Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.151
Peer-review started: November 5, 2023
First decision: December 5, 2023
Revised: December 20, 2023
Accepted: January 17, 2024
Article in press: January 17, 2024
Published online: February 26, 2024
Processing time: 112 Days and 16.7 Hours
Osteoporosis is a common metabolic bone disorder induced by an imbalance between osteoclastic activity and osteogenic activity. During osteoporosis, bone mesenchymal stem cells (BMSCs) exhibit an increased ability to differentiate into adipocytes and a decreased ability to differentiate into osteoblasts, resulting in bone loss. Jumonji domain-containing 1C (JMJD1C) has been demonstrated to suppress osteoclastogenesis.
To examine the effect of JMJD1C on the osteogenesis of BMSCs and the potential underlying mechanism.
BMSCs were isolated from mouse bone marrow tissues. Oil Red O staining, Alizarin red staining, alkaline phosphatase staining and the expression of adipo
The osteogenic and adipogenic differentiation potential of BMSCs isolated from mouse bone marrow samples was evaluated. JMJD1C mRNA and protein expression was upregulated in BMSCs after osteoblast induction, while p-nuclear factor-κB (NF-κB) and inflammatory cytokines were not significantly altered. Knockdown of JMJD1C repressed osteogenic differentiation and enhanced NF-κB activation and inflammatory cytokine release in BMSCs. Moreover, JMJD1C expression decreased during BMM osteoclast differentiation.
The JMJD1C/NF-κB signaling pathway is potentially involved in BMSC osteogenic differentiation and may play vital roles in the pathogenesis of osteoporosis.
Core Tip: Jumonji domain-containing 1C (JMJD1C) is a marker gene for osteoporosis disease. JMJD1C promotes osteogenic differentiation of bone mesenchymal stem cells (BMSCs). JMJD1C inhibited osteoclast differentiation of bone marrow-derived macrophages. JMJD1C knockdown promotes nuclear factor-κB activation in BMSCs during osteogenic differentiation.
- Citation: Li JY, Wang TT, Ma L, Zhang Y, Zhu D. Silencing of Jumonji domain-containing 1C inhibits the osteogenic differentiation of bone marrow mesenchymal stem cells via nuclear factor-κB signaling. World J Stem Cells 2024; 16(2): 151-162
- URL: https://www.wjgnet.com/1948-0210/full/v16/i2/151.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i2.151
Osteoporosis is a common bone disease worldwide characterized by low bone mineral density that can cause fractures in affected bones[1]. This condition is prevalent among elderly individuals, particularly women of postmenopausal age[2]. Osteoporosis is elicited by the disequilibrium of osteoblastic bone formation and osteoclastic bone resorption[3]. Current therapies for osteoporosis mainly focus on the regulation of bone remodeling; nevertheless, these treatments have certain adverse effects and limitations[4]. Further elucidation of the molecular mechanisms underlying osteoporosis is important for developing effective therapeutic strategies for this disease.
Bone mesenchymal stem cells (BMSCs), progenitor cells that can be isolated from bone marrow, can differentiate into various types of cells, such as adipocytes and osteoblasts[5]. As the progenitor cells of adipocytes and osteoblasts, BMSCs play a critical role in bone homeostasis[6]. Recent methods and current developments in utilizing BMSCs for the repair of bone fractures resulting from osteoporosis have been reported in many studies[7]. Moreover, previous studies have reported that osteoporotic BMSCs exhibit impaired osteogenic differentiation potential[8-10].
Jumonji domain-containing (JMJD) proteins, which have histone lysine demethylase (KDM) activities, constitute a large protein family with more than 30 members[11]. One subfamily of JMJD proteins comprises KDM3A/JMJD1A, KDM3B/JMJD1B and KDM3C/JMJD1C[12]. In oral inflammatory lesions, JMJD1C deficiency results in elevated alveolar bone loss. Moreover, loss of JMJD1C accelerates bone marrow-derived macrophage (BMM) differentiation into osteoclasts in vitro[13]. Database analysis revealed that JMJD1C expression is downregulated in bone marrow stromal cells (BMSCs) from patients with osteoporosis. JMJD1C levels are upregulated in osteogenic induction medium and BMSC growth medium supplemented with modified extracellular matrix[14]. However, whether JMJD1C is involved in osteoblast differentiation of BMSCs during osteoporosis is still unclear.
Nuclear factor-κB (NF-κB) is a transcription factor that modulates the expression of many genes implicated in the immune response and inflammation[15]. Previous findings also revealed that NF-κB is an essential factor that contributes to impaired bone formation during osteoporosis[16]. Moreover, NF-κB suppression improved osteopenia and promoted bone formation in a mouse model of osteoporosis[17]. Moreover, repression of the NF-κB pathway induces osteogenic differentiation in MSCs[18]. Importantly, complete knockout and transient knockdown of JMJD1C led to the activation of the NF-κB subunit p65 in both mouse and human macrophages[13].
Here, this study explored the role of JMJD1C in BMSC osteogenic differentiation and the potential underlying mechanisms. We demonstrated that JMJD1C expression was increased while NF-κB signaling activation was not affected during BMSC osteogenic differentiation. Silencing of JMJD1C suppressed osteogenic differentiation and promoted NF-κB activation in BMSCs. Therefore, this study might lead to the identification of an innovative target for treating osteo
C57BL/6 mice (8 wk old) obtained from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., were housed in pathogen-free facilities under a light/dark cycle of 12/12 h. The animal experiments were approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated with Capital Medical University. Primary BMSCs were isolated as described previously. In brief, after anesthesia, the mice were sacrificed via cervical dislocation. Bone marrow cells were collected from the tibiae and femurs of the mice. The cells were maintained in α-MEM (Gibco, Grand Island, NY, United States) supplemented with 1% penicillin/streptomycin (Gibco) and 10% fetal bovine serum (FBS) (Gibco) in an incubator with 5% CO2 at 37 °C. When the cells reached 80% confluence, they were digested and cultured. Cells (5-9 passages) were collected for the next assays. BMMs from mice were cultured in α-MEM supplemented with macrophage colony-stimulating factor (M-CSF, 5 ng/mL), 1% penicillin/streptomycin and 10% FBS. The BMSCs were randomly divided into five groups: Control (without any treatment), osteoblast induction (maintained in osteogenic induction medium for the indicated time periods), adipocyte induction (cultured in adipogenic induction medium for 14 d), short hairpin RNAs (shRNAs)-NC (transfected with shRNA-NC for 48 h) and shRNA-JMJD1C (transfected with shRNA-JMJD1C for 48 h). In addition, the BMMs were randomly divided into two groups: Control (BMMs without any treatment) and osteoclast induction [BMMs treated with receptor activator of nuclear factor-kappa Β ligand (RANKL) for 7 d] groups.
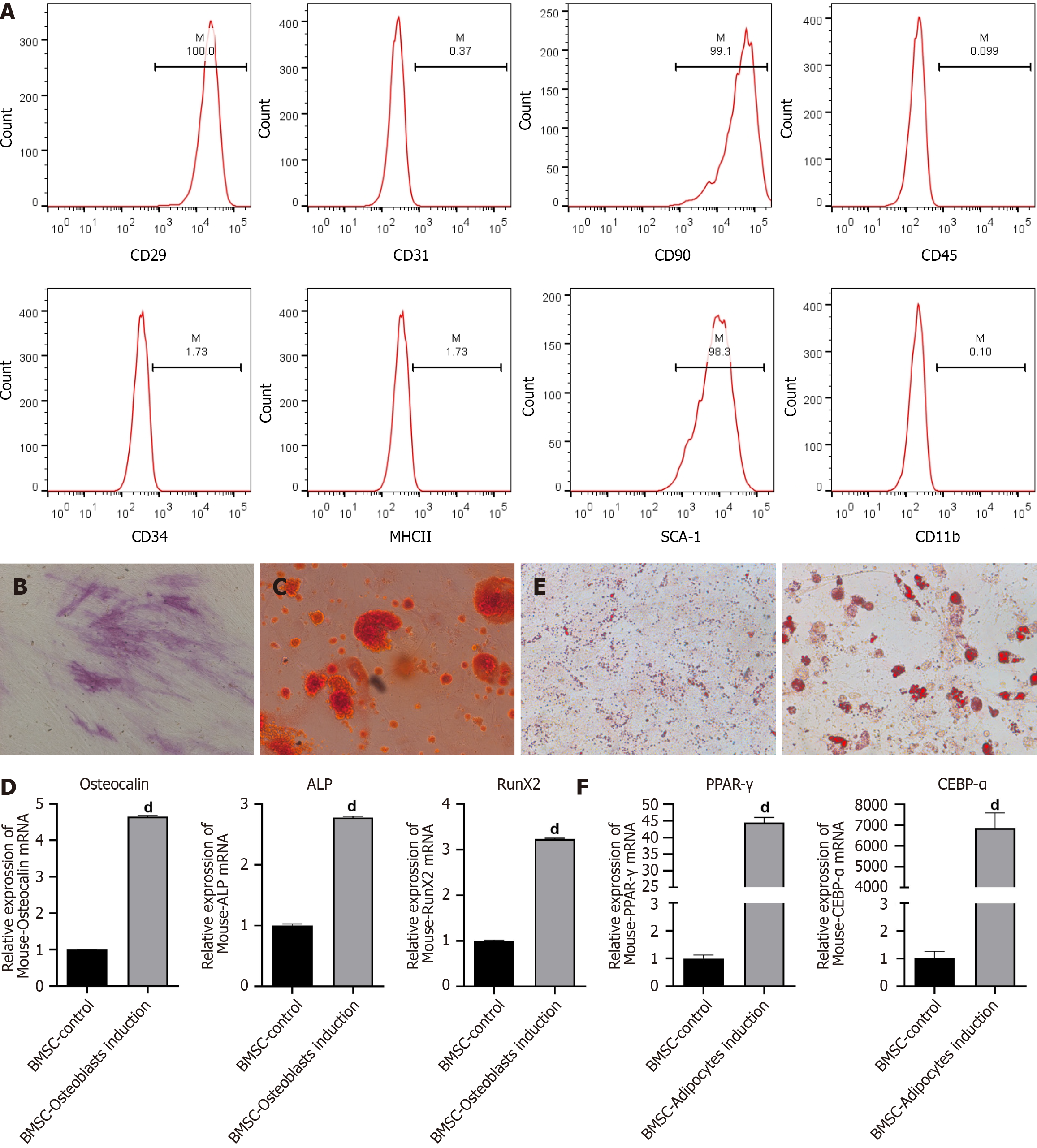
BMSC surface markers were detected via flow cytometry. Briefly, after being rinsed by phosphate buffered saline (PBS), the BMSCs were collected with trypsin. After centrifugation at 1500 rpm, the cells were incubated for 0.5 h with primary antibodies against CD29, CD31, CD34, CD45, CD90, MHCII, SCA-1 and CD11b (Biolegend, California, San Diego, United States and Becton Dickinson, Franklin Lakes, NJ, United States). Afterward, flow cytometry (Becton Dickinson, Franklin Lakes, NJ, United States) was used to assess the cells.
BMSCs were maintained in osteogenic induction medium (α-MEM supplemented with 10-7 M dexamethasone, 10 mmol/L β-glycerophosphate, 0.2 mmol/L ascorbic acid, 1% penicillin/streptomycin and 10% FBS) to induce osteogenic differentiation. The osteogenic induction medium was replaced every 3 d.
In accordance with the manufacturer’s instructions, alkaline phosphatase (ALP) staining of cells was performed using a BCIP/NBT ALP color development kit (Beyotime, Jiangsu, China) following 7 d of osteogenic induction. Briefly, after rinsing with PBS, the BMSCs were fixed in paraformaldehyde (4%). Afterward, the cells were treated with BCIP/NBT staining solution. The color development reaction was terminated using deionized water. A light microscope (Olympus, Tokyo, Japan) was used to observe the results.
According to the manufacturer’s protocol, osteogenic differentiation was evaluated by Alizarin red staining following osteogenic induction for 21 d. In brief, the cells were washed with PBS. After fixation with paraformaldehyde (4%), 500 μL of Alizarin red staining solution was added to the cells (1%, Sigma, St. Louis, MO, United States). Fifteen minutes later, the staining solution was discarded. A light microscope (Olympus) was used to observe the results after the cells were washed three times with PBS.
For induction of adipogenic differentiation, BMSCs (2.5 × 106 cells per well) were cultured for 14 d in 6-well plates with adipogenic induction medium (1 μmol/L dexamethasone, 0.5 mmol/L 3-isobutyl-1-methylxanthine, 5 μg/mL insulin, and 10% FCS in α-MEM). The culture medium was changed every other day.
After adipogenesis induction for 14 d, mature adipocytes were distinguished from preadipocytes using Oil Red O staining. In brief, after rinsing with PBS, the cells were fixed in paraformaldehyde (4%) at room temperature for 30 min. The cells were then washed with PBS and stained with Oil Red O. Twenty minutes later, a light microscope (Olympus) was used to observe the cells.
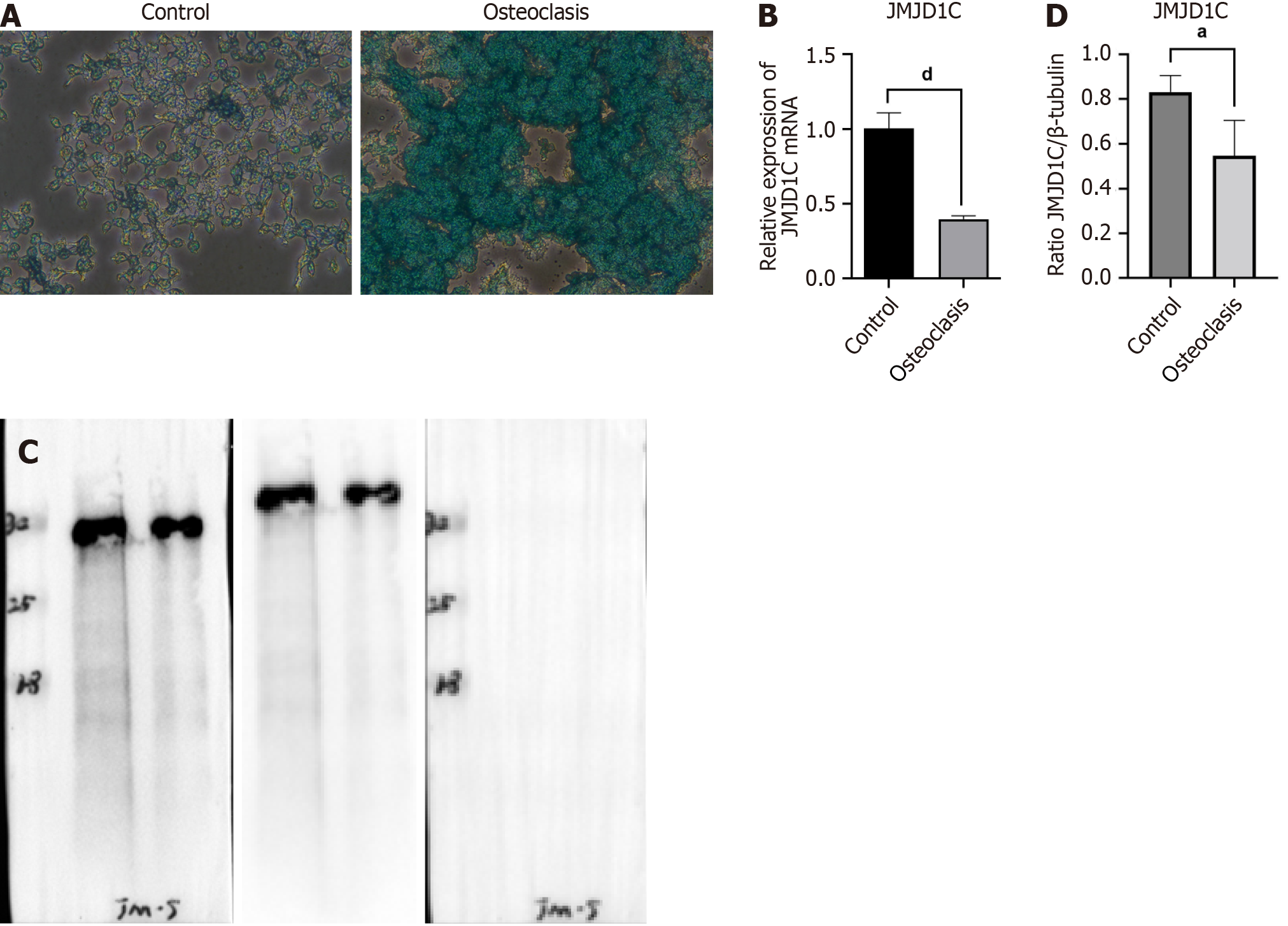
For osteoclast differentiation, 10 ng/mL RANKL obtained from R&D Systems (Minneapolis, MN, United States) was used to treat the BMMs for 7 d.
Osteoclast differentiation of BMMs was confirmed by using tissue-resistant acid phosphatase (TRAP) staining. In brief, the cells were fixed in paraformaldehyde (4%). TRAP staining was performed instantly in the dark for 30 min at 37 °C. The images were obtained by using an optical microscope (Olympus). TRAP-positive cells containing at least 3 nuclei were regarded as osteoclasts.
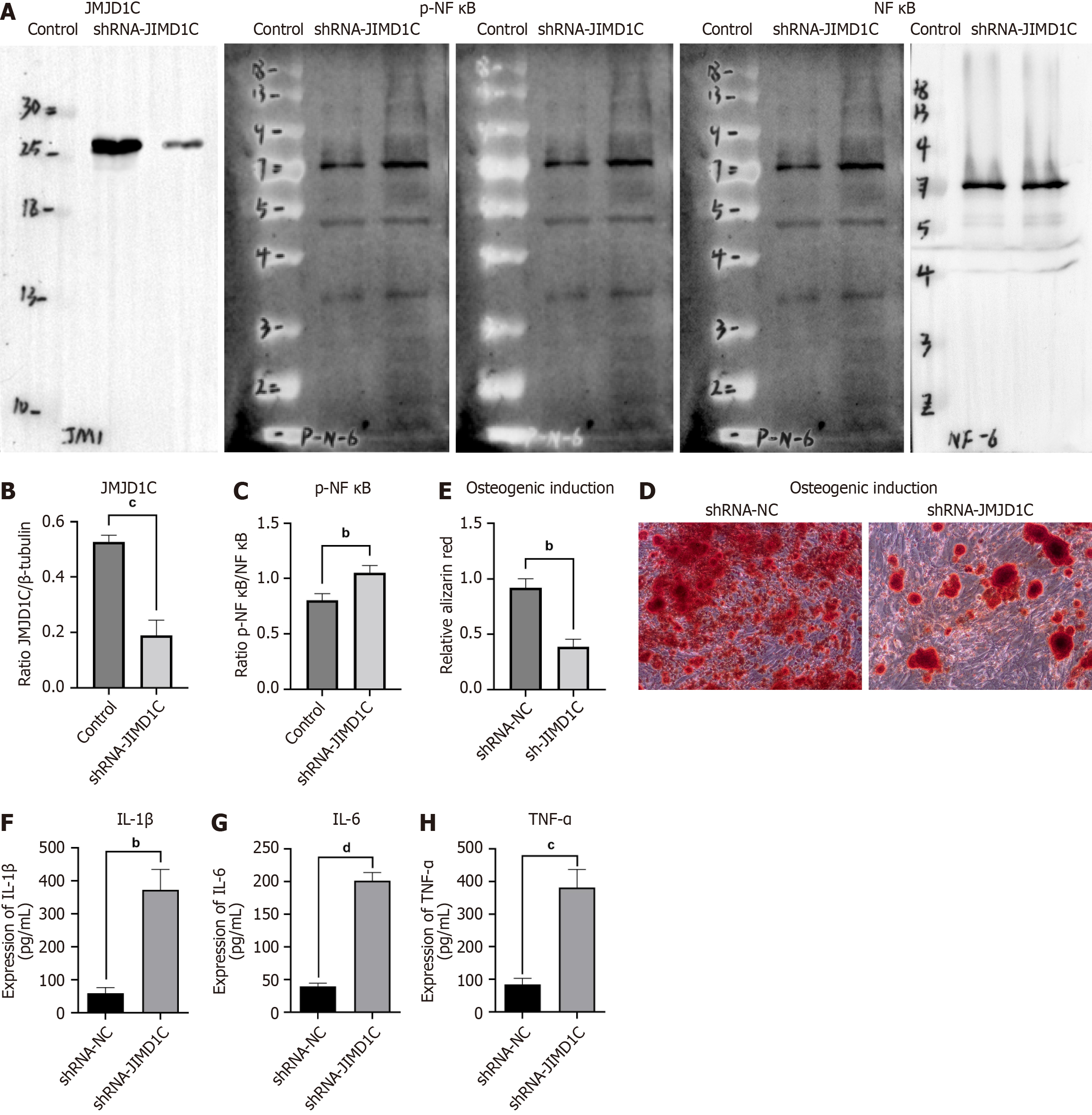
A lentivirus containing short hairpin RNA against JMJD1C (sh-JMJD1C) was obtained from Sangon Biotech (Shanghai, China) for JMJD1C knockdown in BMSCs, and the lentivirus was used to transfect the cells following the manufacturer’s instructions for viral infection. The cells were collected for subsequent assays after 48 h of transfection.
TRIzol (Invitrogen, Carlsbad, CA, United States) was used to extract total RNA. A PrimeScript RT reagent kit (TaKaRa, Tokyo, Japan) was used to transcribe the extracted RNA into cDNA. A CFX96™ Real Time RT-PCR System was used for analysis with SYBR Premix Ex Taq™ II (TaKaRa). Glyceraldehyde 3-phosphate dehydrogenase was used for normalization. The synthesized primers are shown in Table 1. The relative gene expression levels were analyzed with the 2-ΔΔCT method.
| Gene | Species | Direction | Sequence | Tm | Product lengths | Accession |
| RunX2 | Mouse | Forward primer | AGAGTCAGATTACAGATCCCAGG | 60.12 | 109 bp | 90-112 |
| Reverse primer | TGGCTCTTCTTACTGAGAGAGG | 59.66 | 198-178 | |||
| Osteocalin | Mouse | Forward primer | CTGACCTCACAGATCCCAAGC | 61.54 | 114 bp | 66-85 |
| Reverse primer | TGGTCTGATAGCTCGTCACAAG | 59 | 179-160 | |||
| ALP | Mouse | Forward primer | CCAACTCTTTTGTGCCAGAGA | 60.2 | 110 bp | 44-64 |
| Reverse primer | GGCTACATTGGTGTTGAGCTTTT | 61.5 | 153-131 | |||
| PPAR-γ | Mouse | Forward primer | GGAAGACCACTCGCATTCCTT | 62.1 | 121 bp | 69-89 |
| Reverse primer | GTAATCAGCAACCATTGGGTCA | 60.6 | 189-168 | |||
| CEBP-α | Mouse | Forward primer | GCGGGAACGCAACAACATC | 61.2 | 124 bp | 840-860 |
| Reverse primer | GTCACTGGTCAACTCCAGCAC | 62.9 | 963-943 | |||
| JMJD1C | Mouse | Forward primer | ACATCACGGCGAAGGTCTC | 60.4 | 87 bp | 872-893 |
| Reverse primer | TGGGACTATTTGCTTGAGCAC | 61.5 | 958-938 | |||
| IL-1β | Mouse | Forward primer | GGTGCTGATGTACCAGTT | 60.5 | 95 bp | 66-86 |
| Reverse primer | TCTATACCACTTCACAAGTCGGA | 61.0 | 186-165 | |||
| IL-6 | Mouse | Forward primer | GAATTGCCATTGCACAACTCTTT | 60.9 | 105 bp | 642-662 |
| Reverse primer | GTCACTGGTCAACTCCAGCAC | 61.3 | 765-746 | |||
| TNF-α | Mouse | Forward primer | GAACCTTTCTGGCCCGTGT | 60.8 | 93 bp | 770-791 |
| Reverse prime | AGAAATCGCAATTCATGTCGCA | 61.2 | 856-336 | |||
| GAPDH | Mouse | Forward primer | GACGTGCCGCCTGGAGA | 62.5 | 95 bp | 8-28 |
| Reverse prime | GAAGAGTGGGAGTTGCTGTTGAA | 62.5 | 102-84 |
RIPA buffer (Thermo Fisher Scientific) with protease and phosphatase inhibitors was used for extraction of total protein at 4 °C for 0.5 h. A BCA protein assay reagent kit (Thermo Fisher Scientific) was used to determine the protein concentrations in the cell lysates. Next, the protein samples were separated via 10% sodium-dodecyl sulfate gel electrophoresis (Bio-Rad, Hercules, CA, United States) before being transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States). Five percent BSA was used to block the membranes at room temperature for 1 h. Then, the membranes were incubated at 4 °C overnight with primary antibodies against JMJD1C (Invitrogen), p-NF-κB (Abcam), NF-κB (Abcam) and β-tubulin (Abcam). Next, the sections were incubated with secondary antibodies. Ultimately, enhanced chemiluminescence reagent (Beyotime) was used to detect the immunoreactive bands. Image Lab (Bio-Rad, Hercules, CA, United States) was used to quantify the band intensity.
In accordance with the manufacturer’s instructions, enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, United States) were used to measure the levels of cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and IL-1β, in the supernatants. A microplate reader was used to determine the absorbance value of each sample.
The data of this study were accessed by GraphPad Prism 8.0 software. Analysis of variance (ANOVA) or Student’s t test was used to analyze the results. P < 0.05 was regarded as significant, and the outcomes are presented as the means ± SD.
First, we separated BMSCs from mouse bone marrow tissues. Flow cytometry was conducted to identify the BMSCs. As displayed in Figure 1A, BMSCs highly expressed specific stem cell markers, including CD29, CD90 and SCA-1 but weakly expressed CD31, CD45, CD34, MHC2 and CD11b. The results indicated that the BMSCs were successfully separated from the mouse bone marrow. Osteogenic differentiation of BMSCs was induced by incubating the cells with osteogenic induction medium. ALP staining revealed a high level of ALP in BMSCs on day 14 (Figure 1B). Alizarin red staining revealed calcium deposition in the BMSCs on day 21 (Figure 1C). The expression of several osteogenesis-related genes, including Osteocalin, Runt-related transcription factor 2 and ALP, increased after osteogenic induction (Figure 1D). These data suggested successful osteogenic differentiation. Next, the BMSCs were maintained in adipogenic induction medium. The results from Oil Red O staining revealed various lipid drops within the differentiated cells after 14 d of adipogenic differentiation (Figure 1E). In addition, the expression of adipogenesis-related genes, including peroxisome proliferator-activated receptor gamma and CCAAT enhancer-binding protein alpha, was significantly increased in BMSCs after adipocyte induction (Figure 1F). These results indicated successful adipogenic differentiation of BMSCs.
To determine the role of JMJD1C in BMSC osteogenic differentiation, we induced osteogenic differentiation in BMSCs. The reverse transcription coupled to the quantitative polymerase chain reaction results revealed that JMJD1C mRNA expression was significantly increased in BMSCs after osteoblast induction (Figure 2A). Consistently, the JMJD1C protein level was increased after osteoblast induction, as shown by western blot analysis (Figure 2B and C). These data indicated that JMJD1C was upregulated during BMSC osteogenic differentiation. In addition, the protein expression of p-NF-κB was not significantly altered in BMSCs after osteoblast induction (Figure 2B and D). Moreover, the cytokine secretion levels of IL-1β, IL-6 and TNF-α were not significantly altered in BMSCs after osteoblast induction (Figure 2E-G).
To further explore the role of JMJD1C in BMSC osteogenesis, we knocked down JMJD1C in BMSCs by infection with a lentivirus harboring sh-JMJD1C. Compared with that in the control group, the JMJD1C protein level was notably lower in the cells transfected with shRNA-JMJD1C (Figure 3A and B), indicating successful knockdown of JMJD1C. Depletion of JMJD1C notably upregulated the protein expression of p-NF-κB (Figure 3A and C), which indicated that JMJD1C knockdown facilitated NF-κB activation in BMSCs. Moreover, knocking down JMJD1C inhibited stem cell osteogenic differentiation (Figure 3D and E). Moreover, the secretion of the cytokines IL-1β, IL-6 and TNF-α was significantly increased (Figure 3F-H). These findings indicated that JMJD1C promotes cell osteogenic differentiation by negatively regulating p-NF-κB expression in BMSCs.
Osteoclast differentiation was induced in BMMs by treatment with RANKL and M-CSF. The number of TRAP+ multinucleated osteoclasts was significantly greater after 7 d of stimulation than that in the control group, as shown by TRAP staining (Figure 4A), which indicated successful osteoclast differentiation of BMMs induced by RANKL and M-CSF. Compared with those in the control group, JMJD1C mRNA and protein levels were notably lower in cells undergoing osteoclast differentiation (Figure 4B-D). Taken together, these data suggested that JMJD1C was downregulated during BMM osteoclast differentiation.
Osteoporosis is a common bone disorder that arises from an imbalance in bone homeostasis[19]. Impaired BMSC osteogenesis, decreased bone formation and increased marrow adiposity were observed in osteoporosis[20]. Herein, BMSCs isolated from the bone marrow of mice were characterized for their ability to differentiate into osteoblasts and adipocytes. JMJD1C was increased while NF-κB activation was not altered in BMSCs during osteogenic differentiation. Moreover, JMJD1C depletion suppressed osteogenic differentiation and facilitated the activation of NF-κB signaling in BMSCs.
During osteoporosis development, BMSCs exhibit an increased ability to differentiate into adipocytes and a decreased capability to differentiate into osteoblasts, leading to an increase in fat accumulation and a decrease in bone formation[21]. An imbalance in BMSC differentiation has been demonstrated to be a critical mechanism underlying osteoporosis pathogenesis. For example, transcriptionally regulating BMSC differentiation by DEPTOR exacerbates the imbalance of bone fat in osteoporosis[22]. In age-related osteoporosis, the adipogenic differentiation of BMSCs is suppressed, and the osteoblastic differentiation of BMSCs is promoted by the overexpression of miR-130a[23]. The promoted BMSC osteogenesis induced by Foxf1 knockdown contributes to the prevention of ovariectomy-elicited bone loss[24]. Investigating the molecular mechanism modulating adipogenic differentiation and osteogenic differentiation in BMSCs is critical for comprehending the development of osteoporosis and identifying innovative treatment approaches. In the present study, BMSCs cultured in osteogenic induction medium exhibited obvious calcium deposition and osteogenic differentiation, which was in line with the findings of previous studies.
Recent studies on JMJD1C have focused predominantly on tumors and cancers[25,26]. In addition, JMJD1C is involved in regulating cell differentiation in many cells. For example, JMJD1C knockdown is sufficient to trigger neural differentiation of human embryonic stem cells[27]; JMJD1C silencing leads to the induction of differentiation of mouse embryonic stem cells[28]. However, whether JMJD1C is closely associated with osteogenic differentiation is still unclear. A previous study confirmed that in murine 3T3-L1 preadipocyte cells, knockdown of JMJD1C can impair adipogenesis[29]. BMMs isolated from JMJD1C knockout mice exhibit enhanced osteoclastogenesis[13]. Furthermore, KDM3B/JMJD1B has been identified as a potential modulator of osteogenic differentiation[30]. Herein, this study revealed that JMJD1C mRNA and protein levels were elevated during the osteogenic differentiation of BMSCs. In addition, JMJD1C expression decreased in BMMs after osteoclast differentiation. Moreover, we found that JMJD1C knockdown suppressed BMSC osteogenic differentiation. Our study demonstrated the involvement of JMJD1C in regulating BMSC osteogenesis.
NF-κB, an essential transcription factor, is implicated in numerous cellular pathophysiological activities. Moreover, many studies have revealed that NF-κB signaling plays a vital role in osteoblast differentiation. For example, taxifolin promotes BMSC osteogenic differentiation partially through the NF-κB pathway[31]. Melatonin alleviates inflammation and facilitates osteoblast differentiation of BMSCs by repressing the NF-κB pathway[32]. Moreover, in BMSCs from systemic lupus erythematosus patients, activated NF-κB suppressed osteogenic differentiation through downregulation of Smad signaling[33]. TRIM38 acts as a negative modulator of NF-κB during the differentiation of osteoblasts, playing an essential role in bone remodeling[34]. Taken together, these findings suggest that NF-κB may play an essential role in osteoporosis development. Here, we discovered that p-NF-κB expression and inflammatory cytokines, including TNF-α, IL-6 and IL-1β, were not altered in BMSCs during osteogenic differentiation.
Recent studies have revealed the involvement of the NFκB pathway in the functional roles of JMJD proteins in various pathological processes. JMJD3 was found to regulate osteoclastogenesis via RANKL and Ephrin receptor B4 signaling[35]. Inhibition of JMJD1A/KDM3A ameliorates hyperglycemia-mediated myocardial injury by modulating NF-κB/p65[36]. JMJD1A/KDM3A gene silencing mitigates the phosphorylation of MAPKs and NF-κB/p65 activation to attenuate vascular smooth muscle cell injury[37]. Furthermore, in mouse BMMs, JMJD1C silencing promoted NF-κB activation and translocation and resulted in enhanced secretion of IL-6, IL-1β and TNF-α[13]. Similarly, in the present study, JMJD1C knockdown in BMSCs upregulated p-NF-κB expression. Thus, our findings further confirm the negative regulatory effect of JMJD1C on the expression of p-NF-κB. Moreover, JMJD1C knockdown promoted the release of inflammatory cytokines (IL-6, IL-1β, and TNF-α) in BMSCs. Additionally, JMJD1C knockdown suppressed BMSC osteogenic differentiation. Taken together, our study suggested that JMJD1C may play a role through the NF-κB pathway during the osteogenic differentiation of BMSCs. However, the role of JMJD1C/NF-κB signaling in BMSC osteogenic differentiation requires further exploration.
In conclusion, during osteogenic differentiation, JMJD1C was upregulated, while NF-κB activation was not altered in BMSCs. JMJD1C knockdown inhibited osteogenic differentiation and enhanced the activation of NF-κB signaling in BMSCs. Therefore, the JMJD1C/NF-κB pathway may modulate the osteoblast differentiation of BMSCs, which is possibly involved in the pathogenesis of osteoporosis.
Osteoporosis, particularly women of postmenopausal age is elicited by the disequilibrium of osteoblastic bone formation and osteoclastic bone resorption. Elucidation of the molecular mechanisms underlying osteoporosis is important for developing effective therapeutic strategies for this disease.
Bone mesenchymal stem cells (BMSCs) have certain characteristics of differentiation into various types of cells, such as adipocytes and osteoblasts. So that, BMSCs are playing a critical role in bone homeostasis and munch more research groups are utilizing BMSCs for the repair of bone fractures resulting from osteoporosis. The researchers found that Jumonji C domain-containing 1C (JMJD1C) deficiency results in elevated alveolar bone loss in oral inflammatory lesions and loss of JMJD1C accelerates bone marrow-derived macrophage (BMM) differentiation into osteoclasts in vitro. Database analysis revealed that JMJD1C expression is downregulated in BMSCs from patients with osteoporosis. Furthermore, researchers revealed that JMJD1C levels are increased in osteogenic induction medium and BMSC growth medium supplemented with modified extracellular matrix.
To investigate whether JMJD1C is involved in osteoblast differentiation of BMSCs during osteoporosis.
We isolated BMSCs from C57/BL6 suckling mice bone marrow tissues. We assessed the differentiation of BMSCs with Oil Red O staining, Alizarin red staining, alkaline phosphatase staining and reverse transcription coupled to the quantitative polymerase chain reaction. We isolated BMMs and incubated with receptor activator of nuclear factor-kappa Β ligand to induce osteoclast differentiation. The tartrate-resistant acid phosphatase staining were used to confirm the effect of osteoclast differentiation. We used enzyme-linked immunosorbent assay to measure the levels of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL)-6 and IL-1β.
JMJD1C mRNA and protein expression was increased in BMSCs after osteoblast induction. Silence JMJD1C repressed osteogenic differentiation and enhanced nuclear factor-κB (NF-κB) activation and inflammatory cytokine release in BMSCs. JMJD1C upregulation decreased during BMM osteoclast differentiation.
We found that the signaling pathway of JMJD1C/NF-κB is potentially involved in BMSC osteogenic differentiation and may play vital roles in the pathogenesis of osteoporosis.
R&D of MSCs (BMSC, adipose-derived SC, human umbilical cord MSC and embryonic SC, etc.) and their preparations in the field of osteoporosis treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Song S, Guo Y, Yang Y, Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. 2022;237:108168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 229] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 2. | Zhang L, Zheng YL, Wang R, Wang XQ, Zhang H. Exercise for osteoporosis: A literature review of pathology and mechanism. Front Immunol. 2022;13:1005665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 3. | Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, Afanador-Restrepo DF, Carcelén-Fraile MDC, López-Ruiz E. Current Status of the Diagnosis and Management of Osteoporosis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 193] [Reference Citation Analysis (0)] |
| 4. | Liang B, Burley G, Lin S, Shi YC. Osteoporosis pathogenesis and treatment: existing and emerging avenues. Cell Mol Biol Lett. 2022;27:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 5. | Guo Q, Guo Q, Xiao Y, Li C, Huang Y, Luo X. Regulation of bone marrow mesenchymal stem cell fate by long non-coding RNA. Bone. 2020;141:115617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Ning K, Liu S, Yang B, Wang R, Man G, Wang DE, Xu H. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol Metab. 2022;58:101450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Arthur A, Gronthos S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 8. | Bidwell JP, Alvarez MB, Hood M Jr, Childress P. Functional impairment of bone formation in the pathogenesis of osteoporosis: the bone marrow regenerative competence. Curr Osteoporos Rep. 2013;11:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Li D, Yuan Q, Xiong L, Li A, Xia Y. The miR-4739/DLX3 Axis Modulates Bone Marrow-Derived Mesenchymal Stem Cell (BMSC) Osteogenesis Affecting Osteoporosis Progression. Front Endocrinol (Lausanne). 2021;12:703167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Li H, Liu P, Xu S, Li Y, Dekker JD, Li B, Fan Y, Zhang Z, Hong Y, Yang G, Tang T, Ren Y, Tucker HO, Yao Z, Guo X. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J Clin Invest. 2017;127:1241-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Manni W, Jianxin X, Weiqi H, Siyuan C, Huashan S. JMJD family proteins in cancer and inflammation. Signal Transduct Target Ther. 2022;7:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 12. | Sui Y, Gu R, Janknecht R. Crucial Functions of the JMJD1/KDM3 Epigenetic Regulators in Cancer. Mol Cancer Res. 2021;19:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Lee JY, Mehrazarin S, Alshaikh A, Kim S, Chen W, Lux R, Gwack Y, Kim RH, Kang MK. Histone Lys demethylase KDM3C demonstrates anti-inflammatory effects by suppressing NF-κB signaling and osteoclastogenesis. FASEB J. 2019;33:10515-10527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Liu H, Yang L, Zhang E, Zhang R, Cai D, Zhu S, Ran J, Bunpetch V, Cai Y, Heng BC, Hu Y, Dai X, Chen X, Ouyang H. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017;56:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Jimi E, Takakura N, Hiura F, Nakamura I, Hirata-Tsuchiya S. The Role of NF-κB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-κB Inhibition "Killing Two Birds with One Stone"? Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Alles N, Soysa NS, Hayashi J, Khan M, Shimoda A, Shimokawa H, Ritzeler O, Akiyoshi K, Aoki K, Ohya K. Suppression of NF-kappaB increases bone formation and ameliorates osteopenia in ovariectomized mice. Endocrinology. 2010;151:4626-4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Chen X, Liu Y, Ding W, Shi J, Li S, Wu M, Wang H. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κB pathway. Cell Death Dis. 2018;9:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Li Z, Li D, Chen R, Gao S, Xu Z, Li N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol Res. 2023;187:106635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 20. | Pierce JL, Begun DL, Westendorf JJ, McGee-Lawrence ME. Defining osteoblast and adipocyte lineages in the bone marrow. Bone. 2019;118:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 22. | Ouyang Z, Kang D, Li K, Liang G, Liu Z, Mai Q, Chen Q, Yao C, Wei R, Tan X, Bai X, Huang B, Li Q. DEPTOR exacerbates bone-fat imbalance in osteoporosis by transcriptionally modulating BMSC differentiation. Biomed Pharmacother. 2022;151:113164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Lin Z, He H, Wang M, Liang J. MicroRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Prolif. 2019;52:e12688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 24. | Shen G, Ren H, Shang Q, Zhao W, Zhang Z, Yu X, Tang K, Tang J, Yang Z, Liang D, Jiang X. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine. 2020;52:102626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 25. | Zhong C, Tao B, Yang F, Xia K, Yang X, Chen L, Peng T, Xia X, Li X, Peng L. Histone demethylase JMJD1C promotes the polarization of M1 macrophages to prevent glioma by upregulating miR-302a. Clin Transl Med. 2021;11:e424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Lynch JR, Salik B, Connerty P, Vick B, Leung H, Pijning A, Jeremias I, Spiekermann K, Trahair T, Liu T, Haber M, Norris MD, Woo AJ, Hogg P, Wang J, Wang JY. JMJD1C-mediated metabolic dysregulation contributes to HOXA9-dependent leukemogenesis. Leukemia. 2019;33:1400-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Wang J, Park JW, Drissi H, Wang X, Xu RH. Epigenetic regulation of miR-302 by JMJD1C inhibits neural differentiation of human embryonic stem cells. J Biol Chem. 2014;289:2384-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Xiao F, Liao B, Hu J, Li S, Zhao H, Sun M, Gu J, Jin Y. JMJD1C Ensures Mouse Embryonic Stem Cell Self-Renewal and Somatic Cell Reprogramming through Controlling MicroRNA Expression. Stem Cell Reports. 2017;9:927-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Buerger F, Müller S, Ney N, Weiner J, Heiker JT, Kallendrusch S, Kovacs P, Schleinitz D, Thiery J, Stadler SC, Burkhardt R. Depletion of Jmjd1c impairs adipogenesis in murine 3T3-L1 cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Munehira Y, Yang Z, Gozani O. Systematic Analysis of Known and Candidate Lysine Demethylases in the Regulation of Myoblast Differentiation. J Mol Biol. 2017;429:2055-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Wang YJ, Zhang HQ, Han HL, Zou YY, Gao QL, Yang GT. Taxifolin enhances osteogenic differentiation of human bone marrow mesenchymal stem cells partially via NF-κB pathway. Biochem Biophys Res Commun. 2017;490:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Hu Y, Xiong Y, Zha K, Tao R, Chen L, Xue H, Yan C, Lin Z, Endo Y, Cao F, Zhou W, Liu G. Melatonin Promotes BMSCs Osteoblastic Differentiation and Relieves Inflammation by Suppressing the NF-κB Pathways. Stem Cells Int. 2023;2023:7638842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 33. | Tang Y, Xie H, Chen J, Geng L, Chen H, Li X, Hou Y, Lu L, Shi S, Zeng X, Sun L. Activated NF-κB in bone marrow mesenchymal stem cells from systemic lupus erythematosus patients inhibits osteogenic differentiation through downregulating Smad signaling. Stem Cells Dev. 2013;22:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Kim K, Kim JH, Kim I, Seong S, Kim N. TRIM38 regulates NF-κB activation through TAB2 degradation in osteoclast and osteoblast differentiation. Bone. 2018;113:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Wang R, Luo H, Yang D, Yu B, Guo J, Shao L, Okamura H, Qiu L. Osteoblast Jmjd3 regulates osteoclastogenesis via EphB4 and RANKL signalling. Oral Dis. 2023;29:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Zhang B, Zhang J, Liu G, Guo X, Liu X, Chen J. KDM3A Inhibition Ameliorates Hyperglycemia-Mediated Myocardial Injury by Epigenetic Modulation of Nuclear Factor Kappa-B/P65. Front Cardiovasc Med. 2022;9:870999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Zhang BF, Jiang H, Chen J, Guo X, Hu Q, Yang S. KDM3A inhibition attenuates high concentration insulininduced vascular smooth muscle cell injury by suppressing MAPK/NFκB pathways. Int J Mol Med. 2018;41:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |