Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.538
Revised: March 12, 2024
Accepted: April 12, 2024
Published online: May 26, 2024
Processing time: 150 Days and 4.5 Hours
Thrombocytopenia 2, an autosomal dominant inherited disease characterized by moderate thrombocytopenia, predisposition to myeloid malignancies and normal platelet size and function, can be caused by 5’-untranslated region (UTR) point mutations in ankyrin repeat domain containing 26 (ANKRD26). Runt related transcription factor 1 (RUNX1) and friend leukemia integration 1 (FLI1) have been identified as negative regulators of ANKRD26. However, the positive regulators of ANKRD26 are still unknown.
To prove the positive regulatory effect of GATA binding protein 2 (GATA2) on ANKRD26 transcription.
Human induced pluripotent stem cells derived from bone marrow (hiPSC-BM) and urothelium (hiPSC-U) were used to examine the ANKRD26 expression pattern in the early stage of differentiation. Then, transcriptome sequencing of these iPSCs and three public transcription factor (TF) databases (Cistrome DB, animal TFDB and ENCODE) were used to identify potential TF candidates for ANKRD26. Furthermore, overexpression and dual-luciferase reporter experiments were used to verify the regulatory effect of the candidate TFs on ANKRD26. Moreover, using the GENT2 platform, we analyzed the relationship between ANKRD26 expression and overall survival in cancer patients.
In hiPSC-BMs and hiPSC-Us, we found that the transcription levels of ANKRD26 varied in the absence of RUNX1 and FLI1. We sequenced hiPSC-BM and hiPSC-U and identified 68 candidate TFs for ANKRD26. Together with three public TF databases, we found that GATA2 was the only candidate gene that could positively regulate ANKRD26. Using dual-luciferase reporter experiments, we showed that GATA2 directly binds to the 5’-UTR of ANKRD26 and promotes its transcription. There are two identified binding sites of GATA2 that are located 2 kb upstream of the TSS of ANKRD26. In addition, we discovered that high ANKRD26 expression is always related to a more favorable prognosis in breast and lung cancer patients.
We first discovered that the transcription factor GATA2 plays a positive role in ANKRD26 transcription and identified its precise binding sites at the promoter region, and we revealed the importance of ANKRD26 in many tissue-derived cancers.
Core Tip: The 5’-untranslated region mutation of ankyrin repeat domain containing 26 (ANKRD26) plays an important role in the pathology of thrombocytopenia 2 (THC2). Considering the predisposition of THC2 patients to myeloid malignancies, further revealing the molecular mechanism of ANKRD26 transcription is warranted. Although Runt related transcription factor 1 and friend leukemia integration 1 have been shown to negatively regulate ANKRD26 expression, no known positive regulators have been reported. Here, we first revealed that GATA binding protein 2 mediates high ANKRD26 expression by binding to its promoter region. We discovered that high ANKRD26 expression was always associated with favorable overall survival. Our study provides insights into the regulatory network of ANKRD26 and the pathological process of THC2.
- Citation: Jiang YZ, Hu LY, Chen MS, Wang XJ, Tan CN, Xue PP, Yu T, He XY, Xiang LX, Xiao YN, Li XL, Ran Q, Li ZJ, Chen L. GATA binding protein 2 mediated ankyrin repeat domain containing 26 high expression in myeloid-derived cell lines. World J Stem Cells 2024; 16(5): 538-550
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/538.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.538
Ankyrin repeat domain containing protein 26 (ANKRD26) acts as a regulator of adipogenesis and is involved in the regulation of feeding behavior[1-3]. The ANKRD26 gene is located on chromosome 10 and shares regions of homology with the primate-specific gene family POTE. According to the Human Protein Atlas database, the ANKRD26 protein is localized to the Golgi apparatus and vesicles, and its expression can be detected in nearly all human tissues[4]. Moreover, UniProt annotation revealed that ANKRD26 is localized in the centrosome and contains coiled-coil domains formed by spectrin helices and ankyrin repeats[5,6].
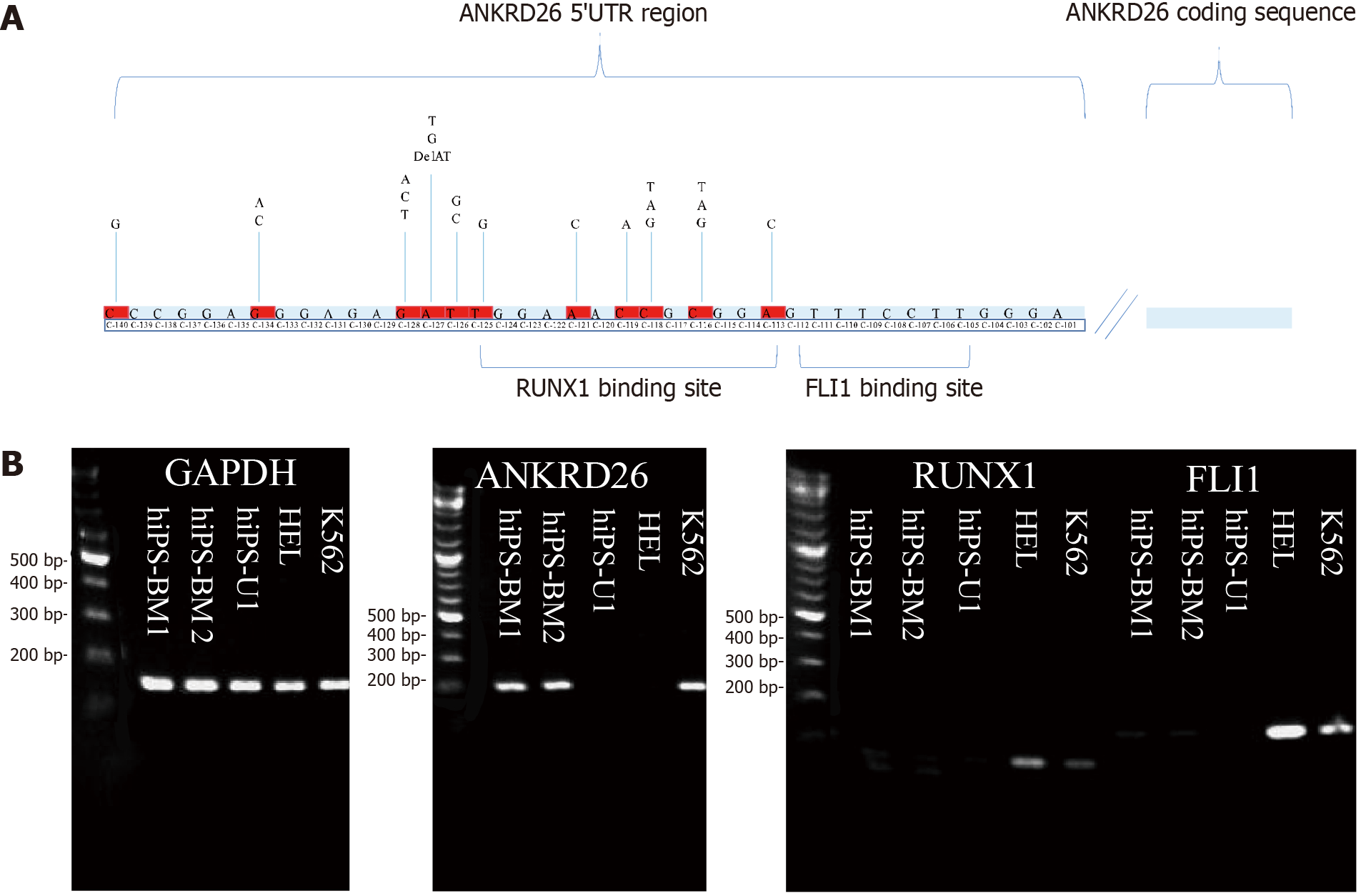
The most common disease related to ANKRD26 is thrombocytopenia 2 (THC2), which is a rare autosomal dominant inherited disease characterized by lifelong mild-to-moderate thrombocytopenia and mild bleeding[7-9]. Caused by the variants in the 5’-untranslated region (UTR) of ANKRD26, THC2 is defined by a decrease in the number of platelets in circulating blood and results in increased bleeding and decreased clotting ability[8,10]. Due to the point mutations that occur in the 5’-UTR of ANKRD26, its negative transcription factors (TFs), Runt related transcription factor 1 (RUNX1) and friend leukemia integration 1 (FLI1), lose their repression effect[11]. The persistent expression of ANKRD26 increases the activity of the mitogen activated protein kinase and extracellular signal regulated kinase 1/2 signaling pathways, which are potentially involved in the regulation of thrombopoietin-dependent signaling and further impair proplatelet formation by megakaryocytes (MKs)[11]. However, the positive regulators of ANKRD26, which might be associated with THC2 pathology, are still unknown.
In this study, we demonstrated that GATA binding protein 2 (GATA2) functions as a positive regulator of ANKRD26 by binding to its promoter region and identified its precise binding sites. Furthermore, we scanned the expression levels of ANKRD26 in human cancers and reported that high ANKRD26 expression was linked to more favorable overall survival. In summary, this is the first study in which the regulatory effect of GATA2 on ANKRD26 in THC2 cells has been investigated. The results of this study will enhance our understanding of the regulatory network of ANKRD26 and the pathological process of THC2.
K562 and HEL cells were cultured in RPMI 1640 medium (Gibco, C11875500BT) supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin. All human induced pluripotent stem cells (hiPSCs) were cultured in Matrigel-coated 6-well cell culture plates with Essential 8 (E8) medium (Cellapy, CA1014500). hiPSCs derived from bone marrow (hiPSC-BM) (SHAMUi001-A, https://hpscreg.eu/cell-line/SHAMUi001-A) were induced as previously described, and hiPSCs derived from the urothelium (hiPSC-U) were obtained from Cellapy (hiPSC-U1)[12].
The following antibodies were used for western blot and chromatin immunoprecipitation (ChIP) analyses: Rabbit anti-ANKRD26 (GeneTex, GTX128255), rabbit anti-GATA2 (Cell Signaling Technology, #79802S), rabbit anti-RUNX1 (Abcam, ab272456), rabbit anti-FLI1 (Abcam, ab133485), mouse anti-GAPDH (Beyotime, AF0006) and rabbit anti-normal IgG (Cell Signaling Technology, #2729P).
Cells were lysed with ice-cold western and IP cell lysis buffer (Beyotime, P0013) containing protease inhibitors. After protein quantification, equal amounts of protein from each sample were separated by sodium-dodecyl sulfate gel electrophoresis (Solarbio, P1200-1/P1200-2) and transferred to polyvinylidene fluoride membranes (Millipore, 0000227526). Then, the membranes were incubated with primary antibodies. The samples were incubated overnight at 4 °C, followed by three washes with TBST. Next, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies [goat anti-rabbit IgG/HRP (Solarbio, SE134) and goat anti-mouse IgG/HRP (Solarbio, SE131)] for 1 h at room temperature. Blots were visualized using SuperSignal™ West Atto Chemiluminescent Substrate (Thermo Scientific, A38555).
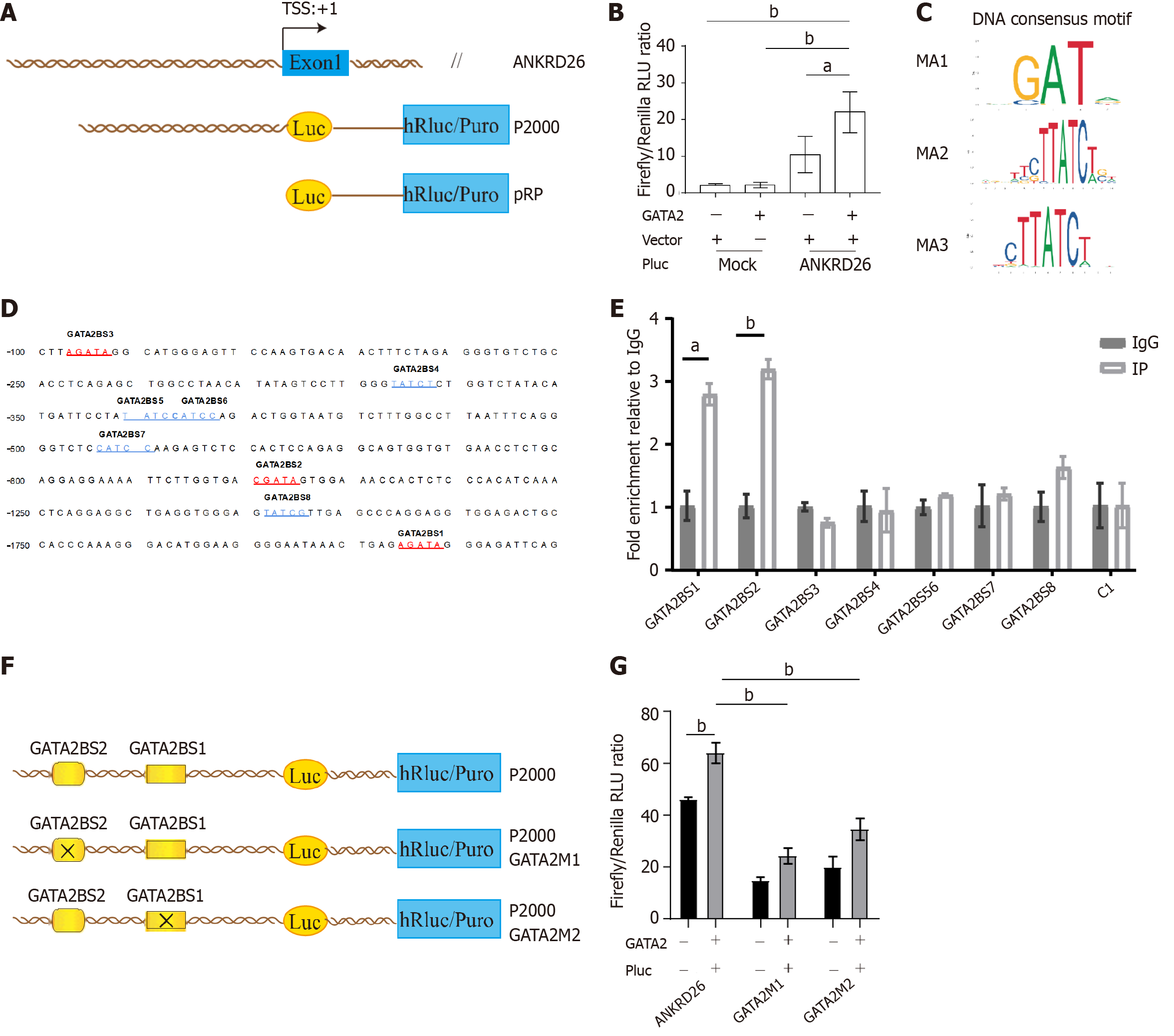
The promoter region (2 kb upstream of the translation start site) of ANKRD26 was amplified by polymerase chain reaction (PCR) and cloned with a luciferase reporter vector (P2000). The promoter regions of the P2000 GATA2M1 and P2000 GATA2M2 vectors were constructed by mutating all the bases 821-825 bp and 1685-1689 bp upstream of the ANKRD26 gene TSS to adenine (A). These two mutated promoters were amplified by PCR and cloned with luciferase reporter vectors. Then, K562 cells were cotransfected with luciferase reporter vectors and the GATA2 overexpression plasmid using Lipofectamine 3000 transfection reagent (Invitrogen, L3000-05). After 48 h of incubation, a dual-luciferase reporter assay was performed using the Dual-Luciferase Reporter Assay System (Promega, E1910). Renilla luciferase activity was used to normalize the transfection efficiency.
ChIP assays were conducted using a SimpleChIPPlus Enzymatic Chromatin IP Kit (Magnetic Beads, Cell Signaling Technology, 9005 S) with an anti-GATA2 antibody (Cell Signaling Technology, #79802S). Assays were performed using chromatin prepared from K562 cells. The cells were first crosslinked with 1% formaldehyde in phosphate buffered saline (PBS) at room temperature for 10 min, quenched with 2.5 M glycine at room temperature for 5 min and washed with ice-cold PBS three times. Sonication was used for DNA fragmentation. The supernatants were immunoprecipitated by incubation with 5 μL of anti-GATA2 (Cell Signaling Technology, #79802S), 10 μL of anti-histone H3 (D2B12) XP rabbit mAb (Cell Signaling Technology, #4620) as a positive control and 2 μL of rabbit anti-normal IgG (Cell Signaling Technology, #2729) as a negative control at 4 °C for 16 h. Then, the immunocomplexes were rotationally incubated with 30 μL of ChIP-Grade Protein G Magnetic Beads for 2 h at 4 °C and then washed three times with low-salt wash buffer and one time with high-salt wash buffer at 4 °C for 5 min per wash. Chromatin was eluted by ChIP elution buffer for 30 min at 65 °C with gentle vortex mixing (1200 rpm), and crosslinking was reversed by treatment with 5 M NaCl and proteinase K overnight at 65 °C. The samples were then incubated with RNase at 37 °C for 1 h. ChIP DNA was purified and subsequently quantified by quantitative real-time PCR (RT-qPCR). The sequences of primers used for ANKRD26 are listed in Supplementary Table 1.
The primer sequences for ANKRD26, GATA2, RUNX1, FLI1 and the housekeeping gene GAPDH were predicted using Primer3 and can be found in Supplementary Table 1. mRNA isolation, reverse transcription, and RT-qPCR were performed as previously described[13]. Total RNA was extracted using TRIzol Reagent (TaKaRa, 9109) according to the manufacturer’s instructions. First-strand cDNA was synthesized from l μg of RNA using the PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, RR047A). qPCR was performed in triplicate in 20-μL reactions containing SYBR Premix Ex Taq II (TaKaRa, RR820A). The reaction protocol was as follows: Heating for 30 s at 95 °C, followed by 40 cycles of amplification (5 s at 95 °C and 30 s at 60 °C).
Total RNA was extracted from bone marrow- and urothelium-derived iPSCs using TRIzol reagent, as previously described[14]. Then, the RNA quality and quantity were analyzed by an Agilent 2100 Bioanalyzer. After the cDNA libraries for all the samples were prepared using the Illumina RNA Prep with Enrichment Kit (Illumina), they were sequenced on the Illumina NovaSeq 6000 platform with the PE150 strategy. Next, the output of sequencing data (fastq format) was cleaned using SOAPnuke and quality controlled by FastQC, as previously described[15]. HISAT2 and Stringtie were used to align the clean reads to the human reference genome (GRCH38) and to profile gene expression for each sample[16]. The fragments per kilobase per million mapped reads (FPKM) method was used to normalize the gene expression in each sample. Differential expression analysis was performed as previously described[15]. HISAT2 and Stringtie were used to align the clean reads to the human reference genome (GRCH38) and to profile gene expression for each sample[16]. The FPKM method was used to normalize the gene expression in each sample. Differential expression analysis was performed as previously described[15].
All the statistical analyses were performed using GraphPad Prism 9.0 software. The mean ± SD method was used to present the values of replicates, and P values were calculated using Student’s t test. P < 0.05 was considered to indicate statistical significance.
Sustained ANKRD26 expression in the late stage of megakaryopoiesis is due to point mutations in the 5’-UTR of ANKRD26, which is in or close to the binding sites of RUNX1 and FLI1 (Figure 1A)[11,17]. To investigate whether there are positive regulators of ANKRD26, we examined its expression, together with its two negative regulators, RUNX1 and FLI1, in two classic THC2 model cell lines, HEL and K562. RUNX1 and FLI1 expression was greater in HEL cells than in K562 cells. ANKRD26 expression was greater in K562 cells than in HEL cells (Figure 1B). Next, we employed hiPSC-BM and hiPSC-U cells to obtain further insights into the regulatory mechanisms of ANKRD26 at an early stage and examined the expression of RUNX1, FLI1 and ANKRD26 in these cells. In the present study, RUNX1 and FLI1 were not detected in these iPSCs, but ANKRD26 was detected only in the hiPSC-BM (Figure 1B). Thus, we proposed that there might be other positive regulators of ANKRD26.
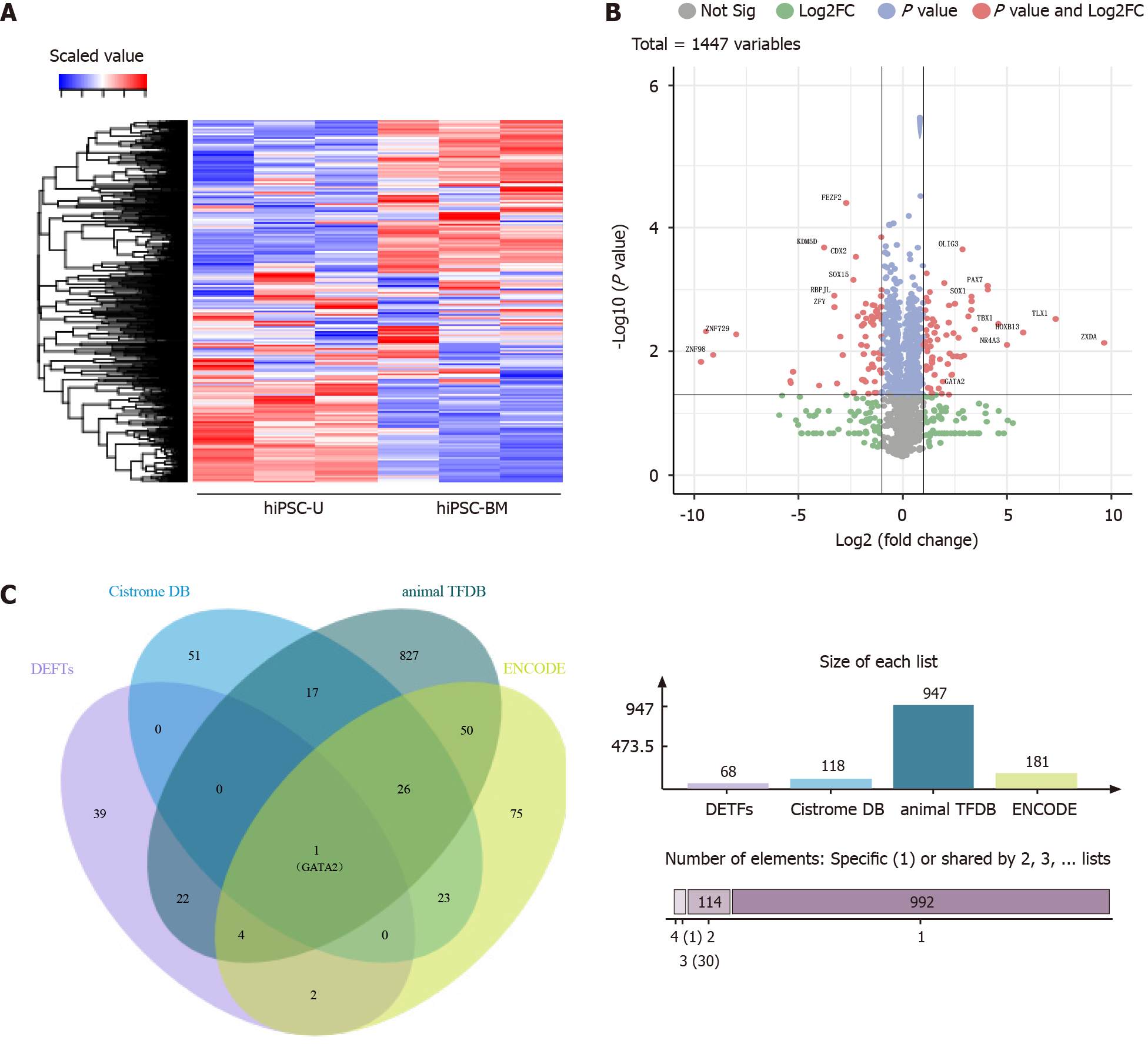
Then, we performed transcriptome sequencing and screened the expression of all TF genes in both kinds of iPSCs. A heatmap (Figure 2A) and a volcano plot (Figure 2B) were used to show the differential expression of TF genes in hiPSC-BM and hiPSC-U. We detected significant differences in the expression of some TF genes (P < 0.05, fold change > 2) in the two types of iPSCs, such as FEZF2, OLIG3, PAX7 and GATA2. In total, 68 TF genes were more highly expressed in hiPSC-BM cells than in hiPSC-U cells. Next, we searched for potential TFs for ANKRD26 in three databases, namely, the Cistrome DB, animal TFDB and ENCODE[18-20]. Interestingly, GATA2 was the only TF identified by our transcriptome sequencing and the three datasets (Figure 2C and Supplementary Table 2), which strongly supports it as a potential regulator of ANKRD26.
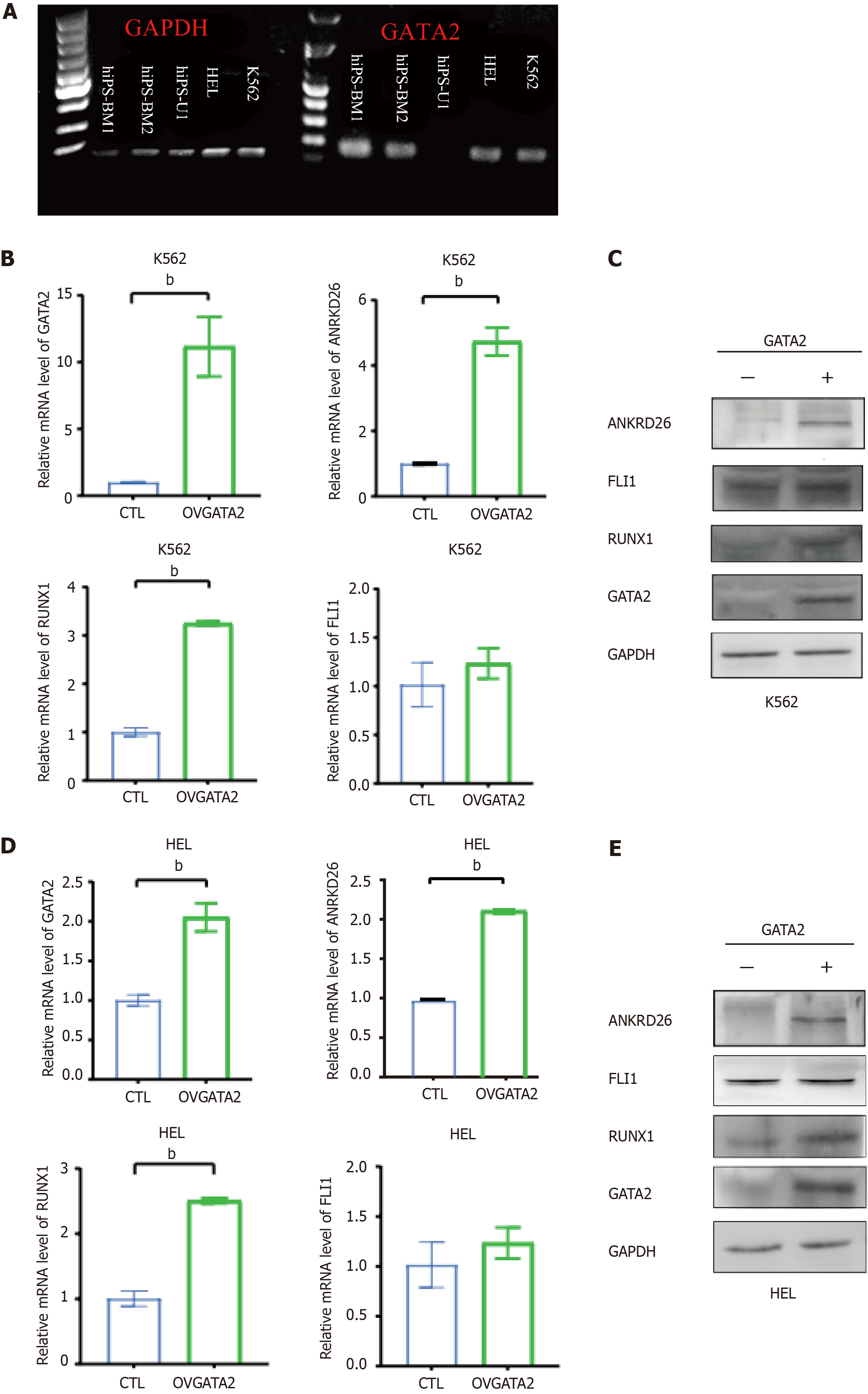
To verify the regulatory effect of GATA2 on the transcription of the ANKRD26 gene, we analyzed the expression of GATA2 and ANKRD26 in HEL, K562, hiPSC-BM and hiPSC-U cells. Although GATA2 was expressed in HEL cells, ANKRD26 was not detected, probably due to the dominant repressive effect of its suppressors RUNX1 and FLI1 (Figures 1B and 3A). In other cells, such as K562 and hiPSC-BM cells, we found that GATA2 was co-expressed with ANKRD26 (Figures 1B and 3A). To study the positive regulatory effect of GATA2 on ANKRD26 expression, we transfected GATA2 plasmids into K562 cells and observed that ANKRD26 expression was significantly increased at both the mRNA and protein levels (Figure 3B and C). Interestingly, the expression of RUNX1 was significantly increased at the mRNA and protein levels (Figure 3B and C), while the expression of FLI1 mRNA and protein was also increased but not significantly different in K562GATA2+ cells (Figure 3B and C). Then, we transfected the GATA2 plasmids into HEL cells and examined the expression levels of GATA2, ANKRD26, RUNX1 and FLI1. Unexpectedly, the expression patterns of these genes in HEL cells following GATA2 overexpression were the same as those in K562 cells following GATA2 overexpression (Figure 3D and E). This might suggest that the positive regulation by the overexpression of GATA2 would take over the repressive effect of RUNX1 and FLI1.
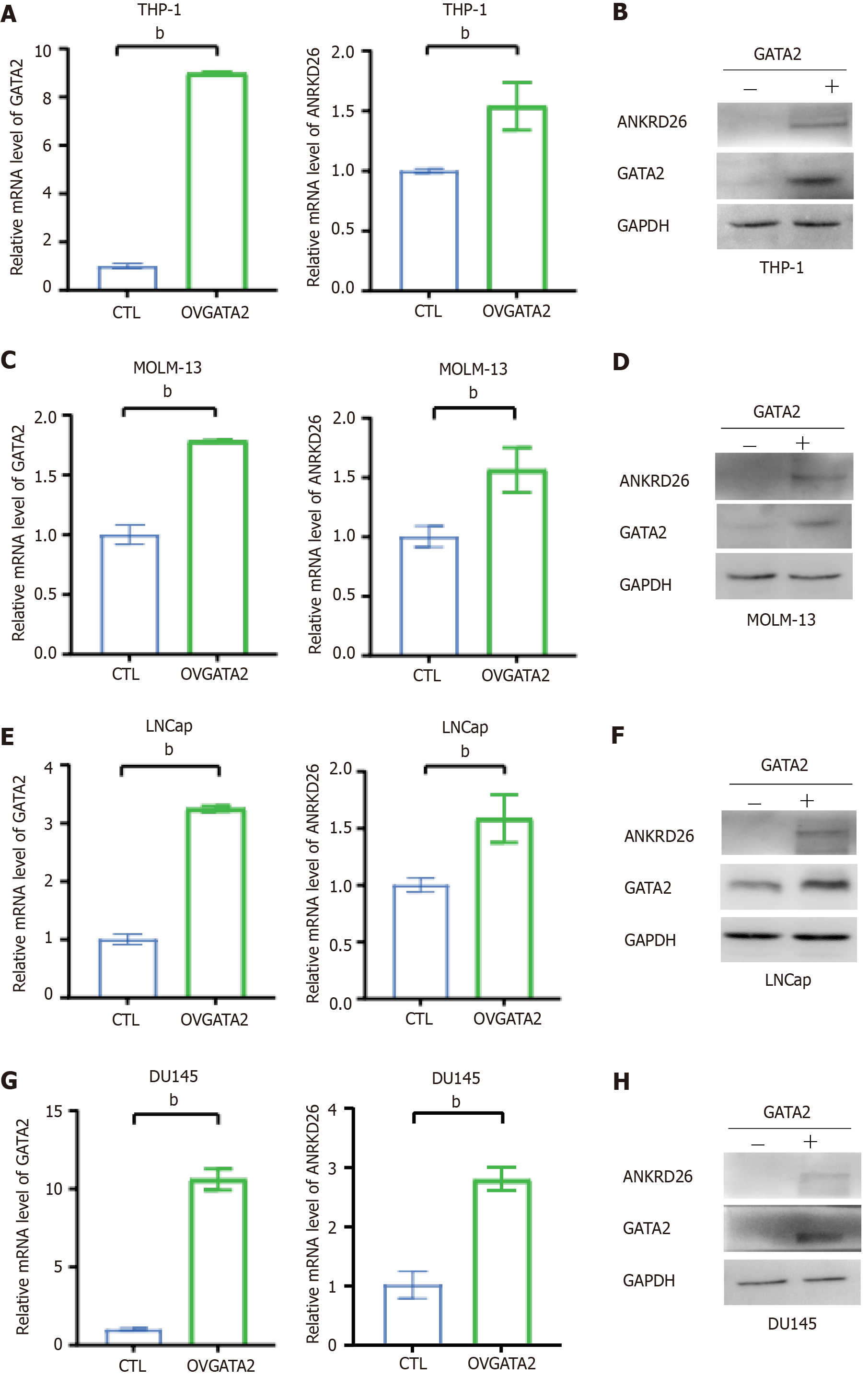
Because the expression of ANKRD26 varied between hiPSC-BMs and hiPSC-Us (Figure 1B), we next transfected the GATA2 plasmids into bone marrow (THP-1 and MOLM13)- and urothelium (LNCaP and DU145)-derived cell lines. The results showed that in these cell lines, overexpressed GATA2 can consistently stimulate the expression of ANKRD26 at both the mRNA and protein levels (Figure 4). Taken together, these findings indicate that GATA2 does not increase the expression of ANKRD26 via the downregulation of RUNX1 or FLI1.
Next, we established a dual-luciferase reporter covering the 2 kb region upstream of the TSS of the ANKRD26 gene (Figure 5A). K562 cells were co-transfected with the GATA2 expression plasmids and these dual-luciferase reporter constructs. Compared with that in the empty vector group, luciferase activity was significantly greater in the GATA2 overexpression group, indicating that GATA2 binds to this region (Figure 5B). Furthermore, using bioinformatics tools, we identified 3 potential consensus binding motifs for GATA2 in the promoter region of ANKRD26 (Figure 5C). These motifs were searched against the 5’-UTR of ANKRD26, and 8 hits were found (Figure 5D). PCR primers encompassing these sites were designed, ChIP assays were performed, and two potential sites were identified (Figure 5E).
To further confirm the binding sites of GATA2 in the ANKRD26 promoter region, we performed a site-directed mutagenesis experiment by modifying the bases in the two binding sites to disrupt the binding of GATA2 (Figure 5F). Then, we found that the luciferase activities of both mutated dual-luciferase reporters were significantly decreased (Figure 5G). Taken together, our findings reveal that GATA2 promotes ANKRD26 transcriptional activity by directly binding to two sites in the ANKRD26 promoter region and that these two sites are indispensable for ANKRD26 gene transcription.
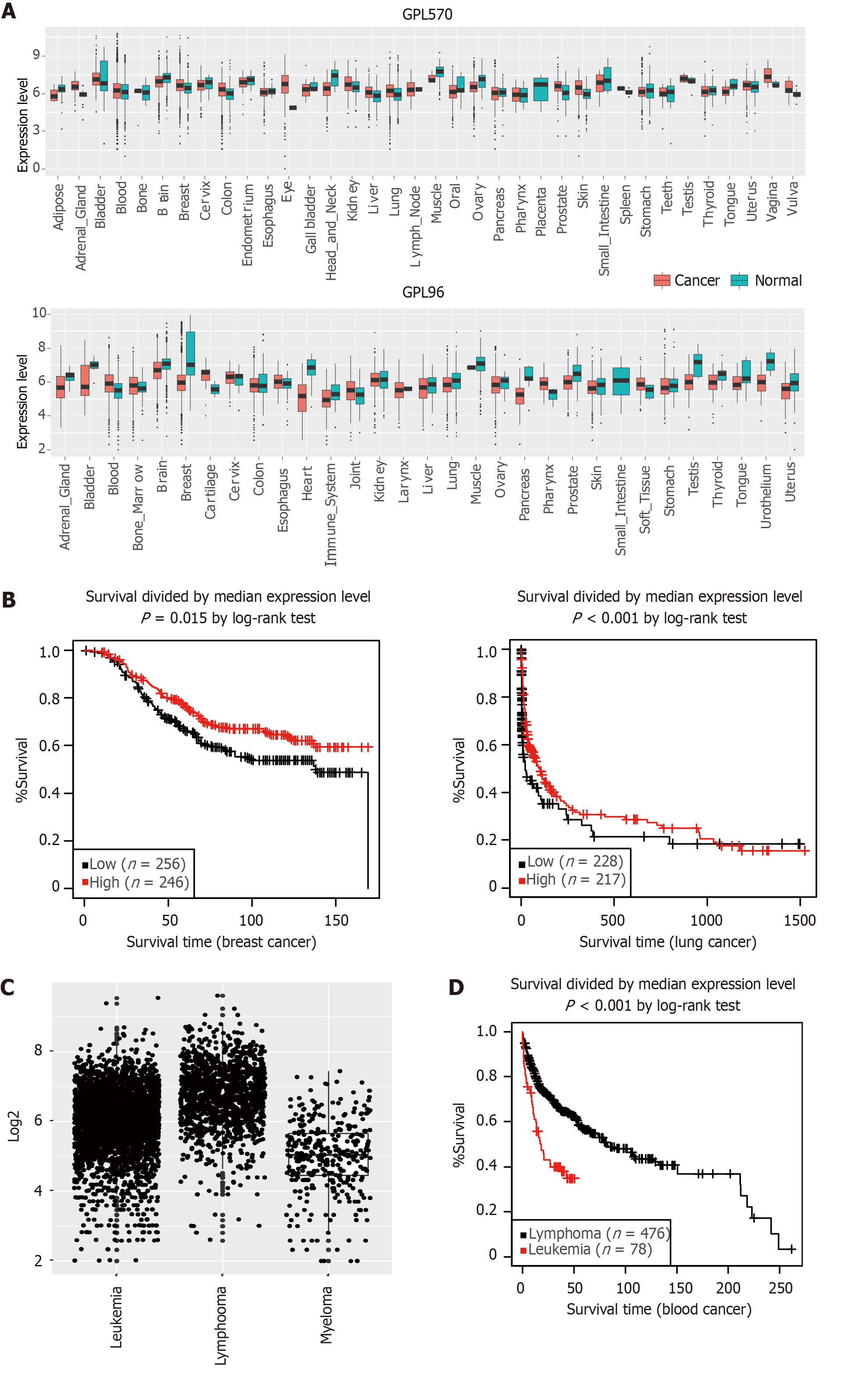
We next explored the expression patterns of ANKRD26 in other human diseases, such as cancers. We downloaded ANKRD26 gene expression profiles (from the GPL570 and GPL96 platforms) from the GENT2 database and compared the expression levels of ANKRD26 in normal and cancer tissues of various cancer types (Figure 6A)[21]. ANKRD26 expression was significantly different (P < 0.05) between cancer and normal tissues of multiple cancer types (15 and 16 cancer types from the GPL570 and GPL96 platforms, respectively), including brain, breast, lung, and blood (Sup
In hematopoietic stem and progenitor cells, FLI1 plays a key role in proliferation and differentiation[22]. RUNX1 is indispensable for the establishment of definitive hematopoiesis, and studies of iPSCs derived from patients with familial platelet disorder with a predisposition to acute myeloid leukemia (FPD/AML) have revealed that RUNX1 mutations also cause megakaryopoiesis defects[23,24]. Bluteau et al[11] reported that RUNX1 and FLI1 are two critical transcription factors that regulate ANKRD26 expression, and losing the binding of these two critical TFs causes persistent ANKRD26 expression, which is the most important factor that contributes to THC2 expression. It is reasonable to assume that ANKRD26 plays an important role in the downstream pathway of RUNX1 because the clinical features of FPD/AML are similar to those of THC2, and RUNX1 is an essential regulatory factor of ANKRD26. To observe the relationship between ANKRD26 and these two TFs (RUNX1 and FLI1) at an early stage, in our study, we measured the expression of ANKRD26, RUNX1 and FLI1 in K562 cells, HEL cells and two tissue-derived iPSCs (hiPSC-BM and hiPSC-U). With the same expression levels of RUNX1 and FLI1, we unexpectedly found that the ANKRD26 expression pattern differed between the two types of tissue-derived iPSCs. We first showed that GATA2 positively regulates ANKRD26 expression. Taken together, these three TFs (GATA2, RUNX1 and FLI1) contribute to the regulation of ANKRD26 expression.
In our study, we first identified the promoting effect of GATA2 on ANKRD26 expression by binding to its promoter region. It has been reported that GATA2 might regulate megakaryopoiesis at the level of MK progenitors[25]. By analyzing the gene expression data in the BloodSpot database (https://www.bloodspot.eu/), we also observed that the expression of GATA2 and ANKRD26 gradually decreased with MK differentiation and that the expression of RUNX1 and FLI1 did not obviously differ (Supplementary Figure 1)[26]. Therefore, we can speculate that GATA2 dominates ANKRD26 regulation at an early stage of differentiation, which is consistent with its expression pattern. However, during the late stage of MK differentiation, GATA2 expression is decreased, and the repressive effect of RUNX1 and FLI1 regulates ANKRD26 expression. In addition, ANKRD26 expression was measured in HEL cells overexpressing GATA2. This finding also proved that the repressive effect of RUNX1 and FLI1 would be disrupted if GATA2 expression was sufficient.
GATA2 was first reported in 1994 and was shown to encode an essential transcriptional regulator in multilineage hematopoiesis[27]. It is a transcriptional activator that regulates endothelin-1 gene expression in endothelial cells and binds to the consensus sequence[28]. In our study, we found that the GATA2 motif MA1 (5’-AGATA-3’) plays a major role in regulating ANKRD26 (Figure 5C). As one of the six GATA family TFs, the pioneering TF GATA2 can facilitate the opening of heterochromatin and the subsequent binding of other TFs and further induce gene expression from previously inaccessible regions of the genome[29].
In addition to affecting THC2, ANKRD26 is related to many other diseases, such as diabetes, epidermodysplasia verruciformis, non-Langerhans cell histiocytosis and psychiatric disorders, although the underlying mechanism has yet to be elucidated[30-35]. Recent studies have shown that ANKRD26 is a distal appendage protein that plays an essential role in centrosome amplification[36,37]. Centrosome amplification is always observed in a variety of human cancers and promotes genome instability and tumor development[38,39]. To explore the role of ANKRD26 in cancer, we analyzed the change in the expression of ANKRD26 in cancers derived from different tissues by using the GENT2 platform. In our study, we showed that there is a significant difference in ANKRD26 expression in many cancer tissues, and patients with higher ANKRD26 expression had a more favorable prognosis in breast and lung cancers. In hepatocytes, ANKRD26 is regarded as an important factor in restricting polyploidization and preventing chronic injury because it can activate the PIDDosome-P53 axis after centrosome amplification[40]. Further investigation into whether ANKRD26 functions as a potential tumor suppressor by inducing apoptosis after centrosome amplification in cancer cells and whether the impairment of proplatelet formation in THC2 patients is caused by excessive ANKRD26, which limits MK polyploidy, is urgently warranted.
In conclusion, we first identified that GATA2 enhanced ANKRD26 expression, and we also identified the precise binding sites of GATA2 on the ANKRD26 promoter region. In addition, we discovered that high ANKRD26 expression was always linked to favorable overall survival. Hence, our study further revealed the transcriptional regulatory network of ANKRD26 and contributed to further exploration of the pathological process of THC2.
The authors thank all the lab members for their helpful advice and suggestions.
| 1. | Liu XF, Bera TK, Kahue C, Escobar T, Fei Z, Raciti GA, Pastan I. ANKRD26 and its interacting partners TRIO, GPS2, HMMR and DIPA regulate adipogenesis in 3T3-L1 cells. PLoS One. 2012;7:e38130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Fei Z, Bera TK, Liu X, Xiang L, Pastan I. Ankrd26 gene disruption enhances adipogenesis of mouse embryonic fibroblasts. J Biol Chem. 2011;286:27761-27768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Acs P, Bauer PO, Mayer B, Bera T, Macallister R, Mezey E, Pastan I. A novel form of ciliopathy underlies hyperphagia and obesity in Ankrd26 knockout mice. Brain Struct Funct. 2015;220:1511-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7696] [Cited by in RCA: 10501] [Article Influence: 1050.1] [Reference Citation Analysis (0)] |
| 5. | Lee Y, Ise T, Ha D, Saint Fleur A, Hahn Y, Liu XF, Nagata S, Lee B, Bera TK, Pastan I. Evolution and expression of chimeric POTE-actin genes in the human genome. Proc Natl Acad Sci U S A. 2006;103:17885-17890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hahn Y, Bera TK, Pastan IH, Lee B. Duplication and extensive remodeling shaped POTE family genes encoding proteins containing ankyrin repeat and coiled coil domains. Gene. 2006;366:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Savoia A, Del Vecchio M, Totaro A, Perrotta S, Amendola G, Moretti A, Zelante L, Iolascon A. An autosomal dominant thrombocytopenia gene maps to chromosomal region 10p. Am J Hum Genet. 1999;65:1401-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Pippucci T, Savoia A, Perrotta S, Pujol-Moix N, Noris P, Castegnaro G, Pecci A, Gnan C, Punzo F, Marconi C, Gherardi S, Loffredo G, De Rocco D, Scianguetta S, Barozzi S, Magini P, Bozzi V, Dezzani L, Di Stazio M, Ferraro M, Perini G, Seri M, Balduini CL. Mutations in the 5' UTR of ANKRD26, the ankirin repeat domain 26 gene, cause an autosomal-dominant form of inherited thrombocytopenia, THC2. Am J Hum Genet. 2011;88:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Noris P, Perrotta S, Seri M, Pecci A, Gnan C, Loffredo G, Pujol-Moix N, Zecca M, Scognamiglio F, De Rocco D, Punzo F, Melazzini F, Scianguetta S, Casale M, Marconi C, Pippucci T, Amendola G, Notarangelo LD, Klersy C, Civaschi E, Balduini CL, Savoia A. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011;117:6673-6680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Tan C, Dai L, Chen Z, Yang W, Wang Y, Zeng C, Xiang Z, Wang X, Zhang X, Ran Q, Guo H, Li Z, Chen L. A Rare Big Chinese Family With Thrombocytopenia 2: A Case Report and Literature Review. Front Genet. 2020;11:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bluteau D, Balduini A, Balayn N, Currao M, Nurden P, Deswarte C, Leverger G, Noris P, Perrotta S, Solary E, Vainchenker W, Debili N, Favier R, Raslova H. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J Clin Invest. 2014;124:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Tan C, Dai L, Yang W, Li F, Wang L, Xiao Y, Wang X, Zhang Y, Wang Y, Zeng C, Xiang Z, Zhang X, Zhang W, Ran Q, Chen M, Li Z, Chen L. Generation of the human induced pluripotent stem cell line (SHAMUi001-A) carrying the heterozygous c.-128G>T mutation in the 5'-UTR of the ANKRD26 gene. Stem Cell Res. 2020;48:102002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Xiang LX, Ran Q, Chen L, Xiang Y, Li FJ, Zhang XM, Xiao YN, Zou LY, Zhong JF, Li SC, Li ZJ. CR6-interacting factor-1 contributes to osteoclastogenesis by inducing receptor activator of nuclear factor κB ligand after radiation. World J Stem Cells. 2020;12:222-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Chen M, Xu R, Ji H, Greening DW, Rai A, Izumikawa K, Ishikawa H, Takahashi N, Simpson RJ. Transcriptome and long noncoding RNA sequencing of three extracellular vesicle subtypes released from the human colon cancer LIM1863 cell line. Sci Rep. 2016;6:38397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Chen M, Mithraprabhu S, Ramachandran M, Choi K, Khong T, Spencer A. Utility of Circulating Cell-Free RNA Analysis for the Characterization of Global Transcriptome Profiles of Multiple Myeloma Patients. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2932] [Cited by in RCA: 7720] [Article Influence: 1286.7] [Reference Citation Analysis (0)] |
| 17. | Vyas H, Alcheikh A, Lowe G, Stevenson WS, Morgan NV, Rabbolini DJ. Prevalence and natural history of variants in the ANKRD26 gene: a short review and update of reported cases. Platelets. 2022;33:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Taing L, Dandawate A, L'Yi S, Gehlenborg N, Brown M, Meyer CA. Cistrome Data Browser: integrated search, analysis and visualization of chromatin data. Nucleic Acids Res. 2024;52:D61-D66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Shen WK, Chen SY, Gan ZQ, Zhang YZ, Yue T, Chen MM, Xue Y, Hu H, Guo AY. AnimalTFDB 4.0: a comprehensive animal transcription factor database updated with variation and expression annotations. Nucleic Acids Res. 2023;51:D39-D45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 20. | ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14554] [Cited by in RCA: 12927] [Article Influence: 994.4] [Reference Citation Analysis (0)] |
| 21. | Park SJ, Yoon BH, Kim SK, Kim SY. GENT2: an updated gene expression database for normal and tumor tissues. BMC Med Genomics. 2019;12:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 22. | Ben-David Y, Gajendran B, Sample KM, Zacksenhaus E. Current insights into the role of Fli-1 in hematopoiesis and malignant transformation. Cell Mol Life Sci. 2022;79:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Connelly JP, Kwon EM, Gao Y, Trivedi NS, Elkahloun AG, Horwitz MS, Cheng L, Liu PP. Targeted correction of RUNX1 mutation in FPD patient-specific induced pluripotent stem cells rescues megakaryopoietic defects. Blood. 2014;124:1926-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Sakurai M, Kunimoto H, Watanabe N, Fukuchi Y, Yuasa S, Yamazaki S, Nishimura T, Sadahira K, Fukuda K, Okano H, Nakauchi H, Morita Y, Matsumura I, Kudo K, Ito E, Ebihara Y, Tsuji K, Harada Y, Harada H, Okamoto S, Nakajima H. Impaired hematopoietic differentiation of RUNX1-mutated induced pluripotent stem cells derived from FPD/AML patients. Leukemia. 2014;28:2344-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Kanapathipillai M. Treating p53 Mutant Aggregation-Associated Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 26. | Gíslason MH, Demircan GS, Prachar M, Furtwängler B, Schwaller J, Schoof EM, Porse BT, Rapin N, Bagger FO. BloodSpot 3.0: a database of gene and protein expression data in normal and malignant haematopoiesis. Nucleic Acids Res. 2024;52:D1138-D1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 27. | Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1092] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 28. | Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;15:4225-4231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Aktar A, Heit B. Role of the pioneer transcription factor GATA2 in health and disease. J Mol Med (Berl). 2023;101:1191-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Bera TK, Liu XF, Yamada M, Gavrilova O, Mezey E, Tessarollo L, Anver M, Hahn Y, Lee B, Pastan I. A model for obesity and gigantism due to disruption of the Ankrd26 gene. Proc Natl Acad Sci U S A. 2008;105:270-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Raciti GA, Bera TK, Gavrilova O, Pastan I. Partial inactivation of Ankrd26 causes diabetes with enhanced insulin responsiveness of adipose tissue in mice. Diabetologia. 2011;54:2911-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Desiderio A, Longo M, Parrillo L, Campitelli M, Cacace G, de Simone S, Spinelli R, Zatterale F, Cabaro S, Dolce P, Formisano P, Milone M, Miele C, Beguinot F, Raciti GA. Epigenetic silencing of the ANKRD26 gene correlates to the pro-inflammatory profile and increased cardio-metabolic risk factors in human obesity. Clin Epigenetics. 2019;11:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Uddin KMF, Amin R, Majumder SN, Aleem MA, Rahaman A, Dity NJ, Baqui MDA, Akter H, Rahman MM, Woodbury-Smith M, Scherer S, Uddin M. An ANKRD26 nonsense somatic mutation in a female with epidermodysplasia verruciformis (Tree Man Syndrome). Clin Case Rep. 2018;6:1426-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Fayiga FF, Reyes-Hadsall SC, Moreno BA, Oh KS, Brathwaite C, Duarte AM. Novel ANKRD26 and PDGFRB gene mutations in pediatric case of non-Langerhans cell histiocytosis: Case report and literature review. J Cutan Pathol. 2023;50:425-429. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Gunturkun MH, Wang T, Chitre AS, Garcia Martinez A, Holl K, St Pierre C, Bimschleger H, Gao J, Cheng R, Polesskaya O, Solberg Woods LC, Palmer AA, Chen H. Genome-Wide Association Study on Three Behaviors Tested in an Open Field in Heterogeneous Stock Rats Identifies Multiple Loci Implicated in Psychiatric Disorders. Front Psychiatry. 2022;13:790566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Burigotto M, Mattivi A, Migliorati D, Magnani G, Valentini C, Roccuzzo M, Offterdinger M, Pizzato M, Schmidt A, Villunger A, Maffini S, Fava LL. Centriolar distal appendages activate the centrosome-PIDDosome-p53 signalling axis via ANKRD26. EMBO J. 2021;40:e104844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Evans LT, Anglen T, Scott P, Lukasik K, Loncarek J, Holland AJ. ANKRD26 recruits PIDD1 to centriolar distal appendages to activate the PIDDosome following centrosome amplification. EMBO J. 2021;40:e105106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 739] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 39. | Holland AJ, Cleveland DW. Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 2012;13:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 40. | Sladky VC, Akbari H, Tapias-Gomez D, Evans LT, Drown CG, Strong MA, LoMastro GM, Larman T, Holland AJ. Centriole signaling restricts hepatocyte ploidy to maintain liver integrity. Genes Dev. 2022;36:843-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Chen T, Zhang H, Liu Y, Liu YX, Huang L. EVenn: Easy to create repeatable and editable Venn diagrams and Venn networks online. J Genet Genomics. 2021;48:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |