Published online Dec 26, 2024. doi: 10.4252/wjsc.v16.i12.1062
Revised: October 15, 2024
Accepted: November 18, 2024
Published online: December 26, 2024
Processing time: 223 Days and 20.6 Hours
Mesenchymal stem cells (MSCs) are capable of self-renewal and differentiation, and extensive studies have demonstrated their therapeutic potential in atherosclerosis (AS).
To conduct a bibliometric analysis of studies on the use of MSC therapy for AS over the past two decades, assess key trends and provide insights for future research directions.
We systematically searched the Web of Science Core Collection database for articles published between 1999 and 2023, yielding a total of 556 articles. Visual representation and bibliometric analysis of information and trends were faci
The analyzed articles were predominantly from 52 countries/regions, with prominent contributions from China and the United States. A cohort of 3057 authors contributed to these publications, with the works of Libby P distinguished by their influence and citation count. Int J Mol Sci has emerged as the journal with the highest publication volume, prominently disseminating influential papers and identifying citation outbreaks. Furthermore, our analysis identified current research hotspots within the field, focusing on vascular progenitor cells, inflammatory mechanisms, and extracellular vesicles. Emerging research frontiers, such as extracellular vesicles and oxidative stress, have been highlighted as areas of burgeoning interest. Finally, we offer perspectives on the status of research and future directions of MSC therapy in AS.
This comprehensive analysis provides valuable insights for advancing scientific research on MSC therapy for AS. By elucidating pivotal trends and research directions, this study aimed to foster innovation and promote the progress of disciplines in this field, thereby contributing to advancing scientific knowledge and clinical practice.
Core Tip: This study provided a comprehensive overview of the research landscape on mesenchymal stem cell therapy in atherosclerosis through detailed bibliometric analysis. It not only collated and presented key studies in this field but also highlighted emerging trends, significant developments, and potential future research directions, providing a valuable foundation for further exploration and application of stem cell therapy in the treatment of atherosclerosis.
- Citation: Cheng X, Li YL, Wang H, Zhang RJ, Fan KY, Qi XT, Zheng GP, Dong HL. Mesenchymal stem cell therapy in atherosclerosis: A bibliometric and visual analysis. World J Stem Cells 2024; 16(12): 1062-1085
- URL: https://www.wjgnet.com/1948-0210/full/v16/i12/1062.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i12.1062
Cardiovascular disease (CVD) is a leading cause of mortality and disability worldwide and poses a significant threat to human health. Statistics indicate that approximately 30% of the annual global deaths can be attributed to CVD. Notably, atherosclerosis (AS) is the predominant cause of CVD and is specifically recognized as atherosclerotic CVD, imposing a substantial economic and social burden[1-4]. Effectively curbing the progression of AS is paramount for reducing the morbidity and mortality associated with CVD. AS manifests as a complex interplay of various risk factors, including hypertension, diabetes mellitus, smoking, alcohol consumption, age, sex, and heredity[5]. Elucidating the intricate mechanism and inhibition of AS formation by considering only a single factor is challenging. A hallmark of this disease is the deposition of lipids in the arterial intima, triggering inflammation and plaque formation. This process leads to profound structural and functional disruption of the arterial wall. Consequently, the cumulative effect of plaque accumulation and rupture primarily results in the narrowing and blockage of blood vessels, resulting in serious consequences, such as stroke, coronary heart disease, and heart attack[6]. Therefore, effective strategies to manage lipid levels, control inflammation, and promote a healthy lifestyle are imperative to prevent and treat AS.
Lowering lipid levels, particularly through statin administration, is the cornerstone of AS treatment[7,8]. Despite the significant advancements achieved with statins[9], several comprehensive controlled clinical studies have revealed their suboptimal efficacy in addressing CVD[10]. Moreover, some patients, especially older adults, face challenges in tolerating statin medications, thereby increasing the risk of discontinuation[11]. In addition to pharmacological approaches, alternative interventions such as endovascular stenting are commonly used to manage arterial stenosis or occlusion[12,13]. However, the prevalence of postprocedural complications, including stent restenosis and thrombosis, is high and, in certain instances, can pose life-threatening risks. Considering these challenges, global efforts are underway to explore novel treatment modalities.
Stem cells, characterized by their ability to self-renew and differentiate, represent a promising avenue for their exploration. These cells not only sustain their numbers by generating identical stem cells but also possess the ability to form specific cell types and tissues. Stem cell therapy has emerged as a well-established research methodology for addressing a diverse array of diseases[14-16]. Mesenchymal stem cells (MSCs), also referred to as pluripotent mesenchymal stromal cells, are the predominant stem cell type investigated in AS. These cells, derived from bone marrow, can be isolated from various tissues, including the blood, skin, white adipose tissue, liver, pancreas, spleen, thymus, skeletal muscle, placenta, and human gingiva[17-19]. MSCs are easily cultivated and expanded in vitro, and their surfaces are characterized by the expression of CD73, CD90, and CD105[20]. MSC therapy has significant therapeutic potential. Numerous studies have demonstrated its ability to stimulate vascular neovascularization[21,22], facilitate endothelial and arterial wall repair[23,24], diminish inflammatory responses, modulate macrophages, and inhibit foam cell formation[25,26]. These findings underscore the innovative prospects of MSC therapy for treating AS.
Bibliometric analysis employs statistical methods to qualitatively and quantitatively assess publications in databases. This method enables the extraction of valuable insights from data, facilitating the summarization and analysis of research progress in specific domains[27]. In recent years, the growing sophistication of statistical science has propelled bibliometric analysis into prominence, particularly in medical research. Through the visualization of networks and comprehensive analysis of the current literature, this approach objectively delineates the developmental history and research focal points of a specific field[28,29], and aids researchers in comprehending future research trends, defining research trajectories, and identifying collaborative opportunities[30,31].
This study presents a scientific and comprehensive analysis of the literature on MSC therapy for atherosclerotic diseases. Using visual networks, we statistically examined bibliometrics over the past two decades (1999-2023). This study is significant as a bibliometric analysis related to MSC therapy for atherosclerotic disease. This study is important for its capacity to meet the pressing needs of researchers in related fields, offering a concise and efficient means of comprehending the present state, research focal points, and trends within the field. Moreover, it assists in establishing attainable work objectives and identifying appropriate research directions.
The Web of Science Core Collection (WoSCC) database, provided by Clarivate Analytics, encompasses over 12000 high-quality international scholarly journals, establishing itself as a comprehensive and authoritative resource[32]. To conduct a bibliometric analysis, we utilized the WoSCC to gather global information, aligning it with established practices in previous studies. The raw data were collected on December 23, 2023, to mitigate potential biases from rapid updates. Employing the search strategy “(TS = (“Stem Cell Treatment”) OR TS = (“Stem Cell Therapy”) OR TS = (“Mesenchymal Stem Cell*”) OR TS = (“Mesenchymal Stromal Cell*”) OR TS = (“Mesenchymal Progenitor Cell*”)) AND (TS = (Atherosclerosis) OR TS = (Atheroscleroses) OR TS = (Atherogenesis))”, with language limited to English, we retrieved a total of 556 articles from WoSCC. Following weighting and data cleaning facilitated by CiteSpace software, 522 articles were retained for subsequent analysis. A schematic representation of the screening process is shown in Figure 1.
The initial step involved downloading raw data from the WoSCC, followed by its subsequent import into the WPS Office software. Three authors independently thoroughly screened the final articles, systematically collecting essential information, including the title, authors, keywords, affiliated institutions, country/region of origin, citation metrics, journal of publication, and publication date. The processed data were subsequently imported into VOSviewer 6.2 R6, CiteSpace 1.6.18[33], and the R package ‘bibliometrix’ for the bibliometric analysis.
CiteSpace is a software tool designed for scientific bibliometric research, with analysis and visualization capabilities to discern academic trends and patterns[34]. Furthermore, it constructs a comprehensive knowledge map of the field and provides a clear overview of a specific knowledge domain. Using various dynamic network analysis techniques, the tool reveals the developmental history, prominent research areas, emerging trends, and frontiers within a specific scientific field[35]. CiteSpace aims to assist researchers in making informed decisions and fostering deeper insights for further research and academic exploration.
Bibliometrix is an open-source R package for conducting bibliometric analysis created by Dr. Massimo Aria and Corrado Cuccurullo from the University of Naples, Italy (https://www.bibliometrix.org)[36]. In the present investigation, the bibliometrix, in conjunction with CiteSpace, was employed to scrutinize co-citation relationships within the literature. This approach facilitates a comprehensive examination of scholarly connections, enabling researchers to gain insights into prevailing academic trends and identify leading papers within their respective fields.
In this study, we employed VOSviewer software, developed by the Center for Science and Technology Studies at Leiden University in the Netherlands, to create and analyze bibliometric networks. It provides bibliometric networks, including co-cited journals, countries/regions of origin, and institutions[37]. Furthermore, we visualized publications and their cross-country interrelationships through R language. Finally, we conducted comprehensive co-occurrence and clustering analyses of authors, cited literature, and keyword co-citations via CiteSpace.
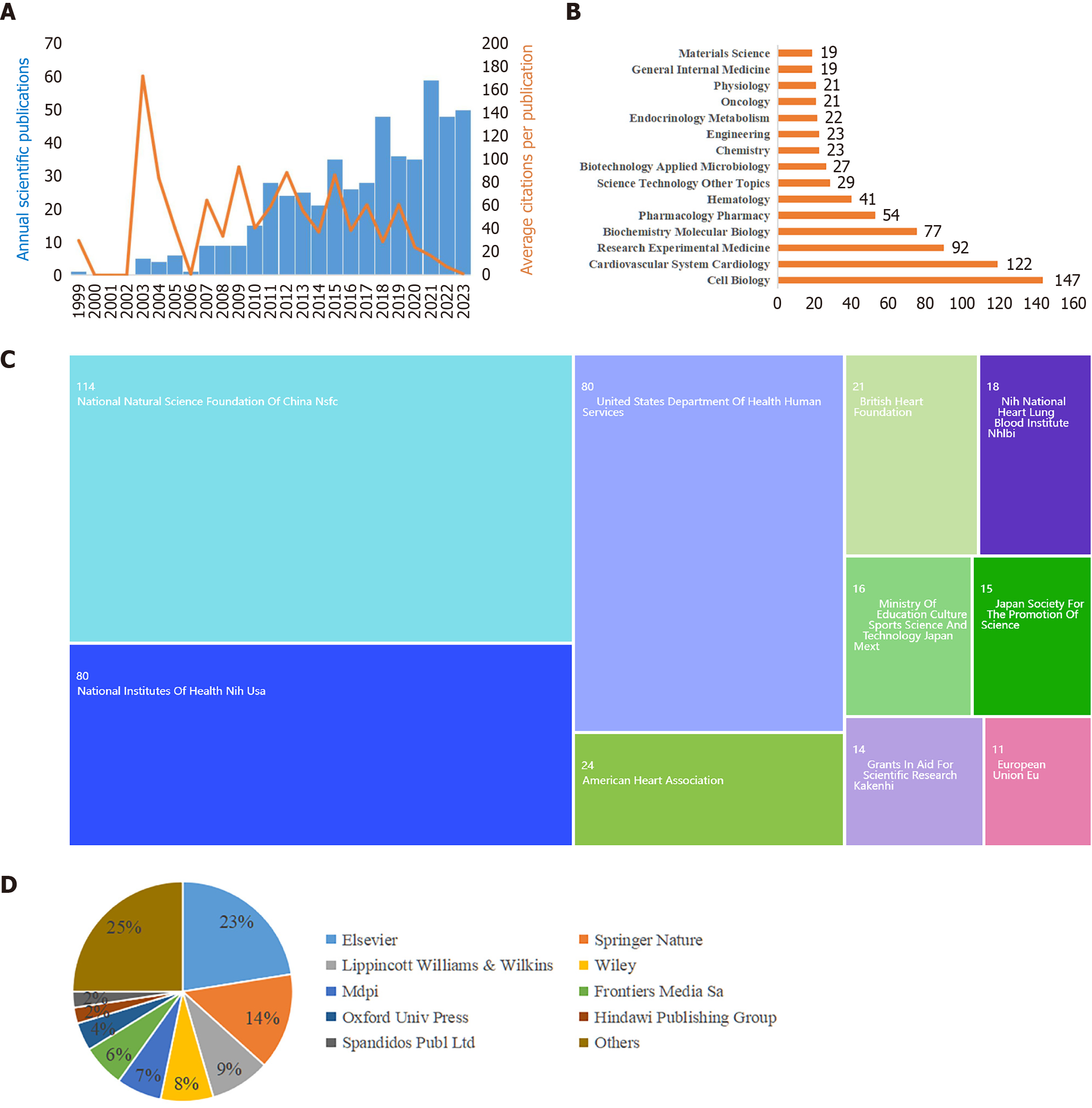
On the basis of the retrieved data, investigations on MSC therapy for AS commenced in 1999. The overall trend in the number of annual publications exhibited a consistent upward trajectory (Figure 2A). Publications peaked in 2021, with 59 articles accounting for 11.3% of the total publications. Furthermore, the mean citation count per article stands at approximately 41, resulting in an average annual citation rate of approximately 2.16. These findings highlight the growing and significant impact of research focused on MSC therapy for AS.
An analysis of the WoSCC database revealed that the 522 articles spanned 53 research areas (Figure 2B). Predominantly, the articles focused on the fields of cell biology (147, 26.44%) and cardiovascular system cardiology (122, 21.94%), with research experimental medicine (92, 16.55%) and biochemistry molecular biology (77, 13.85%). Within this dataset, 197 articles (37.74%) were classified as review articles. Regarding funding sources (Figure 2C), the National Natural Science Foundation of China (114, 20.5%) emerged as the primary funding agency. The National Institutes of Health, United States (80, 14.39%), the United States Department of Health and Human Services (80, 14.39%), and the American Heart Association (24, 4.32%) subsequently played substantial roles. Additionally, in the publishing landscape (Figure 2D), Elsevier occupied the foremost position (125, 22.48%), followed by Springer Nature (79, 14.21%) and Lippincott Williams & Wilkins (49, 8.81%).
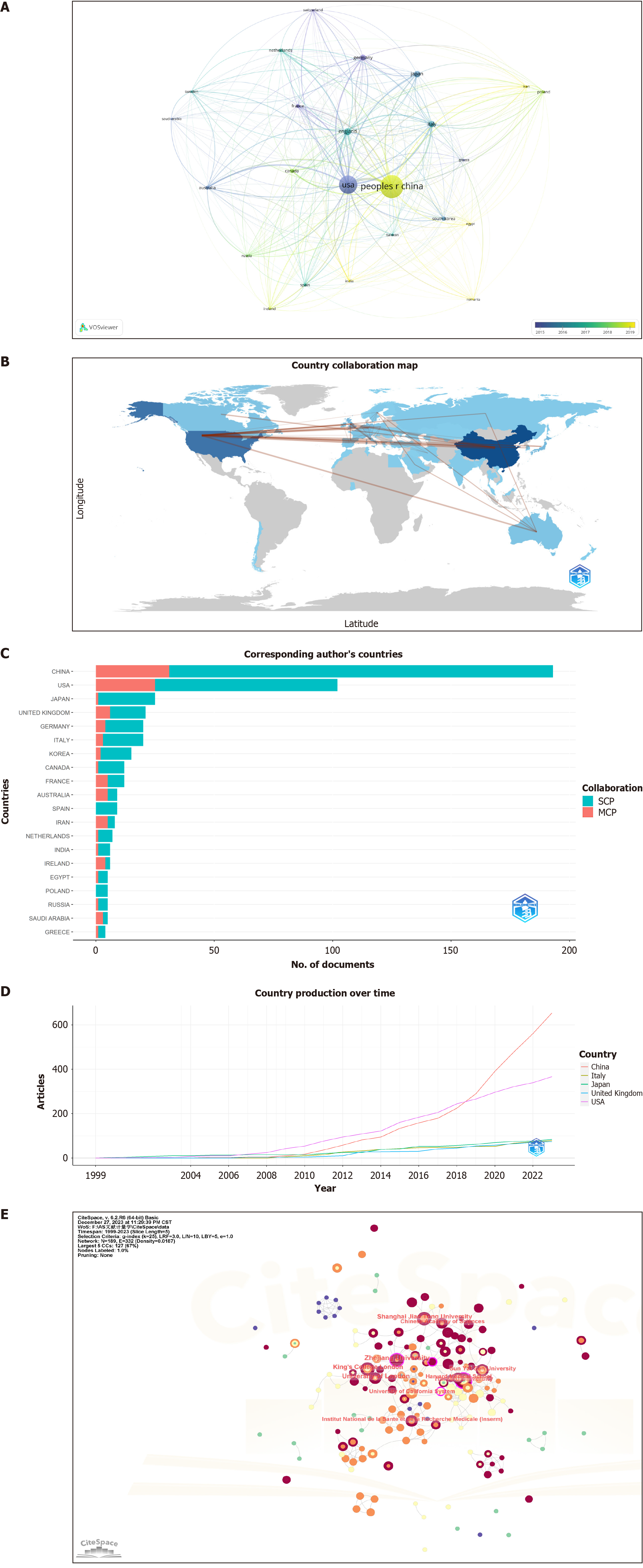
Using VOSviewer for analysis (Figure 3A, Table 1), 522 articles were identified from 52 countries/regions of origin, with 24 contributing at least five publications each. China stands out as the leading contributor in this field, with 195 articles, followed by the United States (140) and the United Kingdom (36). Among the top ten countries, China was the sole representative from the developing world. However, in terms of cumulative citations, the United States had the highest number of citations (9989), followed by China (4797). A bibliometric analysis (Figure 3B) highlighted collaborations between China and the United States, as well as China and the United Kingdom as the most prevalent. An examination of the corresponding authors revealed that China had a significant lead over other countries in both single-country and multi-country corresponding author publications (Figure 3C). Notably, since 2019, China has surpassed the United States in the number of published papers (Figure 3D).
| Rank | Country/region | Documents | Citations | Institution | Documents | Centrality |
| 1 | China | 195 | 4797 | Zhejiang University | 16 | 0.19 |
| 2 | United States | 140 | 9989 | University of London | 14 | 0.03 |
| 3 | United Kingdom | 36 | 2778 | Harvard University | 12 | 0.13 |
| 4 | Japan | 32 | 1071 | Shanghai Jiao Tong University | 11 | 0.05 |
| 5 | Italy | 31 | 1584 | King’s College London | 11 | 0.02 |
| 6 | Germany | 27 | 1251 | Sun Yat Sen University | 10 | 0.04 |
| 7 | Canada | 17 | 524 | Harvard Medical School | 9 | 0.09 |
| 8 | Korea | 17 | 231 | Institut National de la Sante et de la Recherche Medicale (Inserm) | 9 | 0.07 |
| 9 | Australia | 16 | 667 | University of California System | 9 | 0.07 |
| 10 | France | 15 | 1553 | Chinese Academy of Sciences | 9 | 0.04 |
CiteSpace analysis revealed that the 522 articles sourced from 804 institutions, with 42 institutions contributing at least five publications each (Figure 3E, Table 1). Zhejiang University occupied the top position in terms of the number of publications (16), followed by the University of London (14) and Harvard University (12). Centrality, also known as intermediary centrality, is a metric in which a higher value indicates a more active and integral role in cooperative relationships with other nodes. A centrality exceeding 0.1 is denoted by a purple circle at the node. In terms of research institution cooperation, Zhejiang University (0.19) and Harvard University (0.13) presented the highest centralities.
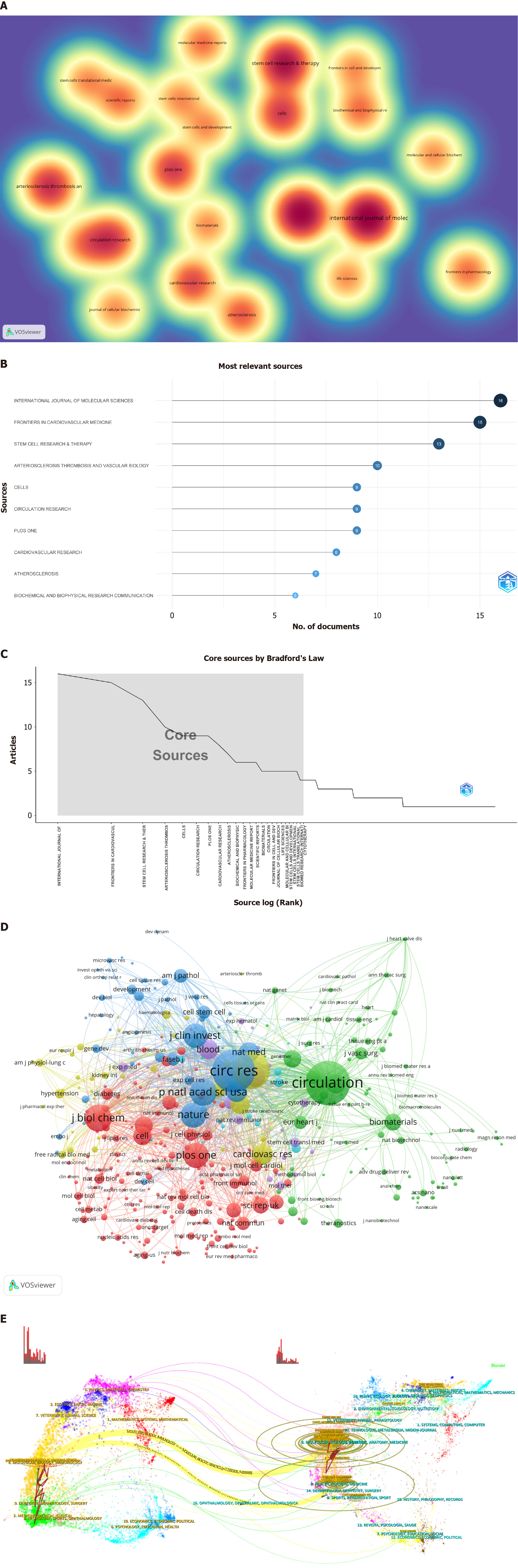
According to the VOSviewer analysis (Figure 4A), 522 of the examined articles were disseminated across 286 journals, with 22 boasting a publication volume of at least five articles each. The preeminent contributor was the International Journal of Molecular Sciences (Int J Mol Sci), with 16 articles and an impact factor (IF) of 5.6, followed by Frontiers in Cardiovascular Medicine (Front Cardiovasc Med), with 15 articles and an IF of 3.6, and Stem Cell Research & Therapy (Stem Cell Res Ther), with 13 articles and an IF of 7.5 (Figure 4B). Of the top ten journals (Table 2), nine ranked at Q2 and above. Bradford’s Law (Figure 4C) further confirmed that the core journals in this field maintained high-quality standards.
| Rank | Journal | Documents | Citations | JCR (2023) | Co-cited journal | Citations | JCR (2023) |
| 1 | Int J Mol Sci | 16 | 227 | Q1, 5.6 | Circulation | 1772 | Q1, 37.8 |
| 2 | Front Cardiovasc Med | 15 | 341 | Q2, 3.6 | Circ Res | 1690 | Q1, 20.1 |
| 3 | Stem Cell Res Ther | 13 | 288 | Q1, 7.5 | Arterioscl Throm Vas | 1347 | Q1, 8.7 |
| 4 | Arterioscl Throm Vas | 10 | 699 | Q1, 8.7 | P Natl Acad Sci Usa | 812 | Q1, 11.1 |
| 5 | Cells-Basel | 9 | 81 | Q2, 6 | J Biol Chem | 809 | Q2, 4.8 |
| 6 | Circ Res | 9 | 1348 | Q1, 20.1 | Nature | 743 | Q1, 64.8 |
| 7 | Plos One | 9 | 209 | Q2, 3.7 | Plos One | 724 | Q2, 3.7 |
| 8 | Cardiovasc Res | 8 | 347 | Q1, 10.9 | J Clin Invest | 684 | Q1, 15.9 |
| 9 | Atherosclerosis | 7 | 275 | Q2, 5.3 | Science | 530 | Q1, 56.9 |
| 10 | Biochem Bioph Res Co | 6 | 270 | Q3, 3.1 | Cardiovasc Res | 520 | Q1, 10.9 |
According to the VOSviewer analysis (Figure 4D, Table 2), there were 3880 co-cited journals and 403 with ≥ 20 citations. Circulation led the pack with 1772 citations and an IF of 37.8, followed by Circulation Research with 1690 citations and an IF of 20.1 and Arteriosclerosis, Thrombosis, and Vascular Biology with 1347 citations and an IF of 8.7. Nine of the top ten co-cited journals were positioned in the JCR Q1 category. Both Nature and Science emerged as prominent co-cited journals in the field, underscoring the high quality of the article citations.
The dual plot overlay of journals provided profound insights into topic distribution, citation trajectories, and shifts in centrality. The citing journals are on the left, while the cited journals are on the right. The curves illustrate the citation links, delineating comprehensive citation relationships. In this dual plot overlay of journals, the vertical axis of the ellipse lengthens with the increasing number of articles published by a journal, whereas the horizontal axis lengthens with the growing number of authors. As shown in Figure 4E, two principal citation pathways were evident, highlighting the frequent citation of articles published in journals related to molecular biology and genetics and those in health, nursing, and medicine by journals specializing in Molecular Biology and Immunology. This visualization also elucidated journals that publish research articles on MSC therapy for AS and the primary domains of co-cited journals.
CiteSpace analysis (Figure 5A, Table 3) revealed that 3057 authors contributed to the publication of these articles. Among them, Xu Qingbo (9) and Orekhov Alexander N (5) had the highest number of published articles. However, no author stands out with an exceptionally high publication count in this field, indicating a lack of collaboration among authors. This observation implied that the field remains in an exploratory phase.
| Rank | Author | Documents | Cited author | Documents | Centrality |
| 1 | Xu Qingbo | 9 | Libby P | 84 | 0.08 |
| 2 | Orekhov Alexander N | 5 | Ross R | 54 | 0.16 |
| 3 | Li Song | 4 | Wang Y | 49 | 0.04 |
| 4 | Li Mincai | 4 | Asahara T | 48 | 0.19 |
| 5 | Colmegna Ines | 3 | Pittenger MF | 48 | 0.04 |
| 6 | Broskey Nicholas T | 3 | Hu YH | 41 | 0.06 |
| 7 | Zhang Wei | 3 | Zhang Y | 40 | 0.05 |
| 8 | Houmard Joseph A | 3 | Li Q | 37 | 0.03 |
| 9 | Song Chunli | 3 | Liu Y | 36 | 0.1 |
| 10 | Yu Jun | 3 | Sata M | 36 | 0.07 |
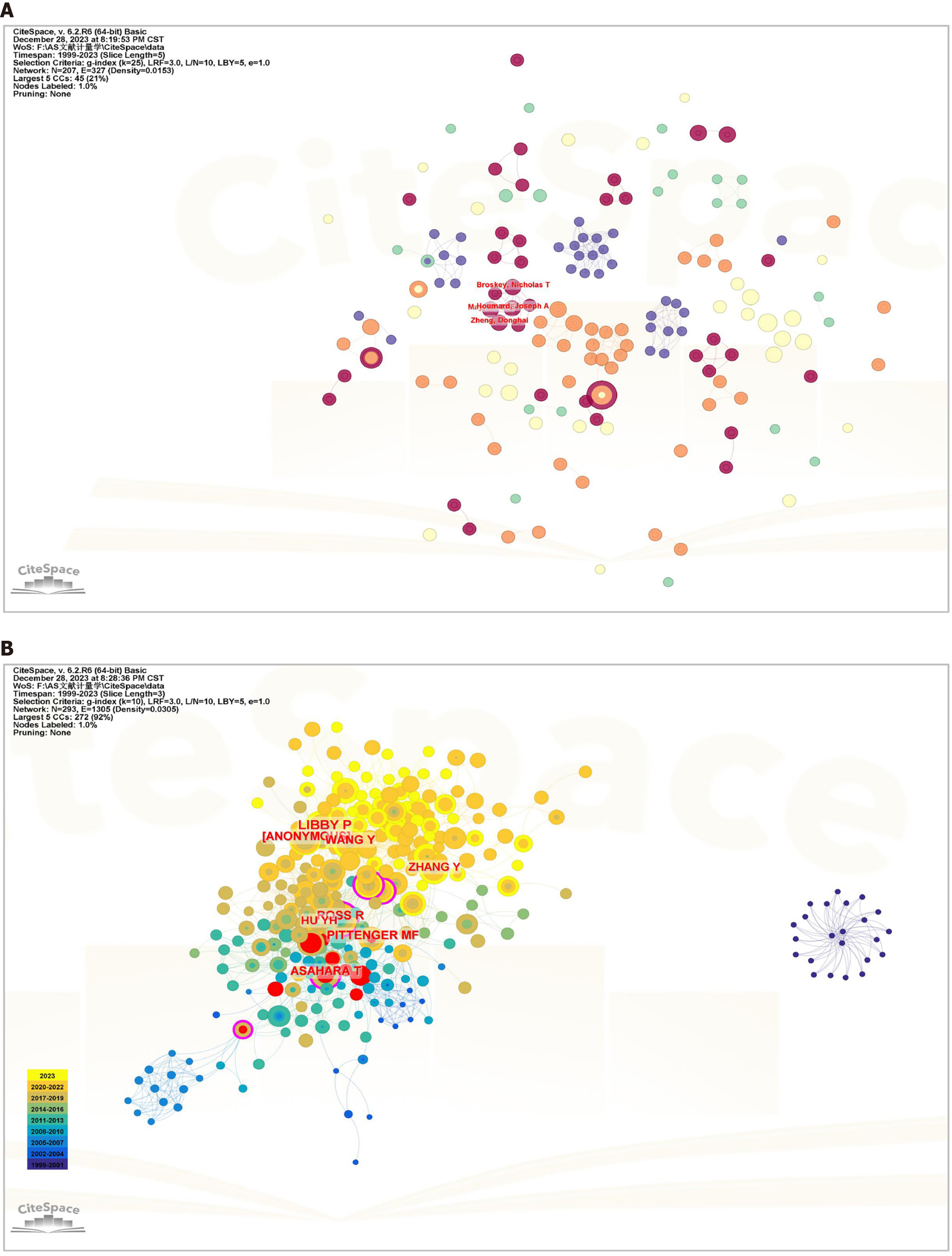
In the context of the CiteSpace analysis (Figure 5B, Table 3), a substantial network of 27637 co-cited authors emerged. The analysis of co-cited authors revealed that two articles by two authors were concurrently cited by a third article. The magnitude of co-citations indicates the proximity of academic interests among researchers and the density of research in the field. Among these authors, 89 had co-citation counts ≥ 20. The leading co-citation count was Libby P (84), followed by Ross R (54) and Wang Y (49). Asahara (0.19) had the highest centrality. Burst detection, a methodology utilized to identify abrupt changes in authors, articles, keywords, and journal citation information, can signify emerging trends and breakthroughs in a field. In this analysis, Asahara T (9.91) demonstrated the highest burst intensity, followed by Asta M (9.58) and Aicher A (7.75) (Supplementary Figure 1).
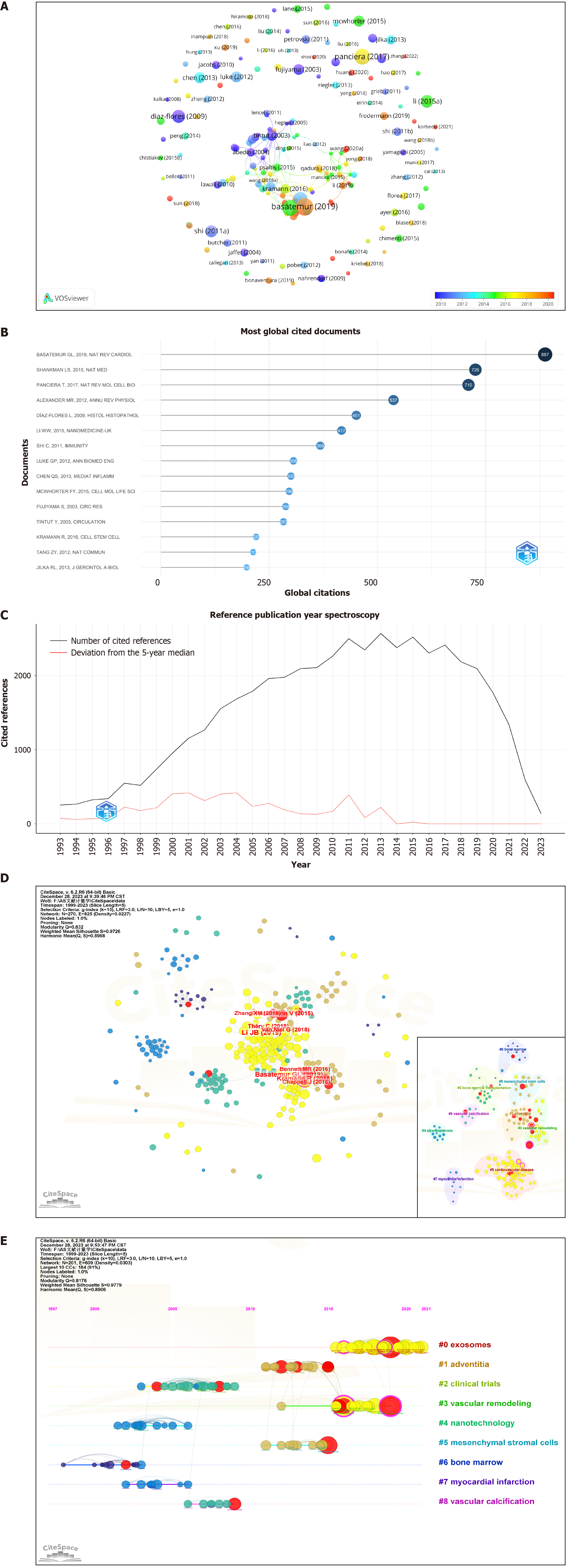
The analysis of 522 articles included a citation examination (Figure 6A and B), revealing 89 articles with ≥ 20 citations. The ten most frequently cited articles are listed in Table 4. Eight were reviews, and two were articles. The article “Vascular Smooth Muscle Cells in Atherosclerosis” in Nature Reviews Cardiology is the most preeminent. This article presents compelling evidence of vascular smooth muscle cell (VSMC) plasticity and summarizes the roles of VSMCs and VSMC-derived cells in the development and progression of atherosclerotic plaques.
| Rank | Title | Journal | Citations | Type | Year | Ref. |
| 1 | Vascular smooth muscle cells in atherosclerosis | Nat Rev Cardiol | 887 | Review | 2019 | [83] |
| 2 | KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis | Nat Med | 726 | Article | 2015 | [84] |
| 3 | Mechanobiology of YAP and TAZ in physiology and disease | Nat Rev Mol Cell Biol | 710 | Review | 2017 | [85] |
| 4 | Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease | Annu Rev Physiol | 537 | Review | 2012 | [86] |
| 5 | Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche | Histol Histopathol | 451 | Review | 2009 | [87] |
| 6 | Gold nanoparticles for photoacoustic imaging | Nanomedicine (Lond) | 417 | Review | 2015 | [88] |
| 7 | Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands | Immunity | 368 | Article | 2011 | [89] |
| 8 | Biomedical applications of photoacoustic imaging with exogenous contrast agents | Ann Biomed Eng | 306 | Review | 2012 | [90] |
| 9 | Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling | Mediators Inflamm | 300 | Review | 2013 | [91] |
| 10 | Physical and mechanical regulation of macrophage phenotype and function | Cell Mol Life Sci | 296 | Review | 2015 | [92] |
A literature co-citation relationship is established when two articles are concurrently cited by a third article. References are widely recognized as repositories of knowledge within a specific field, and the significance of a paper is frequently measured by the frequency of its citations. When Bibliometrix and CiteSpace were used for analysis (Figure 6C, Table 5), 522 articles in question collectively cited a total of 37953 articles, with 111 articles co-cited ten or more times The most frequently cited article, titled “Exosomes Derived from Mesenchymal Stem Cells Attenuate the Progression of Atherosclerosis in ApoE-/- Mice via miR-let7 Mediated Infiltration and Polarization of M2 Macrophage”, was published in Biochemical and Biophysical Research Communications. This study demonstrated that exosomes from MSCs inhibited plaque infiltration of macrophages in mice, suggesting a potential therapeutic avenue for AS treatment and reducing the clinical risk of coronary artery disease.
| Rank | Title | Journal | Citations | Type | Year | Ref. |
| 1 | Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage | Biochem Biophys Res Commun | 26 | Article | 2019 | [93] |
| 2 | Vascular smooth muscle cells in atherosclerosis | Nat Rev Cardiol | 22 | Review | 2019 | [83] |
| 3 | Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease | Cell Stem Cell | 21 | Article | 2016 | [94] |
| 4 | Mesenchymal stem cells reduce murine atherosclerosis development | Sci Rep | 16 | Article | 2015 | [65] |
| 5 | Shedding light on the cell biology of extracellular vesicles | Nat Rev Mol Cell Biol | 14 | Review | 2018 | [95] |
| 6 | Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines | J Extracell Vesicles | 14 | Guidelines | 2018 | [96] |
| 7 | Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models | Circ Res | 13 | Article | 2016 | [97] |
| 8 | Vascular smooth muscle cells in atherosclerosis | Circ Res | 13 | Review | 2016 | [98] |
| 9 | Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis | Front Immunol | 13 | Article | 2018 | [66] |
| 10 | Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction | Theranostics | 12 | Article | 2018 | [99] |
| 11 | Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness | Cardiovasc Res | 12 | Review | 2018 | [100] |
| 12 | Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice | Cardiovasc Res | 12 | Article | 2019 | [101] |
| 13 | Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19 | Cardiovasc Res | 12 | Article | 2020 | [102] |
Burst in cited literature is defined as a sudden increase in the citation count over a given period. CiteSpace analysis identified 15 articles with burst intensity data (Supplementary Figure 2). Among them, the article titled “Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development” presented the highest burst intensity of 6.48. This pioneering study revealed that MSC treatment not only modulated the inflammatory response but also substantially diminished both inflammation and dyslipidemia in mice. These findings suggest that MSCs are robust candidates for the treatment of AS.
The co-cited literature was subjected to clustering analysis via CiteSpace software (Figure 6D) to identify valuable research topics. Nine topics were delineated via the latent semantic indexing algorithm (Q = 0.8176, S = 0.9779). A Q value ranging from 0 to 1 indicates a clear cyclic structure, with a value > 0.3 denoting clarity. A S value > 0.5 suggests reason
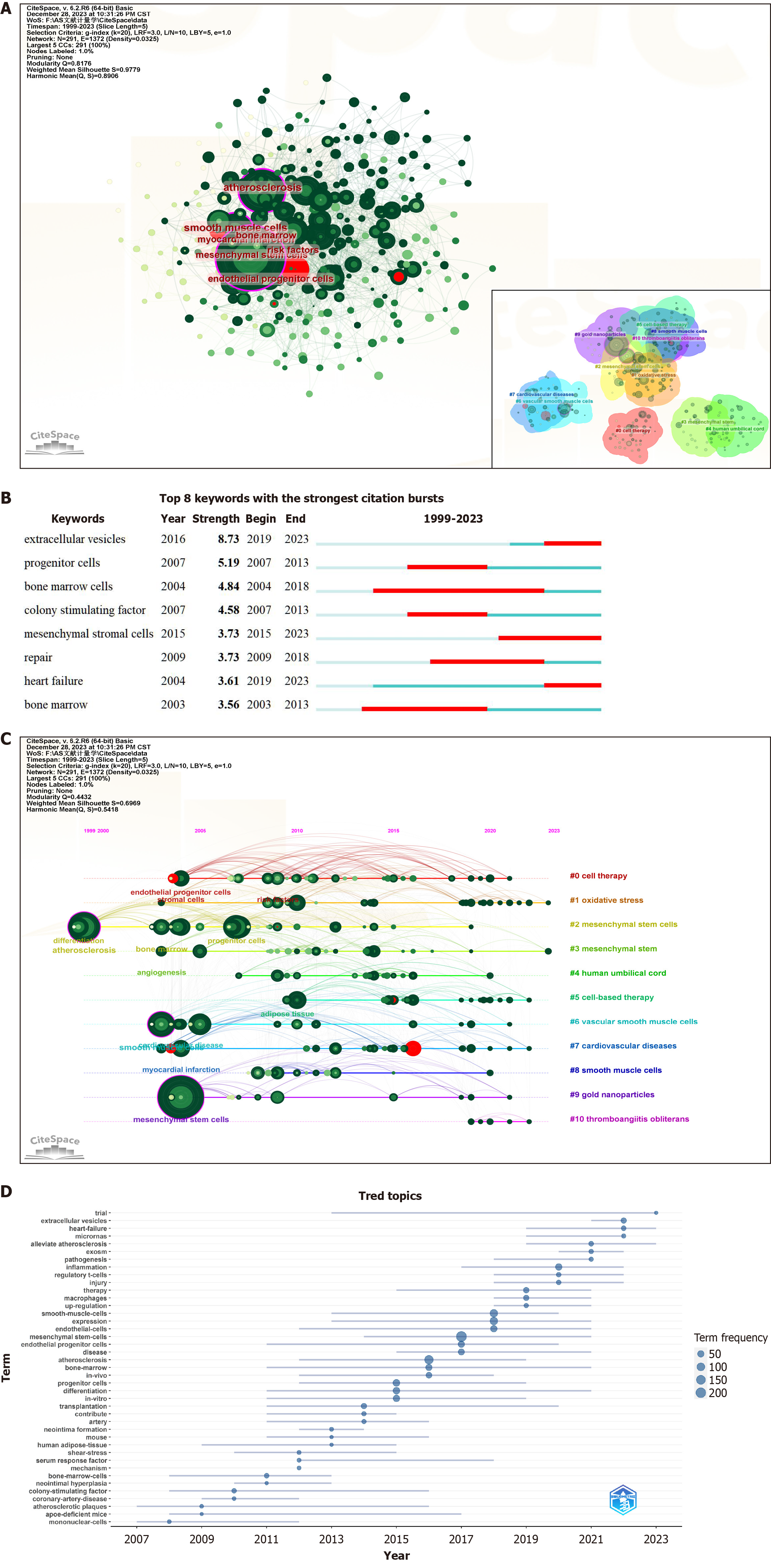
Keywords serve as crucial reflections of an article’s topic and research content. A rapid comprehension of the prevailing status and focal points of MSC therapy for AS can be achieved through mapping keyword co-occurrence. Using CiteSpace software (Figure 7A, Table 6), a comprehensive collection of 2781 keywords was extracted, with 40 exhibiting a frequency of ≥ 20. Table 6 presents the 25 keywords with the highest frequencies of occurrence. Among these cells, “mesenchymal stem cells” (260) and “atherosclerosis” (132) are predominant. After the exclusion of less meaningful keywords, the more frequently appearing terms included “smooth muscle cells”, “endothelial cells”, “myocardial infarction”, “inflammation”, “bone marrow”, “endothelial progenitor cells”, “oxidative stress”, and “extracellular vesicles”. These keywords underwent burst testing (Figure 7B), revealing that “extracellular vesicles” presented the highest burst intensity, persisting to the present day.
| Rank | Keywords | Counts | Centrality | Year |
| 1 | Mesenchymal stem cells | 260 | 0.11 | 2004 |
| 2 | Atherosclerosis | 132 | 0.1 | 1999 |
| 3 | Smooth muscle cells | 96 | 0.15 | 2003 |
| 4 | Expression | 86 | 0.04 | 2007 |
| 5 | Stem cells | 72 | 0.05 | 2004 |
| 6 | Progenitor cells | 64 | 0.06 | 2007 |
| 7 | Endothelial cells | 63 | 0.05 | 2005 |
| 8 | Differentiation | 58 | 0.06 | 1999 |
| 9 | In vitro | 52 | 0.05 | 2004 |
| 10 | Myocardial infarction | 52 | 0.06 | 2004 |
| 11 | Inflammation | 52 | 0.03 | 2010 |
| 12 | Bone marrow | 51 | 0.07 | 2003 |
| 13 | Endothelial progenitor cells | 48 | 0.05 | 2004 |
| 14 | Stromal cells | 48 | 0.1 | 2004 |
| 15 | Oxidative stress | 45 | 0.03 | 2010 |
| 16 | Proliferation | 42 | 0.04 | 2005 |
| 17 | Cardiovascular disease | 39 | 0.07 | 2004 |
| 18 | Disease | 37 | 0.01 | 2009 |
| 19 | Mechanisms | 36 | 0.05 | 2007 |
| 20 | Extracellular vesicles | 34 | 0.02 | 2016 |
| 21 | In vivo | 32 | 0.01 | 2009 |
| 22 | Vascular calcification | 31 | 0.02 | 2004 |
| 23 | Angiogenesis | 30 | 0.08 | 2003 |
| 24 | Gene expression | 28 | 0.02 | 2009 |
| 25 | Activation | 28 | 0.02 | 2012 |
The latent semantic indexing algorithm clustering of keywords through CiteSpace resulted in 11 clusters (Figure 7A), denoted as follows: #0 cell therapy; #1 oxidative stress; #2 MSCs; #3 MSCs; #4 human umbilical cord; #5 cell-based therapy; #6 VSMCs; #7 CVDs; #8 smooth muscle cells; #9 gold nanoparticles; and #10 thromboangiitis obliterans. An examination of the timeline graphs of these keyword clusters (Figure 7C and D) revealed that #1 oxidative stress and #3 MSCs are currently popular research topics.
AS is a chronic progressive vascular disease characterized by complex formation and pathogenic mechanisms. The initiation of AS is often attributed to intima-media injury, which can arise from a singular factor or a combination of factors, including hypertension, diabetes, infection, and smoking[38]. Following intimal injury, lipid components such as cholesterol and low-density lipoprotein (LDL) are deposited at the injury site, forming lipid plaques and thickening of the arterial wall[39]. Lipotoxicity can induce cellular dysfunction and cell death when lipids abnormally accumulate in nonadipose tissues[40]. Lipotoxicity can damage macrophages, leading to premature death of these phagocytes, as manifested by defective clearance of apoptotic cells[41-43]. The accumulation of residual apoptotic cells triggers chronic inflammation and leads to pathological states including AS[44,45]. Interestingly, residual apoptotic cell debris tends to be converted into lipid intermediates, further aggravating lipid deposition, forming a vicious cycle and producing an environment of persistent inflammation. Additionally, these substances undergo oxidation in the inflammatory milieu. Oxidized LDL not only damages the vascular endothelium but also triggers an immune response within the arteries. This immune response promotes monocyte migration and adhesion to endothelial cells[46], induces the production and release of proinflammatory cytokines, and induces persistent infiltration of immune cells into atherosclerotic plaques. Some monocytes further differentiate into diseased macrophages, contributing to the phagocytosis of oxidized lipids and the formation of foam cells[47-49]. To subsequently stabilize the progressively enlarging plaque, smooth muscle cells proliferate and generate collagen, forming a fibrous cap and a stiffer arterial wall. Ultimately, plaque rupture may occur in the arterial wall, leading to a serious vascular event[50,51]. AS encompasses a complex pathological process involving multiple components including intimal damage, inflammation, cholesterol deposition, fibrous tissue proliferation, and plaque rupture.
MSC transplantation technology has gradually emerged as a novel therapeutic approach. Additionally, insights from mechanobiology suggest that the therapeutic efficacy of MSCs can be further enhanced by manipulating mechanical forces at the cellular level[52]. Ongoing animal experimental studies have demonstrated that MSCs exert diverse effects, such as anti-inflammatory and lipid-lowering effects[53,54], promoting vascular repair[55], and enhancing plaque stability in an animal model of AS[56]. This innovative approach holds promise as a new avenue for AS treatment.
As mentioned above, MSC therapy has recently emerged as a novel treatment for AS. This study is the first bibliometric analysis of global studies on using MSC therapy for AS. We reviewed the literature on MSC therapy for AS in the last 20 years. China and the United States have greatly contributed to this field’s research. Libby P’s work is most prominent in terms of influence and citation, with Int J Mol Sci having a greater impact. Research hotspots have focused on vascular progenitor cells, inflammatory mechanisms and extracellular vesicles, and emerging research frontiers, such as cell vesicles and oxidative stress, have gradually attracted increasing attention. These findings provide researchers with a systematic and intuitive overview of general trends in the field.
Regulation of vascular endothelial cells by MSCs: Bone marrow MSCs have the potential to regulate vascular endothelial progenitor cell homing repair or directly influence endothelial cell function. Transplantation of bone marrow MSCs has been shown to significantly increase the proliferation and migration of functional endothelial progenitor cells, thereby improving overall endothelial function[57-59]. Vascular endothelial cells, positioned as barriers between the vessel wall and bloodstream, play pivotal roles in maintaining vascular tone, regulating permeability, and engaging in biological responses, such as inflammation and immunity[59-61]. Additionally, coculturing bone marrow MSCs with ox-LDL-stimulated endothelial cells activates the endothelial nitric oxide synthase system, promoting nitric oxide production by increasing interleukin-8 (IL-8) and macrophage inflammatory protein-2 expression, thereby enhancing endothelial cell function[62]. Furthermore, bone marrow MSCs can activate the β-catenin-mediated Wnt signaling pathway through the secretion of Wnt proteins, subsequently reducing endothelial cell apoptosis by mitigating oxidative stress[63,64].
Lipid-lowering effects of MSCs: MSC transplantation effectively ameliorates dyslipidemia in a mouse model of AS[56]. Frodermann et al[65] conducted a study involving the transplantation of bone marrow MSCs into a constructed mouse model lacking the LDL receptor, significantly reducing serum cholesterol and LDL levels after four weeks. Zhang et al[66] transplanted gingival MSCs into ApoE-/- mice and reported decreased serum cholesterol and LDL levels. This lipid-lowering effect of MSCs may be attributed to the downregulation of the scavenger receptors SR-A1 and CD36, coupled with the upregulation of the cholesterol transporter-associated protein ATP-binding cassette transporter A1[66].
Impact of MSCs on inflammation and immunomodulation: In their study, Frodermann et al[65] established the pivotal role of MSC transplantation in inflammation and immunomodulation. MSCs can suppress proinflammatory factors, including tumor necrosis factor-α and IL-6[67], reducing chemokine C-C motif ligand 2 levels and circulating monocyte counts. Concurrently, MSCs increase the levels of anti-inflammatory cytokines such as transforming growth factor-β, IL-1, and IL-10[68]. In vitro investigations have demonstrated that MSCs sourced from diverse tissues (e.g., human gingiva and cardiac adipose tissue) can facilitate the reprogramming of macrophages from a proinflammatory phenotype (M1) to an anti-inflammatory phenotype (M2)[69-71]. The anti-inflammatory efficacy of adipose-derived MSCs has been attributed to the paracrine release of immunomodulatory factors[72].
T lymphocytes, which are pivotal in mediating the adaptive immune response in AS, are modulated by bone marrow MSCs. This modulation promotes the establishment of a favorable immune composition of T cells within atherosclerotic plaques, thereby stabilizing the plaques and reducing the risk of rupture[64]. Notably, regulatory T cells (Tregs) play essential roles in maintaining immune tolerance and homeostasis, as evidenced in several studies[73-75]. Bone marrow MSC transplantation has been shown to increase the production of IL-10, transforming growth factor-β, and prostaglandin E2 in atherosclerotic mice[76]. This intervention upregulates Foxp3 on the surface of CD4+ T lymphocytes, fostering Treg differentiation, increasing the proportion of Treg cells, and diminishing the ability of effector T cells to produce inflammatory cytokines in mice[77]. Additionally, in vitro studies have revealed that bone marrow MSCs inhibit T-cell proliferation by disrupting the cell cycle, suppressing the activation and proliferation of proinflammatory T helper type 1 and T helper type 17 cells and enhancing Treg activity[68], thereby establishing a favorable immune distribution in plaques and mitigating inflammatory responses.
Modulation of plaque stability by MSCs: The effect of MSCs on the stability of AS plaques has been thoroughly investigated. In a study conducted by Wang et al[67], bone marrow MSC transplantation in a rabbit AS model revealed a noteworthy reduction in inflammatory factors within plaques following a single MSC infusion. Furthermore, after four weeks of MSC treatment, the expression levels of nuclear factor-kappaB and matrix metalloproteinases (MMPs) were significantly diminished in plaques. MMPs, particularly MMP2 and MMP9, play crucial roles in late-stage AS by contributing to plaque formation through matrix degradation and rupture. Histological examination further demonstrated an increased presence of neoplastic endothelial cells, collagen fibers, and BrdU-positive cells within atherosclerotic plaques. Moreover, MSC microvesicles enhance plaque stability by suppressing the NLR family pyrin domain containing 3-mediated macrophage apoptosis[78].
Investigations into stem cell therapy for atherosclerotic diseases have focused predominantly on bone marrow MSCs. These cells are widely accessible and amenable to isolation and cultivation. However, understanding the various factors influencing the biological function of bone marrow MSCs remains an ongoing endeavor that requires further elucidation. Comparative studies evaluating bone marrow MSCs from different sources with variations in their immune and inflammatory regulatory capacities are essential for their application in AS treatment. Additionally, while recent studies have underscored the pivotal paracrine role of bone marrow MSCs[79,80], research on specific molecules such as peptides, small noncoding RNAs, or other bioactive products is lacking, indicating a potential avenue for novel therapeutic approaches.
Despite notable advancements in applying MSC therapies to treat atherosclerotic vascular disease, much of the research remains in the early clinical trial stages[81,82], and significant challenges persist in achieving clinical translation. Primarily, the inherent limitations of animal models in fully mirroring the human condition cast uncertainty on the translational efficacy of stem cell-based therapies on the basis solely of animal findings. Furthermore, the clinical effectiveness of these therapies is contingent on several factors, including safety, cell viability, cell source, administration frequency, and delivery route. While current research has predominantly emphasized the potential benefits, aspects such as safety, side effects, and long-term therapeutic viability have received comparatively less attention. Consequently, further investigation is imperative to advance the clinical implementation of this promising technology.
Nevertheless, there are certain limitations associated with this study. First, owing to the limitations of CiteSpace software, the literature search scope of this study was limited to the WoSCC database. This may have resulted in omitting some articles, causing potential selection bias and analysis error. Additionally, we included only papers written in English and excluded a small number of papers published in other languages. Finally, the dynamic nature of new research means that influential and recently published studies may not have been fully considered. In future research, we will further analyze and interpret the data by using other tools, supplementing new studies, etc.
MSCs have important research value and wide application prospects in the field of atherosclerotic diseases. With the help of bibliometric and visual analyses, we identified global research trends in MSC therapy for atherosclerotic vascular disease over the past two decades. Our comprehensive review helps researchers identify key authors, journals, and potential collaborators, offering insights into research hotspots. Notably, the keywords and locality clustering analysis highlighted pivotal research frontiers, particularly emphasizing “oxidative stress” and “extracellular vesicles”. Analyzing these results will help researchers understand the current state of research and provide promising directions for future research to ultimately achieve meaningful clinical outcomes. Future research should pay more attention to the different aspects of MSC therapy for AS, aiming to achieve early clinical conversion, improve the quality of life and prolong the survival time of patients.
| 1. | Kwan TW, Wong SS, Hong Y, Kanaya AM, Khan SS, Hayman LL, Shah SH, Welty FK, Deedwania PC, Khaliq A, Palaniappan LP; American Heart Association Council on Epidemiology and Prevention; Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Genomic and Precision Medicine. Epidemiology of Diabetes and Atherosclerotic Cardiovascular Disease Among Asian American Adults: Implications, Management, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2023;148:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 2. | Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 852] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 3. | Chinese Society of Cardiology of Chinese Medical Association; Cardiovascular Disease Prevention and Rehabilitation Committee of Chinese Association of Rehabilitation Medicine; Cardiovascular Disease Committee of Chinese Association of Gerontology and Geriatrics; Thrombosis Prevention and Treatment Committee of Chinese Medical Doctor Association. [Chinese guideline on the primary prevention of cardiovascular diseases]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:1000-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Yang X, Li J, Hu D, Chen J, Li Y, Huang J, Liu X, Liu F, Cao J, Shen C, Yu L, Lu F, Wu X, Zhao L, Wu X, Gu D. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation. 2016;134:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 422] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 5. | Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 1910] [Article Influence: 318.3] [Reference Citation Analysis (0)] |
| 6. | Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1608-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 7. | Peloso GM, Natarajan P. Insights from population-based analyses of plasma lipids across the allele frequency spectrum. Curr Opin Genet Dev. 2018;50:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Liang J, Li W, Liu H, Li X, Yuan C, Zou W, Qu L. Di'ao Xinxuekang Capsule Improves the Anti-Atherosclerotic Effect of Atorvastatin by Downregulating the SREBP2/PCSK9 Signalling Pathway. Front Pharmacol. 2022;13:857092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Strandberg TE. Role of Statin Therapy in Primary Prevention of Cardiovascular Disease in Elderly Patients. Curr Atheroscler Rep. 2019;21:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Yeoh A, Cheung R, Ahmed A, Chitnis AS, Do A, Wong RJ. Cardiovascular Disease Risk and Statin Use Among Adults with Metabolic Dysfunction Associated Fatty Liver Disease. Am J Med. 2023;136:669-676.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Lee SH, Lee YJ, Heo JH, Hur SH, Choi HH, Kim KJ, Kim JH, Park KH, Lee JH, Choi YJ, Lee SJ, Hong SJ, Ahn CM, Kim BK, Ko YG, Choi D, Hong MK, Jang Y, Kim JS. Combination Moderate-Intensity Statin and Ezetimibe Therapy for Elderly Patients With Atherosclerosis. J Am Coll Cardiol. 2023;81:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Prince M, Tafur JD, White CJ. When and How Should We Revascularize Patients With Atherosclerotic Renal Artery Stenosis? JACC Cardiovasc Interv. 2019;12:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Banerjee S, Sarode K, Mohammad A, Brilakis ES. Drug-coated balloon and stent therapies for endovascular treatment of atherosclerotic superficial femoral artery disease. Curr Cardiol Rep. 2015;17:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, Ma TL. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front Cell Dev Biol. 2022;10:812262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Duan Y, Lyu L, Zhan S. Stem Cell Therapy for Alzheimer's Disease: A Scoping Review for 2017-2022. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Lee LX, Li SC. Hunting down the dominating subclone of cancer stem cells as a potential new therapeutic target in multiple myeloma: An artificial intelligence perspective. World J Stem Cells. 2020;12:706-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 17. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 18. | Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, Mao N. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 20. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12678] [Article Influence: 704.3] [Reference Citation Analysis (2)] |
| 21. | Aziz NS, Yusop N, Ahmad A. Importance of Stem Cell Migration and Angiogenesis Study for Regenerative Cell-based Therapy: A Review. Curr Stem Cell Res Ther. 2020;15:284-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310:H455-H465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Jang S, Collin de l'Hortet A, Soto-Gutierrez A. Induced Pluripotent Stem Cell-Derived Endothelial Cells: Overview, Current Advances, Applications, and Future Directions. Am J Pathol. 2019;189:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Wang X, Wang R, Jiang L, Xu Q, Guo X. Endothelial repair by stem and progenitor cells. J Mol Cell Cardiol. 2022;163:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Abdolmohammadi K, Mahmoudi T, Alimohammadi M, Tahmasebi S, Zavvar M, Hashemi SM. Mesenchymal stem cell-based therapy as a new therapeutic approach for acute inflammation. Life Sci. 2023;312:121206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Cheng X, Han X, Cheng X, Jiang S, Lin Y, Zhang Z, Lu L, Qu B, Chen Y, Zhang X. Global research landscape and trends of lung cancer immunotherapy: A bibliometric analysis. Front Immunol. 2022;13:1032747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Wu K, Liu Y, Liu L, Peng Y, Pang H, Sun X, Xia D. Emerging Trends and Research Foci in Tumor Microenvironment of Pancreatic Cancer: A Bibliometric and Visualized Study. Front Oncol. 2022;12:810774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Ismail II, Saqr M. A Quantitative Synthesis of Eight Decades of Global Multiple Sclerosis Research Using Bibliometrics. Front Neurol. 2022;13:845539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Zhang N, Li C, Chen J, Liu X, Wang Z, Ni J. Research hotspots and frontiers about role of visual perception in stroke: A bibliometric study. Front Neurol. 2022;13:958875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Yang Z, Fan Z, Wang D, Li H, He Z, Xing D, Lin J. Bibliometric and visualization analysis of stem cell therapy for meniscal regeneration from 2012 to 2022. Front Bioeng Biotechnol. 2023;11:1107209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Qin YF, Ren SH, Shao B, Qin H, Wang HD, Li GM, Zhu YL, Sun CL, Li C, Zhang JY, Wang H. The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front Immunol. 2022;13:931783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 33. | Liu X, Zhao S, Tan L, Tan Y, Wang Y, Ye Z, Hou C, Xu Y, Liu S, Wang G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron. 2022;201:113932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 34. | Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. 2006;57:359-377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2057] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 35. | Zhang Q, Zeng Y, Zheng S, Chen L, Liu H, Chen H, Zhang X, Zou J, Zheng X, Wan Y, Huang G, Zeng Q. Research hotspots and frotiers of stem cells in stroke: A bibliometric analysis from 2004 to 2022. Front Pharmacol. 2023;14:1111815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2268] [Article Influence: 283.5] [Reference Citation Analysis (0)] |
| 37. | Tang R, Zhang S, Ding C, Zhu M, Gao Y. Artificial Intelligence in Intensive Care Medicine: Bibliometric Analysis. J Med Internet Res. 2022;24:e42185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 38. | Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 1000] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 39. | Singh S, Siva BV, Ravichandiran V. Advanced Glycation End Products: key player of the pathogenesis of atherosclerosis. Glycoconj J. 2022;39:547-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 40. | Schaffer JE. Lipotoxicity: Many Roads to Cell Dysfunction and Cell Death: Introduction to a Thematic Review Series. J Lipid Res. 2016;57:1327-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Gao M, Tang M, Ho W, Teng Y, Chen Q, Bu L, Xu X, Zhang XQ. Modulating Plaque Inflammation via Targeted mRNA Nanoparticles for the Treatment of Atherosclerosis. ACS Nano. 2023;17:17721-17739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 42. | Bock FJ, Riley JS. When cell death goes wrong: inflammatory outcomes of failed apoptosis and mitotic cell death. Cell Death Differ. 2023;30:293-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 43. | Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50 Suppl:S382-S387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 44. | Hayden MR, Tyagi SC. Intimal redox stress: accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Atheroscleropathy. Cardiovasc Diabetol. 2002;1:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Kojima Y, Weissman IL, Leeper NJ. The Role of Efferocytosis in Atherosclerosis. Circulation. 2017;135:476-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 46. | Nasser MI, Zhu S, Huang H, Zhao M, Wang B, Ping H, Geng Q, Zhu P. Macrophages: First guards in the prevention of cardiovascular diseases. Life Sci. 2020;250:117559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol. 2022;22:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 295] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 48. | Ilatovskaya DV, Halade GV, DeLeon-Pennell KY. Adaptive immunity-driven inflammation and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2019;317:H1254-H1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 50. | Yurdagul A Jr. Crosstalk Between Macrophages and Vascular Smooth Muscle Cells in Atherosclerotic Plaque Stability. Arterioscler Thromb Vasc Biol. 2022;42:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 51. | Abela OG, Ahsan CH, Alreefi F, Salehi N, Baig I, Janoudi A, Abela GS. Plaque Rupture and Thrombosis: the Value of the Atherosclerotic Rabbit Model in Defining the Mechanism. Curr Atheroscler Rep. 2016;18:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Tassinari R, Olivi E, Cavallini C, Taglioli V, Zannini C, Marcuzzi M, Fedchenko O, Ventura C. Mechanobiology: A landscape for reinterpreting stem cell heterogeneity and regenerative potential in diseased tissues. iScience. 2023;26:105875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Li Q, Sun W, Wang X, Zhang K, Xi W, Gao P. Skin-Derived Mesenchymal Stem Cells Alleviate Atherosclerosis via Modulating Macrophage Function. Stem Cells Transl Med. 2015;4:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Ma J, Chen L, Zhu X, Li Q, Hu L, Li H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2021;53:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 55. | Tao X, Sun M, Chen M, Ying R, Su W, Zhang J, Xie X, Wei W, Meng X. HMGB1-modified mesenchymal stem cells attenuate radiation-induced vascular injury possibly via their high motility and facilitation of endothelial differentiation. Stem Cell Res Ther. 2019;10:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Yao G, Qi J, Li X, Tang X, Li W, Chen W, Xia N, Wang S, Sun L. Mesenchymal stem cell transplantation alleviated atherosclerosis in systemic lupus erythematosus through reducing MDSCs. Stem Cell Res Ther. 2022;13:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 57. | Li Z, Yang A, Yin X, Dong S, Luo F, Dou C, Lan X, Xie Z, Hou T, Xu J, Xing J. Mesenchymal stem cells promote endothelial progenitor cell migration, vascularization, and bone repair in tissue-engineered constructs via activating CXCR2-Src-PKL/Vav2-Rac1. FASEB J. 2018;32:2197-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2:467-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Shao Y, Saredy J, Yang WY, Sun Y, Lu Y, Saaoud F, Drummer C 4th, Johnson C, Xu K, Jiang X, Wang H, Yang X. Vascular Endothelial Cells and Innate Immunity. Arterioscler Thromb Vasc Biol. 2020;40:e138-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 60. | Yang Y, Wang D, Zhang C, Yang W, Li C, Gao Z, Pei K, Li Y. Piezo1 mediates endothelial atherogenic inflammatory responses via regulation of YAP/TAZ activation. Hum Cell. 2022;35:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Joffre J, Hellman J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid Redox Signal. 2021;35:1291-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 62. | Lin YL, Yet SF, Hsu YT, Wang GJ, Hung SC. Mesenchymal Stem Cells Ameliorate Atherosclerotic Lesions via Restoring Endothelial Function. Stem Cells Transl Med. 2015;4:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Wang L, Qing L, Liu H, Liu N, Qiao J, Cui C, He T, Zhao R, Liu F, Yan F, Wang C, Liang K, Guo X, Shen YH, Hou X, Chen L. Mesenchymal stromal cells ameliorate oxidative stress-induced islet endothelium apoptosis and functional impairment via Wnt4-β-catenin signaling. Stem Cell Res Ther. 2017;8:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Lin Y, Zhu W, Chen X. The involving progress of MSCs based therapy in atherosclerosis. Stem Cell Res Ther. 2020;11:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Frodermann V, van Duijn J, van Pel M, van Santbrink PJ, Bot I, Kuiper J, de Jager SC. Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development. Sci Rep. 2015;5:15559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, Su W, Qian X, Olsen N, Zheng SG. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front Immunol. 2018;9:878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 67. | Wang SS, Hu SW, Zhang QH, Xia AX, Jiang ZX, Chen XM. Mesenchymal Stem Cells Stabilize Atherosclerotic Vulnerable Plaque by Anti-Inflammatory Properties. PLoS One. 2015;10:e0136026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Mu Y, Xu W, Liu J, Wang Y, Chen J, Zhou Q. Mesenchymal stem cells moderate experimental autoimmune uveitis by dynamic regulating Th17 and Breg cells response. J Tissue Eng Regen Med. 2022;16:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 70. | Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 281] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 71. | Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, Yamato H, Yotsumoto K, Tanaka U, Taketomi T, Uchiumi T, Le AD, Shi S, Nishimura F. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 72. | Mikłosz A, Chabowski A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J Clin Endocrinol Metab. 2023;108:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 73. | Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015;35:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 74. | Rohm I, Atiskova Y, Drobnik S, Fritzenwanger M, Kretzschmar D, Pistulli R, Zanow J, Krönert T, Mall G, Figulla HR, Yilmaz A. Decreased regulatory T cells in vulnerable atherosclerotic lesions: imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediators Inflamm. 2015;2015:364710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Ding JW, Zheng XX, Zhou T, Tong XH, Luo CY, Wang XA. HMGB1Modulates the Treg/Th17 Ratio in Atherosclerotic Patients. J Atheroscler Thromb. 2016;23:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Zhang D, Lin Y, Li Y, Zhao D, Du M. Mesenchymal stem cells enhance Treg immunosuppressive function at the fetal-maternal interface. J Reprod Immunol. 2021;148:103366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Khosravi M, Bidmeshkipour A, Cohen JL, Moravej A, Hojjat-Assari S, Naserian S, Karimi MH. Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells by mesenchymal stem cells is associated with modulation of ubiquitination factors and TSDR demethylation. Stem Cell Res Ther. 2018;9:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Lin Y, Liu M, Chen E, Jiang W, Shi W, Wang Z. Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis. Cell Biol Int. 2021;45:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Xiao X, Xu M, Yu H, Wang L, Li X, Rak J, Wang S, Zhao RC. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther. 2021;6:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 80. | Guo Z, Zhao Z, Yang C, Song C. Transfer of microRNA-221 from mesenchymal stem cell-derived extracellular vesicles inhibits atherosclerotic plaque formation. Transl Res. 2020;226:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Gupta PK, Shivashankar P, Rajkumar M, Mahapatra SS, Desai SC, Dhar A, Krishna V, Raviraja NS, Bhat S, Viswanathan P, Kannan S, Abraham J, Boggarapu H, Manjuprasad MS, Udaykumar K. Label extension, single-arm, phase III study shows efficacy and safety of stempeucel® in patients with critical limb ischemia due to atherosclerotic peripheral arterial disease. Stem Cell Res Ther. 2023;14:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 82. | Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, Textor SC. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J Am Soc Nephrol. 2017;28:2777-2785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 83. | Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 717] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 84. | Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 913] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 85. | Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 944] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 86. | Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 582] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 87. | Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 247] [Reference Citation Analysis (0)] |
| 88. | Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond). 2015;10:299-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 89. | Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 90. | Luke GP, Yeager D, Emelianov SY. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann Biomed Eng. 2012;40:422-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 91. | Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 305] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 92. | McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72:1303-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 93. | Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, Qiu W, Gao X, Qian M, Xu J, Wang Z, Li G. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 94. | Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell. 2016;19:628-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 95. | van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3060] [Cited by in RCA: 5611] [Article Influence: 801.6] [Reference Citation Analysis (0)] |
| 96. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7682] [Article Influence: 1097.4] [Reference Citation Analysis (1)] |
| 97. | Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res. 2016;119:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 98. | Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1592] [Article Influence: 176.9] [Reference Citation Analysis (0)] |
| 99. | Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-6177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 402] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 100. | Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 743] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 101. | Takafuji Y, Hori M, Mizuno T, Harada-Shiba M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice. Cardiovasc Res. 2019;115:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 102. | Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Cheng K, Qian L, Yang Y. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |