Published online Dec 26, 2024. doi: 10.4252/wjsc.v16.i12.1086
Revised: October 5, 2024
Accepted: November 11, 2024
Published online: December 26, 2024
Processing time: 95 Days and 17.5 Hours
Myocardial fibrosis, a condition linked to several cardiovascular diseases, is associated with a poor prognosis. Stem cell therapy has emerged as a potential treatment option and the application of stem cell therapy has been studied extensively. However, a comprehensive bibliometric analysis of these studies has yet to be conducted.
To map thematic trends, analyze research hotspots, and project future directions of stem cell-based myocardial fibrosis therapy.
We conducted a bibliometric and visual analysis of studies in the Web of Science Core Collection using VOSviewer and Microsoft Excel. The dataset included 1510 articles published between 2001 and 2024. Countries, organizations, authors, references, keywords, and co-citation networks were examined to identify evolving research trends.
Our findings revealed a steady increase in the number of publications, with a projected increase to over 200 publications annually by 2030. Initial research focused on stem cell-based therapy, particularly for myocardial infarction and heart failure. More recently, there has been a shift toward cell-free therapy, involving extracellular vesicles, exosomes, and microRNAs. Key research topics include angiogenesis, inflammation, apoptosis, autophagy, and oxidative stress.
This analysis highlights the evolution of stem cell therapies for myocardial fibrosis, with emerging interest in cell-free approaches. These results are expected to guide future scientific exploration and decision-making.
Core Tip: This study presents a comprehensive bibliometric analysis of stem cell-based therapies for myocardial fibrosis from 2001 to 2024, highlighting significant trends and emerging research directions. The analysis reveals a growing interest in cell-free therapies, including extracellular vesicles, exosomes, and microRNAs. The focus of research is shifting from angiogenesis, inflammation, apoptosis, autophagy, and oxidative stress to autophagy. These findings provide valuable insights into future research priorities in myocardial fibrosis treatment.
- Citation: Ding JY, Meng TT, Du RL, Song XB, Li YX, Gao J, Ji R, He QY. Bibliometrics of trends in global research on the roles of stem cells in myocardial fibrosis therapy. World J Stem Cells 2024; 16(12): 1086-1105
- URL: https://www.wjgnet.com/1948-0210/full/v16/i12/1086.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i12.1086
Cardiovascular diseases (CVDs) are the leading causes of morbidity and mortality worldwide and account for a significant portion of healthcare costs[1,2]. The CVD incidence and prevalence are increasing worldwide. The number of CVD cases has risen from 271 million in 1990 to 775 million in 2022, and CVD-related deaths have increased from 12.1 million in 1990 to 19.8 million in 2022. It is estimated that this number could reach 24.0 million by 2030[3-5].
Myocardial fibrosis is linked to a number of CVDs and conditions, including myocardial infarction (MI), heart failure (HF), hypertension, viral myocarditis, hypertrophic cardiomyopathy, and cardiomyopathies[6-11]. Myocardial fibrosis is a common wound-healing response that occurs following cardiac tissue stress or injury; it is characterized by an imbalance in the extracellular matrix[12,13]. Myocardial fibrosis occurs when fibroblasts become myofibroblasts in the injured heart in order to repair damage; however, excessive myocardial fibrosis reduces heart function and leads to HF with a poor prognosis[14,15]. As standard drugs or surgery cannot repair damaged myocardial tissue or reverse cardiac fibrosis, there is currently no treatment for myocardial fibrosis[16,17]. In this context, stem cells, which have the potential to differentiate into myocardial cells, have emerged as a promising treatment option for myocardial fibrosis[18-20]. The effects of stem cell therapy include attenuated remodeling, improved global and regional functions, decreased scar size, and increased myocardium viability[21].
In bibliometric analyses, the quality and quantity of scientific literature are evaluated. This approach is used in con
The data were obtained from the Web of Science Core Collection (WOSCC), a curated collection of high-quality scientific information. Studies published from the inception of the database until 2024 were included. All journals that were included were selected for coverage, and details, such as authors, abstracts, keywords, and cited references, were recorded for each paper. When using the Web of Science database, “TS” will return the title, abstract, and author key
For the bibliometric analysis, the retrieved publications were imported into VOSviewer version 1.6.18[23] (Science and Technology Research Center, Leiden University, Leiden, The Netherlands) and Microsoft Excel 2019. Maps for visualization were created using VOSviewer, and the countries, organizations, authors, references, and keywords were captured.
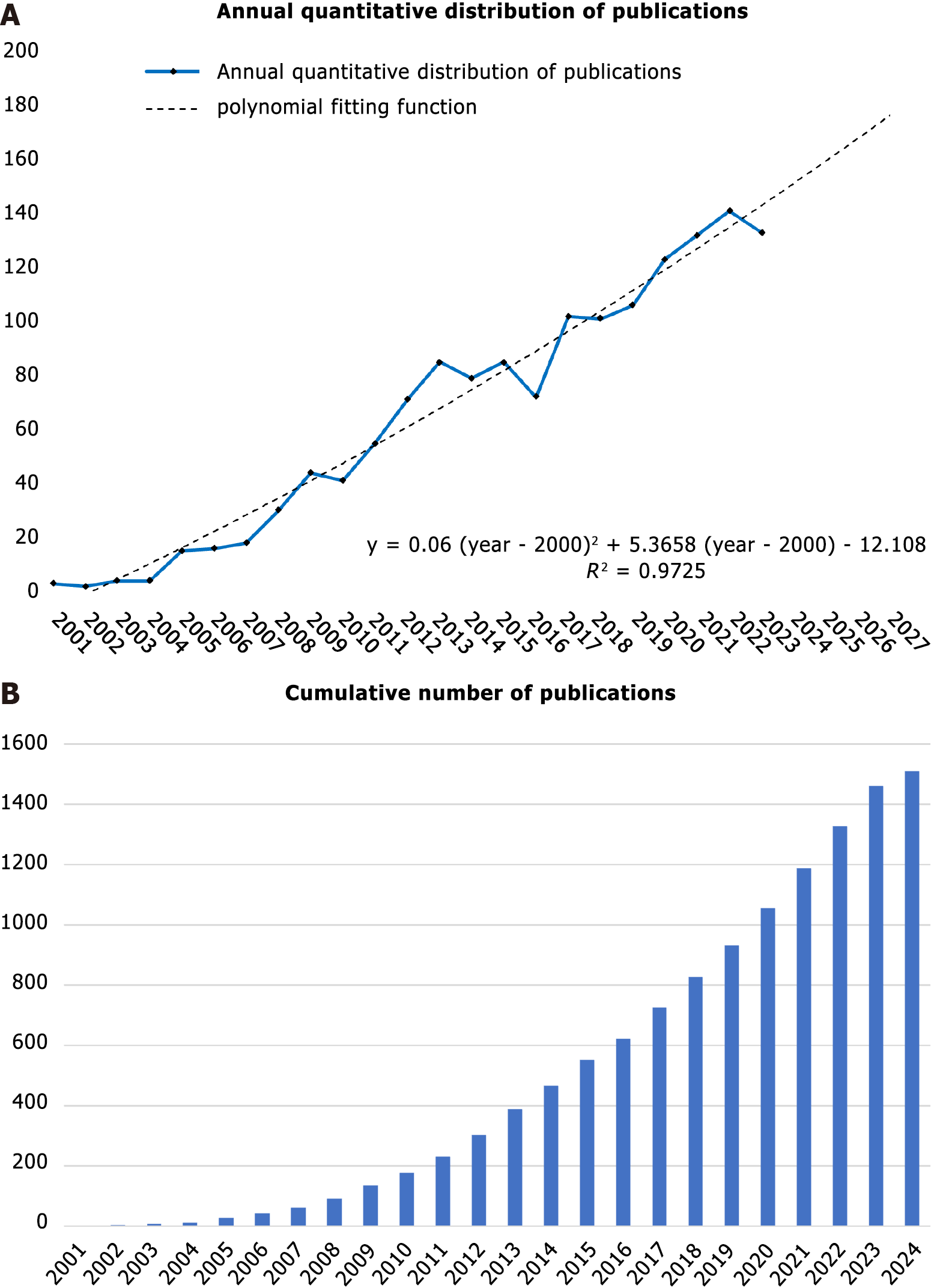
A total of 3088 studies on myocardial fibrosis and stem cells published prior to June 15, 2024 were retrieved from the WOSCC. After eliminating inappropriate articles, 1510 publications meeting the inclusion criteria were retained. Among them, 280 (18.5%) were reviews and 1230 (81.5%) were original studies. As shown in Figure 1, there was a general upward trend in the number of publications in the field. However, a change in the trend was observed in 2007. Before 2007, the number of publications annually grew slowly; thereafter, despite an occasional decline, the number of publications per year grew rapidly overall (Figure 1A; the solid line represents the annual quantitative distribution, and the dotted line represents the polynomial fitting function). The curve could be fitted by the equation y = 0.06 × (x - 2000)2 + 5.3658 × (x - 2000) - 12.108 (the Y-axis represents the number of articles and the X-axis represents the year). If the current trend continues, the number of publications will exceed 200 annually in 2030. The increase in the number of articles published annually suggests that researchers are placing increasing emphasis on this topic (Figure 1B).
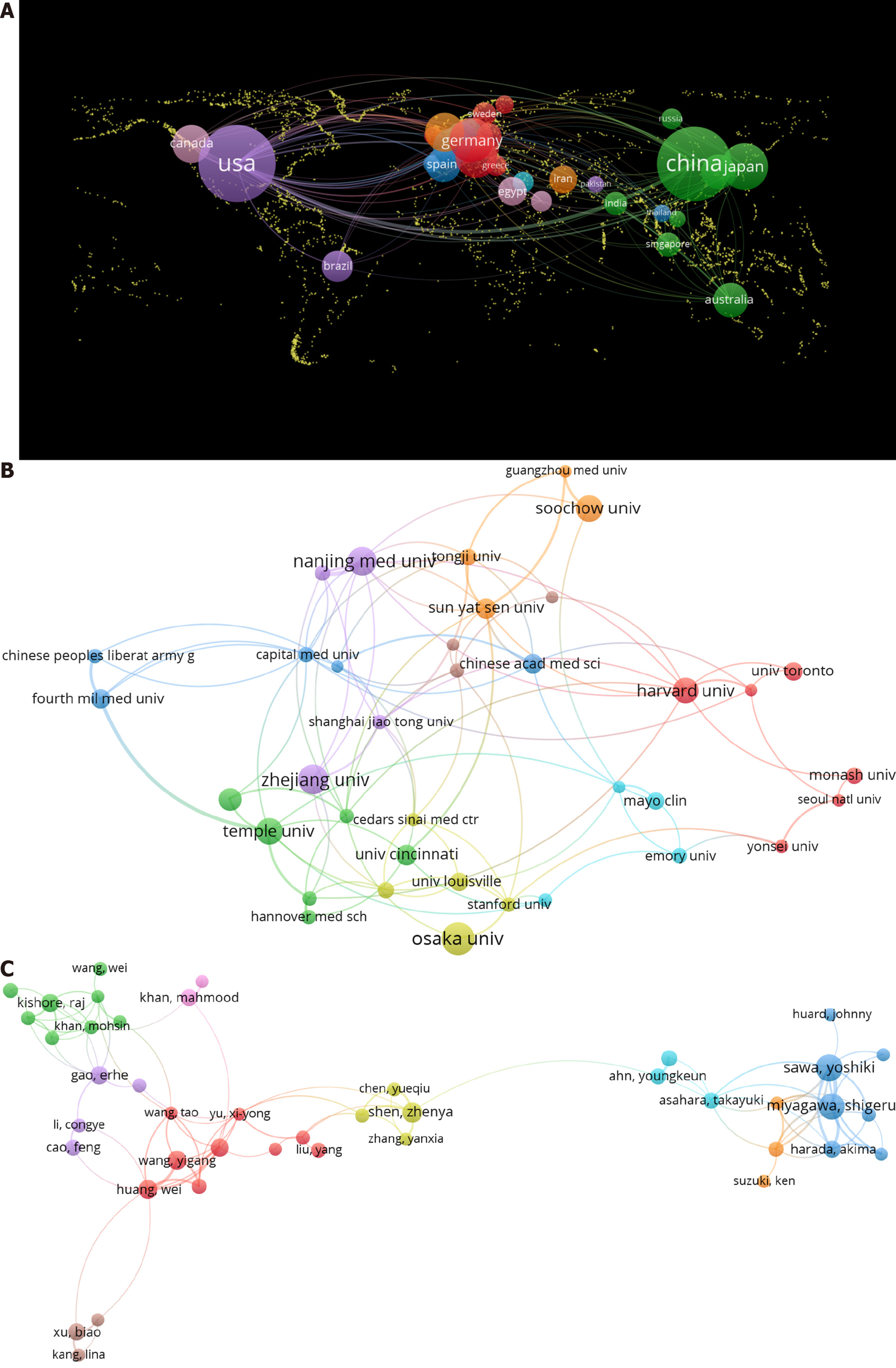
We utilized a geographic visualization analysis (Figure 2A) to investigate the global distribution of studies. Articles identified in the literature search were published in 63 countries. When generating a country collaboration map using VOSviewer, we set the minimum number of documents published by a country to three. In total, 40 countries met the thresholds and were included in the analysis. The United States had the most publications (529, 35.03%), followed by China (478, 31.66%) and Japan (118, 7.81%) (Table 1). There were three times more citations of articles in the United States (30060) than those in China (9811), indicating that the United States had a greater influence than that of China. For example, the article “Bone marrow cells regenerate infarcted myocardium” published in 2001 in Nature from the United States had an impact factor (IF) of 50.5 and citations of 4956 citations across all databases. This study represents a major milestone in the field, demonstrating that the transplantation of bone marrow cells into infarcted mice has the potential to regenerate myocardial tissue, thereby improving outcomes in coronary artery disease[24]. In addition, the total link strength of the United States was 296, ranking first in partnerships with 43 countries, indicating extensive cooperation between researchers in the United States and other countries. The United States had the closest cooperation with China.
| Rank | Country | Publications (%) | Citations | Total link strength |
| 1 | United States | 529 (35.03) | 30060 | 280 |
| 2 | China | 478 (31.66) | 9811 | 151 |
| 3 | Japan | 118 (7.81) | 4970 | 48 |
| 4 | Germany | 99 (6.56) | 4147 | 97 |
| 5 | Italy | 82 (5.43) | 2245 | 74 |
| 6 | United Kingdom | 66 (4.37) | 3035 | 68 |
| 7 | Canada | 65 (4.30) | 3804 | 37 |
| 8 | France | 60 (3.97) | 1734 | 59 |
| 9 | South Korea | 47 (3.11) | 1508 | 18 |
| 10 | Spain | 47 (3.11) | 1664 | 40 |
A total of 1907 organizations conducted studies on the role of stem cells in myocardial fibrosis therapy. When generating an organization collaboration map using VOSviewer, we set the minimum number of documents published by an organization to 12. In total, 43 organizations met the thresholds and were included in the analysis. Among the top 10 organizations, five were from China, four were from the United States, and one was from Japan. Osaka University (Japan) had the most publications in this research area (i.e., 32 papers), followed by Zhejiang University (China) with 29 papers and Nanjing Medical University (China) with 28 papers (Figure 2B and Table 2). Harvard University had 4308 citations, ranking first among the top 10 organizations with the highest number of publications, thus demonstrating a substantial impact on the field. In 2009, Harvard University published an article titled “Growth factors, matrices, and forces combine and control stem cells” in Science (IF: 44.7, cited: 2455), highlighting the key roles of stem cells in the repair and homeostasis of myocardial fibrosis[25]. In terms of collaboration, the Chinese Academy of Medical Sciences collaborated most frequently with Peking Union Medical College. Emory University collaborated closely with the Georgia Institute of Technology, and Nanjing Medical University collaborated closely with Nanjing University. National institutions frequently collaborated; however, international collaborations were limited and should be strengthened in the future.
| Rank | Organization | Country | Documents | Citations | Total link strength |
| 1 | Osaka University | Japan | 32 | 934 | 3 |
| 2 | Zhejiang University | China | 29 | 495 | 10 |
| 3 | Nanjing Medical University | China | 28 | 542 | 20 |
| 4 | Soochow University | China | 26 | 867 | 6 |
| 5 | Temple University | United States | 26 | 930 | 16 |
| 6 | Harvard University | United States | 25 | 4308 | 18 |
| 7 | The Ohio State University | United States | 22 | 669 | 5 |
| 8 | Sun Yat-sen University | China | 20 | 544 | 15 |
| 9 | University ff Cincinnati | United States | 20 | 1345 | 9 |
| 10 | Chinese Academy of Medical Sciences | China | 19 | 452 | 19 |
A total of 9623 authors were involved in research on myocardial fibrosis and stem cells. When generating an author collaboration map using VOSviewer, we set the minimum number of documents published by an author to six. In total, 97 authors met the thresholds and were included in the analysis. Seven of the top 10 co-authors were from the United States, two from Japan, and one from China. Yoshiki Sawa from Osaka University, Japan, had the highest number of publications at 27, followed by Shigeru Miyagawa (Japan) with 25 publications and Zhen-Ya Shen (China) with 15 publications (Table 3). Figure 2C shows a network visualization map; co-authors often had stable collaborative relationships and formed research groups with similar research directions.
| Rank | Author | Country | Affiliation | Documents | Total link strength |
| 1 | Yoshiki Sawa | Japan | Osaka University | 27 | 61 |
| 2 | Shigeru Miyagawa | Japan | Osaka University | 25 | 61 |
| 3 | Zhen-Ya Shen | China | Soochow University | 15 | 22 |
| 4 | Dinender K Singla | United States | University of Central Florida | 15 | 0 |
| 5 | Wei Huang | United States | University of Cincinnati Medical Center | 14 | 34 |
| 6 | Yi-Gang Wang | United States | University of Cincinnati Medical Center | 13 | 30 |
| 7 | Muhammad Ashraf | United States | University of Cincinnati Medical Center | 12 | 25 |
| 8 | Er-He Gao | United States | Temple University | 12 | 11 |
| 9 | Raj Kishore | United States | Temple University | 12 | 17 |
| 10 | Michael E Davis | United States | Emory University | 11 | 8 |
A multi-person team from the Osaka University Graduate School of Medicine included leaders in the field (e.g., Yoshiki Sawa and Shigeru Miyagawa). Their research concentrated on discovering innovative methods to boost regeneration, reduce fibrosis, and improve functional recovery in various heart diseases. They found that transplanting stem cell sheets into the host myocardium effectively reduced fibrosis, attenuated ventricular remodeling, promoted stem cell migration, and enhanced neovascularization in the endocardium[26-29]. Additionally, they demonstrated the therapeutic effects and preservation of heart function through the use of mesenchymal stem cells (MSCs) in vivo[30-32]. However, cooperation in this field was mostly limited to authors within the same institution, with a lack of collaborations among authors globally.
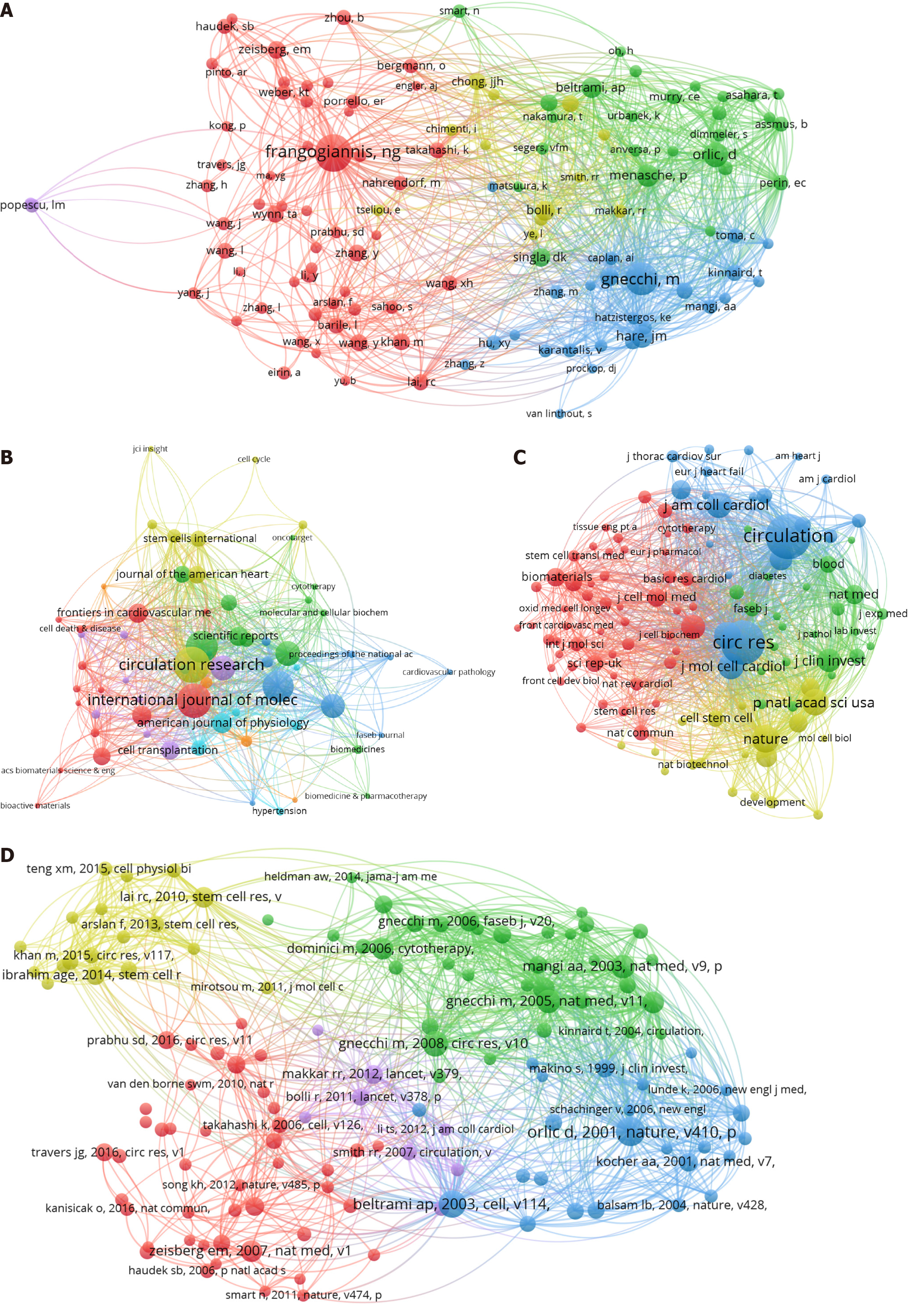
Author co-citation refers to the simultaneous citation of an article by two or more authors. When generating a co-cited author network using VOSviewer, we set the minimum number of citations of a co-cited author to 45. In total, 120 co-cited authors met the thresholds and were included in the analysis. The top 10 co-cited authors included seven from the United States, one from Italy, one from France, and one from the United Kingdom. Nikolaos G Frangogiannis from the Albert Einstein College of Medicine (United States) ranked first (302 citations), followed by Massimiliano Gnecchi (248 citations) from the University of Pavia (Italy), and Donald Orlic (164 citations) from the National Human Genome Research Institute/National Institutes of Health (United States) (Figure 3A and Table 4).
| Rank | Author | Country | Affiliation | Citations |
| 1 | Nikolaos G Frangogiannis | United States | Albert Einstein College of Medicine | 302 |
| 2 | Massimiliano Gnecchi | Italy | University of Pavia | 248 |
| 3 | Donald Orlic | United States | National Human Genome Research Institute/NIH | 164 |
| 4 | Philippe Menasché | France | Université Sorbonne Paris Cité | 141 |
| 5 | Joshua M Hare | United States | University of Miami Miller | 129 |
| 6 | Antonio Paolo Beltrami | United Kingdom | University of Bristol | 121 |
| 7 | Roberto Bolli | United States | University of Louisville | 120 |
| 8 | Dinender K Singla | United States | University of Central Florida | 113 |
| 9 | Mark F Pittinger | United States | University of Maryland | 108 |
| 10 | Elisabeth M Zeisberg | United States | Harvard Medical School | 102 |
Notably, Singla was in the top 10 co-authors and top 10 co-cited authors, implying that he has a major influence in this field. He analyzed the roles of stem cells and exosomes in cardiac repair and discovered that transplantation alone does not significantly enhance cardiac function. Furthermore, he found that factors secreted by stem cells play a crucial role in reducing apoptosis and fibrosis[33]. Singla[34] concluded that exosomes derived from stem cells exhibit significant anti-apoptotic, anti-fibrotic, and pro-angiogenic potential and enhance cardiac differentiation to repair damaged tissue.
The articles included in this study were published in 489 journals. When generating a journal network using VOSviewer, we set the minimum number of documents published in a journal to five. In total, 63 journals met the thresholds and were included in the analysis. The distribution of journals is shown in Figure 3B, and the top 10 journals are listed in Table 5. The most productive journal was Circulation Research, with 48 papers, followed by International Journal of Molecular Sciences (45 papers) and Stem Cell Research & Therapy (41 papers). Circulation had the highest average IF among the top 10 journals (35.5), followed by Circulation Research (16.5) and Cardiovascular Research (10.2).
| Rank | Journal | IF | JIF quartile | Documents |
| 1 | Circulation Research | 16.5 | Q1 | 48 |
| 2 | International Journal of Molecular Sciences | 4.9 | Q1 | 45 |
| 3 | Stem Cell Research & Therapy | 7.1 | Q1 | 41 |
| 4 | PLoS One | 2.9 | Q1 | 40 |
| 5 | Cardiovascular Research | 10.2 | Q1 | 30 |
| 6 | Circulation | 35.5 | Q1 | 30 |
| 7 | Journal of Molecular and Cellular Cardiology | 4.9 | Q2 | 29 |
| 8 | American Journal of Physiology-Heart and Circulatory Physiology | 4.1 | Q1 | 24 |
| 9 | Frontiers in Cardiovascular Medicine | 2.8 | Q2 | 22 |
| 10 | Frontiers in Cell and Developmental Biology | 4.6 | Q2 | 22 |
A citation analysis can be used to evaluate journal importance, which depends primarily on the number of citations. When generating a co-cited journal network using VOSviewer, we set the minimum number of citations of a co-cited journal to 150. In total, 113 co-cited journals met the thresholds and were included in the analysis. The top 10 journals with the highest number of co-citations are summarized in Table 6. All of the top 10 co-cited journals were cited more than 1000 times. Circulation ranked first with the most citations (5091), followed by Circulation Research (4977) and Nature (1938). Among the top 10 co-cited journals, Nature had the highest average IF of 50.5, followed by Circulation (35.5) and Journal of the American College of Cardiology (21.7). According to the journal IF quartile analysis, the majority of the top 10 cited journals and top 10 co-cited journals were distributed in the Q1 region (Figure 3C).
| Rank | Co-cited journal | Citations | IF | JIF quartile | Total link strength |
| 1 | Circulation | 5091 | 35.5 | Q1 | 373781 |
| 2 | Circulation Research | 4977 | 16.5 | Q1 | 397335 |
| 3 | Nature | 1938 | 50.5 | Q1 | 169834 |
| 4 | Proceedings of the National Academy of Sciences of the United States of America-Physical Sciences | 1890 | 9.04 | Q1 | 162817 |
| 5 | Cardiovascular Research | 1832 | 10.2 | Q1 | 148737 |
| 6 | Journal of the American College of Cardiology | 1754 | 21.7 | Q1 | 140925 |
| 7 | Journal of Molecular and Cellular Cardiology | 1694 | 4.9 | Q2 | 134825 |
| 8 | PLoS One | 1552 | 2.9 | Q1 | 142529 |
| 9 | Journal of Clinical Investigation | 1438 | 13.3 | Q1 | 124120 |
| 10 | American Journal of Physiology-Heart and Circulatory Physiology | 1311 | 4.1 | Q1 | 99389 |
Two papers establish a co-citation relationship when they appear simultaneously on the reference list of a third paper. Frequently co-cited references indicate the status of development in the research field and can provide advanced support and guidance for scientific decision-making. When generating a co-cited reference network using VOSviewer, we set the minimum number of citations of a co-cited reference to 25. In total, 130 co-cited references met the thresholds and were included in the analysis. The most co-cited article “Bone marrow cells regenerate infarcted myocardium” was published in Nature in 2001 and had 109 citations[24]. This study revealed that lineage-negative (Lin)- c-kitPOS cells can generate new myocardial tissue in the infarcted area of the ventricle. Next, the co-cited article “Adult cardiac stem cells are multipotent and support myocardial regeneration” was published in Cell in 2003 and had 97 citations[35]. This paper also demonstrated that Lin- c-kitPOS cells have self-renewal properties, clonogenic capacity, and multipotency and opened new opportunities for myocardial repair. The third top co-cited article “Paracrine mechanisms in adult stem cell signaling and therapy” in Circulation Research was published in 2008 and had 85 citations[36]. The contributions and mechanisms of stem cell-paracrine signaling in cardiac repair and regeneration were reviewed in this article. In addition, two articles among the top five co-cited references were published by Massimiliano Gnecchi[36,37], further supporting his contributions and influence in the field (Figure 3D and Table 7).
| Rank | Title | First author | Journal | IF | Publication year | Citations |
| 1 | Bone marrow cells regenerate infarcted myocardium | Donald Orlic | Nature | 50.5 | 2001 | 109 |
| 2 | Adult cardiac stem cells are multipotent and support myocardial regeneration | Antonio P Beltrami | Cell | 45.5 | 2003 | 97 |
| 3 | Paracrine mechanisms in adult stem cell signaling and therapy | Massimiliano Gnecchi | Circulation Research | 16.5 | 2008 | 85 |
| 4 | Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts | Abeel A Mangi | Nature Medicine | 58.7 | 2003 | 77 |
| 5 | Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells | Massimiliano Gnecchi | Nature Medicine | 58.7 | 2005 | 75 |
| 6 | Endothelial-to-mesenchymal transition contributes to cardiac fibrosis | Elisabeth M Zeisberg | Nature Medicine | 58.7 | 2007 | 70 |
| 7 | Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart | Catalin Toma | Circulation | 35.5 | 2002 | 66 |
| 8 | Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement | Massimo Dominici | Cytotherapy | 3.7 | 2006 | 66 |
| 9 | Multilineage potential of adult human mesenchymal stem cells | Mark F Pittenger | Science | 44.7 | 1999 | 66 |
| 10 | Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury | Ruenn Chai Lai | Stem Cell Research | 0.8 | 2010 | 64 |
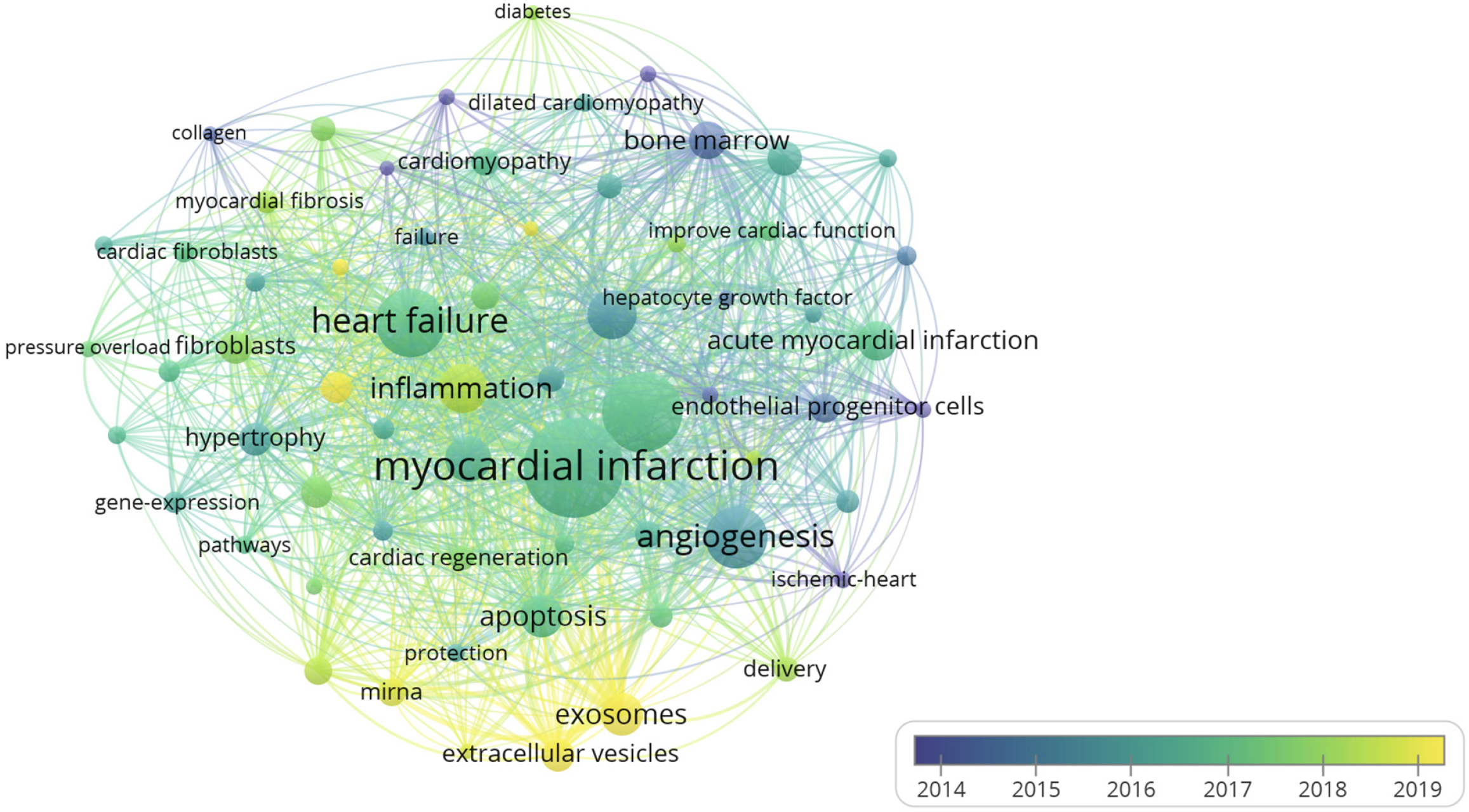
Different versions of words with the same meaning were combined, such as synonyms (e.g., “engraftment” and “transplantation”), different spelling and hyphenation (e.g., “myocardial-infarction” and “myocardial infarction”), abbreviated terms (e.g., “MSCs” and “mesenchymal stem cells”), and singular/plural terms (e.g., “pathway” and “pathways”), for the analysis of keywords. When generating a keyword network using VOSviewer, we set the minimum number of occurrences of a keyword to 30. In total, 64 keywords met the thresholds and were included in the analysis. The top 20 keywords in this study are listed in Table 8. Among the top 20 keywords, “myocardial infarction” and “heart failure” were associated with heart disease; “mesenchymal stem cells”, “exosome”, “bone marrow”, “extracellular vesicles”, and “miRNA” were associated with stem cell therapy; and “angiogenesis”, “inflammation”, “apoptosis”, “autophagy”, and “oxidative stress” were associated with the mechanism of myocardial fibrosis and stem cell therapy.
| Rank | Keyword | Occurrences | Average appearing year |
| 1 | Myocardial infarction | 532 | 2016.69 |
| 2 | Mesenchymal stem cells | 383 | 2016.85 |
| 3 | Heart failure | 301 | 2016.82 |
| 4 | Angiogenesis | 258 | 2015.52 |
| 5 | Progenitor cells | 182 | 2015.55 |
| 6 | Inflammation | 180 | 2018.28 |
| 7 | Cardiomyocytes | 160 | 2016.38 |
| 8 | Apoptosis | 148 | 2016.86 |
| 9 | Exosomes | 146 | 2020.77 |
| 10 | Acute myocardial infarction | 125 | 2016.85 |
| 11 | Bone marrow | 123 | 2014.54 |
| 12 | Cardiac function | 102 | 2016.23 |
| 13 | Hypertrophy | 101 | 2015.93 |
| 14 | Extracellular vesicles | 99 | 2021.16 |
| 15 | Fibroblasts | 99 | 2017.80 |
| 16 | Autophagy | 93 | 2019.15 |
| 17 | Oxidative stress | 93 | 2017.59 |
| 18 | Endothelial progenitor cells | 85 | 2014.65 |
| 19 | Cardiomyopathy | 79 | 2016.71 |
| 20 | miRNA | 78 | 2018.73 |
Keywords with different average appearing years were used to create an overlay visualization map (Figure 4). Early research focused on the effects of stem cell-based therapies (such as stem cell transendocardial injections, cell infusions, cell sheets, and cell patches). With the development of new treatment methods for heart diseases, cell-free therapy with the extracellular vesicle (EV)-mediated transfer of exosomes and selected microRNAs (miRNAs) between stem cells and fibrotic tissues has gained attention. In early research, the most commonly studied mechanisms were angiogenesis, inflammation, apoptosis, and oxidative stress; however, autophagy gained increasing attention in later studies.
In this study, we used bibliometric mapping to analyze several parameters, including countries, organizations, references, and keywords, providing insight into the current direction of research on stem cells in myocardial fibrosis and predicting future trends[38]. The growing number of publications observed in this study indicates that stem cell therapies for myocardial fibrosis have become a significant focus of research. The United States, China, and Japan contributed the most papers to this field. In particular, the United States had a major impact, with several landmark articles[24,25,39]; it ranked first in total citations, with three times more citations than those for the second-ranked country. There is already significant collaboration within institutions and across the country. However, moving forward, there is a pressing need to bolster international partnerships to address global challenges more effectively.
Yoshiki Sawa, the most prolific author, found that transplanting stem cell sheets into the myocardium improves myocardial fibrosis, reduces remodeling, induces stem cell migration, and increases neovascularization[26-29]. Dinender K Singla is among the top 10 prolific authors and top 10 co-cited authors, indicating his substantial influence in this field. Singla[34] evaluated the roles of stem cells and exosomes in cardiac repair and found that while transplantation does not significantly improve heart function, substances secreted by stem cells inhibit apoptosis and fibrosis[33]. He also discovered that stem cell-derived exosomes enhance cardiac differentiation and repair injured tissues, in addition to their anti-apoptotic, anti-fibrotic, and pro-angiogenic properties.
The keyword analysis indicated that myocardial fibrosis is closely related to two myocardial conditions: MI and HF. MI naturally causes myocardial fibrosis when fibroblasts transform into myofibroblasts, characterized by an imbalance in extracellular matrix synthesis, deposition, and degradation[12,13]. Initially, fibrotic tissue repairs the damaged part of the heart and protects it from rupture; however, the progression of myocardial fibrosis reduces heart function, leading to HF and a poor prognosis[14,15]. Preclinical studies have also implicated myocardial fibrosis in the pathophysiology of HF[40-44], resulting from the persistent exacerbation of myocardial fibers, recurrent myocardial ischemia, and hypoxia[45]. In patients with a preserved ejection fraction, myocardial fibrosis in HF is associated with high mortality and hospitalization rates[46]. In a cohort study, a highly modified Look-Locker inversion recovery-extracellular volume, reflecting diffuse myocardial fibrosis, was independently associated with a higher rate of first HF hospitalization and a poor HF prognosis during a 9-month follow-up[47]. These two diseases are also the primary conditions that stem cell therapy seeks to treat.
Stem cells possess regeneration potential and can differentiate into smooth muscle cells, endothelial cells, and cardiomyocytes (CMs)[18,19]. In ischemic cardiomyopathy (ICM), a combination of simultaneous revascularization and stem cell therapy appears to be the most effective approach to date for halting the progression of HF after MI[48]. The keyword analysis identified several stem cell types used to treat myocardial fibrosis, including MSCs[31,49,50] and bone marrow-derived stem cells (BMCs)[51-53]. These stem cells attenuate CVD, alleviate myocardial fibrosis, and contribute to cardiac functional recovery[54,55]. MSC injections stimulated neoangiogenesis, increased contractility, reduced fibrosis, and enhanced the quality of life in patients with chronic ICM in several clinical trials[52,56-59]. Intramyocardial MSC injection in patients reduced infarct size, indicating an improvement in myocardial fibrosis[60]. The improvements in regional contractility in the scarred myocardium were followed by reverse remodeling, evidenced by decreased end-diastolic and end-systolic volumes[60]. Transendocardial injection of either 20 million or 100 million allogeneic human MSCs (hMSCs) in 30 patients reduced scar size; however, the higher dose resulted in a greater improvement in cardiac function than that for the lower dose[61]. These findings suggest that MSC therapy may help in the treatment of myocardial fibrosis and lead to better structural recovery and cardiac function in the infarcted myocardium. Consequently, among all types of stem cells, MSCs are emerging as the most promising for treating heart diseases[62,63]. Intracoronary autologous transplantation of BMCs has been found to increase ventricular contractility and restore contractile function to previously nonviable scars[64]. In addition, infarct size reduction and alleviation of cardiac fibrosis have been observed[51,53,65]. Importantly, in a clinical trial, BMC transplantation has not been shown to produce aberrant cardiac rhythms in humans[52], and preoperative mobilization of BMCs followed by revascularization surgery has been deemed safe[66]. In the phase 3 randomized controlled trial PERFECT study, bone marrow hematopoietic stem cells, through SH2B3/LNK gene manipulation, significantly improved myocardial repair and preserved cardiac function, possibly by reducing left ventricular fibrosis[67]. However, mixed results have been obtained. In a single-blind randomized controlled trial, intracoronary autologous MSC transplantation did not improve cardiac function significantly following acute MI[68]. Additionally, low engraftment rates[69,70] and concerns over tumorigenic potential[71] have limited the therapeutic applicability of stem cells for CVD.
The overlay visualization of co-occurring keywords showed that early regeneration research focused on cell-based therapies, such as stem cell injections[72-75], cell infusions[76], cell sheet transplantations[77-79], and cell patches[80]. Recently, stem cell-free therapies, including exosome-, EV-, and miRNA-based therapies, have emerged as candidate therapeutic approaches for myocardial fibrosis. Stem cell-free therapy can avoid some risks of tumor formation[81], as it exerts indirect effects. Stem cell-derived EVs include microvesicles and exosomes, which are cell-derived membrane structures originating from the endosomal system or the plasma membrane[82,83]. There is growing preclinical evidence that stem cells (such as BMCs, MSCs, and human induced pluripotent stem cells) protect against cardiomyopathy through paracrine mechanisms rather than direct differentiation into CMs or new cardiac muscle[84-87]. In a randomized dose-finding clinical trial, transendocardial MSC injections in patients with ICM demonstrated that lower doses lead to more significant improvements in cardiac function and infarct size[88]. The beneficial effect is believed to be linked to reduced myocardial fibrosis and the increased release of paracrine factors.
Exosomes play significant roles in the diagnosis[89,90] and therapy[91-94] of myocardial fibrosis and are promising biotherapeutics and drug delivery vectors[95]. Following cardiac injury, exosomes derived from exogenous stem cells regulate apoptosis, proliferation, angiogenesis, and fibrosis in the infarcted heart[96]. The release of paracrine factors from stem cells creates functional microvascular networks with red blood cell perfusion, which protects the myocardium and may contribute to the mechanism underlying the repair of damaged myocardial tissues[97]. Hypoxia-inducible factor-1 overexpression in exosomes from MSCs decreased myocardial fibrosis by promoting neovessel development in rats with MI[98]. Furthermore, a minimally invasive spray based on MSC exosomes and biomaterials was used to minimize myocardial fibrosis in a rat model of acute MI[99]. Citro et al[100] suggested that hMSCs and human induced pluripotent stem cell-CMs exhibit potent autocrine and paracrine mechanisms that promote cardiac function and prevent myocardial fibrosis in male athymic nude rats. Cell-free therapies and stem cell-mediated paracrine signaling could be significant breakthroughs in the development of cardiac fibrosis treatments. In addition, the concept of selective miRNA transmission between cells via stem cell-derived EVs, which shuttle miRNAs between cells, is appealing[101-103]. MSCs partially reduced MI size by delivering cytoprotective miRNAs[104]. EVs containing several antifibrotic and cardiac-specific miRNAs reversed the phenotype of activated fibroblasts in vitro, reduced fibrosis in vivo, and improved overall cardiac function[105].
As shown in Table 8, the current focal areas of research on the mechanisms related to the pathogenesis and development of myocardial fibrosis include “angiogenesis”, “inflammation”, “apoptosis”, “autophagy”, and “oxidative stress”. Stem cell therapy can counteract multiple pathological mechanisms during the regeneration of myocardial cells, thereby improving myocardial fibrosis and cardiac function.
Angiogenesis refers to the formation of new blood vessels from pre-existing vessels during the early stage of vasculogenesis[106]. Ischemic CVD is associated with angiogenesis and has been treated using angiogenic strategies[107,108]. Certain cell populations, including cardiac-resident macrophages[109], heterogeneous podoplanin-expressing cells[110], and natural killer cells[111], can promote angiogenesis while also inhibiting fibrosis in ischemic heart disease. EVs and exosomes secreted by stem cells can alleviate myocardial fibrosis and improve cardiac function by enhancing angiogenesis[112-114]. However, emerging evidence suggests that a vigorous angiogenic response often results in fibrosis due to a high apoptosis burden[115-118]. Consequently, stem cell therapy may exert only moderate pro-angiogenic effects.
An injectable oxidized hyaluronic acid-polylysine hydrogel was developed to conveniently load adipose-derived MSC exosomes. These exosomes attenuated inflammation in the early phase of MI and, in the later stage, reduced myocardial fibrosis, promoted angiogenesis, and restored electrophysiological function[119]. Reducing inflammation creates a more suitable environment for stem cell therapy, increasing graft cell numbers and thereby treating myocardial fibrosis more effectively[120,121].
Apoptosis is regulated at the genetic level, ensuring the efficient and orderly removal of damaged cells[122]. Cardiac apoptosis plays an important role in the formation of fibrotic scars and severe impairment of heart function[123,124]. Transplanting hMSCs and endothelial colony-forming cells into NOD/SCID mice with acute MI decreased CM apoptosis significantly, which was associated with significant reductions in scar size, myocardial fibrosis, and cardiac remodeling, ultimately improving cardiac function during rehabilitation[125]. Transplanting MSCs with molecular capsules significantly improved the cardiac microenvironment, increased the survival of transplanted cells, promoted angiogenesis, reduced CM apoptosis, and decreased myocardial fibrosis, thereby enhancing cardiac repair[126].
Myocardial ischemia can stimulate autophagy, an intracellular bulk degradation process of cytoplasmic components that leads to cell death[127,128]. While moderate autophagy can protect cardiac myocytes[127-129], excessive autophagy contributes to the progression of myocardial fibrosis[130-134]. Several studies have demonstrated that inhibiting autophagy in CMs can protect the heart from hypertrophy and fibrosis[131,135-139]. In vivo, pretreatment with bradykinin prior to human cardiac progenitor cell transplantation effectively promoted cardiac function and reduced myocardial fibrosis[140]. Bradykinin suppressed H2O2-induced cell autophagy by increasing P62 expression while decreasing Beclin1, ATG5, and LC3II/I expression[140].
In rats, inhibiting oxidative stress can alleviate HF and myocardial fibrosis[141]. When sirtuin3-overexpressing hMSCs, which have increased antioxidant capacity, were transplanted into adult rats with MI, the infarct size diminished, the number of apoptotic cells decreased, cardiac function improved, and the survival rate of transplanted cells increased[142]. MSCs exhibit high oxidative stress resistance by regulating the redox microenvironment, which involves increasing heme-oxygenase-1 expression, decreasing the 8-OHdG concentration in tissues, and inhibiting reactive oxygen species-induced apoptosis[143-146]. In our study, the map of co-occurring keywords revealed that early research on the pathogenesis of myocardial fibrosis primarily focused on angiogenesis, inflammation, apoptosis, and oxidative stress. Recently, an increasing number of studies have focused on autophagy to uncover the mechanisms of action and targets of stem cell therapy.
This is the first bibliometric study of stem cell therapy and myocardial fibrosis. Our visual analysis provides insights into research hotspots and developmental trends in these fields. However, this study has some limitations. First, only papers from the WOSCC database were considered, excluding those from PubMed and EMBASE. WOSCC covers most high-impact articles indexed in PubMed and has distinct advantages over other platforms, such as its broad multidisciplinary coverage, advanced search and filtering options, and robust citation tracking tools, making it particularly suitable for interdisciplinary research and bibliometric studies. Second, papers on stem cells and myocardial fibrosis that were not published in English were excluded from the analysis. Nonetheless, most high-impact articles were published in English, and focusing on English-language publications ensures the inclusion of high-quality research, minimizes translation challenges and inaccuracies, and improves the rigor and precision of our analysis through manual screening of potentially irrelevant articles. Third, our study acknowledges the potential influence of citation bias in the field, particularly positive publication bias, where articles with promising results may be cited more frequently than studies with non-confirmatory or negative findings. For example, the high-impact study by Orlic et al[24], “Bone marrow cells regenerate infarcted myocardium”[24], was initially influential but later challenged by subsequent studies, such as those by Murry et al[147] and Balsam et al[148], published in the same journal (Nature). This highlights an important limitation in citation-based studies, where non-confirmatory findings may not achieve similar citation counts and could be underrepresented in analyses. We recognize this limitation and suggest that future research consider methods to systematically account for both positive and negative findings to provide a more comprehensive view of the field. Despite these limitations, our comprehensive review of stem cell therapy in myocardial fibrosis offers valuable guidance. Future research should focus on optimizing the production and isolation of EVs, ensuring their scalability for clinical applications, and assessing their long-term efficacy and safety in clinical trials. Moreover, artificial intelligence and machine learning can play a crucial role in accelerating research on myocardial fibrosis and stem cell therapies. These technologies could help optimize stem cell culture conditions, determine the most effective stem cell sources for individual patients, and enhance the design of clinical trials through predictive modeling.
In summary, myocardial fibrosis and stem cells have been a major focus of research for over 20 years, as evidenced by both publications and citations. The United States is the leading country, and Harvard University is the dominant organization in this field. The high-frequency keyword analysis indicated that early research focused on stem cell-based therapy; recently, cell-free therapy using EVs, exosomes, and selected miRNAs has gained increasing attention as an emerging treatment strategy. Angiogenesis, inflammation, apoptosis, and oxidative stress were the most prevalent mechanisms evaluated in early research, while autophagy became more prominent later. These findings highlight current research hotspots and can guide future studies. We conclude that new cell-free therapeutic options for myocardial fibrosis and stem cell-mediated paracrine protection may be key topics in the future.
| 1. | Perk J. The 2016 version of the European Guidelines on Cardiovascular Prevention. Eur Heart J Cardiovasc Pharmacother. 2017;3:9-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Luo F, Wang T, Zeng L, Zhu S, Cao W, Wu W, Wu H, Zou T. Diagnostic potential of circulating LncRNAs in human cardiovascular disease: a meta-analysis. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6994] [Cited by in RCA: 6265] [Article Influence: 1253.0] [Reference Citation Analysis (0)] |
| 4. | Liu C, Du L, Wang S, Kong L, Zhang S, Li S, Zhang W, Du G. Differences in the prevention and control of cardiovascular and cerebrovascular diseases. Pharmacol Res. 2021;170:105737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Mensah GA, Fuster V, Murray CJL, Roth GA; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J Am Coll Cardiol. 2023;82:2350-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 536] [Article Influence: 268.0] [Reference Citation Analysis (0)] |
| 6. | Teekakirikul P, Padera RF, Seidman JG, Seidman CE. Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics. J Cell Biol. 2012;199:417-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Urban ML, Manenti L, Vaglio A. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Piek A, de Boer RA, Silljé HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21:199-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 9. | Teekakirikul P, Zhu W, Huang HC, Fung E. Hypertrophic Cardiomyopathy: An Overview of Genetics and Management. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Travers JG, Tharp CA, Rubino M, McKinsey TA. Therapeutic targets for cardiac fibrosis: from old school to next-gen. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 11. | Sozzi FB, Gherbesi E, Faggiano A, Gnan E, Maruccio A, Schiavone M, Iacuzio L, Carugo S. Viral Myocarditis: Classification, Diagnosis, and Clinical Implications. Front Cardiovasc Med. 2022;9:908663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | McCormick ME, Rojas M, Moser-Katz T, Tzima E, Reader JS. Natural aminoacyl tRNA synthetase fragment enhances cardiac function after myocardial infarction. PLoS One. 2014;9:e109325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Sun Y, Liu J, Xu Z, Lin X, Zhang X, Li L, Li Y. Matrix stiffness regulates myocardial differentiation of human umbilical cord mesenchymal stem cells. Aging (Albany NY). 2020;13:2231-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 572] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 15. | Fan Z, Guan J. Antifibrotic therapies to control cardiac fibrosis. Biomater Res. 2016;20:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 17. | Rentrop KP, Feit F. Reperfusion therapy for acute myocardial infarction: Concepts and controversies from inception to acceptance. Am Heart J. 2015;170:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Shen H, Zhou E, Wei X, Fu Z, Niu C, Li Y, Pan B, Mathew AV, Wang X, Pennathur S, Zheng L, Wang Y. High density lipoprotein promotes proliferation of adipose-derived stem cells via S1P1 receptor and Akt, ERK1/2 signal pathways. Stem Cell Res Ther. 2015;6:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 728] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 20. | Alonaizan R, Carr C. Cardiac regeneration following myocardial infarction: the need for regeneration and a review of cardiac stromal cell populations used for transplantation. Biochem Soc Trans. 2022;50:269-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marbán L, Marbán E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Wallin JA. Bibliometric methods: pitfalls and possibilities. Basic Clin Pharmacol Toxicol. 2005;97:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Waltman L, van Eck NJ, Noyons EC. A unified approach to mapping and clustering of bibliometric networks. J Informetr. 2010;4:629-635. [RCA] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 3547] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 25. | Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2247] [Cited by in RCA: 1897] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 26. | Alshammary S, Fukushima S, Miyagawa S, Matsuda T, Nishi H, Saito A, Kamata S, Asahara T, Sawa Y. Impact of cardiac stem cell sheet transplantation on myocardial infarction. Surg Today. 2013;43:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Kamata S, Miyagawa S, Fukushima S, Nakatani S, Kawamoto A, Saito A, Harada A, Shimizu T, Daimon T, Okano T, Asahara T, Sawa Y. Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: analysis of layer-specific regional cardiac function. Cell Transplant. 2014;23:1305-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Kawamura M, Miyagawa S, Fukushima S, Saito A, Toda K, Daimon T, Shimizu T, Okano T, Sawa Y. Xenotransplantation of Bone Marrow-Derived Human Mesenchymal Stem Cell Sheets Attenuates Left Ventricular Remodeling in a Porcine Ischemic Cardiomyopathy Model. Tissue Eng Part A. 2015;21:2272-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Kawamura M, Paulsen MJ, Goldstone AB, Shudo Y, Wang H, Steele AN, Stapleton LM, Edwards BB, Eskandari A, Truong VN, Jaatinen KJ, Ingason AB, Miyagawa S, Sawa Y, Woo YJ. Tissue-engineered smooth muscle cell and endothelial progenitor cell bi-level cell sheets prevent progression of cardiac dysfunction, microvascular dysfunction, and interstitial fibrosis in a rodent model of type 1 diabetes-induced cardiomyopathy. Cardiovasc Diabetol. 2017;16:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Imanishi Y, Saito A, Komoda H, Kitagawa-Sakakida S, Miyagawa S, Kondoh H, Ichikawa H, Sawa Y. Allogenic mesenchymal stem cell transplantation has a therapeutic effect in acute myocardial infarction in rats. J Mol Cell Cardiol. 2008;44:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Mori D, Miyagawa S, Kido T, Hata H, Ueno T, Toda K, Kuratani T, Oota M, Kawai K, Kurata H, Nishida H, Sawa Y. Adipose-derived mesenchymal stem cells preserve cardiac function via ANT-1 in dilated cardiomyopathy hamster model. Regen Ther. 2021;18:182-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Sougawa N, Miyagawa S, Kawamura T, Matsuura R, Harada A, Sakai Y, Mochizuki-Oda N, Sato-Nishiuchi R, Sekiguchi K, Sawa Y. Combined administration of laminin-221 and prostacyclin agonist enhances endogenous cardiac repair in an acute infarct rat heart. Sci Rep. 2021;11:22243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Singla DK. Stem cells in the infarcted heart. J Cardiovasc Transl Res. 2010;3:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Singla DK. Stem cells and exosomes in cardiac repair. Curr Opin Pharmacol. 2016;27:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2438] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 36. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1645] [Cited by in RCA: 1543] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 37. | Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1261] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 38. | Aksoy U, Küçük M, Versiani MA, Orhan K. Publication trends in micro-CT endodontic research: a bibliometric analysis over a 25-year period. Int Endod J. 2021;54:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 40. | Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 711] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 41. | Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1890] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 42. | Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 518] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 43. | Leong DP, Chakrabarty A, Shipp N, Molaee P, Madsen PL, Joerg L, Sullivan T, Worthley SG, De Pasquale CG, Sanders P, Selvanayagam JB. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J. 2012;33:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 884] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 45. | Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 46. | Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017;2:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 47. | Li F, Xu M, Fan Y, Wang Y, Song Y, Cui X, Fu M, Qi B, Han X, Zhou J, Ge J. Diffuse myocardial fibrosis and the prognosis of heart failure with reduced ejection fraction in Chinese patients: a cohort study. Int J Cardiovasc Imaging. 2020;36:671-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Nair N, Gongora E. Stem cell therapy in heart failure: Where do we stand today? Biochim Biophys Acta Mol Basis Dis. 2020;1866:165489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Li L, Zhang Y, Li Y, Yu B, Xu Y, Zhao S, Guan Z. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl Int. 2008;21:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Huang W, Wang T, Zhang D, Zhao T, Dai B, Ashraf A, Wang X, Xu M, Millard RW, Fan GC, Ashraf M, Yu XY, Wang Y. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21:778-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 52. | Katritsis DG, Sotiropoulou P, Giazitzoglou E, Karvouni E, Papamichail M. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004;109:2386-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 691] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 56. | Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 827] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 57. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 995] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 58. | Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 59. | Haack-Sørensen M, Friis T, Mathiasen AB, Jørgensen E, Hansen L, Dickmeiss E, Ekblond A, Kastrup J. Direct intramyocardial mesenchymal stromal cell injections in patients with severe refractory angina: one-year follow-up. Cell Transplant. 2013;22:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 61. | Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Golpanian S, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid F, Vidro-Casiano M, Saltzman RG, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 62. | Kobayashi K, Suzuki K. Mesenchymal Stem/Stromal Cell-Based Therapy for Heart Failure- What Is the Best Source? Circ J. 2018;82:2222-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 563] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 64. | Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101-I107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q. Potent Paracrine Effects of human induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells Attenuate Doxorubicin-induced Cardiomyopathy. Sci Rep. 2015;5:11235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Dato GM, Sansone F, Omedé P, Zingarelli E, Flocco R, Punta G, Parisi F, Forsennati PG, Bardi GL, Del Ponte S, Casabona R, Tarella C. Preoperative mobilization of bone marrow-derived cells followed by revascularization surgery: early and long-term outcome. Int J Artif Organs. 2012;35:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Wolfien M, Klatt D, Salybekov AA, Ii M, Komatsu-Horii M, Gaebel R, Philippou-Massier J, Schrinner E, Akimaru H, Akimaru E, David R, Garbade J, Gummert J, Haverich A, Hennig H, Iwasaki H, Kaminski A, Kawamoto A, Klopsch C, Kowallick JT, Krebs S, Nesteruk J, Reichenspurner H, Ritter C, Stamm C, Tani-Yokoyama A, Blum H, Wolkenhauer O, Schambach A, Asahara T, Steinhoff G. Hematopoietic stem-cell senescence and myocardial repair - Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3. EBioMedicine. 2020;57:102862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Zhang R, Yu J, Zhang N, Li W, Wang J, Cai G, Chen Y, Yang Y, Liu Z. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: single-blind, multicenter, randomized controlled trial. Stem Cell Res Ther. 2021;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Fukushima S, Sawa Y, Suzuki K. Choice of cell-delivery route for successful cell transplantation therapy for the heart. Future Cardiol. 2013;9:215-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Clavellina D, Balkan W, Hare JM. Stem cell therapy for acute myocardial infarction: Mesenchymal Stem Cells and induced Pluripotent Stem Cells. Expert Opin Biol Ther. 2023;23:951-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Wu R, Hu X, Wang J. Concise Review: Optimized Strategies for Stem Cell-Based Therapy in Myocardial Repair: Clinical Translatability and Potential Limitation. Stem Cells. 2018;36:482-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Stępniewski J, Tomczyk M, Andrysiak K, Kraszewska I, Martyniak A, Langrzyk A, Kulik K, Wiśniewska E, Jeż M, Florczyk-Soluch U, Polak K, Podkalicka P, Kachamakova-Trojanowska N, Józkowicz A, Jaźwa-Kusior A, Dulak J. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Sirish P, Thai PN, Lee JH, Yang J, Zhang XD, Ren L, Li N, Timofeyev V, Lee KSS, Nader CE, Rowland DJ, Yechikov S, Ganaga S, Young N, Lieu DK, Yamoah EN, Hammock BD, Chiamvimonvat N. Suppression of inflammation and fibrosis using soluble epoxide hydrolase inhibitors enhances cardiac stem cell-based therapy. Stem Cells Transl Med. 2020;9:1570-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Guan G, Huo D, Li Y, Zhao X, Li Y, Qin Z, Sun D, Yang G, Yang M, Tan J, Zeng W, Zhu C. Engineering hiPSC-CM and hiPSC-EC laden 3D nanofibrous splenic hydrogel for improving cardiac function through revascularization and remuscularization in infarcted heart. Bioact Mater. 2021;6:4415-4429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Liu J, Liang X, Li M, Lin F, Ma X, Xin Y, Meng Q, Zhuang R, Zhang Q, Han W, Gao L, He Z, Zhou X, Liu Z. Intramyocardial injected human umbilical cord-derived mesenchymal stem cells (HucMSCs) contribute to the recovery of cardiac function and the migration of CD4(+) T cells into the infarcted heart via CCL5/CCR5 signaling. Stem Cell Res Ther. 2022;13:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Bolli RAR, Arshia A, Hassan SA, Dasari C, Nong Y, Guo Y, Tomlin AA, Li Q. Cardiac Mesenchymal Cells Cultured at Physiologic Oxygen Tension Have Superior Therapeutic Efficacy in Heart Failure Caused by Myocardial Infarction. Front Cell Dev Biol. 2021;9:662415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T, Sakata R, Yamashita JK. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, Sougawa N, Kawamura T, Daimon T, Shimizu T, Okano T, Toda K, Sawa Y. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013;128:S87-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 79. | Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 80. | Hamdi H, Planat-Benard V, Bel A, Neamatalla H, Saccenti L, Calderon D, Bellamy V, Bon M, Perrier MC, Mandet C, Bruneval P, Casteilla L, Hagège AA, Pucéat M, Agbulut O, Menasché P. Long-term functional benefits of epicardial patches as cell carriers. Cell Transplant. 2014;23:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3060] [Cited by in RCA: 5570] [Article Influence: 795.7] [Reference Citation Analysis (0)] |
| 83. | Stahl PD, Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology (Bethesda). 2019;34:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 84. | Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 85. | Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front Physiol. 2016;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 86. | Dougherty JA, Kumar N, Noor M, Angelos MG, Khan M, Chen CA, Khan M. Extracellular Vesicles Released by Human Induced-Pluripotent Stem Cell-Derived Cardiomyocytes Promote Angiogenesis. Front Physiol. 2018;9:1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Ye M, Ni Q, Qi H, Qian X, Chen J, Guo X, Li M, Zhao Y, Xue G, Deng H, Zhang L. Exosomes Derived from Human Induced Pluripotent Stem Cells-Endothelia Cells Promotes Postnatal Angiogenesis in Mice Bearing Ischemic Limbs. Int J Biol Sci. 2019;15:158-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 903] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 89. | Olson EN. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci Transl Med. 2014;6:239ps3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 90. | Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner DM, Jakob P, Nakagawa S, Blankenberg S, Engelhardt S, Thum T, Weber C, Meder B, Hajjar R, Landmesser U. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2018;39:2704-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 91. | El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 92. | Ibrahim AGE, Li C, Rogers R, Fournier M, Li L, Vaturi SD, Antes T, Sanchez L, Akhmerov A, Moseley JJ, Tobin B, Rodriguez-Borlado L, Smith RR, Marbán L, Marbán E. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat Biomed Eng. 2019;3:695-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Milano G, Biemmi V, Lazzarini E, Balbi C, Ciullo A, Bolis S, Ameri P, Di Silvestre D, Mauri P, Barile L, Vassalli G. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc Res. 2020;116:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 94. | Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 875] [Article Influence: 218.8] [Reference Citation Analysis (0)] |
| 95. | Fan J, Ren M, He Y. Diagnostic and Therapeutic Properties of Exosomes in Cardiac Fibrosis. Front Cell Dev Biol. 2022;10:931082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 96. | Fan C, Zhang E, Joshi J, Yang J, Zhang J, Zhu W. Utilization of Human Induced Pluripotent Stem Cells for Cardiac Repair. Front Cell Dev Biol. 2020;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 98. | Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 99. | Yao J, Huang K, Zhu D, Chen T, Jiang Y, Zhang J, Mi L, Xuan H, Hu S, Li J, Zhou Y, Cheng K. A Minimally Invasive Exosome Spray Repairs Heart after Myocardial Infarction. ACS Nano. 2021;15:11099-11111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 100. | Citro L, Naidu S, Hassan F, Kuppusamy ML, Kuppusamy P, Angelos MG, Khan M. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS One. 2014;9:e116281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 102. | Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 103. | Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 104. | Charles CJ, Li RR, Yeung T, Mazlan SMI, Lai RC, de Kleijn DPV, Lim SK, Richards AM. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization With MRI in a Porcine Model. Front Cardiovasc Med. 2020;7:601990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Prieto-Vila M, Yoshioka Y, Kuriyama N, Okamura A, Yamamoto Y, Muranaka A, Ochiya T. Adult cardiomyocytes-derived EVs for the treatment of cardiac fibrosis. J Extracell Vesicles. 2024;13:e12461. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 106. | Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF, Bergers G, Bikfalvi A, Bischoff J, Böck BC, Brooks PC, Bussolino F, Cakir B, Carmeliet P, Castranova D, Cimpean AM, Cleaver O, Coukos G, Davis GE, De Palma M, Dimberg A, Dings RPM, Djonov V, Dudley AC, Dufton NP, Fendt SM, Ferrara N, Fruttiger M, Fukumura D, Ghesquière B, Gong Y, Griffin RJ, Harris AL, Hughes CCW, Hultgren NW, Iruela-Arispe ML, Irving M, Jain RK, Kalluri R, Kalucka J, Kerbel RS, Kitajewski J, Klaassen I, Kleinmann HK, Koolwijk P, Kuczynski E, Kwak BR, Marien K, Melero-Martin JM, Munn LL, Nicosia RF, Noel A, Nurro J, Olsson AK, Petrova TV, Pietras K, Pili R, Pollard JW, Post MJ, Quax PHA, Rabinovich GA, Raica M, Randi AM, Ribatti D, Ruegg C, Schlingemann RO, Schulte-Merker S, Smith LEH, Song JW, Stacker SA, Stalin J, Stratman AN, Van de Velde M, van Hinsbergh VWM, Vermeulen PB, Waltenberger J, Weinstein BM, Xin H, Yetkin-Arik B, Yla-Herttuala S, Yoder MC, Griffioen AW. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21:425-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 461] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 107. | Conway RE, Rojas C, Alt J, Nováková Z, Richardson SM, Rodrick TC, Fuentes JL, Richardson NH, Attalla J, Stewart S, Fahmy B, Barinka C, Ghosh M, Shapiro LH, Slusher BS. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis. 2016;19:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Gao JH, Wen SL, Feng S, Yang WJ, Lu YY, Tong H, Liu R, Tang SH, Huang ZY, Tang YM, Yang JH, Xie HQ, Tang CW. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis. 2016;19:501-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 109. | Revelo XS, Parthiban P, Chen C, Barrow F, Fredrickson G, Wang H, Yücel D, Herman A, van Berlo JH. Cardiac Resident Macrophages Prevent Fibrosis and Stimulate Angiogenesis. Circ Res. 2021;129:1086-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 110. | Cimini M, Kishore R. Role of Podoplanin-Positive Cells in Cardiac Fibrosis and Angiogenesis After Ischemia. Front Physiol. 2021;12:667278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther. 2021;6:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 112. | Xuan W, Wang L, Xu M, Weintraub NL, Ashraf M. miRNAs in Extracellular Vesicles from iPS-Derived Cardiac Progenitor Cells Effectively Reduce Fibrosis and Promote Angiogenesis in Infarcted Heart. Stem Cells Int. 2019;2019:3726392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Wu Q, Wang J, Tan WLW, Jiang Y, Wang S, Li Q, Yu X, Tan J, Liu S, Zhang P, Tiang Z, Chen Z, Foo RS, Yang HT. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020;11:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 114. | Hu J, Chen X, Li P, Lu X, Yan J, Tan H, Zhang C. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovasc Diagn Ther. 2021;11:348-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 115. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1144] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 116. | Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 291] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 117. | Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27:6285-6298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 118. | Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 119. | Ren Y, Wang W, Yu C, Wang Y, Qiu Y, Yue Z, Yu Q, Lu J, Che P, Li J, Sun H. An injectable exosome-loaded hyaluronic acid-polylysine hydrogel for cardiac repair via modulating oxidative stress and the inflammatory microenvironment. Int J Biol Macromol. 2024;275:133622. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Murtuza B, Suzuki K, Bou-Gharios G, Beauchamp JR, Smolenski RT, Partridge TA, Yacoub MH. Transplantation of skeletal myoblasts secreting an IL-1 inhibitor modulates adverse remodeling in infarcted murine myocardium. Proc Natl Acad Sci U S A. 2004;101:4216-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Khodayari S, Khodayari H, Amiri AZ, Eslami M, Farhud D, Hescheler J, Nayernia K. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell Physiol Biochem. 2019;53:887-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 122. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10243] [Cited by in RCA: 9533] [Article Influence: 529.6] [Reference Citation Analysis (0)] |
| 123. | Liu T, Song D, Dong J, Zhu P, Liu J, Liu W, Ma X, Zhao L, Ling S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front Physiol. 2017;8:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 124. | Chen L, Zhang D, Yu L, Dong H. Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury. Bioengineered. 2019;10:121-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 125. | Tripathi H, Domingues A, Donahue R, Cras A, Guerin CL, Gao E, Levitan B, Ratajczak MZ, Smadja DM, Abdel-Latif A, Tarhuni WM. Combined Transplantation of Human MSCs and ECFCs Improves Cardiac Function and Decrease Cardiomyocyte Apoptosis After Acute Myocardial Infarction. Stem Cell Rev Rep. 2023;19:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 126. | Wu T, Zhang X, Liu Y, Cui C, Sun Y, Liu W. Wet adhesive hydrogel cardiac patch loaded with anti-oxidative, autophagy-regulating molecule capsules and MSCs for restoring infarcted myocardium. Bioact Mater. 2023;21:20-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 127. | Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 128. | Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1235] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 129. | Wu T, Liu W. Functional hydrogels for the treatment of myocardial infarction. NPG Asia Mater. 2022;14. [DOI] [Full Text] |
| 130. | Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1729] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 131. | Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 624] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 132. | Marchi S, Corricelli M, Trapani E, Bravi L, Pittaro A, Delle Monache S, Ferroni L, Patergnani S, Missiroli S, Goitre L, Trabalzini L, Rimessi A, Giorgi C, Zavan B, Cassoni P, Dejana E, Retta SF, Pinton P. Defective autophagy is a key feature of cerebral cavernous malformations. EMBO Mol Med. 2015;7:1403-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 133. | Patschan D, Schwarze K, Henze E, Patschan S, Müller GA. Endothelial autophagy and Endothelial-to-Mesenchymal Transition (EndoMT) in eEPC treatment of ischemic AKI. J Nephrol. 2016;29:637-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 134. | Zou J, Liu Y, Li B, Zheng Z, Ke X, Hao Y, Li X, Li X, Liu F, Zhang Z. Autophagy attenuates endothelial-to-mesenchymal transition by promoting Snail degradation in human cardiac microvascular endothelial cells. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Zhang C, Wang F, Zhang Y, Kang Y, Wang H, Si M, Su L, Xin X, Xue F, Hao F, Yu L, Xu J, Liu Y, Xue M. Celecoxib prevents pressure overload-induced cardiac hypertrophy and dysfunction by inhibiting inflammation, apoptosis and oxidative stress. J Cell Mol Med. 2016;20:116-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 136. | Liu R, Zhang HB, Yang J, Wang JR, Liu JX, Li CL. Curcumin alleviates isoproterenol-induced cardiac hypertrophy and fibrosis through inhibition of autophagy and activation of mTOR. Eur Rev Med Pharmacol Sci. 2018;22:7500-7508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 137. | Gao W, Zhou Z, Liang B, Huang Y, Yang Z, Chen Y, Zhang L, Yan C, Wang J, Lu L, Wen Z, Xian S, Wang L. Inhibiting Receptor of Advanced Glycation End Products Attenuates Pressure Overload-Induced Cardiac Dysfunction by Preventing Excessive Autophagy. Front Physiol. 2018;9:1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Zhu W, Wu RD, Lv YG, Liu YM, Huang H, Xu JQ. BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed Pharmacother. 2020;121:109368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 139. | Zhang L, He J, Wang J, Liu J, Chen Z, Deng B, Wei L, Wu H, Liang B, Li H, Huang Y, Lu L, Yang Z, Xian S, Wang L. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 2021;12:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 140. | Wu C, Zhou XX, Li JZ, Qiang HF, Wang Y, Li G. Pretreatment of cardiac progenitor cells with bradykinin attenuates H(2)O(2)-induced cell apoptosis and improves cardiac function in rats by regulating autophagy. Stem Cell Res Ther. 2021;12:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Li J, Ding H, Li Y, Zhou H, Wang W, Mei Y, Zhang R. Alarin alleviated cardiac fibrosis via attenuating oxidative stress in heart failure rats. Amino Acids. 2021;53:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Zhang DY, Zhang CF, Fu BC, Sun L, Wang XQ, Chen W, Liu W, Liu KY, Du GQ, Ma CY, Jiang SL, Li RK, Tian H. Sirtuin3 protects aged human mesenchymal stem cells against oxidative stress and enhances efficacy of cell therapy for ischaemic heart diseases. J Cell Mol Med. 2018;22:5504-5517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 143. | Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 144. | Liu H, McTaggart SJ, Johnson DW, Gobe GC. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Jin G, Qiu G, Wu D, Hu Y, Qiao P, Fan C, Gao F. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int J Mol Med. 2013;31:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 146. | Calió ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA, Simões Mde J, Lisbôa-Nascimento T, Ferreira AT, Bertoncini CR. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med. 2014;70:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 147. | Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1490] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 148. | Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1200] [Article Influence: 57.1] [Reference Citation Analysis (0)] |