Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.58
Peer-review started: November 23, 2023
First decision: December 17, 2023
Revised: December 27, 2023
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: February 26, 2024
Processing time: 95 Days and 0.8 Hours
In this editorial, we offer our perspective on the groundbreaking study entitled “Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells”, recently published in World Journal of Stem Cells. Despite over three decades of research on the clinical application of mesenchymal stem cells (MSCs), only a few therapeutic products have made it to clinical use, due to multiple preclinical and clinical challenges yet to be addressed. The study proved the hypoxia and inflammatory factor preconditioning led to higher immunosuppressive effects of MSCs without damaging their biological characteristics, which revealed the combination of inflammatory factors and hypoxic preconditioning offers a promising approach to enhance the function of MSCs. As we delve deeper into the intricacies of pretreat
Core Tip: We offer our perspective on the groundbreaking study titled “Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells”, and recently published in the World Journal of Stem Cells.
- Citation: Wan XX, Hu XM, Xiong K. Multiple pretreatments can effectively improve the functionality of mesenchymal stem cells. World J Stem Cells 2024; 16(2): 58-63
- URL: https://www.wjgnet.com/1948-0210/full/v16/i2/58.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i2.58
Stem cells (SCs) are characterized by multipotency, asymmetric division and having the capacity of self-renewal[1-3]. SCs can directly or indirectly stimulate resident cells, regulate inflammation, release biological molecules and remodel extracellular matrix (ECM) to benefit various diseases[4-8]. SC can be classified into totipotent, pluripotent and monopotent SCs according the different differentiation potentials. There is now a consensus that the clinical use of SC holds great promise for the treatment of a wide range of intractable diseases. As a result, there are various clinical trials to explore the clinical use of SC in many diseases. Mesenchymal SCs (MSCs) are multipotent stromal cells, which have good self-renewal and pluripotent differentiation potentials. The potential for MSCs as a cell-based therapy in treating moderate immunologic disorders and regeneration has been well clarified, and MSCs are the most chosen SCs in clinical treatment[9]. MSCs can be derived from various tissues, including bone marrow, adipose tissue, Wharton’s Jell, dental pulp, menstrual blood and umbilical cord blood gleaned efficiently[10,11]. Although MSCs derived from different tissues share the same basic functional characteristics, there are still differences in their functional strengths, such as cell size, proliferative potential, secreted cytokines, and immunosuppression. MSCs can change the micro-environment in the tissues and increase cell differentiation and regeneration ability, the function of MSCs in the treatment of immunologic disorders has been well established. So, MSCs are used clinically to try to treat many diseases including vascular diseases, immune diseases and wounds.
MSCs have emerged as a prominent cell source for SC therapy, owing to their renewable nature, immunomodulatory properties, minimal risk of tumorigenesis and lack of ethical constraints[12]. Many subtypes of MSCs, such as adipose-derived MSCs, human umbilical MSCs (HUMSCs), and bone marrow MSCs are eligible for clinical use, without significant functional abnormalities or side effects[11,13,14]. MSCs are primary plastic adherent cells with immense proliferative potential and the ability to self-renew and differentiate. These cells can be easily cultured in vitro, and MSCs do not express CD45, CD34, CD14, CD11b, CD79a, CD19, or major histocompatibility complex (MHC) class II, which can curtail immune rejection and facilitate clinical transplantation of MSCs[15-17]. MSCs are widely employed in traumatic tissue repair, immune disorders, and cancer therapy. The proliferative capacity and immunogenicity of MSCs from different human populations vary greatly, and the osteogenic potential of MSCs derived from diabetic patients is decreased than health volunteers[18], while MSCs isolated from patients with autoimmune diseases have morphological and some functional abnormalities[19], which implies that the normal MSCs transplants may restore the MSCs function and alleviate the disease.
The clinical application of MSCs has been conducted for more than thirty years[15,20], and there are thousands of clinical trials are currently being conducted (http://clinicaltrials.gov). However, only a few MSC-based therapeutic products have been approved for clinical use. There are still many key preclinical and clinical challenges to be solved, which are mainly in the areas of cell preparation methods, consistency, efficiency, reproducibility, processing time, scalability and purity of MSCs. So future investigations into the pretreatment of MSCs have the potential to yield improved outcomes and facilitate the implementation of clinical MSCs.
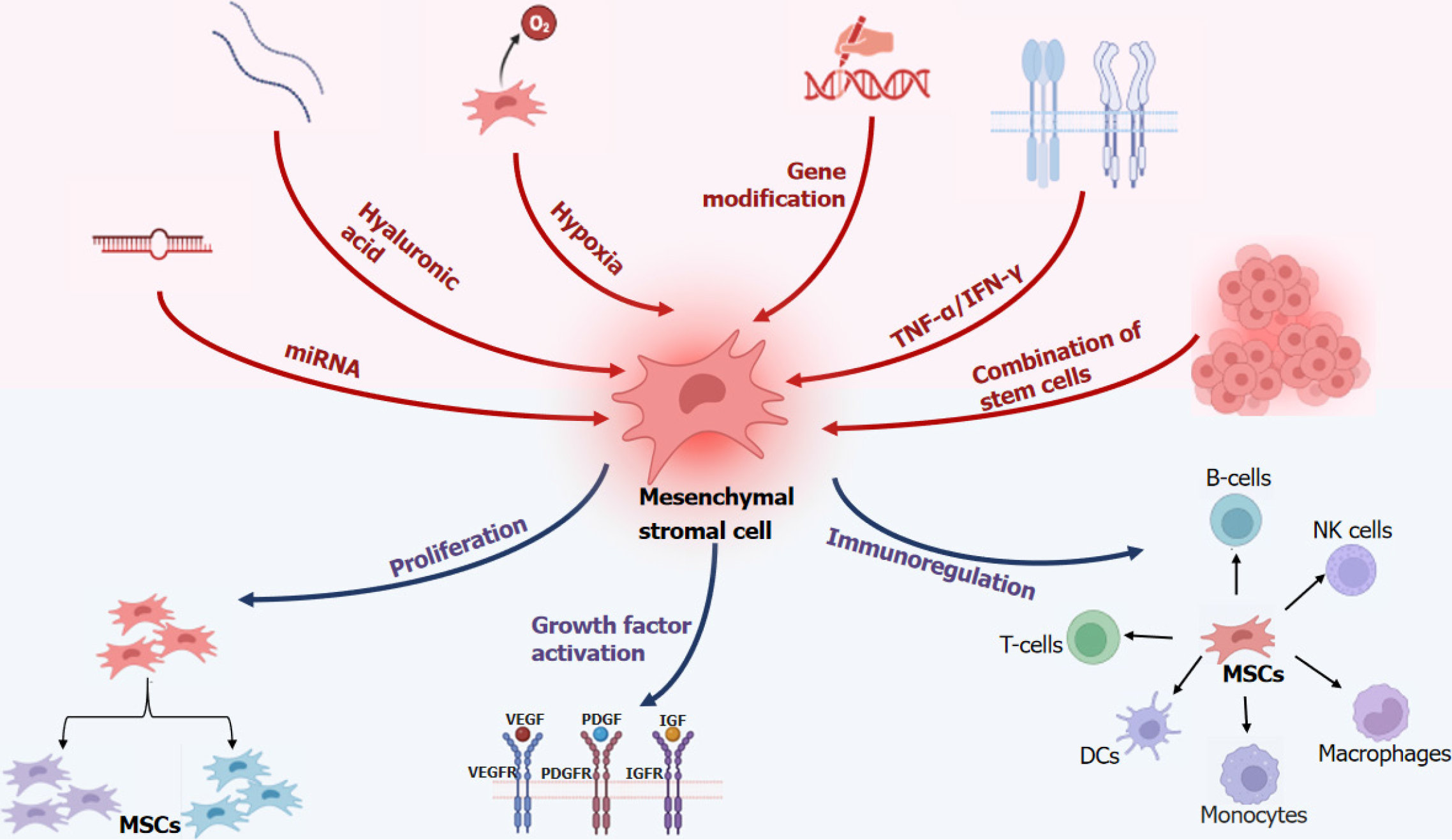
MSCs release a complex array of active ingredients that are influenced by the host microenvironment (inflammatory state, hypoxia, and ECM), resulting in highly variable factors that shape their distinct functions[15,21]. To achieve superior therapeutic effects in clinical applications, researchers have made advancements in treating MSCs using various methods, including genetic engineering, SC conjugation, drug or cytokine interventions, and so on. For examples, pretreatment of 3-methyladenin could inhibit autophagy in MSCs[22]; platelet derived growth factor BB (PDGF-BB) pretreatment could promote MSC migration and inhibit hydrogen peroxide induced MSC apoptosis via PI3K/Akt pathway[23]. The MSCs extracted from nlrp3-KO mice promoted osteogenic differentiation without affecting the phenotypes of MSC[24]. In the diabetic retinopathy rat model, the retinal vessel formation, retinal function and uveitis was found improved when transplanted with tacrolimus-pretreated MSCs[25]. These innovations provide new and exciting options for the clinical application of MSCs (Figure 1).
Previously, our laboratory attempted various methods to enhance the function of MSCs. One possible approach would be the overexpression of the c-jun plasmid in HUMSCs to expedite wound healing by amplifying PDGFA and hepatocyte growth factor levels in diabetic rats’ wound tissues, thereby fostering angiogenesis and re-epithelialization at the wound bed[26]. Another possible solution would be the coating MSCs with ECM, which we demonstrated, could promote wound healing in diabetic rats by stimulating vascular endothelial growth factor-α, PDGF, and epidermal growth factor expression[27]. Additionally, we compared the healing rates of multiple separate wounds in diabetic foot patients injected with MSCs combined with endothelial colony-forming cells (ECFCs) and hyaluronic acid. The self-controlled wounds were only treated with conventional therapy and covered by hydrocolloid dressings, and our findings demonstrated that the combination of umbilical cord MSCs, ECFCs, and hyaluronic acid can safely accelerate the healing of refractory diabetic foot ulcers[28].
Now it has been demonstrated that laser pretreatment can also enhance the function of MSCs. Chen et al[29] proved low-level controllable blue light emitting diodes irradiation can enhance the activity of intracellular calcium levels to enhance the osteogenic differentiation of MSCs derived from human dental pulp. Wen et al[30] encapsulated MSCs in the Prussian blue nanoparticles and methacrylated gelatin hydrogels, and MSCs complex was exposed to a 1.0 W/cm2 of 808 nm laser for 10 min for the implantation. The laser pre-treatment could improve the viability of MSCs and accelerate the regeneration of muscle. Wang et al[31] found low-level laser irradiation treatment of HUMSCs could significantly increase the erythrocyte count and number of myelopoiesis clones. All these results indicate that laser pretreatment could be a new strategy to further enhance the clinical use of MSCs.
Generally, there is no academic consensus on the outcomes of hypoxic intervention in MSCs. Numerous studies have shown that MSCs cultured continuously under low oxygen (1%-5%) conditions typically exhibit enhanced proliferative potential and well-maintained stemness of MSCs[32,33]. Hypoxia significantly induces MSCs to secrete angiogenic and anti-inflammatory cytokines in large amounts and enhances the migratory capacity of MSCs[32]. However, a number of experiments have also demonstrated that the apoptosis rate of MSCs increased in hypoxic environments, suggesting that the effect of hypoxia on MSCs needs further investigation[34]. Interferon-γ (IFN-γ) enhances the anti-inflammatory and therapeutic fibrotic properties of MSCs and promotes their survival[35]; theoretically, the combination of IFN-γ and hypoxic culture could enhance the therapeutic function of MSCs.
The immunocompatibility between donors and recipients is a key factor in reducing the risk of immune rejection, but it is influenced by environmental inflammatory molecules, which can induce a significant expression of MHC-II in MSCs. Further reducing the immunogenicity of MSCs is another important way to improve the efficacy of MSCs. It’s evident that IFN-γ and IFN-γ/transforming growth factor β1 licensing enhance the immunomodulatory effect of MSCs on T cell proliferation[35]. Tumor necrosis factor-α preconditioning can improve the therapeutic efficacy of MSCs in atherosclerosis[36]. In the study of “Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord MSCs”, the researchers pretreated UC-MSCs with hypoxia (2% O2) exposure and inflammatory factors (interleukin-1β, tumor necrosis factor-α, IFN-γ)[37]. After 24 h of exposure, they found that the pretreatment caused UC-MSCs to become elongated without affecting viability, proliferation, or size. Additionally, they demonstrated that this pretreatment enhanced the expression of genes and proteins related to immune regulation and increased peripheral blood mononuclear cell and natural killer (NK) cell proliferation rates while inhibiting NK cell-induced toxicity to varying degrees. With detailed experimental data, this article further revealed that hypoxia combined with inflammatory factor preconditioning can enhance the function of MSCs, providing a new scheme for the clinical application of MSCs.
The combination of inflammatory factors and hypoxic preconditioning is an effective approach to enhance the function of MSCs. The concentration of hypoxia, the type and concentration of inflammatory factors, and the duration of preconditioning have a significant impact on MSC’s function. In-depth investigations in this domain will yield improved outcomes and facilitate the utilization of clinical MSCs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran; Priya A, India S-Editor: Wang JJ L-Editor: A P-Editor: Zhao YQ
| 1. | Tan X, Wang XQ, Zhang C, Zhao XL, Yao H, Chen G, Ma YY, Wen Q, Gao L, Kong PY, Shen Y, Zhang X, Lou SF. Donor-derived CD19 CAR-T Cells versus Chemotherapy Plus Donor Lymphocyte Infusion for Treatment of Recurrent CD19-positive B-ALL After Allogeneic Hematopoietic Stem Cell Transplantation. Curr Med Sci. 2023;43:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Zhang Q, Wan XX, Hu XM, Zhao WJ, Ban XX, Huang YX, Yan WT, Xiong K. Targeting Programmed Cell Death to Improve Stem Cell Therapy: Implications for Treating Diabetes and Diabetes-Related Diseases. Front Cell Dev Biol. 2021;9:809656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Hu XM, Zhang Q, Zhou RX, Wu YL, Li ZX, Zhang DY, Yang YC, Yang RH, Hu YJ, Xiong K. Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications. World J Stem Cells. 2021;13:386-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Jokela TA, LaBarge MA. Integration of mechanical and ECM microenvironment signals in the determination of cancer stem cell states. Curr Stem Cell Rep. 2021;7:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Yang M, Cui Y, Song J, Cui C, Wang L, Liang K, Wang C, Sha S, He Q, Hu H, Guo X, Zang N, Sun L, Chen L. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem Biophys Res Commun. 2021;546:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Zhang Q, Pan RR, Wu YT, Wei YM. MicroRNA-146a Promotes Embryonic Stem Cell Differentiation towards Vascular Smooth Muscle Cells through Regulation of Kruppel-like Factor 4. Curr Med Sci. 2023;43:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Wan XX, Zhang DY, Khan MA, Zheng SY, Hu XM, Zhang Q, Yang RH, Xiong K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front Endocrinol (Lausanne). 2022;13:859638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Yang R, Yang S, Zhao J, Hu X, Chen X, Wang J, Xie J, Xiong K. Progress in studies of epidermal stem cells and their application in skin tissue engineering. Stem Cell Res Ther. 2020;11:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Cao TT, Chen H, Pang M, Xu SS, Wen HQ, Liu B, Rong LM, Li MM. Dose optimization of intrathecal administration of human umbilical cord mesenchymal stem cells for the treatment of subacute incomplete spinal cord injury. Neural Regen Res. 2022;17:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | de Castro LL, Lopes-Pacheco M, Weiss DJ, Cruz FF, Rocco PRM. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J Mol Med (Berl). 2019;97:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Liu J, Wan XX, Zheng SY, He HH, Khan MA, Feng YX, Xiao JG, Chen Y, Hu XM, Zhang Q, Xiong K. Mesenchymal Stem Cell Transplantation in Type 1 Diabetes Treatment: Current Advances and Future Opportunity. Curr Stem Cell Res Ther. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | de Pedro MÁ, López E, González-Nuño FM, Pulido M, Álvarez V, Marchena AM, Preußer C, Szymański W, Pogge von Strandmann E, Graumann J, Sánchez-Margallo FM, Casado JG, Gómez-Serrano M. Menstrual blood-derived mesenchymal stromal cells: impact of preconditioning on the cargo of extracellular vesicles as potential therapeutics. Stem Cell Res Ther. 2023;14:187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Yu S, Yu S, Liu H, Liao N, Liu X. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. 2023;14:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Paganelli A, Rossi E, Magnoni C. The Dark Side of Adipose-Derived Mesenchymal Stromal Cells in Cutaneous Oncology: Roles, Expectations, and Potential Pitfalls. Stem Cells Dev. 2022;31:593-603. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Moravcikova E, Meyer EM, Corselli M, Donnenberg VS, Donnenberg AD. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytometry A. 2018;93:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Uder C, Brückner S, Winkler S, Tautenhahn HM, Christ B. Mammalian MSC from selected species: Features and applications. Cytometry A. 2018;93:32-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Wang G, Wu HL, Liu YP, Yan DQ, Yuan ZL, Chen L, Yang Q, Gao YS, Diao B. Pre-clinical study of human umbilical cord mesenchymal stem cell transplantation for the treatment of traumatic brain injury: safety evaluation from immunogenic and oncogenic perspectives. Neural Regen Res. 2022;17:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Xia SL, Ma ZY, Wang B, Gao F, Guo SY, Chen XH. A gene expression profile for the lower osteogenic potent of bone-derived MSCs from osteoporosis with T2DM and the potential mechanism. J Orthop Surg Res. 2022;17:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Wu F, She Z, Li C, Mao J, Luo S, Chen X, Tian J, Wen C. Therapeutic potential of MSCs and MSC-derived extracellular vesicles in immune thrombocytopenia. Stem Cell Res Ther. 2023;14:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Lu B, Lerman LO. MSC therapy for diabetic kidney disease and nephrotic syndrome. Nat Rev Nephrol. 2023;19:754-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Du HC, Jiang L, Geng WX, Li J, Zhang R, Dang JG, Shu MG, Li LW. Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity. Biomed Res Int. 2017;2017:2578017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Cen S, Wang P, Xie Z, Yang R, Li J, Liu Z, Wang S, Wu X, Liu W, Li M, Tang S, Shen H, Wu Y. Autophagy enhances mesenchymal stem cell-mediated CD4(+) T cell migration and differentiation through CXCL8 and TGF-β1. Stem Cell Res Ther. 2019;10:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Sun Z, Cai Y, Chen Y, Jin Q, Zhang Z, Zhang L, Li Y, Huang L, Wang J, Yang Y, Lv Q, Han Z, Xie M, Zhu X. Ultrasound-targeted microbubble destruction promotes PDGF-primed bone mesenchymal stem cell transplantation for myocardial protection in acute Myocardial Infarction in rats. J Nanobiotechnology. 2023;21:481. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Chen J, Xie S, Qiu D, Xie M, Wu M, Li X, Zhang X, Wu Q, Xiong Y, Wu C, Ren J, Peng Y. The NLRP3 molecule influences the therapeutic effects of mesenchymal stem cells through Glut1-mediated energy metabolic reprogramming. J Adv Res. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Jo HH, Goh YS, Kim HJ, Kim DH, Kim H, Hwang J, Jung JS, Kang N, Park SE, Park KM, Lee HJ. Tacrolimus Improves Therapeutic Efficacy of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Diabetic Retinopathy by Suppressing DRP1-Mediated Mitochondrial Fission. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z. c-Jun Overexpression Accelerates Wound Healing in Diabetic Rats by Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020;2020:7430968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Wang L, Wang F, Zhao L, Yang W, Wan X, Yue C, Mo Z. Erratum to "Mesenchymal Stem Cells Coated by the Extracellular Matrix Promote Wound Healing in Diabetic Rats". Stem Cells Int. 2019;2019:9581478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Zhao L, Guo Z, Chen K, Yang W, Wan X, Zeng P, He H, Luo Y, Xiao Q, Mo Z. Combined Transplantation of Mesenchymal Stem Cells and Endothelial Colony-Forming Cells Accelerates Refractory Diabetic Foot Ulcer Healing. Stem Cells Int. 2020;2020:8863649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Chen J, Sang Y, Li J, Zhao T, Liu B, Xie S, Sun W. Low-level controllable blue LEDs irradiation enhances human dental pulp stem cells osteogenic differentiation via transient receptor potential vanilloid 1. J Photochem Photobiol B. 2022;233:112472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Wen J, Zhao Z, Fang F, Xiao J, Wang L, Cheng J, Wu J, Miao Y. Prussian Blue Nanoparticle-Entrapped GelMA Gels Laden with Mesenchymal Stem Cells as Prospective Biomaterials for Pelvic Floor Tissue Repair. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Wang H, Tu WJ, Xiao C, Dong MX, Ye YT, Deng J, Wang Y, Sha H, Liu Q. Nrf2 played an important role in radiation protection effect of low-level laser exposed on umbilical cord mesenchymal stem cell. Tissue Cell. 2020;63:101329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Xiang C, Xie QP. Protection of mouse pancreatic islet function by coculture with hypoxia pretreated mesenchymal stromal cells. Mol Med Rep. 2018;18:2589-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Hwang OK, Noh YW, Hong JT, Lee JW. Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor. Tissue Eng Regen Med. 2020;17:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Xing Y, Hou J, Guo T, Zheng S, Zhou C, Huang H, Chen Y, Sun K, Zhong T, Wang J, Li H, Wang T. microRNA-378 promotes mesenchymal stem cell survival and vascularization under hypoxic-ischemic conditions in vitro. Stem Cell Res Ther. 2014;5:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Wang J, Donohoe E, Canning A, Moosavizadeh S, Buckley F, Brennan MÁ, Ryan AE, Ritter T. Immunomodulatory function of licensed human bone marrow mesenchymal stromal cell-derived apoptotic bodies. Int Immunopharmacol. 2023;125:111096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Sekenova A, Li Y, Issabekova A, Saparov A, Ogay V. TNF-α Preconditioning Improves the Therapeutic Efficacy of Mesenchymal Stem Cells in an Experimental Model of Atherosclerosis. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Li H, Ji XQ, Zhang SM, Bi RH. Hypoxia and inflammatory factor preconditioning enhances the immunosuppressive properties of human umbilical cord mesenchymal stem cells. World J Stem Cells. 2023;15:999-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |