Published online Apr 26, 2024. doi: 10.4252/wjsc.v16.i4.389
Peer-review started: November 9, 2023
First decision: December 17, 2023
Revised: January 12, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: April 26, 2024
Processing time: 167 Days and 14.7 Hours
Osteoporosis (OP) has become a major public health problem worldwide. Most OP treatments are based on the inhibition of bone resorption, and it is necessary to identify additional treatments aimed at enhancing osteogenesis. In the bone marrow (BM) niche, bone mesenchymal stem cells (BMSCs) are exposed to a hypoxic environment. Recently, a few studies have demonstrated that hypoxia-inducible factor 2alpha (HIF-2α) is involved in BMSC osteogenic differentiation, but the molecular mechanism involved has not been determined.
To investigate the effect of HIF-2α on the osteogenic and adipogenic differentiation of BMSCs and the hematopoietic function of hematopoietic stem cells (HSCs) in the BM niche on the progression of OP.
Mice with BMSC-specific HIF-2α knockout (Prx1-Cre;Hif-2αfl/fl mice) were used for in vivo experiments. Bone quantification was performed on mice of two genotypes with three interventions: Bilateral ovariectomy, semilethal irradiation, and dexamethasone treatment. Moreover, the hematopoietic function of HSCs in the BM niche was compared between the two mouse genotypes. In vitro, the HIF-2α agonist roxadustat and the HIF-2α inhibitor PT2399 were used to investigate the function of HIF-2α in BMSC osteogenic and adipogenic differentiation. Finally, we investigated the effect of HIF-2α on BMSCs via treatment with the mechanistic target of rapamycin (mTOR) agonist MHY1485 and the mTOR inhibitor rapamycin.
The quantitative index determined by microcomputed tomography indicated that the femoral bone density of Prx1-Cre;Hif-2αfl/fl mice was lower than that of Hif-2αfl/fl mice under the three intervention conditions. In vitro, Hif-2αfl/fl mouse BMSCs were cultured and treated with the HIF-2α agonist roxadustat, and after 7 d of BMSC adipogenic differentiation, the oil red O staining intensity and mRNA expression levels of adipogenesis-related genes in BMSCs treated with roxadustat were decreased; in addition, after 14 d of osteogenic differentiation, BMSCs treated with roxadustat exhibited increased expression of osteogenesis-related genes. The opposite effects were shown for mouse BMSCs treated with the HIF-2α inhibitor PT2399. The mTOR inhibitor rapamycin was used to confirm that HIF-2α regulated BMSC osteogenic and adipogenic differentiation by inhibiting the mTOR pathway. Consequently, there was no significant difference in the hematopoietic function of HSCs between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice.
Our study showed that inhibition of HIF-2α decreases bone mass by inhibiting the osteogenic differentiation and increasing the adipogenic differentiation of BMSCs through inhibition of mTOR signaling in the BM niche.
Core Tip: This manuscript explores the role of hypoxia-inducible factor 2alpha (HIF-2α) in bone mesenchymal stem cell (BMSC) osteogenic differentiation in the bone marrow niche, a role that is still unclear and controversial, via in vivo and in vitro experiments. We verified that downregulation of HIF-2α inhibits osteogenesis in vivo by generating mice with BMSC-specific HIF-2α knockout and applying interventions such as bilateral ovariectomy, semilethal irradiation, and treatment with dexamethasone. In vitro, we found that downregulation of HIF-2α can inhibit osteogenesis and increase adipogenesis by suppressing the mechanistic target of rapamycin signaling pathway, which may lead to the identification of drug target genes for the clinical treatment of osteoporosis.
- Citation: Wang LL, Lu ZJ, Luo SK, Li Y, Yang Z, Lu HY. Unveiling the role of hypoxia-inducible factor 2alpha in osteoporosis: Implications for bone health. World J Stem Cells 2024; 16(4): 389-409
- URL: https://www.wjgnet.com/1948-0210/full/v16/i4/389.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i4.389
Osteoporosis (OP) is a disorder associated with a decrease in bone mineral density (BMD), a low bone mass and increased bone fragility, and it increases the risk of fragility fractures. The economic and social burden of fragility fractures is massive, previously estimated at 37 billion euros per year in 27 European countries alone[1]. OP has become an important public health problem in China. From December 2017 to August 2018, the China Osteoporosis Prevalence Study enrolled a representative sample of 20416 participants aged 20 years or older from mainland China, and the overall prevalence of OP was 20.6% among women aged 40 years or older and 5.0% among men aged 40 years or older. The prevalence of OP among postmenopausal women was 32.1%, and the prevalence of OP among men aged 50 years or older was 6.9%. The prevalence of vertebral fracture was 10.5% among men and 9.7% among women[2]. Therefore, it is important for us to investigate the mechanism of OP.
Bone is a highly dynamic tissue with continuous remodeling at the surface that supports body weight and maintains mineral homeostasis. Bone homeostasis requires coordinated activities between osteoblasts and osteoclasts. Osteoblasts are responsible for the deposition of bone matrix on the bone surface, and osteoclasts are responsible for bone resorption[3]. Age-related (type II) OP is a common and debilitating condition driven in part by the loss of bone marrow (BM) mesenchymal stromal cells and their osteoblast progeny, leading to reduced bone formation. Current pharmacological regimens targeting age-related OP do not directly treat the disease by increasing bone formation but instead use bisphosphonates to reduce bone resorption - a treatment approach designed for postmenopausal (type-I) OP[4]. It is important to identify an OP treatment focused on increasing bone formation.
Bone mesenchymal stem cells (BMSCs) are multipotent marrow stromal cells. Upon exposure to different extracellular stimuli, different sets of signaling pathways and transcription factors are activated or inhibited in BMSCs, leading to distinct fates of differentiation into cells of different lineages, such as osteoblasts (bone-forming cells), chondrocytes (cartilage cells) and marrow adipocytes (fat cells). The two other major functions of BMSCs include functioning as cellular components of the hematopoietic stem cell (HSC) niche and immunosuppression[5]. In the BM, BMSCs are exposed to a hypoxic environment. Spencer et al[6] performed direct in vivo measurements of local oxygen tension (pO2) in the BM of live mice. Using two-photon phosphorescence lifetime microscopy, the authors determined that the absolute pO2 of the BM was quite low (< 32 mmHg). Cellular adaptation to hypoxia is mediated in part by hypoxia-inducible factors (HIFs). HIFs are composed of two subunits, an O2-labile α subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed β subunit (HIF-1β, also known as aryl hydrocarbon receptor nuclear translocator)[7]. Tissue-specific deletion of HIF1α, HIF2α, or the HIF-α degradation machinery component proline hydroxylase domain 2 (Phd2) or von Hippel-Lindau (Vhl) results in a variety of skeletal phenotypes that underscore the complexity of skeletal HIF-α signaling during development and disease[8]. Briefly, hypoxia and HIF-α promote skeletal mesenchymal condensation and limb development, promoting chondrogenesis by increasing Sox9 expression and reducing osteogenesis through Runx2 inhibition[9]. Most of the results showed that HIF-1α represents a potential therapeutic target for preventing osteoclast activation and bone loss in postmenopausal patients[10]. Recently, a few studies have demonstrated that HIF-2α is involved in BMSC osteogenic differentiation, but the molecular mechanism and role of HIF-2α in hematopoietic function in the BM niche have not been determined.
In this study, we generated mice with BMSC-specific HIF-2α knockout (Prx1-Cre;Hif-2αfl/fl mice) to study the oste
Hif-2αfl/fl mice (008407-Epas1tm1Mcs/J) were purchased from the Jackson Laboratory. Hif-2αfl/fl mice were crossed with Prx-1-Cre transgenic mice (kindly provided by Professor Meng Zhao (Sun Yat-sen University, Guangzhou, China) to produce mice with conditional knockout of HIF-2α in BMSCs (Prx-1-Cre;Hif-2αfl/fl mice). Age- and sex-matched WT littermates (Hif-2αfl/fl) were used as controls. The primers used for genotyping are listed in Table 1. Hif-2αfl/fl and Prx-1-Cre;Hif-2αfl/fl mice were subjected to ovariectomy, dexamethasone (DEX) treatment, or semilethal irradiation. Mice of the same age (6-8 wk), sex, and genotype were randomly grouped for subsequent experiments (the investigators were not blinded). No sex-related differences were observed in the Prx-1-Cre;Hif-2αfl/fl mice. All animals were maintained under specific pathogen-free conditions and fed a standard diet.
| Primers | Sequence | Amplicon |
| HIF2α | F: 5’-GAGAGCAGCTTCTCCTGGAA-3’ | WT182 bp |
| R: 5’-TGTAGGCAAGGAAACCAAGG-3’ | MT220 bp | |
| Prx-1 | F: 5’-GCGGTCTGGCAGTAAAAACTATC-3’ | 100 bp |
| R: 5’-GTGAAACAGCATTGCTGTCACTT-3’ |
The harvested bones were fixed for 48 h in 4% paraformaldehyde and then dehydrated in 80% ethanol. By using a microcomputed tomography (μCT) 100 scanner (isotropic voxel size of 10 μm, 70 kVp, 200 μA, integration time of 200 ms; Scanco Medical, Switzerland), femora were analyzed as previously described[11]. Determination of the trabecular and cortical bone volume (BV) fractions [BV/total volume (TV)], BMD, thickness (trabecular thickness), trabecular number and trabecular separation was performed using established analysis protocols, and the μCT parameters were reported according to international guidelines.
For OP induction by ovariectomy, 8-wk-old virgin female mice were anesthetized with 10 μL/g 0.6% pentobarbital sodium, the fur was shaved, and the skin was disinfected with betadine. A dorsal midline incision was made, and the periovarian fat pad was gently grasped to exteriorize the ovary. The fallopian tube was then clamped, and the ovary was removed by cutting above the clamped site. The uterine horn was returned to the abdomen, and the same process was repeated on the other side. After surgery, antibiotic water (250 mL sterile water + 2.5 mL of enrofloxacin) was provided for one week, and the mice were closely monitored until they resumed full activity[12].
Two-month-old Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl male mice were treated with daily intraperitoneal injection of phosphate buffered saline or 20 mg/kg DEX for 28 d[12].
Both Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl male mice received whole-body X-irradiation (4.5 Gy, semilethal dose; Rs2000, Rad Source). After surgery, the mice were provided antibiotic water (250 mL sterile water + 2.5 mL enrofloxacin) for 1 wk[13]. Peripheral blood (PB) was collected from individual mice in heparin-coated capillary tubes to measure white blood cell (WBC) counts on days 0, 7, 14, 21, 28, and 42 after semilethal irradiation.
5-fluorouracil (5-FU) was administered intraperitoneally to Prx1-Cre;Hif-2αfl/fl andHif-2αfl/fl mice once a week for 2 wk at a dose of 150 mg/kg body weight[14].
PB was collected from the tail vein and analyzed using a ProCyte Dx Hematology Analyzer (IDEXX).
Enzymatic digestion of BM was performed as described previously[15]. Briefly, intact marrow plugs were flushed from the long bones and subjected to two rounds of enzymatic digestion at 37 °C for 20 min each. The digestion buffer contained 3 mg/mL type I collagenase, 4 mg/mL dispase, and 1 U/mL DNase I in HBSS supplemented with calcium and magnesium. The cells were resuspended in staining medium (HBSS + 2% fetal bovine serum) to terminate digestion.
BM cells were collected from the bilateral femora of the mice and subjected to fibroblast colony-forming unit (CFU-F) assays as previously described[16]. Briefly, 106 cells were cultured for 7 d. The cells were stained with 0.1% crystal violet, and colony-forming units were counted under a microscope[13].
BMSCs were identified by the expression of PDGFRα[17] and CD51[18]. For classification of HSCs, long-term HSCs were defined as Lin-Sca1+c-KIT+ (LSK) CD34-FLK2- cells; short-term HSCs, as LSKCD34+FLK2 cells-; multipotent progenitors, as LSKCD150-CD48- cells; and HSCs, as LSKCD150+CD48- cells. For classification of hematopoietic progenitor cells (HPCs), common myeloid progenitors were defined as Lin-c-Kit+Sca-1-CD16/32medCD34+ cells; common lymphoid progenitors, as Lin-c-KitlowSca-1lowCD127+ cells; and granulocyte-monocyte progenitors, as Lin-c-Kit+Sca-1-CD16/32highCD34+ cells; and megakaryocyte-erythroid progenitors, as Lin-c-Kit+Sca-1-CD16/32med/LowCD34- cells.
For cell cycle analysis, BM cells freshly harvested from femora and tibiae were stained with antibodies conjugated to various fluorochromes, fixed and permeabilized with 0.2% Triton X-100, stained with an Alexa Fluor 488-conjugated anti-Ki-67 antibody, and further incubated with DAPI (0.1 mg/mL)[14].
Equal numbers of cultured cells from Hif-2αfl/fl and Prx1-Cre;Hif-2αfl/fl mice were replated to ensure that there was no difference in the density of the cells of the different genotypes. On the second day of culture, the medium was replaced with adipogenic (7 d) or osteogenic (7 d or 14 d) differentiation medium. The osteogenic differentiation medium consisted of MEM supplemented with 10 mmol/L β-glycerol phosphate disodium salt, 10 nM DEX (D1756) and 50 μM L-ascorbic acid[19]. Fourteen days later, the percentage of colonies that contained osteoblasts was quantified by alizarin red staining (1%, pH = 4.2; Solarbio). Adipogenic differentiation was induced by the addition of α-MEM containing 10% fetal calf serum, insulin (1 μg/mL; Sigma), 1 μM DEX (Sigma), and 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma). The differentiation of adipocytes was monitored by Oil red O staining[11].
Total RNA was extracted from cells and tissues according to the Trizol reagent RNA extraction protocol (Magen). cDNA was obtained by reverse transcription of 1 μg of RNA according to the manufacturer’s instructions (Takara). Gene expression levels were measured using real-time polymerase chain reaction (RT-PCR) with a CFX96 Touch. The primers used are listed in Table 2. Actin was used as an internal control.
| Genes | Primers types | Sequence (5’-3’) |
| HIF-2α | Forward | CTCCCACCTGGACAAAGC |
| Reverse | GTCGCAAGGATGAGTGAAGT | |
| HIF-2α exon2 | Forward | ACAAAGCCTCCATCATGCG |
| Reverse | CGTCTTGGGTCACCACAG | |
| Runx2 | Forward | TCCACAAGGACAGAGTCAGATTACAG |
| Reverse | CAGAAGTCAGAGGTGGCAGTGTCATC | |
| Col1α1 | Forward | TGTGTGCGATGACGTGCAAT |
| Reverse | GGGTCCCTCGACTCCTACA | |
| OCN | Forward | AGACTCCGGCGCTACCTT |
| Reverse | AAGCAGGGTCAAGCTCACAT | |
| CEBPa | Forward | CAAGAACAGCAACGAGTACCG |
| Reverse | GTCACTGGTCAACTCCAGCAC | |
| Lpl | Forward | GCGTAGCAGGAAGTCTGACCAA |
| Reverse | AGCGTCATCAGGAGAAAGGCGA | |
| Adipo-nectin | Forward | TGTTCCTCTTAATCCTGCCCA |
| Reverse | CCAACCTGCACAAGTTCCCTT | |
| PI3K | Forward | ACACCACGGTTTGGACTATGG |
| Reverse | GGCTACAGTAGTGGGCTTGG | |
| AKT | Forward | TGGGTCAAGGAACAGAAGCA |
| Reverse | TCACACTGACCACTGACACA | |
| mTOR | Forward | CGGGACTCTTTACACTGCG |
| Reverse | CCTTCAGGCTCAACCAACA | |
| Actin | Forward | TTGCTGACAGGATGCAGAAGGAGA |
| Reverse | ACTCCTGCTTGCTGATCCACATCT |
Total protein was extracted from cells and tissues with radioimmunoprecipitation assay buffer. Equal amounts of extracted proteins were separated via 12.5% sodium-dodecyl sulfate gel electrophoresis and transferred to a polyvinylidene fluoride membrane. After blocking with 5% nonfat milk in Tris-buffered saline with Tween 20 (TBST, pH = 7.6) for 1 h at room temperature, the membranes were incubated with primary antibodies [specific for mechanistic target of rapamycin (mTOR), AKT, and β-actin] overnight at 4 °C and then incubated with secondary antibodies (rabbit, 1:10000) for 1 h at room temperature. Western blot images were acquired with a ChemiDoc XRS imaging system (Bio-Rad).
All the statistical analyses were performed with GraphPad Prism (version 8.0). The statistical significance of differences between two groups was determined using unpaired Student’s t test (two-sided), unless otherwise specified in the figure legends. In all the studies, the data are shown as the mean ± SD values, and statistically significant differences are indicated in the figures; aP < 0.05, bP < 0.01, cP < 0.001.
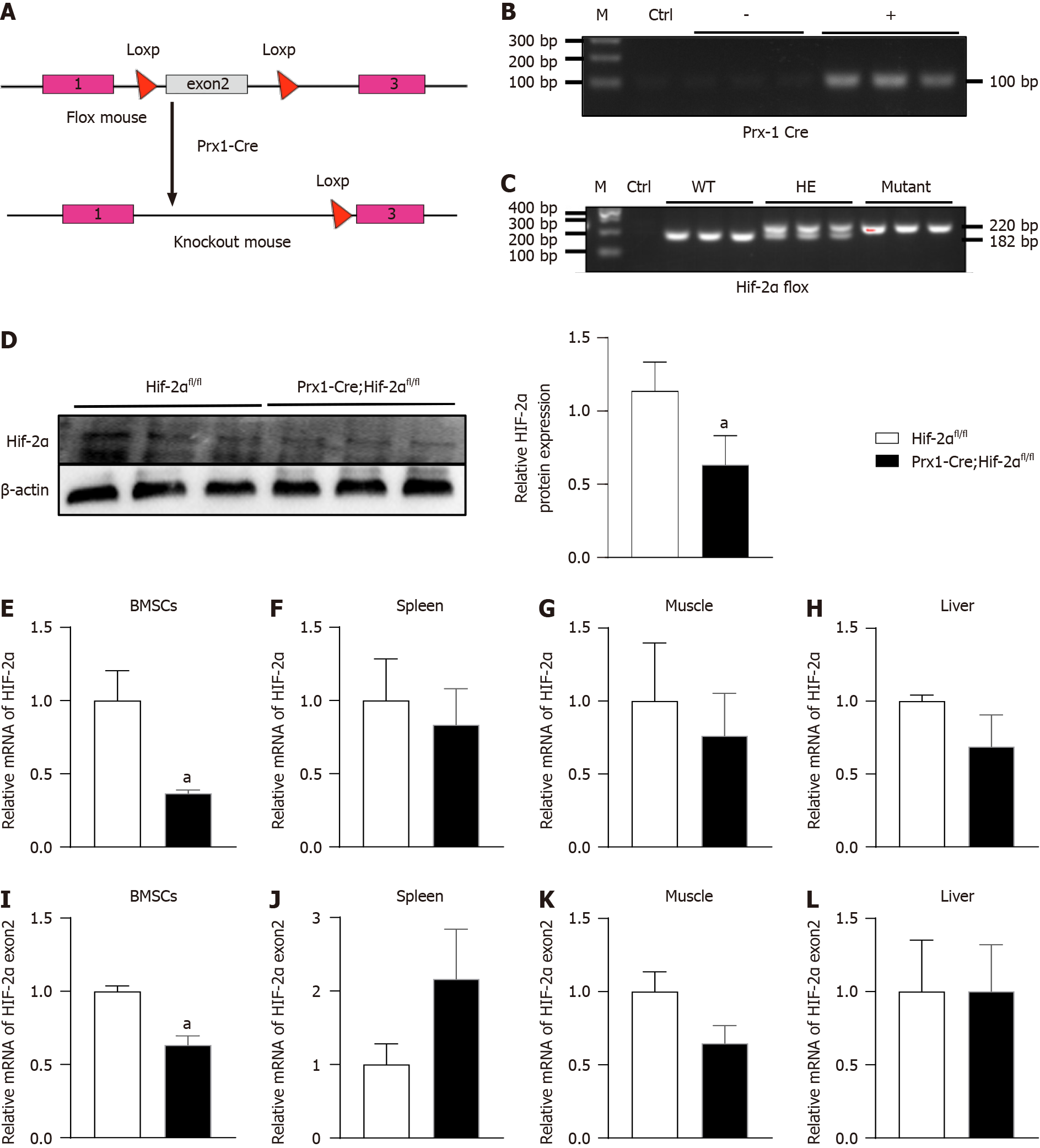
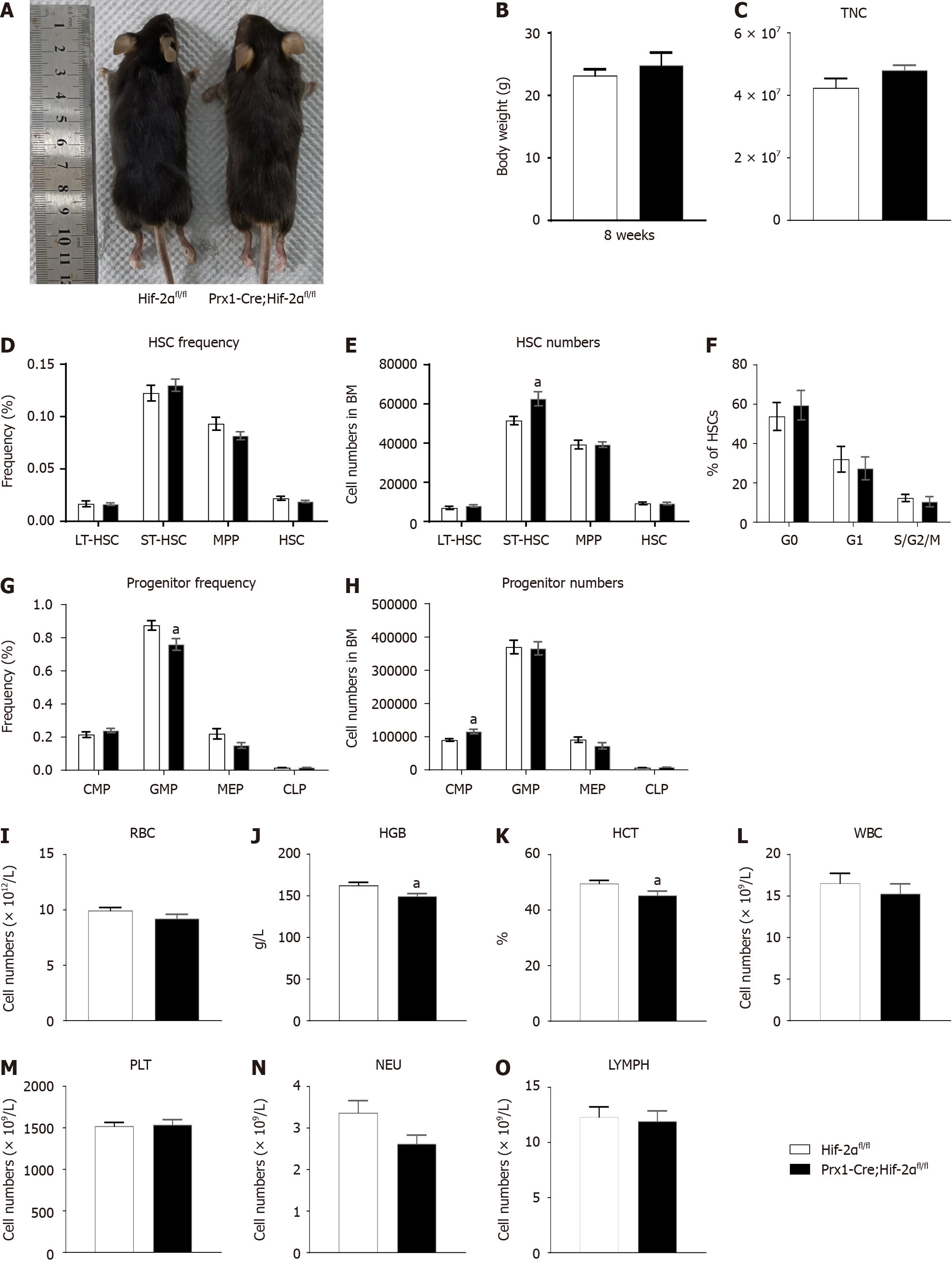
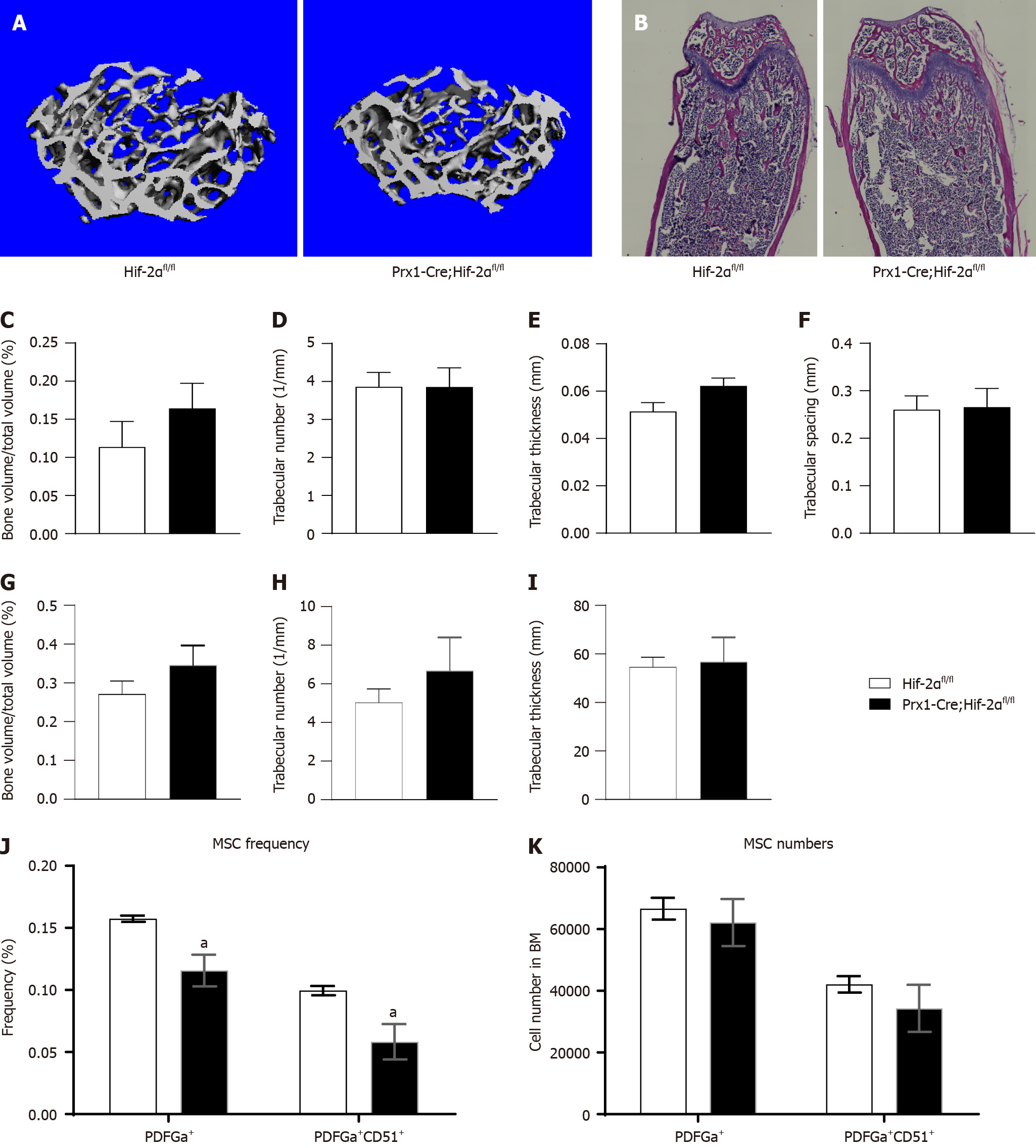
To assess the physiological function of HIF-2α in early osteoblast lineage cells, we generated HIF-2α CKO mice with Prx-1-Cre transgenic mice (Figure 1A), which express Cre recombinase in BMSCs. Briefly, we mated floxed HIF-2α mice with Prx1-Cre transgenic mice to generate Prx1-Cre;Hif-2αwt/fl mice, which were mated with Hif-2αfl/fl mice to generate Hif-2αfl/fl mice, Prx1-Cre;Hif-2αwt/fl mice, and Prx1-Cre;Hif-2αfl/fl mice. Littermate mice were used in all the experiments. The genotyping primers used are listed in Table 1, and the DNA genotyping results are shown in Figure 1B and C. Mice with the above genotypes were born at normal Mendelian ratios and did not exhibit significant weight or developmental differences (Figure 2A and B). To confirm the deletion of HIF-2α in Prx1-Cre;Hif-2αfl/fl BMSCs, the HIF-2α protein expression was analyzed in BMSCs, and HIF-2α was found to be efficiently deleted in most BMSCs from Prx1-Cre;Hif-2αfl/fl mice (Figure 1D). Moreover, quantitative RT-PCR analysis of total RNA extracted from BMSCs and from the spleen, muscle, and liver showed that the expression levels of HIF-2α mRNA (Figure 1E) and HIF-2α exon 2 (Figure 1I) were significantly lower in the BMSCs of Prx1-Cre;Hif-2αfl/fl mice than in those of Hif-2αfl/fl mice, but there were no differences in these levels in the spleen (Figure 1F and J), muscle (Figure 1G and K) or liver (Figure 1H and L) between the two genotypes of mice. Next, we used μCT and hematoxylin and eosin staining to evaluate the effects of HIF-2α deletion on bone morphometry. μCT analysis revealed that there were no differences in bone mass between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice (Figure 3A and C-F). Hematoxylin and eosin staining[20] also showed that there were no differences in bone mass between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice (Figure 3B and G-I). Furthermore, as BMSCs are the major source cells for bone formation, we determined the number of BMSCs in Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice. Fluorescence-activated cell sorting (FACS) analysis showed that the frequency of BMSCs was significantly reduced in Prx1-Cre;Hif-2αfl/fl mice (Figure 3J and K).
As there was a difference in the frequency of BMSCs between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice, we used 3 different interventions to modulate the bone mass in these mice. μCT analysis revealed an osteopenic phenotype in Prx1-Cre;Hif-2αfl/fl mice after each of the 3 interventions.
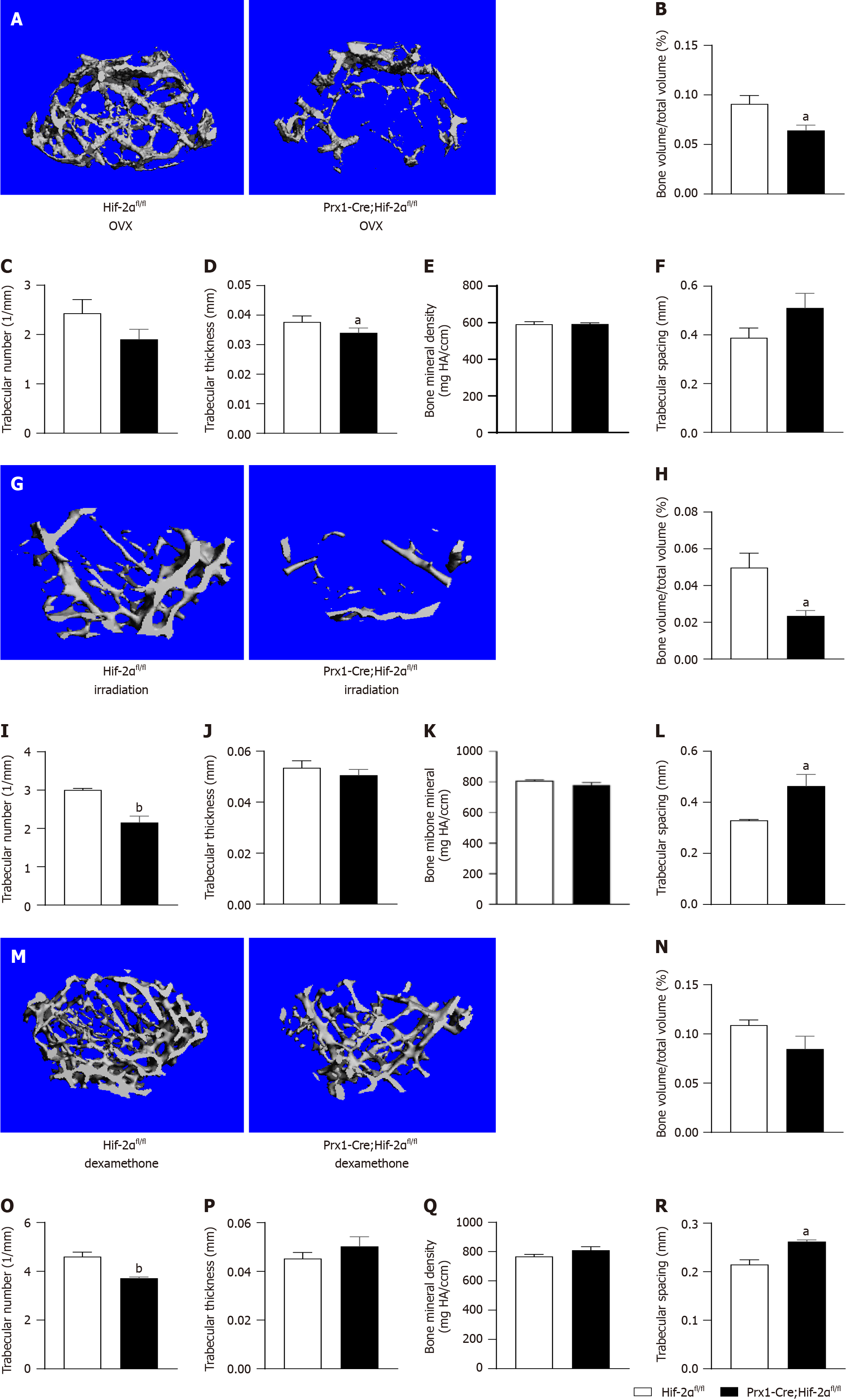
First, 4 wk after bilateral ovariectomy, clinical postmenopausal OP was simulated in female mice. Differences in bone phenotypes between the two genotypes of mice were quantified by μCT. Figure 4A shows the three-dimensional μCT reconstructions of the distal femora of mice of the different genotypes. Quantitative analysis indicated that, compared with those in Hif-2αfl/fl mice, the trabecular BV (Figure 4B) and trabecular thickness (Figure 4D) was decreased, the trabecular number (Figure 4C) and the bone mineral density (Figure 4E) was decreased, and the trabecular separation (Figure 4F) was increased in Prx1-Cre;Hif-2αfl/fl mice. The above results suggest that after bilateral ovariectomy, the bone mass of Prx1-Cre;Hif-2αfl/fl mice decreased significantly compared with that of Hif-2αfl/fl mice.
Second, semilethal irradiation was used to induce OP in mice of different genotypes. Differences in bone phenotypes between mice of the two genotypes were quantified by μCT. Figure 4G shows the three-dimensional μCT reconstructions of the distal femora of mice of the different genotypes. Analysis of the quantitative indicators suggested that the trabecular BV (BV/TV, as shown in Figure 4H) and trabecular number (Figure 4I) were decreased and the trabecular separation (Figure 4L) was increased in Prx1-Cre;Hif-2αfl/fl mice compared with Hif-2αfl/fl mice and that the trabecular thickness (Figure 4J) and the bone mineral density (Figure 4K) was decreased in Prx1-Cre;Hif-2αfl/fl mice compared to WT mice. Taken together, these results suggest that after semilethal irradiation, the bone mass of Prx1-Cre;Hif-2αfl/fl mice decreased significantly compared with that of Hif-2αfl/fl mice.
Mice of the different genotypes were treated with DEX, and after 4 wk, the differences in bone phenotypes between the two genotypes were quantified via μCT. Figure 4M shows the three-dimensional μCT reconstructions of the distal femora of mice of the different genotypes, and analysis of the quantitative indicators indicated a decrease in the trabecular number (Figure 4O) and an increase in the trabecular separation (Figure 4R) in Prx1-Cre;Hif-2αfl/fl mice compared with Hif-2αfl/fl mice. Similarly, compared with that in Hif-2αfl/fl mice, the trabecular BV (BV/TV, as shown in Figure 4N) in Prx1-Cre;Hif-2αfl/fl mice was decreased. There were no differences in the trabecular thickness (Figure 4P) or BMD (Figure 4Q) between the two genotypes of mice. The above results suggested that, after DEX treatment, the bone mass of Prx1-Cre;Hif-2αfl/fl mice decreased significantly compared to that of WT mice.
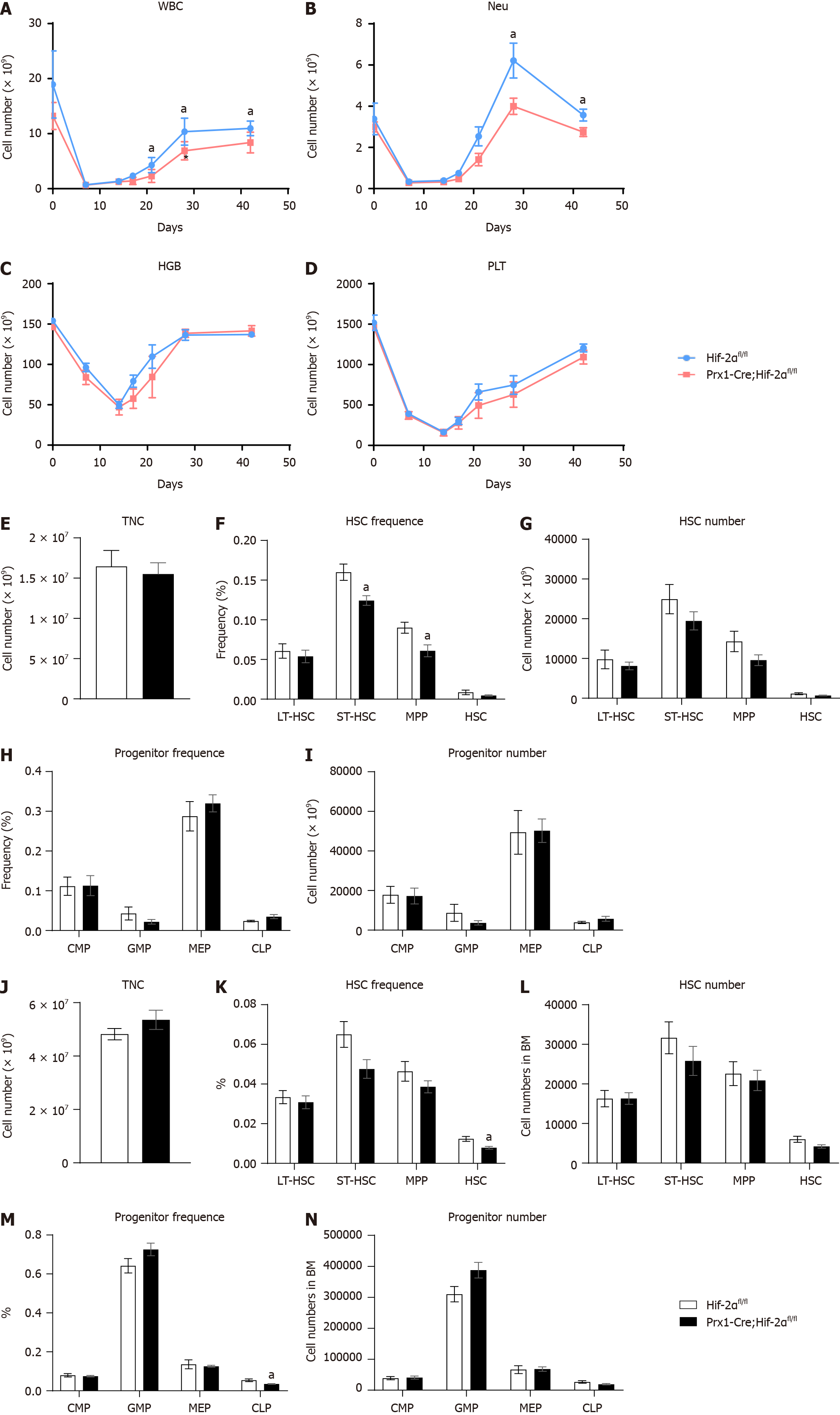
As the above results revealed the difference in bone mass between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice, the effect of HIF-2α in the BM microenvironment on the hematopoietic functions of HSCs and HPCs was deemed worthy of investigation. Under naive conditions, tail vein blood of Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice was analyzed via a PB test. The results indicated that the concentration of hemoglobin (HGB, as shown in Figure 2J) in Prx1-Cre;Hif-2αfl/fl mice was 149 g/L, the HGB concentration in Hif-2αfl/fl mice was 163 g/L, the hematocrit (HCT, as shown in Figure 2K) in Prx1-Cre;Hif-2αfl/fl mice was 45%, and the HCT in Hif-2αfl/fl mice was 49.6%, but no significant differences were observed in the RBC (Figure 2I), leukocyte (WBC, as shown in Figure 2L), neutrophil/granulocyte (NEU, as shown in Figure 2N), lymphocyte (LYMPH, as shown in Figure 2O) and platelet (PLT, as shown in Figure 2M) counts between the two genotypes of mice.
Furthermore, we isolated the total nucleated cell (TNC) population from the BM of Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice for analysis. First, we analyzed the TNC number (as shown in Figure 2C) and found no significant difference between the two genotypes. Then, we analyzed the total number (Figure 2E) and proportion (Figure 2D) of HSCs in Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice and found that there were no significant differences. Furthermore, we analyzed the cell cycle distribution of HSCs from Prx1-Cre;Hif-2αfl/fl mice and from Hif-2αfl/fl mice and found no significant differences (Figure 2F). Moreover, we analyzed the total number (Figure 2H) and proportion (Figure 2G) of HPCs in Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice, and the results did not reveal significant differences. In general, the BMSC population in Prx1-Cre;Hif-2αfl/fl mice was significantly reduced under naive conditions, but there were no significant differences in the BM HSC and HPC populations in Prx1-Cre;Hif-2αfl/fl mice compared with Hif-2αfl/fl mice; thus, genetic deletion of HIF-2α in BMSCs had no effect on the hematopoietic microenvironment.
Under naive conditions, there were no significant differences in the hematopoietic phenotype between Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice. To further clarify the difference in the hematopoietic microenvironment between Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice, mice received two different interventions (including semilethal irradiation and 5-FU injection), and hematopoietic differences were evaluated. First, we observed hematopoietic changes after BM injury induced by semilethal irradiation. First, we collected PB from Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice, and blood analysis indicated that beginning at the third week after irradiation, the recovery of WBCs (Figure 5A) and NEUs (Figure 5B) in Prx1-Cre;Hif-2αfl/fl mice was significantly slower than that in Hif-2αfl/fl mice. However, there were no statistically significant differences in HGB concentration (Figure 5C) or PLT count (Figure 5D) between Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice.
Moreover, the HSCs and HPCs of Hif-2αfl/fl and Prx1-Cre;Hif-2αfl/fl mice were analyzed during the second week after semilethal irradiation. The results suggested that there was no significant difference in the TNC number (Figure 5E). Then, we analyzed the total number (Figure 5G) and proportion (Figure 5F) of HSCs and HPCs (Figure 5H and I) between the two genotypes of mice. There were no significant differences.
We subsequently administered an intervention that caused BM injury in mice, i.e., intraperitoneal injection of 5-FU, and then analyzed HSCs and HPCs in the BM of Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice. The results still indicated that there was no significant difference in the number of TNCs in Prx1-Cre;Hif-2αfl/fl mice compared with Hif-2αfl/fl mice (Figure 5J). We subsequently analyzed the total number and proportion of HSCs and HPCs in the two genotypes of mice, and we found that the total number of HSCs (as shown in Figure 5L), the proportion of HSCs (as shown in Figure 5K), the total number of HPCs (as shown in Figure 5N) and the proportion of HPCs (as shown in Figure 5M) in Prx1-Cre;Hif-2αfl/fl mice did not significantly differ from those in Hif-2αfl/fl mice. Combined with the results of analysis of the hematopoietic phenotypes of the two mouse genotypes after irradiation, these results suggested that the hematopoietic recovery ability of Prx1-Cre;Hif-2αfl/fl mice after BM injury was not significantly different from that of Hif-2αfl/fl mice.
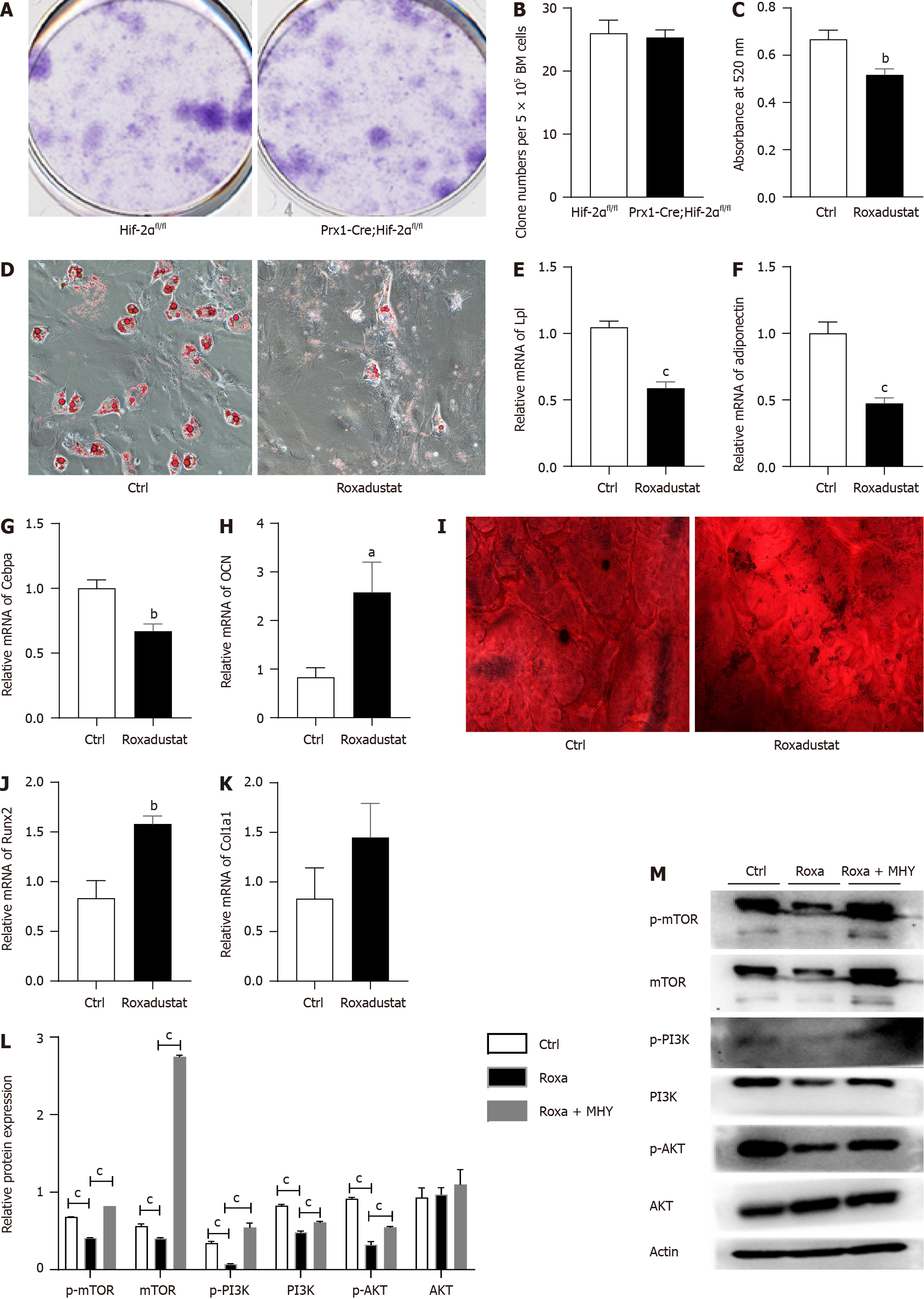
BMSCs from Prx1-Cre;Hif-2αfl/fl mice and Hif-2αfl/fl mice were isolated to evaluate the fibroblast colony formation ability of the BMSCs and determine their proliferation ability. There was no difference in the CFU-F between Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice. Representative images of CFU-Fs in Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice are shown in Figure 6A. The quantification of the CFU-Fs formed by the BMSCs is shown in Figure 6B.
Because of the long duration of cell differentiation, there were no differences in the percentages of BMSCs isolated from Prx1-Cre;Hif-2αfl/fl and Hif-2αfl/fl mice before compared with after the interventions (bilateral ovariectomy, sublethal irradiation, or DEX treatment); therefore, in the remaining studies, we used BMSCs from naive mice. Hif-2αfl/fl mouse BMSCs were cultured and divided into the roxadustat and control groups according to whether they were treated with the HIF-2α agonist roxadustat. After 7 d of adipogenic differentiation, the adipogenic capacity of the roxadustat group was decreased. Oil red O staining indicated that the number of lipid droplets in the BMSCs of the roxadustat group was lower than that in the control group (Figure 6D), and quantification of oil red O staining indicated that lipid droplet formation was reduced in the BMSCs of the roxadustat group (Figure 6C). qPCR analysis revealed that roxadustat treatment significantly downregulated adipogenesis-related genes, such as Lpl (Figure 6E), adiponectin (Figure 6F) and CEBPa (Figure 6G), in BMSCs. These results suggest that activation of HIF-2α can reduce the adipogenic differentiation capacity of BMSCs.
After 14 d of osteogenic differentiation, the osteogenic differentiation capacity of the BMSCs in the roxadustat group was increased. Alizarin red staining indicated that the osteogenic differentiation capacity of the roxadustat group was increased compared with that of the control group (Figure 6I). Similarly, compared with those in the control group, the mRNA levels of osteogenesis-related genes, such as OCN (Figure 6H), Runx2 (Figure 6J) and Col1α1 (Figure 6K), were significantly increased in the roxadustat group. These results suggest that activation of HIF-2α can lead to an increased osteogenic differentiation capacity of BMSCs.
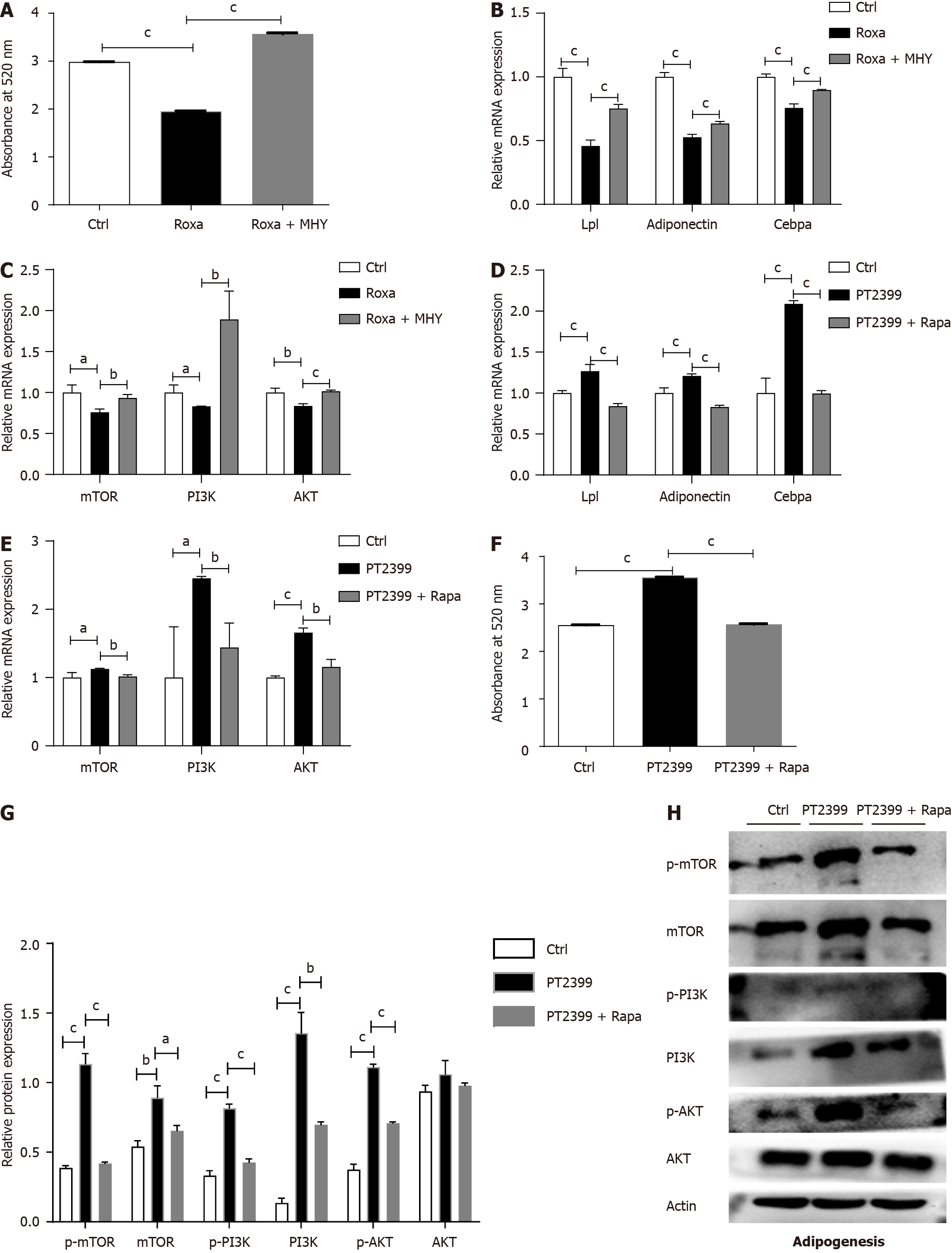
To further investigate whether HIF-2α affects the osteogenic/adipogenic differentiation of BMSCs through the mTOR signaling pathway, we extracted protein and mRNA from BMSCs in the roxadustat group and the control group after adipogenic differentiation. The results suggested that, during the process of adipogenic differentiation, at the translational level, the levels of the key proteins of the mTOR signaling pathway - the p-mTOR protein and the downstream proteins p-PI3K and p-AKT (as shown in Figure 6M) - were significantly reduced in the roxadustat group (Figure 6L). Moreover, the mTOR activator MHY1485 was used to verify whether it could rescue the decreased adipogenic differentiation capacity of BMSCs. The results proved that the mTOR activator MHY1485 increased adipogenic capacity of BMSCs (Figure 7A and B) and the mRNA levels of the mTOR signaling pathway components (Figure 7C). Moreover, the protein levels of the mTOR signaling pathway components were increased (Figure 6L and M).
On the other hand, we used the HIF-2α inhibitor PT2399 and the mTOR inhibitor rapamycin to confirm that HIF-2α regulates adipogenesis by inhibiting the mTOR pathway. We divided cultured BMSCs into three different groups: The control group, the PT2399 group, and the PT2399 + rapamycin group. The results showed that the PT2399 + rapamycin treatment group exhibited a decreased adipogenic differentiation capacity, which was increased in the PT2399 group. Oil red O staining and quantitative analysis indicated that lipid droplet formation was increased in the PT2399-treated BMSCs (Figure 7F), and that after BMSCs were treated with PT2399 and rapamycin, their adipogenic differentiation capacity decreased. At the transcriptional level, the expression levels of genes related to adipogenesis in BMSCs were significantly upregulated in the PT2399 group and downregulated in the PT2399 + rapamycin group (Figure 7D). Similarly, the expression of key mRNAs in the mTOR signaling pathway was significantly decreased in the PT2399 + rapamycin group compared to the PT2399 group (Figure 7E). Then, we extracted protein from BMSCs in the PT2399 group and the PT2399 + rapamycin group after adipogenic differentiation. The results suggested that, during the process of adipogenic differentiation, the levels of the mTOR and p-mTOR proteins and the downstream proteins PI3K, p-PI3K and p-AKT (as shown in Figure 7H) were significantly increased in the PT2399 group but decreased in the PT2399 + rapamycin group (Figure 7G).
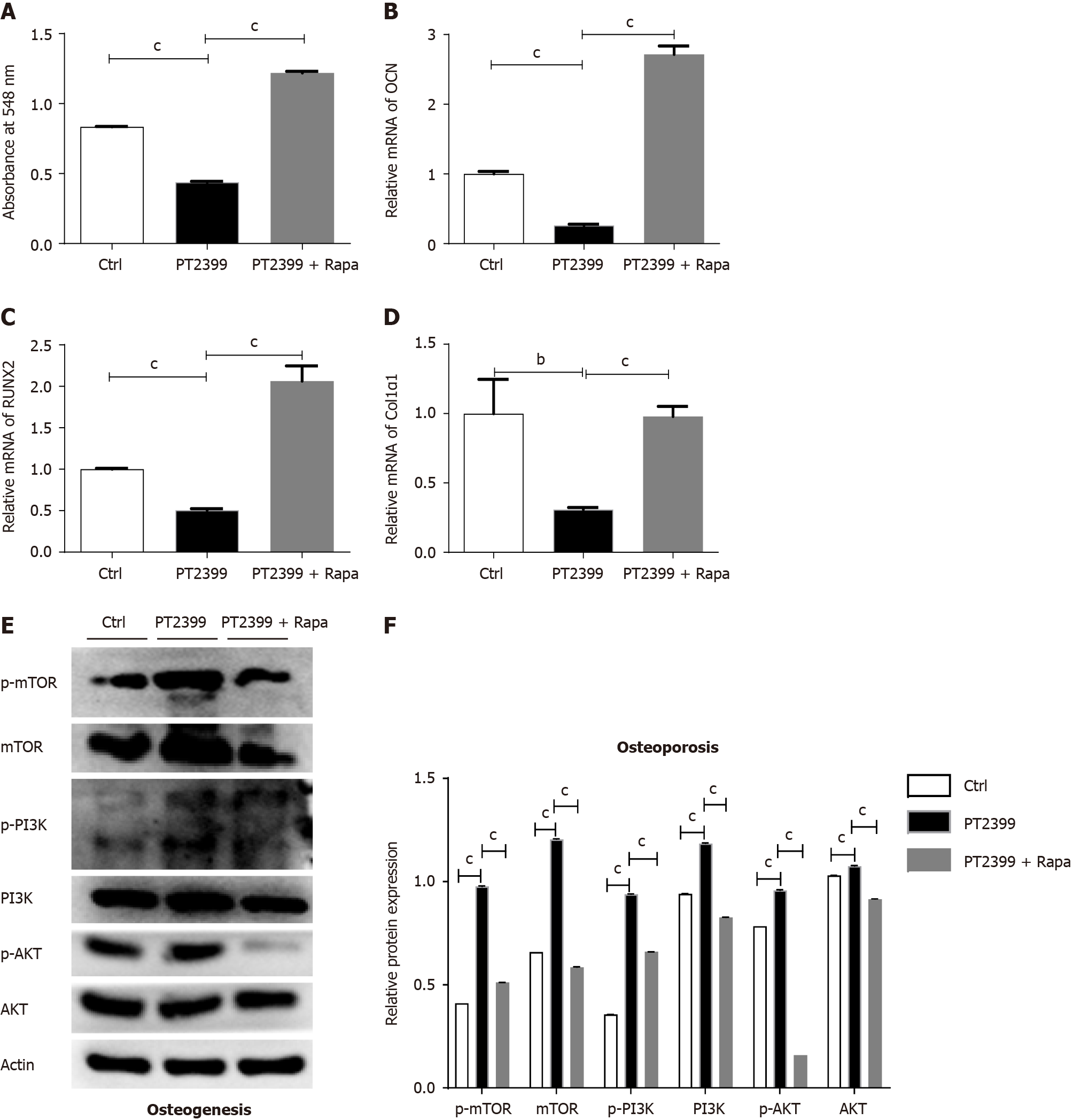
Furthermore, we verified the relevant results regarding the osteogenic differentiation of BMSCs using the HIF-2α inhibitor PT2399 and the mTOR inhibitor rapamycin. Similarly, we divided the cultured BMSCs into three different groups: The control group, the PT2399 group, and the PT2399 + rapamycin group. The results showed that rapamycin rescued the decreased osteogenic differentiation ability of the PT2399 group. Alizarin red staining was used to quantify the osteogenic differentiation capacity of the cells, and the results indicated that the osteogenic differentiation capacity was decreased in the PT2399 group (Figure 8A); moreover, for the in BMSCs treated with PT2399 and rapamycin, the osteogenic differentiation capacity was increased. At the transcriptional level, the expression of genes related to osteogenesis in BMSCs, such as OCN (Figure 8B), RUNX2 (Figure 8C) and Col1α1 (Figure 8D), was significantly downregulated in the PT2399 group and upregulated in the PT2399 + rapamycin group. Then, after osteogenic differentiation, we extracted protein from the BMSCs in the PT2399 group and the PT2399 + rapamycin group. The results suggested that, during the process of osteogenic differentiation, the levels of the mTOR and p-mTOR proteins and the downstream proteins PI3K, p-PI3K, p-AKT and AKT (as shown in Figure 8E) were significantly increased in the PT2399 group but decreased in the PT2399 + rapamycin group (Figure 8F).
We successfully generated mice with BMSC-specific HIF-2α knockout, which were used for our subsequent studies. Notably, during the process of generating the Prx1-Cre;Hif-2αfl/fl mice, we found that female mice harboring the Prx1-Cre gene could not be mated. Upon further investigation, we found that the birth rate of the mice was very low and that the stillbirth rate was high. We have not further clarified and studied the mechanism of this phenomenon, but upon observing this phenomenon, we avoided using females with the prx1-Cre transgene as for breeding; therefore, we expanded the mouse population by mating female Hif-2αfl/fl mice with male Prx1-Cre;Hif-2αfl/fl mice. This phenomenon has not been explained by the relevant literature in other studies and could be investigated in our future research.
Many studies have explored the role of HIF-1α in bone homeostasis and have shown that HIF-1α can promote osteogenesis. It was reported that mice lacking Hif-1α exhibited a significant reduction in trabecular BV, a reduced bone formation rate, and altered cortical bone architecture. In contrast, mice lacking HIF-2α had only a modest decrease in trabecular BV[21]. It has also been suggested that HIF-1α stabilization in osteoblast precursors of postnatal mice markedly increases the osteoblast number and bone mass[22]. Recently, several studies have demonstrated that HIF-2α is involved in BMSC osteogenic differentiation. However, the molecular mechanism and role of HIF-2α in hematopoietic function in the BM niche have not been determined. Although the two α subunits are structurally similar and recognize the same DNA elements, the target genes regulated by HIF-1α and HIF-2α are not identical. Cheng et al[23] generated conditional Phd2 knockout (cKO) mice from osteoblast lineage cells by crossing floxed Phd2 mice with a Col1α2-i Cre line to investigate the function of Phd2 in vivo. The cKO mice developed short stature and experienced premature death at 12 to 14 wk of age. Compared to WT mice, cKO mice had a reduced bone mineral content, bone area, and BMD in the femora and tibiae but not in the vertebrae. The TV, BV and BV fraction (BV/TV) of femoral trabeculae in cKO mice were significantly decreased[23]. A recent study by Lee et al[24] suggested that HIF-2α deficiency promoted osteoblast differentiation and inhibited osteoclast differentiation by increasing bone mass. Merceron et al[25] demonstrated that HIF-2α is an inhibitor of osteoblast formation and increased bone mass. Guo et al[26] conditionally knocked out HIF-2α in leptin receptor-expressing cells and their progeny in mice and found that radiation therapy in littermate control mice reduced bone mass, but HIF-2α conditional knockout mice maintained a bone mass comparable to that of nonirradiated control animals.
Taken together, our findings in this study support the role of HIF-2α in promoting osteogenesis, especially in combination with interventions such as bilateral ovariectomy, semilethal irradiation, and DEX treatment. Moreover, the Prx1-Cre;Hif-2αfl/fl phenotype did not significantly differ in terms of BMD in mice under the naive condition, but the BMD in mice subjected to the various stimulation interventions was indicative of a significant decrease in osteogenesis in Prx1-Cre;Hif-2αfl/fl mice. We hypothesized that the concentration of HIF-2α in the BM of mice under stress stimulation was elevated, leading to an alteration in BMD in these mice. Our results are consistent with those of previous studies. Shomento et al[21] used Osteocalcin-Cre mice and HIF-2α Flox mice to generate mice with osteoblast-specific HIF-2α knockout, and the results suggested that KO mice had reduced BMD. Wu et al[27] selected osx-cre-labeled osteoblasts and PHD mutant mice to generate mice with osteoblast-specific PHD knockout, which exhibited increased BMD. Wang et al[28] overexpressed HIFα in osteoblasts of mice by selective deletion of the Vhl, resulting in increased BMD.
Taken together, our results suggest that HIF-2α deletion in the BM microenvironment does not significantly affect HSC or HPC function in mice. These results indicate that the HIF-2α gene in the BM microenvironment does not affect the stromal niche of HSCs, only bone formation. Our results are consistent with those of previous studies. Wu et al[27] selected Osx-cre-labeled osteoblasts and PHD mutant mice to generate mice with osteoblast-specific PHD knockout (KO). The KO mice exhibited increased BMD without disruption of hematopoietic homeostasis[27]. However, notably, although the deletion of HIF-2α had no significant effect on HSC function, PB analysis revealed that the hemoglobin content and hematocrit value were significantly lower in Prx1-Cre;Hif-2αfl/fl mice than in WT mice, suggesting that other mechanisms may lead to the decreased hemoglobin content observed in Prx1-Cre;Hif-2αfl/fl mice. Rankin et al[29] reported that pharmacological or genetic inhibition of prolyl hydroxylases 1/2/3 in osteoprogenitors elevated EPO expression in bone and increased the hematocrit value, an effect believed to be caused by increased EPO expression and modulation of erythropoiesis in osteoblasts. Our results are consistent with the above results. Therefore, to further study the causes of the decreased hemoglobin content in Prx1-Cre;Hif-2αfl/fl mice observed in this study, further studies can be carried out focusing on EPO.
Recently, accumulating evidence has highlighted the role of mTOR signaling in regulating bone homeostasis, which provides insight into the pathogenesis of OP. It is widely believed that the inhibition of mTOR signaling can promote osteoblastic differentiation, but this remains a controversial issue[30]. Rapamycin is an inhibitor of mTOR signaling, and a high-throughput screening assay showed that rapamycin can promote osteoblastic differentiation-treatment of osteoblast precursors with rapamycin alone or in combination with bone morphogenetic protein (BMP)-2 increased the levels of the phospho-Smad 1/5/8 proteins and the transcription of Runx-2, Osx and Smad-7, consistent with a role in promoting osteoblastic differentiation[31]. Moreover, rapamycin effectively stimulates the osteoblastic differentiation of human embryonic stem cells by inhibiting rapamycin-sensitive mTOR signaling and promoting BMP/Smad signaling[32]. Similarly, blockade of mTOR signaling by osteoblast-specific knockout or rapamycin treatment rescues the osteopenia phenotype in fibrillin-1-deficient mice by augmenting the osteogenic differentiation and inhibiting the adipogenic differentiation of BMSCs[33]. These findings are consistent with the above results, suggesting that the activation of HIF-2α in BMSCs inhibits the mTOR signaling pathway, resulting in a reduction in the adipogenic differentiation and an increase in the osteogenic differentiation of BMSCs. Therefore, we believe that HIF-2α inhibits adipogenic differentiation and pro
Under physiological conditions, HIF-2α is hydroxylated by the PHD protein, leading to Vhl-dependent ubiquitination and protease-dependent degradation[34]. Therefore, the PHD inhibitor roxadustat (FG-4592) activates HIF-2α by increasing HIF-2α stability[35]. The drug is currently on the market in China and is approved for the treatment of renal anemia. In addition, several randomized phase II and III trials have shown that roxadustat significantly reduces plasma total cholesterol levels in patients with chronic kidney disease[36-38]. Moreover, roxadustat has potential use as an antiatherosclerotic treatment. Several studies have shown that roxadustat has an inhibitory effect on obesity-induced atherosclerosis, which depends primarily on the regulation of HIF-2α and ceramide metabolism in adipocytes[39]. In addition to its use for treating CKD-related anemia, roxadustat is potentially useful for the treatment of carcinoma, neurological diseases, ocular diseases, tissue injuries, and obesity[40]. However, studies on roxadustat and osteogenesis are rare. Roxadustat has been suggested to promote fracture repair in vivo by increasing the number of BMSCs and promoting chondrogenesis. In vitro, roxadustat significantly improved BMSC proliferation and migration by increasing the concentrations of intracellular calcium ions and NO and simultaneously decreasing the level of reactive oxygen species[41]. Hulley et al[42] suggested that roxadustat could inhibit osteoclast activity and subsequent bone resorption after coculture with osteoblasts. Our in vitro experiments revealed that roxadustat could promote the osteogenic differentiation of BMSCs and suggested that roxadustat might have a therapeutic effect on promoting osteogenesis in the context of OP. More studies could be carried out to verify the above speculation.
This study has several limitations. We have not verified the effect of HIF-2α on osteoclasts. Since bone homeostasis is a balance between osteoblast and osteoclast activity, the relationship between osteogenesis and osteoclasia should be assessed while observing the phenotype of bone, which is also the focus of our next study.
In conclusion, through in vivo and in vitro experiments, we verified that HIF-2α promoted the osteogenic differentiation and inhibited the adipogenic differentiation of BMSCs by inhibiting the mTOR signaling pathway. Moreover, HIF-2α had no effect on the number or function of HSCs or HPCs in the BM microenvironment. In vivo experiments showed that OP in Prx1-Cre;Hif-2αfl/fl mice was aggravated by bilateral ovariectomy, sublethal irradiation, and DEX treatment. Through in vitro experiments, it was confirmed that HIF-2α can inhibit the mTOR signaling pathway, leading to multiple factors, such as the promotion of BMSC osteogenic differentiation, inhibition of BMSC adipogenic differentiation, inhibition of erythropoiesis, and promotion of the anti-injury function of WBCs to regulate the BM niche, thus participating in the occurrence and progression of OP.
Recently, several studies have demonstrated that hypoxia-inducible factor 2alpha (HIF-2α) is involved in bone mesenchymal stem cell (BMSC) osteogenic differentiation. However, the molecular mechanism involved remains unclear.
An exploration of osteoporosis (OP) treatments aimed at increasing bone formation is needed.
Research on the effects of HIF-2α on the osteogenic and adipogenic differentiation of BMSCs and hematopoietic function in the bone marrow niche.
In vivo, we generated mice with BMSC-specific HIF-2α knockout and induced OP in these mice via three interventions: Bilateral ovariectomy, semilethal irradiation, and treatment with dexamethasone.
In vivo, the bone mass of KO mice was decreased compared with that of WT mice. In vitro, downregulation of HIF-2α inhibited osteogenesis and increased adipogenesis by suppressing the mechanistic target of rapamycin (mTOR) signaling pathway.
In conclusion, through in vivo and in vitro experiments, we verified that inhibition of HIF-2α can decrease the osteogenic differentiation and increase the adipogenic differentiation of BMSCs by inhibiting the mTOR signaling pathway.
In future research, as many patients with chronic kidney disease also have OP, we will verify whether the HIF-2α agonist roxadustat can successfully treat mice with OP induced via ovariectomy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ariga K, Japan; El-Akabawy G, Egypt; Roomi AB, Iraq; Ventura C, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Fuggle NR, Curtis EM, Ward KA, Harvey NC, Dennison EM, Cooper C. Fracture prediction, imaging and screening in osteoporosis. Nat Rev Endocrinol. 2019;15:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, Zhao N, Lin Q, Chen L, Lin H, Jin X, Dong Z, Ren Z, Hou Z, Zhang Y, Zhong J, Cai S, Liu Y, Meng R, Deng Y, Ding X, Ma J, Xie Z, Shen L, Wu W, Zhang M, Ying Q, Zeng Y, Dong J, Cummings SR, Li Z, Xia W. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw Open. 2021;4:e2121106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 3. | Tang L, Wu M, Lu S, Zhang H, Shen Y, Shen C, Liang H, Ge H, Ding X, Wang Z. Fgf9 Negatively Regulates Bone Mass by Inhibiting Osteogenesis and Promoting Osteoclastogenesis Via MAPK and PI3K/AKT Signaling. J Bone Miner Res. 2021;36:779-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Kiernan J, Davies JE, Stanford WL. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl Med. 2017;6:1930-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Wan Y. Bone marrow mesenchymal stem cells: fat on and blast off by FGF21. Int J Biochem Cell Biol. 2013;45:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 746] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 7. | Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr Opin Cell Biol. 2012;24:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Yellowley CE, Genetos DC. Hypoxia Signaling in the Skeleton: Implications for Bone Health. Curr Osteoporos Rep. 2019;17:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Mangiavini L, Merceron C, Araldi E, Khatri R, Gerard-O'Riley R, Wilson TL, Rankin EB, Giaccia AJ, Schipani E. Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev Biol. 2014;393:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Kaluz S, Tan C, Van Meir EG. Taking a HIF pill for old age diseases? Aging (Albany NY). 2018;10:290-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Picke AK, Campbell GM, Blüher M, Krügel U, Schmidt FN, Tsourdi E, Winzer M, Rauner M, Vukicevic V, Busse B, Salbach-Hirsch J, Tuckermann JP, Simon JC, Anderegg U, Hofbauer LC, Saalbach A. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Yue R, Shen B, Morrison SJ. Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Wenxi D, Shufang D, Xiaoling Y, Liming Y. Panax notoginseng saponins suppress radiation-induced osteoporosis by regulating bone formation and resorption. Phytomedicine. 2015;22:813-819. [PubMed] [DOI] [Full Text] |
| 14. | Ni F, Yu WM, Wang X, Fay ME, Young KM, Qiu Y, Lam WA, Sulchek TA, Cheng T, Scadden DT, Qu CK. Ptpn21 Controls Hematopoietic Stem Cell Homeostasis and Biomechanics. Cell Stem Cell. 2019;24:608-620.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Suire C, Brouard N, Hirschi K, Simmons PJ. Isolation of the stromal-vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. Blood. 2012;119:e86-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Yin LM, Jiang HF, Wang X, Qian XD, Gao RL, Lin XJ, Chen XH, Wang LC. Effects of sodium copper chlorophyllin on mesenchymal stem cell function in aplastic anemia mice. Chin J Integr Med. 2013;19:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, Matsuzaki Y. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483-2496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 597] [Cited by in RCA: 655] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 18. | Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 406] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 19. | Li Y, Fu H, Wang H, Luo S, Wang L, Chen J, Lu H. GLP-1 promotes osteogenic differentiation of human ADSCs via the Wnt/GSK-3β/β-catenin pathway. Mol Cell Endocrinol. 2020;515:110921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Huebner AK, Schinke T, Priemel M, Schilling S, Schilling AF, Emeson RB, Rueger JM, Amling M. Calcitonin deficiency in mice progressively results in high bone turnover. J Bone Miner Res. 2006;21:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Shomento SH, Wan C, Cao X, Faugere MC, Bouxsein ML, Clemens TL, Riddle RC. Hypoxia-inducible factors 1alpha and 2alpha exert both distinct and overlapping functions in long bone development. J Cell Biochem. 2010;109:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673-8678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 23. | Cheng S, Xing W, Pourteymoor S, Mohan S. Conditional disruption of the prolyl hydroxylase domain-containing protein 2 (Phd2) gene defines its key role in skeletal development. J Bone Miner Res. 2014;29:2276-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lee SY, Park KH, Yu HG, Kook E, Song WH, Lee G, Koh JT, Shin HI, Choi JY, Huh YH, Ryu JH. Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice. Bone Res. 2019;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Merceron C, Ranganathan K, Wang E, Tata Z, Makkapati S, Khan MP, Mangiavini L, Yao AQ, Castellini L, Levi B, Giaccia AJ, Schipani E. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Guo W, Hoque J, Garcia Garcia CJ, Spiller KV, Leinroth AP, Puviindran V, Potnis CK, Gunn KA, Newman H, Ishikawa K, Fujimoto TN, Neill DW, Delahoussaye AM, Williams NT, Kirsch DG, Hilton MJ, Varghese S, Taniguchi CM, Wu C. Radiation-induced bone loss in mice is ameliorated by inhibition of HIF-2α in skeletal progenitor cells. Sci Transl Med. 2023;15:eabo5217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Wu C, Rankin EB, Castellini L, Alcudia JF, LaGory EL, Andersen R, Rhodes SD, Wilson TL, Mohammad KS, Castillo AB, Guise TA, Schipani E, Giaccia AJ. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015;29:817-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 569] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 29. | Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, Giaccia AJ. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Shen G, Ren H, Qiu T, Zhang Z, Zhao W, Yu X, Huang J, Tang J, Liang D, Yao Z, Yang Z, Jiang X. Mammalian target of rapamycin as a therapeutic target in osteoporosis. J Cell Physiol. 2018;233:3929-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Darcy A, Meltzer M, Miller J, Lee S, Chappell S, Ver Donck K, Montano M. A novel library screen identifies immunosuppressors that promote osteoblast differentiation. Bone. 2012;50:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, Cho YS. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010;19:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, Brombacher F, Sun L, Shi S. mTOR inhibition rescues osteopenia in mice with systemic sclerosis. J Exp Med. 2015;212:73-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3711] [Article Influence: 154.6] [Reference Citation Analysis (0)] |
| 35. | Beuck S, Schänzer W, Thevis M. Hypoxia-inducible factor stabilizers and other small-molecule erythropoiesis-stimulating agents in current and preventive doping analysis. Drug Test Anal. 2012;4:830-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Cai GY, Hao L, Leong R, Liu C, Neff T, Szczech L, Yu KP. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 37. | Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 38. | Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, Aiello JR, Novak JE, Lee T, Leong R, Roberts BK, Saikali KG, Hemmerich S, Szczech LA, Yu KP, Neff TB. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin J Am Soc Nephrol. 2016;11:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 39. | Zhang X, Zhang Y, Wang P, Zhang SY, Dong Y, Zeng G, Yan Y, Sun L, Wu Q, Liu H, Liu B, Kong W, Wang X, Jiang C. Adipocyte Hypoxia-Inducible Factor 2α Suppresses Atherosclerosis by Promoting Adipose Ceramide Catabolism. Cell Metab. 2019;30:937-951.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 40. | Su K, Li Z, Yu Y, Zhang X. The prolyl hydroxylase inhibitor roxadustat: Paradigm in drug discovery and prospects for clinical application beyond anemia. Drug Discov Today. 2020;25:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Chen C, Yan S, Qiu S, Geng Z, Wang Z. HIF/Ca(2+)/NO/ROS is critical in roxadustat treating bone fracture by stimulating the proliferation and migration of BMSCs. Life Sci. 2021;264:118684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Hulley PA, Papadimitriou-Olivgeri I, Knowles HJ. Osteoblast-Osteoclast Coculture Amplifies Inhibitory Effects of FG-4592 on Human Osteoclastogenesis and Reduces Bone Resorption. JBMR Plus. 2020;4:e10370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |