Published online Apr 26, 2024. doi: 10.4252/wjsc.v16.i4.375
Peer-review started: December 30, 2023
First decision: January 17, 2024
Revised: February 14, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: April 26, 2024
Processing time: 116 Days and 21.6 Hours
The repair of bone tissue damage is a complex process that is well-orchestrated in time and space, a focus and difficulty in orthopedic treatment. In recent years, the success of mesenchymal stem cells (MSCs)-mediated bone repair in clinical trials of large-area bone defects and bone necrosis has made it a candidate in bone tissue repair engineering and regenerative medicine. MSCs are closely related to macrophages. On one hand, MSCs regulate the immune regulatory function by influencing macrophages proliferation, infiltration, and phenotype polarization, while also affecting the osteoclasts differentiation of macrophages. On the other hand, macrophages activate MSCs and mediate the multilineage differentiation of MSCs by regulating the immune microenvironment. The cross-talk between MSCs and macrophages plays a crucial role in regulating the immune system and in promoting tissue regeneration. Making full use of the relationship between MSCs and macrophages will enhance the efficacy of MSCs therapy in bone tissue repair, and will also provide a reference for further application of MSCs in other diseases.
Core Tip: Bone regeneration has always been a challenge and priority in the treatment of orthopedic diseases. The interaction between mesenchymal stem cells (MSCs) and macrophages mediating the multilineage differentiation potential and immunomodulatory capabilities plays a crucial role in bone tissue repair and remodeling. Therefore, we reviewed the interactions between MSCs and macrophages, summarized the roles and potential of MSCs and macrophages in bone tissue regeneration, and looked forward to how to better utilize their relationship to enhance the efficacy of MSCs therapy in orthopedic diseases such as bone defects and osteoarthritis.
- Citation: Zhang FF, Hao Y, Zhang KX, Yang JJ, Zhao ZQ, Liu HJ, Li JT. Interplay between mesenchymal stem cells and macrophages: Promoting bone tissue repair. World J Stem Cells 2024; 16(4): 375-388
- URL: https://www.wjgnet.com/1948-0210/full/v16/i4/375.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i4.375
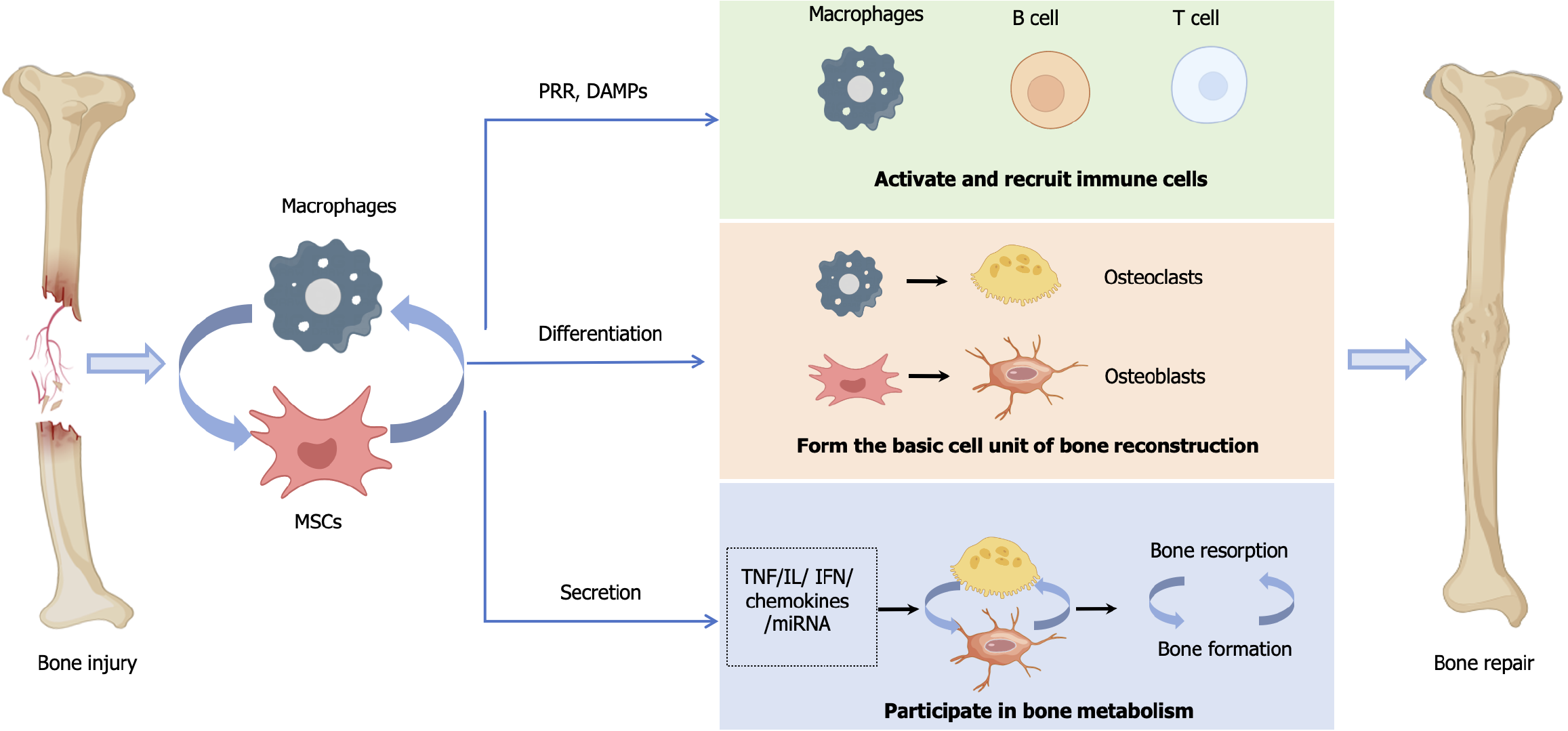
The destruction of bone tissue caused by violent injuries and chronic inflammation is the core of various orthopedic conditions, such as bone defects, osteoarthritis, and osteoporosis. The repair of bone tissue damage is a complex process that is spatiotemporally well orchestrated. It has long been the focus of but also a difficulty in orthopedic treatment. Inflammation, tissue repair, and tissue remodeling are three important stages of tissue regeneration, involving strictly regulated participation of various types of cells. The immune system plays a core role in tissue repair and regeneration, and the immune response to tissue damage determines the speed and outcome of tissue healing[1]. Mesenchymal stem cells (MSCs) and macrophages are the sources of bone tissue. Moreover, MSCs and macrophages play important collaborative roles in immune regulation and in bone tissue regeneration as regulators and effectors of the inflammatory response[2]. MSCs mediate the phenotypic polarization of macrophages that are involved in immune regulation of the body. The pro-inflammatory and anti-inflammatory factors secreted by polarized macrophages mediate the biological functions of MSCs such as adhesion, migration, immune activity, and osteogenic activity. On these bases, the osteogenesis mediated by MSCs and the bone resorption mediated by macrophages-derived osteoclasts (OCs) together maintain bone homeostasis and bone remodeling[3].
MSCs, versatile and plastic effector cells, play multiple roles in bone tissue repair and regeneration. On the one hand, as pluripotent stem cells, MSCs have the abilities to self-renew and differentiate into multiple lineages. Under the influence of injury signals and chemokines, MSCs migrate to the site of injury, differentiate directionally, and secrete various growth factors, such as transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor, stromal cell-derived factor-1 (SDF-1), and fibroblast growth factor, to promote cell homing and stimulate tissue regeneration[4,5]. Factors in the injured environment, such as interferon gamma (IFN-γ) and tumor necrosis factor-α (TNF-α), stimulate the multilineage differentiation potential of MSCs, including osteogenic, adipogenic, and angiogenic differentiation, forming the various cells required for tissue repair[5]. On the other hand, as the first line of defense of the innate immune system, MSCs maintain the stability of the tissue microenvironment by controlling the inflammatory cascade[6]. MSCs can be activated by biomarkers in the inflammatory environment and respond to these signals through homing or paracrine effects, secreting soluble factors, such as prostaglandin E2 (PGE2), TGF-β1, indoleamine 2,3-dioxygenase (IDO), interleukin (IL)-6, and IL-10, as well as proteins and RNAs that control macrophages differentiation and promote the anti-inflammatory phenotypic transformation of macrophages to inhibit uncontrolled immune responses[4,5,7-11].
Macrophages, as important innate immune cells, are closely related to cells involved in bone tissue repair and regeneration[12]. In addition to being responsible for clearing invading organisms, as well as cells and cell fragments after tissue damage, macrophages also demonstrate functional plasticity[13,14]. Macrophages play different roles at various stages of tissue repair, regulating inflammation by secreting pro-inflammatory and anti-inflammatory factors, and simultaneously secreting various biologically active factors such as chemokines, matrix metalloproteinases (MMPs), growth factors, and osteogenesis-related proteins, which affect the recruitment, migration, proliferation, and differentiation of various tissue repair-related cells, including MSCs[15-19]. The reduction of macrophages can lead to the disruption of the tissue microenvironment and the extension of tissue healing time[20]. Due to the critical role of macrophages in tissue repair, they have emerged as a potential target for therapeutic tissue regeneration strategies.
In this review, based on the importance of the immune response in the repair of bone tissue damage, we explored the dual role of MSCs and macrophages and their mutual regulation to maintain different functional phenotypes in immune regulation and bone regeneration. We then discussed the prospects of these cells in bone tissue repair.
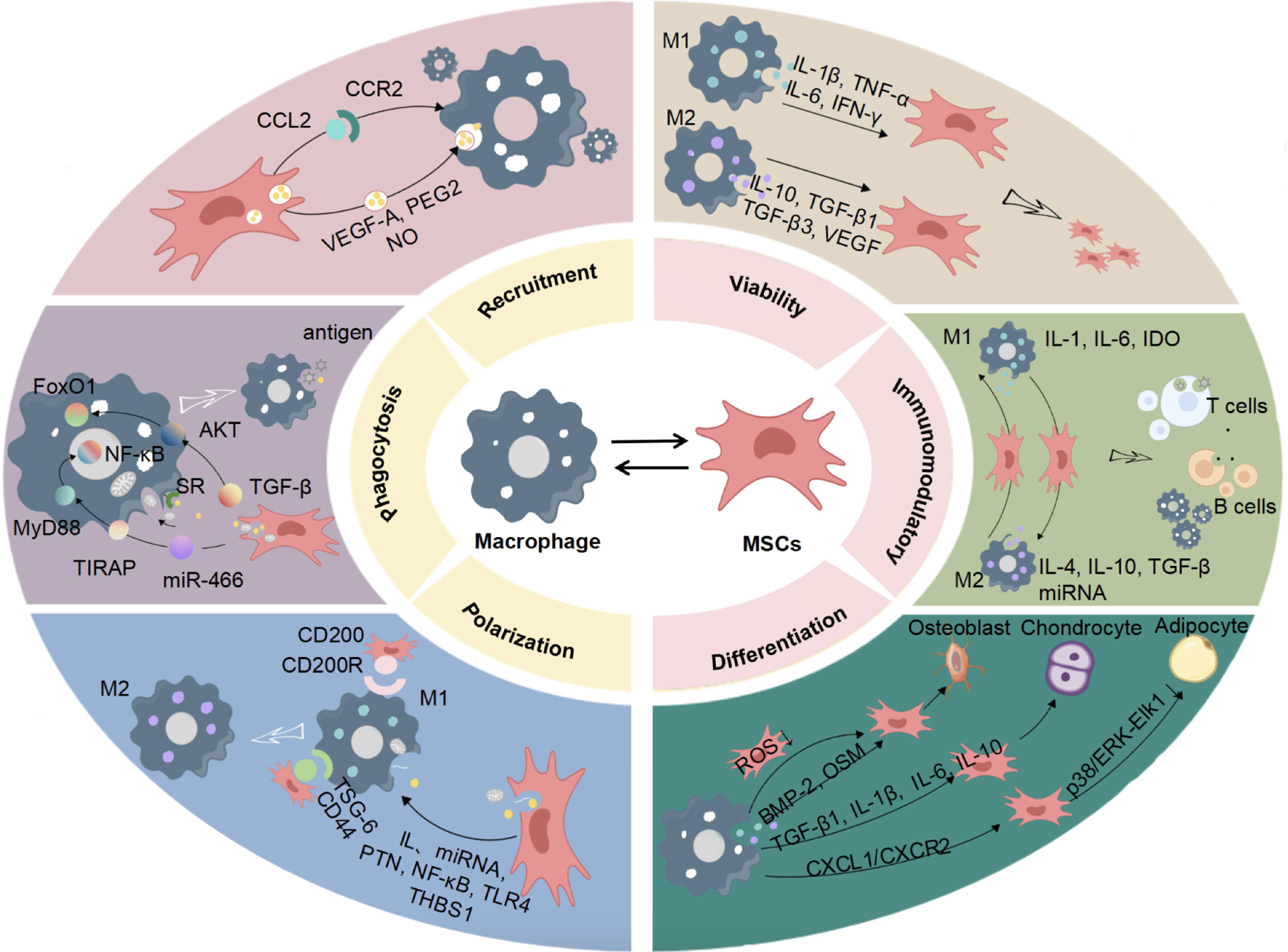
MSCS can interact with macrophages in mutual regulation of the host microenvironment. MSCs can promote the basic functions of macrophages and mediate the polarization of macrophages phenotypes. Macrophages can secrete different active factors to regulate the immune microenvironment and affect the biological functions of MSCs through their own polarization (Figure 1)[4,21-43].
Macrophages are an important part of the human immune system. Their polarization states and biological function changes are closely related to the occurrence and development of various diseases[12]. Proteins, RNA, chemokines, and other regulatory factors produced by MSCs enter macrophages through endocytosis, regulating macrophages genes expression, functional phenotypes, and also physiological and pathological states[44,45]. By mediating macrophages phenotype and function, MSCs participate in regulating inflammation, tissue healing, immune diseases, tumor growth, and other biological processes, playing an important role in the treatment of orthopedic diseases, respiratory diseases, urological diseases, etc.[17].
MSCs promote macrophages recruitment by interacting with macrophages. Chemokines are considered to be chemotactic agents for immune cells, and macrophages play a key role in inflammatory responses by migrating along an increased chemokine gradient signal. C-C motif chemokine ligand 2 (CCL2) is an inflammatory chemokine that regulates the recruitment of immune cells during inflammation[46]. The release of CCL2 by MSCs and its binding to C-C motif chemokine receptor 2 (CCR2) may increase intracellular calcium ion influx through the Janus kinase (JAK)/signaling and transcription activator (STAT)1/STAT3 signaling pathway, activate important inflammatory signaling cascades, and promote the recruitment and infiltration of macrophages[47,48]. MSCs express various bioactive factors, such as VEGF, PGE2, and nitric oxide (NO) through exosomes, which may regulate macrophages recruitment via endocytosis[22,23]. Additionally, apoptosis of MSCs can inhibit macrophages recruitment. Research conducted by Zheng et al[49] revealed that apoptotic MSCs release apoptotic vesicles, which are engulfed by macrophages and induce macrophages repro
MSCs can secrete a variety of active ingredients to regulate the phagocytic ability of macrophages. TGF-β secreted by MSCs can stimulate the polarization of macrophages toward the M2 type and enhance their phagocytic ability through AKT/forkhead box transcription factor O1 signaling pathway[24]. MiR-466 secreted by MSCs, increases the phagocytic ability of macrophages by downregulating the Toll-interleukin-1 receptor domain-containing adaptor protein (TIRAP)-Myeloid differentiation primary response gene 88 (MyD88)-nuclear factor kappa-B (NF-κB) signaling pathway[25]. Scavenger receptor (SR), a receptor with extensive ligand binding ability, is related to the phagocytic function of macrophages. After interacting with macrophages, MSCs can upregulate the expression of SR in macrophages, enhance their phagocytic ability for microbes, and reduce further stimulation of immune cells by invading microbes[26]. In addition, MSCs mitochondria also play an important role in regulating macrophages function. Studies have found that MSCs can transfer mitochondria to macrophages through tunneling nanotube like structures, which not only promotes the oxidative phosphorylation of macrophages but also enhances their phagocytic ability[50].
MSCs primarily regulate macrophages through paracrine effects, direct cell contact, mitochondrial transfer, and cell burial. Mechanisms mediated by paracrine factors, such as cytokines and hormones are the main pathways by which MSCs participate in tissue damage repair. Macrophages, derived from monocytes, are involved in innate immunity and cell-mediated immunity in the body and can be polarized into M1 and M2 subtypes. Increasing evidence suggests that extracellular vesicles secreted by MSCs play a key role in regulating the balance of M1/M2 macrophages, but the exact mechanism remains unclear[51]. Pleiotropic hormones (PTNs) are critical regulators of macrophages differentiation, and MSCs maintain the M2-like phenotype by secreting PTNs to regulate the syndecan-3 receptor-mediated cell adhesion molecule pathway[30]. Liu et al[31] found that thrombospondin-1 secreted by MSCs could induce the activation of TGF-β and stimulate the polarization of macrophages toward the M2 phenotype. In addition, other microRNAs (miRNAs), through extracellular vesicles, affect the levels of transcription, post-transcriptional modifications, and metabolism in macrophages, regulating their function and phenotype, and participating in physiological and pathological processes. MSC-derived small extracellular vesicles can transfer miR-223-3p, miRNA-124-3p, miRNA-125a, etc., to macrophages, change gene expression and biological activity, and promote the polarization of macrophages from M1 to M2 anti-inflammatory phenotype[28,52,53]. Abe et al[54] found that MSCs could release paracrine factors through cell-cell contact, further inducing the polarization of macrophages from M1 to M2, and significantly reducing the expression of pro-inflammatory factors such as IL-6 and TNF-α[29,54]. Lu et al[55] found that extracellular vesicles derived from MSCs could promote the polarization of M2 macrophages after transferring mitochondria to macrophages. Ghahremani Piraghaj et al[56] found that the apoptotic vesicles of MSCs were engulfed by macrophages, promoting M2 polarization of macrophages, reducing the production of TNF-α and NO, and increasing the level of IL-10.
The functional phenotype and secretory profile of MSCs, as well as their availability, are regulated by the microenvironment, which determines their tissue repair capabilities. Multiple studies have shown that while MSCs regulate macrophages, the change in the polarization phenotype of macrophages also affects the biological activity and function of MSCs.
Homing is one of the three major mechanisms of MSCs. In the damaged microenvironment, various chemokines, adhesion factors, and growth factors bind to receptors on the surface of MSCs, driving MSCs to migrate directionally to inflamed or damaged areas and participate in tissue repair. Multiple chemokines are indispensable for MSCs homing. Various chemokine receptors are present on MSCs, such as CCR4, CCR7, CCR9, C chemokine receptor type 6 (CXCR6), and SDF1α[57-59]. The most studied is the SDF-1/CXCR4 axis, whose main functions include regulating the trans
During the organizational repair process, the interaction between MSCs and immune cells is necessary to activate the immune regulatory activity of MSCs. On the one hand, the immunomodulatory ability of MSCs is triggered by the inflammatory environment. The changes in macrophages functional phenotypes play an essential regulatory role in the inflammatory environment. Inflammatory factors released by M1 macrophages, such as IFN-γ, can activate the immune regulatory properties of MSCs, promoting the regulatory effect of MSCs on immune cells, such as secreting IDO to inhibit T cell proliferation and induce T cell apoptosis[35]. Anti-inflammatory factors produced by M2 macrophages, such as IL-10, can enhance the promoter activity of TNF-α, a pro-inflammatory cytokine, in MSCs, reducing the expression of inflammatory factors such as IL-6 and IFN-γ[27]. On the other hand, MSCs demonstrate poor survival in inflammatory areas, mainly through paracrine chemokines, growth factors, and cytokines, or by direct contact, to regulate macrophages polarization, resulting in anti-inflammatory or immunosuppressive effects, and playing an immune regulatory role. Exosomes derived from MSCs can significantly enrich macrophages for activating the HMGB1/toll-like receptor 4 (TLR4)/NF-κB signaling pathway, and promoting the conversion of macrophages to M2 macrophages[67,68]. M2 macrophages secrete anti-inflammatory factors, such as IL-4, IL-10, and TGF-β, to prevent excessive activation of inflammatory responses[34,36].
Pittenger and colleagues successfully induced the differentiation of MSCs into osteoblasts (OBs), chondrocytes, and adipocytes in vitro[38]. Subsequently, the potential of MSCs to differentiate into muscle, ligament, endotheliocytes, and hepatocytes, among other cell types, was discovered[38,51,69-71]. Multiple studies have shown that macrophages play a crucial role in the MSC differentiation process. Firstly, macrophages provide a certain protective effect on the multilineage differentiation potential of MSCs. Mitochondrial metabolic damage in MSCs can lead to differentiation disorders. Teissier et al[72] found that co-culture of M0 macrophages with mitochondrially impaired MSCs could correct the redox imbalance in MSCs, restore their metabolic homeostasis, and promote their differentiation. Additionally, macrophages have an inducing effect on MSC differentiation. Current research mainly focuses on the impact of macrophages on the osteogenic, chondrogenic, and adipogenic differentiation of MSCs. Macrophages secrete certain anti-inflammatory factors, pro-inflammatory factors, and osteogenic factors such as bone morphogenic protein-2 (BMP-2) and oncostatin-M (OSM), which can mediate the osteogenic differentiation of MSCs[39,73]. Recent studies have also shown that oxidative stress plays a key role in the regulation of MSCs by macrophages. Macrophages can reduce the level of reactive oxygen species in MSCs similarly to the antioxidant N-acetyl cysteine, regulating their osteogenic differentiation[74]. Cao et al[40] discovered that co-culture of macrophages with MSCs could inhibit the expression of C-X-C motif chemokine ligand 1 (CXCL1) and its receptor CXCR2, reduce the phosphorylation of p38/ERK-ELK1 during the early stage of adipogenesis, and regulate the adipogenic differentiation of MSCs. Physical stimuli and changes in chemical composition can enhance the chondrogenic differentiation of MSCs in vitro and in vivo by controlling macrophages activation. Wang et al[41] found that mechanical stimulation could promote the polarization of macrophages toward the M2 phenotype, which then secreted TGF-β1 to promote MSC-based chondrogenesis. Hu et al[42] found that magnesium deficiency can increase the secretion of inflammatory factors, such as IL-1β and IL-6 by M1 macrophages, inhibiting MSCs chondrogenesis. Due to the roles of macrophages in bone, cartilage, and fat formation, they are considered potential targets for the treatment of various related diseases, such as bone defects, osteoarthritis, and vascular diseases.
The process of bone tissue damage includes an inflammatory response, which creates the basis for subsequent bone formation, mineralization, resorption, and bone replacement. In the process of damage repair, the synergistic effect of MSCs and macrophages mainly involves intervention in the occurrence and regression of inflammation after damage and regulation of bone formation and remodeling. The polarization of M1/M2 macrophages is the basis of the inflammatory response, and MSCs can regulate the balance of M1/M2 macrophages to participate in the regulation of inflammation progression and regression[75]. During bone formation and remodeling, the mutual regulation between MSCs and macrophages plays an important role in balancing OBs and OCs and maintaining bone homeostasis. At the same time, the dynamic balance between MSC-drives osteogenic differentiation-mediated bone regeneration and OCs-mediated bone resorption also participates in the bone repair and remodeling process. The interaction between MSCs and macrophages creates a negative feedback loop, which plays an important role in bone tissue repair.
The occurrence of inflammation is a necessary link in the repair of bone tissue damage. Activation of the innate and adaptive immune system is beneficial for the body to make a sufficient response to bone tissue damage[76,77]. The temporary inflammatory response after damage helps the body to mobilize the immune system and to activate the damage repair mechanism, which is conducive to wound healing. After tissue damage, cells autonomously conduct inflammatory signal transduction. Macrophages are large secretory cells that act as inflammation effectors and can shape the post-damage tissue environment by secreting a variety of bioactive factors. They play pro-anabolic roles throughout the entire stage of bone tissue damage repair and are key cells in the repair of bone tissue damage[78]. MSCs are key regulators of inflammation. Under the action of damage-associated molecular patterns, MSCs exhibit a pro-inflammatory phenotype[79]. After receiving stimulation signals through pattern recognition receptors, MSCs activate transcriptional effectors such as NF-κB and TLR4 for immune regulation[80]. For example, NF-κB can bind to the receptor activator of NF-κB (RANK) ligand (RANKL) receptor, stimulate the activation of classical M1 macrophages, and release a variety of inflammatory factors, while recruiting various immune cells to enhance the immune response[81,82].
Prolonged inflammatory responses are not conducive to the repair of injuries, as they can damage the osteogenic ability of MSCs and lead to secondary osteopathies such as osteoporosis, bone necrosis, and hyperostosis[83]. Therefore, improving the inflammatory state of the organizational microenvironment is the precursor and basis for the recovery of tissue structure and function. Inflammatory signal transduction stimulation can cause changes in the biological activities and epigenetics of various cells, which is a key adaptation of cells to tissue damage. Once inflammatory factors in the microenvironment reach a certain level, MSCs are activated to inhibit the secretion of pro-inflammatory factors by M1 macrophages in various ways, promoting the secretion of multiple anti-inflammatory factors by M2 macrophages, effectively preventing excessive activation of inflammation, and ensuring the smooth progress of tissue repair and remodeling. On the one hand, MSCs and macrophages interact with each other through direct receptor binding. Li et al[27] found that pro-inflammatory M1 macrophages can interact directly with MSCs, promoting the production of TNF-α-stimulated gene 6 protein (TSG-6) and CD200 expression in MSCs. Simultaneously, CD200 on MSCs can interact with CD200 receptor on macrophages, promoting the polarization of M2 macrophages and enhancing the inhibitory effect on inflammation[27]. On the other hand, MSCs and macrophages also regulate each other through paracrine effects. The anti-inflammatory protein TGS-6 is a multifunctional protein associated with inflammation that has anti-inflammatory and tissue-protective properties and plays a critical role in inflammatory cell migration, cell proliferation, and deve
Bone repair and remodeling are processes involving “activation–resorption–formation” of the basic multicellular units[86]. OBs derived from MSCs and OCs derived from macrophages are two major cell types involved in bone tissue repair, playing essential roles in maintaining bone homeostasis[87]. OBs are the key cells for bone formation. MSCs and skeletal stem cells differentiate into OB precursor cells (pre-OBs), while macrophages and endosteal OBs participate in bone mineralization side by side. Mature OBs embed in the gaps of the mineralized matrix and become bone cells. OCs are the key cells for bone resorption. Inflammatory factors and chemokines can attract circulating macrophages to migrate to the inflammatory site and become resident macrophages in bone (also known as bone macrophages)[88]. OBs-derived macrophages secrete colony-stimulating factor (M-CSF), which binds to CSF 1 receptor (CSF1R, also known as c-FMS) on the macrophages surface, activating the induction signal for OC differentiation, RANKL/RANK[89]. Key factors, such as M-CSF and RANKL, can activate the expression of the primary switch regulatory factor for OC generation, i.e., the transcription factor nuclear factor of activated T cells (NFATc1), promoting the migration of monocytes/macrophages, which are OC precursor cells (pre-OCs), to the bone surface for activation and differentiation into mature OCs[90-92].
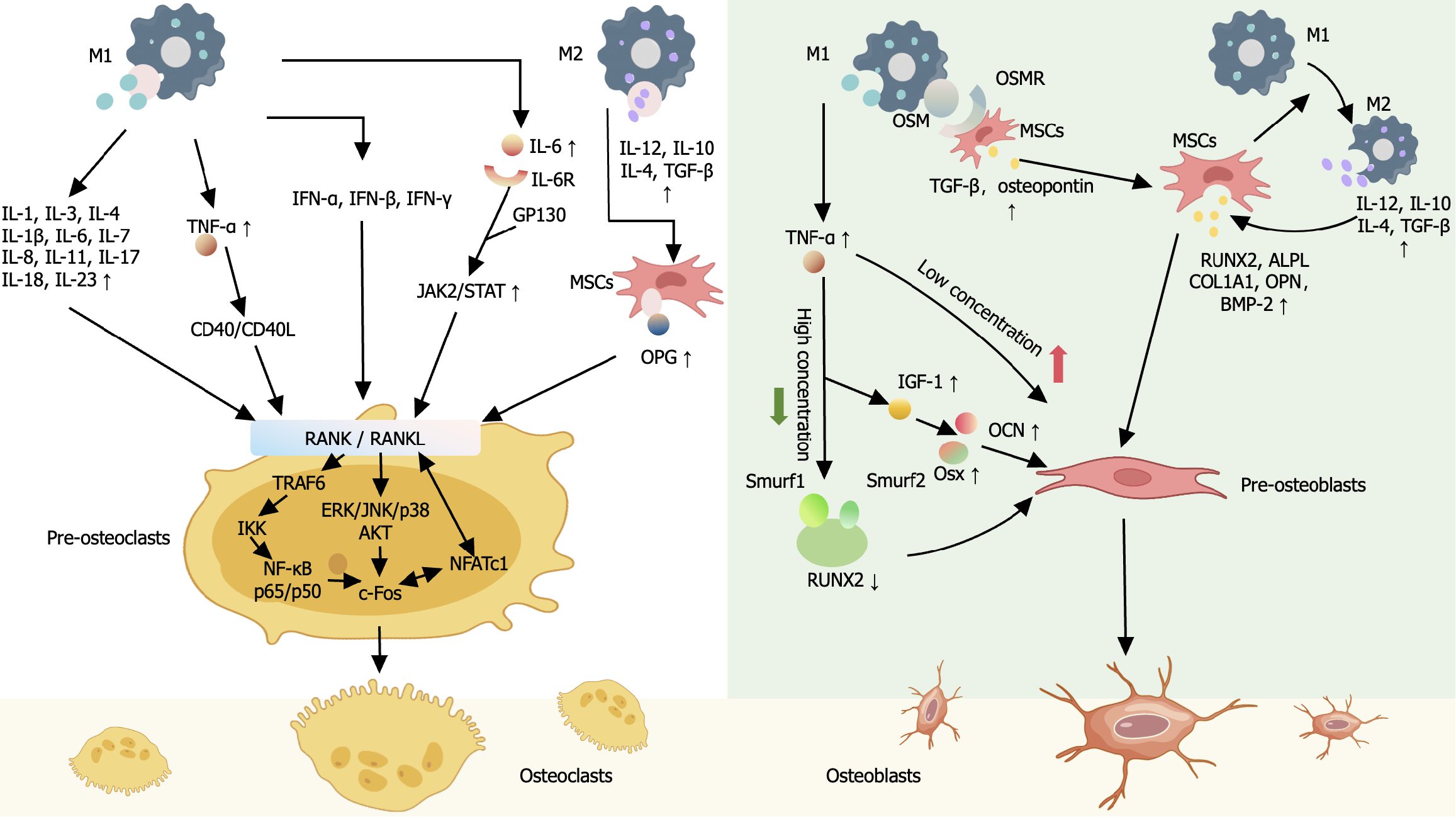
MSCs and macrophages not only serve as the sources of OBs and OCs for tissue repair but also interact closely with each other through common cellular precursors and molecular mediators of the immune system and bone tissue. This shapes the various bioactive factors required for the bone tissue repair microenvironment, including osteogenic-related factors, TNF family, IL family, IFN family, chemokines, etc., and participating in metabolism related to bone resorption and bone formation (Figure 2)[73,93-107].
In the acute phase of inflammation, the interaction between MSCs and M1 macrophages releases various pro-inflammatory factors and chemokines, mainly involved in the differentiation and maturation of pre-OCs. RANKL serves as a bridge between the inflammatory response and bone resorption[93]. In the presence of RANKL, multiple pro-inflammatory cytokines synergistically coordinate to enhance the genesis of OCs. MSCs-secreted NF-κB binds to the OCs differentiation-inducing RANKL to control the expression of key factors in pre-OCs differentiation. Inflammatory factors, such as TNF-α, IL-1, IL-1β, IL-6, IL-7, IL-8, IL-11, IL-17, IL-18, IL-23, etc., secreted by M1, can directly or indirectly affect OBs to promote RANKL expression, participating in OBs differentiation of MSCs and OCs differentiation of bone macrophages[93-99]. Among them, TNF-α and IL-6 are key regulatory factors. TNF-α acts on signaling pathways such as that of RANKL/RANK, downstream NF-κB, JNK, p38, AKT, etc., to reduce the expression of OCs-specific genes and proteins, promoting the activation of bone macrophages and the differentiation of OCs[81]. After binding to IL-6R and gp130, IL-6 activates the phosphorylation of JAK/STAT[108]. The phosphorylated STAT3 translocate to the nucleus, where it activates the expression of RANKL, directly participating in the OC differentiation of bone macrophages, or indirectly influencing OC differentiation through its action on TNF-α[109,110]. At the same time, these factors also provide key aspects of the feedback regulation mechanism of RANKL induction in bone macrophages. IL-4 inhibits the transcription factor NFATc1, weakening RANKL-induced OC formation[104]. IFNs, such as IFN-α, IFN-β, and IFN-γ induce rapid degradation of RANK-associated protein TNF receptor-associated factor 6 or mediate FAS/FASL signaling, strongly inhibiting the activation of RANKL-induced transcription factors NF-κB and c-fos, thus inhibiting excessive OC formation[101]. TNF-α activates RANKL-related CD40/CD40L signaling to promote the production of the OC genesis inhibitory factor osteoprotegerin (OPG) and to reduce bone resorption[100]. IL-3 can also down-regulates TNF-α-induced c-fos nuclear translocation to inhibit OCs formation and bone resorption[94].
In addition, the pro-inflammatory factors secreted by M1 macrophages are also related to the early and mid-stage osteogenic effects of MSCs. The absence of TNF-α and IL-6 has been proven to delay the differentiation of MSCs. TNF-α can selectively mediate the osteogenic differentiation of OBs. Low concentrations of TNF-α stimulate the differentiation of pre-OBs into OBs, while high concentrations of TNF-α regulate the degradation of RUNT-related transcription factor 2 (RUNX2) protein by signal molecules drosophila mothers against decapentaplegic homologue ubiquitination regulatory factor 1 (SMURF1) and SMURF2 to block the expression of RUNX2, and simultaneously inhibit the expression of insulin-like growth factor-1 and osterix and osteocalcin, inhibiting OB differentiation[106]. The effect of IL-6 on bone marrow-derived OCs stimulates the release of “bone messengers”, which act through the cortical bone cell network to stimulate the formation of OBs on the bone membrane. Cyclooxygenase-2 and PGE2 regulate the production of the IL-6 family cytokine OSM in classically activated inflammatory M1 macrophages[73]. OSM binds to the OSM receptor on MSCs, then activates the STAT3-CCAAT-enhancer-binding protein delta-C/core binding factor alpha 1 differentiation pathway, mediates the recruitment and proliferation of MSCs, stimulates the maturation of OBs from MSCs, and drives the mineralization of OBs. TGF-β and OPN enhance the activity of alkaline phosphatase (ALP) in MSCs, promoting the maturation and mineralization of the matrix[107].
However, recent studies have shown that the microenvironment that forms during the resolution stage of inflammation has a more important impact on bone formation and remodeling, which can significantly increase the ratio of OBs/OCs and can promote bone formation. On the one hand, MSCs promote M2 macrophages polarization, and release inflammatory factors, chemokines, and other active factors to regulate bone homeostasis indirectly. Anti-inflammatory factors secreted by M2 macrophages, such as IL-4, IL-10, TGF-β, and IL-12, can promote the expression of OBs and inhibit the activity of OCs by regulating the expression of osteogenic markers such as RUNX2, ALP ligand, and collagen1A1, and adjusting the OPG/RANKL signaling pathway[103,106]. Chemokines, such as CXCL3, CXCL6, and CXCL14, are important mediators promoting osteogenic differentiation and bone integration of MSCs. These chemokines regulate pathways downstream of cytoskeletal actin in MSCs and promote the expression of distal-less homeobox 5 and RUNX2 by activating CXCR2 and CCR1[30]. On the other hand, macrophages induce MSCs to release osteogenic factors, such as BMP-2, activating signal molecules drosophila mothers against decapentaplegic homologue in OBs to promote the osteogenic function of MSCs[111]. These studies suggest that MSCs and macrophages play an important role in bone tissue repair (Figure 3)[30,31,43,81,88,93,100].
The multilineage differentiation potential of MSCs and their susceptibility to microenvironmental regulation make their roles in bone tissue less clear. Modulating immune cells to enhance the osteogenic effects of MSCs is emerging as a new method to address current challenges in this field. Co-transplantation of macrophages and MSCs may be a potential effective treatment for bone repair. Current research attempts to exploit the characteristics of mutual regulation and microenvironment sensitivity between MSCs and macrophages, exploring their potential applications in acute or chronic bone tissue injuries such as fractures and osteoarthritis. Co-culture of MSCs with macrophages can enhance the immunomodulatory and osteogenic ability of MSCs. Some studies have found that preconditioning can further enhance the regulatory ability of MSCs on macrophages. For example, Philipp et al[29] discovered that the immunosuppressive effect of MSCs mediated by IFN-γ and IL-1β pretreatment mainly involved the active component IL-6, which stimulates the polarization of macrophages toward the M2 phenotype, promoting the expression of CD86, inducible nitric oxide synthase protein, and IL-10 secretion by macrophages, thus significantly enhancing the immune regulatory capabilities of MSCs. Furthermore, conditions such as hypoxia, lipopolysaccharide treatment, and heat shock pretreatment can enhance the impacts of macrophages on the immune regulatory capacity and osteogenic activity of MSCs, although these treatments may have some inhibitory effects on MSC survival[112]. The depletion of macrophages can lead to a decrease in MSCs, and the use of IL-13 and IL-4 to induce an increase in the number of M2 macrophages can promote MSC osteogenic differentiation and accelerate bone repair[113,114].
Methods of effectively regulating the crosstalk between MSCs and macrophages through biomaterial scaffolds to promote bone healing are becoming a new research focus. The implantation of living cells wrapped in 3D-bioprinted scaffolds can effectively improve the inflammatory microenvironment within the body, further accelerating bone repair. Yu et al[115] discovered that implanting a dual-channel bioprinting scaffold, formed by wrapping macrophages with 8% methacrylamidated gelatin/1% methacrylamidated hyaluronic acid and wrapping MSCs with 3% alginate/0.5 mg/mL graphene oxide, into model rat calvarial defects, can effectively promote the polarization of M2 macrophages in the early bone defect environment, avoid excessive inflammatory responses, and further promote bone repair. The results indicated that this method is more effective than using single-cell MSC scaffolds[115]. Furthermore, some studies have chosen to use special coatings to modify implanted scaffolds or hydrogels, etc., to regulate the biomaterial scaffolds to activate macrophages in the body and promote the differentiation of osteogenic phenotypes. For example, Majrashi et al[116] implanted dexamethasone, which has both anti-inflammatory and osteogenic differentiation promoting effects, into 3D scaffolds. According to the principle that early inflammation benefits MSC-based osteogenesis and that excessive inflammatory responses harm this process, they used excipient-sucrose acetate isobutyrate to achieve sustained release of dexamethasone, regulate the activation of macrophage phenotypes, and control the inflammatory microenvironment within the tissue to promote tissue regeneration. In the early stage of bone tissue injury repair, M1 macrophages can secrete IL-6 to promote osteogenesis. As inflammation progresses, dexamethasone can promote the polarization of M2 macrophages and enhance the osteogenic effect of MSCs by jointly secreting BMP-2 with M2 macrophages[116]. Niu et al[117] wrapped hydrogels containing MSCs with a unique macrophage-affinitive glucomannan polysaccharide and found that, after implantation into mice, it effectively promoted the adherence and activation of macrophages, and induced the release of osteogenic genes in MSCs, thereby effectively promoting bone tissue regeneration[117]. In addition, numerous studies are currently innovating biomaterial compositions for bone tissue engineering, which will provide a foundation for enhancing the application of MSCs and macrophages in bone tissue repair and remodeling[118-121].
A comprehensive understanding of the roles of MSCs and macrophages in immunomodulation and bone regeneration is beneficial for the optimal application of cellular therapy in tissue repair and regeneration. We here discussed the important roles of both MSCs and macrophages in the immune microenvironment of bone injury and bone tissue regeneration by summarizing their mutual regulatory interactions. However, the regulatory mechanisms at play between MSCs and macrophages are complex, and the repair and regeneration of bone tissue is a complicated, dynamic process. Our research and discussion are not comprehensive, and with further study, new breakthroughs in their interactive mechanisms may emerge. Furthermore, through further exploration of potential therapeutic models based on the crosstalk between MSCs and macrophages, we have described that special pretreatment of MSCs and macrophages, or the combined use of new biological material carriers (such as hydrogels and 3D scaffolds) for the MSC and macrophage co-transplantation treatment model, may represent effective therapies that can promote the immunomodulatory capacity of MSCs or endogenous tissue regeneration. This approach could provide new methods and strategies for the repair and regeneration of clinical bone defects or other soft tissue injuries. However, this field is currently mostly in the basic research stage, and the efficacy and safety of these approaches will require verification in future.
I would like to thank all people who helped me in the process of writing this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gutiérrez-Cuevas J, Mexico S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2865] [Cited by in RCA: 2961] [Article Influence: 329.0] [Reference Citation Analysis (0)] |
| 2. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1059] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 3. | Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 825] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 4. | Liu H, Yang R, Zhao S, Zhou F, Liu Y, Zhou Z, Chen L, Xie J. Collagen scaffolds derived from bovine skin loaded with MSC optimized M1 macrophages remodeling and chronic diabetic wounds healing. Bioeng Transl Med. 2023;8:e10467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 5. | Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 465] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 7. | Yuan Y, Ni S, Zhuge A, Li L, Li B. Adipose-Derived Mesenchymal Stem Cells Reprogram M1 Macrophage Metabolism via PHD2/HIF-1α Pathway in Colitis Mice. Front Immunol. 2022;13:859806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Cao X, Duan L, Hou H, Liu Y, Chen S, Zhang S, Wang C, Qi X, Liu N, Han Z, Zhang D, Han ZC, Guo Z, Zhao Q, Li Z. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE(2)-mediated M2 macrophage polarization. Theranostics. 2020;10:7697-7709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci Rep. 2016;6:38308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 12. | Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3473] [Article Influence: 289.4] [Reference Citation Analysis (0)] |
| 13. | Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2080] [Article Influence: 231.1] [Reference Citation Analysis (0)] |
| 15. | Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1127] [Cited by in RCA: 1078] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 16. | Rodríguez-Morales P, Franklin RA. Macrophage phenotypes and functions: resolving inflammation and restoring homeostasis. Trends Immunol. 2023;44:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 17. | Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 18. | Pirraco RP, Reis RL, Marques AP. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med. 2013;7:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, Schwarzenberger P, van den Berg WB, Lubberts E. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005;52:3239-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519-4532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Zhu S, Liu M, Bennett S, Wang Z, Pfleger KDG, Xu J. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J Cell Physiol. 2021;236:7211-7222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1571] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 23. | Saldaña L, Vallés G, Bensiamar F, Mancebo FJ, García-Rey E, Vilaboa N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci Rep. 2017;7:14618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J, Chen J, Yang Y, Xie J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Shi MM, Zhu YG, Yan JY, Rouby JJ, Summah H, Monsel A, Qu JM. Role of miR-466 in mesenchymal stromal cell derived extracellular vesicles treating inoculation pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Clin Transl Med. 2021;11:e287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, Becchetti F, Andaloro A, Moretta L, Bottino C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 2016;34:1909-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 27. | Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D, Du M. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 28. | Hou L, Zhu Z, Jiang F, Zhao J, Jia Q, Jiang Q, Wang H, Xue W, Wang Y, Tian L. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circPWWP2A/miR-223-3p/NLRP3 axis. Ecotoxicol Environ Saf. 2023;251:114537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 29. | Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Wei J, Yu X, Chen L, Ren R, Dong Y, Wang S, Zhu M, Ming N, Zhu Z, Gao C, Xiong W. CXCL chemokines-mediated communication between macrophages and BMSCs on titanium surface promotes osteogenesis via the actin cytoskeleton pathway. Mater Today Bio. 2023;23:100816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Liu J, Lai X, Bao Y, Xie W, Li Z, Chen J, Li G, Wang T, Huang W, Ma Y, Shi J, Zhao E, Xiang AP, Liu Q, Chen X. Intraperitoneally Delivered Mesenchymal Stem Cells Alleviate Experimental Colitis Through THBS1-Mediated Induction of IL-10-Competent Regulatory B Cells. Front Immunol. 2022;13:853894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 33. | Hengartner NE, Fiedler J, Schrezenmeier H, Huber-Lang M, Brenner RE. Crucial role of IL1beta and C3a in the in vitro-response of multipotent mesenchymal stromal cells to inflammatory mediators of polytrauma. PLoS One. 2015;10:e0116772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Yu D, Zhao Y, Wang H, Kong D, Jin W, Hu Y, Qin Y, Zhang B, Li X, Hao J, Li G. IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis. Stem Cell Res Ther. 2021;12:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 36. | Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-6440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 3273] [Article Influence: 467.6] [Reference Citation Analysis (0)] |
| 37. | Jiang Z, Zhang J. Mesenchymal stem cell-derived exosomes containing miR-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle. 2021;20:993-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 39. | Zhang Y, Böse T, Unger RE, Jansen JA, Kirkpatrick CJ, van den Beucken JJJP. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017;369:273-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 40. | Cao D, Ma F, Ouyang S, Liu Z, Li Y, Wu J. Effects of macrophages and CXCR2 on adipogenic differentiation of bone marrow mesenchymal stem cells. J Cell Physiol. 2019;234:9475-9485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Wang L, Li S, Xiao H, Zhang T, Liu Y, Hu J, Xu D, Lu H. TGF-β1 derived from macrophages contributes to load-induced tendon-bone healing in the murine rotator cuff repair model by promoting chondrogenesis. Bone Joint Res. 2023;12:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Hu T, Xu H, Wang C, Qin H, An Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci Rep. 2018;8:3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Franquesa M, Hoogduijn MJ, Bestard O, Grinyó JM. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol. 2012;3:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 44. | Biswas S, Mandal G, Roy Chowdhury S, Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X, Conejo-Garcia JR, Bhattacharyya A. Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer. J Immunol. 2019;203:3447-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 45. | Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY). 2020;12:25138-25152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 46. | Shinohara I, Tsubosaka M, Toya M, Lee ML, Kushioka J, Murayama M, Gao Q, Li X, Zhang N, Chow SK, Matsumoto T, Kuroda R, Goodman SB. C-C Motif Chemokine Ligand 2 Enhances Macrophage Chemotaxis, Osteogenesis, and Angiogenesis during the Inflammatory Phase of Bone Regeneration. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Toya M, Zhang N, Tsubosaka M, Kushioka J, Gao Q, Li X, Chow SK, Goodman SB. CCL2 promotes osteogenesis by facilitating macrophage migration during acute inflammation. Front Cell Dev Biol. 2023;11:1213641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 48. | Rahimi P, Wang CY, Stashenko P, Lee SK, Lorenzo JA, Graves DT. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology. 1995;136:2752-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, Wu D, Ye Q, Xiang L, Qiu X, Liu S, Deng Z, Zhou J, Shi S, Jin Y. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021;10:e12109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 50. | Yuan Y, Yuan L, Li L, Liu F, Liu J, Chen Y, Cheng J, Lu Y. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39:913-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 51. | Jiang X, Yang J, Lin Y, Liu F, Tao J, Zhang W, Xu J, Zhang M. Extracellular vesicles derived from human ESC-MSCs target macrophage and promote anti-inflammation process, angiogenesis, and functional recovery in ACS-induced severe skeletal muscle injury. Stem Cell Res Ther. 2023;14:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 53. | Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. 2021;170:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 54. | Abe Y, Ochiai D, Sato Y, Kanzaki S, Ikenoue S, Kasuga Y, Tanaka M. Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Lu D, Jiao X, Jiang W, Yang L, Gong Q, Wang X, Wei M, Gong S. Mesenchymal stem cells influence monocyte/macrophage phenotype: Regulatory mode and potential clinical applications. Biomed Pharmacother. 2023;165:115042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 56. | Ghahremani Piraghaj M, Soudi S, Ghanbarian H, Bolandi Z, Namaki S, Hashemi SM. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. 2018;212:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Altemus J, Dadgar N, Li Y, Lightner AL. Adipose tissue-derived mesenchymal stem cells' acellular product extracellular vesicles as a potential therapy for Crohn's disease. J Cell Physiol. 2022;237:3001-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 59. | Wang M, Chen F, Wang J, Chen X, Liang J, Yang X, Zhu X, Fan Y, Zhang X. Calcium phosphate altered the cytokine secretion of macrophages and influenced the homing of mesenchymal stem cells. J Mater Chem B. 2018;6:4765-4774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Yu Y, Wu RX, Gao LN, Xia Y, Tang HN, Chen FM. Stromal cell-derived factor-1-directed bone marrow mesenchymal stem cell migration in response to inflammatory and/or hypoxic stimuli. Cell Adh Migr. 2016;10:342-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 62. | Wang Y, Xu J, Zhang X, Wang C, Huang Y, Dai K. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8:e2715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 63. | Qi Y, Zhu T, Zhang T, Wang X, Li W, Chen D, Meng H, An S. M1 macrophage-derived exosomes transfer miR-222 to induce bone marrow mesenchymal stem cell apoptosis. Lab Invest. 2021;101:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 64. | Chen YC, Lai YS, Hsuuw YD, Chang KT. Withholding of M-CSF Supplement Reprograms Macrophages to M2-Like via Endogenous CSF-1 Activation. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 65. | Lin F, Xue D, Xie T, Pan Z. HMGB1 promotes cellular chemokine synthesis and potentiates mesenchymal stromal cell migration via Rap1 activation. Mol Med Rep. 2016;14:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Lin F, Zhang W, Xue D, Zhu T, Li J, Chen E, Yao X, Pan Z. Signaling pathways involved in the effects of HMGB1 on mesenchymal stem cell migration and osteoblastic differentiation. Int J Mol Med. 2016;37:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Correction to: Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm Bowel Dis. 2023;29:1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Biguetti CC, Cavalla F, Silveira EV, Tabanez AP, Francisconi CF, Taga R, Campanelli AP, Trombone APF, Rodrigues DC, Garlet GP. HGMB1 and RAGE as Essential Components of Ti Osseointegration Process in Mice. Front Immunol. 2019;10:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 70. | Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol Med. 2017;23:831-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 71. | Kang JY, Oh MK, Joo H, Park HS, Chae DH, Kim J, Lee HR, Oh IH, Yu KR. Xeno-Free Condition Enhances Therapeutic Functions of Human Wharton's Jelly-Derived Mesenchymal Stem Cells against Experimental Colitis by Upregulated Indoleamine 2,3-Dioxygenase Activity. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Teissier V, Gao Q, Shen H, Li J, Li X, Huang EE, Kushioka J, Toya M, Tsubosaka M, Hirata H, Alizadeh HV, Maduka CV, Contag CH, Yang YP, Zhang N, Goodman SB. Metabolic profile of mesenchymal stromal cells and macrophages in the presence of polyethylene particles in a 3D model. Stem Cell Res Ther. 2023;14:99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, Richards CD, Chevalier S, Rédini F, Heymann D, Gascan H, Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 74. | Schulert GS, Fall N, Harley JB, Shen N, Lovell DJ, Thornton S, Grom AA. Monocyte MicroRNA Expression in Active Systemic Juvenile Idiopathic Arthritis Implicates MicroRNA-125a-5p in Polarized Monocyte Phenotypes. Arthritis Rheumatol. 2016;68:2300-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Xu J, Kong L, Oliver BA, Li B, Creasey EA, Guzman G, Schenone M, Carey KL, Carr SA, Graham DB, Deguine J, Xavier RJ. Constitutively active autophagy in macrophages dampens inflammation through metabolic and post-transcriptional regulation of cytokine production. Cell Rep. 2023;42:112708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 76. | Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 518] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 77. | Cooke JP. Inflammation and Its Role in Regeneration and Repair. Circ Res. 2019;124:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 78. | Granulocyte-macrophage colony-stimulation factor and infection. Infection. 1992;20 Suppl 2:S79-134. [PubMed] |
| 79. | Deng Z, Ren Y, Park MS, Kim HKW. Damage associated molecular patterns in necrotic femoral head inhibit osteogenesis and promote fibrogenesis of mesenchymal stem cells. Bone. 2022;154:116215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Li Y, Dong Y, Ran Y, Zhang Y, Wu B, Xie J, Cao Y, Mo M, Li S, Deng H, Hao W, Yu S, Wu Y. Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res Ther. 2021;12:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, Yamato H, Yotsumoto K, Tanaka U, Taketomi T, Uchiumi T, Le AD, Shi S, Nishimura F. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 82. | Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366-47372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 83. | Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, Mylvaganam S, Grynpas M, Alman BA. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30:1090-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 84. | Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston A, Chiovitti J, Keating A, Kapoor M, Ogilvie-Harris DJ, Syed KA, Gandhi R, Mahomed NN, Marshall KW, Sussman MS, Naraghi AM, Viswanathan S. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl Med. 2019;8:746-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 85. | Giri J, Das R, Nylen E, Chinnadurai R, Galipeau J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020;30:1923-1934.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 86. | Siddiqui JA, Partridge NC. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology (Bethesda). 2016;31:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 87. | Hu K, Shang Z, Yang X, Zhang Y, Cao L. Macrophage Polarization and the Regulation of Bone Immunity in Bone Homeostasis. J Inflamm Res. 2023;16:3563-3580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 88. | Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262:56-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 89. | Mun SH, Park PSU, Park-Min KH. The M-CSF receptor in osteoclasts and beyond. Exp Mol Med. 2020;52:1239-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 90. | Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010;42:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 91. | Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1070] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 92. | Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1914] [Cited by in RCA: 2033] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 93. | Huang R, Wang X, Zhou Y, Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Res. 2017;5:17019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 94. | Piprode V, Singh K, Kumar A, Josh SR, Wani MR. IL-3 inhibits rat osteoclast differentiation induced by TNF-α and other pro-osteoclastogenic cytokines. J Biosci. 2021;46. [PubMed] |
| 95. | Fukawa Y, Kayamori K, Tsuchiya M, Ikeda T. IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma. Int J Mol Sci. 2022;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 96. | Zhao JJ, Wu ZF, Yu YH, Wang L, Cheng L. Effects of interleukin-7/interleukin-7 receptor on RANKL-mediated osteoclast differentiation and ovariectomy-induced bone loss by regulating c-Fos/c-Jun pathway. J Cell Physiol. 2018;233:7182-7194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | Ganesan R, Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol. 2017;91:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | Mae M, Alam MI, Yamashita Y, Ozaki Y, Higuchi K, Ziauddin SM, Montenegro Raudales JL, Sakai E, Tsukuba T, Yoshimura A. The Role of Cytokines Produced via the NLRP3 Inflammasome in Mouse Macrophages Stimulated with Dental Calculus in Osteoclastogenesis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Liang M, Ma Q, Ding N, Luo F, Bai Y, Kang F, Gong X, Dong R, Dai J, Dai Q, Dou C, Dong S. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019;10:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 100. | Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 101. | Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1023] [Cited by in RCA: 996] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 102. | Johnson RW, McGregor NE, Brennan HJ, Crimeen-Irwin B, Poulton IJ, Martin TJ, Sims NA. Glycoprotein130 (Gp130)/interleukin-6 (IL-6) signalling in osteoclasts promotes bone formation in periosteal and trabecular bone. Bone. 2015;81:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Chen S, Liang H, Ji Y, Kou H, Zhang C, Shang G, Shang C, Song Z, Yang L, Liu L, Wang Y, Liu H. Curcumin Modulates the Crosstalk Between Macrophages and Bone Mesenchymal Stem Cells to Ameliorate Osteogenesis. Front Cell Dev Biol. 2021;9:634650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 104. | Kamel Mohamed SG, Sugiyama E, Shinoda K, Hounoki H, Taki H, Maruyama M, Miyahara T, Kobayashi M. Interleukin-4 inhibits RANKL-induced expression of NFATc1 and c-Fos: a possible mechanism for downregulation of osteoclastogenesis. Biochem Biophys Res Commun. 2005;329:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Chen H, Fang C, Zhi X, Song S, Gu Y, Chen X, Cui J, Hu Y, Weng W, Zhou Q, Wang Y, Jiang H, Li X, Cao L, Su J. Neobavaisoflavone inhibits osteoclastogenesis through blocking RANKL signalling-mediated TRAF6 and c-Src recruitment and NF-κB, MAPK and Akt pathways. J Cell Mol Med. 2020;24:9067-9084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Vallés G, Bensiamar F, Maestro-Paramio L, García-Rey E, Vilaboa N, Saldaña L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res Ther. 2020;11:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Guihard P, Boutet MA, Brounais-Le Royer B, Gamblin AL, Amiaud J, Renaud A, Berreur M, Rédini F, Heymann D, Layrolle P, Blanchard F. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am J Pathol. 2015;185:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 108. | Choe JY, Park KY, Park SH, Lee SI, Kim SK. Regulatory effect of calcineurin inhibitor, tacrolimus, on IL-6/sIL-6R-mediated RANKL expression through JAK2-STAT3-SOCS3 signaling pathway in fibroblast-like synoviocytes. Arthritis Res Ther. 2013;15:R26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Li CH, Xu LL, Jian LL, Yu RH, Zhao JX, Sun L, Du GH, Liu XY. Stattic inhibits RANKL-mediated osteoclastogenesis by suppressing activation of STAT3 and NF-κB pathways. Int Immunopharmacol. 2018;58:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 110. | Hu L, Liu R, Zhang L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int Immunopharmacol. 2022;111:109095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 111. | Wu S, Ma J, Liu J, Liu C, Ni S, Dai T, Wang Y, Weng Y, Zhao H, Zhou D, Zhao X. Immunomodulation of Telmisartan-Loaded PCL/PVP Scaffolds on Macrophages Promotes Endogenous Bone Regeneration. ACS Appl Mater Interfaces. 2022;14:15942-15955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 112. | Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 113. | Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 429] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 114. | Kozicky LK, Sly LM. Depletion and Reconstitution of Macrophages in Mice. Methods Mol Biol. 2019;1960:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | Yu K, Huangfu H, Qin Q, Zhang Y, Gu X, Liu X, Zhou Y. Application of Bone Marrow-Derived Macrophages Combined with Bone Mesenchymal Stem Cells in Dual-Channel Three-Dimensional Bioprinting Scaffolds for Early Immune Regulation and Osteogenic Induction in Rat Calvarial Defects. ACS Appl Mater Interfaces. 2022;14:47052-47065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 116. | Majrashi M, Kotowska A, Scurr D, Hicks JM, Ghaemmaghami A, Yang J. Sustained Release of Dexamethasone from 3D-Printed Scaffolds Modulates Macrophage Activation and Enhances Osteogenic Differentiation. ACS Appl Mater Interfaces. 2023;15:56623-56638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 117. | Niu Y, Li Q, Xie R, Liu S, Wang R, Xing P, Shi Y, Wang Y, Dong L, Wang C. Modulating the phenotype of host macrophages to enhance osteogenesis in MSC-laden hydrogels: Design of a glucomannan coating material. Biomaterials. 2017;139:39-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 118. | Demarquay C, Moussa L, Réthoré G, Milliat F, Weiss P, Mathieu N. Embedding MSCs in Si-HPMC hydrogel decreased MSC-directed host immune response and increased the regenerative potential of macrophages. Regen Biomater. 2022;9:rbac022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 119. | Nativel F, Smith A, Boulestreau J, Lépine C, Baron J, Marquis M, Vignes C, Le Guennec Y, Veziers J, Lesoeur J, Loll F, Halgand B, Renard D, Abadie J, Legoff B, Blanchard F, Gauthier O, Vinatier C, Rieux AD, Guicheux J, Le Visage C. Micromolding-based encapsulation of mesenchymal stromal cells in alginate for intraarticular injection in osteoarthritis. Mater Today Bio. 2023;19:100581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 120. | Doron G, Wood LB, Guldberg RE, Temenoff JS. Poly(ethylene glycol)-Based Hydrogel Microcarriers Alter Secretory Activity of Genetically Modified Mesenchymal Stromal Cells. ACS Biomater Sci Eng. 2023;9:6282-6292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 121. | Chen Z, Wu C, Gu W, Klein T, Crawford R, Xiao Y. Osteogenic differentiation of bone marrow MSCs by β-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35:1507-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |