Published online Apr 26, 2024. doi: 10.4252/wjsc.v16.i4.444
Peer-review started: January 15, 2024
First decision: January 29, 2024
Revised: February 11, 2024
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: April 26, 2024
Processing time: 100 Days and 13.2 Hours
Leukemia stem cells (LSCs) are found to be one of the main factors contributing to poor therapeutic effects in acute myeloid leukemia (AML), as they are protected by the bone marrow microenvironment (BMM) against conventional therapies. Gossypol acetic acid (GAA), which is extracted from the seeds of cotton plants, exerts anti-tumor roles in several types of cancer and has been reported to induce apoptosis of LSCs by inhibiting Bcl2.
To investigate the exact roles of GAA in regulating LSCs under different microenvironments and the exact mechanism.
In this study, LSCs were magnetically sorted from AML cell lines and the CD34+CD38- population was obtained. The expression of leucine-rich pentatricopeptide repeat-containing protein (LRPPRC) and forkhead box M1 (FOXM1) was evaluated in LSCs, and the effects of GAA on malignancies and mitochondrial function were measured.
LRPPRC was found to be upregulated, and GAA inhibited cell proliferation by degrading LRPPRC. GAA induced LRPPRC degradation and inhibited the activation of interleukin 6 (IL-6)/janus kinase (JAK) 1/signal transducer and activator of transcription (STAT) 3 signaling, enhancing chemosensitivity in LSCs against conventional chemotherapies, including L-Asparaginase, Dexamethasone, and cytarabine. GAA was also found to downregulate FOXM1 indirectly by regulating LRPPRC. Furthermore, GAA induced reactive oxygen species accumulation, disturbed mitochondrial homeostasis, and caused mitochondrial dysfunction. By inhibiting IL-6/JAK1/STAT3 signaling via degrading LRPPRC, GAA resulted in the elimination of LSCs. Meanwhile, GAA induced oxidative stress and subsequent cell damage by causing mitochondrial damage.
Taken together, the results indicate that GAA might overcome the BMM protective effect and be considered as a novel and effective combination therapy for AML.
Core Tip: Gossypol acetic acid (GAA) inhibited janus kinase 1/signal transducer and activator of transcription 3 signaling activated by interleukin 6 in leukemia stem cells (LSCs). GAA sensitizes to chemoagent, including cytarabine, Dexamethasone and L-Asparaginase in LSCs. GAA decreased leucine-rich pentatricopeptide repeat-containing protein and subsequent downregulated forkhead box M1, which are critical and necessary for stemness of LSCs. GAA induces mitochondrial dysfunction via inducing reactive oxygen species accumulation. GAA might eliminate the effects of inflammatory environment on LSCs.
- Citation: Ai CJ, Chen LJ, Guo LX, Wang YP, Zhao ZY. Gossypol acetic acid regulates leukemia stem cells by degrading LRPPRC via inhibiting IL-6/JAK1/STAT3 signaling or resulting mitochondrial dysfunction. World J Stem Cells 2024; 16(4): 444-458
- URL: https://www.wjgnet.com/1948-0210/full/v16/i4/444.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i4.444
Acute myeloid leukemia (AML) is a hematopoietic disorder characterized by uncontrolled proliferation of immature, abnormal blast cells and loss of functional blood cells, resulting in remarkably chemoresistant disease and poor prognosis[1]. Therapy failure is common in many cases of risk karyotype AML, and most patients develop chemotherapy relapses, depending on the mechanisms of recurrence[2].
Rare subsets labeled as the CD34+CD38- population are considered leukemia stem cells (LSCs), which possess remarkable proliferative potential[3,4], as also reported in other studies[5,6]. It is well-established that primary chemotherapeutic agents might eliminate AML blast cells but not LSCs, indicating that LSCs could be responsible for chemoresistance in AML[7,8]. Moreover, original AML behaves differently compared to LSCs[9,10]. Therefore, investigating LSCs is critical for eliminating cancer cells in AML.
Bone marrow microenvironment (BMM) is a key component of leukemia pathogenesis by exerting protective effects on LSC against various types of stress, such as oxidative and hypoxic stress[11]. Therefore, novel therapeutic strategies have been developed targeting BMM. Inactivation of interleukin 6 (IL-6) via inhibition of janus kinase 1/signal transducer and activator of transcription 3 (JAK1/STAT3) signaling leads to the inhibition of LSC survival[12,13]. In chronic myeloid leukemia, which is characterized by TKI-resistance, inhibition of JAK1/STAT3 signaling has been found to reduce LSC survival and impair its stemness[14]. Recently, it has also been reported that activation of the JAK1 kinase and subsequent STAT3 signaling contributes to poor therapeutic outcomes and prognosis in chronic myeloid leukemia cells[15]. However, the development of effective and efficient BMM-targeted chemotherapeutic agents is still largely needed.
GAA is a traditional Chinese herbal medicine widely used clinically to treat different diseases with significant antitumor effects[16]. Previous studies have reported that GAA exerts anti-tumor effects by inhibiting cell proliferation via blocking cell cycle progression and inducing cell apoptosis. Yang et al[17] reported that, in colorectal cancer, GAA treatment specifically degrades leucine-rich pentatricopeptide repeat-containing protein (LRPPRC), a key regulator of mitochondrial homeostasis and function, thereby acting as an anti-tumor agent. This prompted us to investigate GAA’s potential anti-tumor effects on LSCs[17].
To investigate this possibility, we analyzed the LRPPRC profile status in LSCs derived from AML cell lines. As expected, LSCs exhibited higher expression levels of LRPPRC than original AML cells. GAA degraded LRPPRC and thus inhibited IL-6/JAK1/STAT3 signaling. Moreover, GAA also downregulated forkhead box M1 (FOXM1), which is positively correlated with LRPPRC, in an IL-6/JAK1/STAT3-independent manner.
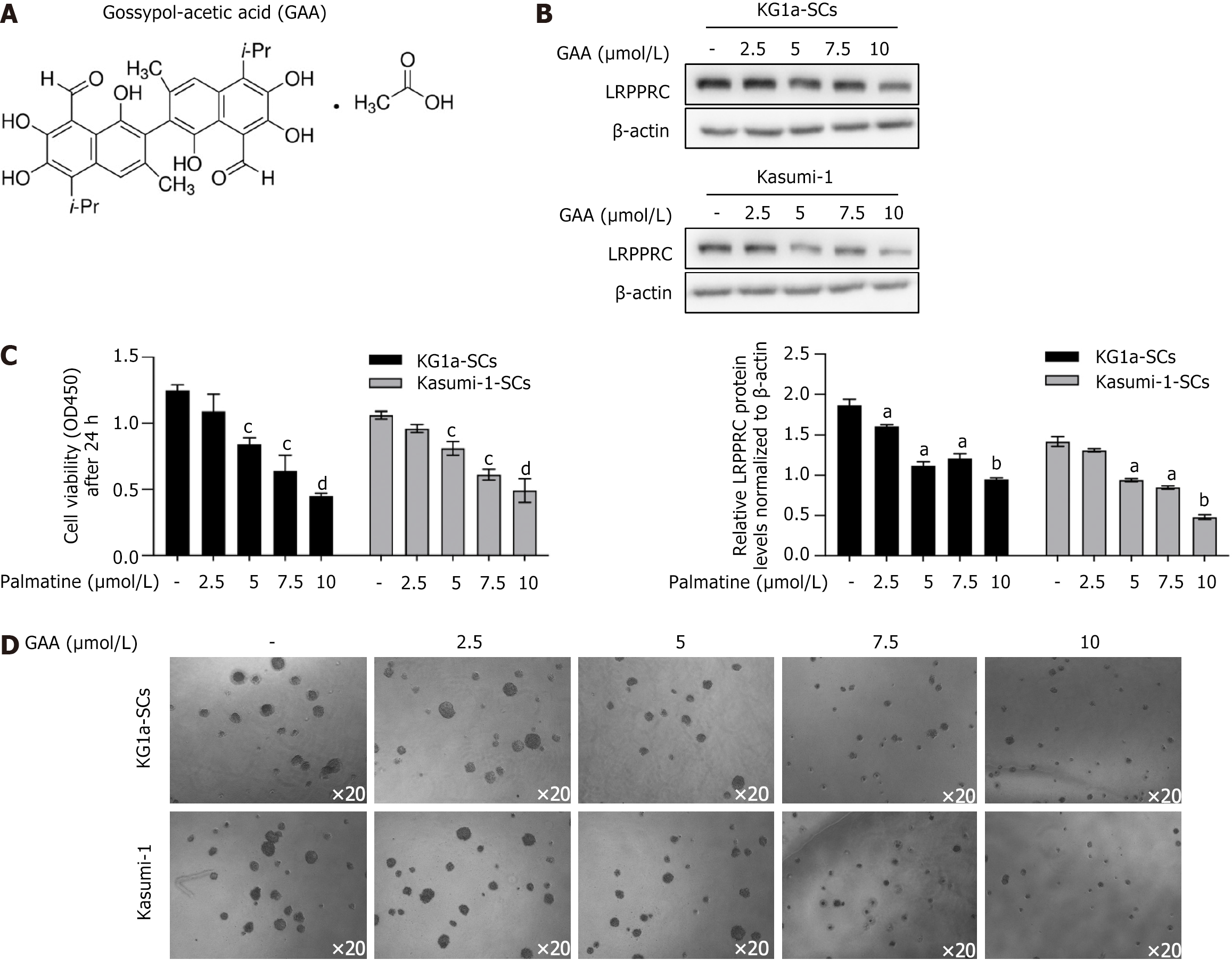
Human AML cell lines Kasumi-1 and KG1a were bought from Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences. Cells were maintained in RPMI1640 medium supplemented with 20% fetal calf serum (Gibco) stored in the incubator (Life Technology, United States) at 37 °C and 5% CO2 saturation humidity. Culture medium was changed for passage every 2-3 d. For GAA treatment, GAA bought from Sigma-Aldrich (Figure 1A) was added into original medium at final concentration of 2.5, 5, 7.5, or 10 μmol/L for 24-h incubation followed by certain detection. For IL-6 incubation, IL-6 bought from Sigma-Aldrich was added into original medium at final concentration of 5, 10, 25, or 50 μmol/L for 6-h incubation followed by certain detection. For S3I-210 incubation, S3I-210 bought from Selleck was added into original medium at final concentration of 10 μmol/L for 24-h incubation followed by certain detection.
Of 5 × 107 cultured cells was collected and washed using 1× Dulbecco’s phosphate-buffered saline (DPBS) for three times. After brief centrifugation, supernatant was discard and cell pellet was resuspended in 10 mL of 1× DPBS. CD34+CD38- cells were selected using a magnetic bead selection protocol (Miltenyi Biotech, Bergisch Gladbach, Germany). Briefly, the CD34+/CD38- population (Kasumi-1 cells) or CD34+/CD38- population (KG-1 cells) was obtained by incubating with CD34-FITC (Biolegend, San Diego, CA United States) and CD38-R-phycoerythrin cyanine7 (CD38-PE-Cy™7; Biolegend) in Cell Staining Buffer (Biolegend).
The GEPIA 2 online analysis tool (http://gepia2.cancer-pku.cn) was employed for gene expression analysis by comparing to The Cancer Genome Atlas dataset. GEPIA 2 provides a variety of functions, including tumor/normal differential expression and correlation analysis. Expression correlation of LRPPRC and FOXM1 in whole blood samples, AML cell lines and LAML samples was analyzed. |R| > 0.1 and P value < 0.05 were set as selection criteria for identifying as statistically significant.
Cells were plated into 96-well plates at 1 × 105 cells/well and allowed to attach overnight. Then, different concentrations of cytarabine (Ara-C) (0, 0.5, 1, 2, 4 μmol/L), Dexamethasone (Dex) (0, 0.5, 1, 2, 4 μmol/L) or L-Asparaginase (L-Asp) (0, 0.5, 1, 2, 4 μmol/L) were added into wells for 24-h culture. CCK-8 reagent (Sigma-Aldrich) was added (10 μL/well) for extra 4 h incubation. The absorbance (A) at 450 nm was measured using a microplate reader (Synergy 2 Multi-Mode Microplate Reader; BioTek, Winooski, VT, United States). By using the following formula: Cell survival rate (%) = [(administration group A - negative control group A)/(non-administration group A - negative control group A)] × 100%, cell survival rate was calculated.
Of 1 × 105 LSCs was suspended in Human Methylcellulose Complete Media (R&D Systems, Inc, Minneapolis, MN, United States) and were added into a 6-well dish. Cells were then cultured in 5% CO2 at 37 °C with more than 95% humidity for 7 d. On day 7, an optical microscope was employed to observe and count the formed colonies.
Of 1 × 106 cells were collected and washed using 1× PBS for three times. Then, 10 μmol/L 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA, Life Technologies, Cat# D-399) was added and incubated for 1 h. Cells were collected and washed using 1× PBS for three times to remove free H2DCFDA. Oxidation of H2DCFDA to the highly fluorescent 2’,7’-dichlorofluorescein is proportional to reactive oxygen species (ROS) generation and the signals were then read at EX485 nm/Em535 nm using a GloMax 96 Microplate Luminometer (Promega). Samples were also detected immediately by using a flow cytometer (Fortessa, BD Biosciences). A minimum of 10000 cells was analyzed per condition.
Total proteins were extracted using Animal Tissue/cells/bacteria total protein isolation kit (DocSense, Chengdu, China) from cells. Then, quantification of protein lysates was measured with a protein BCA assay Kit (Bio-Rad) and fractionated by 10% sodium-dodecyl sulfate gel electrophoresis and transferred to a nitrocellulose membrane, followed by blockage using 5% non-fat milk in TBST for 60 min at 25 °C and then incubated with primary antibody diluted in blocking buffer at 4 °C overnight: Anti-LRPPRC/GP130 antibody (ab259927, 1:1000); anti-FOXM1 antibody (ab207298, 1:2000); anti-Peroxiredoxin 3/PRDX3 antibody (ab128953, 1:2000); anti-SOD2/MnSOD antibody (ab68155, 1:1000); anti-catalase antibody (ab209211, 1:1000); anti-cleaved caspase-3 antibody (ab32042, 1:1000); anti-cleaved PARP1 antibody (ab32064, 1:1000); anti-JAK1 antibody (ab133666, 1:2000); anti-JAK1 (phosphor Y1022 + Y1023) antibody (ab138005, 1:1000); anti-STAT3 antibody (ab68153, 1:2000); anti-STAT3 (phosphor Y705) antibody (ab267373, 1:1000); anti-beta actin antibody (ab8226, 1:4000). Washed membranes were then incubated with horseradish peroxidase-coupled Goat Anti-Rabbit IgG H&L secondary antibody (1:50000; abcam, ab6721) for 1 h at room temperature and signal was imaged using enhanced chemiluminescence according to the manufacturer’s instructions (ECL kit, Life Technologies).
Mitochondrial membrane potentials were observed in cells stained with JC-1 dye bought from Life Technology by employing a confocal microscope (LSM880, Zeiss, Jena, Germany). Specifically, cells were harvested, washed with ice-cold PBS. Then JC-1 was added for 20 min incubation at 37 °C. Green florescence emission in its monomer forma and red florescence emission in its polymer forms were collected separately. Cells were analyzed by performing flow cytometry using a 3 Laser Navios flow cytometers (Beckman Coulter, Brea, CA, United States).
Before ATP analyzing, cells were cultured in original medium for 24 h and then collected and washed using 1× PBS. Then CellTiterGlo kit was employed to detect ATP content by using luciferase chemiluminescence Multiskan spectrum microplate reader (Thermo Electron Corporation, Waltham, MA, United States).
All data are presented as mean ± SD. Statistical differences between different groups were analyzed by one way ANOVA post hoc Dunnett T-test using Prism 6 (GraphPad Software, San Diego, CA, United States). The correlation of gene expression was evaluated in the GEPIA database using Spearman’s correlation analysis. Value of P < 0.05, P < 0.01, P < 0.001 was considered statistically significant.
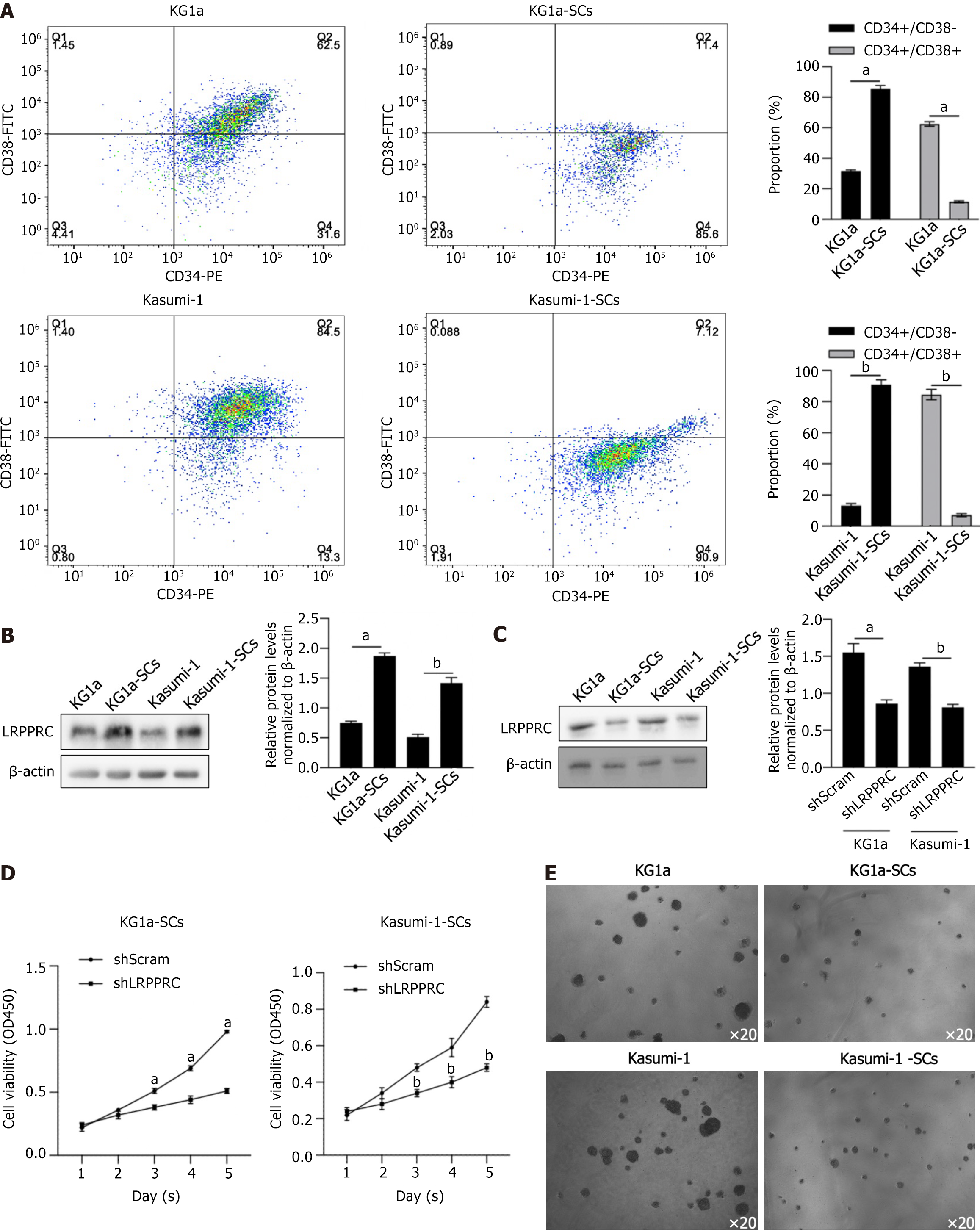
To evaluate the potential roles of LRPPRC in SCs derived from KG1a and Kasumi-1, we adopted magnetic bead sorting to enrich LSCs from these two cell lines, separately, to investigate whether LRPPRC expresses differentially in LSCs compared to AML cells. LSCs were selected by being co-cultured with PE-labeled CD34 and FITC-labeled CD38. As shown in Figure 2A, over 85% of cells were CD34+CD38-, which was regarded as LSCs after magnetic bead sorting followed by flow cytometry assay, which was significantly higher than those in KG1a or Kasumi-1 cells. This result indicated successful enrichment of LSCs from KG1a and Kasumi-1 cells. Then, total protein was fractionated and detected by western blot to evaluate LRPPRC expression levels. As shown in Figure 2B, LRPPRC was detected to be upregulated in KG1a-SCs and Kasumi-1-SCs significantly, demonstrating that LSCs maintained significant higher LRPPRC levels.
Based on the widely investigated oncogenic role, we further conjectured that LRPPRC expression could affect LSCs to alter the in vitro biological functions and subsequently affecting leukemia progression. We transfected short hairpin RNA targeting to LRPPRC mRNA and efficiently knockdown LRPPRC expression in LSCs after 48-h transfection (Figure 2C). We then evaluate the effects of LRPPRC knockdown in LSCs by detecting cell viability from day 1 to 5 and colony assays. Expectedly, LRPPRC knockdown significantly decreased cell viability in LSCs from day 1 to 5 and inhibited colony formation (Figure 2D and E).
It is reported that GAA acts as an anti-tumor agent, via targeting to LRPPRC and resulted in protein degradation[16], we treated KG1a-SCs and Kasumi-1-SCs with GAA with different concentrations ranged from 2.5-10 μmol/L for 24 h. By performing western blot, it is presented that 5-10 μmol/L GAA treatment significantly decreased LRPPRC protein levels after 24 h via promoting protein degradation (Figure 1B), without affecting LRPPRC transcription (data not shown). By considering that LRPPRC acts as a oncogenic factor in several kinds of cancer[18,19], we then evaluate the effect of GAA on KG1a-SCs and Kasumi-1-SCs cell viability and colony formation. As shown in Figure 1C, GAA treatment ranged from 5-10 μmol/L significantly decreased cell viability. In colony assay, long-term GAA treatment also inhibited colony formation ability in KG1a-SCs and Kasumi-1-SCs (Figure 1D).
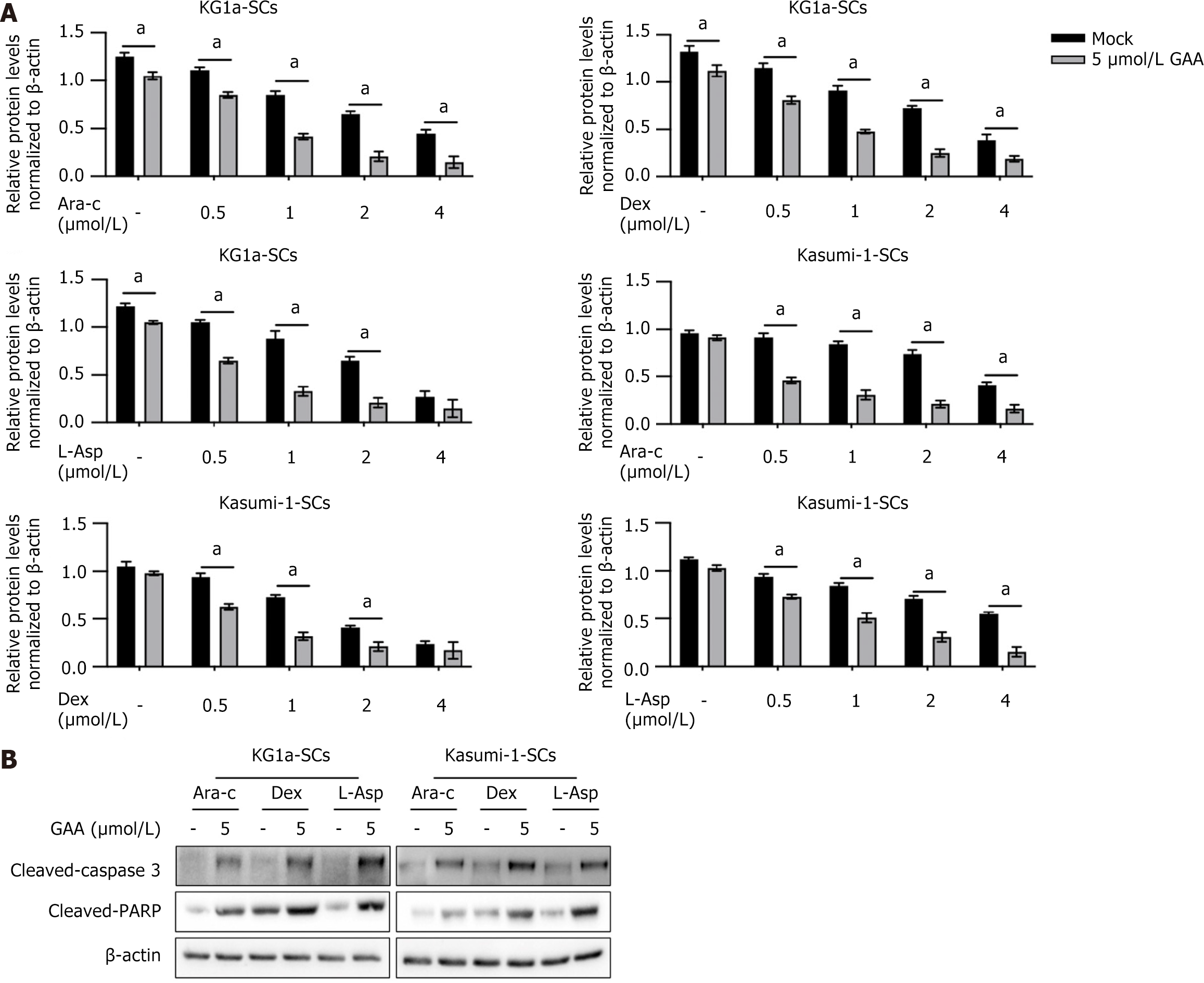
LRPPRC is reported to be critical for chemoresistance in cancer cells[17], and promotes us to confirm whether GAA treatment affects chemoresistance in KG1a-SCs and Kasumi-1-SCs. Three commonly used chemotherapeutic agents, including Ara-c, Dex, or L-Asp, were used to treat KG1a-SCs and Kasumi-1-SCs with or without addition of 5 μmol/L of GAA. As it is shown in Figure 3A, presence of GAA significantly decreased cell viability with the presence of GAA. It is also observed that co-treatment of chemoagent and GAA obviously increased cleaved-caspase 3 and cleaved-PARP, demonstrating that GAA might increase chemosensitivity in KG1a-SCs and Kasumi-1-SCs. To further confirm whether GAA promotes apoptosis induced by chemoagent, we then detected cleaved-caspase 3 and cleaved-PARP after chemotreatment. Expectedly, addition of 5 μmol/L GAA obviously increased cleaved-caspase 3 and cleaved-PARP with the presence of 1 μmol/L Ara-c, Dex or L-Asp (Figure 3B).
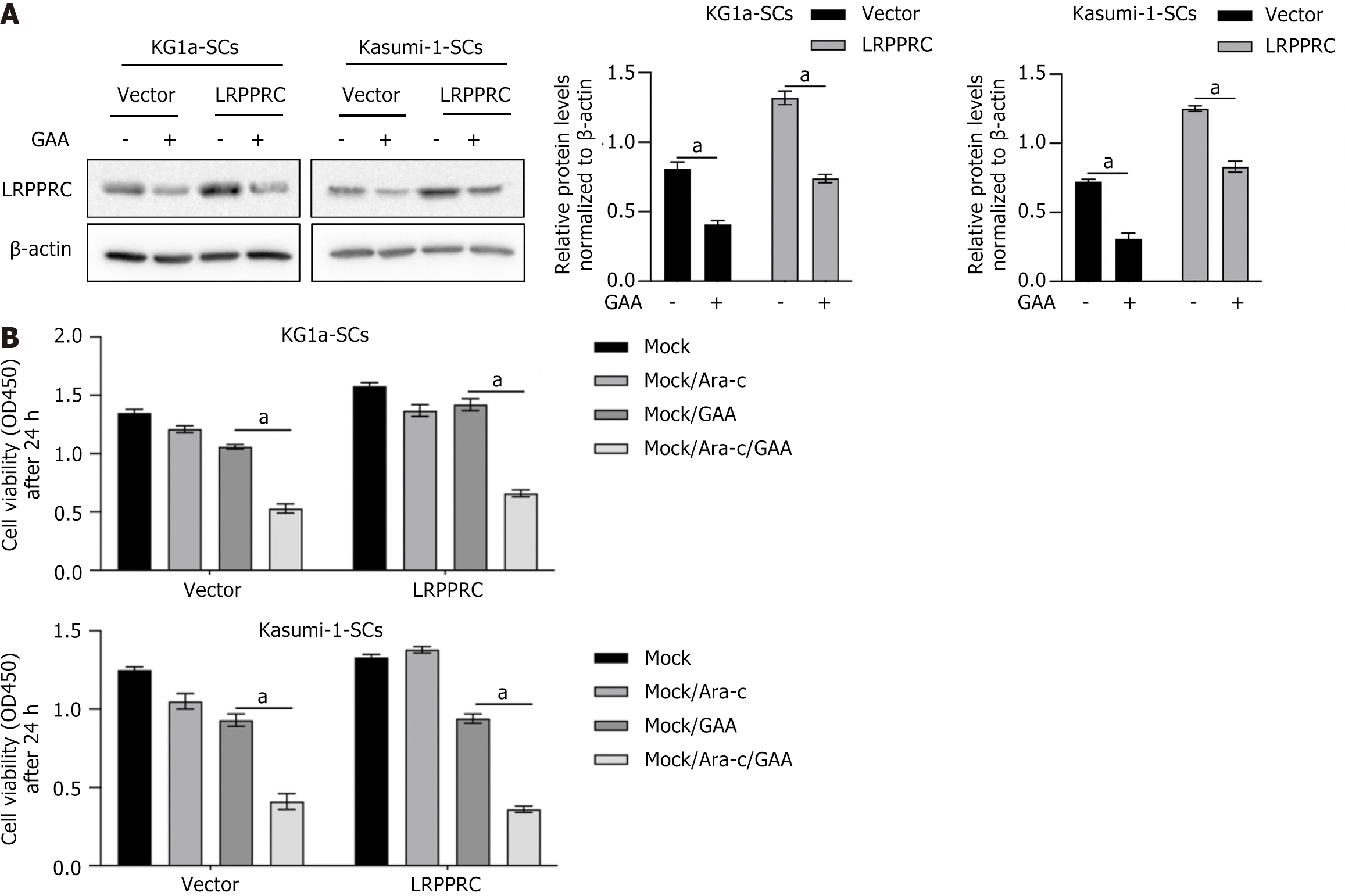
To confirm whether the impact of GAA on LSCs is due to degrade LRPPRC or not, LRPPRC was overexpressed before GAA treatment. As it is shown in Figure 4A, LRPPRC overexpression significantly reversed degradation of LRPPRC caused by GAA treatment. As expected, in LRPPRC overexpressed cells, chemosensitivity was significantly decreased (Figure 4B), indicating that the effect of GAA on promoting chemosensitivity is mainly via inducing LRPPRC degra
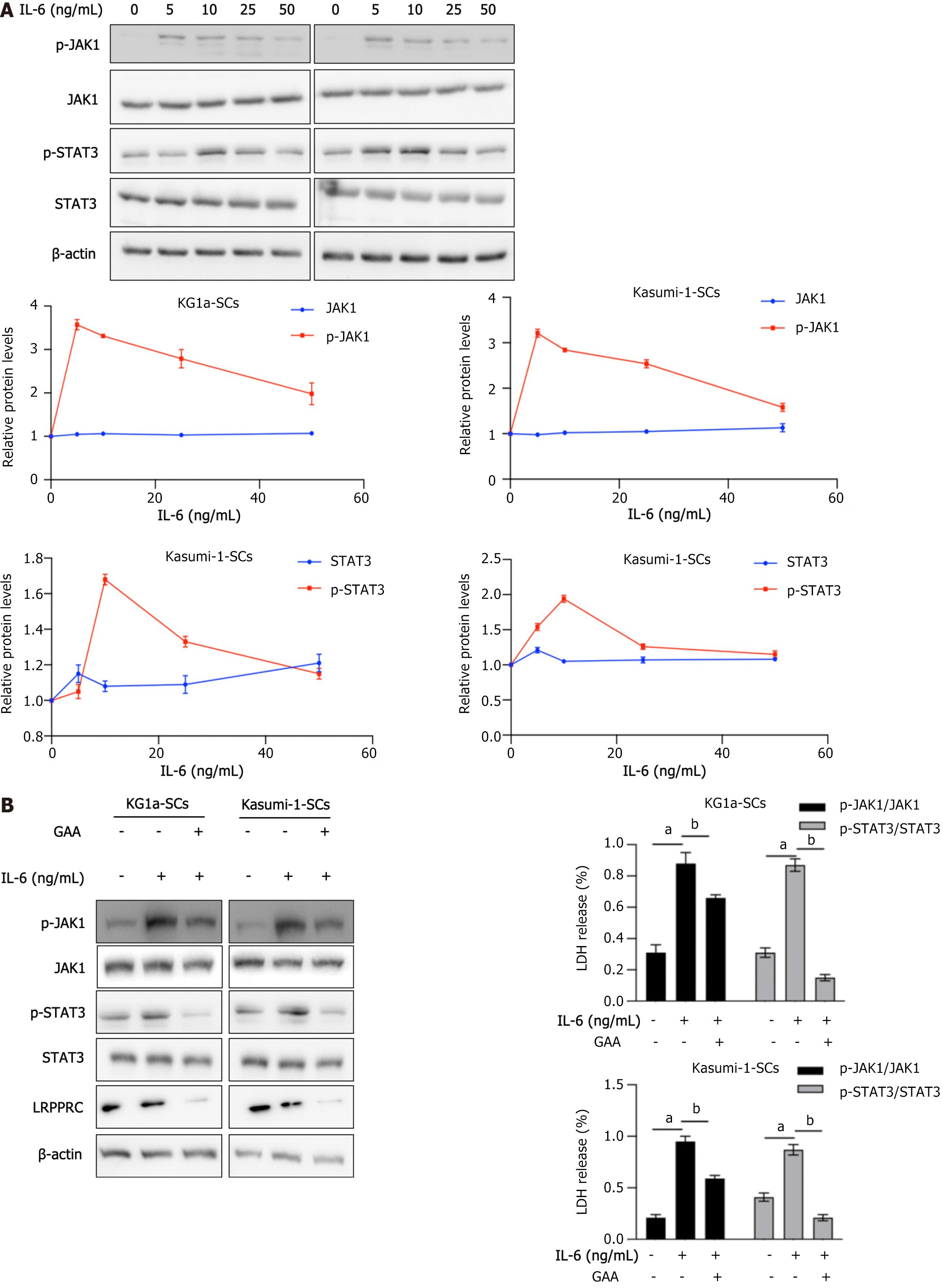
IL-6 is a pleiotropic inflammatory cytokine that has a central role in inflammatory response and malignancy[20]. In classic IL-6 signaling, binding of IL-6 to IL-6R on the cell surface is critical to initiate intracellular signaling. By considering that GAA affects IL-6/STAT3 signaling in human non-small cell lung cancer cells[21,22], we detected whether GAA treatment affects IL-6/JAK1/STAT3 signaling or not. As it is presented in Figure 5A, after 4-h incubation with different concentration of IL-6 ranged from 5-50 ng/mL, phosphorylation of JAK1 (p-JAK1) and phosphorylation of STAT3 (p-STAT3) were obviously activated, without disturbing p-JAK1 and p-STAT3 protein levels. Thus, we further added 10 ng/mL IL-6 to KG1a-SCs or Kasumi-1-SCs pretreated with 5 μmol/L GAA for 24 h, for 4-h incubation. As shown in Figure 5B, IL-6 treatment activated JAK1/STAT3 signaling, without affecting p-JAK1, p-STAT3 or LRPPRC protein levels. Expectedly, pretreatment of GAA significantly inhibited JAK1/STAT3 stimulated by IL-6.
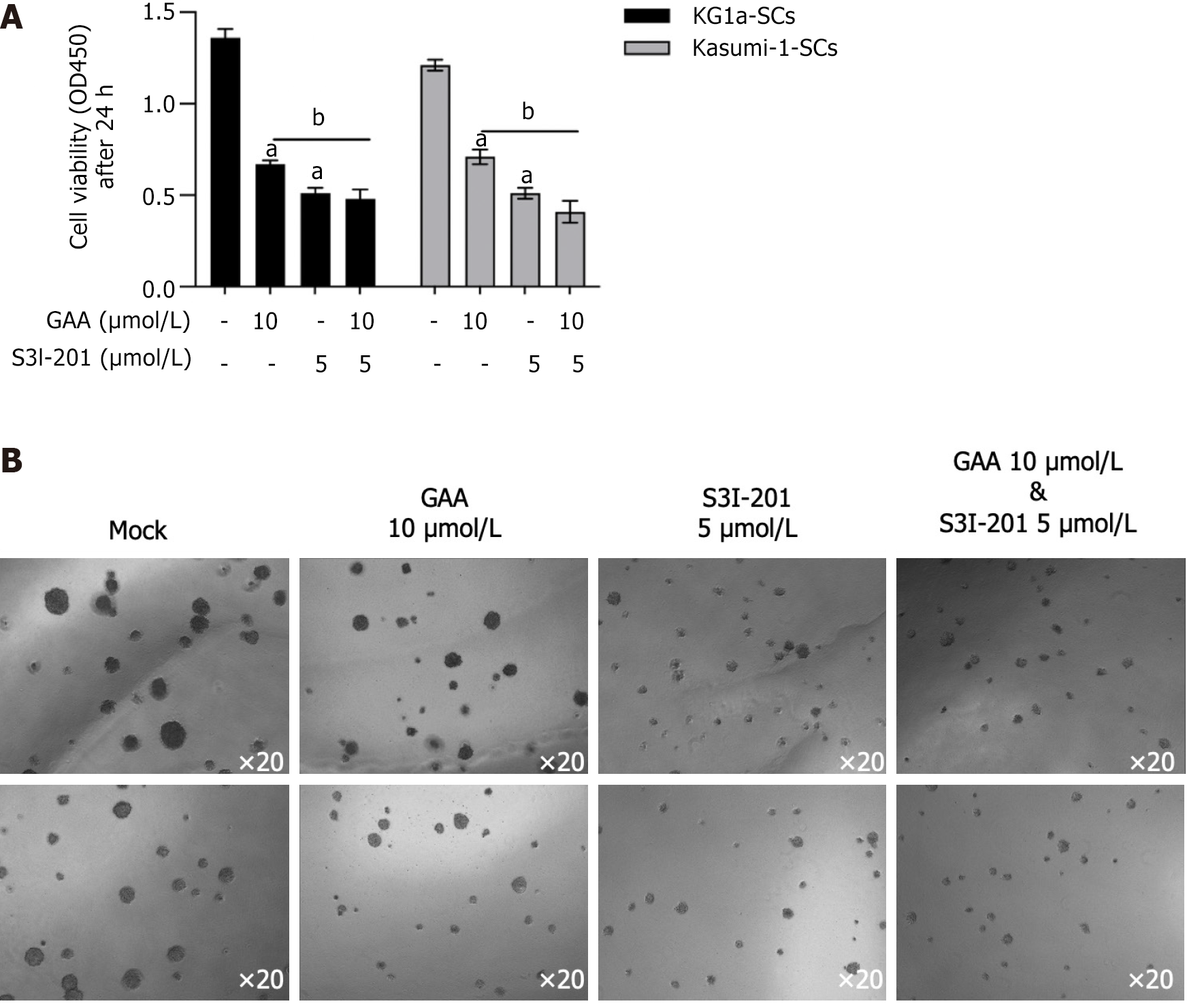
The roles of IL-6 in BMM controls leukemic multipotent progenitor cell fate and contributes to maintenance of self-renewal capacity of LSCs[23,24]. To further evaluate whether IL-6/JAK-1/STAT3 signaling regulation of self-renewal capacity of LSCs is reversed by GAA, we employed 5 μmol/L S3I-201, a pharmacological inhibitor of STAT3 signaling. As shown in Figure 6A and B, addition of GAA or S3I-201 respectively inhibited cell viability and colony formation. Notably, addition of S3I-201 to KG1a-SCs or Kasumi-SCs pretreated with GAA further decreased malignancies. These results indicated that IL-6/JAK1/STAT3 signaling is partially inhibited by GAA and results in loss of self-renewal in LSCs.
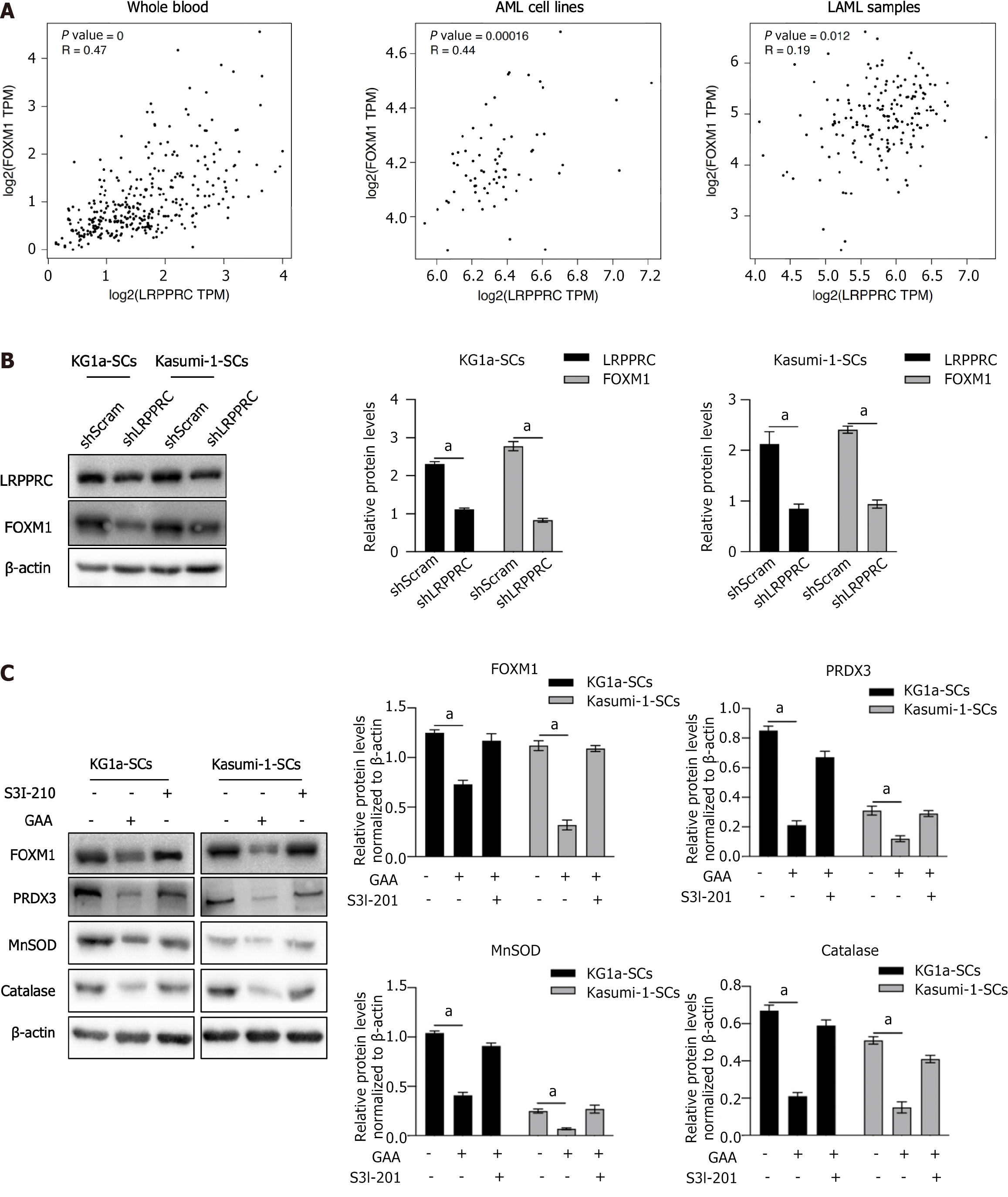
FOXM1 is a crucial regulator of cancer development and found often to be overexpressed in AML[25]. It is also reported to be tightly involved in regulating LSC survival[26]. By considering that LRPPRC is reported to tightly regulate FOXM1[27,28], we employed GEPIA database online tool to analyze correlation of LRPPRC with FOXM1 in whole blood, AML cell lines and AML pathological samples. As it is shown in Figure 7A, FOXM1 and LRPPRC are transcriptionally and positively correlated in whole blood (r = 0.47; P < 0.001), AML cell lines (r = 0.44; P = 0.00016) and LAMA samples (r = 0.19; P = 0.012). LRPPRC knockdown or overexpression decreased or increased FOXM1 (Figure 7B), indicating that LRPPRC positively regulates FOXM1 in KG1a-SCs and Kasumi-1-SCs. We then measured the expression of FOXM1 downstream ROS scavenger genes, including PRDX3, MnSOD, and catalase. To confirm whether LRPPRC affects FOXM1 via IL-6/JAK1/STAT3 signaling, S3I-210 was employed. As shown in Figure 7C, without being affected by S3I-210, GAA treatment significantly decreased FOXM1 and its downstream target genes, indicating that LRPPRC regulates FOXM1 and its downstream genes in an IL-6/JAK1/STAT3-independent manner.
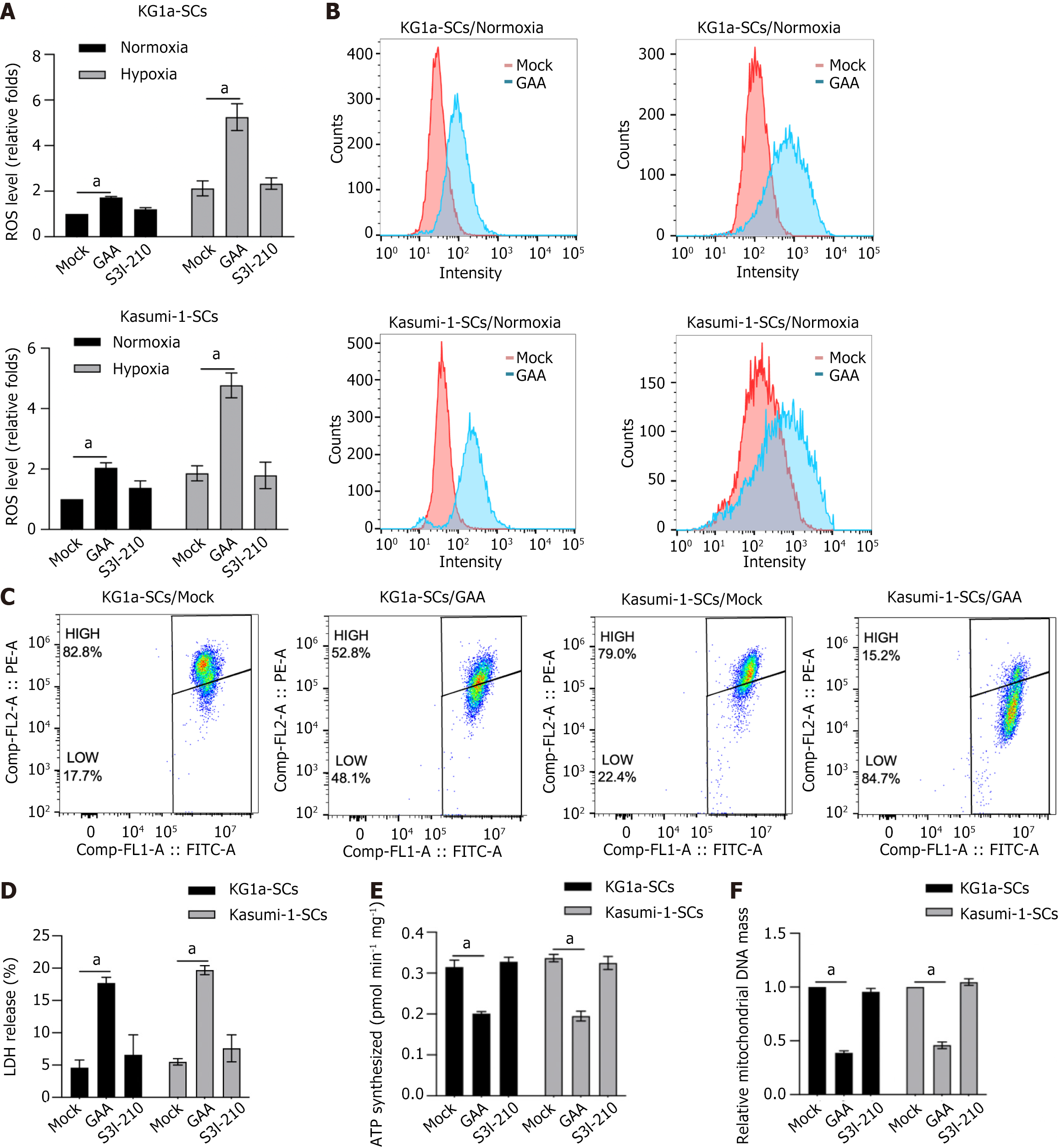
In our precious findings, LRRPRC was presented to be a mitochondrial protector against oxidative stress[17]. This promotes us to evaluate the effect of GAA on mitochondrial function. As presented in Figure 8A, GAA treatment, but not S3I-210, significantly induced ROS accumulation, especially under hypoxic condition. By performing flow cytometry, it is also observed that both in hypoxia and normoxia condition, GAA treatment obviously increased ROS levels in KG1a-SCs and Kasumi-1-SCs (Figure 8B). Then we evaluate the effect of GAA treatment on mitochondrial homeostasis by performing JC-1 staining. Expectedly, GAA treatment significantly increased JC-1 FITC-positive proportion in KG1a-SCs and Kasumi-1-SCs, indicating that GAA might affect mitochondrial homeostasis and potentially result in mitochondrial dysfunction (Figure 8C). Analysis of LDH leakage presented that GAA treatment significantly increased cell membrane impact (Figure 8D). We further confirmed whether disturbance of mitochondrial homeostasis induced by GAA induces mitochondrial dysfunction by detecting mitochondrial DNA (mtDNA) content (Figure 8E) and ATP synthesis (Figure 8F). Expectedly, GAA treatment significantly decreased mtDNA content and ATP synthesis, indicating that GAA might regulate stemness of LSCs via inducing mitochondrial dysfunction.
Our study has established the critical role of GAA in the regulation of cell proliferation, chemosensitivity, and self-renewal of LSCs derived from AML cells. Our results show that GAA exerts anti-tumor effects by inhibiting the IL-6/JAK1/STAT3 signaling pathway through promoting the degradation of LRPPRC, which is a critical component of IL-6/JAK1/STAT3 signaling. In addition, we have identified the role of GAA in degrading LRPPRC, resulting in the downregulation of FOXM1, which plays a critical role in the regulation of survival, quiescence, and self-renewal of LSCs. Furthermore, considering that LRPPRC regulates redox homeostasis, mitochondrial homeostasis, gene expression, and energy metabolism[29,30], we also found that GAA induces oxidative stress, loss of mitochondrial homeostasis, mitochondrial mass, and energy metabolism.
Bone marrow samples were used to predict a novel functional profile for predicting prognosis in pediatric patients with AML[31]. It has been demonstrated that the sensitivity of AML cells to ligand-induced activation of STAT3 might be considered as a predictor of event-free survival and overall survival in pediatric patients with AML. IL-6 activates the JAK1/STAT3 signaling and thus acts as a key cytokine[32]. GAA was reported to inhibit IL-6/JAK1/STAT3 signaling and result in suppressing the STAT3-DNA binding activity and its regulatory roles in its downstream target genes, including Bcl-2 and Bcl-xL[27]. In the present study, we found that GAA pretreatment inhibited activation of JAK1/STAT3 signaling stimulated by IL-6. GAA inhibited phosphorylation of JAK1 and STAT3 after IL-6 was added, indicating that GAA might affect IL-6 and IL-6 receptor interaction. However, further investigation is necessary to reveal the exact molecular mechanism.
FOXM1 is a critical member of forkhead box transcription factor family, reported to be involved in regulating multiple physiological processes. In contrast to other family members of forkhead box transcription factor family, FOXM1 is mainly expressed and activated in proliferating cells due to its role in promoting cell cycle progression[33,34]. Many studies have implicated FOXM1 in tumorigenesis as it is overexpressed in a variety of human malignancies. For instance, gene expression profiles in a number of carcinomas[35]. High expression of FOXM1 in glioblastoma correlates with the tumorigenicity of the glioma cells[36]. In prostate cancer, FOXM1 overexpression is also highly correlated with metastasis[37]. Importantly, FOXM1 is reported to be required for survival, quiescence and self-renewal of LSCs in vivo via activation of Wnt/β-catenin signaling pathway by directly binding to β-catenin and subsequently stabilizing β-catenin protein. By considering its promoting effects on LSCs, FOXM1 is considered as a potential therapeutic target for selectively eliminating LSCs[24]. In this study, we found LRPPRC is upregulated in LSCs compared to AML cells. More importantly, in whole blood sample, AML cell lines and LAML samples, LRPPRC and FOXM1 are positively correlated transcriptionally. It is observed that, in LSCs, LRPPRC is positively associated with FOXM1 and thus modified its downstream target genes, including PRDX3, MnSOD and catalase. However, IL-6/JAK1/STAT3 signaling is irrelevant with FOXM1 expression, indicating that GAA affects LSCs survival and proliferation, at least partially, via two different manners.
There is growing evidence that mitochondria play an important role in tumor growth as they regulate apoptosis and ROS production[38]. ROS are by-products of oxidative respiration, which can be generated in the tricarboxylic acid cycle. mtDNA, a small circular genome, encodes 13 proteins of the ETC machinery. Dysregulated mtRNA could lead to mitochondrial protein alterations, influencing ETC activity and leading to excess ROS production. FOXM1 is required for redox homeostasis and survival of several cancers via the regulation of tricellular ROS accumulation[39]. In our results, GAA treatment significantly induced ROS accumulation, especially under hypoxic conditions, but not by inhibiting IL-6/JAK1/STAT3 signaling. Moreover, we found that GAA treatment decreased mitochondrial membrane potential, causing disturbance of mitochondrial homeostasis, cell membrane impact, energy metabolism, and mitochondrial mass. However, it is still unclear whether LRPPRC degradation caused by GAA or subsequent downregulation of FOXM1 is the main cause of mitochondrial dysfunction, which needs further investigation. Moreover, in this study, we failed to evaluate the effects of LRPPRC in nude mice, which is worth to be performed in further investigation.
In summary, our study provides the first evidence of GAA used as a potential chemoagent specifically targeting LSCs by blocking BMM and LRPPRC in AML cells. GAA blocks pro-inflammatory cytokines and also induces mitochondrial dysfunction via the LRPPRC/FOXM1 axis. Thus, this study suggests GAA as a novel chemoagent targeting LSCs for the treatment of AML.
The presence of leukemia stem cells (LSCs) is one of the major contributors of poor prognosis and outcome of myeloid leukemia. By considering the anti-chemoagent property of LSCs, it is still a major challenge of developing chemoagent targeting to this sub-population of acute myeloid leukemia (AML).
The subpopulation of AML is characterized by remarkable anti-agent property, which leads to poor therapeutic effects. Instead of searching chemoagent exerting cytotoxic effect against LSCs, developing chemoagent or molecule exerting anti-stemness might be a promising strategy.
We aimed to evaluate the effects of gossypol acetic acid (GAA) on stemness of LSCs. We also evaluated the malignent behaviors after GAA treatment.
LSCs were magnetically sorted from AML cell lines and the CD34+CD38- population was obtained. The expression of leucine-rich pentatricopeptide repeat-containing protein (LRPPRC) and forkhead box M1 was evaluated in LSCs, and the effects of GAA on malignancies and mitochondrial function were measured.
GAA treatment decreased LRPPRC, and thus inhibiting interleukin 6/janus kinase 1/signal transducer and activator of transcription 3 signaling. As a result, GAA treatment decreased stemness and enhanced chemosensitivity in LSCs.
Our results indicate that GAA might overcome the bone marrow microenvironment protective effect and be considered as a novel and effective combination therapy for AML.
It is worth investigating how GAA results degradation of LRPPRC. Meanwhile, it is worth to evaluate the effects of GAA in AML mice model to evaluate the therapeutic effect in vivo.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, India S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 2. | Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3010] [Cited by in RCA: 4311] [Article Influence: 479.0] [Reference Citation Analysis (0)] |
| 3. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4858] [Article Influence: 173.5] [Reference Citation Analysis (1)] |
| 4. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3316] [Cited by in RCA: 3384] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 5. | Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, Canty AJ, Danska JS, Bohlander SK, Buske C, Minden MD, Golub TR, Jurisica I, Ebert BL, Dick JE. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 821] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 6. | Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Récher C, Mansat-De Mas V, Delabesse E, Danet-Desnoyers G, Carroll M. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J Clin Invest. 2011;121:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 7. | Hanekamp D, Denys B, Kaspers GJL, Te Marvelde JG, Schuurhuis GJ, De Haas V, De Moerloose B, de Bont ES, Zwaan CM, de Jong A, Depreter B, Lammens T, Philippé J, Cloos J, van der Velden VHJ. Leukaemic stem cell load at diagnosis predicts the development of relapse in young acute myeloid leukaemia patients. Br J Haematol. 2018;183:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Ho TC, LaMere M, Stevens BM, Ashton JM, Myers JR, O'Dwyer KM, Liesveld JL, Mendler JH, Guzman M, Morrissette JD, Zhao J, Wang ES, Wetzler M, Jordan CT, Becker MW. Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood. 2016;128:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Boyd AL, Aslostovar L, Reid J, Ye W, Tanasijevic B, Porras DP, Shapovalova Z, Almakadi M, Foley R, Leber B, Xenocostas A, Bhatia M. Identification of Chemotherapy-Induced Leukemic-Regenerating Cells Reveals a Transient Vulnerability of Human AML Recurrence. Cancer Cell. 2018;34:483-498.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Vu LP, Kharas MG. Targeting the Residual Leukemia Cells after Chemotherapy. Cancer Cell. 2018;34:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, Ferraro F, Shterental S, Lin CP, Gilliland DG, Scadden DT, Armstrong SA, Williams DA. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Welner RS, Amabile G, Bararia D, Czibere A, Yang H, Zhang H, Pontes LL, Ye M, Levantini E, Di Ruscio A, Martinelli G, Tenen DG. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell. 2015;27:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 324] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Florian S, Sonneck K, Hauswirth AW, Krauth MT, Schernthaner GH, Sperr WR, Valent P. Detection of molecular targets on the surface of CD34+/CD38-- stem cells in various myeloid malignancies. Leuk Lymphoma. 2006;47:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Kuepper MK, Bütow M, Herrmann O, Ziemons J, Chatain N, Maurer A, Kirschner M, Maié T, Costa IG, Eschweiler J, Koschmieder S, Brümmendorf TH, Müller-Newen G, Schemionek M. Stem cell persistence in CML is mediated by extrinsically activated JAK1-STAT3 signaling. Leukemia. 2019;33:1964-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Zhou W, Sun G, Zhang Z, Zhao L, Xu L, Yuan H, Li S, Dong Z, Song Y, Fang X. Proteasome-Independent Protein Knockdown by Small-Molecule Inhibitor for the Undruggable Lung Adenocarcinoma. J Am Chem Soc. 2019;141:18492-18499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Yang Y, Yuan H, Zhao L, Guo S, Hu S, Tian M, Nie Y, Yu J, Zhou C, Niu J, Wang G, Song Y. Targeting the miR-34a/LRPPRC/MDR1 axis collapse the chemoresistance in P53 inactive colorectal cancer. Cell Death Differ. 2022;29:2177-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Wang L, Luo J, Li Y, Lu Y, Zhang Y, Tian B, Zhao Z, Hu QY. Mitochondrial-Associated Protein LRPPRC is Related With Poor Prognosis Potentially and Exerts as an Oncogene Via Maintaining Mitochondrial Function in Pancreatic Cancer. Front Genet. 2021;12:817672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Liu ZX, Li LM, Sun HL, Liu SM. Link Between m6A Modification and Cancers. Front Bioeng Biotechnol. 2018;6:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 20. | Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. 2014;44:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 21. | Ren T, Shan J, Qing Y, Qian C, Li Q, Lu G, Li M, Li C, Peng Y, Luo H, Zhang S, Zhang W, Wang D, Zhou SF. Sequential treatment with AT-101 enhances cisplatin chemosensitivity in human non-small cell lung cancer cells through inhibition of apurinic/apyrimidinic endonuclease 1-activated IL-6/STAT3 signaling pathway. Drug Des Devel Ther. 2014;8:2517-2529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Ren T, Shan J, Li M, Qing Y, Qian C, Wang G, Li Q, Lu G, Li C, Peng Y, Luo H, Zhang S, Yang Y, Cheng Y, Wang D, Zhou SF. Small-molecule BH3 mimetic and pan-Bcl-2 inhibitor AT-101 enhances the antitumor efficacy of cisplatin through inhibition of APE1 repair and redox activity in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:2887-2910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Stevens AM, Miller JM, Munoz JO, Gaikwad AS, Redell MS. Interleukin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017;1:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegué E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Chesnokov MS, Borhani S, Halasi M, Arbieva Z, Khan I, Gartel AL. FOXM1-AKT Positive Regulation Loop Provides Venetoclax Resistance in AML. Front Oncol. 2021;11:696532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Sheng Y, Yu C, Liu Y, Hu C, Ma R, Lu X, Ji P, Chen J, Mizukawa B, Huang Y, Licht JD, Qian Z. FOXM1 regulates leukemia stem cell quiescence and survival in MLL-rearranged AML. Nat Commun. 2020;11:928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Cui J, Wang L, Ren X, Zhang Y, Zhang H. LRPPRC: A Multifunctional Protein Involved in Energy Metabolism and Human Disease. Front Physiol. 2019;10:595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Wei WS, Wang N, Deng MH, Dong P, Liu JY, Xiang Z, Li XD, Li ZY, Liu ZH, Peng YL, Li Z, Jiang LJ, Yao K, Ye YL, Lu WH, Zhang ZL, Zhou FJ, Liu ZW, Xie D, Yu CP. LRPPRC regulates redox homeostasis via the circANKHD1/FOXM1 axis to enhance bladder urothelial carcinoma tumorigenesis. Redox Biol. 2021;48:102201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Kühl I, Kukat C, Ruzzenente B, Milenkovic D, Mourier A, Miranda M, Koolmeister C, Falkenberg M, Larsson NG. POLRMT does not transcribe nuclear genes. Nature. 2014;514:E7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 31. | Redell MS, Ruiz MJ, Gerbing RB, Alonzo TA, Lange BJ, Tweardy DJ, Meshinchi S; Children's Oncology Group. FACS analysis of Stat3/5 signaling reveals sensitivity to G-CSF and IL-6 as a significant prognostic factor in pediatric AML: a Children's Oncology Group report. Blood. 2013;121:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Schmidt T, Kharabi Masouleh B, Loges S, Cauwenberghs S, Fraisl P, Maes C, Jonckx B, De Keersmaecker K, Kleppe M, Tjwa M, Schenk T, Vinckier S, Fragoso R, De Mol M, Beel K, Dias S, Verfaillie C, Clark RE, Brümmendorf TH, Vandenberghe P, Rafii S, Holyoake T, Hochhaus A, Cools J, Karin M, Carmeliet G, Dewerchin M, Carmeliet P. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell. 2011;19:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 660] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 34. | Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875-10894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 275] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, Sawaya R, Huang S. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 37. | Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, Michalopoulos G, Becich M, Monzon FA. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 38. | Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 1230] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 39. | Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |