Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.462
Revised: March 8, 2024
Accepted: March 25, 2024
Published online: May 26, 2024
Processing time: 150 Days and 1.7 Hours
Diabetes mellitus (DM), an increasingly prevalent chronic metabolic disease, is characterised by prolonged hyperglycaemia, which leads to long-term health consequences. Although much effort has been put into understanding the pathogenesis of diabetic wounds, the underlying mechanisms remain unclear. The advent of single-cell RNA sequencing (scRNAseq) has revolutionised biological research by enabling the identification of novel cell types, the discovery of cellular markers, the analysis of gene expression patterns and the prediction of developmental trajectories. This powerful tool allows for an in-depth exploration of pathogenesis at the cellular and molecular levels. In this editorial, we focus on progenitor-based repair strategies for diabetic wound healing as revealed by scRNAseq and highlight the biological behaviour of various healing-related cells and the alteration of signalling pathways in the process of diabetic wound healing. ScRNAseq could not only deepen our understanding of the complex biology of diabetic wounds but also identify and validate new targets for inter
Core Tip: Understanding the mechanism of diabetic wound healing is crucial for the development of novel therapeutic strategies. In this editorial, we focus on advances in the biological behaviour of various healing-related cells and the alteration of signalling pathways in the process of diabetic wound healing. Single-cell RNA sequencing (scRNAseq) has emerged as a powerful tool to explore cellular heterogeneity, reveal new cell subpopulations and predict developmental trajectories. Summarising the current results of scRNAseq in diabetic wounds has provided new insights into progenitor-based repair strategies and possible therapeutic targets.
- Citation: Xiang Z, Cai RP, Xiao Y, Huang YC. Single-cell sequencing technology in diabetic wound healing: New insights into the progenitors-based repair strategies. World J Stem Cells 2024; 16(5): 462-466
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/462.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.462
The prevalence of diabetes mellitus (DM), currently affecting an estimated 550 million individuals, continues to escalate[1]. Diabetic foot ulcer (DFU) is a common and severe complication in patients with diabetes, characterised by its stubbornness, difficulty in treating, and high recurrence rate. It mainly manifests as peripheral neuropathy, lower limb arterial sclerosis and local infection, which profoundly impact the patient’s quality of life and disease prognosis. Without proper treatment or inadequate management, it can easily lead to lower limb paralysis, disability and even amputation. Approximately 19% to 34% of diabetes patients worldwide will develop DFU, and about 20% of DFU cases will require lower limb amputation[2]. Current treatment approaches for DFU predominantly encompass wound debridement, offloading strategies, glycemic control, and the management of infections[3]. Recently, several innovative therapeutic modalities have surfaced, including hyperbaric oxygen therapy, application of dressings, negative pressure wound therapy, growth factor therapy, stem cell-based therapy and application of tissue-engineered skin[4]. Despite the deve
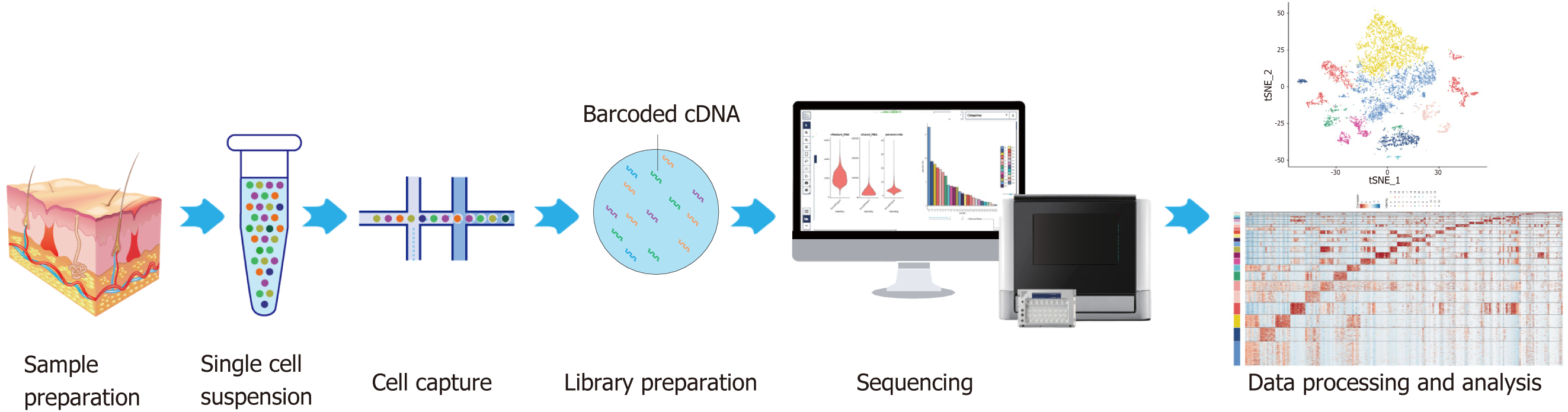
ScRNAseq represents a technique for amplifying the entire transcriptome at the individual cell level. This process involves the reverse transcription of mRNA into cDNA, subsequent amplification of the cDNA, and high-throughput sequencing[5]. As a quintessential instrument for single-cell analysis (Figure 1), scRNAseq facilitates unbiased, high-throughput investigations requiring minimal initial sample volumes. It allows for the detection of cell-specific attributes and intercellular variances through cell mapping, delves into the cooperative functions of cells, and examines the heterogeneity within tissues[6]. Thus, using scRNAseq to investigate progenitor-based repair strategies may yield novel insights into the mechanisms of diabetic wound healing.
Traditionally, it is believed that endothelial cells, epithelial cells, fibroblasts, keratinocytes, macrophages, inflammatory cells and tissue stem cells are the main contributors to wound healing. ScRNAseq has shown that endothelial cells, fibroblasts, epithelial cells, keratinocytes, monocytes, macrophages, B cells, T cells and tissue stem cells are significantly present in both non-healing and healing DFU wounds. By applying scRNAseq, we can gain a clearer understanding of the biological behaviour of various cell types during diabetic wound healing. The following content presents five applications of scRNAseq in studying diabetic wounds and unveiling cellular behaviour and molecular changes during the healing process (Table 1). The samples, primarily from DFUs, non-DFUs and healthy subjects, exhibited cell quantities ranging from 21819 to 174962, facilitating the identification of cell populations, such as fibroblasts, keratinocytes, macrophages, vascular endothelial cells and the discovery of associated signal pathways.
| Objective | Major findings | Cell quantity | Changes at molecular level | Changes at cellular level | Signaling pathway changes | Ref. |
| Of 11 healthy subjects, 9 DFU healings, and 5 DFU non-healings | Some targeting genes, such as ANPEP, BID, CYBA, CYBB, FCER1G, ITGA1, and PLAUR were found in DFUs | 94325 | ANPEP, BID, CYBA, CYBB, FCER1G, ITGA1, PLAUR, CD19, ITGAM, and HLA-DR up-regulated in patients with DFUs | Macrophages, white blood cells, and monocytes increased in DFUs; pluripotent stem cells and stromal cells increased in DMs | - | [7] |
| Of 10 non-DMs and 17 DMs (11 DFUs and 6 non-DFUs) | The CCL2-ACKR1 signal pathway may be closely associated with DFU wound healing | - | CXCL11, MMP1, HS3ST2, CALML, LCK, LDLRAD2, S10OA1, and MAMDC2 up-regulated | Tissue stem cells and endothelial cells increased in DFUs | CCL, PROS, EDN, PERIOSTIN, and PARs activated in healing DFU tissues; FGF, SEMA3, MK, PIN, and TGF activated in DFUs | [8] |
| Of 10 non-DMs, 6 DMs, and 11 DFUs (7 healers; 4 Non-healers) | A new type of fibroblast named HE-Fibro was found in the DFUs | 174962 | IL7R, TCF7, CCR7, IL1B, S100A, HIF1A, TNF, STAT5a/b, TLR7, TLR9, IL17R/C, IL6, PLA2G2A, FOS, TNFAIP6, MMP1, and CHI3L1 up-regulated, while CD44, TGFβ1, CCL5, NFKBIA, SOX4, TGFβ1, and NANOG down-regulated in DFU-healers; NKG7, GNLY, CCL5, KLRD1, DAB2, CD163, TYMP, and ANXA1 up-regulated in DFU-non-healers | CCR7+, LEF1+ naive T cells, M1 macrophage, IL17+ cells, and HE-fibroblasts increased in DFU-healers; M2 macrophage increased in DFU-non-healers | IL-6, IL-8, CD28 signaling pathways, iCOS-iCOSL pathways inhibited in DFU-healers; RhoGDI, EIF2 signaling pathways; IL6, HIF1A, ILK signaling pathways activated in DFU-healers | [9] |
| Of 1 non-DM, 2 T2DMs | Some differentially expressed genes were found as keratinocyte-related gene, such as LUCAT1, MAL2 and MXD1 | 21819 | ARG1, PHYH, PKLR, PHKG1, ADH4, AQP9, HADH, PC, and ARG2 up-regulated in T2DM patients | - | Oxidative phosphorylation pathway, antigen processing and presentation pathway, tight junction pathway, amyotrophic lateral sclerosis pathway, vasopressin-regulated water reabsorption pathway activated in T2DM patients | [10] |
| DMs, non-DMs, STZ-induced diabetic mice | RAB17 in DFU-HDMECs may be the key factor of angiogenic capacity in DFUs | - | RAB17, CD200, HIF-1α and VEGF-A down-regulated in DFU patients | - | Hallmark-KRAS-signaling-on activated in DFU HDMECs; hallmark-angiogenesis, hallmark-epithelial-mesenchymal-transition, hallmark-inflammatory-responses, and hallmark-TNFα-signaling-via-NFκB inhibited in DFU HDMECs | [11] |
Through a cluster analysis of the data from the GEO database, Li et al[7] and Wang et al[8] found that the proportions of macrophages, leukocytes and monocytes were higher in patients with DFUs, which indicated a higher level of inflammation; also, the elevated proportions of pluripotent stem cells and stromal cells were observed in patients with DM, which indicated a higher level of dryness. These findings were in line with research by Theocharidis et al[9], who noted that DFU healers had a higher presence of naive and early differentiated progenitor T-lymphocytes, while non-healers had more cytotoxic natural killer T cells at the systemic level. Additionally, the proportion of M1 macrophages (classically activated macrophages that promote inflammation) was higher in DFU-healer than in DFU-non-healers, compared to that of M2 macrophages (alternatively activated macrophages with anti-inflammatory properties)[9].These results implied that suppressing systemic immuno-inflammatory responses while activating local responses in the wound environment could facilitate diabetic wound healing. Liao et al[10] found highly expressed keratinocyte genes in the diabetic wound, including SFN, LYPD3, S100A8, KRT1, KRT10, KRT6A, KRT5, and KRT16, underscoring the crucial role of keratinocytes in diabetic wound healing. By analysing the skin specimens of DFU patients and healthy controls using scRNAseq, Du et al[11] found that human dermal microvascular endothelial cells (HDMECs) isolated from DFU patients showed considerably impaired tube formation compared to those from healthy controls; they also found that the significantly under-expressed RAB17 in DFU-HDMECs may be the key factor leading to the impaired angiogenic capacity in DFUs. Moreover, it was proven in the diabetic mouse wound-healing model that the STZ-induced diabetic mice injected with an RAB17-overexpressing rAAV vector had a higher wound perfusion and a significant acceleration of wound closure[11]. Theocharidis et al[9] profiled 174962 single cells from the foot, forearm and peripheral blood mononuclear cells using scRNAseq; based on the differential expression of genes, the fibroblast population was divided into 14 subclusters, in which an unique population of fibroblasts overexpressing matrix metalloproteinase 1 (MMP1), MMP3, MMP11, hypoxia inducible factor 1-alpha (HIF1A), chitinase 3-like protein 1 and tumor necrosis factor (TNF)-alpha-stimulated gene-6 was defined as a new type of fibroblast, namely HE-Fibro. HE-Fibro were found to preferentially locate at the wound bed compared to the wound edge or unwounded skin and increase M1 macrophage polarisation in the DFU patients with healing wounds, suggesting that particular subtypes of fibroblasts play pivotal roles in the healing process of DFUs and targeting these specific fibroblast subtypes may represent a viable therapeutic strategy[9]. The differential gene ex
The advent and integration of scRNAseq into the study of diabetic wound healing have afforded unprecedented insights into cellular functions, pathophysiological processes and the intricate microenvironment of wounds. This technology enables the precise delineation of cellular subpopulations, the elucidation of pivotal molecular mechanisms and the identification of novel therapeutic targets. Future longitudinal studies that build a map of the diabetic wound healing timeline, and combine scRNAseq and spatial transcription may provide a better way to explore tissue regeneration and repair mechanisms. Collectively, the application of scRNAseq in diabetic wounds has provided new insights into the mechanism of diabetic wound healing and possible directions for further treatment.
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 484] [Article Influence: 242.0] [Reference Citation Analysis (0)] |
| 2. | McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. 2023;46:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 391] [Article Influence: 195.5] [Reference Citation Analysis (1)] |
| 3. | Bardill JR, Laughter MR, Stager M, Liechty KW, Krebs MD, Zgheib C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022;138:73-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Huang F, Lu X, Yang Y, Li Y, Kuai L, Li B, Dong H, Shi J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv Sci (Weinh). 2023;10:e2203308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 138] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Liu L. Applications of single cell RNA sequencing to research of stem cells. World J Stem Cells. 2019;11:722-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Armand EJ, Li J, Xie F, Luo C, Mukamel EA. Single-Cell Sequencing of Brain Cell Transcriptomes and Epigenomes. Neuron. 2021;109:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 7. | Li Y, Ju S, Li X, Li W, Zhou S, Wang G, Cai Y, Dong Z. Characterization of the microenvironment of diabetic foot ulcers and potential drug identification based on scRNA-seq. Front Endocrinol (Lausanne). 2022;13:997880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Wei D, Li S, Tang Q, Lu G, Gu S, Lu L, Liang F, Teng J, Lin J, Yu Y, Fang D, Huang Z. Healing mechanism of diabetic foot ulcers using single-cell RNA-sequencing. Ann Transl Med. 2023;11:210. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Theocharidis G, Thomas BE, Sarkar D, Mumme HL, Pilcher WJR, Dwivedi B, Sandoval-Schaefer T, Sîrbulescu RF, Kafanas A, Mezghani I, Wang P, Lobao A, Vlachos IS, Dash B, Hsia HC, Horsley V, Bhasin SS, Veves A, Bhasin M. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022;13:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 215] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 10. | Liao B, Ouyang Q, Song H, Wang Z, Ou J, Huang J, Liu L. Characteristic analysis of skin keratinocytes in patients with type 2 diabetes based on the single-cell levels. Chin Med J (Engl). 2022;135:2461-2466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Du H, Li S, Lu J, Tang L, Jiang X, He X, Liang J, Liao X, Cui T, Huang Y, Liu H. Single-cell RNA-seq and bulk-seq identify RAB17 as a potential regulator of angiogenesis by human dermal microvascular endothelial cells in diabetic foot ulcers. Burns Trauma. 2023;11:tkad020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |