apoptosis, late apoptosis, and total apoptosis rate (Figure 4A and B). However, the addition of different concentrations of ginsenoside Rg1 could suppress the apoptosis of HSCs cells from AA mice (Figure 4A and B). The expression of Caspase3, Caspase9, and Bax in HSCs cells from AA mice was significantly increased, while the expression of the anti-apoptotic molecule Bcl2 was inhibited. Undoubtedly, the addition of ginsenoside Rg1 rescued the apoptosis of HSCs cells from AA mice (Figure 4C).
To evaluate the effects of ginsenoside Rg1 on inflammation regulation in HSCs from AA mice, the levels of IL-10, IL-6, TNF-α, and IL-1β in the supernatant were quantified. As indicated in Figure 5A-D, the ginsenoside Rg1 group exhibited reduced expression of IL-6, TNF-α, and IL-1β, while the expression of IL-10 was increased by ginsenoside Rg1. Additionally, the western blot analysis of these cytokines in the HSCs from AA mice also showed the same pattern (Figure 5E).
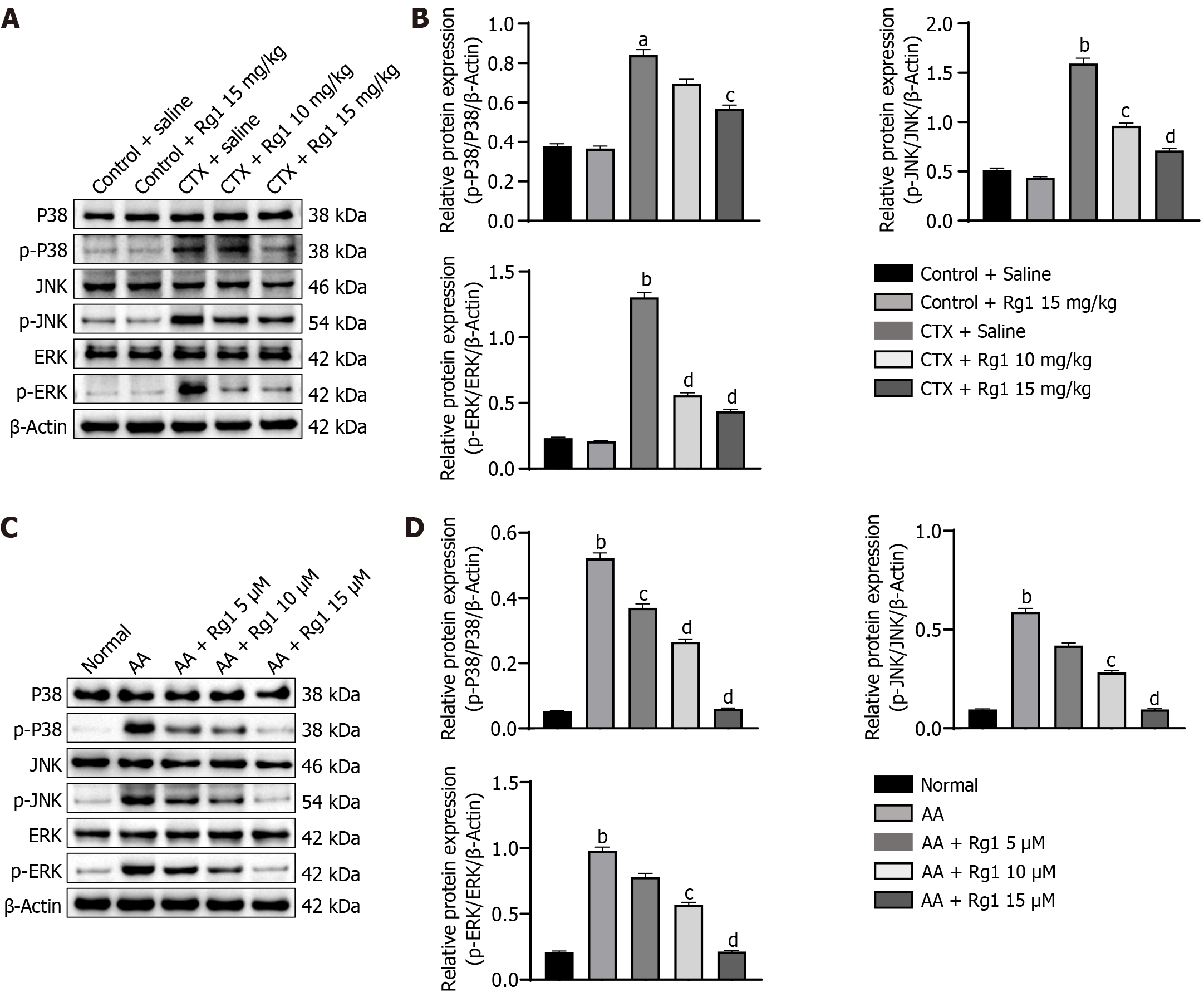
Western blot was used to determine the protein levels of p-p38, p38, p-JNK, JNK, p-ERK, and ERK, respectively. Compared with the control group, the protein expressions of p-p38, p38, p-JNK, JNK, and p-ERK, ERK were significantly increased in the CTX group. However, Rg1 (15 mg/kg) reduced the expression level of p-p38, p38, p-JNK, JNK, and p-ERK, ERK is compared with the CTX group (Figure 6A and B). Additionally, the ginsenoside Rg1 has the same effects in vitro, ginsenosides Rg1 can significantly inhibit the CTX-induced increase in p-p38/p38, p-JNK/JNK, and p-ERK/ERK (Figure 6C and D). From above, we suggested that ginsenoside Rg1 alleviates CTX-induced myelosuppression by inhibiting the MAPK signaling pathway in vivo and in vitro.
AA is recognized as a prevalent, intractable blood disorder affecting childhood. Those diagnosed with AA suffer from anemia due to the dysfunction of body marrow HSCs and/or bone marrow hematopoietic microenvironment[28]. This impairment leads to the substitution of the hematopoietic red pulp with adipose tissue, culminating in a diminished blood cell count[28]. In Chinese traditional medicine, AA is considered to be attributed to “acute consumptive disease” and “bone marrow deficiency”[29]. Increasingly, studies have revealed the effectiveness of traditional Chinese herbal medicine in treating AA[30-32].
This study successfully established a myelosuppressive C57 mice model via injection with CTX. CTX is a commonly used chemotherapeutic agent, which inhibits bone marrow hematopoiesis to induce AA[33]. We observed significant weight loss in mice after 7 and 13 d of CTX administration. In addition, CTX has also decreased the bone marrow cell number, as well as thymus and spleen indices. Routine blood analyses revealed marked decreases in the levels of WBC, lymphocytes, RBC, HGB, and PLT in the CTX group. Ginsenoside Rg1 has been utilized for many years in the treatment of AA and bone marrow damage. It has demonstrated efficacy in mitigating hematopoietic deficiencies and delaying the senescence of hematopoietic stem/progenitor cells, actions attributed to modulation of the SIRT6/nuclear factor-kappaB signaling pathway[34,35], sustains HSC and regulates HSC proliferation in the bone marrow to prevent HSC senescence and recovery of the hematopoietic cells by regulating the SIRT1-forkhead box O3 and SIRT3-superoxide dismutase-2 signaling pathways[36,37]. In this study, ginsenoside Rg1, especially the injection with 15 mg/kg, significantly alleviated CTX-induced myelosuppression in mice. Additionally, ginsenoside Rg1 can significantly alleviate the apoptosis blockade induced by CTX and promote HSCs self-renewal, both in vivo and in vitro. Taken together, these results suggested that ginsenoside Rg1 can ameliorate AA (Figure 7).
HSCs have been shown to play an essential role in hematopoiesis. The homeostasis of HSCs determines the fate of various hematopoietic/blood cells. In mice, HSCs are broadly defined by the Lin - Sca-1 + c-Kit + immunophenotype and divided into two types: Long-term HSC and short-term HSC[38]. The primitive HSCs give rise to committed progenitors, which further differentiate into mature blood cells. In the present study, we observed that CTX significantly affected bone marrow cells by flow cytometry, decreasing the numbers of common myeloid progenitor, granulocyte-macrophage progenitor, megakaryocyte-erythroid progenitor and common lymphoid progenitor. In addition, CTX also increased the apoptosis of HSCs, which was consistent with previous studies. Rg1 ameliorated hematopoietic dysfunction in mice by reversing CTX-induced bone marrow cell damage. According to previous reports, alterations in the ratio of M1/M2 macrophages and levels of interferon-γ and TNF-α can result in the development of AA[39]. Toll-like receptors are responsible for the identification of M1 macrophages, which then release pro-inflammatory cytokines like IL-6, IL-12, and TNF-α, ultimately contributing to the progression of inflammation[40]. During our investigation, a notable reduction in IL-10 expression was observed following CTX treatment, alongside an elevation in the levels of IL-6, TNF-α, and IL-1β. These observations were consistent with previously discussed theories. Remarkably, the introduction of ginsenoside Rg1 led to the reversal of these changes, underscoring its potential efficacy as a therapeutic agent for AA.
When GTP was bound to Ras, Ras was activated and recruited Raf from the cytoplasm to the cell membrane, which ultimately activated MAPK. After MAPK was activated, it transduced into the nucleus and activated transcription factors. Studies have shown that G-CSF combined with GCSFR can promote neutrophil production and differentiation of bone marrow precursor cells into granulocytes through the Ras/MEK/ERK signaling pathway[41,42]. The results of animal experiments showed that G-CSF mediated proliferation of bone marrow cells could be inhibited by MEK kinase inhibitor UO126[43]. In contrast, MAPK activation enhanced the proliferation of G-CSF stimulated state-negative myeloid cells. P38-MAPK was one of the subclasses of MAPK, which can directly participate in cell apoptosis, cytokine production, cytoskeleton recognition and transcriptional regulation[44]. Previous studies have shown that cytoskeletal proteins can directly participate in the regulation of the p38 pathway, play an important role in cell differentiation, migration, proliferation and apoptosis, increase the expression level of tyrosinase genes, and play an important role in bone marrow suppression[45,46]. In this study, we observed a significant upregulation in the ratios of phosphorylated to total forms of p38, JNK, and ERK proteins in the CTX-treated group. Interestingly, treatment with ginsenoside Rg1 markedly reversed the expression levels of these signaling proteins, highlighting its potential in modulating key pathways involved in the stress response and inflammation.
Our research indicated that ginsenoside Rg1 has the potential to become an effective drug for treating AA; however, our research also has certain limitations. Additional in vitro and in vivo studies were needed to clarify the specific effects of ginsenoside Rg1 on the treatment of AA and the target population. Furthermore, drug development is a lengthy and intricate process. While we have shown a certain therapeutic effect of ginsenoside Rg1 on AA model mice, more human clinical trials are still necessary to confirm the efficacy of ginsenoside Rg1.
This research proposed that ginsenoside Rg1 mitigates myelosuppression in mice by targeting and inhibiting the MAPK signaling pathway. This mechanism offers a theoretical foundation for the clinical application of ginsenoside Rg1 in the treatment of AA, paving the way for future research and development in this area.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Rotondo JC, Italy S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S
| 1. | DeZern AE, Churpek JE. Approach to the diagnosis of aplastic anemia. Blood Adv. 2021;5:2660-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Furlong E, Carter T. Aplastic anaemia: Current concepts in diagnosis and management. J Paediatr Child Health. 2020;56:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Kordasti S, Costantini B, Seidl T, Perez Abellan P, Martinez Llordella M, McLornan D, Diggins KE, Kulasekararaj A, Benfatto C, Feng X, Smith A, Mian SA, Melchiotti R, de Rinaldis E, Ellis R, Petrov N, Povoleri GA, Chung SS, Thomas NS, Farzaneh F, Irish JM, Heck S, Young NS, Marsh JC, Mufti GJ. Deep phenotyping of Tregs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood. 2016;128:1193-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Castro-Malaspina H. Aplastic anemia: current concepts on pathogenesis and therapy. Nouv Rev Fr Hematol (1978). 1993;35:183-186. [PubMed] |
| 5. | Scheinberg P. Acquired severe aplastic anaemia: how medical therapy evolved in the 20th and 21st centuries. Br J Haematol. 2021;194:954-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Peslak SA, Olson T, Babushok DV. Diagnosis and Treatment of Aplastic Anemia. Curr Treat Options Oncol. 2017;18:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 8. | Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem Toxicol. 2017;107:362-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H, Zhao H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121:2994-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 10. | Chen J, Zhang X, Liu X, Zhang C, Shang W, Xue J, Chen R, Xing Y, Song D, Xu R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol. 2019;856:172418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, Huang FQ, Liu B, Zhou W, Qi LW, Li J. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11:1703-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 12. | Li L, Wang Y, Guo R, Li S, Ni J, Gao S, Gao X, Mao J, Zhu Y, Wu P, Wang H, Kong D, Zhang H, Zhu M, Fan G. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 13. | Zhen N, Jin L, Ma J, Zhu J, Gu S, Wang J, Pan Q, Ni X, Xu M. Ginsenoside Rg1 impairs homologous recombination repair by targeting CtBP-interacting protein and sensitizes hepatoblastoma cells to DNA damage. Anticancer Drugs. 2018;29:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Yang HY, Liu ML, Luo P, Yao XS, Zhou H. Network pharmacology provides a systematic approach to understanding the treatment of ischemic heart diseases with traditional Chinese medicine. Phytomedicine. 2022;104:154268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Raghavendran HR, Sathyanath R, Shin J, Kim HK, Han JM, Cho J, Son CG. Panax ginseng modulates cytokines in bone marrow toxicity and myelopoiesis: ginsenoside Rg1 partially supports myelopoiesis. PLoS One. 2012;7:e33733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Suzuki M, Shimizu R, Yamamoto M. Transcriptional regulation by GATA1 and GATA2 during erythropoiesis. Int J Hematol. 2011;93:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Falcicchia C, Tozzi F, Arancio O, Watterson DM, Origlia N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 18. | Zheng Y, Han Z, Zhao H, Luo Y. MAPK: A Key Player in the Development and Progression of Stroke. CNS Neurol Disord Drug Targets. 2020;19:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Xiong L, Tang W, Tang L, Wang B. Hypoxic culture enhances the expansion of rat bone marrow-derived mesenchymal stem cells via the regulatory pathways of cell division and apoptosis. In Vitro Cell Dev Biol Anim. 2018;54:666-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Feng L, Huang Q, Huang Z, Li H, Qi X, Wang Y, Liu Z, Liu X, Lu L. Optimized Animal Model of Cyclophosphamide-induced Bone Marrow Suppression. Basic Clin Pharmacol Toxicol. 2016;119:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Spencer NJ, Fryer AA, Farmer AD, Duff CJ. Blood test monitoring of immunomodulatory therapy in inflammatory disease. BMJ. 2021;372:n159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Grosset AA, Loayza-Vega K, Adam-Granger É, Birlea M, Gilks B, Nguyen B, Soucy G, Tran-Thanh D, Albadine R, Trudel D. Hematoxylin and Eosin Counterstaining Protocol for Immunohistochemistry Interpretation and Diagnosis. Appl Immunohistochem Mol Morphol. 2019;27:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Selliah N, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc Cytom. 2019;87:e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Lian Y, Niu X, Cai H, Yang X, Ma H, Ma S, Zhang Y, Chen Y. Clinicopathological significance of c-MYC in esophageal squamous cell carcinoma. Tumour Biol. 2017;39:1010428317715804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 685] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 27. | Tabatabaei MS, Ahmed M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol Biol. 2022;2508:115-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 28. | Young NS. Aplastic Anemia. N Engl J Med. 2018;379:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (60)] |
| 29. | Zhu N, Wu D, Ye B. The Progress of Traditional Chinese Medicine in the Treatment of Aplastic Anemia. J Transl Int Med. 2018;6:159-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Yang H, Xu X, Jiang X, Yao Z. Treatment of menorrhagia due to aplastic anemia by hysteroscopic resection of endometrial functional layer and levonorgestrel-releasing intra-uterine system: Three case reports. Medicine (Baltimore). 2019;98:e15156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Wu D, Shen Y, Ye B, Fang B, Lin S, Chen Z, Jiang H, Feng C, He HL, Gao Y, Liu Y, Zhu J, Wu L, Shao K, Keding S, Zhou Y. Efficacy and advantages of modified Traditional Chinese Medicine treatments based on "kidney reinforcing" for chronic aplastic anemia: a randomized controlled clinical trial. J Tradit Chin Med. 2016;36:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Lu YY, Du ZY, Li Y, Wang JL, Zhao MB, Jiang Y, Guo XY, Tu PF. Effects of Baoyuan decoction, a traditional Chinese medicine formula, on the activities and mRNA expression of seven CYP isozymes in rats. J Ethnopharmacol. 2018;225:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Yang L, Duan F, Su D, Li Y, Ma L, Shi B, He X, Ma R, Ding C, Sun S, Yao X. The effects of CTX damage or inhibition of bone marrow hematopoiesis and GM-CSF stimulation of bone marrow hematopoiesis on the peripheral blood TCRβ CDR3 repertoire of BALB/c mice. Immunopharmacol Immunotoxicol. 2020;42:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Cai SZ, Zhou Y, Liu J, Li CP, Jia DY, Zhang MS, Wang YP. Alleviation of ginsenoside Rg1 on hematopoietic homeostasis defects caused by lead-acetate. Biomed Pharmacother. 2018;97:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Tang YL, Zhou Y, Wang YP, Wang JW, Ding JC. SIRT6/NF-κB signaling axis in ginsenoside Rg1-delayed hematopoietic stem/progenitor cell senescence. Int J Clin Exp Pathol. 2015;8:5591-5596. [PubMed] |
| 36. | Tang YL, Zhou Y, Wang YP, He YH, Ding JC, Li Y, Wang CL. Ginsenoside Rg1 protects against Sca-1(+) HSC/HPC cell aging by regulating the SIRT1-FOXO3 and SIRT3-SOD2 signaling pathways in a γ-ray irradiation-induced aging mice model. Exp Ther Med. 2020;20:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Zeng Y, Hu W, Jing P, Chen X, Wang Z, Wang L, Wang Y. The regulation of ginsenoside Rg1 upon aging of bone marrow stromal cell contribute to delaying senescence of bone marrow mononuclear cells (BMNCs). Life Sci. 2018;209:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Agger K, Nishimura K, Miyagi S, Messling JE, Rasmussen KD, Helin K. The KDM4/JMJD2 histone demethylases are required for hematopoietic stem cell maintenance. Blood. 2019;134:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Mu H, Jia H, Lin ZH, Zheng HH, Wang L, Liu H. [Role of imbalance of M1/M2 subsets of bone marrow macrophages in the pathogenesis of immune-mediated aplastic anemia in mice]. Zhonghua Xue Ye Xue Za Zhi. 2021;42:945-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 40. | Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013;946:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Peng H, Yu Y, Gu H, Qi B, Yu A. MicroRNA-483-5p inhibits osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting the RPL31-mediated RAS/MEK/ERK signaling pathway. Cell Signal. 2022;93:110298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Tsubaki M, Satou T, Itoh T, Imano M, Yanae M, Kato C, Takagoshi R, Komai M, Nishida S. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol. 2012;361:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Yip RKH, Rimes JS, Capaldo BD, Vaillant F, Mouchemore KA, Pal B, Chen Y, Surgenor E, Murphy AJ, Anderson RL, Smyth GK, Lindeman GJ, Hawkins ED, Visvader JE. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat Commun. 2021;12:6920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 44. | Chang SF, Li HC, Huang YP, Tasi WJ, Chou YY, Lu SC. SB203580 increases G-CSF production via a stem-loop destabilizing element in the 3' untranslated region in macrophages independently of its effect on p38 MAPK activity. J Biomed Sci. 2016;23:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Decean HP, Brie IC, Tatomir CB, Perde-Schrepler M, Fischer-Fodor E, Virag P. Targeting MAPK (p38, ERK, JNK) and inflammatory CK (GDF-15, GM-CSF) in UVB-Activated Human Skin Cells with Vitis vinifera Seed Extract. J Environ Pathol Toxicol Oncol. 2018;37:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Koga Y, Hisada T, Ishizuka T, Utsugi M, Ono A, Yatomi M, Kamide Y, Aoki-Saito H, Tsurumaki H, Dobashi K, Yamada M. CREB regulates TNF-α-induced GM-CSF secretion via p38 MAPK in human lung fibroblasts. Allergol Int. 2016;65:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |